Background: Phosphate and iron depletion by the vertebrate host can trigger bacterial virulence.
Results: PhoB-PhoR and Fur sense phosphate and iron, respectively, to regulate expression of T3SS and T6SS in E. tarda through EsrC and by negative cross-talk among each other.
Conclusion: T3SS and T6SS are regulated differently depending on the type of environmental factor.
Significance: Understanding the regulation of bacterial virulence is crucial for controlling the pathogenicity of bacteria.
Keywords: Bacterial Pathogenesis, DNA-Protein Interaction, Gene Regulation, Iron, Protein Phosphorylation, PhoB-PhoR, Ferric Uptake Regulator, Type III and VI Secretion Systems
Abstract
Inorganic phosphate (Pi) and iron are essential nutrients that are depleted by vertebrates as a protective mechanism against bacterial infection. This depletion, however, is sensed by some pathogens as a signal to turn on the expression of virulence genes. Here, we show that the PhoB-PhoR two-component system senses changes in Pi concentration, whereas the ferric uptake regulator (Fur) senses changes in iron concentration in Edwardsiella tarda PPD130/91 to regulate the expression of type III and VI secretion systems (T3SS and T6SS) through an E. tarda secretion regulator, EsrC. In sensing low Pi concentration, PhoB-PhoR autoregulates and activates the phosphate-specific transport operon, pstSCAB-phoU, by binding directly to the Pho box in the promoters of phoB and pstS. PhoB also binds with EsrC simultaneously on the promoter of an E. tarda virulence protein, evpA, to regulate directly the transcription of genes from T6SS. In addition, PhoB requires and interacts with PhoU to activate esrC and suppress fur indirectly through unidentified regulators. Fur, on the other hand, senses high iron concentration and binds directly to the Fur box in the promoter of evpP to inhibit EsrC binding to the same region. In addition, Fur suppresses transcription of phoB, pstSCAB-phoU, and esrC indirectly via unidentified regulators, suggesting negative cross-talk with the Pho regulon. Physical interactions exist between Fur and PhoU and between Fur and EsrC. Our findings suggest that T3SS and T6SS may carry out distinct roles in the pathogenicity of E. tarda by responding to different environmental factors.
Introduction
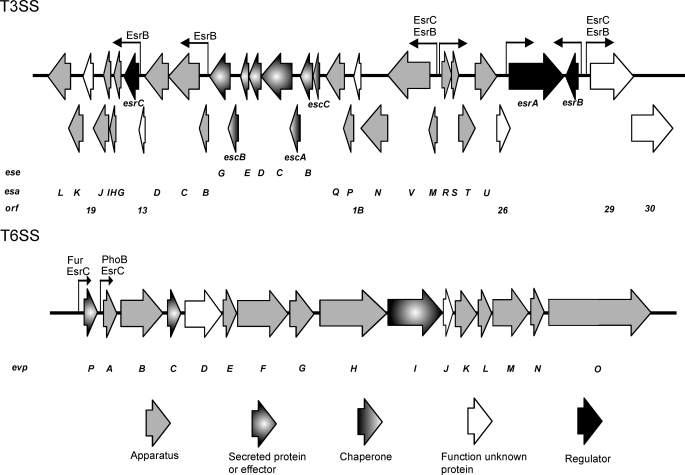
Edwardsiella tarda is a bacterial pathogen with a broad host range; it infects not only freshwater and marine life but is also an emerging agent for gastrointestinal infection in humans (1–3). Using transposon tagging and proteomics, several genes/proteins were identified and ranked according to their importance on E. tarda pathogenicity (4, 5). Among them, type III and VI secretion systems (T3SS and T6SS) were determined as the two most important virulence mechanisms in E. tarda (6, 7). We previously showed that E. tarda PPD130/91 utilizes a PhoP-PhoQ two-component system (TCS)2 to detect the host based on changes in temperature, Mg2+ concentration, and the presence of antimicrobial peptide to regulate the expression of virulence genes of T3SS and T6SS through an E. tarda secretion regulator, EsrB (8). EsrB is also the response regulator of another TCS, EsrA-EsrB, which directly activates expression of esrC (9). EsrC has significant sequence similarity to the AraC family of transcriptional regulators and has been shown to regulate the expression of the T6SS gene clusters (also known as E. tarda virulence proteins), such as evpP, evpA, and evpC, and certain genes of the T3SS, such as E. tarda secretion effectors eseB and eseD and open reading frames orf29 and orf30, but not the E. tarda secretion apparatus esaC. In contrast, EsrB directly regulates esaC, orf29, and orf30 independently of EsrC, but it can only regulate T6SS via EsrC (9). The exact binding sites of EsrB and EsrC on the T3SS and T6SS of E. tarda, however, remain to be determined.
Inorganic phosphate (Pi) and iron are two scarce nutrients found in the environment that are essential for bacterial growth. Bacterial pathogens have developed complex sensing, signaling, regulatory, and transport systems to acquire these nutrients (10, 11). Vertebrates sequester iron from invading pathogens as a means of nutritional immunity using serum proteins, such as transferrin, that bind to iron with very high affinity. Bacterial pathogens on the other hand have evolved to detect iron depletion as a sign that they are within a vertebrate host and to elicit the expression of virulence genes (12). Similarly, Pi depletion due to physiological stress, such as surgical injury-induced intestinal Pi depletion, also serves to alert pathogens to infection opportunities (13).
Pi is sensed and regulated in bacteria by the Pho regulon, which in turn is controlled by the PhoB-PhoR TCS. PhoR, unlike other sensor histidine kinases, does not have a large periplasmic domain but has an extended cytoplasmic domain. The proposed function of this extended cytoplasmic domain is to sense internal signals that repress the kinase function of the PhoR (14). PhoB is a response regulator that binds to DNA and regulates transcription of genes, such as the high affinity phosphate-specific transport (Pst) system, to acquire Pi. The Pst system contains four proteins: PstS, PstC, PstA, and PstB. These proteins form an ATP binding cassette transporter that is essential for the high affinity capture of Pi and its transport to the cytosol. These proteins are encoded with the accessory protein PhoU in the polycistronic pstSCAB-phoU operon. When Pi is in excess (>4 μm), the Pst system, possibly PstB, forms a repression complex with PhoR that prevents activation of PhoB. Pi limitation on the other hand activates PhoR, leading to its autophosphorylation and the transfer of a phosphoryl group to the receiver domain of PhoB (11). Phosphorylation causes a dramatic conformational change that releases the DNA binding domain to bind to Pho regulon promoters (15). When shifted to a Pi-rich medium, the activation signal is interrupted by PhoR, which acts as a phosphatase on phospho-PhoB. The accessory protein PhoU is essential for the repression of the Pho regulon at high Pi conditions: it binds to PhoR, PhoB, or a PhoR-PhoB complex as a chaperone and promotes dephosphorylation of phospho-PhoB (11, 16). PhoB binds to promoters that share an 18-bp Pho box of the sequence 5′-CTGTCAT A(A/T)A(T/A) CTGT(C/A)A(C/T)-3′ (17). Virulence genes are among those of the Pho regulon. In Vibrio cholerae, PhoB regulates virulence genes by directly and negatively controlling the expression of a key upstream transcriptional regulator, tcpPH, of the ToxR regulon, which is essential for colonization of the small intestine (18).
The ferric uptake regulator (Fur) is a dimeric metal-dependent DNA-binding protein. Each monomer contains two domains: the N-terminal DNA binding domain and the C-terminal dimerization domain. The crystal structure of V. cholerae Fur contains two metal binding sites occupied by Zn2+. Zn2+ can be removed from V. cholerae Fur by EDTA, and this form does not bind to promoter DNA (19). Fur binds to promoter DNA containing a 19-bp Fur box with the consensus sequence 5′-GATAATGATAATCATTATC-3′; this sequence can be described as three adjacent hexamers of the sequence 5′-GATAAT-3′ with the third hexamer being imperfect (an F-F-F configuration) or as two hexamers in the forward orientation separated by 1 base pair from a third hexamer in the reverse orientation (an F-F-X-R configuration) (20). Microarray data on the Fur regulon of Yersinia pestis showed that it constitutes a total of 34 putative operons that are potential iron-responsive targets of Fur. Fur is a global regulator; it acts as both an activator and a repressor to control iron and non-iron functions (21). In Y. pestis, Fur works together with an AraC-like transcriptional regulator, YbtA, to regulate the ybt locus for the production of yersiniabactin, a high affinity iron uptake peptide that enables the pathogen to multiply for virulence under iron-depleted conditions (22).
In this study, we showed that high Pi and iron concentrations significantly suppressed the secretion and expression of proteins from the T3SS and T6SS of E. tarda PPD130/91. We also showed that these effects of Pi and iron are additive. A novel PhoB-PhoR TCS and the iron-sensing Fur protein were identified in E. tarda PPD130/91 as an activator and a repressor, respectively, of the T3SS and T6SS. Using an electrophoretic mobility shift assay (EMSA) and a LacZ reporter gene assay on deletion mutants of phoB, phoU, and fur under different Pi and iron concentrations, PhoB and Fur were found to regulate T3SS and T6SS either by direct promoter binding or indirectly through EsrC but not EsrB. Using yeast two-hybrid experiments, we confirmed the physical interactions of PhoB-PhoU, Fur-PhoU, and Fur-EsrC and provided new insights into the complex regulatory network of virulence in E. tarda by Pi and iron signaling.
