Background: Rap1b is a small G protein that is a key regulator for platelet activation.
Results: Agonist-induced Rap1b activation plays a role in platelet secretion, and integrin outside-in signaling-mediated Rap1b activation is important in platelet spreading on fibrinogen and clot retraction.
Conclusion: There are dual activation mechanisms of Rap1 that play distinct roles in platelet function.
Significance: Learning two novel functions of Rap1b in platelets.
Keywords: Adhesion, Integrin, Platelets, Protein Kinase C, Secretion, Src, Clot Retraction, Outside-in, Rap1b
Abstract
Rap1b is activated by platelet agonists and plays a critical role in integrin αIIbβ3 inside-out signaling and platelet aggregation. Here we show that agonist-induced Rap1b activation plays an important role in stimulating secretion of platelet granules. We also show that αIIbβ3 outside-in signaling can activate Rap1b, and integrin outside-in signaling-mediated Rap1b activation is important in facilitating platelet spreading on fibrinogen and clot retraction. Rap1b-deficient platelets had diminished ATP secretion and P-selectin expression induced by thrombin or collagen. Importantly, addition of low doses of ADP and/or fibrinogen restored aggregation of Rap1b-deficient platelets. Furthermore, we found that Rap1b was activated by platelet spreading on immobilized fibrinogen, a process that was not affected by P2Y12 or TXA2 receptor deficiency, but was inhibited by the selective Src inhibitor PP2, the PKC inhibitor Ro-31-8220, or the calcium chelator demethyl-1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis. Clot retraction was abolished, and platelet spreading on fibrinogen was diminished in Rap1b-deficient platelets compared with wild-type controls. The defects in clot retraction and spreading on fibrinogen of Rap1b-deficient platelets were not rescued by addition of MnCl2, which elicits αIIbβ3 outside-in signaling in the absence of inside-out signaling. Thus, our results reveal two different activation mechanisms of Rap1b as well as novel functions of Rap1b in platelet secretion and in integrin αIIbβ3 outside-in signaling.
Introduction
Rap1b, the small GTPase from the Ras family, is a key regulator for integrin activation and platelet aggregation (1–4). Platelets lacking Rap1b show defects in αIIbβ3-dependent aggregation in vitro and in thrombosis and hemostasis in vivo (3). Rap1 has been shown to be important in regulating integrin-dependent cell adhesion in response to cytokines and growth factors in many cell types (5–7). Rap1b activation results in association of Rap1b with the Rap1-GTP-interacting adaptor molecule and talin, leading to integrin-talin interaction and thereby promotes integrin activation (8, 9). The Ca2+ and diacylglycerol-regulated guanine nucleotide exchange factor I (CalDAG-GEFI),2 an upstream guanine nucleotide exchange factor for Rap 1 and Rap 2, is also crucial in regulating β1, β2, and β3 integrin activation (10–12). Mice deficient in CalDAG-GEFI, have a similar phenotype as Rap1b-deficient mice (11, 12).
Platelet secretion is essential for thrombosis (13). Three main types of secretory granules have been described in platelets, dense granules, α-granules, and lysosomes (14). Dense granules contain small molecules such as ADP, serotonin, and calcium. α-Granules contain proteinaceous components such as fibrinogen, von Willebrand factor, coagulation factors, cytokines, and growth factors, and lysosomes contain a host of proteolytic enzymes. Granule secretion requires fusion between plasma and granule (vesicle) membranes, which is mediated by protein complexes of vesicle-soluble N-ethylmaleimide-sensitive fusion protein attachment receptor (v-SNAREs) proteins and target membrane v-SNARE receptors (t-SNAREs) (14, 15). The interaction between SNARE proteins is controlled by SNARE regulators (such as Munc 18 and Munc 13 families), which are sensitive to second messengers such as Ca2+ or are phosphorylated by kinases. Munc 13-4 has been recently shown to be crucial for platelet secretion (16). Munc 13-4 regulates dense granule release via its interaction with the small GTP binding protein, Rab27b, a member of the Ras subfamily of GTPases (17). Mice deficient in Rab27b demonstrate reduced numbers of dense granules and impaired dense granule secretion (18). Other small GTPases such as Rac1 and Cdc42 have also been shown to be involved in the regulation of platelet secretion (19, 20). Although it is unknown whether Rap1b plays a role in platelet secretion, platelets from CalDAG-GEFI-deficient mice are defective in secretion in response to agonists (21), suggesting a possibility that Rap1b may also be involved in the regulation of platelet secretion.
αIIbβ3 has a low affinity for its ligands such as fibrinogen and von Willebrand factor in resting platelets, but becomes a high affinity receptor for these ligands upon activation by physiological agonists, a process named “inside-out signaling.” Ligand binding to αIIbβ3 triggers outside-in signaling that leads to stable platelet adhesion, spreading, and clot retraction (22, 23). The small G proteins Rac, Rho, and Cdc42 each have been shown to play a critical role in integrin outside-in signaling (24, 25). Rac and Cdc42 appear to regulate lamellipodia and filopodia formation, respectively (25). RhoA GTPase promotes cell contractility (26). Outside-in signaling, mediated by αIIbβ3, also involves Src family kinases (27, 28) and PKC (29, 30). It has been shown that αIIbβ3-mediated platelet spreading requires c-Src-dependent inhibition of the RhoA signaling pathway (31, 32). PKC θ and β are constitutively associated with αIIbβ3 and are important for platelet spreading on fibrinogen (29, 30).
In this study, we present data showing that there are two distinct Rap1b activation mechanisms. In addition to the known agonist-stimulated activation of Rap1b, which involves ADP receptor P2Y12 and TXA2 receptor (TP) signaling, integrin outside-in signaling results in Rap1b activation that is independent of P2Y12 and TP signaling. More importantly, we found that a primary role of agonist-induced Rap1b activation is to mediate platelet granule release, thus amplifying platelet responses and stimulating the second wave of platelet aggregation. Distinct from agonist-induced Rap1b activation, integrin-mediated Rap1b activation is important in outside-in signaling responses leading to clot retraction and platelet spreading. Thus, our study reveals dual activation mechanisms of Rap1 that play distinct roles in platelet function.
EXPERIMENTAL PROCEDURES
Materials
α-Thrombin was purchased from Enzyme Research Laboratories (South Bend, IN). PAR4 peptide AYPGKF was custom-synthesized at Biomatik USA, LLC (Wilmington, DE). ADP, the P2Y12 receptor antagonist 2-Methylthioadenosine 5′-monophosphate triethylammonium salt hydrate, the ADP scavenger apyrase (grade III), a mouse monoclonal antibody against β-actin, and rhodamine-phalloidin were from Sigma. AR-C69931MX was from the Medicines Co. (Parsippany, NJ). Luciferase/luciferin reagent and collagen were from Chrono-log (Havertown, PA). RalGDS-RBD fused to GST was a generous gift from Dr. Johannes L. Bos, University Medical Center, Utrecht, the Netherlands. The Src family kinase inhibitor PP2, the PKC inhibitor Ro-31-8220, and the TXA2 analog U46619 were purchased from Calbiochem. Calcium chelator dimethyl-BAPTA, Fura-2/AM, Oregon Green 488-conjugated fibrinogen, and Pluronic F-127 were from Invitrogen. Fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse P-selectin and integrin β3 antibodies were from BD Pharmingen. A rabbit polyclonal antibody against Rap1b and a rabbit polyclonal antibody against PAR4 were purchased from Santa Cruz Biotechnology. Phospho-specific rabbit monoclonal antibodies against phosphorylated Akt residue Ser473, p38 residues Thr180/Tyr182, and ERK residues Thr202/Tyr204 were from Cell Signaling Technologies. A rat monoclonal antibody against PF4 was from R&D System (Minneapolis, MN). A rat monoclonal antibody against GPVI was purchased from emfret Analytics (Eibelstadt, Germany).
