Abstract
The ubiquitin/proteasome pathway plays critical roles in virtually all aspects of cell biology. Enzymes of the ubiquitin pathway add (ligases) or remove (deubiquitinases) ubiquitin tags to or from their target proteins in a selective fashion. USP2a is a member of a subfamily of deubiquitinases, called ubiquitin-specific cysteine proteases (USPs). Although USP2a has been reported to be a bona fide oncogene that regulates the stability of MDM2, MDMX, and FAS, it is likely that there are other unidentified substrates for USP2a. In this study, we show that USP2a mediates mitotic progression by regulating the stability of Aurora-A. Through cell-based screening of a USP siRNA library, we discovered that knockdown of USP2a reduced the protein levels of Aurora-A. USP2a interacts with Aurora-A directly in vitro and in vivo. In addition, Aurora-A is a substrate for USP2a in vitro and in vivo. Our study provides a novel mechanism for the role of USP2a in mediating the stability of Aurora-A.
Keywords: Cell Cycle, Deubiquitination, Protein Degradation, Protein-Protein Interactions, siRNA, Aurora-A, USP2a, Ubiquitination
Introduction
The posttranslational modification of proteins through ubiquitination/deubiquitination plays critical roles in virtually every aspect of cell biology (1, 2). Ubiquitin tags are added to (by E3 ligases) or removed from (by deubiquitinases) their target proteins in a selective fashion (1). At least 530 human genes have been identified to encode enzymes involved in the conjugation and deconjugation of ubiquitin (3, 4). Of these genes, 95 are thought to encode functional deubiquitinases (Dub) (4). The ubiquitin-specific proteases (USPs)2 are cysteine proteases that are part of the Dub family. 58 putative USPs have been identified in the human genome to date (4). Despite the large number of USPs identified, relatively little is known about their physiological roles or their substrates (4). USP2a is a member of the USP family that was originally identified as an inducible USP enzyme in rat testis (5). Recent data indicate that aberrant USP2a activity is associated with the formation and progression of human tumors and that USP2a is overexpressed in >40% of prostate tumors (6). In addition, USP2a is a bona fide oncogene as demonstrated by its ability to transform NIH-3T3 cells (6).
USP2a interacts with and stabilizes MDM2, MDMX, and fatty acid synthase (FAS) (7–9). MDM2 is an E3 ligase that regulates the protein level of p53 (10). p53 is a protein with pleiotropic function that plays critical roles in preserving the integrity of the genome and is the most frequently mutated protein in human cancer (11). FAS, a key metabolic enzyme that catalyzes the synthesis of long chain fatty acids, is highly expressed in a variety of human cancers, including cancers from breast, colon, ovary, lung, and prostate, suggesting that FAS may also play a role in tumorigenesis (12, 13).
Aurora kinases are serine/threonine kinases that regulate mitotic events, including centrosome maturation, mitotic spindle formation, and chromosome segregation to cytokinesis (14–17). The three members of the Aurora kinase family in metazoans share extensive structure and sequence similarities (14–16). However, they show distinct localizations and functions during mitosis. Aurora-A localizes to centrosomes and is essential for centrosome duplication and maturation (14–17). Overexpression of Aurora-A leads to genomic instability and neoplastic transformation (18–20). Cells depleted of Aurora-A by siRNA arrest at mitosis (21, 22) and display a G2 delay in synchronized cells (22). Aurora-B is localized to centromeres in early mitosis, relocates to the central spindle in anaphase and the spindle midzone during telephase, and finally migrates to the midbody during cytokinesis (14–16, 23). Aurora-B functions as a chromosome passenger protein involved in chromosome condensation, kinetochore-microtubule attachment, chromosome alignment in metaphase, and midbody function during cytokinesis (14, 15). Like Aurora-A, Aurora-C is also associated with the centrosomes, but its function in mitosis is not well defined (15, 16).
Although USP2a has been reported to play important roles in tumorigenesis by regulating the stability of MDM2 and FAS, it is likely that there are other unidentified substrates for USP2a. In this study, we show that Aurora-A is a substrate for USP2a and USP2a mediates the stability of Aurora-A.
MATERIALS AND METHODS
Chemicals
All chemicals were from Sigma. Protein concentration was determined using the BCA method (Pierce).
Cell Lines
MIA PaCa-2, 786-O, MDA-MB-231, HeLa, HCT116, and H1299 cells were obtained from the American Type Culture Collection (Rockville, MD).
Plasmids and Proteins
cDNA for USP2a in pCMV-xl6 was obtained from Origene Technologies (Rockville, MD), which was used as a template to make pcDNA3-USP2a. pcDNA3-USP2a-res was generated to be resistant to USP2a siRNA#1 using the QuikChange site-directed mutagenesis kit by changing the third base in every codon in the siRNA sequence (Stratagene). For expression in bacteria, USP2a cDNA encoding amino acids 258–605 was cloned into the pET29A vector. The USP2a-C276A mutation was generated using the QuikChange mutagenesis kit. Aurora-A with a C-terminal Myc tag, Aurora-B with a C-terminal Myc tag, MDM2 with a C-terminal Myc tag, and USP7 with a C-terminal Myc-tag were cloned into the pcDNA3 vector. Ubiquitin with an N-terminal His tag was cloned into the pcDNA3 vector. Full-length Aurora-A and Aurora-B with His tag were obtained from Invitrogen.
