Background: TRPM7 channels are key regulators of cell growth and proliferation.
Results: A natural compound from a Hawaiian soft coral blocks TRPM7 currents and inhibits proliferation.
Conclusion: Waixenicin A represents the first potent and relatively specific inhibitor of TRPM7 ion channels.
Significance: Waixenicin A or structural analogs may have cancer-specific therapeutic potential.
Keywords: Cancer Therapy, Cell Cycle, Cell Division, TRP Channels, Tumor Therapy, TRPM7 Inhibitor, Proliferation, Waixenicin A
Abstract
Transient receptor potential melastatin 7 (TRPM7) channels represent the major magnesium-uptake mechanism in mammalian cells and are key regulators of cell growth and proliferation. They are expressed abundantly in a variety of human carcinoma cells controlling survival, growth, and migration. These characteristics are the basis for recent interest in the channel as a target for cancer therapeutics. We screened a chemical library of marine organism-derived extracts and identified waixenicin A from the soft coral Sarcothelia edmondsoni as a strong inhibitor of overexpressed and native TRPM7. Waixenicin A activity was cytosolic and potentiated by intracellular free magnesium (Mg2+) concentration. Mutating a Mg2+ binding site on the TRPM7 kinase domain reduced the potency of the compound, whereas kinase deletion enhanced its efficacy independent of Mg2+. Waixenicin A failed to inhibit the closely homologous TRPM6 channel and did not significantly affect TRPM2, TRPM4, and Ca2+ release-activated Ca2+ current channels. Therefore, waixenicin A represents the first potent and relatively specific inhibitor of TRPM7 ion channels. Consistent with TRPM7 inhibition, the compound blocked cell proliferation in human Jurkat T-cells and rat basophilic leukemia cells. Based on the ability of the compound to inhibit cell proliferation through Mg2+-dependent block of TRPM7, waixenicin A, or structural analogs may have cancer-specific therapeutic potential, particularly because certain cancers accumulate cytosolic Mg2+.
Introduction
TRPM6 and TRPM7 are members of the melastatin-like transient receptor potential (TRPM)4 subfamily. They possess an ion channel and a functional α-kinase domain and have been implicated in Mg2+ homeostasis (1–3). Originally, TRPM6 was thought to regulate systemic (4, 5) and TRPM7 cellular Mg2+ homeostasis (6). However, recent findings demonstrate that TRPM7 also is critically involved in systemic Mg2+ regulation of mammalian organisms, with the channel function facilitating cellular Mg2+ influx and the kinase activity regulating Mg2+ absorption (6). Both activities are interdependent in that Mg2+ enters through the channel pore and the kinase domain requires Mg2+ ions to function, whereas both Mg2+ and Mg-ATP provide a negative feedback loop by regulating channel activity through binding at specific sites on the kinase domain (2, 7, 8).
Suppression of divalent ion conductance via TRPM7 (9) protects neurons from reperfusion injury after cerebral ischemia (10, 11). When silencing TRPM7, Ca2+ influx and neuronal cell death following ischemic injury are blocked, and neuronal recovery is enhanced (12), linking TRPM7 to neurodegenerative diseases (10, 12–15). TRPM7 has also been implicated as a regulator of cell proliferation (16–20), inducing cell cycle arrest if blocked. This is based on channel function in Mg2+ transport because cell growth can be restored by Mg2+ supplementation (2, 8, 18, 21, 22). Mg2+ is involved in essentially every step of cell proliferation, with cancerous cell growth representing the most detrimental effect of deregulated proliferation. Interestingly, cancerous tissue acts as a Mg2+ trap by accumulating Mg2+ at the expense of plasma or surrounding tissues (23, 24). In vivo experiments revealed a 60% reduction in primary tumor growth and angiogenesis in Mg2+-deficient mice compared with Mg2+-sufficient controls. When reintroducing Mg2+ into the diet of hypomagnesemic mice, tumors rapidly regained proliferation capacity and became 40% larger than those grown in Mg2+-sufficient mice (24). It is tempting to speculate that TRPM7 is involved in these processes.
TRPM7 is expressed abundantly in a variety of human carcinoma cells including gastric adenocarcinoma, breast cancer, and human head and neck carcinoma cells. Suppression of TRPM7 by siRNA and/or non-selective inhibitors has been shown to inhibit the growth of these cell types (18, 19, 21). Overexpression of TRPM7 was detected in breast cancer tissues, and TRPM7 expression levels correlate with their proliferative potential (18). Moreover, TRPM7 has been shown to regulate migration of human nasopharyngeal carcinoma cells (25), indicating a potential role in metastasis.
Currently, there is no known specific inhibitor for TRPM7. Although several compounds have been reported to affect TRPM7, including 2-aminoethyl-diphenylborinate (1), lanthanum (La3+), gadolinium (Gd3+), SKF-96365 (9, 26–28), spermine (27), carvacrol (29), and 5-lipoxygenase inhibitors (30), they lack either potency or specificity or both and are therefore of limited use. Concentrations between 50 and 500 μm of the cell-permeant compound 2-aminoethyl-diphenylborinate have been shown to reduce TRPM7 currents, while enhancing TRPM6 currents, providing an experimental tool to distinguish between both channel types (1). However, 2-aminoethyl-diphenylborinate can also affect Ca2+ release-activated Ca2+ current (CRAC) channels as well as a number of other ion channels in a dose-dependent manner (31–33), thus disqualifying it as specific inhibitor for TRPM7. La3+, Gd3+, and SKF-96365 block TRPM7 but also inhibit other Ca2+-permeable channels, including CRAC channels (9, 26–28). The polyamine spermine has been shown to distinguish between CRAC and TRPM7 channels, blocking only monovalent TRPM7 currents at micromolar concentrations (27). However, spermine and polyamines also inhibit K+ and other cation channels (34–37). Recently, carvacrol was shown to inhibit TRPM7 but also lacks specificity as it inhibits several TRPC and TRPM channels (29) and activates TRPV3 and TRPA1 channels. The 5-lipoxygenase inhibitors, nordihydroguaiaretic acid, AA861, and MK886, have also been shown to affect TRPM7 channels in the micromolar range and independently of lipoxygenase activity (30), but they also affect K+ and Cl− channels (38–41). A pharmacological modulator for the TRPM7 ion channel could be beneficial not only as an experimental tool but also therapeutically in cardiac, neuropathological, or anti-cancer treatment. We therefore screened an in-house library of marine-derived natural products and identified waixenicin A as a highly potent and relatively selective inhibitor for TRPM7 that effectively suppressed cell growth and proliferation.
