Background: Bacterial peptidyl-tRNA hydrolase is essential in recycling of ribosome-dissociated peptidyl-tRNAs.
Results: Comparing minimalist substrates and using NMR mapping, the RNA-binding site of the hydrolase is characterized.
Conclusion: Interaction between the hydrolase and tRNA involves features common to all elongator tRNAs.
Significance: Knowledge of a bacterial peptidyl-tRNA hydrolase·substrate complex may drive the search for enzyme inhibitors.
Keywords: Enzyme Mechanisms, Escherichia coli, NMR, Protein Synthesis, RNA-Protein Interaction, Transfer RNA (tRNA)
Abstract
In a cell, peptidyl-tRNA molecules that have prematurely dissociated from ribosomes need to be recycled. This work is achieved by an enzyme called peptidyl-tRNA hydrolase. To characterize the RNA-binding site of Escherichia coli peptidyl-tRNA hydrolase, minimalist substrates inspired from tRNAHis have been designed and produced. Two minisubstrates consist of an N-blocked histidylated RNA minihelix or a small RNA duplex mimicking the acceptor and TψC stem regions of tRNAHis. Catalytic efficiency of the hydrolase toward these two substrates is reduced by factors of 2 and 6, respectively, if compared with N-acetyl-histidyl-tRNAHis. In contrast, with an N-blocked histidylated microhelix or a tetraloop missing the TψC arm, efficiency of the hydrolase is reduced 20-fold. NMR mapping of complex formation between the hydrolase and the small RNA duplex indicates amino acid residues sensitive to RNA binding in the following: (i) the enzyme active site region; (ii) the helix-loop covering the active site; (iii) the region including Leu-95 and the bordering residues 111–117, supposed to form the boundary between the tRNA core and the peptidyl-CCA moiety-binding sites; (iv) the region including Lys-105 and Arg-133, two residues that are considered able to clamp the 5′-phosphate of tRNA, and (v) the positively charged C-terminal helix (residues 180–193). Functional value of these interactions is assessed taking into account the catalytic properties of various engineered protein variants, including one in which the C-terminal helix was simply subtracted. A strong role of Lys-182 in helix binding to the substrate is indicated.
Introduction
Premature termination of translation with concomitant drop off of metabolically inactive peptidyl-tRNAs may cause aminoacyl-tRNA starvation in a cell (1–3). Counter-reaction is ensured by the action of a peptidyl-tRNA hydrolase (Pth),2 which cleaves the ester bond between the C-terminal end of the polypeptide and the 3′-terminal ribose in peptidyl-tRNA (4, 5). Pth activity has been described in bacteria as well as in archaea and eukaryotes. However, two types of Pth occur. One type (Pth1) is found in all cells except the archaeal ones. Another type (Pth2) is specific to archaea but is also found in eucaryotes (6, 7). In eucaryotes, both Pth1 and Pth2 coexist (6, 7). Pth1 has been found to be essential in bacteria (1–3, 8) but not in Saccharomyces cerevisiae (2, 6, 7). Therefore, the bacterial enzyme offers itself as an interesting potential target for new antibacterial drugs (9). In this context, the characterization of the interface between Pth1 and its substrate is important to guide the search for enzyme inhibitors.
Biochemical studies have shown that N-blocking of the α-amino group of tRNA-esterified amino acids is required to obtain hydrolysis by a Pth. However, in bacteria, initiator tRNAfMet esterified with a formyl-methionyl moiety escapes the action of the Pth1 hydrolase. A few years ago, we proposed that recognition of its peptidyl-tRNA substrates by Escherichia coli Pth1 involves an interaction with the phosphate at the 5′-end of the polynucleotide (10). Site-directed mutagenesis of the protein and enzymatic assays with substrates having or not having the 5′-phosphate indicated two cationic residues (Lys-105 and Arg-133) as the likely receptor site of this phosphate. These residues are very close to each other in the crystallographic enzyme three-dimensional structure (8) and are nearly fully conserved in the available bacterial Pth sequences (Pfam family PF01195). Bacterial initiator tRNAfMet differs from elongator tRNAs by the occurrence in the former of a defect in base pairing at position 1–72 of the acceptor helix. Possibly, by hindering docking of the tRNAfMet 5′-phosphate to the hydrolase, this 1–72 mismatch guards fMet-tRNAfMet against removal of its formylmethionine moiety (10–12).
Based on the position of the above-predicted 5′-phosphate receptor site and on that of the putative catalytic center of the protein, docking of a peptidyl-tRNA molecule to the surface of crystalline E. coli Pth was attempted (10). The hand-built model suggested that only the peptidyl moiety and the acceptor and TψC stems of the substrate interact with the protein. The anticodon arm would remain free in the solvent. At the present time, this working model could not be experimentally verified. In this study, to make progress in the knowledge of complex formation between bacterial Pth and an RNA, various small RNAs derived from E. coli tRNAHis were prepared, esterified with histidine, N-blocked, and assayed as substrates of E. coli Pth. The choice of the tRNAHis system was dictated by the property of E. coli histidyl-tRNA synthetase to be one rare enzyme capable of efficiently aminoacylating small helical RNAs derived from the acceptor end of its cognate tRNA (13, 14). This capacity can be explained by the presence in tRNAHis of a unique extra acceptor base pair, G−1–C73, which is an essential feature to direct aminoacylation by histidyl-tRNA synthetase (13, 15). In the tRNAHis structure, this extra acceptor base pair covers the 5′-phosphate at the +1 position, which we assume is necessary for recognition by Pth. However, as shown before (15), recognition of the +1 phosphate in tRNAHis by E. coli Pth resembles that of the 5′-terminal phosphate in the other elongator tRNA molecules, and therefore, peptidyl-tRNAHis behaves as a normal substrate of Pth.
After functional comparison of the various prepared small RNAs, a duplex RNA of 13 base pairs mimicking the acceptor and TψC stems of tRNAHis was chosen to map by NMR the tRNA-binding site on the Pth molecule. Several putative protein-RNA contacts detected in this way were functionally validated by site-directed mutagenesis experiments. Finally, to recapitulate all data, NMR-driven docking of the duplex to the hydrolase was undertaken using ambiguous distance restraints.
EXPERIMENTAL PROCEDURES
RNA Preparations
In vitro transcriptions of minihelixHis, microhelixHis, and tetraloopHis (Fig. 1) were performed using purified T7 RNA polymerase, as described (16, 17). The template oligonucleotides were 5′-TGG GGT GGC TAA TGG GAT TCG AAC CCA CTA GCC ACC TAT AGT GAG TCG TAT TA-3′ (minihelixHis), 5′-TGG GGT GGC TAA TTC GAA TAG CCA CCT ATA GTG AGT CGT ATT A-3′ (microhelixHis),and 5′-TGG GGT GCC GAA GCA CCT ATA GTG AGT CGT ATT A-3′ (tetraloopHis). In all cases, the sequence of the top strand, complementary to the template strand in the promoter region, was 5′-TAA TAC GAC TCA CTA TAG-3′. After phenol extraction, RNA transcripts were purified by chromatography on a GF05 (IBF, France) gel filtration column (1.1 × 27 cm), followed by a preparative electrophoresis on a denaturing 18% (w/v) polyacrylamide, 8 m urea gel. Transcripts were then excised from the gel, precipitated with ethanol, and suspended in a solution of 5 mm sodium acetate (pH 4.8), 0.1 mm EDTA, and 10 mm MgCl2. The concentrations of minihelixHis, microhelixHis, and tetraloopHis were estimated using molar absorbance at 260 nm coefficients of 0.345, 0.245, and 0.156 μm−1·cm−1, respectively. These coefficients were obtained with the help of the OligoCalc calculator (18), assuming that the double-stranded regions of these RNAs were as shown in Fig. 1.