Branched DNA Signal Amplification Assay
QuantiGene Plex 2.0 Assay kit, Plex Set, and Handheld Magnetic Washer were obtained from Panomics/Affymetrix (Santa Clara, CA). The Plex Set contains capture beads and a set of probes for targets including AURKA, AURKB, USP2, USP20, MDM2, and ACTB. The VorTemp56 incubator shaker was purchased from Midwest Scientific (St. Louis, MO). The branched DNA assay was carried out according to instructions provided by the vendor. Briefly, cells were lysed at concentration of 400 cells/μl for 30 min at 50 °C. 80 μl of cell lysate was loaded onto the hybridization plate containing blocking reagent, proteinase K, and Plex Set followed by incubation in the incubator shaker for 18–22 h at 54 °C, 600 rpm. Then, the samples were transferred to a Magnetic Separation Plate. Sequential hybridization with 100 μl of 2.0 Pre-Amplifier, 100 μl of 2.0 Amplifier, and 100 μl of biotinylated label probe were performed for 1 h at 50 °C. Then, 100 μl of streptavidin-conjugated phycoerythrin was added to the samples, and the plate was incubated for 30 min at room temperature. The plate was read on a Luminex 200 instrument (Millipore) according to the manufacturer's instructions. The level of streptavidin-conjugated phycoerythrin fluorescence is proportional to the amount of mRNA transcripts captured by the beads. The mRNA levels were normalized against the housekeeping gene ACTB.
Western Blot Analysis
Cells from 10-cm dishes were harvested and lysed in 200 μl of buffer A (20 mm Hepes, pH 7.5, 10 mm NaCl, 20 mm NaF, 1 mm EDTA, 1 mm EGTA, 5 mm sodium pyrophosphate, 2 mm sodium vanadate, 10 mm β-glycerophosphate, and 1% Nonidet P-40) on ice for 30 min. The samples were centrifuged at 12,000 × g at 4 °C for 10 min. Cell lysates were subjected to SDS-PAGE and Western blot analysis. Anti-actin and anti-MDM2 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-FAS antibody was from BD Biosciences. Monoclonal anti-Aurora-A antibody was from Cell Signaling Technology (Beverly, MA). Anti-Myc antibody (clone 9E10) was from Roche Applied Science. Western blot analysis was performed using enhanced chemiluminescence (ECL) detection reagents (Amersham Biosciences) according to the manufacturer's instructions.
Screening of USP siRNA Library
The USP siRNA library for 58 USPs (four ON-TARGET-plus siRNAs for each USP) was obtained from Dharmacon (Lafayette, CO). For cell-based USP siRNA screening, the four ON-TARGET-plus siRNAs for each USP were pooled with an equal concentration of 20 μm. Luciferase siRNA for a control (sequence: AACGUACGCGGAAUACUUCGA) was also from Dharmacon. Transfection of MIA PaCa-2 cells were carried out with a final concentration of 30 nm siRNA for 4 days.
siRNA Transfection
For transfection of MIA PaCa-2 cells, 0.6 × 106 cells were plated in a 10-cm dish on day 0. On day 1, 50 μl of Lipofectamine 2000 (Invitrogen) was added to 1.5 ml of Opti-MEM (Invitrogen) and incubated at room temperature for 5 min (solution A). Then 6 μl of the siRNAs were added to 1.5 ml of Opti-MEM (solution B). Solution A and solution B were mixed and incubated at room temperature for 20 min. The medium in the plate was reduced to 1 ml, and the Lipofectamine 2000-siRNA mixture was laid onto the cells. Four hours after incubation at 37 °C in a CO2 incubator, the medium containing transfection solution was replaced with complete medium. The cells were harvested, and Western blot analysis was carried out.
Immunoprecipitation
20 μg of anti-Myc antibody, 30 μl of anti-USP2a antibody, or 30 μl of control antibody was incubated with a 20-μl bed volume of protein A/G-agarose beads (Santa Cruz Biotechnology) in 200 μl of PBS at room temperature for 1 h. The beads were washed three times with 1 ml of PBS and then incubated with 0.5 mg of cell lysate at 4 °C for 4 h in radioimmune precipitation assay (RIPA) buffer (Cell Signaling Technology). The beads were next washed with 1 ml of RIPA buffer three times, resuspended in 20 μl of loading buffer, and subjected to Western blot analysis.
Ubiquitination of Aurora-A in Vitro
The ubiquitination of recombinant His-tagged Aurora-A was carried out using an ubiquitination kit (Boston Biochem). Briefly, 1 μg of His-tagged Aurora-A was incubated at 37 °C for 4 h with a ubiquitin conjugation system consisting of 200 μg of HeLa S-100, 5 μm MG-132, 4 μm ubiquitin aldehyde, 600 μm ubiquitin, and 5 μl of energy regeneration solution in a total volume of 50 μl (Boston Biochem). The ubiquitinated Aurora-A was purified with Ni-NTA-resin by incubating the resin with the reaction mixture at room temperature for 30 min followed by washing five times with 1 ml of 50 mm Hepes buffer, pH 7.6.
In Vitro Translation of USP2a and USP7
Recombinant USP2a and USP7 proteins were generated using the TnT-coupled transcription/translation system according to the vendor's instruction (Promega). Briefly, a reaction mixture of 400 μl of solution containing 280 μl of rabbit reticulocyte lysate, 8 μl of amino acid mixture, 16 μl of TnT reaction buffer, 8 μl of T7 polymerase, 4 μg of pcDNA3-USP2a or pcDNA3-USP7 was incubated in 30 °C for 2 h. The resulting solution was used directly in the pulldown assay.
USP2a Activity Assay
Ubiquitinated Aurora-A on Ni-NTA acid beads was incubated with 0.5 μg of USP2a or USP2a-C276A in 30 μl of buffer B (50 mm Tris-HCl, pH 8.0, 4 mm MgCl2, 1 mm DTT, and 150 mm imidazole) at 37 °C for 2 h. The reaction mixtures were centrifuged at 13,000 rpm at room temperature for 1 min. The supernatants were subjected to Western blot analysis for Aurora-A, ubiquitin, and USP2a.