EXPERIMENTAL PROCEDURES
Cloning of TCS PhoB-PhoR and Iron Sensor Fur in E. tarda PPD130/91
Bacterial genomic DNA was extracted using the Wizard genomic DNA purification kit (Promega, Madison, WI). PCR amplification (2 min at 94 °C, 30 cycles each of 10 s at 94 °C, 30 s at 56 °C, and 1 min at 72 °C, and a final extension of 5 min at 72 °C) was carried out using the Advantage 2 polymerase mixture (Clontech) with degenerate primers based on the sequences of related bacteria, such as E. tarda EIB202 (23), Y. pestis KIM (24), Salmonella typhimurium LT2 (25), and Escherichia coli CFT073 (26). The PCR products were cloned with the pGEM-T Easy vector system (Promega) and transformed into E. coli DH5α cells (Table S1). The cloned fragments were sequenced using the PRISMTM 3100 automated DNA sequencer with the ABI Prism Big Dye termination cycle sequence kit (Applied Biosystems, Foster City, CA). This approach identified full-length sequences of phoB, phoR, and fur along with flanking sequences comprising 1000 bp upstream of phoB to 1200 bp downstream of phoR and 1200 bp upstream to 1100 bp downstream of fur.
LacZ Reporter Gene System
For the construction of the ersB-LacZ, esrC-LacZ, phoB-LacZ, and fur-LacZ reporter gene systems, the primer pairs derived from E. tarda PPD130/91 genomic DNA, pRWesrb, pRWesrc, pRWphoB, and pRWfur, were used to amplify the putative promoter regions of esrB, esrC, phoB, and fur, respectively (Table S2). The PCR products were digested with restriction enzymes and then ligated into the pRW50 plasmid (27). The ligation mixtures were introduced into either the wild-type or mutant E. tarda by electroporation. The transformants were screened for resistance to both colistin (12.5 μg/ml) and tetracycline (10 μg/ml). For the construction of phoU-LacZ, an internal fragment of phoU was amplified with the primer pVIKphoU, digested, and then ligated into the same sites of the pVIK112 plasmid (28). The plasmid was then transferred from S17-1 λpir into wild-type or mutant E. tarda by conjugation and was selected for by kanamycin (50 μg/ml) and colistin resistance. For the β-galactosidase assays, E. tarda cells were grown in phosphate-free DMEM overnight at 30 °C with the addition of phosphate (20 mm KH2PO4) and/or iron (10 μm FeSO4) as indicated. The overnight cultures (5%) were inoculated into fresh medium and grown at the same conditions until the cell density reached 0.5 as measured by optical density at 600 nm (A600). Cells were permeabilized as described previously (8).
EMSA
EsrB (from residue Ser-143 to Phe-214), EsrC (from residue Ala-123 to Gly-230), full-length Fur, and PhoB (from residue Met-126 to Phe-229) at various concentrations were mixed with the indicated promoter DNA fragments (2 μg) labeled at the 5′-end with 6-carboxyfluorescein tag (1st BASE, Singapore) in reaction mixtures (25 μl) containing 20 mm Tris-HCl, pH 7.5, 50 mm KCl, 5 mm MgCl2, and 5% (v/v) glycerol. 150 μm FeCl3 was added in the reaction mixtures containing Fur as indicated. The mixtures were incubated at 25 °C for 2 h before loading onto each lane of a 5% native polyacrylamide gel for electrophoresis (0.5× Tris borate-EDTA buffer; 1 mA/cm for 4 h).
Preparation of Extracellular Proteins (ECPs) and Total Cellular Proteins (TCPs) and Western Blot Analysis
Overnight cultures of E. tarda grown in phosphate-free DMEM in the presence or absence of iron (10 μm FeSO4) and/or phosphate (20 mm KH2PO4) (A550 = 0.8) were diluted 1:200 into fresh DMEM and incubated for 24 h at the indicated conditions. For the isolation of TCPs, whole broth containing the cells was sonicated on ice for 5 min with a delay every 1 min. The total protein was precipitated by 10% trichloroacetic acid (TCA) for 1 h at 4 °C, washed three times with −20 °C acetone, and then finally air-dried. TCP pellets were solubilized in Ready Prep reagent 3 (5 m urea, 2 m thiourea, 2% (w/v) CHAPS, 2% (w/v) SB 3–10, 40 mm Tris, and 0.2% (w/v) Bio-Lyte 3/10 ampholyte (Bio-Rad)) and stored at −80 °C until analysis. The extraction of ECPs and Western blot analysis were performed in the same way as described previously (8). The protein concentrations were determined with a Bio-Rad protein assay kit using bovine serum albumin as the standard.
Generation of ΔphoB, phoUi, and Δfur Mutants
An insertional mutant of phoU (phoUi) was constructed with the suicide plasmid pRE112 (29). For the construction of phoUi, an internal fragment of phoU was amplified from E. tarda PPD130/91 genomic DNA with the primer pair phoUmut-for/phoUmut-rev, which contains a KpnI restriction enzyme site. The PCR product was digested by restriction enzymes and ligated into the same sites of the pRE112 plasmid. The resulting plasmid was then transformed into E. coli MC1061 λpir. After sequencing, the recombinant plasmid was transformed into E. coli SM10 λpir. These transformants were used for conjugation with wild-type E. tarda to obtain defined mutants by selecting colonies resistant to both chloramphenicol (30 μg/ml) and colistin (12.5 μg/ml). The insertion of the plasmid into chromosomal DNA was confirmed by sequence analysis. Overlap extension PCR (30) was used to generate in-frame deletion mutants of phoB (ΔphoB) and fur (Δfur). For the construction of ΔphoB, two PCR fragments were generated from E. tarda genomic DNA with the primer pairs phoBmut-for/phoBmut-irev and phoBmut-rev/phoBmut-ifor. The resulting products generated an 820-bp fragment upstream and 850-bp fragment containing the downstream region of phoB, respectively. An 18-bp overlap in the sequences permitted amplification of a 1670-bp product during a second PCR with primers phoBmut-for and phoBmut-rev using the first two PCR products as the template. The primers introduced EcoRI and HindIII restriction sites, and the second PCR created a truncation from amino acid residues 15 to 230 of PhoB. The PCR product was cloned into the pGEM-T Easy vector, and the sequence was verified. The ΔphoB fragment was excised and ligated into the same sites of pRE112 (29). The resulting plasmid was then transformed into MC1061 λpir. After sequencing, the recombinant plasmid was transformed into E. coli SM10 λpir. The single crossover mutants were obtained by conjugal transfer into E. tarda PPD130/91. Double crossover mutants were obtained by plating onto 10% sucrose tryptic soy agar plates. Deletion mutants were confirmed by PCR and verified by sequencing. The Fur deletion mutant (Δfur) was constructed following the same protocol. The primers used for the construction were furmut-for, furmut-irev, furmut-rev, and furmut-ifor. These primers introduced KpnI as the restriction enzyme cloning site. This approach created a deletion from amino acid residues 15 to 100 of Fur.
Cloning, Expression, and Purification of EsrB, EsrC, Fur, and PhoB
EsrB (from residue Ser-143 to the end), EsrC (from residue Ala-123 to the end), full-length Fur, and PhoB (from residue Met-126 to the end) were amplified using primer pairs esrBm-for/esrBm-rev, esrCm-for/esrCm-rev, furm-for/furm-rev, and phoBm-for/phoBm-rev, respectively, derived from E. tarda PPD130/91genomic DNA. The PCR products were subcloned into pET-M, a modified pET-32a vector (Novagen, Darmstadt, Germany), and then transformed into E. coli BL21(DE3) cells for expression to generate EsrBm, EsrCm, Furm, and PhoBm, respectively. Overnight cultures of these cells were inoculated into LB broth with 100 μg/ml ampicillin and grown until A600 reached 0.6. Protein expression was induced with 0.4 mm of isopropyl 1-thio-β-d-galactopyranoside, and the cells were grown at 25 °C overnight. Cells were harvested by centrifugation and then resuspended in binding buffer (1 mm PMSF, 500 mm NaCl, 20 mm Tris-HCl, and 5 mm imidazole, pH 7.9). The cell suspensions were subjected to sonication followed by centrifugation to obtain the soluble proteins, which were applied to a charged nickel-nitrilotriacetic acid column (Qiagen) and eluted with binding buffer containing 500 mm imidazole. Eluted fractions were analyzed by 12.5% SDS-PAGE. Fractions containing Furm were pooled and dialyzed against buffer containing 20 mm Tris-HCl, pH 7.5, 50 mm KCl, 5 mm MgCl2, and 5% (v/v) glycerol. The sample was further purified by a Superdex 75 gel filtration column (GE Healthcare) equilibrated with the dialysis buffer. For purification of EsrBm and EsrCm, eluent fractions from the nickel-nitrilotriacetic acid column were dialyzed against buffer of 20 mm phosphate buffer, pH 7.5, 200 mm NaCl, and 5% (v/v) glycerol, followed by gel filtration using the same dialysis buffer. For purification of PhoBm, the composition of dialysis and gel filtration buffer was 20 mm phosphate buffer, pH 6.5, 500 mm NaCl, and 5% (v/v) glycerol. All the protein fractions were pooled, concentrated, and stored at −80 °C until further use.