EXPERIMENTAL PROCEDURES
For detailed methods, see the supplemental “Experimental Procedures.”
Animal Material and Cell Line Origin
Freeze-dried samples of Sarcothelia edmondsoni (formerly known as Anthelia edmondsoni) were extracted in methanol and reconstituted in methanol/ethyl acetate/t-butyl methyl ether (60:30:10) (MET) and diluted in Krebs-Ringer-HEPES buffer (135 mm NaCl, 5 mm KCl, 1.5 mm MgCl2, 1.5 mm CaCl2, 20 mm HEPES, and 5.6 mm glucose). HEK293 cell lines were obtained from Dr. Andrew Scharenberg's laboratory (TRPM2, TRPM7-wt, and mutants) and Dr. Jean-Pierre Kinet's laboratory (TRPM4). rat basophilic leukemia cells (RBL1) cells were acquired through ATCC. Human Jurkat T-lymphocytes were obtained from Dr. Kinet's laboratory. No authentication was performed by the authors other than testing for ion channel expression through electrophysiological measurements.
Isolation of Waixenicin A
Fractionation of the soft coral extract was guided by LC-MS data (electrospray ionization; ion trap analyzer; Thermo Finnigan LCQ Deca XP) obtained for the extract peak corresponding to the highest TRPM7 activity. The extract was subjected to vacuum liquid chromatography and fraction eluting with dichloromethane/methanol (95:5) concentrated the targeted compound. Repeated semi-preparative reversed phase HPLC led to the isolation of waixenicin A (see Fig. 1E), which was identified by comparison of NMR data and optical rotation with literature values (42). Purity of our waixenicin A sample was determined to be >95%, and lyophilized waixenicin A was dissolved in 60 μl of methanol (100 μm stock solution) stored at −20 °C.
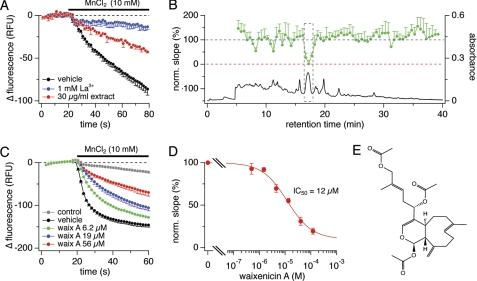
FIGURE 1.
Screening assay identifies waixenicin A as TRPM7 inhibitor. A, decrease in relative fluorescence units (RFU) following 10 mm MnCl2 application in HEK293-TRPM7. Vehicle was negative control (black, n = 10). La3+ (blue, n = 10) and the extract (red, n = 2) reduced Mn2+-induced fluorescence quench. Error bars represent S.D. B, HPLC chromatogram (UV absorbance at 220–240 nm) of extract fractionation (black) and bioassay profile for the fractions (green) plotted as normalized slopes of fluorescence quench against retention time. Error bars represent S.D. C, HEK293-TRPM7 cells were incubated with waixenicin A for 15 min before 10 mm MnCl2 application: uninduced HEK293 control (gray, n = 8), vehicle (black, n = 8), waixenicin A at 6.2 μm (green, n = 3), 19 μm (blue, n = 3), and 56 μm (red, n = 3). D, maximum slopes of fluorescence quench normalized to vehicle control, plotted against concentration, and approximated by dose-response fit (n = 3–8). Error bars represent S.D. E, chemical structure of waixenicin A (relative configuration shown).
Statistical Analysis
Data represent the mean of individual experiments ± S.E. and Student's t test assessed p < 0.05 as statistically significant.
RESULTS
Waixenicin A Is TRPM7 Inhibitor Isolated from Hawaiian Soft Coral Sarcothelia edmondsoni
We screened an in-house chemical library of 1100 marine organism-derived extracts in a high-throughput assay system that measures the fluorescence quench of intracellular fura-2 by Mn2+ ions in HEK293 cells overexpressing murine TRPM7 (43). We identified the organic extract of the soft coral S. edmondsoni (synonym: Anthelia edmondsoni) as a strong inhibitor of TRPM7-mediated Mn2+ influx at a concentration of 30 μg/ml (Fig. 1A). The assay was further employed to identify the major active component by bioassay-linked fractionation (44). Fig. 1B shows the HPLC chromatogram and bioassay profile for the resulting 70 fractions. The highest activity concentrated in fractions eluting at 16.5–18 min, corresponding to the UV peak at 17.1 min. The active peak was characterized by HPLC coupled to a mass spectrometer (LC-MS), leading to the isolation and identification of waixenicin A (Fig. 1E), a known metabolite from S. edmondsoni (42). Waixenicin A inhibited TRPM7-mediated Mn2+ quench in a dose-dependent manner (Fig. 1C) and demonstrated an IC50 of the maximal slope of the Mn2+ quench of 12 μm (Fig. 1D).
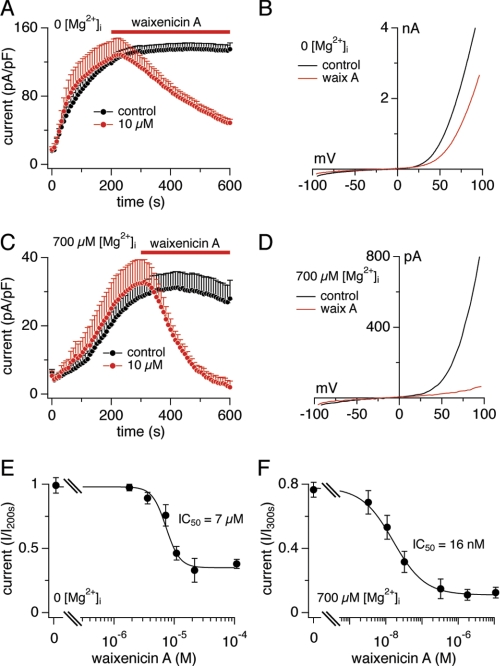
Analysis of waixenicin A in patch clamp experiments (Fig. 2) confirmed the inhibitory effect on TRPM7. To activate TRPM7 currents, intracellular Mg2+ and Mg-ATP were washed out by perfusion with Mg2+- and ATP-free internal solution. Whole-cell currents were elicited by voltage ramps from −100 to +100 mV delivered at 0.5 Hz, and current amplitudes were extracted at +80 mV and plotted versus time. TRPM7 currents reached a plateau of ∼130 pico-Ampere per pico-Farad within 200 s, whereas application of 10 μm waixenicin A for 300 s inhibited TRPM7 by ∼50% (Fig. 2, A and B). The dose-response analysis of waixenicin A-mediated inhibition of TRPM7 revealed an IC50 of 7 μm (Fig. 2E). Outward and inward currents were affected similarly (supplemental Fig. S1A). In the presence of physiological 700 μm free internal Mg2+ ([Mg2+]i), TRPM7 currents were smaller, leveling off at ∼30 pA/pF (Fig. 2, C and D), and 10 μm waixenicin A completely blocked the current. The dose-response curve obtained with 700 μm [Mg2+]i dramatically shifted the IC50 from 7 μm in 0 [Mg2+]i to 16 nm (Fig. 2F). Inward and outward currents were blocked similarly (supplemental Fig. S1B).