FIGURE 1.
RNA molecules used in this study. tRNAHis is from E. coli. The sequence of duplexHis is based on that of the minihelixHis. However, in the duplex the following was done: (i) U65 (as numbered in native tRNAHis) was changed to a C to improve the stability of the duplex; (ii) the G52–C62 base pair was changed to C52–G62 to prevent hybridization of two duplex molecules through their free 3′-ends, and (iii) the G1–C72 base pair was changed to C1–G72 to prevent formation of a triple helix in NMR conditions (see text). These changes are shown in red. At the base of the loop of the tetraloopHis, a G-C base pair (in cyan) was introduced to reinforce the tetraloop stability. As shown at the bottom of the figure, two tetraloopHis molecules (in black and in green) may associate to form a triple helix. Resulting Hoogsteen G-C+ base pairs in the triple helix are indicated by stars.
E. coli tRNAHis was obtained from an overproducing E. coli strain, as already described (15). E. coli tRNAHis and analogs (minihelixHis, microhelixHis, and tetraloopHis) were aminoacylated for 30 min at 28 °C, in a reaction mixture containing 20 mm Tris-HCl (pH 7.5), 7 mm MgCl2, 0.1 mm EDTA, 2 mm ATP, 10–50 μm l-[14C]histidine (327 Ci/mol), 1–8 μm purified histidyl-tRNA synthetase (15), and 5–50 μm of the RNA substrate. After acetylation with acetic anhydride, the N-blocked aminoacylated RNA samples were purified through Chelex 100 (Bio-Rad) and GF05 (IBF) chromatographies (15). Diacetyl-[14C]Lys-tRNALys (50 Ci/mol) was prepared as described previously (8).
When used in NMR experiments, the tetraloopHis (5′-GGU GCU UCG GCA CCC CA-3′) was chemically synthesized on an Amersham Biosciences LKB Gene Assembler Plus apparatus, as described previously (19). The final yield was higher than 95%.
To prepare duplexHis, oligoribonucleotides 5′-CGC ACU AGC CAG CCC A-3′ and 5′-GCU GGC UAG UGC G-3′ were chemically synthesized as described above for the tetraloopHis. Their concentrations were calculated using molar absorbance coefficients of 0.185 and 0.155 μm−1·cm−1 at 260 nm, respectively (18). The two complementary oligonucleotides were dialyzed against H2O, mixed at a concentration of 100 μm each, lyophilized, and stored at −20 °C. Before use, oligonucleotides were dissolved in NMR buffer (50 mm sodium acetate (pH 6.0), 200 mm NaCl) at a final concentration of 1040 μm each, incubated for 1 h at 60 °C, and slowly cooled down to room temperature.
To aminoacylate the above duplex with histidyl-tRNA synthetase, phosphorylation at the two 5′-ends was ensured by a 30-min incubation at 37 °C in the presence of 50 mm Tris-HCl (pH 8.0), 10 mm MgCl2, 5 mm dithiothreitol, 2 mm ATP and 0.5 units/μl of T4 polynucleotide kinase (Roche Applied Science). Aminoacylation and acetylation of this duplex were performed as described above for tRNAHis.
Production and Purification of Wild-type and Mutant Pths
The gene of a histidine-tagged Pth was produced using PCR with the pUCpth plasmid (20) as template and oligonucleotides 5′-GGT GGT CAT ATG ACG ATT AAA TTG ATT GTC GG-3′ and 5′-CCG CTC GAG TTA TTG CGC TTT AAA GGC GT-3′ as primers. Mismatches in the sequences of the primers were introduced to create both an NdeI and a XhoI site (underlined). The resulting amplified fragment was digested with NdeI and XhoI. The digestion product was inserted into plasmid pET15blpa (21), between the NdeI and XhoI sites, to give plasmid pET15blpa-Pth. The gene of a histidine-tagged Pth lacking 14 residues at its C terminus (PthΔCter) was produced by a similar procedure using oligonucleotides 5′-GGT GGT CAT ATG ACG ATT AAA TTG ATT GTC GG-3′ and 5′-CCG CTC GAG TTA GCC ATC TGT AAA CCA CA-3′ as primers. The resulting plasmid was named pET15blpa-PthΔCter. To introduce mutations into the pth gene harbored by pET15blpa-Pth, DpnI-mediated site-directed mutagenesis (22) was employed. Sequences of the mutated pth genes were systematically fully verified. Plasmids expressing the Pth variants were introduced into K37ΔrecAλDE3 (23) cells. Transformants were grown in 1 liter of 2× TY-rich medium. Induction of the expression of the Pth variants and protein purification by affinity chromatography were performed as described previously for the production of His-tagged Pth H20A (24).
Nontagged wild-type Pth was expressed in JM101TR cells harboring the pUCpth plasmid and purified by successive chromatographies on DEAE-Sephacel, SP-Sepharose, and HI-Propyl chromatographies, as described previously (20). 15N-Labeled His-tagged H20A and H20A R133A Pth variants were produced in K37ΔrecAλDE3 cells and purified by affinity chromatography as described previously in the case of the 15N-labeled His-tagged H20A variant (24). Homogeneity of the samples was estimated to be higher than 95% by SDS-PAGE analysis. To check the absence of contaminating RNA hydrolase in the 15N-labeled His-tagged H20A preparation, tRNAHis (50 μm) was incubated for 15 days at 17 °C, in the NMR buffer conditions (50 mm sodium acetate (pH 6.0), 200 mm NaCl), in the presence of 75 μm of the purified enzyme. Then the polynucleotide was precipitated with ethanol, centrifuged, and assayed for aminoacylation by histidyl-tRNA synthetase as described previously (15). The tRNAHis was fully aminoacylatable after this treatment.
Enzymatic Assays
With the small RNAs, Pth catalytic efficiency measurements were performed at 28 °C in 100-μl assays containing 20 mm Tris-HCl (pH 7.5), 10 mm MgCl2, 0.1 mm EDTA, 0.06 to 0.15 μm of the N-acetyl-His-RNA substrate under study and catalytic amounts of Pth (2–30 nm). In the case of each RNA, it was verified that, at fixed enzyme concentrations, the initial rates of hydrolysis were directly proportional to the substrate concentration in the assay. This indicated that, whatever the used substrate, its concentration was much below its Km value and that we were allowed to calculate the catalytic efficiency (kcat/Km) of the enzyme from the following equation: v/[E]0 = (kcat/Km)[S]0, where v is the initial rate of hydrolysis, [E]0 is the total enzyme concentration, and [S]0 is the initial substrate concentration in the assay.