Yeast Two-hybrid Analysis
The yeast two-hybrid analysis was done using a Gal4 DNA binding domain encoding bait vector (pBGKT7) and a Gal4 activation domain encoding prey vector (pGADT7-AD) in the yeast strain AH109. The fusion constructs were amplified by PCR and cloned into both vectors. All fusion constructs were made as full-length fusions except the histidine sensor kinase protein PhoR (from residue Trp-33 to the end) where the putative transmembrane region was removed. The resulting bait and prey plasmids were co-transformed into the yeast strain AH109 as recommended by the manufacturer's protocol (YeastmakerTM Yeast Transformation System 2 user manual; Clontech) with some modifications. Briefly, in the final transformation step, the yeast cells were resuspended in 50 μl of 0.9% (w/v) NaCl solution of which 10 μl was transferred into the appropriate plates. Bait and prey hybrid proteins were tested for self-activation by co-expression of the bait hybrid proteins with the empty prey vector and of the prey hybrid proteins with the empty bait vector. A fusion construct of Arabidopsis histidine phosphotransfer 1 in the prey vector and Arabidopsis response regulator 4 in the bait vector was used as a positive control (31). The transformants were selected on synthetic dropout agar plates (SD) lacking Trp and Leu. All confirmations were performed on medium stringency SD lacking Trp, Leu, and His and high stringency SD lacking Trp, Leu, His, and adenine. All yeast clones were incubated for 3 days at 30 °C. To investigate the effect of iron, 150 μl of FeCl3 was added both in the plates and in the final resuspension solution (0.9% NaCl).
RT-PCR Studies
RNA was extracted with RNAprotectTM Bacteria Reagent and an RNeasy® Mini kit (Qiagen), treated with RNase-free DNase I (Roche Diagnostics), and purified with RNeasy Mini spin columns. Four pairs of primers were designed to confirm the transcription of phoBR as a single operon. Likewise, six pairs of primers were designed based on the genomic DNA sequence of E. tarda PPD130/91 to determine whether pstSCAB-phoU genes were transcribed as a single operon.
Accession Numbers
The DNA and protein sequences of PhoB-PhoR and Fur of E. tarda PPD130/91 were deposited in GenBankTM under submission numbers BankIt1470497 Seq1 JN543949 and BankIt1471150 Seq1 JN543950, respectively.
RESULTS
Identification of PhoB-PhoR Two-component System and Fur in E. tarda PPD130/91
Using the genome sequences of E. tarda EIB202 (23) and related bacteria as templates, degenerate primers were designed based on conserved nucleotide sequences to identify the phoB and phoR genes of E. tarda PPD130/91 (GenBank submission number BankIt1470497 Seq1 JN543949). Alignment of the sequences of PhoB and PhoR of E. tarda PPD130/91 with those of Y. pestis KIM (24), S. typhimurium LT2 (25), and E. coli CFT073 (26) showed identities of around 85 and 70%, respectively. High sequence identities of 99 and 100% were observed in the sequences of PhoB and PhoR, respectively, between E. tarda PPD130/91 and E. tarda EIB202.
To determine whether phoB and phoR are transcribed as a single operon, RT-PCR experiments were performed on RNA isolated from E. tarda PPD130/91 using pairs of primers designed to span two adjacent genes. The result showed that phoB and phoR, flanked by sbcD and ppX, were transcribed together as a single phoBR operon (supplemental Fig. S1). Similar experiments were performed on the pstSCAB-phoU operon, which is a known target of PhoB, and showed that these five genes are transcribed as a single operon in E. tarda PPD130/91 (supplemental Fig. S2). The result agrees with previous findings that these five genes of E. coli, pstSCAB and phoU, are transcribed as a single mRNA molecule and controlled by a promoter located upstream of pstS (32).
In E. coli, the 18-bp consensus Pho box 5′-CTGTCAT A(A/T)A(T/A) CTGT(C/A)A(C/T)-3′ was found at PhoB-activated promoters in genes regulated by Pi, including phoB and pstS (17). Inspection of promoter regions of the phoBR and pstSCAB-phoU operons of E. tarda PPD130/91 showed the presence of a putative Pho box of the sequence 5′-TTTTCAT AAAT CTGTCAT-3′ between nt −42 and −25 upstream of the start codon of phoB. Another putative Pho box of the sequence 5′-CTGTCAT CAAA CTGCCTT-3′ was also found between nt −89 and −72 upstream of the start codon of pstS. EMSA experiments confirmed the direct binding of PhoB to DNA fragments derived from the promoter regions of phoBR and pstSCAB-phoU operons of E. tarda PPD130/91 (supplemental Fig. S3), suggesting that PhoB autoregulates its own expression as well as the expression of the pstSCAB-phoU operon for both the high affinity Pi transporter and the PhoU protein. A Fur box was not found in the promoter upstream of the phoBR or pstSCAB-phoU operon in E. tarda.
The fur gene of E. tarda PPD130/91 (GenBank submission number BankIt1471150 Seq1 JN543950) was identified based on the sequence of fur in E. tarda EIB202 (23). The Fur protein of E. tarda PPD130/91 contains 111 residues, and its amino acid sequence is identical to that of Fur in E. tarda EIB202. The length of the Fur protein of E. tarda is much shorter than that of Fur found in E. coli (145 residues), Y. pestis (148 residues), and S. typhimurium (150 residues). An alignment of these sequences showed that the N-terminal helices, α1 and α2, in the DNA binding domain of the crystal structure of V. cholerae Fur are missing in Fur of E. tarda (19). The DNA recognition helix, α4, is still intact in Fur of E. tarda, and the aligned region shares a sequence identity close to 90% with Fur proteins from other bacterial species. Pho and Fur boxes were both absent from the promoter upstream of fur in E. tarda.
PhoB and Fur Bind Directly to Promoters in T6SS but Not T3SS
To determine whether PhoB and Fur regulate the virulence of E. tarda directly through the T3SS and T6SS, all of the promoters found in the T3SS and T6SS were assessed for the presence of Pho and Fur boxes. Putative promoters on the T3SS and T6SS of E. tarda were predicted using promoter prediction software (Neural Network Promoter Prediction) set at high stringency (cutoff score of 0.95). PCR primers were then designed to confirm transcription of genes or gene clusters as a single mRNA molecule using RT-PCR experiments. The results showed that the T3SS of E. tarda PPD130/91 was transcribed as seven genes or gene clusters, each regulated by a single promoter upstream. These seven genes or gene clusters were esrC-esaL, esaB-orf13, esaM-eseG, esaR-easU, esrA, esrB, and orf29-orf30 (supplemental Fig. S4) (6). In contrast, the whole T6SS of E. tarda PPD130/91 was transcribed as a single gene cluster from evpP to evpO (supplemental Fig. S4) (7). Inspection of the promoter region of the evpA gene of T6SS revealed a putative Pho box with the sequence 5′-CTGTCAT CAAA GAGAACT-3′ located upstream between nt −496 and −479 of evpA. In contrast, inspection of the promoter region of the evpP gene of T6SS revealed a putative Fur box with the sequence 5′-GATAACGCAATGATTGATA-3′ located upstream between nt −174 and −156 of the start codon of evpP. These results agreed with the previous findings of a putative Fur box of the same sequence in E. tarda EIB202 and that transcription of evpP was regulated in an iron-dependent manner as verified by in vitro Fur titration assays (33). Interestingly, neither a Pho box nor a Fur box was observed in any of the promoter regions identified in the T3SS of E. tarda PPD130/91.
To verify the direct binding of PhoB and Fur to promoter regions of the T6SS, DNA binding domains of PhoB (34) and full-length Fur were cloned, expressed, and purified for EMSA using labeled DNA fragments derived from promoter regions of evpA and evpP. The results confirmed the direct binding of PhoB to a 510-bp DNA fragment (from nt −507 to +3) derived from the promoter region upstream of evpA as well as the direct binding of Fur to a 402-bp DNA fragment (from nt −391 to +11) derived from the promoter region upstream of evpP. The binding of Fur to the promoter of evpP was iron-dependent as no binding was observed in the absence of 150 μm FeCl3 (data not shown). Furthermore, Fur bound to the promoter of evpP with a relatively higher affinity than PhoB bound to the promoter region of evpA (Fig. 1A).
FIGURE 1.
EMSA on promoter regions in T3SS and T6SS of E. tarda PPD130/91 using DNA binding domains of EsrB, EsrC, PhoB, and full-length Fur. A, EMSA using DNA fragments derived from promoter regions of evpP (401 bp; nt −391 to +11) and evpA (510 bp; nt −507 to +3) in T6SS of E. tarda PPD130/91. B, EMSA using DNA fragments derived from promoter regions of esrC (425 bp; nt −419 to +6), esaB (456 bp; nt −447 to +9), esaM (533 bp; nt −530 to +3), esaR (456 bp; nt −450 to +6), esrA (455 bp; nt −449 to +6), esrB (470 bp; nt −467 to +3), and orf29 (406 bp; nt −400 to + 6) in T3SS of E. tarda PPD130/91. Numbers above each panel represent concentrations (μm) of each protein used in the assay. Labels on the left and right of each panel represent the type of DNA or protein-DNA complexes formed based on their respective molecular weights. For the assay involving Fur, 150 μm FeCl3 was included in the reaction mixture.
EsrC Binds Promoters in Both T3SS and T6SS, but EsrB Binds Promoters Only in T3SS
As neither a Pho box nor a Fur box was found on the T3SS, if PhoB and Fur were to regulate T3SS, then it must occur indirectly through other transcription regulators. Previously, we found that the PhoP-PhoQ TCS indirectly regulates T3SS and T6SS through EsrB (8). EsrB and EsrC were both encoded within the T3SS and found to regulate T3SS and T6SS (9), but the exact locations of their binding sites on T3SS and T6SS have yet to be determined. Hence, EMSA experiments were performed to verify the direct binding of the DNA binding domains of EsrB (35) and EsrC (36) to the identified promoter regions derived from T3SS and T6SS of E. tarda PPD130/91.