FIGURE 2.
Mg2+ dependence of waixenicin A block. Error bars represent S.E. A, TRPM7 current densities in HEK293-TRPM7 without (black, n = 8) and with 10 μm waixenicin A (red, n = 9). B, I/V relationships are representative currents obtained at 500 s. C, TRPM7 current densities in the presence of 700 μm [Mg2+]i without (black, n = 10) and with waixenicin A (red, n = 8). Same analysis as described in A. D, I/V relationships are representative currents obtained at 600 s. E, waixenicin A was applied as described in A. Currents were extracted at +80 mV at 500 s, normalized to current at 200 s, plotted against concentration, and approximated by dose-response fit (n = 8–10). F, waixenicin A was applied as described in C. Currents were extracted at +80 mV at 600 s, normalized to current at 300 s, and analyzed as described in E (n = 5–13).
Additional removal of extracellular Mg2+ resulted in a 2-fold increase of TRPM7 currents compared with 2 mm extracellular Mg2+ (supplemental Fig. S2A), indicating that Mg2+ influx through TRPM7 contributes to reduced channel activity. 10 μm waixenicin A was less effective in suppressing TRPM7 in extracellular Mg2+-free conditions, causing 30% inhibition compared with 50% when extracellular Mg2+ was present. This suggests that waixenicin A synergizes with intracellular Mg2+ in suppressing channel activity.
Blocking Potency of Waixenicin A Is Regulated by Intracellular Mg2+
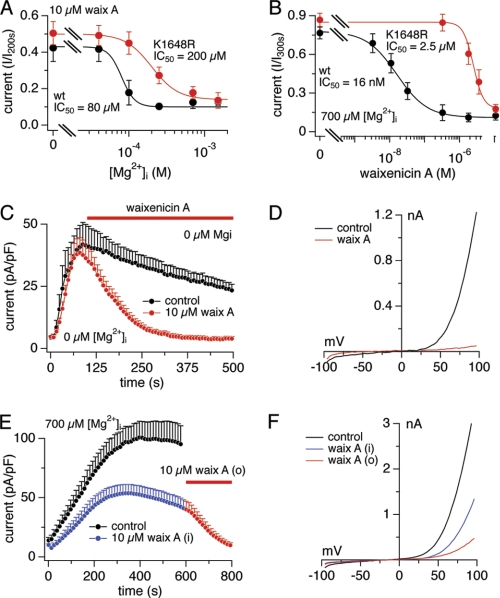
We investigated the Mg2+ dependence of waixenicin A by applying 10 μm waixenicin A at various internal Mg2+ concentrations. We observed an IC50 of 80 μm [Mg2+]i for both outward (Fig. 3A) and inward TRPM7 currents (supplemental Fig. S1C). Thus, 10 μm waixenicin A exhibited an equivalent blocking potency as millimolar levels of Mg-ATP as the IC50 values for [Mg2+]i in the presence of 2–6 mm Mg-ATP are 110–250 μm (7). This would suggest that Mg2+ facilitates waixenicin A binding to TRPM7 or that the compound increases efficacy of block by free internal Mg2+.
FIGURE 3.
Waixenicin A inhibits TRPM7 kinase domain mutants. Error bars represent S.E. A, dose-response curve for [Mg2+]i in waixenicin A-treated HEK293 cells overexpressing TRPM7-wt and TRPM7-K1648R. Current amplitudes at 600 s were normalized to maximal current, averaged, plotted against [Mg2+]i, and approximated by a dose-response fit (n = 6–10). B, data acquisition and analysis as in Fig. 2C. Normalized currents of TRPM7-wt cells (black) taken from Fig. 2F and TRPM7-K1648R cells (red) are plotted against concentration and approximated by dose-response fit (n = 6–9). C, TRPM7-ΔK current densities in zero [Mg2+]i without (black, n = 7) and with 10 μm waixenicin A (waix A; red, n = 8). D, I/V relationships are representative currents obtained at 500 s. E, TRPM7-wt currents in the presence of 700 μm [Mg2+]i without (black, n = 17) and with 10 μm waixenicin A applied first intracellularly (blue/red, n = 14) and then extracellularly (red, n = 14). F, I/V relationships are representative currents obtained at 600 s (black and blue) and 800 s (red).
The Lys-1648 residue within the TRPM7 kinase domain represents one of the inhibitory sites for Mg2+ and Mg-ATP (2, 7, 8). A stable cell line inducibly overexpressing the K1648R mutant has a reduced Mg2+ sensitivity with an IC50 for Mg2+ of ∼3 mm compared with ∼700 μm for TRPM7-wt (8). We first assessed the dose-response behavior of waixenicin A, whereas [Mg2+]i was clamped to 700 μm. The dose-response relationships in Fig. 3B reflect normalized current amplitudes at +80 mV extracted after 300 s of waixenicin A exposure. They demonstrate that the K1648R mutant has a 150-fold reduced sensitivity to the Mg2+-dependent inhibition of waixenicin A compared with TRPM7-wt (IC50 = 2.5 μm versus 16 nm, respectively). We next assessed the efficacy of 10 μm waixenicin A to inhibit TRPM7-K1648R currents while varying [Mg2+]i. 10 μm waixenicin A (Fig. 3A) shifted waixenicin A sensitivity to Mg2+-dependent inhibition of the K1648R mutant by almost 3-fold compared with TRPM7-wt (IC50 = 200 μm versus 80 μm, respectively). Together, these results suggest that the Mg2+ binding site on the kinase domain contributes to the inhibitory potency of waixenicin A.
