Background: Peroxiredoxin IV is an H2O2 sensor that mediates protein thiol oxidation in endoplasmic reticulum.
Results: An alternatively transcribed form of peroxiredoxin IV is found only in the spermatid cytoplasm of postpubertal mouse testis.
Conclusion: This cytosolic peroxiredoxin IV appears to function also as an H2O2 sensor during spermiogenesis.
Significance: Identification of proteins that are oxidized by this new peroxiredoxin IV is crucial for understanding sperm maturation.
Keywords: Antioxidants, Peroxidase, Reactive Oxygen Species (ROS), Spermatogenesis, Thiol, Peroxiredoxin, Thiol Oxidation
Abstract
2-Cysteine (Cys) peroxiredoxins (Prxs), which include mammalian Prxs I–IV, possess two conserved Cys residues that are readily oxidized by H2O2 to form a disulfide. In the case of Prx I–III, the disulfide is reduced by thioredoxin, thus enabling these proteins to function as peroxidases. Prx IV was shown previously to be synthesized as a 31-kDa polypeptide with an NH2-terminal signal peptide that is subsequently cleaved to generate a 27-kDa form of the protein that is localized to the endoplasmic reticulum. A form of Prx IV, larger than 27 kDa revealed by immunoblot analysis was suggested to represent the unprocessed, 31-kDa form, but this larger form was detected only in spermatids of the postpubertal testis. We now show that the larger form of Prx IV (here designated Prx IV-L) detected in the testis is actually a product of alternative transcription of the Prx IV gene that is encoded by newly identified exon 1A together with exons 2–7 that are shared with the 27-kDa form (designated Prx IV-S). Prx IV-L was detected in spermatids but not in mature sperm, it could form disulfide-linked dimers but not higher order oligomers via oxidation, and it was resistant to hyperoxidation unless additional reductant was added, suggesting that its peroxidase activity is limited in vivo. Phylogenetic analysis showed that the Prx IV-S gene is present in all vertebrates examined, whereas the Prx IV-L gene was detected only in placental mammals. We suggest that Prx IV-L functions as an H2O2 sensor that mediates protein thiol oxidation required for the maturation of spermatozoa in placental mammals.
Introduction
Members of the peroxiredoxin (Prx)3 family of peroxidases are present in all organisms and reduce peroxides with a catalytic cysteine (Cys) residue serving as the site of oxidation by peroxides (1). Six mammalian Prx isoforms (Prx I–VI) have been identified to date and can be divided into three subgroups: typical 2-Cys (Prx I– IV), atypical 2-Cys (Prx V), and 1-Cys (Prx VI) subgroups (1–3). The 2-Cys Prx enzymes exist as homodimers and possess two conserved Cys residues. In the catalytic cycle of 2-Cys Prxs, peroxides oxidize the NH2-terminal Cys-SH (designated the peroxidatic Cys (CP-SH)) to Cys sulfenic acid (Cys-SOH). The unstable sulfenic acid then reacts with the COOH-terminal conserved Cys-SH (the resolving Cys (CR-SH)) of the other subunit in the homodimer to form a disulfide. The resulting disulfide is subsequently reduced by thioredoxin to complete the catalytic cycle (4). As a result of the slow rate of its reaction with CR-SH, CP-SOH is occasionally further oxidized to Cys sulfinic acid (Cys-SO2H) (5). This hyperoxidation reaction, which results in inactivation of peroxidase function, is reversed by sulfiredoxin (6, 7).
Prx IV cDNA was initially cloned from a human cell line and was predicted to encode a 272-amino acid protein with a calculated molecular mass of 31 kDa (8). Prx IV was detected in the culture medium when expressed in COS-1 cells, and this secreted form of the protein had an apparent molecular mass of ∼27-kDa and lacked the 36 amino acids beginning with the initiating methionine residue (9, 10). It was thus proposed that the 36 NH2-terminal residues of Prx IV function as a signal sequence for secretion and that, when not processed, the NH2-terminal signal peptide anchors the intact 31-kDa form of the protein to the membrane of the endoplasmic reticulum (ER) as a result of its hydrophobic nature. Indeed, a recent study confirmed that the 27-kDa form of Prx IV is produced as the result of cleavage of the NH2-terminal 36 residues of the encoded protein (11). This study also showed, however, that the 27-kDa form of Prx IV is retained within the ER despite the fact that it lacks a recognized ER retention motif (11, 12). Both the 27- and 31-kDa forms of Prx IV thus appear to be localized to the ER, whereas other 2-Cys Prx isoforms are located in the cytoplasm (Prxs I and II) or mitochondria (Prx III) (4).
Immunoblot analysis readily detected the 27-kDa form of Prx IV in most cultured cell types examined but failed to detect the 31-kDa form (11, 13). The 27-kDa form was also detected in most rodent tissues, but both the 27- and 31-kDa forms were detected only in the testis (14). Cleavage of the NH2-terminal signal peptide thus appeared to be inhibited only in the testis. Further analysis showed that the 27-kDa form of Prx IV was present in the mouse testis at all ages, whereas the 31-kDa form was observed only in spermatids from around puberty (14). The Prx IV gene was mapped to the X chromosome in both humans and rodents (10, 15), and Prx IV knock-out (KO) mice were recently generated by deletion of exon 1 of the gene (16). The Prx IV KO mice are fertile, but the males manifest testicular atrophy and increased lipid peroxidation in the testis as well as accelerated spermatogenic cell death under conditions of oxidative stress (16).
In the present study, we found by immunoblot analysis that the putative 31-kDa form of Prx IV was still detected in the testis of the Prx IV KO mice, whereas the 27-kDa form was not observed in any of the KO mouse tissues, including the testis. Further analysis indicated, however, that the protein corresponding to the persisting immunoreactive band actually had a molecular mass of ∼29.5 kDa, which is smaller than the 31 kDa estimated for the unprocessed form of mouse Prx IV (which comprises 274 amino acids compared with 272 residues for the human protein). This 29.5-kDa form of Prx IV, which was assumed previously to be the unprocessed form of Prx IV present in the ER membrane, is in fact an alternatively transcribed cytosolic isoform whose expression is restricted to spermatids of eutherian mammals. On the basis of our characterization of this male germ cell-specific isoform of Prx IV, we propose that it functions as an H2O2 sensor for protein disulfide formation during spermiogenesis.
EXPERIMENTAL PROCEDURES
Antibodies
Rabbit polyclonal antibodies to the COOH-terminal region (common to Prx IV-L and Prx IV-S) of human Prx IV (KPGSETIIPDPAGKLKYFDK) were described previously (8). Rabbit polyclonal antibodies specific for Prx IV-L were prepared by injection of two peptides, MDHRCRSRGMSHSR and REGEEWERELPRQR, that correspond to amino acids 1–14 and 36–49, respectively, of mouse Prx IV-L. Rabbit polyclonal antibodies to Prx I, to Prx II, to Prx III, to Prx V, to Prx VI, and to hyperoxidized 2-Cys Prx (1) as well as those to thioredoxin-glutathione reductase (17) were described previously. Rabbit polyclonal antibodies to glutathione peroxidase 4 and to catalase were obtained from Young-In Frontier (Seoul, Korea). Rabbit polyclonal antibodies to β-actin (A9718) and to α-tubulin (T5168) were obtained from Sigma-Aldrich, and that to protein disulfide isomerase (PDI) (H-160) was from Santa Cruz Biotechnology.
Animals
Male C57BL/6J mice were obtained from The Jackson Laboratory (Bar Harbor, ME), and Prx IV KO mice were described previously (16). All mice were housed in a temperature-controlled animal room with a 12-h light, 12-h dark cycle. The study conformed to institutional guidelines for the treatment of animals in research.
RT-PCR Analysis
Total RNA was isolated from mouse tissues with the use of TRIzol reagent (Invitrogen). Portions (0.36 μg) of the RNA were subjected to RT with the use of a SuperScript II reverse transcriptase kit (Invitrogen), and the cDNA products (1 μl) were subjected to PCR with gene-specific primer sets. The PCR products were fractionated by agarose gel electrophoresis and visualized by staining with ethidium bromide. The primers for the Prx IV gene were 5′-ACTTCCGTTCCATGTTGAGC-3′ (primer a, exon 1A sequence), 5′-TCCCTGCATCTAAGCAAAGC-3′ (primer b, exon 1B sequence), and 5′-TGACCTTTATTGAGAAGGTCAA-3′ (primer c, exon 7 sequence).