Mice
Mice deficient in Rap1b (3), P2Y12 (33), TP (34), and integrin β3 (35) were generated as described previously. Littermate wild-type mice from heterozygous breeding were used as controls. Mice were bred and maintained in the University of Kentucky Animal Care Facility following institutional and National Institutes of Health guidelines after approval by the Animal Care Committee.
Preparation of Mouse Platelets
Washed platelets from knock-out mice and wild-type controls were prepared as described previously (36). Briefly, blood was collected from the abdominal aorta of isofluorane-anesthetized mice (8–10 weeks) using a 1/7 volume of ACD (85 mm trisodium citrate, 83 mm dextrose, and 21 mm citric acid) as anticoagulant. For each experiment, blood was pooled from three to five mice of each genotype. Platelets were then washed twice with CGS (0.12 m sodium chloride, 0.0129 m trisodium citrate, 0.03 m d-glucose, pH 6.5), resuspended in modified Tyrode's solution (12 mm NaHCO3, 138 mm NaCl, 5.5 mm glucose, 2.9 mm KCl, 2 mm MgCl2, 0.42 mm NaH2PO4, 10 mm HEPES, pH 7.4) at 3 × 108/ml, and incubated for 1 h at 22 °C before use.
Detection of Platelet ATP Secretion and Aggregation
Platelet secretion of granule ATP was determined as previously described in a Lumi-Aggregometer (Chrono-log, Havertown, PA) at 37 °C with stirring (1000 rpm) after addition of the luciferin-luciferase reagent and platelet agonists collagen or α-thrombin (37). Statistical significance between groups was determined using a Student t test. Platelet aggregation was measured by detecting changes in light transmission.
P-selectin Expression
Washed platelets (3 × 108/ml) from Rap1b-deficient mice or wild-type controls were incubated with various concentrations of thrombin in the presence of an FITC-conjugated anti-mouse P-selectin antibody for 30 min at 22 °C and fixed by adding paraformaldehyde (1% final concentration). P-selectin expression was analyzed by flow cytometry.
Measurement of Mouse Platelet Serotonin
Washed platelets (5 × 108/ml, 250 μl) from Rap1b+/+ or Rap1b−/− mice were solubilized by adding 60 μl of 6.0 m TCA. After centrifugation at 12,000 rpm for 2 min at room temperature, the TCA extract was transferred to a tube containing 1 ml of 0.05% O-phalal-dehyde. Samples were boiled for 10 min, cooled on ice, and then washed twice with chloroform. Serotonin release was measured using an MDS fluorescence spectrophotometer (MDS Analytical Technologies, Sunnyvale, CA) with an excitation wavelength of 360 nm and an emission wavelength of 475 nm.
Platelet Electron Microscopy
Washed platelets (1 × 109/ml) from Rap1b-deficient mice or wild-type controls were stimulated with 0.1 unit/ml thrombin at 22 °C for 5 min. Platelet electron microscopy was analyzed as described previously (38). Numbers of α granules in all platelets from five randomly chosen fields of Rap1b+/+ and Rap1b−/− resting samples were counted.
Rap1b Activation
Washed platelets from different knock-out mice or wild-type controls were resuspended in modified Tyrode's solution at 3 × 108/ml and incubated at 22 °C for 1 h before use. Platelets were then stimulated with various agonists and immediately lysed with addition of an equal volume of ice-cold lysis buffer (Tris, 100 mm; NaCl, 400 mm; MaCl2, 5 mm; Nonidet P-40, 2%; glycerol, 20%; PMSF, 2 mm; aprotinin, 220 Kallikrein inhibitor unit/ml; Na3VO4, 1 mm; leupeptin, 1 μg/ml, pH 7.4). A sample containing 50 μl lysate was added with an equal volume of 2 × SDS-sample buffer for the determination of total Rap1b levels. Active Rap1 was precipitated by glutathione-linked Agarose beads prebound to RalGDS RBD fused to GST. Precipitated GTP-Rap1 was detected by SDS-PAGE and immunoblotting with rabbit polyclonal anti-Rap1b-specific antibodies.
Calcium Mobilization
Intra-platelet calcium was measured using Fura-2/AM as described previously (38). Briefly, washed mouse platelets were incubated with 12.5 μm Fura-2/AM and 0.2% Pluronic F-127 (Invitrogen) for 45 min at 37 °C. After washing with CGS once more, platelets were resuspended to 3 × 108/ml in Tyrode's solution. Continuous fluorescent measurements were analyzed by excitation at 340 nm and 380 nm, and emission was measured at 509 nm using a model LS55 luminescence spectrometer (PerkinElmer Life Sciences). The intracellular Ca2+ level was expressed as relative fluorescence calculated based on the ratio of emissions simultaneously using FL WinLab4.0 software (PerkinElmer Life Sciences).
Immunoblot Detection of Akt, p38, and ERK Phosphorylation in Platelets
Washed platelets (3 × 108/ml) from Rap1b+/+ and Rap1b−/− mice were added with 0.025 unit/ml thrombin or 1.0 μg/ml collagen and incubated at 37 °C in the aggregometer for 2 or 5 min. Platelets were solubilized by adding equal volume of 2 × SDS sample buffer containing 2 mm sodium orthovanadate, 20 mm NaF, and protease inhibitor mixture (Sigma). Lysates were analyzed by SDS-PAGE using 4% to 15% gradient gels and immunoblotted using phospho-specific rabbit monoclonal antibodies against p38, p42/44, and Akt as described previously (39). Enhanced chemiluminescence (GE Healthcare, Piscataway, NJ) was used for visualization of antibody reactions.
Immunoblot Detection of PF4, PAR4, and GPVI in Platelets
Washed platelets (3 × 108/ml) from Rap1b+/+ and Rap1b−/− mice were solubilized by adding an equal volume of 2 × SDS sample buffer containing protease inhibitor mixture (Sigma). Lysates were analyzed by SDS-PAGE using 4% to 15% gradient gels and immunoblotted using a rat monoclonal antibody against PF4, a rabbit polyclonal antibody against PAR4, or a rat monoclonal antibody against GPVI. The total amount of PF4 in platelet lysates was measured using the anti-mouse ELISA kit from R & D Systems (Minneapolis, MN) following the manufacturer's instructions.
Integrin β3 Expression
Washed platelets from Rap1b+/+ and Rap1b−/− mice were incubated with a rat FITC-conjugated anti-mouse P-selectin antibody for 30 min at 22 °C and fixed by adding paraformaldehyde (1% final concentration). Integrin β3 expression was analyzed by flow cytometry.