Generation of USP2a Antibody
Rabbit anti-USP2a antibody was generated by AnaSpec (San Jose, CA) using the peptide CLRPKSNPENLDHLPDDEKGRQ-NH2 as an immunogen. Antibody was purified using protein A/G-agarose.
Generation of USP2a and USP2a-C276A Recombinant Proteins
BL21 (DE3) cells were transfected with plasmid PET15-USP2a or PET15-USP2a-C276A. The cells were grown in LB medium at 37 °C. Isopropyl 1-thio-β-d-galactopyranoside was added to a final concentration of 1 m when the A600 of the bacteria culture reached 0.6, and bacteria were cultured for another 4 h. Proteins were purified using Ni-NTA beads.
Immunofluorescence
Cells were cultured in Lab-Tek-2 chamber slides (Nalge Nunc International, Rochester, NY) at 4 × 104/chamber. After incubation with compound A or B for 24 h, the cells were fixed and permeabilized with methanol:acetone (50:50) for 20 min and blocked with blocking solution (3% BSA in PBS) for another 20 min. The cells were then incubated sequentially with the following antibodies for 2 h in blocking buffer with three times of washes in between: rabbit polyclonal anti-γ-tubulin antibody (1:500) (Sigma), donkey anti-rabbit IgG(H+L) conjugated with Alexa Fluor 555 (1:500) (Invitrogen), and monoclonal anti-α-tubulin-FITC antibody (1:100) (Sigma). Finally, the cells were covered with mounting medium Prolong Gold anti-fade reagent with DAPI (Invitrogen), sealed with coverslips, counted, and photographed with an Axiovert 200M microscope (Carl Zeiss Inc, Chester, VA). All procedures were done at room temperature.
RESULTS
USP2a siRNA Reduces the Protein Levels of USP2a and Aurora-A
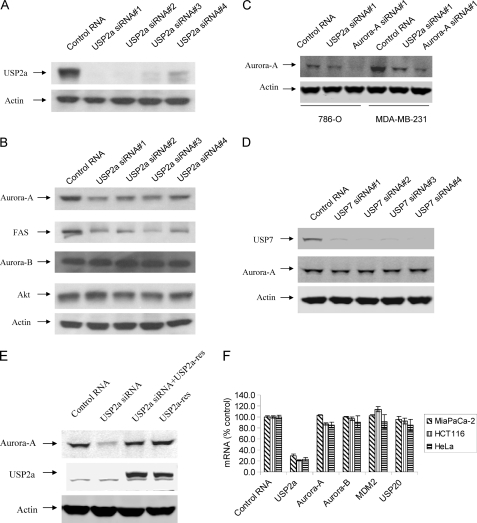
Aurora-A is an oncogenic kinase that plays critical roles in cell transformation. It has been documented that Aurora-A is degraded through the action of the anaphase-promoting complex/cyclosome (24, 25). However, it is not known whether Aurora-A is regulated by any USP. To investigate whether USP activity mediates the stability of Aurora-A, we transfected a USP siRNA library against 56 USPs into MIA PaCa-2 cancer cells and examined the protein level of Aurora-A 96 h after transfection. Each transfection experiment consisted of a pool of four ON-TARGET-plus individual siRNAs per USP target. From the screen, we discovered that several USP siRNAs, including USP2a, USP8, and USP20, reduced the protein level of Aurora-A (supplemental Fig. S1). Because USP2a has been reported to be an oncogene, we focused our study on USP2a. To verify these results, we tested all four individual USP2a siRNAs separately in MIA PaCa-2 cells. Because the USP2a antibody generated in our laboratory did not recognize the endogenous USP2a protein, we transfected USP2a siRNA with pCMV-xl6-USP2a in MIA PaCa-2 cells. As shown in Fig. 1A, all four ON-TARGET-plus USP2a siRNAs reduced USP2a protein. Knockdown of USP2a resulted in the reduction of the expression of Aurora-A, but not Akt or Aurora-B (Fig. 1B). Consistent with previously published results, knockdown of USP2a also reduced the protein levels of FAS (Fig. 1B). A similar effect of USP2a knockdown on Aurora-A was observed for USP2a siRNA in other cancer cell lines. As shown in Fig. 1C, both USP2a siRNA and Aurora-A siRNA reduced Aurora-A protein in renal cell carcinoma 786-O and triple negative breast cancer cell line MDA-MB-231. As controls, USP7 siRNAs did not reduced Aurora-A protein even though they reduced USP7 protein (Fig. 1D). To verify that the effect of USP2a siRNA on the protein level of Aurora-A is specific, we generated pcDNA3-USP2a-res that is resistant to USP2a siRNA#1. As shown in Fig. 1E, co-transfection of pcDNA3-USP2a-res with USP2a siRNA#1 rescued the effect of USP2a siRNA#1 on the protein level of Aurora-A (Fig. 1E).
FIGURE 1.