Immunoblot Analysis
Epididymal sperm were collected from male mice by mincing the cauda epididymides and allowing the sperm to swim out into PBS. The sperm were then collected by centrifugation at 800 × g for 5 min at room temperature and were either homogenized as described below or solubilized in a solution containing 0.1 m 2-mercaptoethanol and 6 m guanidine hydrochloride (18). Other mouse tissues were homogenized in ice-cold lysis buffer (20 mm Tris-HCl, pH 8.0, 137 mm NaCl, 10% (v/v) glycerol, 1% (v/v) Nonidet P-40, and 2 mm EDTA containing protease inhibitors (Roche Applied Science)), and the protein concentration of the homogenates was determined with the Bradford assay (Bio-Rad). The sperm extract and tissue homogenates were fractionated by SDS-PAGE, and the separated proteins were transferred to a PVDF membrane (Amersham Biosciences). The membrane was exposed to 0.5% (w/v) skim milk in TBS containing 0.2% (v/v) Tween 20 (TBST), incubated overnight at 4 °C with primary antibodies, washed with TBST, and incubated for 1 h at room temperature with HRP-conjugated secondary antibodies. Immune complexes were then detected with ECL Plus chemiluminescence reagents (Amersham Biosciences).
Matrix-assisted Laser Desorption Ionization-Time-of-flight Mass Spectrometry (MALDI-TOF MS) Analysis of Prx IV Proteins
Lysates of the testis from 20-week-old WT or Prx IV KO mice were prepared with nondenaturing lysis buffer. The lysates were cleared of material that binds to protein G- or protein A-Sepharose (Amersham Biosciences) and were then incubated (with continuous rotation) at 4 °C overnight with rabbit antibodies to the COOH-terminal region of Prx IV and then for 2 h in the additional presence of protein G/A-Sepharose beads. The beads were then washed five times with the lysis buffer, boiled in 2× SDS sample buffer, and subjected to SDS-PAGE on a 14% gel. The gel was stained with silver, and the bands corresponding to Prx IV-L and Prx IV-S were excised, exposed to a solution containing 30 mm K4FeCN6 and 100 mm sodium thiosulfate to remove the stain, and washed. For facilitation of tryptic digestion, the gel pieces were dehydrated by treatment with acetonitrile adjusted to pH 8.0 with 200 mm NH4HCO3. Each gel piece was then incubated for 16–18 h at 37 °C with 10–20 μl of 25 mm NH4HCO3 containing sequencing grade methylated trypsin (20 ng/ml; Promega). Generated peptides were extracted with 30 μl of a solution containing 60% (v/v) acetonitrile and 0.1% (v/v) trifluoroacetic acid, dried completely with the use of a SpeedVac, and analyzed by MALDI-TOF MS with a Voyager ion mirror reflector mass spectrometer (Applied Biosystems). Mass spectra were interpreted with the use of the MS-Fit program. Only significant hits in a single MASCOT-searchable peak were accepted and aligned with Prx IV protein sequences.
Primary Testicular Cell Culture
Leydig, Sertoli, and spermatogenic cells were isolated from 20-week-old mice and cultured as described previously (19) with slight modification. Testes were aseptically removed, decapsulated mechanically, and incubated (with gentle shaking) for 20 min at room temperature in Krebs-Ringer bicarbonate medium containing 2.5 mg/ml type 1 collagenase (Worthington) and DNase (1 mg/ml). Collagenase activity was terminated by dilution of the incubation mixture 5-fold with Krebs-Ringer bicarbonate medium, released interstitial cells were separated by filtration through a nylon cell strainer (pore size, 100 μm; BD Transduction Laboratories), and any seminiferous tubule elements that passed through the filter were allowed to settle for 1 min before collection of the supernatant for enrichment of each testicular cell type by Percoll (GE Healthcare) density gradient centrifugation. For isolation of Sertoli and spermatogenic cells, the seminiferous tubules captured by the cell strainer were washed with PBS and then incubated (with gentle shaking) for 15 min at 32.5 °C with PBS containing 0.25% trypsin (Invitrogen). Trypsin activity was terminated by the addition of FBS to a final concentration of 10%, and the resulting cell suspension was passed through the nylon cell strainer to remove cell aggregates. The cells in the filtrate were isolated by centrifugation and suspended at a density of 5 × 106/ml in F-12/L-15 culture medium (1:1 mixture of Ham's F-12 and Leibovitz's L-15) supplemented with sodium bicarbonate (1 mg/ml), penicillin G (100 units/ml), streptomycin sulfate (100 μg/ml), 15 mm Hepes-NaOH (pH 7.3), and 10% FBS. The cells were then incubated for 24 h in plastic culture plates at 32.5 °C (the optimal temperature for testicular spermatogenic cells) and under a humidified atmosphere of 5% CO2 in air. Cells that attached to the plates were defined as Sertoli cells, and the nonadherent cells were collected as a spermatogenic cell-enriched fraction.
Immunohistochemical Analysis
Twenty-week-old male mice were anesthetized by intraperitoneal injection of a mixture of tiletamine, zolazepam, and xylazine, and the testes and epididymides were perfused via the heart with PBS before their removal and fixation overnight first with Bouin's fixative and then with 4% (v/v) formalin in PBS. The fixed tissue was embedded in paraffin and sectioned at a thickness of 3.5 μm. The sections were exposed to 10 mm sodium citrate, pH 6.0, to CAS-Block reagent (Invitrogen), and to 2.5% normal horse serum supplied with the ImmPRESS reagent kit (Vector Laboratories). They were then incubated at 4 °C for 16–18 h with primary antibodies, washed with PBS, and incubated with HRP-conjugated secondary antibodies. Immune complexes were detected at room temperature with the use of a DAB (diaminobenzidine) substrate kit (Vector Laboratories), and slides were counterstained with Mayer's hematoxylin.
Subcellular Fractionation
Testes from 20-week-old C57BL/6J mice were homogenized with a Dounce homogenizer in 5 volumes of an ice-cold solution containing 0.25 m sucrose, 5 mm Tris-HCl, pH 7.2, 1 mm MgCl2, and a protease inhibitor mixture (Roche Applied Science). The homogenate was filtered through a nylon cell strainer (pore size, 100 μm; BD Transduction Laboratories), and the filtrate was centrifuged at 1,000 × g for 20 min at 4 °C. The resulting supernatant was centrifuged at 10,000 × g for 20 min at 4 °C, and the new supernatant was centrifuged at 105,000 × g for 2 h at 4 °C. The final supernatant was saved as a cytosolic fraction, and the pellets obtained at 1,000 × g, 10,000 × g, and 105,000 × g were also saved. Each pellet was washed with the homogenization buffer and resuspended in the lysis buffer. The resuspended pellets and the cytosolic fraction were subjected to immunoblot analysis.
Proteinase K Digestion
The pellets obtained at 10,000 × g and 105,000 × g during subcellular fractionation of testis homogenates described in the previous section were washed twice with the homogenization buffer without protease inhibitors and then diluted with the same solution to a protein concentration of 1 mg/ml. The samples were incubated on ice for 30 min with proteinase K (Roche Applied Science) at concentrations of 10–40 μg/mg protein and in the absence or presence of 0.5% (v/v) Triton X-100. The reaction was then terminated by the addition of PMSF to a final concentration of 10 mm, and the samples were subjected to immunoblot analysis.
Comparative Analyses of Prx IV
Forty-seven vertebrate and other representative eukaryotic genomes with substantial sequence coverage generated by the Entrez Genome Project at the NCBI were used to analyze the distribution of organisms that contain Prx IV. For analysis of the distribution of Prx IV-S and Prx IV-L gene sequences, the human and zebrafish Prx IV sequences were used as initial queries to search for homologous sequences in these genomes with TBLASTN and default parameters (47). Orthologous proteins were defined on the basis of the bidirectional best hit test (20) and phylogenetic analysis. The presence of Prx IV-L was verified by that of exon 1A. Instances of Prx IV-L were further examined by BLASTN searches of expressed sequence tag (EST) data sets. Multiple sequence alignments were performed using ClustalW (21) with default parameters, and ambiguous alignments in highly variable regions were excluded. Phylogenetic trees were prepared with TreePuzzle (22) and drawn with TreeView.