The results for T3SS showed that EsrB bound directly to promoters of esrC, esaB, esaM, and orf29 with affinity for esaB > orf29 > esrC ∼ esaM, whereas EsrC bound directly to promoters of esaM and orf29 with affinity for esaM > orf29. The promoters of esaM and orf29 could be bound simultaneously by both EsrB and EsrC, but the affinities for EsrB were higher in both cases as compared with EsrC. The promoters of esaR, esrA and esrB were not bound by either EsrB or EsrC (Fig. 1B). In T6SS, EsrC bound directly to the promoters of both evpP and evpA with affinity for evpA > evpP, but EsrB did not bind to any of these promoters in T6SS (Fig. 1A). Interestingly, EsrC and PhoB could bind simultaneously to the promoter of evpA with EsrC binding with higher affinity. Fur on the other hand inhibited the binding of EsrC to the promoter of evpP and bound with a higher affinity than EsrC in the presence of iron (Fig. 1A). The locations of the binding sites for EsrB, EsrC, PhoB, and Fur on the T3SS and T6SS of E. tarda PPD130/91 are summarized in Fig. 2.
FIGURE 2.
Locations of binding sites for EsrB, EsrC, PhoB, and Fur at promoter regions of T3SS and T6SS in E. tarda PPD130/91. The bent arrows represent putative promoter regions with their orientation representing the direction of transcription. The label(s) above each promoter region indicates the presence of a DNA binding site(s) for the respective regulator(s). The sizes of the arrows do not reflect the scale of the corresponding genes.
Expression and Secretion of T3SS and T6SS Proteins Are Dependent on Pi and Iron Concentration
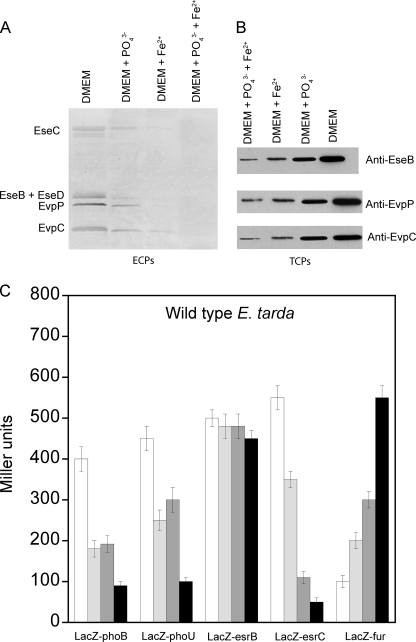
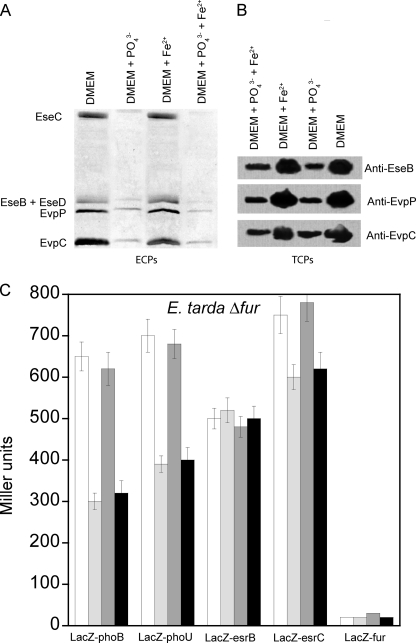
The effects of Pi and iron concentrations on the expression and secretion of proteins from the T3SS and T6SS were determined by detecting the amount of secreted ECPs and expression of TCPs. At high Pi and iron concentrations, the amount of secreted ECPs from both T3SS (EseB, EseC, and EseD) and T6SS (EvpC and EvpP) was significantly reduced with a seemingly additive inhibitory effect from high levels of both Pi and iron (Fig. 3A). A similar trend was observed for the amount of TCPs, showing that high Pi and iron concentrations suppressed protein expression from both systems (Fig. 3B).
FIGURE 3.
Effects of Pi and iron concentration on secretion and expression of proteins from T3SS and T6SS in wild-type E. tarda PPD130/91. The effects of Pi and iron concentrations on the amounts of ECPs (A) and TCPs (B) from T3SS (EseB, EseC, and EseD) and T6SS (EvpC and EvpP) were determined by growing wild-type E. tarda in DMEM or the same medium containing 20 mm KH2PO4 and/or 10 μm FeSO4. The results were determined using Western blotting. C, the effects of Pi and iron concentration on the β-galactosidase activities of the reporter genes phoB-LacZ, phoU-LacZ, esrB-LacZ, esrC-LacZ, and fur-LacZ in wild-type E. tarda PPD130/91 were determined by culturing the cells in DMEM (white bar) or the same medium containing 20 mm KH2PO4 (light gray bar), 10 μm FeSO4 (dark gray bar), or 20 mm KH2PO4 + 10 μm FeSO4 (black bar). Values represent mean ± S.E., n = 3, by one experiment.
To further investigate this regulation, the transcription levels of genes of selected regulators, namely phoB, phoU, esrB, esrC, and fur, in wild-type E. tarda PPD130/91 were determined using a LacZ reporter gene assay under different Pi and iron concentrations (Fig. 3C). At high concentrations of Pi and iron, an additive inhibitory effect was observed with the transcription levels of both phoB and phoU reduced by around 50%. Both phoBR and pstSCAB-phoU operons are regulated directly by PhoB, so we expected to see suppression of transcription at a high Pi concentration. Surprisingly, both phoB and phoU responded to high iron concentrations. As inspection of their promoters showed no sign of a Fur box, this suggested that these promoters were regulated by Fur indirectly via unidentified regulators and may indicate cross-talk between the Pi and iron signaling networks.
Pi and iron concentrations had no effect on the transcription level of esrB. However, the transcription level of esrC was highly sensitive to Pi and especially iron with high Pi and iron concentrations suppressing expression by about 40 and 80%, respectively (Fig. 3C). This result suggests that the effects of Pi and iron are regulated through EsrC instead of EsrB in E. tarda PPD130/91 for both T3SS and T6SS. As Pho and Fur boxes are not present in the promoter upstream of esrC, we propose that the expression level of esrC is regulated by PhoB and Fur indirectly through unidentified regulators.
The transcription level of fur was regulated oppositely as compared with phoB, phoU, and esrC with respect to Pi and iron concentrations. fur transcription increased by ∼100% at high Pi concentration and by 200% at high iron concentration with synergistic effects at high concentrations of both (Fig. 3C). If PhoB, PhoU, and EsrC are considered as activators, then Fur is a repressor of the T3SS and T6SS genes in E. tarda. Again, as neither a Pho box nor a Fur box is present in the promoter upstream of fur, the transcription level of fur is likely to be regulated by PhoB and Fur indirectly via unidentified regulators. The findings also support the possibility of negative cross-talk between Pi and iron signaling networks in the regulation of T3SS and T6SS genes in E. tarda PPD130/91.
PhoB-PhoR Senses Pi Concentration and Regulates T3SS and T6SS with or via EsrC
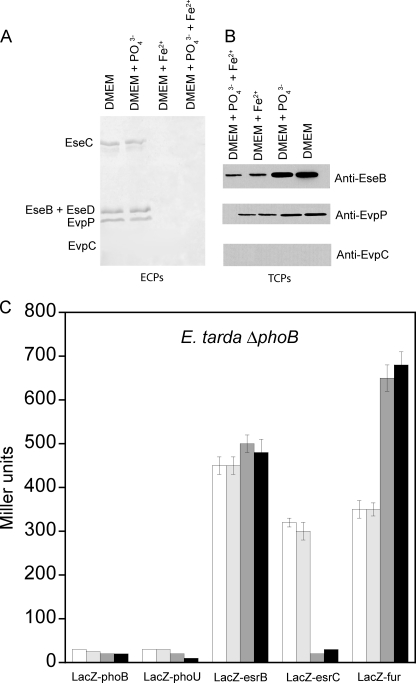
With the phoB gene deleted (E. tarda ΔphoB), we next determined the effects of Pi and iron concentrations on the expression and secretion of proteins from T3SS and T6SS. The results showed that secretion of ECPs from both T3SS and T6SS by E. tarda ΔphoB was no longer sensitive to Pi concentration but was still suppressed at high iron concentration (Fig. 4A). The same trend was also observed for TCPs (Fig. 4B), suggesting that PhoB conveys Pi signals to regulate virulence gene expression and secretion in E. tarda PPD130/91. Interestingly, evpC could not be secreted or expressed without PhoB, but this was not the case for evpP. These results indicate that PhoB is required for T6SS gene expression from evpA to evpO, whereas EsrC is essential for expression of every gene in T6SS because deletion of esrC completely abolished expression of both evpP and evpC from T6SS (supplemental Fig. S5). This agrees with our finding that PhoB and EsrC activate the expression of genes from evpA and evpO of T6SS by binding simultaneously and directly to the promoter upstream of evpA (Fig. 1A). The evpP gene alone is controlled by a separate promoter that does not have a Pho box but does contain a Fur box.
FIGURE 4.