Michaelian parameters (kcat and Km) of His-tagged wild-type and mutant enzymes were obtained by varying diacetyl-[14C]Lys-tRNALys concentration between 0.25 and 30 μm in the above assay. Ki determinations were performed at 17 °C in the NMR buffer conditions (50 mm sodium acetate (pH 6.0), 200 mm NaCl). The inhibitor under study (tRNAHis or duplexHis) was added at a final concentration between 0.25 and 130 μm to an assay mixture containing 0.15–12 μm diacetyl-[14C]Lys-tRNALys and 2–40 nm Pth.
In all cases, after a 10-min incubation, reactions were quenched by the addition of 340 μl of ethanol, 14 μl of sodium acetate (3 m) (pH 4.8), and 20 μl of carrier RNA from yeast at a 4 mg/ml concentration. Samples were centrifuged, and soluble radioactivity in the supernatants was measured by scintillation counting (11). Ki, Km, kcat, and kcat/Km values were derived from fits of the theoretical Michaelis equation to the experimental values, using the Levenberg-Marquardt algorithm. Confidence limits on the fitted values were obtained by 100 Monte Carlo simulations followed by least squares fitting, using the experimental standard deviations on individual measurements (25).
NMR Experiments
The base-pairing of the tetraloopHis and duplexHis oligonucleotides was checked by examining the imino-proton region of their one-dimensional spectra recorded with a jump-and-return water suppression sequence (26). TetraloopHis spectra were recorded on a 500 μm sample at different pH values (between 5.6 and 7.4) and temperatures (between 6 and 70 °C). Spectra of the duplex were recorded on a 130 μm sample in the conditions used for the protein/RNA interactions (17 °C, 50 mm sodium acetate buffer at pH 6.0, 200 mm NaCl). All these experiments were performed on a 500 MHz Inova (Varian) spectrometer.
Protein/tRNA titration experiments were performed by recording HSQC spectra on a 130 μm 15N-uniformly labeled sample of His-tagged H20A Pth (50 mm sodium acetate (pH 6.0), 200 mm NaCl), to which aliquots of a concentrated duplexHis solution were progressively added. Seven points were realized corresponding to RNA/protein ratios of 0, 0.1, 0.2, 0.4, 0.6, 0.8, and 1.0. The 15N spectral width and offset frequency were set to 70 and 140 ppm, respectively, so that the backbone HN-N and arginine side chain Hϵ-Nϵ correlations could be observed without folding. All spectra were recorded at 17 °C on a Bruker Advance II 600 MHz spectrometer equipped with a TXI cryoprobe and analyzed using the Sparky software.
Structure Calculation
The Pth coordinates were retrieved from Protein Data Bank code 2PTH (chain A). The missing hydrogen atoms were added; the His-20 residue was changed into an alanine residue, and the energy of the protein was minimized using a CNS script derived from the generate.inp procedure. The RNA duplexHis coordinates were built using the XDNA software. We generated a type A DNA helix, added the 2′-hydroxyl groups, and converted the thymines in uracils by removing the 5-methyl groups. The geometry of the duplex was further rectified by minimizing its energy in the presence of a set of restraints derived from the HADDOCK dna-rna_restraint.def file. The PTH·duplex complex was built by assuming that the residues displaying a chemical shift variation larger than 0.02 ppm in the presence of 0.4 RNA equivalent and located at the surface of the protein should be in contact with the RNA, with the exception of the five glycine residues of the 106–115 loop (whose chemical shift variations could be due to a structural reorganization of this flexible loop). This condition was imposed by introducing in the optimization procedure, for each concerned residue, a restraint stating that at least one of the heavy atoms of the residue must be at a distance below 4 Å of a heavy atom of the RNA by using the CNS NOE soft-square potential with the R-6 mean. The complex formation was initiated with the RNA duplex randomly disposed in a half-sphere with respect to the Pth, at a distance (∼50 Å) sufficient to avoid any contact between them. However, the RNA orientation was imposed so that the CCA arm was directed toward the presumed active site of the Pth. Then the complex was submitted to three stages of Cartesian dynamics (at 300, 100, and 0 K) in the presence of the intermolecular restraints, using the standard Charm 19 force field but without electrostatic interaction. The energy of the complex was finally minimized using the same force field. To avoid large deformations of the protein and of the duplex, the atoms of the protein were submitted to harmonic restraints (100 kcal·mol−1·Å−2 on all heavy atoms for the unaffected residues and 10 kcal·mol−1·Å−2 on the backbone atoms for the affected ones), and the geometry of the RNA was maintained by an ensemble of distance (for the base pair hydrogen bonds), planar (for the base pair plans), and dihedral (for the sugar-phosphate backbone geometry) restraints derived from the standard dna-rna-restraint.def file. The harmonic constraints on the protein were gradually suppressed during the last minimization to allow the system to relax freely.
RESULTS AND DISCUSSION
Comparison of Potential Pth Substrates
According to the available data (10), E. coli Pth is predicted to recognize small RNA molecules recapitulating the acceptor and TψC stems of an elongator tRNA. To validate this hypothesis, we have studied the action of Pth on minimal N-blocked aminoacylated substrates derived from E. coli tRNAHis.
Mini- and microhelixHis based on the acceptor and TψC stem sequences of E. coli tRNAHis (13, 27) were synthesized by in vitro T7 transcription (Fig. 1) (17). Because of the high thermodynamic stability of RNA tetraloop motifs found in ribosomal RNAs, we also synthesized a tetraloopHis with the 3′-CCA end, the last 4 bp of the acceptor stem of tRNAHis and, at the base of the loop, an additional G-C base pair to reinforce the tetraloop stability (28). All these potential substrates of histidyl-tRNA synthetase contained the equivalent of the extra acceptor stem base pair G−1–C73. Relative to that measured with full-length tRNAHis, the catalytic efficiencies (kcat/Km) of aminoacylation of these substrates by the synthetase were decreased by 2 and 3 orders of magnitude in the case of the mini- or microhelixHis, respectively, and by 5 orders of magnitude in the case of the tetraloopHis (13, 28). In our hands, at the highest histidyl-tRNA synthetase concentration assayed, aminoacylation of the minihelix and microhelix reached 75 and 60%, respectively, at the steady state. In the case of the tetraloop, likely because of a poor recognition by histidyl-tRNA synthetase, only ∼4% histidylation could be obtained.