Platelet Spreading on Fibrinogen
Washed platelets (2 × 107/ml) from Rap1b+/+ and Rap1b−/− mice were resuspended in Tyrode's solution. Chamber slides (Lab-Tek, Nunc, Thermo-Fisher Scientific, Rochester, NY) were coated with 50 μg/ml fibrinogen and blocked with 5% bovine serum albumin (BSA) in phosphate-buffered saline (PBS). Platelets were added to wells (100 μl/well) and allowed to spread for 45, 60, and 90 min at 37 °C. At each time point, slides were immediately rinsed to detach non-adherent platelets and fixed with 1% paraformaldehyde. Samples were blocked with 5% BSA in PBS and incubated with Rhodamine-conjugated phalloidin. Images were collected with a Leica DM IRB microscope using 60× objective (Leica Microsystems, Wetzlar, Germany), and platelet size was quantified by using MetaMorph® microscopy automation and image analysis software. Statistical significance between groups was determined using a Student t test.
Platelet-mediated Clot Retraction
Platelet-poor plasma from healthy donors was anti-coagulated with 0.38% sodium citrate and mixed with washed murine platelets to a concentration of 3 × 108/ml. Plasma was induced to coagulate with 0.4 unit/ml thrombin. The clots were allowed to retract at 37 °C and were photographed at various time points after addition of thrombin. Two-dimensional sizes of retracted clots on photographs were quantified using MetaMorph® microscopy automation and image analysis software, and retraction was expressed as percentage retraction (final clot size/initial clot size). Statistical significance was determined using a Student t test.
RESULTS
A Role of Rap1b in Platelet Secretion from Dense Granules
Rap1b is activated by platelet agonists (21, 40–47), and Rap1b activation plays an important role in promoting platelet aggregation (3). However, platelets lacking Rap1b have a reduced aggregation in response only to low concentrations of thrombin or collagen, but not to high concentrations of the agonists (data not shown) (3), suggesting that agonist-induced Rap1b activation might stimulate an amplification mechanism of platelet aggregation, such as granule secretion. To test this hypothesis, we examined the effect of Rap1b deficiency on agonist-induced dense granule secretion. ATP secretion during aggregation in response to low dose thrombin was diminished in Rap1b-deficient platelets compared with wild-type platelets (Fig. 1, A and C). ATP secretion induced by collagen was also reduced in Rap1b-deficient platelets (Fig. 1, B and C). Thus, Rap1b indeed plays a role in dense granule secretion.
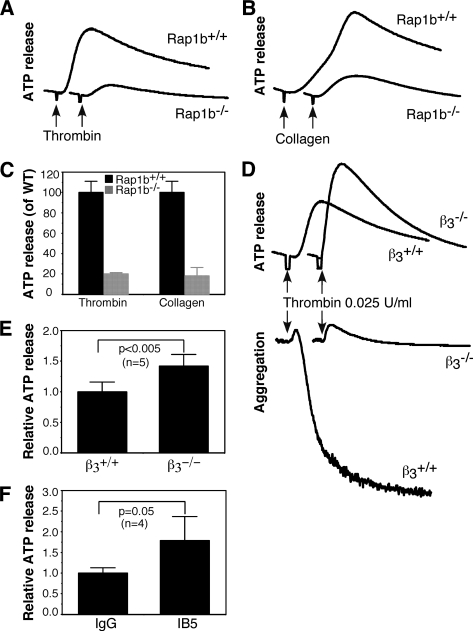
FIGURE 1.
The role of Rap1b and integrin αIIbβ3 in secretion from dense granules. A–C, washed platelets from Rap1b−/− and Rap1b+/+ mice were stimulated with 0.025 unit/ml thrombin (A) or 1 μg/ml collagen (B), and ATP release was measured in a Lumi-Aggregometer (Chrono-log, Havertown, PA). Quantification of peak platelet ATP release (% of wild-type control) is shown in C. D and E, washed platelets from β3−/− and β3+/+ mice were stimulated with 0.025 unit/ml thrombin, and platelet aggregation and ATP release were recorded. Quantification of peak platelet ATP release (% of wild-type control) is shown in E. Statistical significance was determined using Student t test. F, washed platelets C57B6 mice were pre-incubated with a hamster anti-mouse integrin β3 monoclonal antibody IB5 (10 μg/ml) or hamster IgG for 5 min, and then stimulated with 0.025 unit/ml thrombin. ATP release was recorded. Values were normalized with respect to platelets treated with rat IgG (mean ± S.D. from 4 separate experiments). Statistical significance was determined using Student t test.
It is well established that Rap1b plays an important role in integrin inside-out signaling (1–3). Thus, the defect in secretion of Rap1b-deficient platelets might be a consequence of impaired integrin activation. However, although thrombin failed to induce aggregation of integrin β3-deficient platelets, ATP secretion in response to low dose thrombin was not defective and was even enhanced in β3-deficient platelets (Fig. 1, D and E), suggesting that thrombin-induced dense granule release is independent of integrin activation. Consistently, secretion of ATP induced by thrombin in wild-type platelets was not inhibited, but enhanced, by pre-treatment of platelets with a hamster monoclonal antibody that blocks murine αIIbβ3 function, 1B5 (48) (Fig. 1F). These results also signify that the defective secretion characteristic of Rap1b-deficient platelets is not a result of diminished integrin activation.
The Role of Rap1b in Platelet Secretion of α-Granules
To address the role of Rap1b in α-granule secretion, we investigated P-selectin expression in Rap1b-deficient platelets in response to thrombin stimulation. P-selectin was examined by flow cytometry using an FITC-labeled rat anti-mouse P-selectin monoclonal antibody. As shown in Fig. 2, P-selectin expression induced by various concentrations of thrombin was diminished in Rap1b-deficient platelets compared with wild-type platelets (Fig. 2, A and B).
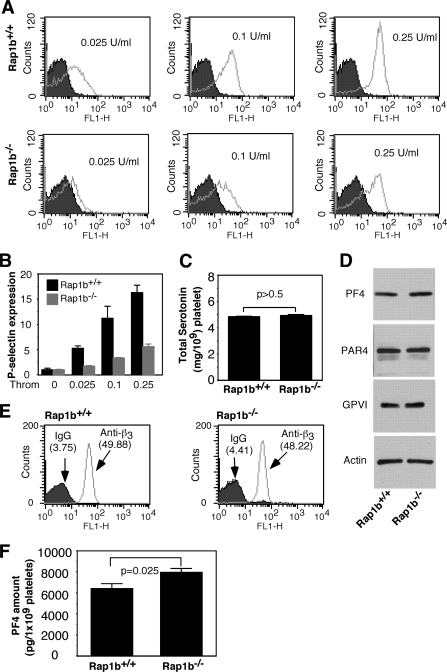
FIGURE 2.
The role of Rap1b in secretion from α granules. A and B, washed platelets from Rap1b−/− and Rap1b+/+ mice were incubated with various concentrations of thrombin for 30 min at 22 °C in the presence of an FITC-labeled monoclonal anti-mouse P-selectin antibody for 30 min and analyzed using flow cytometry (A). The P-selectin expression index (mean of fluorescence intensity) with a certain concentration of thrombin/mean of fluorescence intensity of unstimulated platelets) is shown (B). C, total serotonin in 1 × 109 Rap1b−/− and Rap1b+/+ platelets from five mice with each genotype was measured by O-phthalaldehyde assay as described under “Experimental Procedures.” D, washed platelets (3 × 108/ml) were solubilized in 1 × SDS sample buffer. PF4, PAR4, and GPVI in platelet lysates were analyzed by Western blot analysis using a rat monoclonal antibody against PF4, a rabbit polyclonal antibody against PAR4, or a rat monoclonal antibody against GPVI, respectively. A mouse monoclonal antibody against β-actin (Sigma) was used to verify equal loading. E, expression of integrin β3 on platelets from Rap1b+/+ or Rap1b−/− mice was analyzed by flow cytometry with a FITC-labeled rat anti-mouse β3 monoclonal antibody. Data shown are representative of two independent experiments. Mean of fluorescence intensity of Rap1b+/+ and Rap1b−/− platelets is shown. F, total PF4 amount in platelet lysates was measured using the anti-mouse ELISA kit from R & D Systems (Minneapolis, MN). Statistical significance was determined using Student t test.