USP2a siRNAs reduce USP2a protein and induce the down-regulation of Aurora-A. A, MIA PaCa-2 cells were transfected with 2 μg of pCMV-xl6-USP2a and with 30 nm either control siRNA or four ON-TARGET-plus USP2a siRNAs for 72 h. The cells were harvested, and Western blot analysis was carried out for USP2a and actin. B, MIA PaCa-2 cells were transfected with 30 nm control siRNA or four USP2a siRNAs for 72 h. The cells were harvested, and Western blot analysis was carried out for Aurora-A, Akt, Aurora-B, FAS, and actin. C, 786-O and MDA-MB-231 cells were transfected with either control RNA, 30 nm USP2a siRNA, or 30 nm Aurora-A siRNA for 72 h. The cells were harvested, and Western blot analysis was carried out for Aurora-A and actin. D, MIA PaCa-2 cells were transfected with 30 nm control siRNA or four USP7 siRNAs for 72 h. The cells were harvested, and Western blot analysis was carried out for Aurora-A and actin. E, MIA PaCa-2 cells were transfected with 30 nm control RNA or 30 nm USP2a siRNA#1 in the absence or presence of 3 μg of pcDNA3-USP2a-res for 72 h. The cells were harvested, and Western blot analysis was carried out for Aurora-A, USP2a, and actin. F, MIA PaCa-2, HCT116, and HeLa cells were transfected with 30 nm USP2a siRNA for 72 h. Cells were harvested, and a branched DNA assay was carried out for USP2a, Aurora-A, Aurora-B, MDM2, and USP20.
To examine whether knockdown of USP2a has any effect on the mRNA level of Aurora-A, we transfected USP2a siRNA into three cancer cell lines and measured the mRNA levels for USP2a, Aurora-A, Aurora-B, MDM2, and USP20. As shown in Fig. 1F, USP2a siRNA reduced the mRNA levels of USP2a in MIA PaCa-2, HCT116, and HeLa cells by 71, 78, and 75%, respectively (p < 0.05 compared with control RNA), but it has little effect on the mRNA levels of Aurora-A, Aurora-B, MDM2, and USP20.
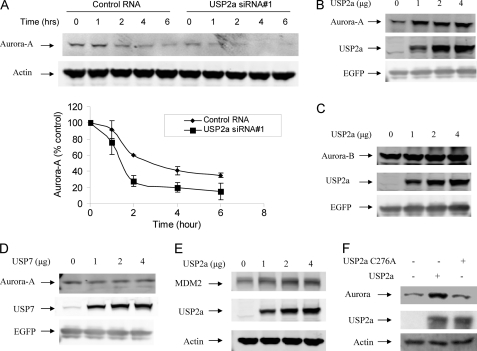
To determine the effect of USP2a knockdown on Aurora-A protein stability, MIA PaCa-2 cells were transfected with control or USP2a siRNA for 72 h. Cycloheximide was added to inhibit protein synthesis. As shown in Fig. 2A, Aurora-A protein has a half-life about 3 h in MIA PaCa-2 cells. USP2a siRNA not only reduced Aurora-A protein, but also reduced the half-life of Aurora-A to about 1.5 h (Fig. 2A).
FIGURE 2.
USP2a regulates the protein levels of Aurora-A. A, MIA PaCa-2 cells were transfected with 30 nm USP2a siRNA for 72 h. The cells were then treated with 50 μm cycloheximide for the indicated time. The cells were harvested, and Western blot analysis was carried out for Aurora-A and actin. The lower panel shows the quantification of the levels of Aurora-A protein. The intensity of protein band was determined using a Bio-Rad GS-800 calibrated densitometer (Bio-Rad) and normalized according to actin. Data are the average of two independent experiments. Error bars represent S.D. B, H1299 cells were transfected with 0.5 μg of pcDNA3-EGFP and 1 μg of pc-DNA3-Aurora-A in the presence of 0, 1, 2, or 4 μg of pCMV-xl6-USP2a for 24 h. The cells were treated with 20 μm cycloheximide for 4 h. Cells were harvested, and the cell lysates were prepared and subjected to Western blot analysis for Aurora-A, USP2a, and EGFP. C, experiments similar to those in B were carried out except 1 μg of pc-DNA3-Myc-Aurora-B was used. D, experiments similar to those in B were carried out except pc-DNA3-Myc-USP7 was used. E, experiments similar to those in B were carried out except 1 μg of pc-DNA3-Myc-MDM2 was used. F, H1299 cells were transfected with 1 μg of pc-DNA3.1 (−) Myc-Aurora-A and 4 μg of pCMV-xl6-USP2a or 4 μg of pcDNA3-USP2a-C276A for 24 h. Cells were harvested, and lysates were prepared and subjected to Western blot analysis for Aurora-A, USP2a, and actin.
To test further whether USP2a regulates the stability of Aurora-A, we expressed USP2a and Aurora-A in H1299 cells by transient transfection. As shown in Fig. 2B, Aurora-A levels increased when more USP2a was included in the transfection experiment. However, USP2a-C276A had no effect on the Aurora-A level, indicating that USP2a catalytic activity was required for this effect (Fig. 2F). Aurora-B protein levels did not change upon UPS2a overexpression, indicating that Aurora-A, but not Aurora-B, was a substrate for USP2a in H1299 cells (Fig. 2, B and C). To examine the specificity for USP2a in regulating the stability of Aurora-A in cells, we did similar transfection with USP7. As shown in Fig. 2D, overexpression of USP7 has little effect on the protein level of Aurora-A. We also did a similar experiment for MDM2, a known substrate for USP2a. MDM2 protein levels increased when more USP2a was included in the transfection experiments (Fig. 2E).