We next tested compound activity in HEK293 cells overexpressing a TRPM7 protein lacking the entire kinase domain (TRPM7-ΔK). Despite the lack of the kinase domain and its Mg2+ binding site, the ΔK mutant has been shown to be even more sensitive to intracellular Mg2+ levels, as kinase truncation appears to expose a second high affinity Mg2+ binding site on the channel (8). Thus, we used Mg2+-free intracellular solutions to elicit TRPM7-ΔK currents (Fig. 3, C and D). Waixenicin A (10 μm) was still able to inhibit TRPM7 conductance completely and the block was even more pronounced than in TRPM7-wt (cf. Fig. 2A and supplemental Fig. S2A). In the complete absence of both intra- and extracellular Mg2+, TRPM7-ΔK currents were larger but were still completely suppressed by 10 μm waixenicin A (supplemental Fig. S2B). Even 300 nm waixenicin A completely inhibited TRPM7-ΔK, suggesting a direct and Mg2+-independent high affinity mode of action.
Waixenicin A was also effective when applied intracellularly in TRPM7-overexpressing HEK293 cells with internal solution containing 700 μm Mg2+ and 10 μm waixenicin A, causing a ∼50% reduction in current (Fig. 3, E and F). Reduced efficacy when applied through the patch pipette is not unexpected for a lipophilic compound due to diffusional escape of waixenicin A across the plasma membrane. Additional extracellular application of 10 μm waixenicin A completely suppressed the current. These results suggest that the primary site of action of waixenicin A is intracellular.
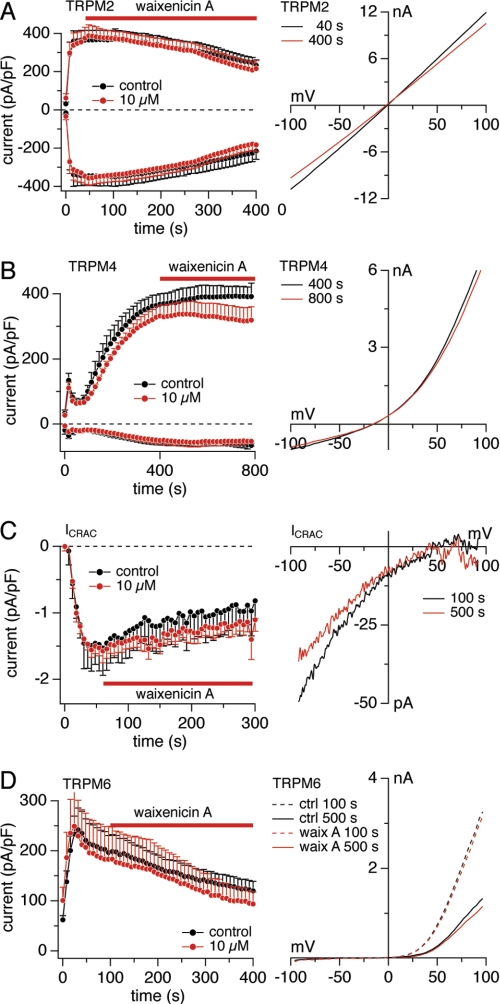
Waixenicin A Is a Relatively Specific TRPM7 Inhibitor
Although a few molecules have been reported to inhibit TRPM7, they all require micromolar concentrations, and none can be considered selective. We tested waixenicin A for selectivity against a panel of other cation channels, such as CRAC and members of the TRP superfamily, TRPM2, TRPM4, and the closest homolog of TRPM7, TRPM6. Although this is not an exhaustive panel of ion channels, they either are expressed in Jurkat and RBL cell lines we have studied in cell proliferation assays (see below) or are particularly interesting, as in the case of TRPM6, which has the highest homology to TRPM7.
The Ca2+-permeable ion channel TRPM2 conducts monovalent and divalent cations (45–48). TRPM2 currents elicited by 100 μm adenosine-diphosphate ribose were recorded in HEK293 cells overexpressing TRPM2 (46). The application of 10 μm waixenicin A had no effect on TRPM2 current amplitudes or I/V relationships extracted before and after waixenicin A treatment (Fig. 4A).
FIGURE 4.
Waixenicin A is specific for TRPM7. Error bars represent S.E. A, current densities at −80/+80 mV elicited by 100 μm intracellular ADP-ribose in HEK293-TRPM2 cells without (black, n = 6) and with 10 μm waixenicin A (red, n = 8). Corresponding average I/V relationships in response obtained before (black, n = 6) and at the end of waixenicin A (waix A) application (red, n = 8). B, current densities at −80/+80 mV elicited by 3 μm [Ca2+]i in HEK293-TRPM4 cells without (black, n = 6) and with 10 μm waixenicin A (red, n = 7). Corresponding I/V relationships are average currents obtained before (black, n = 6) and at the end of waixenicin A application (red, n = 7). C, native CRAC current densities at −80 mV elicited by 20 μm inositol 1,4,5-trisphosphate in RBL1 cells without (black, n = 6) and with 10 μm waixenicin A (red circles, n = 6). Corresponding I/V relationships are average currents obtained before (black, n = 6) and at the end of waixenicin A application (red, n = 6). D, current densities at +80 mV elicited by Mg2+-free solution in HEK293-TRPM6 cells without (black, n = 11) and with 10 μm waixenicin A application (red, n = 9). E, representative I/V relationships extracted at 100 s (dashed lines) and 500 s (solid lines) from untreated controls (ctrl; black) and waixenicin A-treated cells (red).
TRPM4 is a Ca2+-activated non-selective monovalent cation channel (49). TRPM4 currents were activated by 3 μm free internal Ca2+ in HEK293 cells overexpressing TRPM4 (49) and exposed to 10 μm waixenicin A. Both current amplitudes and I/V relationships remained unaffected by waixenicin A (Fig. 4B).
Because CRAC channels have similar pharmacological properties as TRPM7 (26, 28) and can be blocked by 2-aminoethyl-diphenylborinate, La3+, Gd3+, and SKF-96365, we investigated the effect of 10 μm waixenicin A on native ICRAC. CRAC currents elicited by 20 μm inositol 1,4,5-trisphosphate in RBL1 cells remained unaffected by waixenicin A (Fig. 4C).
TRPM6 is the closest subfamily relative of TRPM7, and they share similar permeation profiles and regulation by intracellular Mg2+ (4, 5, 50). We transfected HEK293 cells with TRPM6 and activated it with internal Mg2+-free solution. Application of 10 μm waixenicin A did not alter TRPM6 current densities or I/V relationships compared with untreated controls (Fig. 4D). Unlike TRPM7, TRPM6 currents inactivated over time in both control and waixenicin A-treated cells. Taken together, our findings suggest that waixenicin A may indeed be relatively specific for TRPM7 as it does not significantly affect any other ion channels tested, including its closest homolog, TRPM6.