The defect in secretion of Rap1b-deficient platelets was not due to reduced granule contents, because the amount of serotonin in Rap1b-deficient platelets was similar to wild-type platelets (Fig. 2C), and the amount of platelet factor 4 (PF4) in Rap1b-deficient platelets was even more than that of wild-type platelets (Fig. 2, D and F). These results demonstrate that granule biogenesis in Rap1b-deficient platelets is not defective. The expression levels of thrombin receptor PAR4, collagen receptor glycoprotein VI (GPVI), and integrin β3 were examined to exclude possibility that reduced secretion of Rap1b-deficient platelets results from its effect on receptor expression. The expression of PAR4, GPVI, and integrin β3 was not affected by Rap1b deficiency (Fig. 2, D and E).
We next performed transmission electron microscopy on thrombin-activated platelets to further confirm the role of Rap1b in platelet secretion. Under resting conditions, wild-type and Rap1b-deficient platelets had normal discoid shapes with similar numbers of granules (Fig. 3, A, B, and I). When stimulated with thrombin (0.1 unit/ml) for 5 min, wild-type platelets showed an irregular appearance with protruding filopodia and lack of granules. Thrombin-stimulated Rap1b−/− platelets also showed irregular appearance, but more Rap1b−/− platelets contained visible α granules than wild-type platelets (Fig. 3, C–H). Dense granules were also more obvious in thrombin-stimulated Rap1b−/− platelets than wild-type platelets.
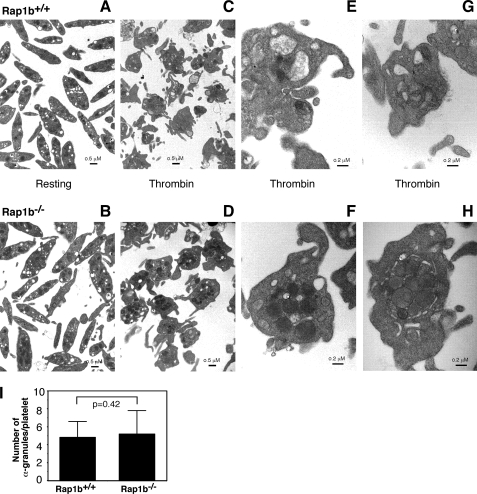
FIGURE 3.
Platelet electron microscopy analysis. A, washed platelets from Rap1b+/+ (A, C, E, and G) and Rap1b−/− (B, D, F, and H) mice were either maintained in resting state or incubated with 0.1 unit/ml thrombin for 5 min at 22 °C. The samples were fixed and analyzed by transmission electron microscopy. A and B represent platelets under resting conditions, and C–H represent platelets stimulated with thrombin. Scale bars represent 0.5 μm for A–D, and 0.2 μm for E–H. I, numbers of α granules in all platelets from five randomly chosen fields of Rap1b−/− (5.20 ± 2.61/platelets, n = 75) and Rap1b+/+ (4.82 ± 1.77/platelets, n = 84) resting samples were counted. Statistical significance was determined using Student t test.
Addition of ADP and Fibrinogen Rescued Aggregation of Rap1b-deficient Platelets
The causal relationship, if any, between the diminished secretion and impaired aggregation of Rap1b−/− platelets in response to low concentrations of agonists was investigated. Specifically, we examined whether or not supplementation with a low concentration of the granule constituents ADP and/or fibrinogen could rescue the aggregation of Rap1b-deficient platelets. As shown in Fig. 4, although addition of ADP (0.5 μm) or fibrinogen (50 μg/ml) alone is insufficient to induce aggregation of washed platelets, each addition of one constituent or the other did partially and significantly restore aggregation of Rap1b platelets in response to either thrombin or collagen (Fig. 4, A and B). Also addition of ADP plus fibrinogen almost completely restored aggregation of Rap1b-deficient platelets in response to thrombin (Fig. 4A) and collagen (Fig. 4B), respectively. These results demonstrate that stimulation of secretion may be an important role of Rap1b and account for the defect in aggregation of Rap1b-deficient platelets in response to low concentrations of agonists.
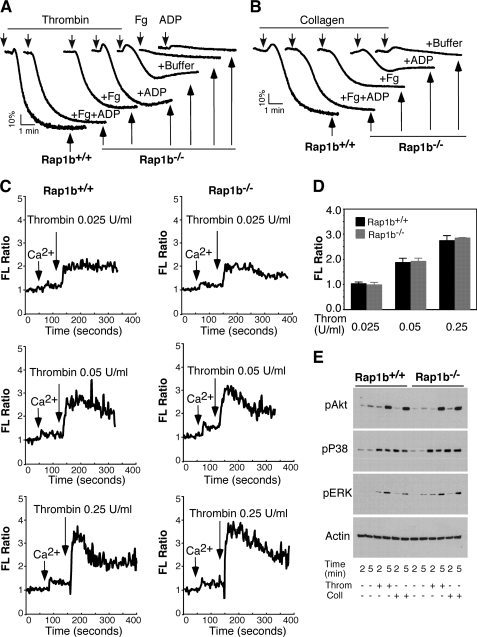
FIGURE 4.
ADP and fibrinogen rescued aggregation of Rap1b-deficient platelets, and the role of Rap1b in calcium mobilization. A and B, washed platelets from Rap1b+/+ (Rap1b+/+) and Rap1b−/− mice (Rap1b−/−) were added with 0.025 unit/ml thrombin (A) or 1.0 μg/ml collagen (B) in the absence (buffer) or presence of ADP (0.5 μm) (+ADP), fibrinogen (50 μg/ml) (+Fg), or both (+Fg+ADP) to induce aggregation. Platelets were also added with fibrinogen (Fg) or ADP (ADP) alone (A). Aggregation traces shown are representative of at least three independent experiments. C and D, washed platelets from Rap1b-deficient or wild-type mice were labeled with 12.5 μm Fura-2/AM/0.2% Pluronic F-127 and resuspended in Tyrode's solution. Platelets were then stimulated with various concentration of thrombin. Changes in the intracellular free calcium level were measured every 2 s and expressed as a ratio of fluorescence (FL) detected at 509 nm emission with an excitation wavelength of 340 nm and 380 nm (C). Statistical data from three experiments are shown (D). E, washed platelets from Rap1b+/+ and Rap1b−/− mice were added with 0.025 unit/ml thrombin (Throm) or 1.0 μg/ml collagen (Coll), and incubated at 37 °C in the aggregometer for 2 or 5 min. Platelets were solubilized in 1 × SDS sample buffer, and phosphorylation of Akt, p38, and ERK was detected by Western blot using phospho-specific rabbit monoclonal antibodies against phosphorylated Ser473 of Akt, Thr180/Tyr182 of p38, and Thr202/Tyr204 of ERK (Cell Signaling Technologies). A mouse monoclonal antibody against β-actin (Sigma) was used to verify equal loading.