RESULTS
Expression of Prx IV in Mouse Tissues
We first examined the expression of Prx IV in 27 tissues from 12-week-old C57BL/6J mice by immunoblot analysis with antibodies generated in response to a synthetic peptide corresponding to a COOH-terminal sequence of human Prx IV. Consistent with previous observations (8, 14, 16, 23), only a 27-kDa immunoreactive protein was detected in all tissues with the exception of the testis, which showed an immunoreactive protein of ∼29.5 kDa in addition to the 27-kDa protein (supplemental Fig. S1A). The larger Prx IV protein was first detected in the testis at 4 weeks of age (around the time of puberty) and was expressed at a similar level in adult and aged mice from 6 weeks to 2 years of age (supplemental Fig. S1B). In contrast, the 27-kDa form of Prx IV was similarly expressed in both young and mature animals (from 2 weeks to 2 years old of age). The expression levels of the other Prx family members in the testis did not change substantially with age (supplemental Fig. S1B), suggesting that the larger form of Prx IV may play a specific physiological role in spermatogenesis. Immunoblot analysis of the liver from the same animals revealed that the abundance of Prx IV (only the 27-kDa form was detected) was not altered during maturation (supplemental Fig. S1B).
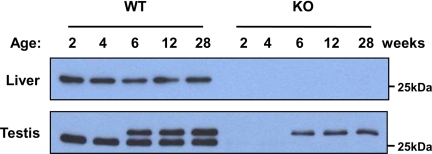
We also performed immunoblot analysis of the liver and testis from Prx IV KO mice, which were generated by deletion of exon 1 of the Prx IV gene. The 27-kDa Prx IV protein was not detected in the liver or testis of the Prx IV KO mice (Fig. 1). Unexpectedly, however, the 29.5-kDa form of Prx IV was detected in the testis of postpubertal and older Prx IV KO mice with the antibodies to the COOH-terminal region of Prx IV, although the intensity of the band was weaker than that in WT animals (Fig. 1). These observations thus suggested that the larger Prx IV protein is not a precursor of the 27-kDa form.
FIGURE 1.
Expression of Prx IV in liver and testis of WT and Prx IV KO mice. The liver and testis from WT and Prx IV KO animals at 2, 4, 6, 12, or 28 weeks of age were subjected to immunoblot analysis with antibodies to the COOH-terminal region of Prx IV. The position of a 25-kDa molecular size standard is indicated.
Prx IV Gene Organization
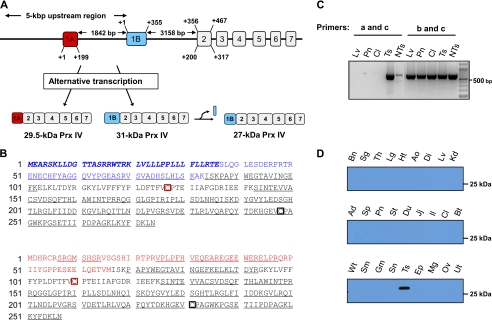
The previously characterized mouse Prx IV gene (Ensembl transcript identification number ENSMUST00000026328; NCBI accession number NM_016764) was aligned with the use of the BLAST and Ensembl search engines (EnsEMBL release 59). A mouse Prx IV isoform with a potential alternative transcription start site was identified in Ensembl with transcript identification number ENSMUST00000112546 and protein identification number ENSMUSP00000108165. This isoform corresponds to a protein of 257 amino acids with an estimated molecular mass of 29,418 Da and a pI of 6.1. The gene identified in Ensembl appears to be transcribed with an alternative first exon (hereafter referred to as exon 1A) located in the promoter region of the previously characterized Prx IV gene; it skips exon 1 (hereafter referred to as exon 1B) of this latter gene and then includes the original exons 2–7 (Fig. 2A). The hydrophobic sequence corresponding to the signal peptide encoded by exon 1B (10) is not encoded by exon 1A. Further analysis of the human database also revealed a transcript (ENST00000418872) and protein (ENSP00000404888) corresponding to an isoform of Prx IV that contains 257 amino acids, including a sequence encoded by exon 1A and lacking that encoded by exon 1B. Database analysis of the mouse Prx IV gene locus by web-based transcription starting site prediction programs suggested the existence of two distinct promoter regions, one located upstream of exon 1A and the other located upstream of exon 1B (supplemental Experimental Procedures).
FIGURE 2.
Identification of alternatively transcribed forms of Prx IV. A, schematic representation of the genomic organization and alternative transcription of the mouse Prx IV gene. The two alternative exons are designated 1A (red box) and 1B (blue box) and encode transcripts for the 29.5- and 31-kDa forms of Prx IV, respectively. Exons 2–7 are shown as gray boxes. The 31-kDa protein is cleaved in the NH2-terminal region to produce a 27-kDa form of Prx IV. The distances between exons 1A, 1B, and 2 are indicated. The 5,000-bp genomic region upstream of exon 1B indicated was subjected to a promoter search (supplemental Experimental Procedures). The map is not to scale. B, amino acid sequences of the proteins encoded by the exon 1B-containing and exon 1A-containing transcripts (31- and 29.5-kDa forms of Prx IV, respectively). Tryptic peptide sequences identified by MALDI-TOF MS analysis are underlined. Amino acids derived from exons 1A and 1B are indicated in red and blue, respectively, and those that constitute the cleaved signal peptide of the 31-kDa protein are shown in italics. The CP and CR residues are boxed in red and black, respectively. C, RT-PCR analysis of Prx IV transcripts in the liver (Lv), pancreas (Pn), colon (Cl), testis of adult mice (Ts), and testis of a 2-day-old neonate (NTs). PCR was performed with pairs of primers specific for exon 1A (primer a) and exon 7 (primer c) or for exon 1B (primer b) and exon 7 (primer c). Size markers are shown in the left-most lane. D, immunoblot analysis of adult mouse tissues with polyclonal antibodies generated in response to two peptides encoded by exon 1A of the Prx IV gene. Bn, brain; Sg, salivary gland; Th, thymus; Lg, lung; Ht, heart; Ao, aorta; Di, diaphragm; Lv, liver; Kd, kidney; Ad, adrenal gland; Sp, spleen; Pn, pancreas; St, stomach; Du, duodenum; Jj, jejunum; Il, ileum; Cl, colon; Bt, brown adipose tissue; Wt, white adipose tissue; Sm, soleus muscle; Gm, gastrocnemius muscle; Sn, skin; Ts, testis; Ep, epididymis; Mg, mammary gland; Ov, ovary; Ut, uterus.
MS Evidence for Alternatively Transcribed Prx IV Isoforms
To determine whether the 29.5-kDa form of Prx IV detected in the mature mouse testis corresponds to the alternatively transcribed form derived from exon 1A, we subjected a lysate of this tissue to immunoprecipitation with the antibodies to the COOH-terminal region of Prx IV. SDS-PAGE and silver staining of the immunoprecipitate revealed two prominent bands corresponding to proteins of 27 and 29.5 kDa (data not shown). The two bands were excised, digested with trypsin, and subjected to MALDI-TOF MS analysis. A single MALDI mass spectrum encompassed 79% of the exon 1A-derived form of Prx IV as well as 74% of the exon 1B-derived form (Fig. 2B). Despite this coverage, however, tryptic peptides corresponding to the first 50 amino acids of exon 1B were not detected (Fig. 2B). This result is consistent with the previous finding that the 36 NH2-terminal amino acids are cleaved from the exon 1B-derived translation product to generate the 27-kDa form of Prx IV (9–11). A tryptic peptide beginning with the 15th amino acid of the 27-kDa form of Prx IV was observed in MALDI-TOF analysis (Fig. 2B). The MS analysis also revealed a tryptic peptide starting at the seventh amino acid of the sequence encoded by exon 1A, which together with four additional peptides covered ∼50% of the exon 1A-derived residues (Fig. 2B). These results indicated that the larger form of Prx IV detected in the testis of mature mice is the product of translation of an exon 1A-containing transcript, which does not encode the NH2-terminal hydrophobic region derived from exon 1B. MS data also confirmed that the smaller (27-kDa) form of Prx IV (hereafter referred to as Prx IV-S) and the larger (29-kDa) form (hereafter referred to as Prx IV-L) differ only in the NH2-terminal sequence derived from exons 1B and 1A, respectively, and that they share the sequences encoded by exons 2–7.