Waixenicin A Blocks Native TRPM7 Currents and Inhibits Cell Proliferation
We next investigated waixenicin A effects on native TRPM7. We used Jurkat T-cell lymphoma and RBL1 cells because these are the cell types in which native TRPM7 has been most extensively characterized, including biophysical channel characteristics, pharmacology, cell cycle, and involvement in cell proliferation and growth (2, 8, 20, 26, 27, 51). We perfused RBL1 cells with 0 or 700 μm intracellular free Mg2+ and exposed them to waixenicin A. In the absence of intracellular Mg2+, native TRPM7 conductance was blocked partly by 10 μm waixenicin A and 300 nm waixenicin A had no effect (supplemental Fig. S3A). In the presence of 700 μm free intracellular Mg2+, however, 300 nm waixenicin A was sufficient to completely block TRPM7 conductance (supplemental Fig. S3B). Increasing waixenicin A concentration to 10 μm accelerated inhibition of TRPM7 currents 2-fold, as determined by slope analysis (300 nm = −0.012 pA/pF/s versus 10 μm = −0.025 pA/pF/s).
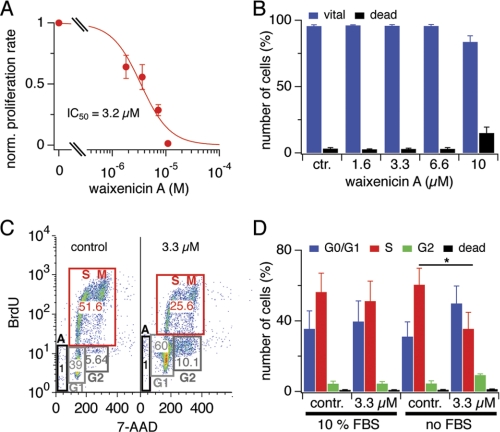
Because TRPM7 has been implicated in cell growth and proliferation, we evaluated the proliferative activity of RBL1 cells incubated for 2 days with waixenicin A. Vital cell counts using trypan blue and mitochondrial dehydrogenase assays revealed a dose-dependent reduction in viable cell numbers (supplemental Figs. S4A and 5A) and observed that only at the highest concentration of 10 μm the number of dead cells increased (supplemental Fig. S4, B and C). We additionally performed annexin V/propidium iodide staining with subsequent flow cytometric analysis of RBL1 cells treated with waixenicin A for 2 days to assess apoptotic cell death (supplemental Fig. S5). Again, only the highest concentration of waixenicin A (10 μm) became toxic (Fig. 5B and supplemental Fig. S5F). Thus, the cell number reduction by waixenicin A below 10 μm was primarily due to inhibition of proliferation rather than toxicity.
FIGURE 5.
Waixenicin A inhibits proliferation of RBL1 cells. Error bars represent S.E. A, normalized proliferation rate of RBL1 cells treated with waixenicin A for 2 days and analyzed via MTT assay (n = 3). The absorbance ratio of untreated controls represents 100% of proliferation rate. B, RBL1 cells were treated as in A, stained with annexin V/phosphatidylinositol, and analyzed via flow cytometry. Bars represent normalized (norm.) cell numbers (n = 3). C, cell cycle analysis of RBL1 cells incubated with or without 10% FBS, 3.3 μm waixenicin A, and BrdU for 6 h. Cells were stained with antiBrdU antibody and 7-AAD and analyzed via flow cytometry. Representative plots of control (contr.) cells and cells treated with 3.3 μm waixenicin A. D, statistical analysis of data set described in E. Bars represent normalized cell numbers (n = 3).
Inhibition of proliferation by waixenicin A in intact cells revealed an IC50 in the low μm range (Fig. 5A and supplemental Fig. S4A), whereas in patch clamp experiments, it was in the nanomolar range (supplemental Fig. S3B). One explanation for this discrepancy could be the presence of serum proteins in growth assays (10% FBS). We performed cell cycle analysis using a BrdU/7-amino-actinomycin D assay to evaluate the impact of serum on waixenicin A activity. RBL1 cells were incubated in DMEM with or without 10% FBS and 3.3 μm waixenicin A was added 10 min before BrdU (Fig. 5, C and D). Cell populations slightly shifted along the y axis due to autofluorescence of waixenicin A (supplemental Fig. S6). The number of waixenicin A-treated cells in the S phase (for gating, see supplemental data) decreased by half (to 25.6 from 51.5% in control), whereas the number of cells in the G1 and G2 phase almost doubled (from 39 to 60% for G1 and from 5.6 to 10.1% for G2) (Fig. 5C). However, 3.3 μm waixenicin A in the presence of 10% FBS was not sufficient to block cells from entering the S phase (p < 0.01) (Fig. 5D). Thus, the efficacy of waixenicin A was significantly higher without serum than in its presence, indicating that serum may partially explain the difference in potency observed in proliferation versus patch clamp assays.
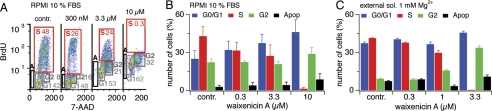
To further assess cell cycle progression, we investigated Jurkat T-cells and confirmed the dose-dependent inhibitory effect of waixenicin A (Fig. 6, A and B). Cells were treated with different concentrations of waixenicin A and exposed to BrdU for 2 h in media containing 10% FBS. Waixenicin A was more potent in Jurkat cells than in RBL1 cells, as 300 nm waixenicin A effectively prevented cells from entering S phase (26 versus 48% in control). Increasing its concentration to 3.3 μm decreased the amount of cells entering the S phase by half to 24%, and 10 μm waixenicin A completely abolished S phase progression. Although the number of Jurkat cells in the S phase decreased with increased waixenicin A concentration, the number of cells in the G1 phase increased, demonstrating dose-dependent growth arrest (Fig. 6B). When using the same external solution as in patch clamp experiments, efficacy was further enhanced, and 3.3 μm waixenicin A completely blocked entry into S phase (Fig. 6C). Taken together, the results establish that waixenicin A selectively blocks heterologous and native TRPM7 currents, exhibits nanomolar potency in the presence of intracellular Mg2+, and arrests cell growth and proliferation with low cytotoxicity in two different tumor cell lines.
FIGURE 6.
Waixenicin A causes growth arrest in Jurkat T-cells. Error bars represent S.E. A, cell cycle analysis of Jurkat cells incubated in RPMI media with 10% FBS and waixenicin A plus BrdU for 2 h (n = 3). Cells were stained with antiBrdU antibody and 7-AAD and analyzed via flow cytometry. B, statistical analysis of the data set as described in A. Bars represent normalized cell numbers (n = 3). C, Jurkat cells were incubated in standard external solution (sol.) containing 1 mm Mg2+ with no FBS. Different waixenicin A-concentrations were applied for 2 h, and cells were analyzed as described in B. Bars represent average cell numbers (n = 3). Apop, apoptotic cells.