USP2a and Aurora-A Interact in Vivo and in Vitro
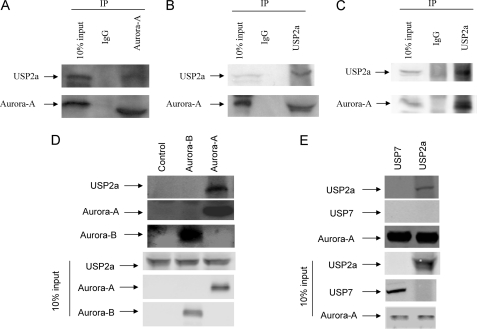
To examine whether USP2a interacts with Aurora-A in cells, we transfected H1299 cells with vectors expressing USP2a and Myc-tagged Aurora-A. The cell lysates were immunoprecipitated with anti-Myc antibody or control IgG. USP2a was present in the immunocomplex of Aurora-A, indicating that USP2a interacted with Aurora-A (Fig. 3A). Similar results were obtained when immunoprecipitation was carried out with an anti-USP2a antibody (Fig. 3B). The inability of USP2a antibody to recognize the endogenous USP2a protein prevented us from examining the interaction between endogenous USP2a and Aurora-A. However, we can still examine the interaction of USP2a with endogenous Aurora-A. H1299 cells were transfected with a vector expressing USP2a. The cell lysates were immunoprecipitated with anti-USP2a antibody or control IgG. Endogenous Aurora-A was present in the immunocomplex of USP2a (Fig. 3C).
FIGURE 3.
USP2a interacts with Aurora-A in vitro and in vivo. A and B, H1299 cells were transfected with 3 μg of pCMV-xl6-USP2a and 2 μg of pcDNA3.1 (−) Myc-His-Aurora-A. A, 24 h after transfection, the cells were treated with 20 μm MG-132 for 4 h and harvested, and cell lysates were prepared. 20 μl of protein A/G-agarose beads was incubated with either control mouse IgG or anti-Myc antibody at room temperature for 30 min. The beads were washed with PBS three times and incubated with 0.5 mg of the H1299 cell lysate in RIPA buffer at 4 °C for 4 h. The beads were washed with RIPA buffer five times and boiled in 20 μl of SDS-loading buffer for 3 min. The supernatant was subjected to Western blot analysis for Myc and USP2a. B, experiments similar to those in A were carried out except that the beads were incubated with either control rabbit IgG or anti-USP2a antibody. C, H1299 cells were transfected with 3 μg of pCMV-xl6-USP2a. Experiments similar to those in B were carried out. D, recombinant USP2a was generated using the TnT-coupled transcription/translation system as described under “Materials and Methods.” 100 μl of recombinant USP2a was incubated with either buffer alone or 3 μg of full-length His-tagged Aurora-A or Aurora-B at 4 °C overnight. 20 μl of Ni-NTA beads were added to the reaction mixture and incubated at 4 °C for 60 min. The beads were washed three times with PBS-Tween 20 (0.1% v/v). The beads were boiled in 20 μl of SDS-loading buffer for 3 min, and the supernatants were subjected to Western blot analysis for Aurora-A, Aurora-B, and USP2a. E, 100 μl of recombinant USP2a or USP7 was incubated with 3 μg of full-length His-tagged Aurora-A at 4 °C overnight. 20 μl of Ni-NTA beads was added to the reaction mixture and incubated at 4 °C for 60 min. The beads were washed three times with PBS-Tween 20 (0.1% v/v). The beads were boiled in 20 μl of SDS-loading buffer for 3 min, and the supernatants were subjected to Western blot analysis for Aurora-A, USP2a, and USP7.
To test whether USP2a interacts with Aurora-A directly in vitro, we mixed in vitro translated USP2a protein with either buffer alone, or His-tagged full-length Aurora-B, or His-tagged full-length Aurora-A. The proteins were then isolated with Ni-NTA beads. As shown in Fig. 3D, Ni-NTA beads were able to pull down both Aurora-B and Aurora-A. However, USP2a was only pulled down by Ni-NTA beads with His-Aurora-A, but not His-Aurora-B. As a control, USP7 was not pulled down by Ni-NTA beads with His-Aurora-A (Fig. 3E).
Aurora-A Is a Substrate for USP2a
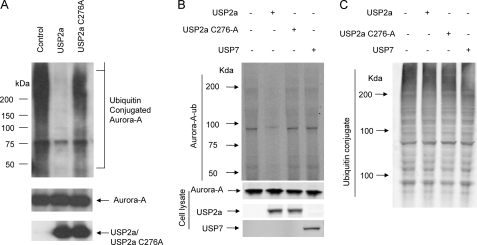
To examine whether USP2a could deubiquitinate Aurora-A in vitro, we first conducted an ubiquitination experiment to conjugate ubiquitin to recombinant Aurora-A protein. The ubiquitinated Aurora-A was purified and incubated with buffer control, USP2a, or a catalytically inactive mutant, USP2a-C276A. As shown in Fig. 4A, USP2a catalyzed the removal of the majority of ubiquitin conjugated to Aurora-A, whereas USP2a-C276A failed to do so.
FIGURE 4.
Aurora-A is a substrate for USP2a in vitro and in vivo. A, recombinant His-tagged full-length Aurora-A was ubiquitinated with a ubiquitination kit as described under “Materials and Methods.” USP2a activity assays were carried out using the purified ubiquitinated Aurora-A with either buffer alone, 0.5 μg of USP2a, or 0.5 μg of USP2a-C276A as described under ”Materials and Methods.” The reaction mixture was subjected to Western blot analysis for ubiquitin, Aurora-A, and USP2a. B, H1299 cells were transfected with 1 μg of pcDNA3-Aurora-A and 1 μg of pcDNA3-His8-ubiquitin alone, or in the presence of 1 μg of the following constructs: pcDNA3, pcDNA3-USP2a, pcDNA3-USP2a-C276A, or pcDNA3-Myc-USP7. Cells were treated with 30 μm MG-132 for 4 h. Cell lysates were prepared, and ubiquitinated proteins were purified using Ni-NTA beads and blotted with antibody for Aurora-A. Cell lysates were also blotted for Aurora-A, USP2a, and USP7 (bottom panel). C, cell lysates prepared in B were also blotted with anti-ubiquitin antibody.