After histidylation and chemical N-acetylation, the above small RNAs were incubated with Pth. As shown in Table 1, the kcat/Km value in hydrolysis of the N-blocked aminoacylated minihelixHis was only 2-fold lower than that of acetyl-His-tRNAHis, supporting the idea that, in complex formation of Pth with a substrate, no more than the acceptor and TψC stem regions of tRNAHis were involved. With the microhelixHis or the tetraloopHis, the loss of the TψC arm resulted in a further 10-fold decrease in the kcat/Km value, as compared with the minihelixHis. This result suggested that a functional interaction with Pth could still be achieved, although the TψC arm has been removed. In agreement with this view, we reported elsewhere that 2′-(3′)-O-(l-[N,N-diacetyl-lysinyl])adenosine, a small mimic of diacetyl-Lys-tRNALys, keeps the capacity to be hydrolyzed by the enzyme (kcat/Km of 5.1·10−5 μm−1·s−1) (24). However, we conclude here that the introduction of the TψC arm in a putative substrate significantly improves the efficiency of catalysis.
TABLE 1.
Enzymatic hydrolysis of N-blocked aminoacylated tRNAHis analogs by E. coli Pth
| Substrate | No. of bases | kcat/Kma |
|---|---|---|
| μm−1·s−1 | ||
| Acetyl-[14C]His-tRNAHis | 76 | 0.10 ± 0.01 |
| Acetyl-[14C]His-minihelixHis | 36 | 0.050 ± 0.003 |
| Acetyl-[14C]His-microhelixHis | 26 | 0.006 ± 0.001 |
| Acetyl-[14C]His-tetraloopHis | 17 | 0.005 ± 0.001 |
| Acetyl-[14C]His-duplexHis | 29 | 0.015 ± 0.002 |
a The catalytic efficiency (kcat/Km) of nontagged wild-type Pth for the indicated substrates was measured at 28 °C in the presence of 20 mm Tris-HCl (pH 7.5), 10 mm MgCl2, and 0.1 mm EDTA. Measured values are given with confidence limits (see “Experimental Procedures”).
Another mimic containing the acceptor and TψC stems of tRNAHis was designed by assembling two complementary oligoribonucleotides. The resulting duplex resembles the above minihelix but without the loop (Fig. 1). To improve stability of the duplex, U65 (as numbered in native tRNAHis) was changed into a C. To prevent hybridization of the 3′-end of one oligonucleotide with the 3′-end of the other oligonucleotide, the G52–C62 base pair was changed into a C52–G62 one. Moreover, to avoid formation of a triple helix in the NMR conditions (see below), the G1–C72 base pair was changed into C1–G72. Such a change is compatible with Pth recognition (11). Base pairs (G−1–C73, U2–A71, and G3–C70) in the acceptor stem of tRNAHis, which are strong identity elements for synthetase recognition (14, 27), were not modified. Provided it had been 5′-phosphorylated, the duplexHis could be aminoacylated by histidyl-tRNA synthetase with a catalytic efficiency 800-fold lower than that measured with tRNAHis. Aminoacylation of the duplex reached nearly 20% at the steady state. Once aminoacylated and acetylated, the duplex RNA behaved as a substrate of Pth, with a kcat/Km value only six times smaller than that of authentic acetyl-His-tRNAHis (Table 1).
To know whether our nonaminoacylated and non-5′-phosphorylated RNA duplex interacted with Pth, we studied its inhibitory effect on the activity of the enzyme. In NMR conditions (17 °C, 50 mm sodium acetate, pH 6.0, 200 mm NaCl) and using diacetyl-lysyl-tRNALys as substrate, a competitive inhibition was found, with a Ki value of 45 μm. This value is close to that measured with nonaminoacylated tRNAHis (15 μm), in the same assay conditions.
Choice of Conditions of the NMR Study
Recently, we used NMR to gain some details of the binding of 3′-(l-[N,N-diacetyl-lysinyl])amino-3′-deoxyadenosine to E. coli Pth (24). To guard this ligand against hydrolysis under the NMR conditions, we used an inactive His-tagged H20A enzyme variant. Here, to study the interaction between Pth and RNAs, we used the same His-tagged H20A variant because assignment of most of the proton resonances of this protein was available (in 50 mm phosphate buffer (pH 6.0) containing 200 mm NaCl, BMRB accession number 17404) (24). The His-20 residue plays an essential role in the hydrolysis reaction (8, 29). However, although deprived of activity, mutants at position 20 keep the capacity to bind a peptidyl-tRNA substrate molecule. Indeed, an H20N mutant binds a fluorescent artificial Pth substrate with a Kd of 0.7 μm, whereas the wild-type enzyme hydrolyzes the same substrate with a Km of 5.5 μm (29).
Because of its small size, the tetraloopHis seemed promising to undertake NMR experiments. However, at pH 6.0 (our NMR conditions), it adopted, at least in part, a triple helix conformation, resulting from its dimerization (Fig. 1), as suggested by the presence in the NMR spectra of imino resonances characteristic of protonated cytidines, indicative of formation of C-G-C+ base triplets with G-C+ Hoogsteen pairing. This effect did not vary with temperatures between 6 and 40 °C. It disappeared at pH >7, the pH condition in which we studied the hydrolysis of acetyl-His-tetraloopHis by Pth. Eventually, we used the duplex RNAHis in which introduction of base pair C1–G72 was expected to preclude dimerization. Observation by NMR of the secondary structure of this duplex indeed confirmed the absence of triple helix formation. This RNA molecule has the advantage to recapitulate the acceptor and TψC stems of tRNAHis and to be small.
Fingerprint of RNA Binding on Pth
The fingerprint of the RNA-binding site on the Pth molecule was obtained by recording 15N HSQC spectra of the protein (130 μm) in the presence of increasing amounts of the duplexHis. Fig. 2 displays overlaid HSQC spectra recorded at 0, 0.4, and 0.8 duplexHis/protein ratios. Most peaks are perfectly superimposed, showing that the spectra were actually recorded in the same conditions. The intensity of all peaks progressively decreased when the duplex/protein ratio increased, the intensities being generally 2–3-fold lower at the 0.8 ratio than in the reference spectrum. Such a systematic decrease in intensity is likely caused by the increase in the system molecular size (22 kDa for the free protein, 32 kDa for the 1:1 complex).
FIGURE 2.
HSQC spectra of His-tagged Pth-H20A in the presence of various concentrations of duplexHis. The 1H-15N HSQC spectrum obtained in the presence of 130 μm Pth alone (in red) is superimposed to those obtained after addition of duplexHis at an RNA/protein ratio of 0.4 (in blue) or 0.8 (in yellow). Experiments were performed at 17 °C, in a 50 mm sodium acetate buffer (pH 6.0) containing 200 mm NaCl. Shown by arrows are the HN–N peaks of several residues sensitive to the presence of the RNA.