DISCUSSION
TRPM7 is a ubiquitous Mg2+- and Ca2+-permeable channel vital for cellular Mg2+ homeostasis and crucial for cell survival (2, 19, 21). Targeted deletion of TRPM7 in DT40 chicken B-cells results in cell cycle arrest in G1/G0 phase and subsequent inhibition of cell proliferation (2, 8), and mice lacking the full-length protein (52) or just the kinase domain of TRPM7 die during early embryonic development (6). Suppressing TRPM7 expression in hippocampal neurons is neuroprotective, as it reduces ischemic cell death, preserves cell function, and prevents ischemia-induced deficits in memory (12). This is likely caused by decreases in TRPM7-mediated Ca2+ or Zn2+ influx (9, 11, 12). At present, there are only relatively unspecific blockers for TRPM7 that also affect other ion channels such as the CRAC channels (9, 26–28) or other TRP channel family members (29). A specific pharmacological approach that could be used to acutely and temporarily suppress TRPM7 function would provide better insights into the role of TRPM7 in malignant cell proliferation and neurotoxicity.
This study establishes waixenicin A, a xenicane diterpenoid from the Hawaiian soft coral S. edmondsoni with no previously reported activity (42), as a highly potent and selective inhibitor of the TRPM7 ion channel. A screening assay based on Mn2+ quenching of fura-2 (43) identified TRPM7 inhibitory activity for the soft coral extract and waixenicin A as the major active component of the extract, whereas patch clamp experiments confirmed waixenicin A as a TRPM7 antagonist. Additionally, it demonstrates that the pharmacological inhibition of TRPM7 by waixenicin A causes growth arrest in G0/G1 phase of the cell cycle.
The inhibitory effects of waixenicin A on TRPM7 are strongly dependent on the prevailing intracellular Mg2+ concentration. This further distinguishes the compound from other known pharmacological TRPM7 inhibitors, which are mostly nonspecific pore blockers and do not have a known Mg2+ dependence or nanomolar potencies (9, 26–28, 30). Mutational analysis involving the channel kinase domain and its previously identified Mg2+ binding site reveal a complex interaction of waixenicin A and Mg2+. Under physiological conditions of 700 μm Mg2+, waixenicin A inhibits TRPM7 with an IC50 of 16 nm, but its potency is greatly reduced when removing Mg2+, resulting in an IC50 of just 7 μm. This indicates that waixenicin A and [Mg2+]i synergize to inhibit the channel. The relevant Mg2+ binding site involved in this mechanism appears to be Lys-1648 within the kinase domain. This residue has been demonstrated to alter the sensitivity of the channel to intracellular Mg2+ and Mg-ATP (7, 8). In this study, we demonstrate that removing this Mg2+ binding site through mutation K1648R indeed caused a dramatic shift in the dose-response curve to waixenicin A from an IC50 of 16 nm in TRPM7-wt to 2.5 μm in the presence of physiological [Mg2+]i levels of 700 μm. Because Mg2+ itself acts as an inhibitor of TRPM7, the synergy between waixenicin A and Mg2+ could be either due to waixenicin A-mediated enhancement of Mg2+ block or that Mg2+ enhances binding affinity of waixenicin A.
Although we have established an interaction between the Lys-1648 residue of the kinase domain and waixenicin A effects, the kinase deletion mutant TRPM7-ΔK suggests that waixenicin A does not necessarily bind to the kinase domain itself, as waixenicin A remains effective in blocking the channel even when the entire kinase domain is absent. In fact, waixenicin A is more potent and effective at blocking TRPM7-ΔK than either wild-type or K1648R channels and does so even in the complete absence of extra- and intracellular Mg2+. This indicates that waixenicin A can bind to TRPM7 outside of the kinase domain with high affinity and block the channel independently of Mg2+ at least in TRPM7 truncation constructs. Although other interpretations cannot be ruled out, the following scenario would most adequately explain our observations: in the absence of Mg2+, the TRPM7-wt channel has a low affinity to waixenicin A because the binding site of the compound is partly masked. At physiological Mg2+ levels, when the Lys-1648 residue is occupied with Mg2+, TRPM7 will be in a conformational state that enables waixenicin A to bind with high affinity and effectively block the channel. Thus, the synergy between waixenicin A and Mg2+ appears to be caused by a change in affinity of TRPM7 for waixenicin A rather than enhancement of Mg2+ block. Removing the entire kinase domain exposes the waixenicin A binding site so that waixenicin A can bind with high affinity and block TRPM7 largely independently of Mg2+.
Waixenicin A suppressed cell growth in various proliferation assays of RBL1 and Jurkat T-cells, complementing previous observations that TRPM7-deficient cells fail to grow and proliferate (20). Waixenicin A induced a dose-dependent reduction of cells in synthesis phase and an accumulation of cells in G0/G1 and G2 phase of the cell cycle. This suggests that the G1/S as well as the G2/M transition is impaired. It has been shown that TRPM7-deficient cells exhibit substantially decreased signaling along the PI3K pathway (20). A key effector in this pathway is protein kinase B (or Akt). Constitutive protein kinase B expression was not sufficient to support TRPM7-independent growth, whereas PI3K expression was sufficient (20). The PI3K pathway is activated during G1/S transition, and PI3K is required for G1/S phase progression in lymphocytes. In particular, PI3K activation is linked to restriction point progression and coincides with G1 events that cumulate in cyclin E/cyclin-dependent kinase-2 activation. Thus, PI3K pathway activation is required for G1/S progression and PI3K inhibition leads to G1 arrest in many cell types (reviewed in Ref. 53). Moreover, the PI3K/protein kinase B pathway may also regulate the efficiency of G2/M phase progression (54), suggesting cross-talk between the PI3K pathway and key regulators of DNA damage checkpoint machinery. It is therefore not surprising that we find fewer cells in S phase, whereas the numbers of cells in G1/G0 and G2 phase increase during waixenicin A treatment. Thus, our data suggest that blocking TRPM7 conductance, resulting in failure of G1/S and G2/M progression, might also negatively affect the PI3K/protein kinase B pathway as previously shown for TRPM7 knock-out DT40 cells (20).