Effects of Pi and iron concentration on secretion and expression of proteins from T3SS and T6SS in E. tarda PPD130/91 ΔphoB mutant. The effects of Pi and iron concentrations on the amounts of ECPs (A) and TCPs (B) from T3SS (EseB, EseC, and EseD) and T6SS (EvpC and EvpP) were determined by growing E. tarda ΔphoB mutant in DMEM or the same medium containing 20 mm KH2PO4 and/or 10 μm FeSO4. The results were determined using Western blotting. C, the effects of Pi and iron concentration on the β-galactosidase activities of the reporter genes phoB-LacZ, phoU-LacZ, esrB-LacZ, esrC-LacZ, and fur-LacZ in E. tarda PPD130/91 ΔphoB mutant were determined by culturing the cells in DMEM (white bar) or the same medium containing 20 mm KH2PO4 (light gray bar), 10 μm FeSO4 (dark gray bar), or 20 mm KH2PO4 + 10 μm FeSO4 (black bar). Values represent mean ± S.E., n = 3, by one experiment.
Using a LacZ reporter gene assay, we next determined the transcription levels of phoB, phoU, esrB, esrC, and fur in E. tarda ΔphoB at different Pi and iron concentrations (Fig. 4C). Transcription of the pstSCAB-phoU operon seems to be solely dependent on PhoB as deletion of phoB completely abolished transcription of phoU. In contrast, deletion of phoB had little effect on the transcription of esrB. The transcription of esrC, however, was insensitive to Pi concentration in E. tarda ΔphoB but was significantly reduced (∼90%) at high iron concentration (Fig. 4C). These results indicate that PhoB is the sole regulator and indirectly confers the effect of Pi concentration to the transcription of esrC. As transcription of esrC could still be observed in E. tarda ΔphoB albeit at a 40% reduction, PhoB is not the only transcription activator of esrC: our previous study identified that PhoP-PhoQ senses temperature and Mg2+ and directly regulates expression of EsrB (8), which in turn activates the transcription of esrC by direct binding to its promoter (Fig. 1B).
The transcription of fur in E. tarda ΔphoB was about 250% higher as compared with that of the wild-type E. tarda and became insensitive to Pi concentration. A further 100% up-regulation was observed in the presence of high iron concentration (Fig. 4C), suggesting that PhoB confers the effect of Pi concentration to the transcription of fur, suppressing its transcription at low Pi concentrations. This negative cross-talk is likely to be indirect via unidentified regulators because the promoter of fur does not have a Pho box.
PhoU Is a Co-activator of PhoB at Low Concentration of Pi and Is Essential for Expression of EsrC
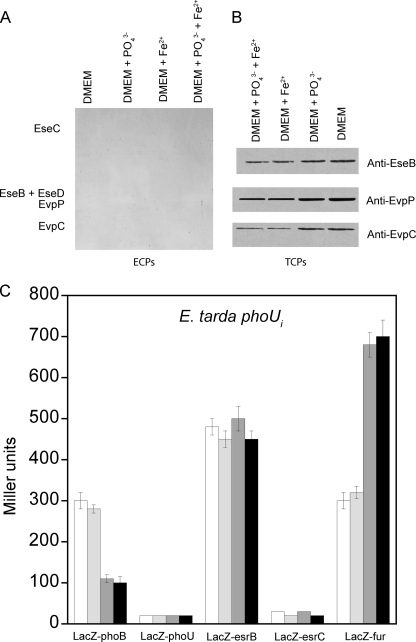
PhoU is essential for the repression of the Pho regulon in high Pi conditions by binding to PhoR or a PhoR-PhoB complex to promote dephosphorylation of phospho-PhoB (16). Based on our ECP and TCP assays on E. tarda phoUi, our results suggest that PhoU is an essential co-activator of PhoB for the expression and secretion of T3SS and T6SS proteins at low Pi conditions. An insertional mutation of phoU in E. tarda PPD130/91 abolished the secretion of proteins from both T3SS and T6SS entirely (Fig. 5A). This absence of secretion was due to significantly reduced expression levels of proteins from T3SS and T6SS that were no longer sensitive to Pi concentrations, although further suppression by Fur was observed at higher iron concentration (Fig. 5B).
FIGURE 5.
Effects of Pi and iron concentration on secretion and expression of proteins from T3SS and T6SS in E. tarda PPD130/91 phoUi mutant. The effects of Pi and iron concentrations on the amounts of ECPs (A) and TCPs (B) from T3SS (EseB, EseC, and EseD) and T6SS (EvpC and EvpP) were determined by growing E. tarda phoUi mutant in DMEM or the same medium containing 20 mm KH2PO4 and/or 10 μm FeSO4. The results were determined using Western blotting. C, the effects of Pi and iron concentration on the β-galactosidase activities of the reporter genes phoB-LacZ, phoU-LacZ, esrB-LacZ, esrC-LacZ, and fur-LacZ in E. tarda PPD130/91 phoUi mutant were determined by culturing the cells in DMEM (white bar) or the same medium containing 20 mm KH2PO4 (light gray bar), 10 μm FeSO4 (dark gray bar), or 20 mm KH2PO4 + 10 μm FeSO4 (black bar). Values represent mean ± S.E., n = 3, by one experiment.
The LacZ reporter gene assay showed that transcription of phoB was reduced by about 25% as compared with wild-type E. tarda in the phoUi mutant; these results support the hypothesis that PhoU is important for the activation of PhoB at low Pi concentrations in this case in the autoregulation of phoB. Because phoB transcription was independent of Pi concentration, this indicates that PhoU is necessary for sensing high Pi concentration for the suppression of phoB. A high iron concentration was found to still suppress transcription of phoB by about 60% in the absence of PhoU (Fig. 5C).
The phoUi mutation had no effect on the transcription level of esrB, suggesting that PhoU did not convey Pi-induced virulence gene expression by regulating esrB transcription. However, esrC transcription absolutely required PhoU as esrC transcription was abolished completely in the phoUi mutant (Fig. 5C). The indirect activation of esrC by PhoB requires PhoU as there was no esrC transcription in the phoUi mutant even though phoB was intact. Deletion of phoB could not abolish expression of esrC completely (Fig. 4C), but the phoUi mutation could, suggesting that another PhoU-dependent regulator(s) in addition to PhoB was also involved in regulating the transcription of esrC.
PhoU was required by PhoB for its indirect suppression of the transcription of fur, and the phoUi mutation increased the level of transcription to a level similar to that of the ΔphoB mutation (Fig. 5C). The phoUi mutation also rendered the transcription of fur independent of Pi concentration but still sensitive to iron concentration, suggesting that PhoB and PhoU cooperate to suppress fur and convey Pi concentration in the regulation of fur transcription.
Fur Senses Iron Concentration and Suppresses T3SS and T6SS Directly or Indirectly through EsrC
When the fur gene in E. tarda PPD130/91 was deleted, the secretion (ECPs) and expression (TCPs) of proteins from both T3SS and T6SS were “derepressed” and significantly up-regulated (Fig. 6, A and B). This shows that Fur represses the expression of T3SS and T6SS proteins even in the absence of iron. The secretion and expression levels of proteins from T3SS and T6SS in E. tarda Δfur became independent of iron concentration but were still sensitive to Pi concentration, confirming that Fur senses iron concentrations to regulate T3SS and T6SS in E. tarda. In addition to evpP, other genes in the T3SS were also regulated by Fur, suggesting that Fur can also indirectly regulate T3SS via unidentified downstream regulators in addition to direct binding of the Fur box at high iron concentrations.
FIGURE 6.
Effects of Pi and iron concentration on secretion and expression of proteins from T3SS and T6SS in E. tarda PPD130/91 Δfur mutant. The effects of Pi and iron concentrations on the amounts of ECPs (A) and TCPs (B) from T3SS (EseB, EseC, and EseD) and T6SS (EvpC and EvpP) were determined by growing E. tarda Δfur mutant in DMEM or the same medium containing 20 mm KH2PO4 and/or 10 μm FeSO4. The results were determined using Western blotting. C, the effects of Pi and iron concentration on the β-galactosidase activities of the reporter genes phoB-LacZ, phoU-LacZ, esrB-LacZ, esrC-LacZ, and fur-LacZ in E. tarda PPD130/91 Δfur mutant were determined by culturing the cells in DMEM (white bar) or the same medium containing 20 mm KH2PO4 (light gray bar), 10 μm FeSO4 (dark gray bar), or 20 mm KH2PO4 + 10 μm FeSO4 (black bar). Values represent mean ± S.E., n = 3, by one experiment.
In E. tarda Δfur mutant, there was a general up-regulation of the transcription levels of phoB (60%), phoU (55%), and esrC (35%), but the transcription level of esrB was unchanged as in the wild-type E. tarda (Fig. 6C). These transcription levels became independent of iron concentration but were still sensitive to Pi concentration, confirming that Fur is the sole iron sensor for suppression of genes from T3SS and T6SS in E. tarda by directly binding the promoter upstream of evpP and indirectly regulating esrC. The indirect suppression of phoB and phoU by Fur via unidentified downstream regulators also confirms a negative cross-talk between the Fur and Pho regulons.