PCR Evidence for Alternatively Transcribed Prx IV Gene Products
To provide further support for the alternative and tissue-specific transcription of the Prx IV gene, we performed RT-PCR analysis of total RNA isolated from mouse tissues with a primer specific for exon 1A (primer a) or for exon 1B (primer b) together with a primer specific for exon 7 (primer c). The sizes of the PCR products derived from primers a and c and from primers b and c are predicted to be 740 and 643 bp, respectively, if two separate transcripts are produced by alternative transcription. Primers a and c generated a PCR product of the expected size with the mature testis but did not give rise to PCR products with other tissues (Fig. 2C). In contrast, primers b and c yielded a PCR product of the predicted size for lung, pancreas, colon, testis, and liver (Fig. 2C), suggesting that Prx IV-L mRNA is specifically produced only in the testis and that Prx IV-S mRNA is generated ubiquitously.
Immunoblot Evidence for Alternatively Transcribed Prx IV Isoforms
We next prepared antibodies specific for the region of Prx IV derived from exon 1A by injecting rabbits with two peptides, MDHRCRSRGMSHSR and REGEEWERELPRQR, that correspond to amino acids 1–14 and 36–49, respectively, of Prx IV-L. Immunoblot analysis of 27 adult mouse tissues with these antibodies revealed that only the testis yielded an immunoreactive band (Fig. 2D), supporting the notion that expression of Prx IV-L is restricted to the adult testis.
Cellular Localization of Prx IV-S and Prx IV-L in Mouse Testis
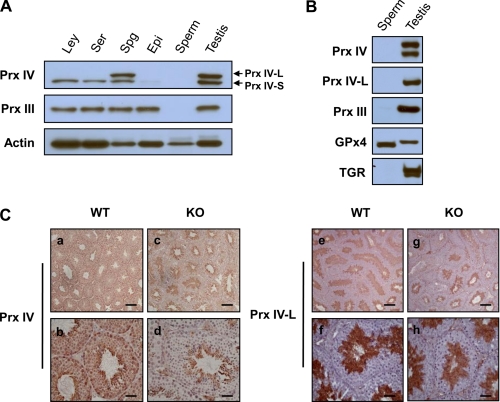
To examine the cellular localization of Prx IV-S and Prx IV-L in the testis, we isolated fractions enriched in Leydig cells, Sertoli cells, or spermatogenic cells from the adult mouse testis and subjected them to immunoblot analysis with the antibodies to the COOH-terminal region of Prx IV. Homogenates of the epididymis and mature sperm were also included in the analysis. Prx IV-S was detected in all three testis cell types, was barely detectable in the epididymis, and was not detected in mature sperm (Fig. 3A). In contrast, Prx IV-L was detected only in the spermatogenic cell-enriched fraction (Fig. 3A).
FIGURE 3.
Cellular localization of Prx IV-L and Prx IV-S in testis. A, Leydig cells (Ley), Sertoli cells (Ser), spermatogenic cells (Spg), the epididymis (Epi), epididymal spermatozoa (sperm), and the testis were isolated from adult C57BL/6J mice and subjected to immunoblot analysis with antibodies to the COOH-terminal region of Prx IV, to Prx III, and to β-actin (loading control). B, epididymal spermatozoa solubilized with 0.1 m 2-mercaptoethanol and 6 m guanidine hydrochloride as well as a testis homogenate prepared from adult C57BL/6J mice were subjected to immunoblot analysis with antibodies to the COOH-terminal region of Prx IV, to Prx IV-L, to Prx III, to glutathione peroxidase 4 (GPx4), and to thioredoxin-glutathione reductase (TGR). C, immunohistochemical localization of Prx IV. Sections of the testis of 20-week-old WT (panels a, b, e, and f) or Prx IV-S KO (panels c, d, g, and h) mice were subjected to immunoperoxidase staining with antibodies to the COOH-terminal region of Prx IV (panels a–d) or to Prx IV-L (panels e–h) and counterstained with hematoxylin. The antibodies to total Prx IV stained all seminiferous tubule cells but not luminal spermatozoa in the WT testis (panels a and b), whereas they stained only spermatogenic cells located closest to the lumen (spermatids) in the KO testis (panels c and d). The antibodies to Prx IV-L stained only spermatids in both WT (panels e and f) and KO (panels g and h) testis. (Scale bars, 200 μm for low magnification and 50 μm for high magnification.)
During the final stage of spermatogenesis, known as spermiogenesis, spermatids begin to grow a tail, the mitochondrial sheath is formed in the sperm midpiece, chromatin becomes condensed, and unnecessary cytosol and organelles are eliminated (24). Two redox-related proteins, glutathione peroxidase 4 and thioredoxin-glutathione reductase, have been studied extensively in association with spermiogenesis (17, 18, 25). Glutathione peroxidase 4 is highly expressed in spermatogenic cells and becomes oxidatively cross-linked to structural components of the sperm midpiece during spermiogenesis (18). Thioredoxin-glutathione reductase, which is expressed at a low level in most tissues but is abundant in spermatids of the postpubertal testis, promotes this cross-linking of glutathione peroxidase 4 by catalyzing disulfide isomerization. After completion of spermiogenesis, thioredoxin-glutathione reductase is released into the residual body (17, 25).
To test whether the apparent absence of Prx IV in sperm (Fig. 3A) was due to cross-linking or to the exclusion of cytosolic components and organelles, we subjected sperm proteins that had been extracted with a denaturing buffer containing mercaptoethanol to immunoblot analysis. As shown previously (17, 18), glutathione peroxidase 4, but not thioredoxin-glutathione reductase, was detected in the sperm extract (Fig. 3B). Prx IV and Prx III (which is located in mitochondria) were not detected in the sperm extract (Fig. 3B), suggesting that they were eliminated during spermiogenesis.
Spermatogenesis takes place in the seminiferous tubules of the testis after puberty. Spermatogonia (immature germ cells) are located around the outer edge (basal lamina) of seminiferous tubules where they proliferate and differentiate into spermatocytes. Spermatocytes undergo two consecutive cycles of meiotic division to produce haploid spermatids, which then undergo morphological differentiation into sperm. All steps of the progression of spermatogonia through spermatocytes and spermatids to sperm occur while the developing gametes are associated with Sertoli cells. As they progress through spermatogenesis, the spermatogenic cells migrate from the outer edge of the seminiferous tubules to a position closer to the lumen. Sperm then escape into the lumen of the seminiferous tubules and subsequently move into the epididymis where they are stored and undergo further maturation. Spermatogenesis depends on testosterone secreted by Leydig cells located between the seminiferous tubules.
To further specify the spermatogenic cell types that express Prx IV, we performed immunohistochemical analysis of paraffin-embedded sections of the testis with the antibodies to the COOH-terminal region of Prx IV as well as with those specific for Prx IV-L. Consistent with previous observations (14), total Prx IV immunoreactivity was detected in all seminiferous tubule cells, being most pronounced in those spermatogenic cells located closest to the lumen, but it was not detected in spermatozoa within the lumen (Fig. 3C). The cells located closest to the lumen have been identified previously as spermatids (14). Total Prx IV immunoreactivity was detected only in spermatogenic cells located closest to the lumen in the testis of Prx IV (Prx IV-S) KO mice (Fig. 3C). Immunostaining of tissue sections obtained from the same animals with the Prx IV-L-specific antibodies revealed immunoreactivity only in spermatogenic cells that appeared to be spermatids in both WT and Prx IV-S KO mice (Fig. 3C). Together, these results thus suggested that Prx IV-L is specifically expressed in spermatids and is eliminated after further differentiation of these cells into spermatozoa.