We found that waixenicin was less potent in long term cell growth assays (low micromolar range) compared with acute inhibitory effects observed in patch clamp experiments (low nanomolar range). Possible factors that might contribute to this discrepancy include cellular sequestration of the waixenicin A, conversion into inactive metabolites, up-regulation of compensatory Mg2+ transport mechanisms that counteract the lowered Mg2+ transport via TRPM7, and/or reduced bioavailability of waixenicin due to serum binding. Indeed, we found that waixenicin A was less potent in the presence of serum, indicating direct binding of waixenicin A to serum proteins, and thereby effectively lowering free waixenicin A concentration. Moreover, serum and growth factors in the medium may activate additional mitogenic signals such as cyclin-dependent kinases, which contribute to cell cycle progression and might offset the inhibition by waixenicin A. Proliferative signals can also be transduced by proto-oncogenes, even in the face of reduced or absent growth factors. Constitutive oncogene activation plays a key role in carcinogenesis and tumor progression. Because constitutive or increased activity of PI3K-dependent pathways present a major means whereby tumor cells achieve uncontrolled proliferation (53), waixenicin A might be particularly effective in targeting oncogenic cell growth. Additionally, tumor cells accumulate Mg2+ at the expense of surrounding cells, and waixenicin A is much more potent in inhibiting TRPM7 conductance in the presence of elevated intracellular Mg2+. Thus, waixenicin A represents a novel natural compound with anti-proliferative activity, whose mechanism is based on a highly potent and seemingly specific inhibition of Mg2+ transport via TRPM7.
We confirmed the specificity of waixenicin A against a number of ion channels found in our cellular model systems of Jurkat and RBL cells, including ICRAC, TRPM2, TRPM4, and TRPM6. Although we cannot rule out potential effects on other channels or proteins, we consider the specificity of TRPM7 as relatively high for the following reasons: (i) blocking efficacy occurs in the low nanomolar range, (ii) this potency is dependent on Mg2+ binding at a strategic residue, Lys-1648, in the kinase domain of TRPM7, (iii) TRPM6, the only other known channel kinase and the most homologous relative of TRPM7 within the TRPM subfamily, remained unaffected by the waixenicin A even at 10 μm. These features make waixenicin A an attractive molecular structure for targeting TRPM7-related pathophysiologies such as cancer (18, 19, 21), ischemia-related neuronal death (10, 11), and cardiac fibrosis and atrial fibrillations (55–57), and they open the door to future structure activity relationship studies of the waixencin A pharmacophore. Such studies would be aimed at elucidating the structural features of waixencin A that are essential for TRPM7 inhibition and making modifications to improve solubility and/or bioavailability. It might also be possible to reduce and/or simplify the structure to facilitate synthetic chemistry of analogs.
Supplementary Material
Acknowledgments
We thank L. Tsue, S. Johne, and M. K. Monteilh-Zoller for technical support, Dr. R. J. M. Bindels for the TRPM6 plasmid, and Dr. S. Kahng for soft coral identification.
This work was supported, in whole or in part, by National Institutes of Health Grants P01GM078195 (to A. F.), P20 RR-016467 (to F. D. H.), and 5G12 RR003061-22 (University of Hawaii Imaging Core). This work was also supported by an American Society of Pharmacognosy Undergraduate Research Award (to W. C. K.) and Austrian Science Fund Grant J2784 (to S. Z.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Experimental Procedures” and Figs. S1–S6.
- TRPM
- transient receptor potential melastatin
- CRAC
- Ca2+-release activated Ca2+ current
- RBL
- rat basophilic leukemia
- pA
- picoAmpere
- pF
- picoFarad.
REFERENCES
- 1. Li M., Jiang J., Yue L. (2006) J. Gen. Physiol. 127, 525–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nadler M. J., Hermosura M. C., Inabe K., Perraud A. L., Zhu Q., Stokes A. J., Kurosaki T., Kinet J. P., Penner R., Scharenberg A. M., Fleig A. (2001) Nature 411, 590–595 [DOI] [PubMed] [Google Scholar]
- 3. Ryazanova L. V., Dorovkov M. V., Ansari A., Ryazanov A. G. (2004) J. Biol. Chem. 279, 3708–3716 [DOI] [PubMed] [Google Scholar]
- 4. Chubanov V., Gudermann T., Schlingmann K. P. (2005) Pflugers Arch. 451, 228–234 [DOI] [PubMed] [Google Scholar]
- 5. Voets T., Nilius B., Hoefs S., van der Kemp A. W., Droogmans G., Bindels R. J., Hoenderop J. G. (2004) J. Biol. Chem. 279, 19–25 [DOI] [PubMed] [Google Scholar]
- 6. Ryazanova L. V., Rondon L. J., Zierler S., Hu Z., Galli J., Yamaguchi T. P., Mazur A., Fleig A., Ryazanov A. G. (2010) Nat. Commun. 1, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Demeuse P., Penner R., Fleig A. (2006) J. Gen. Physiol. 127, 421–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schmitz C., Perraud A. L., Johnson C. O., Inabe K., Smith M. K., Penner R., Kurosaki T., Fleig A., Scharenberg A. M. (2003) Cell 114, 191–200 [DOI] [PubMed] [Google Scholar]
- 9. Monteilh-Zoller M. K., Hermosura M. C., Nadler M. J., Scharenberg A. M., Penner R., Fleig A. (2003) J. Gen. Physiol. 121, 49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aarts M., Iihara K., Wei W. L., Xiong Z. G., Arundine M., Cerwinski W., MacDonald J. F., Tymianski M. (2003) Cell 115, 863–877 [DOI] [PubMed] [Google Scholar]
- 11. Inoue K., Branigan D., Xiong Z. G. (2010) J. Biol. Chem. 285, 7430–7439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sun H. S., Jackson M. F., Martin L. J., Jansen K., Teves L., Cui H., Kiyonaka S., Mori Y., Jones M., Forder J. P., Golde T. E., Orser B. A., Macdonald J. F., Tymianski M. (2009) Nat. Neurosci. 12, 1300–1307 [DOI] [PubMed] [Google Scholar]
- 13. Nicotera P., Bano D. (2003) Cell 115, 768–770 [DOI] [PubMed] [Google Scholar]