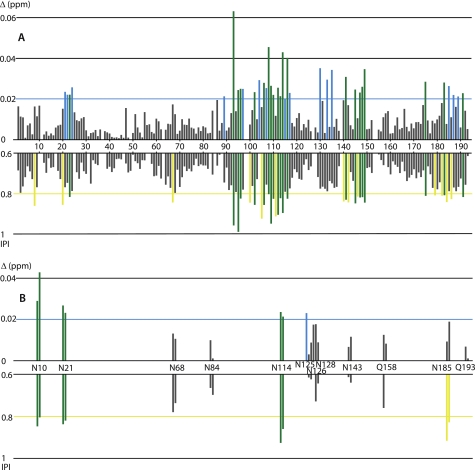
Despite the complexity of the spectra, it was possible to analyze the position and intensity modifications of nearly all HN-N correlations. Peak shifts were quantified by the distance Δδ = ((ΔδHN)2 + (ΔδN/10)2)1/2 that combines hydrogen and nitrogen chemical shift variations. Intensity variations were measured by the intensity perturbation index (IPI) (Iref − IRNA)/Iref, where Iref and IRNA are the peak heights in the reference spectrum (no RNA) and in a given RNA/protein spectrum, respectively. The chemical shift and intensity variations between the reference and the fourth (RNA/Pth ratio of 0.4) HSQC spectra are reported in Fig. 3. The variations were more pronounced at a 1:1 stoichiometric ratio, but the large reduction in some intensities rendered the analysis more difficult. As shown in Fig. 3, 21 backbone HN-N and 3 side-chain Hγ/δ-Nγ/δ correlations (in green) present a chemical shift variation and an intensity perturbation larger than the corresponding mean values plus one standard deviation (0.02 and 0.8 ppm, respectively). Fifteen backbone and one side-chain correlation (in blue) have a chemical shift variation larger than 0.02 ppm but an intensity perturbation smaller than 0.8. Finally, 2 Hγ-Nγ belonging to asparagine 185 and 13 backbone HN-N (in yellow) have a chemical shift variation smaller than 0.02 ppm but an intensity variation larger than 0.8. The observed concomitant chemical shift and intensity variations are indicative of rapid to intermediate exchange (on the chemical shift variation scale). In Fig. 4, we reported on the surface of the x-ray structure of E. coli Pth the positions of the 49 residues for which the chemical shift variation and/or the intensity perturbation index of their backbone HN-N were larger than one standard deviation. These residues form a cluster whose overall surface is compatible with the docking of one RNA duplex molecule.
FIGURE 3.
Chemical shift and intensity variations of HN–N peaks of Pth-H20A upon addition of duplexHis. For each residue (1–193) of the protein, the figure shows the chemical shift variation (Δδ = ((ΔδHN)2 + (ΔδN/10)2)1/2) and the IPI upon addition of duplexHis at an RNA/protein ratio of 0.4. Residues for which the Δδ value is higher than 0.02 ppm are in blue. Residues for which the IPI value is higher than 0.8 are in yellow. Residues for which both the Δδ value is higher than 0.02 ppm and the IPI value is higher than 0.8 are in green. A, amide protons of the protein backbone. In the histogram, a Δδ value of 0 was given to the proline residues and to the superimposed peaks for which the detection of the chemical shift variation was impossible. B, amide protons of the asparagines and glutamine side chains.
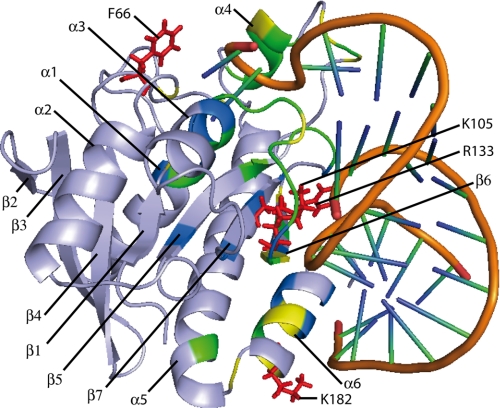
FIGURE 4.
Pth-H20A residues affected by RNA binding. A, on the molecular surface of Pth (PDB accession number 2PTH, chain A) on which hydrogen atoms have been added, residues for which the Δδ value is higher than 0.02 ppm are in blue; residues for which the IPI value is higher than 0.8 are in yellow, and residues for which both the Δδ value is higher than 0.02 ppm and the IPI value is higher than 0.8 are in green. The protein is displayed on two faces differing by 180°. The locations of several surface residues whose resonances are affected by RNA binding are shown by arrows. B, schematic representations of the protein with the same orientations and colors as in A, showing α-helices and β-strands. The figure was generated with PyMol (DeLano Scientific, San Carlos, CA).
Strong spectral variations are observed for Asn-10 (mainly the side chain), Ala-20 (which substitutes His-20), Met-67, Asp-93, and Asn-114, all of which are inside the catalytic center (8, 10, 29), suggesting that, although it lacks an esterified amino acid at its end, the CCA arm can reach the active site or affect it at a distance. A second group of amino acids, at the vicinity of the five above residues, is also affected. In this group, Ala-22, Gly-23, Ala-24, and Trp-25 are located at one end of helix α1, and Asn-21 links this helix to catalytic His-20. These five residues are not at the surface of the protein, raising the possibility that the helix α1 is submitted to motion/deformation in response to RNA binding.
The spectral properties of residues Asp-96 and Gly-111 are strongly affected. These residues are believed to delineate the boundary between the peptidyl region and the main core region of a bound Pth substrate (24, 30). The neighboring residues (Asp-93, Leu-95, and 112–117) are also sensitive to RNA binding. A response of residues Asp-96 and Gly-111 agrees with the idea that, upon RNA binding, deformation of this enzyme region opens the channel connecting the peptide and the tRNA binding regions (30, 31).
The loop-helix that covers the active site includes amino acids 140–149. All of them, except Asn-143 and Leu-144, are strongly affected, suggesting that the conformation of this flexible region is modified upon RNA binding. Indeed, motions of this loop-helix region were proposed to occur in Mycobacterium tuberculosis Pth (30, 31). These motions are interpreted to collaborate to the opening-closing of the enzyme active channel.
Beyond the active site, there is a region (residues 97–110, His-130, Arg-133, and Gly-135) that also responds to RNA binding. It is formed by the strands β6 and β7 and the loops around them. This region includes residues Lys-105 and Arg-133, which are believed to form a receptor site for the 5′-phosphate of an elongator tRNA molecule (10). Between bases −1 and +1, duplexHis displays a phosphate group that mimics this 5′-phosphate. To assess an involvement of the side chain of residue Arg-133 in the binding of the duplexHis molecule, we examined the portion of the HSQC spectrum corresponding to the side chain Hϵ protons of all arginine residues. One peak was strongly modified by the addition of the RNA (Fig. 5A). At an RNA/protein ratio of 0.4, its IPI was 0.8, and its Δδ was 0.027 ppm. This peak vanished at an RNA/protein ratio of 0.8. To verify that this peak does correspond to the Arg-133 Nϵ-Hϵ correlation, we compared the HSQC spectrum of the R133A/H20A double mutant to that of the H20A single mutant. As we expected, the spectrum of the double mutant lacked that peak (Fig. 5B), thereby giving support to the idea that Arg-133 side chain is involved in RNA recognition.
FIGURE 5.