PhoU Interacts with PhoB and Fur, whereas Fur Interacts with EsrC
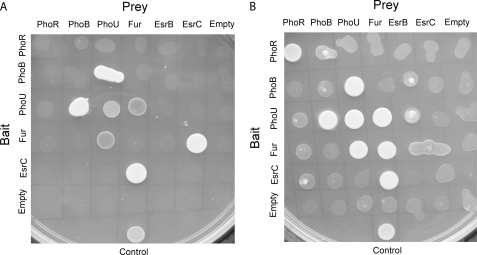
To investigate whether there are direct physical interactions among regulators of the Fur and Pho regulons and of the T3SS and T6SS, we performed yeast two-hybrid experiments on phoR, phoB, phoU, fur, esrB, and esrC using stringent quadruple dropout conditions and under limiting Pi and iron concentrations. Fig. 7A shows the results of the yeast two-hybrid experiments. PhoU interacted with both PhoB and Fur, suggesting that it is an important protein in both Pi and iron signaling. Furthermore, the transcription of phoB was reduced in phoUi mutant (Fig. 5C), supporting our finding that PhoU could be a co-activator of PhoB at low Pi concentration. On the other hand, the interaction between PhoU and Fur may be inhibitory and involved in the negative cross-talk between Fur and the Pho regulon. The yeast two-hybrid result also suggested that PhoU interacted with itself to form a dimer (Fig. 7A); this agrees with the crystal structure of Thermotoga maritima PhoU2 protein by Liu et al. (37) in which two monomers (six helices each) form a dimer through the crystallographic dyad, resulting in a 12-helix bundle. Interestingly, in addition to PhoU, Fur also interacted directly with EsrC (Fig. 7A), suggesting that Fur may also inhibit protein expression from T3SS and T6SS of E. tarda through its direct interaction with EsrC; this is in addition to Fur competing with EsrC for binding to the Fur box upstream of evpP (Fig. 1A) and suppressing the transcription of esrC indirectly through other regulators (Fig. 6C).
FIGURE 7.
Yeast two-hybrid assay to determine interactions among PhoR, PhoB, PhoU, Fur, EsrB, and EsrC in E. tarda PPD130/91. Yeast two-hybrid results using high stringency SD lacking Trp, Leu, His and adenine (A) and medium stringency SD lacking Trp, Leu and His (B) are shown. The positive control comprised a fusion construct of Arabidopsis histidine phosphotransfer 1 in the prey vector or activation domain vector and Arabidopsis response regulator 4 in the bait vector or DNA binding domain vector. Empty vectors were used as negative controls. All full-length protein constructs were used (PhoB, PhoU, Fur, EsrB, and EsrC) except where the putative transmembrane region of PhoR was removed in the final construct.
Using a less stringent condition with only a triple dropout, the results showed that both PhoR and Fur formed dimers (Fig. 7B). This is probably the first direct evidence that PhoR can interact with itself, and the dimerization of Fur agrees with the crystal structure of Fur from V. cholerae (19). A weak interaction was also observed between PhoU and EsrB (Fig. 7B). Our findings show that there are regulators in addition to PhoB that require PhoU to regulate transcription of esrC. EsrB binds to the promoter of esrC directly to activate its transcription (8). EsrB alone, however, seems unable to activate esrC as no transcription of esrC was observed in phoUi mutant with intact esrB (Fig. 5C), suggesting that EsrB may require an interaction with PhoU to activate esrC.
An earlier study proposed that PhoU may act as a negative regulator of the Pho regulon by interacting with PhoR for dephosphorylation of PhoB at high Pi concentration (38). This interaction was not observed in our yeast two-hybrid assay (Fig. 7B). A fluorescence resonance energy transfer (FRET) assay also failed to show an interaction between PhoU and PhoB or PhoR in E. coli (39). An intact Pst system may be required for formation of this inhibitory complex. Although a weak interaction was observed between PhoB and PhoR in our yeast two-hybrid experiment (Fig. 7B) and in the FRET assay (39), the binding is not conclusive.
DISCUSSION
Binding Sites of EsrB and EsrC on T3SS and T6SS of E. tarda PPD130/91
In this study, we mapped the locations of the DNA binding sites of EsrB and EsrC on the type III and VI secretion systems (T3SS and T6SS) of E. tarda PPD130/91 (Fig. 2). Both esrB and esrC are encoded in the T3SS, but EsrB acts upstream of EsrC and binds directly to the promoters in T3SS, including esrC. EsrB did not bind to any of the promoters on T6SS and only regulates T6SS indirectly through EsrC. On the other hand, EsrC binds to promoters in both the T3SS and T6SS, although the affinity is weaker compared with EsrB in the promoters that bind to both. These findings are further verified by the expression profiles of TCP in ΔesrB and ΔesrC mutants of E. tarda. Deletion of esrB abolished expression of proteins from both T3SS and T6SS, whereas deletion of esrC abolished expression only from T6SS (supplemental Fig. S5), confirming that EsrB regulates T6SS through EsrC, whereas EsrC plays a supporting role for EsrB by enhancing the expression of certain genes in T3SS.
EsrB and EsrC did not seem to bind to consensus sequences, such as PhoB and Fur. In Salmonella, a common motif, TTAAT, was identified in the SsrB binding sites at three promoters of ssrA, ssrB, and srfH. This motif, however, is not present in SsrB binding sites identified at SPI2 genes sseA, ssaB, ssaG, and ssaM (40), suggesting that this may not be the consensus DNA binding motif of SsrB. This TTAAT motif is also found upstream of orf29 from nt −216 to −211 but is not found in other promoters that bound EsrB. In addition, EsrC is unique to E. tarda, and homologous protein was not observed in T3SS of other bacteria, including S. typhimurium.
PhoB-PhoR and PhoU Activate T3SS and T6SS through EsrC and Negative Cross-talk to Fur
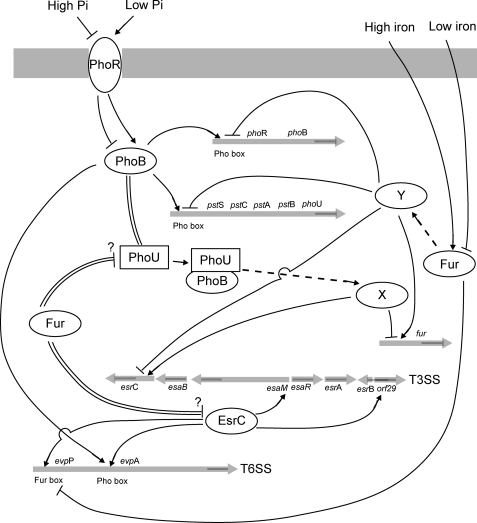
Encoded by the pstSCAB-phoU operon, the PhoB-PhoR TCS and PhoU do not solely regulate the expression of the Pst system for acquiring nutrient Pi; they are also essential for regulating virulence by activating T3SS and T6SS at low Pi concentrations through EsrC. Fig. 8 details a schematic representation of the regulatory network of PhoB-PhoR and Fur on T3SS and T6SS of E. tarda PPD130/91 through EsrC. The involvement of PhoB-PhoR and the pstSCAB-phoU operon in the regulation of virulence is also observed in other pathogenic bacteria. In extraintestinal pathogenic E. coli, the Pho regulon is controlled jointly by PhoB-PhoR and the Pst system for the transfer of a phosphoryl group to lipid A. The pst mutant displays an increased sensitivity to different cationic antimicrobial peptides and vancomycin (41). The pstSCAB-phoU operon is required for adherence in the atypical enteropathogenic E. coli strain E128012 and bacterial colonization in Citrobacter rodentium by causing increased expression of an unidentified Pho-regulated adhesin (42).
FIGURE 8.
Schematic diagram to summarize regulatory network of PhoB-PhoR and Fur on T3SS and T6SS of E. tarda PPD130/91 through EsrC. The TCS PhoB-PhoR senses low Pi concentration for autoregulation and activation of pstSCAB-phoU through direct binding of a Pho box upstream of the promoters of phoB and pstS. PhoB binds simultaneously with EsrC on the promoter upstream of evpA to regulate transcription of genes from T6SS. PhoB requires and also interacts with PhoU (double line) to activate esrC and suppress fur indirectly through unidentified regulators (dotted line; through regulator “X”) in T3SS and T6SS through EsrC. EsrC binds promoters upstream of esaM and orf29 of T3SS and evpP and evpA of T6SS. Fur senses high iron concentration and binds directly to a Fur box upstream of evpP to inhibit binding of EsrC to the same promoter. Additionally, Fur suppresses transcription of phoB, pstSCAB-phoU, and esrC indirectly through unidentified regulators (dotted line; through regulator “Y”). Fur shows negative cross-talk with the Pho regulon and physically interacts (double line) with PhoU and EsrC; however, whether this interaction is an inhibitory mechanism remains to be determined.
In the current study, we found that PhoB and EsrC simultaneously bind to the promoter upstream of evpA to regulate transcription of genes from evpA to evpO in the T6SS of E. tarda. EsrC had a higher affinity for this promoter, but PhoB binding was necessary for the transcription. Unlike other PhoB-regulated promoters, the Pho box upstream of evpA is located at a distance (between nt −496 and −479) from the initiation site compared with those of phoB (between nt −42 and −25) and pstS (between nt −89 and −74). This may explain why the simultaneous binding of EsrC was required for activation of this promoter in E. tarda. Binding of PhoB to promoters containing the Pho box causes bending to the promoter region, and it was postulated that the PhoB and Pho box complex interacts with the σ70 subunit of RNA polymerase to control transcription initiation (34, 43).