Calcium Mobilization in Response to Thrombin Is Not Affected by Rap1b Deficiency
Agonist-induced increase of intra-platelet calcium concentrations is a key signaling event for platelet secretion and aggregation. Therefore, we examined the change of calcium levels in Rap1b-deficient platelets in response to thrombin treatment to determine whether or not Rap1b plays a role in agonist-stimulated calcium mobilization. Washed platelets from Rap1b-deficient or wild-type mice were labeled with the calcium probe, Fura-2/AM. Intra-platelet calcium concentrations in thrombin-treated wild-type and Rap1b-deficient platelets were measured. The increases in intracellular calcium concentrations induced by thrombin in Rap1b-deficient platelets were similar to the increases in wild-type platelets (Fig. 4, C and D). Thus, calcium mobilization in Rap1b-deficient platelets is not defective.
Deficiency of Rap1b Does Not Affect Agonist-induced Phosphorylation of Akt, ERK, and p38 MAPK
Several signaling pathways, including PI3K/Akt, ERK, and p38 MAP kinase pathways have been shown to be important in regulating platelet secretion. However, agonist-induced phosphorylation of Akt, p38, and ERK was not affected by Rap1b deficiency (Fig. 4E). These data suggest that these signaling cascades initiated in activated Rap1b-deficient platelets do not grossly differ from that in the wild-type platelets.
Platelet Adhesion to Fibrinogen Induces P2Y12 and TP-independent Rap1b Activation
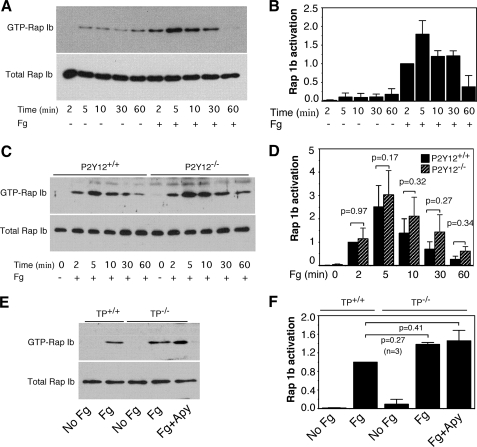
Ligand binding to integrins in nucleated cells can activate Rap1b, and integrin-mediated Rap1b activation in such cells plays a critical role in regulating cell adhesion and spreading. Rap1b can be activated by integrin α2β1 outside-in signaling in platelets (49). Thus, we hypothesized that fibrinogen binding to αIIbβ3 might also be able to induce Rap1b activation in platelets. To test this hypothesis, platelets from C57B6 mice were added to fibrinogen-coated tissue culture dishes. Precipitation of active Rap1b with GST-linked RalGDS RBD beads demonstrated that Rap1b was activated by platelet adhesion to immobilized fibrinogen (Fig. 5, A and B). Rap1b activation reached maximum by 5 min after platelet adhesion to fibrinogen-coated wells.
FIGURE 5.
Outside-in signaling mediated Rap1b activation. A, washed platelets from C57B6 mice were added to fibrinogen-coated dishes and incubated at 37 °C for various lengths of time. GTP-bound Rap1 was precipitated with GST-RalGDS RBD bound to glutathione-agarose beads. B, densitometry measurements from results in A. Values were normalized with respect to platelets stimulated for 2 min for each immunoblot and are expressed as relative phosphorylation (mean ± S.D. from three separate experiments). C, washed platelets from wild-type or P2Y12-deficient mice were added to fibrinogen-coated dishes and incubated at 37 °C for various lengths of time. GTP-bound Rap1 was precipitated with GST-RalGDS RBD bound to glutathione-agarose beads. D, densitometry measurements from results in C. Values were normalized with respect to wild-type platelets stimulated with fibrinogen for 2 min for each immunoblot and expressed as relative phosphorylation (mean ± S.D. from three separate experiments). Statistical significance was determined using Student's t test. E, washed platelets from wild-type or TP-deficient mice were preincubated with buffer or apyrase (1 unit/ml) for 5 min, and then added to fibrinogen-coated dishes and incubated at 37 °C for 5 min. GTP-bound Rap1 was precipitated with GST-RalGDS RBD bound to glutathione-agarose beads. F, densitometry measurements from results in E. Values were normalized with respect to wild-type platelets stimulated with fibrinogen for each immunoblot and expressed as relative phosphorylation (mean ± S.D. from three separate experiments). Statistical significance was determined using Student's t test.
ADP receptor P2Y12/Gi plays an important role in Rap1b activation elicited by platelet agonists (supplemental Fig. S1) (42–44). We also found that Rap1b activation induced by collagen or ADP was diminished in the platelets lacking TXA2 receptor, TP (supplemental Fig. S2), suggesting a role of TXA2/TP in agonist-induced Rap1b activation. To determine whether Rap1b activation elicited by integrin αIIbβ3 outside-in signaling involves P2Y12 and TP, platelets from P2Y12- or TP-deficient mice were added to fibrinogen-coated dishes, and Rap1b activation was examined. Rap1b activation induced by immobilized fibrinogen was not affected by P2Y12 deficiency (Fig. 5, C and D), suggesting that P2Y12 is not required for Rap1b activation elicited by αIIbβ3 outside-in signaling. Immobilized fibrinogen-elicited Rap1b activation in TP-deficient platelets in the presence or absence of the ADP scavenger apyrase was similar to that of wild-type platelets (Fig. 5, E and F). These results demonstrate that ADP/P2Y12 and TXA2/TP are not required for Rap1b activation induced by αIIbβ3 outside-in signaling.
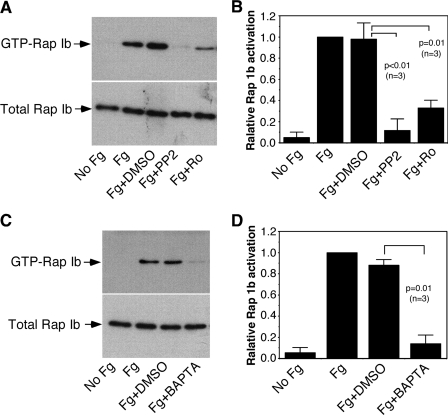
Rap1b Activation Elicited by αIIbβ3 Outside-in Signaling Requires Src Family Kinases, PKC, and Ca2+ Mobilization
Both Src family kinases (SFKs) and PKC play important roles in αIIbβ3 outside-in signaling. To establish whether or not Rap1b activation induced by αIIbβ3 outside-in signaling is downstream from these components, the effects of the selective Src inhibitor PP2 and the PKC inhibitor Ro-31-8220 on Rap1b activation induced by platelet adhesion to immobilized fibrinogen were examined. Rap1b activation induced by adhesion to immobilized fibrinogen was inhibited by PP2 and Ro-31-8220, respectively (Fig. 6, A and B). Rap1b activation induced by platelet adhesion was also inhibited by dimethyl-BAPTA (Fig. 6, C and D), suggesting a role of Ca2+ mobilization in αIIbβ3-mediated outside-in signaling-mediated Rap1b activation.
FIGURE 6.