Region of the 15N-HSQC spectra corresponding to the Hϵ protons of the Pth arginine side chains. A, effect of duplexHis addition. Spectra were recorded in the presence of 130 μm Pth in the absence of RNA (in red) or in the presence of RNA at an RNA/protein ratio of 0.4 (blue) or 0.8 (yellow). B, effect of the R133A mutation. The spectrum of the H20A mutant is in red and that of the H20A/R133A double mutant is in green. The peaks of two Hϵ protons, resonating at lower frequencies, are not visible in the figure. These peaks are not significantly modified either by the addition of duplexHis or by the R133A mutation.
Close to the above region, most of the residues composing the C-terminal helix α6 (residues 180–189) were affected by RNA binding. This observation is in agreement with the idea that this helix makes contacts with the TψC stem of tRNA (10).
Site-directed Mutagenesis
Among the above regions of Pth responding to the presence of the RNA duplex, three of them have already been extensively characterized by site-directed mutagenesis (8, 10). These three regions are as follows: (i) that of Lys-105 and Arg-133, the putative anchor site of the 5′-phosphate of tRNA; (ii) that of the helix-loop region covering the active site, and (iii) that of the active site itself. For instance, using diacetyl-Lys-tRNALys as substrate, we observed a 2.5-fold increase in the Km value of the R133A mutant. With the R19A, K103A, K105A, and K142A mutants, Km values became immeasurable (increases higher than 4-fold) (10). NMR mapping experiments implicate two more regions as follows: (i) the C-terminal helix, and (ii) the region around Leu-95 and the bordering residues (111–117). A K117A mutant has already been studied (10). It increases the Km value for the substrate 3.5-fold. Here, we substituted His-92, Leu-95, and Asp-96 by an alanine in a His-tagged Pth context. With the L95A mutation, the kcat value was decreased 25-fold if compared with that measured using the control wild-type tagged enzyme (Table 2). However, the Km value for the diacetyl-Lys-tRNALys substrate did not change. This suggests that the Leu-95 residue is involved in catalysis and not in substrate binding. With the D96A variant, the kcat and Km values were each about two times smaller than those with wild-type Pth. The effect of the H92A mutation on Michaelian parameters was small. Because of the high conservation of Arg-131 and because of its chemical shift variation (0.0191 ppm) just below the threshold of 0.02 ppm, we also produced the R131A mutation. The catalytic efficiency of this variant drops to 1.2% of the value measured with the wild-type enzyme. However, in view of the HSQC spectrum corresponding to the side-chain Hϵ protons of arginine residues, a direct interaction of Arg-131 with the RNA moiety of the substrate is unlikely. Indeed, in this spectrum, Arg-133 was the only residue displaying a marked change of its Hϵ proton resonance peak. We thus favor the idea that Arg 131 is structurally important in the expression of the activity of the hydrolase rather than being involved in substrate recognition. One possibility is that Arg 131 interacts with the glycine-rich 106–115 loop.
TABLE 2.
Catalytic parameters of various E. coli His-tagged Pth variants
For each mutant enzyme, kcat and Km values for diacetyl-lysyl-tRNALys were measured at 28 °C in the presence of 20 mm Tris-HCl (pH 7.5), 10 mm MgCl2, and 0.1 mm EDTA. Measured values are given with confidence limits (see “Experimental Procedures”). Relative kcat/Km values are given as percentages of the value with the wild-type enzyme.
| Enzyme | kcat | Km | Relative kcat/Km |
|---|---|---|---|
| s−1 | μm | % | |
| Wild type | 1.06 ± 0.18 | 1.8 ± 0.3 | 100 |
| H92A | 0.65 ± 0.06 | 3.7 ± 0.7 | 30 |
| L95A | 0.043 ± 0.010 | 1.8 ± 0.3 | 4.0 |
| D96A | 0.41 ± 0.06 | 0.70 ± 0.25 | 99 |
| R131A | 0.069 ± 0.022 | 9.4 ± 2.6 | 1.2 |
| K182A | 0.16 ± 0.03 | 15 ± 4 | 1.8 |
| N185A | 0.60 ± 0.10 | 3.0 ± 0.8 | 34 |
| PthΔCter | 0.17 ± 0.02 | 15 ± 4 | 1.9 |
In the C-terminal helix region, we introduced either a K182A or an N185A mutation. The N185A change reduced the catalytic efficiency by only three times. In contrast, the kcat/Km value of K182A was decreased 55-fold. The Km value of this mutant, 8-fold higher than that of the wild-type, significantly contributes to this decrease. In previous studies, we prepared the R186A mutant and showed that its catalytic efficiency was decreased 2-fold (10). These results suggest that the C-terminal helix of Pth participates in the docking of the RNA moiety in the substrate. To reinforce this conclusion, we designed and produced a Pth variant in which the C-terminal helix (residues 180–193) is removed. The kcat and Km values of this truncated Pth for the substrate were very similar to those of the K182A mutant (Table 2). This comparison confirms the idea that the C-terminal helix is important for recognition of the substrate and suggests that, in the helix, the main involved residue is Lys-182.
CONCLUSION
Efforts made for nearly 10 years now converge toward a quasi-complete model of the complexation of E. coli Pth to its peptidyl-tRNA substrate. In early crystallographic studies, the structure of crystalline Pth was found similar to that of the central core of an aminopeptidase from Aeromonas proteolytica (8, 10). This similarity guided us to probe the role in catalysis of several residues by site-directed mutagenesis (8, 10). His-20, Asp-93, and His-113, which are strongly conserved among the known Pth sequences, were shown important for the activity of Pth. A subsequent study by Goodall et al. (29) confirmed the importance of His-20. With the assistance of Asp-93, this residue would catalyze the hydrolysis of peptidyl-tRNA by accepting a proton from a proximal water molecule, which then attacks the electrophilic carbon of the ester bond (29). The water molecule would be held by Asn-114 (24).
Together with Asn-10 and Met-67, His-20, Asp-93, and His-113 delineate a channel that, in the crystal, is occupied by the C-end of a neighboring Pth molecule (8). Hence, several main chain atoms of the three residues (Lys-191*–Ala-192*–Gln-193*) at the C-end of one Pth polypeptide (the donor, residues marked in the text with an asterisk) establish contacts inside the active site of another Pth molecule (the recipient, residues not marked). Electrostatic bonds occur between the terminal oxygens of Gln-193* and the amide nitrogens of Asn-114 and Asn-68, between the main chain oxygen of Ala-192* and the amide nitrogen of Asn-10, and between the main chain oxygen of Lys-191* and the main chain nitrogen of Asn-68 (10, 24). Such a set of interactions involving only main chain atoms of the donor protein is compatible with the idea that the recipient enzyme can accommodate any peptide sequence. In this view, the crystal structure may reflect complex formation between an enzyme molecule and its peptide product.