Other than direct DNA binding, PhoB was found to bind to PhoU and use it to indirectly activate transcription of esrC at low Pi concentrations (Fig. 8). The transcription of esrC was not regulated solely by PhoB but involved another regulator, e.g. EsrB, which may also require PhoU for its action. PhoU was found to be an inhibitor of the Pho regulon by interacting with PhoR/PhoB and in high Pi concentrations releasing dephosphorylated PhoB. Using a promoter-swapping technique, PhoU was shown to control the activity of PstSCAB2 transporter as well as its abundance within the cell (44). When internal Pi levels are elevated, PhoU inhibits transport by the PstSCAB2 transporter by shifting the biochemical activity of PhoR from autokinase to phospho-PhoB phosphatase activity. It was proposed that PhoU may bind intracellular Pi and then interact with one of the PstSCAB proteins to inhibit its activity (44). The crystal structure of a “PhoU-like” phosphate uptake regulator from Aquifex aeolicus suggests that PhoU may bind to the complex of PhoR and phosphorylated PhoB to dephosphorylate and release PhoB (45). The role of PhoU at limiting Pi concentration, however, had not been completely understood. In our study, we showed that PhoB interacted with PhoU to activate expression of esrC indirectly when the Pi concentration was low. This agrees with findings in the extraintestinal pathogenic E. coli strain CFT073 that PhoU is a urovirulence factor and indirectly affects the expression of virulence-related genes. The phoU mutant was significantly outnumbered by the wild-type strain in competitive colonization experiments, and the attenuation was not due to a general growth defect (46).
This study also presents evidence of negative cross-talk between PhoB and Fur with PhoB indirectly suppressing the transcription of fur along with the requirement of an intact PhoU protein. PhoB and Fur also function in an opposite manner in the regulation of esrC with PhoB activating it, whereas Fur suppresses it (Fig. 8). A similar trend was also observed in the T3SS encoded on SPI1 of S. typhimurium in which HilA is a direct regulator of SPI1 structural genes. Iron signaling was mediated by Fur, which activates hilA transcription in an HilD-dependent manner (47). In contrast to Fur, PhoB was proposed to repress hilA and the expression of invasion genes under low extracellular Pi conditions in S. typhimurium (48). The Pho regulon was also found to overlap and interact with several other control circuits in Sinorhizobium meliloti, e.g. oxidative stress response and iron homeostasis (49), suggesting that cross-talk between the Pho regulon and Fur is highly possible.
Fur Represses T3SS and T6SS through EsrC and Negative Cross-talk to Pho Regulon
Fur plays an important role in regulating the uptake of iron, which is an essential nutrient and highly related to oxidative stress. However, Fur is also essential for sensing iron concentration to activate or suppress the expression of virulence genes. Vertebrates sequester iron from invading pathogens as a mean of “nutritional immunity” using the serum protein transferrin, which binds iron with very high affinity (Ka = 1036). Many bacterial pathogens sense this iron depletion as a signal that they are within a vertebrate host and elicit the expression of virulence genes that allow them to circumvent iron withholding (12). For instance, Staphylococcus aureus senses alterations in the iron status via Fur and alters the abundance of a large number of virulence factors, such as cytolysins and a subset of immunomodulatory proteins, to survive neutrophils attack (50). Fur is also a regulator of the E. tarda hemolysin (Eth) system in which EthA and EthB (which is regulated by iron) are noted virulence elements widely distributed in pathogenic E. tarda. ethB expression decreases under high expression levels of Fur and is enhanced upon the activation of Fur (51); this is in agreement with earlier work showing that Fur acts as a repressor under iron repletion conditions and is inactivated under conditions of iron starvation to permit the expression of Fur-regulated genes (52).
In this study, we found that Fur binds directly to a Fur box in the promoter upstream of evpP. It bound with a higher affinity than EsrC and consequently inhibited its binding (Fig. 1A). However, Fur was unable to bind the Fur box in iron-depleted conditions, explaining why Fur only suppressed evpP transcription by EsrC at high iron concentrations in E. tarda PPD130/91 (Fig. 8). Our result agrees with the findings of Wang et al. (33) that evpP in E. tarda EIB202 was regulated by esrB (likely through EsrC) and iron. However, in that study, transcription of evpP was positively regulated by either EsrB or the iron concentration in media. The activity of the evpP promoter in iron-rich media was 2.18-fold higher than that in iron-depleted media; these contrasting observations remain to be explained. Unlike other T6SS genes, evpP has a separate promoter that is not regulated directly by PhoB, suggesting that the function of EvpP may be unique. EvpP was shown to mediate hemolytic activity, mucus adhesion, serum resistance, and internalization of host cells by E. tarda, and mutation in evpP caused marked attenuation in virulence and rapid elimination of E. tarda in vivo (33).
Our results reveal that Fur does not autoregulate its own expression in E. tarda PPD130/91 as a Fur box was not found in the promoter upstream of fur. In Pseudomonas aeruginosa, autoregulation of fur has also not been observed (53). However, the autoregulation of fur has been observed in other bacterial strains and in completely different ways. In E. tarda TX1 isolated from diseased fish, a Fur box was identified from nt −61 to −79 of the initiation translation site, and Fur negatively regulated its own expression (54). In Vibrio vulnificus, however, fur gene expression was positively regulated by Fur when the iron concentration was limited (55). Later, the group identified a Fur-protected region from nt −142 to −106 with respect to the transcription start site of the fur gene in iron-depleted conditions using footprinting analysis (56).
In addition to directly regulating T6SS genes, Fur suppressed phoB, phoU (negative cross-talk to Pho regulon), and esrC indirectly in an iron concentration-dependent manner through downstream regulators that are yet to be identified. EsrC in turn regulates the expression of genes in T3SS and T6SS of E. tarda (Fig. 8). The DNA binding function of Fur alone cannot explain its essential role, and many other regulators are involved in mediating the activity of Fur. In P. aeruginosa, Fur does not directly repress regA (toxR) and toxA; rather, it requires PvdS, an extracytoplasmic function class alternative σ factor, to mediate iron control of both regA and ptxR for toxA expression to produce exotoxin A (53). In E. tarda PPD130/91, a Fur box was found in the promoter region of pspF, which codes a phage shock protein operon transcriptional activator, that is σ54-dependent and could function to mediate signals from Fur. In Y. pestis, Fur functions together with YbtA to regulate the production of yersiniabactin. YbtA is an AraC family transcriptional regulator that binds the consensus sequence ACCCGwwwCGGGT (w = A or T) (22). Other than regulation at the transcription level, in this study, Fur physically interacted with PhoU and EsrC. These interactions could be part of the inhibitory mechanisms of Fur on the function of EsrC and the activation of the Pho regulon.
Conclusions
In this study, we demonstrated that the PhoB-PhoR two-component system senses fluctuations in Pi concentration, whereas Fur senses fluctuations in iron concentration. Both of these signaling pathways affect the regulation of the type III and VI secretion systems (T3SS and T6SS) in E. tarda PPD130/91 by EsrC. Although previous work proposed that PhoU interacts with PhoR at high Pi concentrations to dephosphorylate PhoB, here we show that PhoU interacts with PhoB and is an important co-activator of PhoB at low Pi concentrations. PhoB and Fur suppress each other indirectly through downstream, unidentified transcription regulators, providing negative cross-talk between the Pho and Fur regulons in the regulation of virulence genes.
EsrB is the main regulator of T3SS and binds promoters exclusively in T3SS, but EsrB can only regulate T6SS by affecting the transcription of esrC. In contrast, EsrC binds to promoters from both T3SS and T6SS. PhoB binds directly and simultaneously with EsrC to activate the promoter of evpA, whereas Fur binds directly and inhibits binding of EsrC to the promoter of evpP. The direct DNA binding cannot completely explain the roles of PhoB and Fur as they regulate each other and indirectly regulate esrC via unidentified downstream regulators. Additionally, Fur may interact with PhoU and EsrC for their regulation.
We previously showed that environmental factors, such as temperature, Mg2+, and antimicrobial peptide were sensed by PhoP-PhoQ in the regulation of T3SS and T6SS through EsrB for detection of the host (8). Here, we reveal that the depletion of nutrients, such as Pi and iron, by the host is sensed by PhoB-PhoR and Fur, respectively, to regulate T3SS and T6SS through EsrC and that this signaling maintains survival of the pathogen inside the host. These results demonstrate that T3SS and T6SS may be regulated differently in response to different environmental factors or cues and suggest that T3SS and T6SS could have distinct roles in the pathogenicity of E. tarda.
Supplementary Material
Acknowledgment
We thank Prof. Prakash Kumar of the National University of Singapore for providing plasmids and media for the yeast two-hybrid studies.
This work was supported by Grant 07/1/21/19/495 from the Biomedical Research Council of the Agency for Science Technology and Research (A*STAR) of Singapore and Ministry of Education Tier 1 Grant R-154-000-498-112 (to Y.-K. M.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S5 and Tables S1 and S2.
- TCS
- two-component system
- Fur
- ferric uptake regulator
- Esr
- E. tarda secretion regulator
- Pst
- phosphate-specific transport
- Evp
- E. tarda virulence protein
- Ese
- E. tarda secretion effector
- Esa
- E. tarda secretion apparatus
- ECP
- extracellular protein
- TCP
- total cellular proteins
- SD
- synthetic dropout agar plates
- nt
- nucleotides
- Eth
- E. tarda hemolysin.