To examine whether Aurora-A is a substrate for USP2a in cells, we transfected H1299 cells with pcDNA3-His8-ubiquitin and pcDNA3-Myc-Aurora-A in the presence of pcDNA3, pcDNA3-USP2a, pcDNA3-USP2a-C276A, of pCDNA3-Myc-USP7. Cell lysates were prepared and incubated with Ni-NTA beads. Proteins on the Ni-NTA beads were subjected to Western blotting with Aurora-A antibody. As shown in Fig. 4B, the ubiquitinated Aurora-A level is much less in H1299 cells transfected with USP2a than that in H1299 cells transfected with either pcDNA3, pcDNA3-USP2a-C276A, or pCDNA3-Myc-USP7. The expression levels for Aurora-A, USP2a, USP2a-C276A, and USP7 in these cell lysates were also determined through Western blot analysis (Fig. 4B, bottom panel). To examine whether overexpression of USP2a altered the overall ubiquitination of proteins, we did Western blot analysis of the cell lysates with anti-ubiquitin antibody. As shown in Fig. 4C, the pattern for ubiquitinated proteins in these cell lysates is quite similar. This indicates that Aurora-A is a substrate for USP2a in cells.
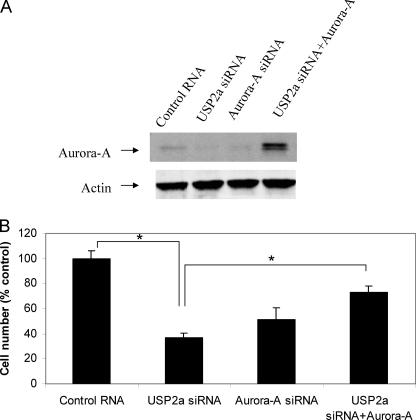
Knockdown of USP2a Inhibits Cell Proliferation, Which Can Be Partially Rescued by Overexpression of Aurora-A
To examine whether knockdown of USP2a inhibits cancer cell proliferation through the reduction of Aurora-A, we transfected MIA PaCa-2 cells with USP2a siRNA in the absence or presence of pcDNA3-Aurora-A, then cell number was counted and normalized to siRNA control. As shown in Fig. 5A, both USP2a siRNA and Aurora-A siRNA reduced Aurora-A protein, whereas co-transfection of pcDNA3-Aurora-A restored Aurora-A protein level in MIA PaCa-2 cell treated with USP2a siRNA. USP2a siRNA inhibited 62% cell proliferation, whereas Aurora-A siRNA inhibited 51% cell proliferation (p < 0.05 compared with control) (Fig. 5B). Overexpression of Aurora-A partially rescued the effect of USP2a siRNA in MIA PaCa-2 cells transfected with USP2a siRNA and pcDNA3-Aurora-A (p < 0.05 for USP2a siRNA compared with USP2a siRNA+Aurora-A) (Fig. 5B).
FIGURE 5.
USP2a siRNA inhibits cell proliferation, which is partially rescued by Aurora-A. MIA PaCa-2 cells were transfected with 20 nm control RNA plus 1 μg of pcDNA3, 20 nm USP2a siRNA#1 plus 1 μg of pcDNA3, 20 nm Aurora-A siRNA plus 1 μg of pcDNA3, or 20 nm USP2a siRNA#1 plus 1 μg of pcDNA3-Aurora-A for 5 days. The cells were harvested. A, Western-blot analysis was carried out for Aurora-A and actin. B, cell number was counted and normalized according to control RNA. *, p < 0.05 (Student's t test). Error bars, S.D.
USP2a Knockdown Induces Abnormal Mitosis
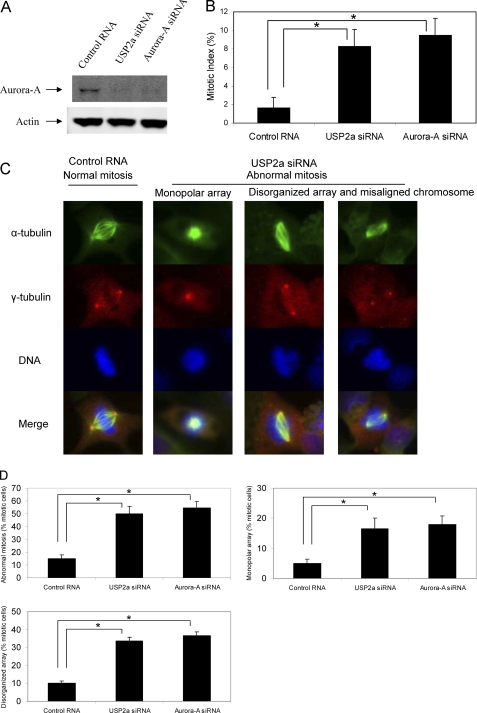
Because USP2a regulates the stability of Aurora-A, we next assessed its role in regulating mitotic progression. We used 786-O cells for mitotic phenotype studies because 786-O cells are one of the cell lines where USP2a siRNA treatment through double transfection results in robust reduction of Aurora-A protein level. We first examined the mitotic cell population in asynchronized 786-O cells transfected with USP2a siRNA or Aurora-A siRNA. As shown in Fig. 6A, both USP2a siRNA and Aurora-A siRNA reduced the protein levels of Aurora-A. The mitotic population of 786-O cells treated with USP2a siRNA or Aurora-A siRNA is 8.3% and 9.7%, respectively, much higher than that (1.7%) in 786-O cells treated with control RNA (p < 0.05 compared with control RNA) (Fig. 6B).
FIGURE 6.