Recently, interaction of a His-tagged H20A Pth mutant with 3′-(l-[N,N-diacetyl-lysinyl])amino-3′-deoxyadenosine, a small nonhydrolysable analog of the substrate, was followed by NMR (24). Docking simulations driven by the chemical shift variations and based on ambiguous interaction restraints gave consistence to contacts of the acetyl-blocked peptide moiety in this analog with Asn-10 and Asn-68. A stacking interaction of Phe-66 with the purine was also indicated. A model of an enzyme-bound substrate could be drawn where the δ2 NH2 groups of Asn-68 and of Asn-10 interact with the main chain carbonyl of the C-terminal residue and with that of the penultimate C-terminal residue in the tRNA-esterified peptide, respectively. In agreement with this model and with the crystallographic data, the enzyme loses its capacity of distinguishing between diacetyl-Lys-tRNALys and Lys-tRNALys upon mutagenesis of Asn-10 (24).
Previous data indicated a clamping of the phosphate at the 5′-end of tRNA by Pth (10). Starting with this constraint, a preliminary hand-made docking of tRNA was then attempted. The main resulting indication was that tRNA recognition might be limited to the only acceptor and TψC arms. Here, by using minimalist substrates displaying these two tRNA regions, this idea is given strong experimental support. Moreover, Ki measurements indicate similar bindings of authentic tRNA or of a small RNA duplex only recapitulating the acceptor and TψC arms of this tRNA. An involvement of the C-terminal helix of the protein in RNA binding is clearly indicated. Our mutagenesis data designate Lys-182 as a prominent actor in this contact. The K182A mutation increases the Km value of the Pth for diacetyl-Lys-tRNALys by a factor of 8 but also reduces the kcat by more than 6-fold. Such an effect on the kcat suggests that the formation of a productive enzyme·substrate complex requires precise positioning of the RNA moiety and that, by interacting with the RNA backbone, Lys-182 participates in this positioning.
A lysine or an arginine are found at position 182 in ∼40% of the available Pth sequences (Pfam family PF01195). Interestingly, a lysine or an arginine is also displayed at position 186 in ∼50% of the sequences. As a result, ∼70% of the sequences have a cationic residue either at position 182 or at position 186, or at both. Therefore, in those enzymes that lack a lysine or an arginine at position 182, functional involvement of a cationic residue at position 186 may be conceived. In E. coli Pth, there is an arginine at position 186. As shown previously (10), this residue can be shifted to an Ala without dramatic effect on activity. This reinforces the idea that, in the E. coli Pth, Lys-182 is the important functional basic residue in the α6 helix. Indeed, we show here that elimination of this lysine upon the removing of the full C-helix has the same consequence on the enzyme catalytic efficiency as the point mutation changing the lysine into an alanine.
The remaining are 30% of the bacterial-like Pth sequences that lack a cationic residue at both the 182 and 186 positions, or a few cases that simply are deprived of the α6 helix. Such cases are represented in procaryotes (for instance Chlamydia muridarum) as well as in eukaryotes (S. cerevisiae). They suggest alternative two- or three-dimensional topologies at the C-end of the corresponding enzymes. More efforts will be necessary to know whether all bacterial-like Pths recognize or not the same tRNA determinants. In this context, the structure of chloroplast RNA splicing 2 (CRS2) factor deserves attention. This protein displays the conserved active cleft residues of a bacterial Pth (Tyr-15, Thr-18, Arg-19, His-20, Asn-21, Met-67, Asn-68, Asp-93, His-113, and Asn-114). It also shows the Asn-10 residue, which interacts with the carbonyl group in the penultimate residue of the peptide, esterified to tRNA. Despite these similarities, expression of CRS2 in E. coli does not complement a pthts mutation, and therefore, CRS2 is not supposed to express Pth activity (32). Actually, CRS2 lacks the RNA 5′-phosphate clamp found in bacterial Pths. However, transplantation of this clamp in CRS2 is not enough to allow pthts complementation. Another cause of the particular behavior of CRS2 might be the occurrence of a basic and aromatic C-tail beyond the C-terminal helix (33). This tail possibly hinders correct binding of tRNA substrates to the CRS2 surface.
Within the 140–149-residue loop-helix, which covers the active site, numerous residues (141, 142, and 145–149) respond to RNA binding. Changes of the conformation of this region upon substrate binding have already been suggested in several reports (10, 30, 31). In particular, NMR relaxation data with M. tuberculosis Pth1 showed that residues in this segment display motion on a fast time scale, indicative of high local flexibility (31). Comparison of the E. coli and M. tuberculosis Pth1 crystal structures revealed that the loop adopts an open conformation in the M. tuberculosis enzyme (30), although it comes close to the active site channel in the peptide-bound E. coli enzyme (8). As a consequence, in the E. coli Pth structure, Lys-142 points toward the empty putative core RNA binding region. In agreement with the importance of the loop-helix, we already observed a pronounced effect of the K142A mutation on the Km value of the E. coli enzyme for its substrate (10). Moreover, in favor of functional significance of this region, two residues composing this domain, Val-149 and Leu-150, are conserved by 97 and 98% in the Pth primary structures, respectively.
Finally, we used the NMR data to build a model of the RNA bound at the surface of the enzyme. This model is supposed to correspond to the complex of Pth with one of its leaving products. By using conservative criteria (we only considered the amino acids undergoing a chemical shift variation larger than 0.02 ppm, located at the surface of the protein and not in the flexible loops), 18 residues of the protein (21, 93, 95–97, 104, 106, 113, 114, 117, 133, 141, 145, 148, 149, 185, 189, and 191) were restrained to be in contact with the RNA (see “Experimental Procedures”). The best solution (corresponding to the minimal restraint energy) is presented in Fig. 6. The docking surface fits in with the previous hand-made complex model. The +1 phosphate at the 5′-end of the shorter RNA strand is in the vicinity of the Lys-105 and Arg-133 cationic residues. The 3′-terminal adenosine of the longer strand does not stack on the side chain of Phe-66. However, through slight movements of the adenosine and/or of the Phe residue, the stacking should be easily achieved. Binding in the active site of a full substrate with a peptide moiety possibly allows such adjustments (24).
FIGURE 6.
Schematic view of the complex between duplexHis and Pth-H20A, based on the best-scored solution of a CNS energy minimization procedure. α-Helices and β-strands are shown with protein residues colored as in Fig. 4B. Side chains of Phe-66, Lys-105, Arg-133, and Lys-182 residues are in red. RNA backbone is in orange.
An important feature of the model of Fig. 6 is the C-terminal helix of the protein making contact with the RNA through its major groove. Previously, an overall resemblance between the hand-made model of the E. coli Pth·tRNA complex and the three-dimensional crystallographic structure of the Bacillus stearothermophilus EF-Tu·GTP·Phe-tRNAPhe complex was noted (10). However, the two protein structures are markedly different. Nevertheless, the possibility that in the two cases a cationic clamp on the protein surface may enable the elongator tRNAs to be distinguished from the initiator one was striking. This study affords experimental evidence that, similarly to EF-Tu, E. coli Pth recognizes the same side of the long helix formed by the acceptor and TψC stems.