The role of Src family kinases, PKC, and Ca2+ in αIIbβ3 outside-in signaling-mediated Rap1b activation. A and B, washed platelets were preincubated with the PKC inhibitor Ro-31-8220 (Ro) (1 μm) or the SFK inhibitor PP2 (10 μm) for 5 min, and then added to fibrinogen-coated dishes and incubated at 37 °C for 5 min. Densitometry measurements from three independent experiments are shown in B. Statistical significance was determined using Student's t test. C and D, washed platelets were preincubated with BAPTA (10 μm) for 5 min, and then added to fibrinogen-coated dishes and incubated at 37 °C for 5 min. Densitometry measurements from three independent experiments are shown in D. Statistical significance was determined using Student t test.
The Role of Rap1b in αIIbβ3-mediated Clot Retraction and Platelet Spreading on Fibrinogen
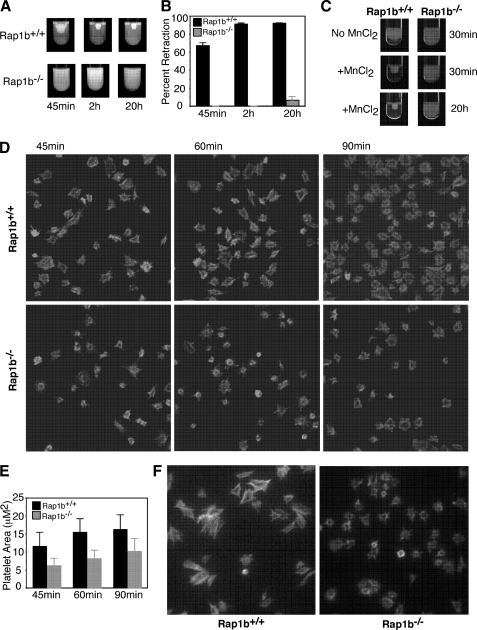
Next, we determined whether Rap1b plays a role in αIIbβ3 outside-in signaling. It is known that platelet-mediated clot retraction requires αIIbβ3-dependent outside-in signaling (32, 50, 51). Thus, the effect of Rap1b deficiency on αIIbβ3 outside-in signaling-dependent clot retraction was examined. As shown in Fig. 7, platelets from Rap1b+/+ mice supported clot retraction in human plasma. The maximal clot retraction occurred at 2 h after addition of thrombin (0.4 unit/ml) (Fig. 7, A and B). In contrast, up to 20 h after addition of thrombin, Rap1b−/− platelets failed to promote clot retraction in human plasma (Fig. 7, A and B).
FIGURE 7.
The role of Rap1b in clot retraction and platelet spreading on fibrinogen. A, platelet-poor plasma was mixed with 3 × 108/ml washed platelets from Rap1b−/− and Rap1b+/+ mice. Coagulation was induced by adding 0.4 unit/ml thrombin, and clots were allowed to retract at 37 °C and photographed. B, two-dimensional retraction of clots in A was measured using MetaMorph® microscopy automation and image analysis software and expressed as percent retraction (mean ± S.D.). Statistical significance was determined using a Student t test. C, platelet-poor plasma from a healthy donor was mixed with 3 × 108/ml washed platelets from Rap1b−/− and Rap1b+/+ mice. Coagulation was induced with 0.4 unit/ml thrombin plus 0.5 mm MnCl2, and clots were allowed to retract at 37 °C and photographed. D, washed platelets from Rap1b−/− and Rap1b+/+ mice were added to fibrinogen-coated slides for 45, 60, and 90 min and fixed with paraformaldehyde to stop spreading. Platelets were subsequently labeled with rhodamine-phalloidin and photographed using a fluorescence microscope. E, quantification of area of 100 randomly selected cells (mean ± S.E.). Statistical analysis performed using Student t test (p < 0.0001 for all three time points). F, washed platelets from Rap1b−/− and Rap1b+/+ mice were added to fibrinogen-coated slides. 0.5 mm MnCl2 was added to platelets immediately. Platelets were incubated at 37 °C for 45 min, and then fixed with paraformaldehyde to stop spreading. Platelets were subsequently labeled with rhodamine-phalloidin and photographed using a fluorescence microscope.
Treatment of platelets with MnCl2 bypasses any need for inside-out signaling to induce αIIbβ3 activation, thereby directly eliciting αIIbβ3 outside-in signaling. Therefore, MnCl2 was used to further evaluate the role of Rap1b in αIIbβ3 outside-in signaling. As shown in Fig. 7, although MnCl2 (0.5 mm) accelerated clot retraction of Rap1b+/+ platelets, MnCl2 failed to restore clot retraction of Rap1b−/− platelets (Fig. 7C).
Platelet spreading on immobilized fibrinogen is a response known to be dependent on αIIbβ3 outside-in signaling. In supporting a role for Rap1b in integrin outside-in signaling, previous studies have shown that Rap1b-deficient platelets are defective in spreading on fibrinogen (3). Consistent with the previous findings, Rap1b-deficient platelets have a significant defect in spreading on fibrinogen in the absence of agonists (Fig. 7, D and E) (p < 0.0001). Rap1b−/− platelets displayed an ability to undergo an extent of filopodia protrusion, whereas formation of lamellipodia was prohibited. Importantly, addition of MnCl2 did not rescue spreading of Rap1b−/− platelets on fibrinogen (Fig. 7F).
DISCUSSION
In this study, we have discovered that there are two distinct mechanisms of Rap1b activation in platelets, each playing a different role in platelet function. We demonstrate that agonist-induced Rap1b activation is important for platelet granule secretion. We also demonstrate that ligand binding to integrin αIIbβ3 induces outside-in signaling-mediated Rap1b activation, which is important for clot retraction and platelet spreading.
Previous studies have highlighted the importance of Rap1b in integrin inside-out signaling (1–3). Our data demonstrate that Rap1b activation induced by different mechanisms is important for different aspects of platelet function. An important role of Rap1b activation induced by platelet agonists appears to be stimulating granule secretion, and some of the secreted materials subsequently amplify and stabilize platelet aggregation. This conclusion is supported by the data showing that: 1) Rap1b-deficient platelets have a defect in the second wave of platelet aggregation that is secretion-dependent (Fig. 4) (3); 2) secretion of both dense granules (ATP release) and α-granules (P-selectin expression) are significantly attenuated in Rap1b-deficient platelets (Figs. 1 and 2); and 3) the defect in aggregation of Rap1b-deficient platelets is corrected by supplementing platelets with low concentrations of the granule constituents ADP and fibrinogen (Fig. 4), suggesting that the inhibitory effect of Rap1b deficiency on aggregation is secondary to diminished granule secretion of ADP and fibrinogen. Platelet dense granule release is critical for thrombus formation in vivo (13). Therefore, the defect in secretion of Rap1b-deficient platelets may contribute to the impaired thrombus formation of Rap1b-deficient mice (3).
Unlike platelets lacking the small GTPase Rab27b that have reduced number of dense granules and decreased secretion from dense granules (18), Rap1b-deficient platelets do not have a defect in granule biogenesis. Rap1b appears to be more important for secretion from α granules than secretion from dense granules, because the defect in ATP release of Rap1b-deficient platelets only occurs in response to low concentrations of agonists. In contrast, P-selectin expression induced by thrombin is dramatically reduced in Rap1b-deficient platelets in response to relatively high concentrations of thrombin (Fig. 2). Our data demonstrating that Rap1b is important for secretion are consistent with CalDAG-GEFI-deficient platelet results showing that dense granule release from CalDAG-GEFI deficient platelets is defective and the defect in secretion is responsible for the impaired aggregation (21). However, the conclusion that Rap1b plays an important role in secretion does not exclude the possibility that Rap1b also plays a role in other aspects of platelet function, for instance in the integrin inside-out signaling (1–3, 11).