We also examined the expression of Prx IV-L in various cell lines derived from the testis: TM3 neonatal mouse Leydig cells, TM4 neonatal mouse Sertoli cells, F9 mouse embryonic testis carcinoma cells, LC540 adult rat Leydig cells, and R2C adult rat Leydig cell tumor cells. Immunoblot analysis revealed that none of these cell lines expressed Prx IV-L at a detectable level, whereas Prx IV-S was detected in all of them (supplemental Fig. S2).
Subcellular Localization of Prx IV Isoforms in Testis
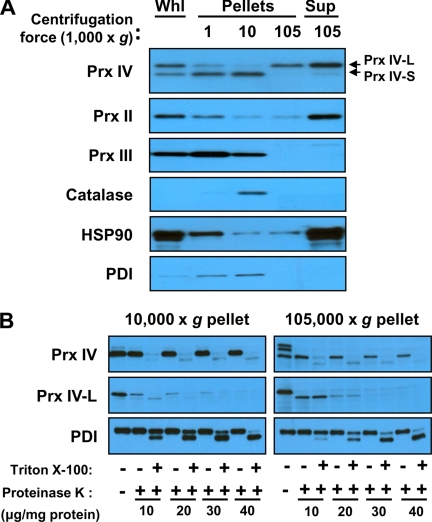
Prx IV-S was identified previously as an ER luminal protein (11, 26). To determine the subcellular localization of Prx IV-L, we fractionated homogenates of the mature testis by differential centrifugation and subjected the resultant fractions to immunoblot analysis with the antibodies to the COOH-terminal region of Prx IV. The fidelity of fractionation was verified by analysis of the cytosolic markers Prx II and heat shock protein 90 as well as of the organellar markers Prx III, catalase, and PDI. Prx II and heat shock protein 90 are localized primarily in the cytosol but are also present in the nucleus, and they interact with various cellular proteins. Prx III, catalase, and PDI are exclusively located in mitochondria, peroxisomes, and the ER, respectively. Prx IV-S was detected in the pellets obtained by consecutive centrifugation of testis homogenates at 1,000 × g and 10,000 × g (Fig. 4A) in which PDI was also detected, consistent with the previously identified ER localization of the 27-kDa form of Prx IV. Most Prx IV-L was detected in the 105,000 × g supernatant, although a small amount was also present in the three pellets, especially in that obtained after centrifugation at 105,000 × g (Fig. 4A).
FIGURE 4.
Subcellular localization of Prx IV-S and Prx IV-L. A, a testis homogenate prepared from 20-week-old C57BL/6J mice was subjected to differential centrifugation at 1,000 × g, 10,000 × g, and 105,000 × g. The whole homogenate (Whl) as well as the pellets obtained at each centrifugation step and the final supernatant (Sup) were subjected to immunoblot analysis with antibodies to the COOH-terminal region of Prx IV and to the indicated proteins. B, the pellets obtained after centrifugation at 10,000 × g and 105,000 × g were incubated with various concentrations of proteinase K in the absence or presence of 0.5% Triton X-100 after which the reaction was stopped by the addition of PMSF and the samples were subjected to immunoblot analysis with antibodies to the COOH-terminal region of Prx IV, to Prx IV-L, and to PDI. HSP, heat shock protein.
To determine whether Prx IV-L detected in the 10,000 × g and 105,000 × g pellets was present within lipid bilayers, we subjected these fractions to digestion with various concentrations of proteinase K in the absence or presence of Triton X-100. Incubation of either the 10,000 × g or 105,000 × g pellets with proteinase K in the absence of Triton X-100 resulted in the proteolysis of Prx IV-L, whereas the ER proteins Prx IV-S and PDI remained intact (Fig. 4B). However, in the presence of Triton X-100, all three proteins underwent proteolysis (Fig. 4B). These results thus indicated that Prx IV-L is not encapsulated within the membrane but rather might be located outside of the membrane like Prx I, which is localized at the cytosolic side of the ER membrane (27).
Insensitivity of Prx IV to Hyperoxidation
Hyperoxidized Prxs I, II, and III are readily detected in tissues or cells that have been exposed to H2O2, suggesting that all of these proteins go through a peroxidase catalytic cycle involving the CP-SOH intermediate (1, 27). The susceptibility of Prx IV, also a member of the 2-Cys Prx subfamily, to hyperoxidation has not been well studied. A recent study indicated that Prx IV (Prx IV-S) also forms a disulfide between Cp-SH and CR-SH but that the resulting disulfide transfers its oxidation state to PDI rather than to thioredoxin, thereby contributing to protein folding in the ER (26). This finding is consistent with the fact that the ER does not contain thioredoxin, which is required for efficient peroxidase function. Hyperoxidized Prxs can be detected by immunoblot analysis with antibodies that recognize a specific sequence surrounding the CP-SO2H moiety (1). Given that the active site sequence (DFTFVCPTEI) is the same for 2-Cys Prxs (Prxs I-IV) and that the sizes of Prx I and Prx II are identical, the sulfinic forms of Prx I and Prx II cannot be differentiated by immunoblot analysis. However, the sizes of Prxs I/II, Prx III, Prx IV-S, and Prx IV-L are sufficiently different for such analysis.
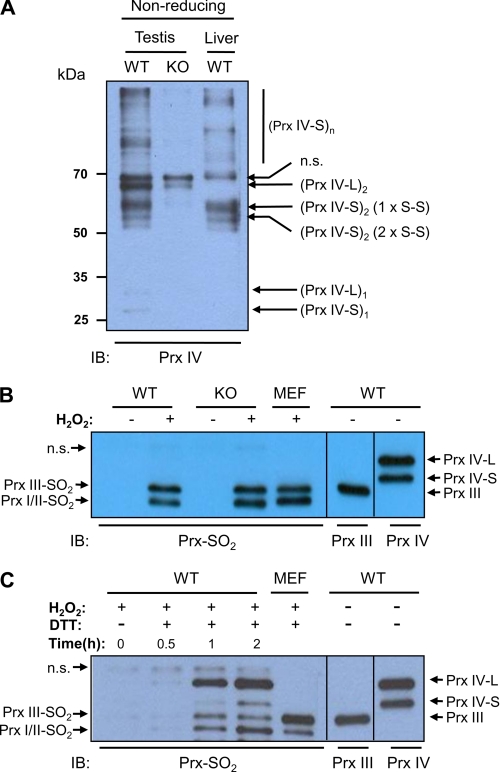
When homogenates of the testis and liver of adult WT mouse were subjected to immunoblot analysis with antibodies to the COOH-terminal region of Prx IV after fractionation by SDS-PAGE under non-reducing conditions, multiple bands were evident (Fig. 5A). It was shown previously that Prx IV-S forms two types of dimer, one type with one CP-S–S-CR disulfide bond and the other type with two CP-S–S-CR disulfide bonds. In addition, it was also shown that Prx IV-S forms a disulfide via a third, noncatalytic cysteine residue, which links subunits within the decamer (11). Formation of the disulfide between noncatalytic cysteines produces oligomers that are larger than dimer (Fig. 5A). In contrast, an immunoblot of the homogenate of Prx IV-S KO mouse testis showed one band corresponding to Prx IV-L dimer, which can be seen also in the blots of WT testis homogenate but not in the blot of WT liver homogenate (Fig. 5A). This result suggests that Prx IV-L exists mainly as a dimer linked by the CP-S–S-CR disulfide bond.
FIGURE 5.