Such a spatial distribution of the tRNA identity elements recognized by Pth explains why ribosome-bound peptidyl-tRNA cannot be a substrate of this enzyme. As indicated by the three-dimensional structure of the 70 S ribosome·tRNA complex (34), L16 ribosomal protein and 23 S rRNA helix H89 prevent access of Pth to its binding site on peptidyl-tRNA when tRNA is engaged at the A- or the P-site. In the case of release factors, which mimic tRNA at the A-site, access to P-site bound peptidyl-tRNA and ensuing ester bond hydrolysis are rendered possible via the inside of the L-corner of the polynucleotide (35). The E. coli yaeJ gene product, which has recently been shown to rescue stalled ribosomes from nonstop mRNAs (36, 37), resembles the catalytic domain 3 of release factors. It probably follows the same way of action.
Acknowledgments
We are grateful to Jean-Louis Leroy and Sandrine Caputo for participation in the chemical synthesis of oligonucleotides, to Jean-Louis Leroy for performing one-dimensional NMR experiments, and to Bruno Klaholz for advice in the handling of the ribosome three-dimensional structure.
Footnotes
- Pth
- peptidyl-tRNA hydrolase
- IPI
- intensity perturbation index.
REFERENCES
- 1. Atherly A. G., Menninger J. R. (1972) Nat. New Biol. 240, 245–246 [DOI] [PubMed] [Google Scholar]
- 2. Menez J., Buckingham R. H., de Zamaroczy M., Campelli C. K. (2002) Mol. Microbiol. 45, 123–129 [DOI] [PubMed] [Google Scholar]
- 3. Sassetti C. M., Boyd D. H., Rubin E. J. (2003) Mol. Microbiol. 48, 77–84 [DOI] [PubMed] [Google Scholar]
- 4. Cuzin F., Kretchmer N., Greenberg R. E., Hurwitz R., Chapeville F. (1967) Proc. Natl. Acad. Sci. U.S.A. 58, 2079–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kössel H., RajBhandary U. L. (1968) J. Mol. Biol. 35, 539–560 [DOI] [PubMed] [Google Scholar]
- 6. Fromant M., Ferri-Fioni M. L., Plateau P., Blanquet S. (2003) Nucleic Acids Res. 31, 3227–3235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rosas-Sandoval G., Ambrogelly A., Rinehart J., Wei D., Cruz-Vera L. R., Graham D. E., Stetter K. O., Guarneros G., Söll D. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 16707–16712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schmitt E., Mechulam Y., Fromant M., Plateau P., Blanquet S. (1997) EMBO J. 16, 4760–4769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Das G., Varshney U. (2006) Microbiology 152, 2191–2195 [DOI] [PubMed] [Google Scholar]
- 10. Fromant M., Plateau P., Schmitt E., Mechulam Y., Blanquet S. (1999) Biochemistry 38, 4982–4987 [DOI] [PubMed] [Google Scholar]
- 11. Dutka S., Meinnel T., Lazennec C., Mechulam Y., Blanquet S. (1993) Nucleic Acids Res. 21, 4025–4030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schulman L. H., Pelka H. (1975) J. Biol. Chem. 250, 542–547 [PubMed] [Google Scholar]
- 13. Francklyn C., Schimmel P. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 8655–8659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Musier-Forsyth K., Schimmel P. (1993) FASEB J. 7, 282–289 [DOI] [PubMed] [Google Scholar]
- 15. Fromant M., Plateau P., Blanquet S. (2000) Biochemistry 39, 4062–4067 [DOI] [PubMed] [Google Scholar]
- 16. Milligan J. F., Groebe D. R., Witherell G. W., Uhlenbeck O. C. (1987) Nucleic Acids Res. 15, 8783–8798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Milligan J. F., Uhlenbeck O. C. (1989) Methods Enzymol. 180, 51–62 [DOI] [PubMed] [Google Scholar]
- 18. Kibbe W. A. (2007) Nucleic Acids Res. 35, W43–W46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Snoussi K., Nonin-Lecomte S., Leroy J. L. (2001) J. Mol. Biol. 309, 139–153 [DOI] [PubMed] [Google Scholar]
- 20. Schmitt E., Fromant M., Plateau P., Mechulam Y., Blanquet S. (1997) Proteins 28, 135–136 [DOI] [PubMed] [Google Scholar]
- 21. Guillon L., Schmitt E., Blanquet S., Mechulam Y. (2005) Biochemistry 44, 15594–15601 [DOI] [PubMed] [Google Scholar]
- 22. Fisher C. L., Pei G. K. (1997) BioTechniques 23, 570–574 [DOI] [PubMed] [Google Scholar]
- 23. Ferri-Fioni M. L., Fromant M., Bouin A. P., Aubard C., Lazennec C., Plateau P., Blanquet S. (2006) J. Biol. Chem. 281, 27575–27585 [DOI] [PubMed] [Google Scholar]
- 24. Giorgi L., Plateau P., O'Mahony G., Aubard C., Fromant M., Thureau A., Grøtli M., Blanquet S., Bontems F. (2011) J. Mol. Biol. 412, 619–633 [DOI] [PubMed] [Google Scholar]
- 25. Dardel F. (1994) Comput. Appl. Biosci. 10, 273–275 [DOI] [PubMed] [Google Scholar]
- 26. Plateau P., Guéron M. (1982) J. Am. Chem. Soc. 104, 7310–7311 [Google Scholar]
- 27. Francklyn C., Shi J. P., Schimmel P. (1992) Science 255, 1121–1125 [DOI] [PubMed] [Google Scholar]
- 28. Shi J. P., Martinis S. A., Schimmel P. (1992) Biochemistry 31, 4931–4936 [DOI] [PubMed] [Google Scholar]
- 29. Goodall J. J., Chen G. J., Page M. G. (2004) Biochemistry 43, 4583–4591 [DOI] [PubMed] [Google Scholar]
- 30. Selvaraj M., Roy S., Singh N. S., Sangeetha R., Varshney U., Vijayan M. (2007) J. Mol. Biol. 372, 186–193 [DOI] [PubMed] [Google Scholar]
- 31. Pulavarti S. V., Jain A., Pathak P. P., Mahmood A., Arora A. (2008) J. Mol. Biol. 378, 165–177 [DOI] [PubMed] [Google Scholar]
- 32. Jenkins B. D., Barkan A. (2001) EMBO J. 20, 872–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ostheimer G. J., Hadjivassiliou H., Hadjivasiliou H., Kloer D. P., Barkan A., Matthews B. W. (2005) J. Mol. Biol. 345, 51–68 [DOI] [PubMed] [Google Scholar]
- 34. Yusupov M. M., Yusupova G. Z., Baucom A., Lieberman K., Earnest T. N., Cate J. H., Noller H. F. (2001) Science 292, 883–896 [DOI] [PubMed] [Google Scholar]
- 35. Korostelev A., Zhu J., Asahara H., Noller H. F. (2010) EMBO J. 29, 2577–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chadani Y., Ono K., Kutsukake K., Abo T. (2011) Mol. Microbiol. 80, 772–785 [DOI] [PubMed] [Google Scholar]
- 37. Handa Y., Inaho N., Nameki N. (2011) Nucleic Acids Res. 39, 1739–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]