Another important finding of our studies is that Rap1b is activated by ligand binding to integrin αIIbβ3 and Rap1b plays an important role in αIIbβ3 outside-in signaling. Almost all agonists can activate Rap1b in platelets, and the mechanisms by which agonists activate Rap1b have been intensively studied (21, 40–47). By using a combination of Gq- and Gi-deficient mice as well as P2Y12 antagonists, two major mechanisms leading to Rap1b activation in response to thrombin have been supported: the Gq/Ca2+ pathway and the P2Y12/Gi/PI3K pathway (21, 42, 44, 45). Here we confirmed an important role of P2Y12 in mediating Rap1b activation in response to platelet agonists by using P2Y12-deficient mice (supplemental Fig. S1). Our results also suggest that the TXA2/TP pathway contributes to Rap1b activation in response to ADP and collagen, but not thrombin (supplemental Fig. S2). In contrast, Rap1b activation induced by immobilized fibrinogen was not affected by P2Y12 or TP deficiency. These results demonstrate that integrin αIIbβ3 outside-in signaling-mediated Rap1b activation is independent of the secondary activation of platelets by ADP and TXA2. Bernardi et al. (49) have previously shown that platelet adhesion to ligands for integrin α2β1 induced a robust and rapid activation of Rap1b. Interestingly, Rap1b activation by α2β1 outside-in signaling is also independent of secreted ADP and TXA2 synthesis (49).
Boylan et al. (52) reported recently that ligand binding to platelet αIIbβ3 induces integrin cytoplasmic domain-dependent phosphorylation of FcγRIIa, resulting in activation of phospholipase C γ2. We show that activation of Rap1b by immobilized fibrinogen requires PKC and Ca2+, suggesting that Rap1b activation by αIIbβ3 outside-in signaling is downstream from phospholipase C γ2. Similar to collagen receptor GPVI signaling (53), activation of SFKs is an early signaling event in αIIbβ3 outside-in signaling, which is upstream of the Syk-phospholipase C γ2 pathway (52–55). Therefore, it is not a surprise that αIIbβ3 outside-in signaling-mediated Rap1b activation was inhibited by the selective inhibitor of SFKs, PP2. Thus, our results have identified a novel integrin-mediated Rap1b activation pathway that is important for αIIbβ3 outside-in signaling. The finding that platelet adhesion to immobilized fibrinogen can activate Rap1b in platelets is also consistent with a previous study that LIBS-6, an integrin αIIbβ3-activating monoclonal antibody, induced Rap1b activation in human platelets (41).
In support of the conclusion that Rap1b plays a role in αIIbβ3 outside-in signaling leading to clot retraction, we show that platelets lacking Rap1b failed to promote clot retraction (Fig. 7). The data showing that MnCl2 failed to correct the defect in clot retraction by Rap1b deficiency further demonstrate the role of Rap1b in αIIbβ3 outside-in signaling. Also, consistent with the previous findings (3), Rap1b-deficient platelets have diminished spreading on fibrinogen. The defect in spreading on fibrinogen of Rap1b-deficient platelets was not corrected by MnCl2 either. These findings are consistent with a previous report showing that platelets lacking CalDAG-GEFI have a defect in integrin α2β1-mediated αIIbβ3 activation (49).
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Cardiovascular Disease Grant P20RR021954 from the National Center for Research Resources. This work was also supported by American Heart Association Midwest affiliate Grand-in-Aid 0855698G (to Z. L.) and 0950118G (to M. C.-W.), and in part by the Centers of Biomedical Research Excellence in Obesity. This material is the result of work supported with the resources and use of the facilities at the Lexington VA Medical Center.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- CalDAG-GEFI
- Ca2+ and diacylglycerol-regulated guanine nucleotide exchange factor I
- GPVI
- glycoprotein VI
- v-SNARE
- vesicle-soluble N-ethylmaleimide-sensitive fusion protein attachment receptor
- TP
- TXA2 receptor
- BAPTA
- 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis
- SFK
- Src family kinase
- PF4
- platelet factor 4
- PAR4
- protease-activated receptor 4
- TXA2
- thromboxane A2.
REFERENCES
- 1. Bertoni A., Tadokoro S., Eto K., Pampori N., Parise L. V., White G. C., Shattil S. J. (2002) J. Biol. Chem. 277, 25715–25721 [DOI] [PubMed] [Google Scholar]
- 2. Han J., Lim C. J., Watanabe N., Soriani A., Ratnikov B., Calderwood D. A., Puzon-McLaughlin W., Lafuente E. M., Boussiotis V. A., Shattil S. J., Ginsberg M. H. (2006) Curr. Biol. 16, 1796–1806 [DOI] [PubMed] [Google Scholar]
- 3. Chrzanowska-Wodnicka M., Smyth S. S., Schoenwaelder S. M., Fischer T. H., White G. C., 2nd (2005) J. Clin. Invest. 115, 680–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang Z., Holly S. P., Larson M. K., Liu J., Yuan W., Chrzanowska-Wodnicka M., White G. C., 2nd, Parise L. V. (2009) J. Thromb. Haemost 7, 693–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arai A., Nosaka Y., Kanda E., Yamamoto K., Miyasaka N., Miura O. (2001) J. Biol. Chem. 276, 10453–10462 [DOI] [PubMed] [Google Scholar]
- 6. Sebzda E., Bracke M., Tugal T., Hogg N., Cantrell D. A. (2002) Nat. Immunol. 3, 251–258 [DOI] [PubMed] [Google Scholar]
- 7. Reedquist K. A., Ross E., Koop E. A., Wolthuis R. M., Zwartkruis F. J., van Kooyk Y., Salmon M., Buckley C. D., Bos J. L. (2000) J. Cell Biol. 148, 1151–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lafuente E. M., van Puijenbroek A. A., Krause M., Carman C. V., Freeman G. J., Berezovskaya A., Constantine E., Springer T. A., Gertler F. B., Boussiotis V. A. (2004) Dev. Cell 7, 585–595 [DOI] [PubMed] [Google Scholar]
- 9. Lee H. S., Lim C. J., Puzon-McLaughlin W., Shattil S. J., Ginsberg M. H. (2009) J. Biol. Chem. 284, 5119–5127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eto K., Murphy R., Kerrigan S. W., Bertoni A., Stuhlmann H., Nakano T., Leavitt A. D., Shattil S. J. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 12819–12824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crittenden J. R., Bergmeier W., Zhang Y., Piffath C. L., Liang Y., Wagner D. D., Housman D. E., Graybiel A. M. (2004) Nat. Med. 10, 982–986 [DOI] [PubMed] [Google Scholar]