- 14. Szydlowska K., Tymianski M. (2010) Cell Calcium 47, 122–129 [DOI] [PubMed] [Google Scholar]
- 15. Tseveleki V., Rubio R., Vamvakas S. S., White J., Taoufik E., Petit E., Quackenbush J., Probert L. (2010) Genomics 96, 82–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abed E., Moreau R. (2007) Cell Prolif. 40, 849–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abed E., Moreau R. (2009) Am. J. Physiol. Cell Physiol. 297, C360–368 [DOI] [PubMed] [Google Scholar]
- 18. Guilbert A., Gautier M., Dhennin-Duthille I., Haren N., Sevestre H., Ouadid-Ahidouch H. (2009) Am. J. Physiol. Cell Physiol. 297, C493–502 [DOI] [PubMed] [Google Scholar]
- 19. Jiang J., Li M. H., Inoue K., Chu X. P., Seeds J., Xiong Z. G. (2007) Cancer Res. 67, 10929–10938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sahni J., Scharenberg A. M. (2008) Cell Metab. 8, 84–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim B. J., Park E. J., Lee J. H., Jeon J. H., Kim S. J., So I. (2008) Cancer Sci. 99, 2502–2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wykes R. C., Lee M., Duffy S. M., Yang W., Seward E. P., Bradding P. (2007) J. Immunol. 179, 4045–4052 [DOI] [PubMed] [Google Scholar]
- 23. Wolf F. I., Trapani V., Cittadini A. (2008) Magnes. Res. 21, 83–91 [PubMed] [Google Scholar]
- 24. Wolf F. I., Cittadini A. R., Maier J. A. (2009) Cancer Treat Rev. 35, 378–382 [DOI] [PubMed] [Google Scholar]
- 25. Chen J. P., Luan Y., You C. X., Chen X. H., Luo R. C., Li R. (2010) Cell Calcium 47, 425–432 [DOI] [PubMed] [Google Scholar]
- 26. Hermosura M. C., Monteilh-Zoller M. K., Scharenberg A. M., Penner R., Fleig A. (2002) J. Physiol. 539, 445–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kerschbaum H. H., Kozak J. A., Cahalan M. D. (2003) Biophys. J. 84, 2293–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kozak J. A., Kerschbaum H. H., Cahalan M. D. (2002) J. Gen. Physiol. 120, 221–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parnas M., Peters M., Dadon D., Lev S., Vertkin I., Slutsky I., Minke B. (2009) Cell Calcium 45, 300–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen H. C., Xie J., Zhang Z., Su L. T., Yue L., Runnels L. W. (2010) PLoS One 5, e11161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peinelt C., Lis A., Beck A., Fleig A., Penner R. (2008) J. Physiol. 586, 3061–3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang S. L., Kozak J. A., Jiang W., Yeromin A. V., Chen J., Yu Y., Penna A., Shen W., Chi V., Cahalan M. D. (2008) J. Biol. Chem. 283, 17662–17671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. DeHaven W. I., Smyth J. T., Boyles R. R., Bird G. S., Putney J. W., Jr. (2008) J. Biol. Chem. 283, 19265–19273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matsuda H., Hayashi M., Okada M. (2010) J. Physiol. 588, 4673–4681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li M., Sun Y., Simard J. M., Wang J. Y., Chai T. C. (2009) Am. J. Physiol. Cell Physiol. 297, C1445–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kucherenko Y. V., Lang F. (2010) J. Membr. Biol. 237, 93–106 [DOI] [PubMed] [Google Scholar]
- 37. Coburn R. F. (2009) J. Cell. Physiol. 221, 544–551 [DOI] [PubMed] [Google Scholar]
- 38. Mastrocola T., De Luca M., Rugolo M. (1991) Biochim. Biophys. Acta 1097, 275–282 [DOI] [PubMed] [Google Scholar]
- 39. Korn S. J., Horn R. (1990) Mol. Pharmacol. 38, 524–530 [PubMed] [Google Scholar]
- 40. Hatton C. J., Peers C. (1997) Br. J. Pharmacol. 122, 923–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gong Y. Z., Ding W. G., Wu J., Tsuji K., Horie M., Matsuura H. (2008) Eur. J. Pharmacol. 600, 18–25 [DOI] [PubMed] [Google Scholar]
- 42. Coval S., Scheuer P., Matsumoto G., Clardy J. (1984) Tetrahedron 40, 3823 [Google Scholar]
- 43. Castillo B., Pörzgen P., Penner R., Horgen F. D., Fleig A. (2010) J. Biomol. Screen 15, 498–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lang G., Mitova M. I., Ellis G., van der Sar S., Phipps R. K., Blunt J. W., Cummings N. J., Cole A. L., Munro M. H. (2006) J. Nat. Prod. 69, 621–624 [DOI] [PubMed] [Google Scholar]
- 45. Lange I., Yamamoto S., Partida-Sanchez S., Mori Y., Fleig A., Penner R. (2009) Sci. Signal 2, ra23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Perraud A. L., Fleig A., Dunn C. A., Bagley L. A., Launay P., Schmitz C., Stokes A. J., Zhu Q., Bessman M. J., Penner R., Kinet J. P., Scharenberg A. M. (2001) Nature 411, 595–599 [DOI] [PubMed] [Google Scholar]
- 47. Starkus J. G., Fleig A., Penner R. (2010) J. Physiol. 588, 1227–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yamamoto S., Shimizu S., Kiyonaka S., Takahashi N., Wajima T., Hara Y., Negoro T., Hiroi T., Kiuchi Y., Okada T., Kaneko S., Lange I., Fleig A., Penner R., Nishi M., Takeshima H., Mori Y. (2008) Nat. Med. 14, 738–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Launay P., Fleig A., Perraud A. L., Scharenberg A. M., Penner R., Kinet J. P. (2002) Cell 109, 397–407 [DOI] [PubMed] [Google Scholar]
- 50. Cao G., Hoenderop J. G., Bindels R. J. (2008) Curr. Opin. Nephrol. Hypertens 17, 373–378 [DOI] [PubMed] [Google Scholar]
- 51. Tani D., Monteilh-Zoller M. K., Fleig A., Penner R. (2007) Cell Calcium 41, 249–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jin J., Desai B. N., Navarro B., Donovan A., Andrews N. C., Clapham D. E. (2008) Science 322, 756–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liang J., Slingerland J. M. (2003) Cell Cycle 2, 339–345 [PubMed] [Google Scholar]
- 54. Shtivelman E., Sussman J., Stokoe D. (2002) Curr. Biol. 12, 919–924 [DOI] [PubMed] [Google Scholar]
- 55. Du J., Xie J., Zhang Z., Tsujikawa H., Fusco D., Silverman D., Liang B., Yue L. (2010) Circ. Res. 106, 992–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sontia B., Montezano A. C., Paravicini T., Tabet F., Touyz R. M. (2008) Hypertension 51, 915–921 [DOI] [PubMed] [Google Scholar]
- 57. Yue L., Xie J., Nattel S. (2011) Cardiovasc. Res. 89, 744–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.