Knockdown of USP2a induces abnormal mitosis. 786-O cells on a 10-cm dish and on chamber slides were transfected with 20 nm control RNA, 20 nm USP2a siRNA#1, or 20 nm Aurora-A siRNA on day 1. The cells were transfected again on day 2 and were left in culture for another 2 days. A, the cells on the 10-cm dish were harvested, and Western blot analysis was carried out for Aurora-A and actin. B, the cells on chamber slides were fixed and stained for α-tubulin (green), γ-tubulin (red), and DNA (blue) as described under “Materials and Methods.” Mitotic index was determined. *, p < 0.05 (Student's t test). Error bars, S.D. C, typical examples of normal and abnormal mitotic cells. D, quantitative assessment of normal and abnormal mitotic spindles. For each treatment condition, >150 mitotic cells were scored. Data are representative of three independent experiments. *, p < 0.05 (Student's t test).
We also observed that most of the mitotic cells treated with USP2a siRNA contained abnormal spindle formation consisting of rosette or monopolar array (Fig. 6C). Some of the mitotic cells treated with USP2a siRNA could form bipolar spindles; however, the bipolar spindles are disorganized, and chromosomes were not aligned at the equators as are those in normal control (Fig. 6C). Quantitative analysis indicated that abnormal spindle formation dramatically increased in USP2a siRNA-treated cells. 16% and 50% abnormal mitosis were observed in control RNA and USP2a siRNA-treated cells, respectively (p < 0.05) (Fig. 6D), a mitotic phenotype that was very similar to cells treated with Aurora-A siRNA (Fig. 6, C and D). Of these abnormal mitotic cells induced by knockdown of UPS2a, about one-third had rosette or monopolar array (Fig. 6D). Similar results were observed for Aurora-A siRNA-treated cells (Fig. 6D).
Overexpression of Aurora-A Rescues the Mitotic Arrest Induced by USP2a siRNA
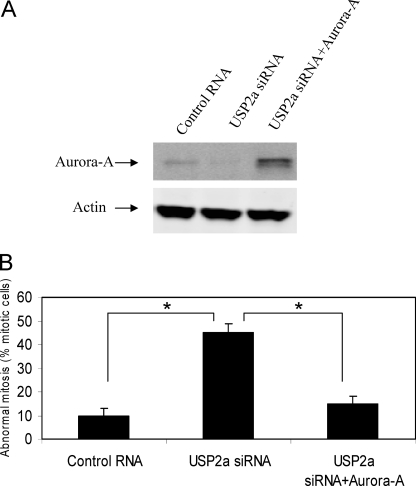
To examine whether knockdown of USP2a induces mitotic arrest through Aurora-A down-regulation, we overexpressed Aurora-A to determine whether it could rescue the mitotic arrest induced by USP2a siRNA. As shown in Fig. 7A, USP2a siRNA reduced Aurora-A protein, whereas co-transfection of pcDNA3-Aurora-A restored Aurora-A protein level in 786-O cell treated with USP2a siRNA. We estimated that 60% of the cells were transfected by co-transfecting with an EGFP construct (data not shown). In the transfected cell population, the mitotic defects can be reversed by the overexpression of Aurora-A (Fig. 7B). This suggests that USP2a mediates mitotic progression through regulating the stability of Aurora-A.
FIGURE 7.
Overexpression of Aurora-A rescues USP2a siRNA-induced abnormal mitosis. 786-O cells on a 10-cm dish and on chamber slides were transfected with 20 nm control RNA plus 1 μg of pcDNA3, 20 nm USP2a siRNA#1 plus 1 μg of pcDNA3, or 20 nm USP2a siRNA#1 plus 1 μg of pcDNA3-Aurora-A on day 1 in the presence of 0.3 μg of pcDNA3-EGFP. The cells were transfected again on day 2 and left for another 2 days. A, the cells on the 10-cm dish were harvested, and Western blot analysis was carried out for Aurora-A and actin. B, the cells on chamber slides were fixed and stained for α-tubulin (green), γ-tubulin (red), and DNA (blue). Abnormal mitosis was scored by examining >150 mitotic cells. *, p < 0.05 (Student's t test). Error bars, S.D.
DISCUSSION
Despite the large number of deubiquitinases identified, relatively little is known about their physiological roles or their substrates. Thus, identification of novel substrates for these deubiquitinases helps to shed light on their physiological function. In this study, we show that USP2a mediates cell cycle progression by regulating the stability of Aurora-A. USP2a can interact directly with Aurora-A in vitro. Aurora-A is also a substrate for USP2a in vitro and inside cells. Aurora-A is a kinase that plays critical roles in centrosome duplication and maturation. Reduced levels of Aurora-A result in the arrest in mitosis and delayed cell cycle progression (21, 22). Our data indicate that USP2a siRNA also delays cell cycle progression, a phenotype similar to the effect of Aurora-A reduction.
Aurora-A is degraded through the action of the anaphase-promoting complex/cyclosome (24, 25). Because USP2a stabilizes Aurora-A by removing ubiquitin from it, the concerted action of anaphase-promoting complex/cyclosome and USP2a determines the level of Aurora-A inside cells through posttranslational modification. Previously, we showed that Aurora-A can also be regulated through transcription, as an Akt inhibitor blocks the transcription of Aurora-A through the ETS element in its promoter region (26). Thus, Aurora-A can be regulated at both the transcriptional and posttranslational levels.
Both USP2a and Aurora-A are overexpressed in a variety of human cancers and the expression level correlates with the tumor malignancy (6, 27, 28). USP2a has been shown to be a bona fide oncogene by its ability to transform NIH-3T3 cells. Given the fact that Aurora-A is also a bona fide oncogene, it is possible that Aurora-A as a substrate for USP2a contributed to the oncogenicity of USP2a.