- 12. Bergmeier W., Goerge T., Wang H. W., Crittenden J. R., Baldwin A. C., Cifuni S. M., Housman D. E., Graybiel A. M., Wagner D. D. (2007) J. Clin. Invest. 117, 1699–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Graham G. J., Ren Q., Dilks J. R., Blair P., Whiteheart S. W., Flaumenhaft R. (2009) Blood 114, 1083–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ren Q., Ye S., Whiteheart S. W. (2008) Curr. Opin. Hematol. 15, 537–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blair P., Flaumenhaft R. (2009) Blood Rev. 23, 177–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ren Q., Wimmer C., Chicka M. C., Ye S., Ren Y., Hughson F. M., Whiteheart S. W. (2010) Blood 116, 869–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shirakawa R., Higashi T., Tabuchi A., Yoshioka A., Nishioka H., Fukuda M., Kita T., Horiuchi H. (2004) J. Biol. Chem. 279, 10730–10737 [DOI] [PubMed] [Google Scholar]
- 18. Tolmachova T., Abrink M., Futter C. E., Authi K. S., Seabra M. C. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 5872–5877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Akbar H., Kim J., Funk K., Cancelas J. A., Shang X., Chen L., Johnson J. F., Williams D. A., Zheng Y. (2007) J. Thromb. Haemost 5, 1747–1755 [DOI] [PubMed] [Google Scholar]
- 20. Pleines I., Eckly A., Elvers M., Hagedorn I., Eliautou S., Bender M., Wu X., Lanza F., Gachet C., Brakebusch C., Nieswandt B. (2010) Blood 115, 3364–3373 [DOI] [PubMed] [Google Scholar]
- 21. Cifuni S. M., Wagner D. D., Bergmeier W. (2008) Blood 112, 1696–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coller B. S., Shattil S. J. (2008) Blood 112, 3011–3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shattil S. J., Kim C., Ginsberg M. H. (2010) Nat. Rev. Mol. Cell Biol. 11, 288–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nobes C. D., Hall A. (1999) J. Cell Biol. 144, 1235–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Legate K. R., Wickström S. A., Fässler R. (2009) Genes Dev. 23, 397–418 [DOI] [PubMed] [Google Scholar]
- 26. Hall A. (2005) Biochem. Soc. Trans. 33, 891–895 [DOI] [PubMed] [Google Scholar]
- 27. Arias-Salgado E. G., Lizano S., Sarkar S., Brugge J. S., Ginsberg M. H., Shattil S. J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 13298–13302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reddy K. B., Smith D. M., Plow E. F. (2008) J. Cell Sci. 121, 1641–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Soriani A., Moran B., de Virgilio M., Kawakami T., Altman A., Lowell C., Eto K., Shattil S. J. (2006) J. Thromb. Haemost. 4, 648–655 [DOI] [PubMed] [Google Scholar]
- 30. Buensuceso C. S., Obergfell A., Soriani A., Eto K., Kiosses W. B., Arias-Salgado E. G., Kawakami T., Shattil S. J. (2005) J. Biol. Chem. 280, 644–653 [DOI] [PubMed] [Google Scholar]
- 31. Arthur W. T., Petch L. A., Burridge K. (2000) Curr. Biol. 10, 719–722 [DOI] [PubMed] [Google Scholar]
- 32. Flevaris P., Stojanovic A., Gong H., Chishti A., Welch E., Du X. (2007) J. Cell Biol. 179, 553–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Foster C. J., Prosser D. M., Agans J. M., Zhai Y., Smith M. D., Lachowicz J. E., Zhang F. L., Gustafson E., Monsma F. J., Jr., Wiekowski M. T., Abbondanzo S. J., Cook D. N., Bayne M. L., Lira S. A., Chintala M. S. (2001) J. Clin. Invest. 107, 1591–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thomas D. W., Mannon R. B., Mannon P. J., Latour A., Oliver J. A., Hoffman M., Smithies O., Koller B. H., Coffman T. M. (1998) J. Clin. Invest. 102, 1994–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hodivala-Dilke K. M., McHugh K. P., Tsakiris D. A., Rayburn H., Crowley D., Ullman-Cullere M., Ross F. P., Coller B. S., Teitelbaum S., Hynes R. O. (1999) J. Clin. Invest. 103, 229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li Z., Zhang G., Le Breton G. C., Gao X., Malik A. B., Du X. (2003) J. Biol. Chem. 278, 30725–30731 [DOI] [PubMed] [Google Scholar]
- 37. Flevaris P., Li Z., Zhang G., Zheng Y., Liu J., Du X. (2009) Blood 113, 893–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ren Q., Barber H. K., Crawford G. L., Karim Z. A., Zhao C., Choi W., Wang C. C., Hong W., Whiteheart S. W. (2007) Mol. Biol. Cell 18, 24–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xiang B., Zhang G., Liu J., Morris A. J., Smyth S. S., Gartner T. K., Li Z. (2010) J. Thromb. Haemost. 8, 2032–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Franke B., Akkerman J. W., Bos J. L. (1997) EMBO J. 16, 252–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Franke B., van Triest M., de Bruijn K. M., van Willigen G., Nieuwenhuis H. K., Negrier C., Akkerman J. W., Bos J. L. (2000) Mol. Cell Biol. 20, 779–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Woulfe D., Jiang H., Mortensen R., Yang J., Brass L. F. (2002) J. Biol. Chem. 277, 23382–23390 [DOI] [PubMed] [Google Scholar]
- 43. Larson M. K., Chen H., Kahn M. L., Taylor A. M., Fabre J. E., Mortensen R. M., Conley P. B., Parise L. V. (2003) Blood 101, 1409–1415 [DOI] [PubMed] [Google Scholar]
- 44. Lova P., Paganini S., Sinigaglia F., Balduini C., Torti M. (2002) J. Biol. Chem. 277, 12009–12015 [DOI] [PubMed] [Google Scholar]
- 45. Lova P., Paganini S., Hirsch E., Barberis L., Wymann M., Sinigaglia F., Balduini C., Torti M. (2003) J. Biol. Chem. 278, 131–138 [DOI] [PubMed] [Google Scholar]
- 46. Campus F., Lova P., Bertoni A., Sinigaglia F., Balduini C., Torti M. (2005) J. Biol. Chem. 280, 24386–24395 [DOI] [PubMed] [Google Scholar]
- 47. Stefanini L., Roden R. C., Bergmeier W. (2009) Blood 114, 2506–2514 [DOI] [PubMed] [Google Scholar]
- 48. Smyth S. S., Reis E. D., Väänänen H., Zhang W., Coller B. S. (2001) Blood 98, 1055–1062 [DOI] [PubMed] [Google Scholar]
- 49. Bernardi B., Guidetti G. F., Campus F., Crittenden J. R., Graybiel A. M., Balduini C., Torti M. (2006) Blood 107, 2728–2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chen Y. P., O'Toole T. E., Shipley T., Forsyth J., LaFlamme S. E., Yamada K. M., Shattil S. J., Ginsberg M. H. (1994) J. Biol. Chem. 269, 18307–18310 [PubMed] [Google Scholar]
- 51. Law D. A., Nannizzi-Alaimo L., Phillips D. R. (1996) J. Biol. Chem. 271, 10811–10815 [DOI] [PubMed] [Google Scholar]
- 52. Boylan B., Gao C., Rathore V., Gill J. C., Newman D. K., Newman P. J. (2008) Blood 112, 2780–2786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Watson S. P., Auger J. M., McCarty O. J., Pearce A. C. (2005) J. Thromb. Haemost. 3, 1752–1762 [DOI] [PubMed] [Google Scholar]
- 54. Obergfell A., Eto K., Mocsai A., Buensuceso C., Moores S. L., Brugge J. S., Lowell C. A., Shattil S. J. (2002) J. Cell Biol. 157, 265–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. de Virgilio M., Kiosses W. B., Shattil S. J. (2004) J. Cell Biol. 165, 305–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.