REFERENCES
- 1. Janda J. M., Abbott S. L. (1993) Clin. Infect. Dis. 17, 742–748 [DOI] [PubMed] [Google Scholar]
- 2. Thune R. L., Stanley L. A., Cooper R. K. (1993) Annu. Rev. Fish Dis. 3, 37–68 [Google Scholar]
- 3. Schlenker C., Surawicz C. M. (2009) Best Pract. Res. Clin. Gastroenterol. 23, 89–99 [DOI] [PubMed] [Google Scholar]
- 4. Tan Y. P., Lin Q., Wang X. H., Joshi S., Hew C. L., Leung K. Y. (2002) Infect. Immun. 70, 6475–6480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Srinivasa Rao P. S., Yamada Y., Tan Y. P., Leung K. Y. (2004) Mol. Microbiol. 53, 573–586 [DOI] [PubMed] [Google Scholar]
- 6. Tan Y. P., Zheng J., Tung S. L., Rosenshine I., Leung K. Y. (2005) Microbiology 151, 2301–2313 [DOI] [PubMed] [Google Scholar]
- 7. Zheng J., Leung K. Y. (2007) Mol. Microbiol. 66, 1192–1206 [DOI] [PubMed] [Google Scholar]
- 8. Chakraborty S., Li M., Chatterjee C., Sivaraman J., Leung K. Y., Mok Y. K. (2010) J. Biol. Chem. 285, 38876–38888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zheng J., Tung S. L., Leung K. Y. (2005) Infect. Immun. 73, 4127–4137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vasil M. L. (2007) Biometals 20, 587–601 [DOI] [PubMed] [Google Scholar]
- 11. Lamarche M. G., Wanner B. L., Crépin S., Harel J. (2008) FEMS Microbiol. Rev. 32, 461–473 [DOI] [PubMed] [Google Scholar]
- 12. Skaar E. P. (2010) PLoS Pathog. 6, e1000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Long J., Zaborina O., Holbrook C., Zaborin A., Alverdy J. (2008) Surgery 144, 189–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Scholten M., Tommassen J. (1993) Mol. Microbiol. 8, 269–275 [DOI] [PubMed] [Google Scholar]
- 15. Arribas-Bosacoma R., Kim S. K., Ferrer-Orta C., Blanco A. G., Pereira P. J., Gomis-Rüth F. X., Wanner B. L., Coll M., Solà M. (2007) J. Mol. Biol. 366, 626–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hsieh Y. J., Wanner B. L. (2010) Curr. Opin. Microbiol. 13, 198–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wanner B. L. (1993) J. Cell. Biochem. 51, 47–54 [DOI] [PubMed] [Google Scholar]
- 18. Pratt J. T., Ismail A. M., Camilli A. (2010) Mol. Microbiol. 77, 1595–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sheikh M. A., Taylor G. L. (2009) Mol. Microbiol. 72, 1208–1220 [DOI] [PubMed] [Google Scholar]
- 20. Lavrrar J. L., McIntosh M. A. (2003) J. Bacteriol. 185, 2194–2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gao H., Zhou D., Li Y., Guo Z., Han Y., Song Y., Zhai J., Du Z., Wang X., Lu J., Yang R. (2008) J. Bacteriol. 190, 3063–3075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anisimov R., Brem D., Heesemann J., Rakin A. (2005) Int. J. Med. Microbiol. 295, 19–28 [DOI] [PubMed] [Google Scholar]
- 23. Wang Q., Yang M., Xiao J., Wu H., Wang X., Lv Y., Xu L., Zheng H., Wang S., Zhao G., Liu Q., Zhang Y. (2009) PLoS One 4, e7646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Deng W., Burland V., Plunkett G., 3rd, Boutin A., Mayhew G. F., Liss P., Perna N. T., Rose D. J., Mau B., Zhou S., Schwartz D. C., Fetherston J. D., Lindler L. E., Brubaker R. R., Plano G. V., Straley S. C., McDonough K. A., Nilles M. L., Matson J. S., Blattner F. R., Perry R. D. (2002) J. Bacteriol. 184, 4601–4611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McClelland M., Sanderson K. E., Spieth J., Clifton S. W., Latreille P., Courtney L., Porwollik S., Ali J., Dante M., Du F., Hou S., Layman D., Leonard S., Nguyen C., Scott K., Holmes A., Grewal N., Mulvaney E., Ryan E., Sun H., Florea L., Miller W., Stoneking T., Nhan M., Waterston R., Wilson R. K. (2001) Nature 413, 852–856 [DOI] [PubMed] [Google Scholar]
- 26. Welch R. A., Burland V., Plunkett G., 3rd, Redford P., Roesch P., Rasko D., Buckles E. L., Liou S. R., Boutin A., Hackett J., Stroud D., Mayhew G. F., Rose D. J., Zhou S., Schwartz D. C., Perna N. T., Mobley H. L., Donnenberg M. S., Blattner F. R. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 17020–17024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lodge J., Fear J., Busby S., Gunasekaran P., Kamini N. R. (1992) FEMS Microbiol. Lett. 74, 271–276 [DOI] [PubMed] [Google Scholar]
- 28. Kalogeraki V. S., Winans S. C. (1997) Gene 188, 69–75 [DOI] [PubMed] [Google Scholar]
- 29. Edwards R. A., Keller L. H., Schifferli D. M. (1998) Gene 207, 149–157 [DOI] [PubMed] [Google Scholar]
- 30. Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. (1989) Gene 77, 51–59 [DOI] [PubMed] [Google Scholar]
- 31. Dortay H., Mehnert N., Bürkle L., Schmülling T., Heyl A. (2006) FEBS J. 273, 4631–4644 [DOI] [PubMed] [Google Scholar]
- 32. Aguena M., Yagil E., Spira B. (2002) Mol. Genet. Genomics 268, 518–524 [DOI] [PubMed] [Google Scholar]
- 33. Wang X., Wang Q., Xiao J., Liu Q., Wu H., Xu L., Zhang Y. (2009) Fish Shellfish Immunol. 27, 469–477 [DOI] [PubMed] [Google Scholar]
- 34. Blanco A. G., Sola M., Gomis-Rüth F. X., Coll M. (2002) Structure 10, 701–713 [DOI] [PubMed] [Google Scholar]
- 35. Carroll R. K., Liao X., Morgan L. K., Cicirelli E. M., Li Y., Sheng W., Feng X., Kenney L. J. (2009) J. Biol. Chem. 284, 12008–12019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rodgers M. E., Schleif R. (2009) Proteins 77, 202–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu J., Lou Y., Yokota H., Adams P. D., Kim R., Kim S. H. (2005) J. Biol. Chem. 280, 15960–15966 [DOI] [PubMed] [Google Scholar]
- 38. Steed P. M., Wanner B. L. (1993) J. Bacteriol. 175, 6797–6809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Baek J. H., Kang Y. J., Lee S. Y. (2007) FEMS Microbiol. Lett. 277, 254–259 [DOI] [PubMed] [Google Scholar]
- 40. Feng X., Walthers D., Oropeza R., Kenney L. J. (2004) Mol. Microbiol. 54, 823–835 [DOI] [PubMed] [Google Scholar]
- 41. Lamarche M. G., Kim S. H., Crépin S., Mourez M., Bertrand N., Bishop R. E., Dubreuil J. D., Harel J. (2008) J. Bacteriol. 190, 5256–5264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cheng C., Tennant S. M., Azzopardi K. I., Bennett-Wood V., Hartland E. L., Robins-Browne R. M., Tauschek M. (2009) Infect. Immun. 77, 1936–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Makino K., Amemura M., Kawamoto T., Kimura S., Shinagawa H., Nakata A., Suzuki M. (1996) J. Mol. Biol. 259, 15–26 [DOI] [PubMed] [Google Scholar]
- 44. Rice C. D., Pollard J. E., Lewis Z. T., McCleary W. R. (2009) Appl. Environ. Microbiol. 75, 573–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Oganesyan V., Oganesyan N., Adams P. D., Jancarik J., Yokota H. A., Kim R., Kim S. H. (2005) J. Bacteriol. 187, 4238–4244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Buckles E. L., Wang X., Lockatell C. V., Johnson D. E., Donnenberg M. S. (2006) Microbiology 152, 153–160 [DOI] [PubMed] [Google Scholar]
- 47. Ellermeier J. R., Slauch J. M. (2008) J. Bacteriol. 190, 476–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lucas R. L., Lostroh C. P., DiRusso C. C., Spector M. P., Wanner B. L., Lee C. A. (2000) J. Bacteriol. 182, 1872–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yuan Z. C., Zaheer R., Morton R., Finan T. M. (2006) Nucleic Acids Res. 34, 2686–2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Torres V. J., Attia A. S., Mason W. J., Hood M. I., Corbin B. D., Beasley F. C., Anderson K. L., Stauff D. L., McDonald W. H., Zimmerman L. J., Friedman D. B., Heinrichs D. E., Dunman P. M., Skaar E. P. (2010) Infect. Immun. 78, 1618–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang F., Zhang M., Hu Y. H., Zhang W. W., Sun L. (2009) J. Microbiol. Biotechnol. 19, 765–773 [PubMed] [Google Scholar]
- 52. Bagg A., Neilands J. B. (1987) Biochemistry 26, 5471–5477 [DOI] [PubMed] [Google Scholar]
- 53. Vasil M. L., Ochsner U. A. (1999) Mol. Microbiol. 34, 399–413 [DOI] [PubMed] [Google Scholar]
- 54. Wang F., Cheng S., Sun K., Sun L. (2008) J. Microbiol. 46, 350–355 [DOI] [PubMed] [Google Scholar]
- 55. Lee H. J., Park K. J., Lee A. Y., Park S. G., Park B. C., Lee K. H., Park S. J. (2003) J. Bacteriol. 185, 5891–5896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lee H. J., Bang S. H., Lee K. H., Park S. J. (2007) J. Bacteriol. 189, 2629–2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.