Insensitivity of Prx IV to hyperoxidation. A, testis and liver of adult WT and Prx IV-S KO mouse were homogenized in a lysis buffer (20 mm Tris-HCl, pH 8.0, 137 mm NaCl, 10% glycerol, 1% Nonidet P-40, 2 mm EDTA, and protease inhibitors) containing 2 mm N-ethylmaleimide plus 2 mm iodoacetic acid. The resulting homogenates were fractionated by SDS-PAGE on a 14% gel under non-reducing conditions and subjected to immunoblot analysis with antibodies to the COOH-terminal region of Prx IV. Monomer, dimer, and oligomers of higher order than dimer are indicated using subscript 1, 2, and n after the parenthesis. Dimers of Prx IV-S with one CP-S–S-CR disulfide bond (1 x S–S) and two CP-S–S-CR disulfide bonds (2 x S–S) are indicated. n.s. denotes a nonspecific band. B, slices of the adult WT and Prx IV-S KO mouse testis were incubated for 30 min at room temperature with or without 5 mm H2O2 after which the tissue was homogenized, fractionated by SDS-PAGE on a 14% gel, and subjected to immunoblot analysis with antibodies to sulfinic 2-Cys Prx (Prx-SO2). Lysate of H2O2-treated mouse embryonic fibroblasts (MEF) was also analyzed as a positive control for hyperoxidized Prxs I/II and Prx III. On the same gel, homogenates of adult WT mouse testis were fractionated and then subjected to immunoblot analysis with antibodies to Prx III and to the COOH-terminal region of Prx IV to mark the positions of Prx III, Prx IV-S, and Prx IV-L. The positions of Prx isoforms are indicated. C, homogenates of adult WT testis were incubated with 1 mm H2O2 in the presence or absence of 1 mm DTT for the indicated times at room temperature after which the homogenates were fractionated by SDS-PAGE on a 14% gel and subjected to immunoblot analysis with antibodies to sulfinic 2-Cys Prx (Prx-SO2). Immunoblot analyses of H2O2-treated mouse embryonic fibroblast (MEF) lysate (for a positive control for hyperoxidized Prxs I/II and Prx III) and of adult WT mouse testis homogenate (for the positions of Prx III, Prx IV-S, and Prx IV-L) were as described in B. n.s. denotes a nonspecific band.
When slices of adult WT and Prx IV-S KO mouse testis were exposed to 5 mm H2O2 and then subjected to immunoblot analysis with the antibodies to sulfinic 2-Cys Prx, neither the hyperoxidized form of Prx IV-S nor that of Prx IV-L was detected, whereas an intense band was observed for Prxs I/II and Prx III (Fig. 5). Given that both Prx IV-S and Prx IV-L are abundant in the testis, the hyperoxidation of even a small proportion of these proteins would be expected to be readily detected. However, when homogenates of WT mouse testis were incubated in the presence of H2O2 and DTT, Prx IV-L underwent hyperoxidation as readily as Prxs I/II and Prx III did (Fig. 5C). These results thus suggest that neither Prx IV-S nor Prx IV-L functions as a peroxidase in the testis, but both have peroxidase activity in the presence of an efficient electron donor such as DTT.
Phylogenetic Analysis of Prx IV Gene
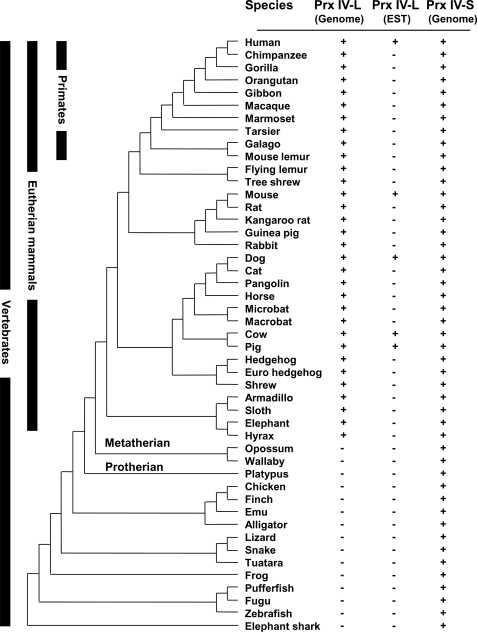
Finally, we examined the evolutionary relations among vertebrate Prx IV gene sequences. The Prx IV-S gene was present in all vertebrates examined (Fig. 6). In addition, several Prx IV-like genes were detected in some insects, including Drosophila melanogaster (not shown). In contrast, the Prx IV-L gene was detected only in placental (eutherian) mammals, not in nonplacental (metatherian and protherian) mammals or in birds, reptiles, amphibians, or fish (Fig. 6). ESTs corresponding to Prx IV-L were detected in human, mouse, dog, cow, and pig. All ESTs were derived from the testis with the exception of a single EST of cow that was of liver origin. Multiple sequence alignments of Prx IV-L confirmed that this protein lacks a signal peptide (supplemental Fig. S3). Overall, computational analysis revealed the presence of Prx IV genes in all vertebrates. The testis-specific Prx IV-L may therefore have evolved from the ancestral ER-resident form of Prx IV specifically in placental mammals through the use of a new exon instead of that encoding the signal peptide. The sequence alignments of Prx IV-L revealed that the amino acid identity among nine mammalian species is 72 and 97% for the sequences encoded by exon 1A and by the remaining exons 2–7, respectively (supplemental Fig. S3).
FIGURE 6.
Occurrence of Prx IV in vertebrates. Forty-seven vertebrate genomes with substantial sequence coverage were selected to illustrate the distribution of Prx IV in vertebrates. In each organism, the presence of Prx IV-L or Prx IV-S forms is indicated. EST databases were also analyzed for the presence of Prx IV-L cDNAs. Prx IV-L orthologs were only detected in eutherian mammals.
DISCUSSION
Recent studies have suggested that mouse Prx IV is synthesized as a 274-amino acid polypeptide (with a calculated molecular mass of ∼31 kDa) with a typical NH2-terminal signal sequence and that cleavage of the 36-amino acid NH2-terminal peptide generates a 27-kDa protein, Prx IV-S. Prx IV-S, which is expressed in almost all cell lines and mouse tissues examined, is retained in the ER despite the absence of the ER retention sequence KDEL. The 27-kDa form of Prx IV is also secreted. Immunoblot analysis of the postpubertal testis revealed a Prx IV protein with a molecular mass of >27 kDa. The larger band was thought to represent the unprocessed, 31-kDa form of Prx IV that is anchored to the ER membrane via its NH2-terminal hydrophobic region before it is secreted into the extracellular space as the 27-kDa form. However, the putative 31-kDa protein was observed reliably only in the postpubertal testis, not in other tissues or cultured cells. A question as to the nature of this 31-kDa form of Prx IV was raised as a result of immunoblot analysis of tissues from Prx IV KO mice that had been generated by deletion of exon 1 (here renamed exon 1B). Such analysis with antibodies to the COOH-terminal region of Prx IV did not detect the 27-kDa band, but the putative 31-kDa band was still apparent in the testis. This unexpected result prompted us to study the nature of the larger form of Prx IV present in the testis.
Our results indicate that the larger band seen in the testis corresponds to a product of alternative transcription of the Prx IV gene: the newly identified exon 1A together with exons 2–7 yields the larger Prx IV protein (here designated Prx IV-L), which consists of 257 amino acids and has a calculated molecular size of 29,418 Da. In contrast, exon 1B together with exons 2–7 yields the 274-amino acid form of Prx IV that is converted to the 27-kDa Prx IV-S. Our conclusion is based on the observations that (i) analysis of the mouse database revealed an alternative transcript that encodes a protein of 257 amino acids, (ii) MALDI-TOF MS analysis of tryptic peptides derived from the larger testis-specific band (Prx IV-L) revealed 79% of the amino acid sequence encoded by exon 1A together with exons 2–7, (iii) RT-PCR analysis of adult mouse tissues with a pair of primers specific for exons 1A and 7 yielded a PCR product of the predicted size only for the testis, and (iv) antibodies targeted to the exon 1A-encoded protein sequence recognized the larger (Prx IV-L) band, but not the 27-kDa (Prx IV-S) band, only in the postpubertal testis. A more accurate estimation of the molecular size of Prx IV-L by SDS-PAGE was 29.5 kDa rather than 31 kDa. Prx IV-L does not contain the NH2-terminal hydrophobic region or cleavage site seen in the precursor of Prx IV-S. Both Prx IV-L and Prx IV-S contain four Cys residues, three of which, including CP and CR, are conserved and are encoded by exons 2–7.