USP2a and Aurora-A interact with each other in vitro and in vivo. Given that there are at least two binding sites on USP2a to interact with MDM2 (7), it is also possible that there are multiple binding sites on USP2a for Aurora-A. Further study is warranted to map the minimal binding sites for each protein.
Supplementary Material
Acknowledgment
We thank Dr. Tim R. Middleton for critically reading the manuscript.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- USP
- ubiquitin-specific protease
- FAS
- fatty acid synthase
- Ni-NTA
- nickel-nitrilotriacetic acid
- RIPA
- radioimmune precipitation assay
- MDM
- murine double minute.
REFERENCES
- 1. Pickart C. M., Eddins M. J. (2004) Biochim. Biophys. Acta 1695, 55–72 [DOI] [PubMed] [Google Scholar]
- 2. Pickart C. M. (2004) Cell 116, 181–190 [DOI] [PubMed] [Google Scholar]
- 3. Wong B. R., Parlati F., Qu K., Demo S., Pray T., Huang J., Payan D. G., Bennett M. K. (2003) Drug Discovery Today 8, 746–754 [DOI] [PubMed] [Google Scholar]
- 4. Nijman S. M., Luna-Vargas M. P., Velds A., Brummelkamp T. R., Dirac A. M., Sixma T. K., Bernards R. (2005) Cell 123, 773–786 [DOI] [PubMed] [Google Scholar]
- 5. Lin H., Keriel A., Morales C. R., Bedard N., Zhao Q., Hingamp P., Lefrançois S., Combaret L., Wing S. S. (2000) Mol. Cell. Biol. 20, 6568–6578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Priolo C., Tang D., Brahamandan M., Benassi B., Sicinska E., Ogino S., Farsetti A., Porrello A., Finn S., Zimmermann J., Febbo P., Loda M. (2006) Cancer Res. 66, 8625–8632 [DOI] [PubMed] [Google Scholar]
- 7. Stevenson L. F., Sparks A., Allende-Vega N., Xirodimas D. P., Lane D. P., Saville M. K. (2007) EMBO J. 26, 976–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Graner E., Tang D., Rossi S., Baron A., Migita T., Weinstein L. J., Lechpammer M., Huesken D., Zimmermann J., Signoretti S., Loda M. (2004) Cancer Cell 5, 253–261 [DOI] [PubMed] [Google Scholar]
- 9. Allende-Vega N., Sparks A., Lane D. P., Saville M. K. (2010) Oncogene. 29, 432–441 [DOI] [PubMed] [Google Scholar]
- 10. Toledo F., Wahl G. M. (2006) Nat. Rev. Cancer 6, 909–923 [DOI] [PubMed] [Google Scholar]
- 11. Hainaut P., Hollstein M. (2000) Adv. Cancer Res. 77, 81–137 [DOI] [PubMed] [Google Scholar]
- 12. Kuhajda F. P. (2000) Nutrition 16, 202–208 [DOI] [PubMed] [Google Scholar]
- 13. Baron A., Migita T., Tang D., Loda M. (2004) J. Cell. Biochem. 91, 47–53 [DOI] [PubMed] [Google Scholar]
- 14. Andrews P. D. (2005) Oncogene 24, 5005–5015 [DOI] [PubMed] [Google Scholar]
- 15. Carmena M., Earnshaw W. C. (2003) Nat. Rev. Mol. Cell Biol. 4, 842–854 [DOI] [PubMed] [Google Scholar]
- 16. Meraldi P., Honda R., Nigg E. A. (2004) Curr. Opin. Genet. Dev. 14, 29–36 [DOI] [PubMed] [Google Scholar]
- 17. Liu Q., Ruderman J. V. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 5811–5816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bischoff J. R., Anderson L., Zhu Y., Mossie K., Ng L., Souza B., Schryver B., Flanagan P., Clairvoyant F., Ginther C., Chan C. S., Novotny M., Slamon D. J., Plowman G. D. (1998) EMBO J. 17, 3052–3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meraldi P., Honda R., Nigg E. A. (2002) EMBO J. 21, 483–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anand S., Penrhyn-Lowe S., Venkitaraman A. R. (2003) Cancer Cell 3, 51–62 [DOI] [PubMed] [Google Scholar]
- 21. Yang H., Burke T., Dempsey J., Diaz B., Collins E., Toth J., Beckmann R., Ye X. (2005) FEBS Lett. 579, 3385–3391 [DOI] [PubMed] [Google Scholar]
- 22. Hirota T., Kunitoku N., Sasayama T., Marumoto T., Zhang D., Nitta M., Hatakeyama K., Saya H. (2003) Cell 114, 585–598 [DOI] [PubMed] [Google Scholar]
- 23. Murata-Hori M., Tatsuka M., Wang Y. L. (2002) Mol. Biol. Cell 13, 1099–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Littlepage L. E., Ruderman J. V. (2002) Genes Dev. 16, 2274–2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Taguchi S., Honda K., Sugiura K., Yamaguchi A., Furukawa K., Urano T. (2002) FEBS Lett. 519, 59–65 [DOI] [PubMed] [Google Scholar]
- 26. Liu X., Shi Y., Woods K. W., Hessler P., Kroeger P., Wilsbacher J., Wang J., Wang J. Y., Li C., Li Q., Rosenberg S. H., Giranda V. L., Luo Y. (2008) Neoplasia 10, 828–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. da Silva S. D., Cunha I. W., Nishimoto I. N., Soares F. A., Carraro D. M., Kowalski L. P., Graner E. (2009) Oral. Oncol. 45, e134–139 [DOI] [PubMed] [Google Scholar]
- 28. Marumoto T., Zhang D., Saya H. (2005) Nat. Rev. Cancer 5, 42–50 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.