Our proteolysis analysis with proteinase K performed in the absence or presence of Triton X-100 supported the previous conclusion that Prx IV-S, like PDI, is a luminal protein of the ER. The newly identified Prx IV-L, however, was found to be predominantly a cytosolic protein. Although a small proportion of Prx IV-L was detected in the pellets obtained by differential centrifugation of a testis homogenate, the Prx IV-L in these pellets was highly susceptible to digestion by proteinase K in the absence of detergent, suggesting that it is not located within the lipid bilayer of the membrane.
Consistent with previous observations, we found that Prx IV-S is expressed in a wide variety of mouse tissues, whereas Prx IV-L was detected only in the testis after puberty. Age-matched comparison revealed that the levels of Prx IV-L protein in the testis of Prx IV-S KO mice are less than those in WT mice, suggesting that deletion of exon 1B might have an effect on the expression of Prx IV-S. Immunohistochemical analysis revealed Prx IV-S to be present in various cell types throughout seminiferous tubules, including Sertoli cells and their associated germ cells (spermatogonia, primary spermatocytes, and spermatids), but it was not detected in luminal spermatozoa. Analysis of the Prx IV-S KO mouse testis indicated that Prx IV-L expression is restricted to spermatids, and analysis with the antibodies specific for the exon 1A-encoded sequence confirmed this observation. Prx IV-L was not detected by immunoblot analysis in a sperm protein extract prepared with a denaturing buffer containing 2-mercaptoethanol. These observations thus suggested that Prx IV-L accumulates in spermatids during spermatogenesis and that it is released into the residual body during spermiogenesis. The accumulation of Prx IV-L in spermatids and its subsequent exclusion from sperm are reminiscent of the expression pattern for thioredoxin-glutathione reductase, which serves as a disulfide isomerase during chromatin condensation and capsule formation in sperm.
Computational analysis of Prx IV indicated that the Prx IV-S gene is present in all vertebrates examined. However, the Prx IV-L gene was detected only in advanced vertebrates with nonmammalian vertebrates (birds, reptiles, amphibians, and fish) appearing not to possess the gene. Furthermore, the Prx IV-L gene was found to be present in placental mammals but not in nonplacental mammals such as the opossum, wallaby, and platypus, suggesting that it may have evolved in the common ancestor of placental mammals.
The functional maturation and capacitation of mammalian spermatozoa are associated with H2O2 production and glutathione depletion that result in increased oxidative stress in the germ cells (24, 28). As a result of this increased oxidative stress, protein thiols are vastly oxidized during spermiogenesis (24, 29–31). Such thiol oxidation is associated with intra- or intermolecular disulfide formation that is required for chromatin condensation and formation of the supramolecular structure of the sperm tail (24, 32, 33). Condensation of spermatid chromatin is accompanied by replacement of histones with protamines, which are basic nuclear proteins with a high Cys content, as well as by subsequent oxidation of protamine thiol groups to form disulfide linkages that result in protein cross-linking (32, 34). The chromatin of sperm is highly compacted to protect the DNA from deleterious environments with insufficient oxidation of protamine thiol groups being associated with damage to sperm DNA (35–37). The reduction of sperm disulfides followed by decondensation of sperm chromatin in readiness for fertilization is accomplished in oocytes, which contain high levels of glutathione (38). The thiol-disulfide transition of sperm proteins is a unique feature of eutherian reproduction (39, 40). Thus, eutherian sperm nuclei can be decondensed in vitro only by treatment with a denaturing agent and thiols, whereas sperm nuclei of nonmammalian vertebrates or of protherian or metatherian mammals are readily decondensed (40, 41). This difference is related to the fact that protamines of nonmammalian vertebrates and noneutherian mammals lack Cys residues (40). In addition, the supramolecular structure of the sperm tail is strengthened by disulfide bridges in eutherian sperm but not in the sperm of other vertebrates (38).
In view of the facts that expression of Prx IV-L is restricted to eutherian mammals and that the thiol-disulfide transition of structural proteins during sperm development occurs only in such mammals, we speculate that Prx IV-L participates in the formation of disulfide linkages during spermiogenesis. This notion is consistent with the recently suggested role of Prx IV-S in protein folding in the ER (26). Oxidation of thiols to disulfide linkages during protein folding in the ER is achieved with the use of H2O2 produced by oxidant-generating sources such as ER oxidation 1, NADPH oxidase, and mitochondria. In the proposed model, CP-SH and CR-SH of Prx IV-S are first oxidized by H2O2 to form a disulfide. The oxidation status of Prx IV-S is then transferred to PDI thiols, thereby converting them to a disulfide, which finally results in the formation of a disulfide in the protein to be folded. Prx IV in the ER physically interacts with PDI. Prx IV-S is thus thought to function as a sensor of H2O2 in PDI-mediated protein folding (12, 26). Similar to the proposed function of Prx IV-S in the ER, Prx IV-L may sense H2O2 in the cytosol and mediate the formation of disulfide linkages in proteins that participate in chromatin condensation and contribute to the supramolecular structure of the sperm tail. A sensor function for 2-Cys Prx has also been demonstrated in yeast (42, 43). Such a function for 2-Cys Prx proteins is attributed to the fact that their structure facilitates the reaction of CP-SH with low levels of H2O2 (4, 44–46) and to their interaction with target molecules such as PDI (12). The presence of Prx IV in the ER and in maturing eutherian male germ cells may thus allow protein disulfide formation without the production of damaging levels of H2O2. Given that most protein sulfhydryl groups are not highly sensitive to oxidation by H2O2, much higher levels of H2O2 would be required for such oxidation without the presence of sensor proteins.
During the catalytic cycle of 2-Cys Prxs, only a small fraction of CP-SOH is further oxidized to CP-SO2H; for example, only seven of 10,000 catalytic cycles result in hyperoxidation of Prx I (5). After CP-SOH forms a disulfide with CR-SH, it is no longer susceptible to further oxidation. Incubation of 2-Cys Prxs with a high concentration of H2O2 in the absence of the reduced form of thioredoxin thus does not result in a substantial level of hyperoxidation; the sulfinic form of Prx I accumulates only when Prx I molecules turn over continuously. Such accumulation is possible because the reduction of sulfinic forms of 2-Cys Prxs by sulfiredoxin is a slow process (kcat = 0.18/min for Prx I and Prx II) (47). In the present study, incubation of testis tissue with a millimolar concentration of H2O2 resulted in hyperoxidation of Prxs I/II and Prx III but not in that of Prx IV-S or Prx IV-L, consistent with the notion that Prx IV-L does not function as a peroxidase but rather as an H2O2 sensor like Prx IV-S. Whereas most thiol groups of various proteins are oxidized to form disulfide linkages during spermiogenesis and sperm maturation, conservation of some residual thiols in the extracellular domains of membrane proteins appears to be necessary for sperm motility and capacitation (48). Furthermore, conservation of protamine thiols is required for the decondensation of sperm nuclei that is initiated by thiol-disulfide exchange after fertilization (34). The conservation of certain thiols under conditions that promote massive oxidation likely requires specific interaction between an H2O2 sensor protein such as Prx IV-L and the potential protein target of oxidation. Further understanding of the role of Prx IV-L will require identification of such target proteins.
Supplementary Material
This work was supported by grants from the National Research Foundation of Korea (National Honor Scientist Program Grant 2009-0052293 and Bio Research and Development Program Grant M10642040001-07N4204-00110 to S. G. R.) and World Class University Program Grant R31-2008-000-10010-0.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Experimental Procedures and Figs. S1–S3.
- Prx
- peroxiredoxin
- ER
- endoplasmic reticulum
- PDI
- protein disulfide isomerase
- EST
- expressed sequence tag.
REFERENCES
- 1. Woo H. A., Jeong W., Chang T. S., Park K. J., Park S. J., Yang J. S., Rhee S. G. (2005) J. Biol. Chem. 280, 3125–3128 [DOI] [PubMed] [Google Scholar]
- 2. Knoops B., Goemaere J., Van der Eecken V., Declercq J. P. (2011) Antioxid. Redox Signal. 15, 817–829 [DOI] [PubMed] [Google Scholar]
- 3. Fisher A. B. (2011) Antioxid. Redox Signal. 15, 831–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rhee S. G., Woo H. A. (2011) Antioxid. Redox Signal. 15, 781–794 [DOI] [PubMed] [Google Scholar]
- 5. Yang K. S., Kang S. W., Woo H. A., Hwang S. C., Chae H. Z., Kim K., Rhee S. G. (2002) J. Biol. Chem. 277, 38029–38036 [DOI] [PubMed] [Google Scholar]
- 6. Woo H. A., Chae H. Z., Hwang S. C., Yang K. S., Kang S. W., Kim K., Rhee S. G. (2003) Science 300, 653–656 [DOI] [PubMed] [Google Scholar]
- 7. Biteau B., Labarre J., Toledano M. B. (2003) Nature 425, 980–984 [DOI] [PubMed] [Google Scholar]
- 8. Jin D. Y., Chae H. Z., Rhee S. G., Jeang K. T. (1997) J. Biol. Chem. 272, 30952–30961 [DOI] [PubMed] [Google Scholar]
- 9. Okado-Matsumoto A., Matsumoto A., Fujii J., Taniguchi N. (2000) J. Biochem. 127, 493–501 [DOI] [PubMed] [Google Scholar]
- 10. Matsumoto A., Okado A., Fujii T., Fujii J., Egashira M., Niikawa N., Taniguchi N. (1999) FEBS Lett. 443, 246–250 [DOI] [PubMed] [Google Scholar]
- 11. Tavender T. J., Sheppard A. M., Bulleid N. J. (2008) Biochem. J. 411, 191–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jessop C. E., Tavender T. J., Watkins R. H., Chambers J. E., Bulleid N. J. (2009) J. Biol. Chem. 284, 2194–2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wong C. M., Chun A. C., Kok K. H., Zhou Y., Fung P. C., Kung H. F., Jeang K. T., Jin D. Y. (2000) Antioxid. Redox Signal. 2, 507–518 [DOI] [PubMed] [Google Scholar]
- 14. Sasagawa I., Matsuki S., Suzuki Y., Iuchi Y., Tohya K., Kimura M., Nakada T., Fujii J. (2001) Eur. J. Biochem. 268, 3053–3061 [DOI] [PubMed] [Google Scholar]
- 15. Haridas V., Ni J., Meager A., Su J., Yu G. L., Zhai Y., Kyaw H., Akama K. T., Hu J., Van Eldik L. J., Aggarwal B. B. (1998) J. Immunol. 161, 1–6 [PubMed] [Google Scholar]
- 16. Iuchi Y., Okada F., Tsunoda S., Kibe N., Shirasawa N., Ikawa M., Okabe M., Ikeda Y., Fujii J. (2009) Biochem. J. 419, 149–158 [DOI] [PubMed] [Google Scholar]
- 17. Su D., Novoselov S. V., Sun Q. A., Moustafa M. E., Zhou Y., Oko R., Hatfield D. L., Gladyshev V. N. (2005) J. Biol. Chem. 280, 26491–26498 [DOI] [PubMed] [Google Scholar]
- 18. Ursini F., Heim S., Kiess M., Maiorino M., Roveri A., Wissing J., Flohé L. (1999) Science 285, 1393–1396 [DOI] [PubMed] [Google Scholar]
- 19. Nagao Y. (1989) In Vitro Cell Dev. Biol. 25, 1088–1098 [DOI] [PubMed] [Google Scholar]
- 20. Tatusov R. L., Galperin M. Y., Natale D. A., Koonin E. V. (2000) Nucleic Acids Res. 28, 33–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thompson J. D., Higgins D. G., Gibson T. J. (1994) Nucleic Acids Res. 22, 4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schmidt H. A., Strimmer K., Vingron M., von Haeseler A. (2002) Bioinformatics 18, 502–504 [DOI] [PubMed] [Google Scholar]
- 23. Fujii J., Ikeda Y. (2002) Redox Rep. 7, 123–130 [DOI] [PubMed] [Google Scholar]
- 24. Maiorino M., Ursini F. (2002) Biol. Chem. 383, 591–597 [DOI] [PubMed] [Google Scholar]
- 25. Gerashchenko M. V., Su D., Gladyshev V. N. (2010) J. Biol. Chem. 285, 4595–4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zito E., Melo E. P., Yang Y., Wahlander Å., Neubert T. A., Ron D. (2010) Mol. Cell 40, 787–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bae S. H., Sung S. H., Cho E. J., Lee S. K., Lee H. E., Woo H. A., Yu D. Y., Kil I. S., Rhee S. G. (2011) Hepatology 53, 945–953 [DOI] [PubMed] [Google Scholar]
- 28. Griveau J. F., Le Lannou D. (1997) Int. J. Androl. 20, 61–69 [DOI] [PubMed] [Google Scholar]
- 29. Grosshans K., Calvin H. I. (1985) Biol. Reprod. 33, 1197–1205 [DOI] [PubMed] [Google Scholar]
- 30. Shalgi R., Seligman J., Kosower N. S. (1989) Biol. Reprod. 40, 1037–1045 [DOI] [PubMed] [Google Scholar]
- 31. Rufas O., Fisch B., Seligman J., Tadir Y., Ovadia J., Shalgi R. (1991) Mol. Reprod. Dev. 29, 282–288 [DOI] [PubMed] [Google Scholar]
- 32. Balhorn R. (1982) J. Cell Biol. 93, 298–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Seligman J., Kosower N. S., Weissenberg R., Shalgi R. (1994) J. Reprod. Fertil. 101, 435–443 [DOI] [PubMed] [Google Scholar]
- 34. Rousseaux J., Rousseaux-Prevost R. (1995) Biol. Reprod. 52, 1066–1072 [DOI] [PubMed] [Google Scholar]
- 35. Kosower N. S., Katayose H., Yanagimachi R. (1992) J. Androl. 13, 342–348 [PubMed] [Google Scholar]
- 36. Lolis D., Georgiou I., Syrrou M., Zikopoulos K., Konstantelli M., Messinis I. (1996) Int. J. Androl. 19, 23–27 [DOI] [PubMed] [Google Scholar]
- 37. Zini A., Kamal K. M., Phang D. (2001) Urology 58, 80–84 [DOI] [PubMed] [Google Scholar]
- 38. Sutovsky P., Schatten G. (1997) Biol. Reprod. 56, 1503–1512 [DOI] [PubMed] [Google Scholar]
- 39. Bedford J. M., Calvin H. I. (1974) J. Exp. Zool. 187, 181–204 [DOI] [PubMed] [Google Scholar]
- 40. Retief J. D., Krajewski C., Westerman M., Winkfein R. J., Dixon G. H. (1995) Proc. Biol. Sci. 259, 7–14 [DOI] [PubMed] [Google Scholar]
- 41. Oliva R., Dixon G. H. (1991) Prog. Nucleic Acid Res. Mol. Biol. 40, 25–94 [DOI] [PubMed] [Google Scholar]
- 42. Vivancos A. P., Castillo E. A., Jones N., Ayté J., Hidalgo E. (2004) Mol. Microbiol. 52, 1427–1435 [DOI] [PubMed] [Google Scholar]
- 43. Veal E. A., Findlay V. J., Day A. M., Bozonet S. M., Evans J. M., Quinn J., Morgan B. A. (2004) Mol. Cell 15, 129–139 [DOI] [PubMed] [Google Scholar]
- 44. Peskin A. V., Low F. M., Paton L. N., Maghzal G. J., Hampton M. B., Winterbourn C. C. (2007) J. Biol. Chem. 282, 11885–11892 [DOI] [PubMed] [Google Scholar]
- 45. Brigelius-Flohé R., Flohé L. (2011) Antioxid. Redox Signal. 15, 2335–2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hall A., Nelson K., Poole L. B., Karplus P. A. (2011) Antioxid. Redox Signal. 15, 795–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chang T. S., Jeong W., Woo H. A., Lee S. M., Park S., Rhee S. G. (2004) J. Biol. Chem. 279, 50994–51001 [DOI] [PubMed] [Google Scholar]
- 48. de Lamirande E., Gagnon C. (1998) Free Radic. Biol. Med. 25, 803–817 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.