Background: The melanocortin-3 (MC3R) and ghrelin (GHSR) receptors are important key components in hypothalamic weight regulation.
Results: MC3R and GHSR di/oligomerize and have an opposite impact on each other's function.
Conclusion: The high basal activity of GHSR is a determinant of heterodimer function, and MC3R may constrain GHSR function.
Significance: Receptor di/oligomerization and its functional relevance contribute to the complex network of hypothalamic weight regulation.
Keywords: 7-Helix Receptor, G-protein-coupled Receptor (GPCR), Molecular Biology, Signal Transduction, Signaling
Abstract
Interaction and cross-talk of G-protein-coupled receptors (GPCRs) are of considerable interest because an increasing number of examples implicate a profound functional and physiological relevance of homo- or hetero-oligomeric GPCRs. The ghrelin (growth hormone secretagogue receptor (GHSR)) and melanocortin-3 (MC3R) receptors are both known to have orexigenic effects on the hypothalamic control of body weight. Because in vitro studies indicate heterodimerization of GHSR and MC3R, we investigated their functional interplay. Combined in situ hybridization and immunohistochemistry indicated that the vast majority of GHSR-expressing neurons in the arcuate nucleus also express MC3R. In vitro coexpression of MC3R and GHSR promoted enhanced melanocortin-induced intracellular cAMP accumulation compared with activation of MC3R in the absence of GHSR. In contrast, agonist-independent basal signaling activity and ghrelin-induced signaling of GHSR were impaired, most likely due to interaction with MC3R. By taking advantage of naturally occurring GHSR mutations and an inverse agonist for GHSR, we demonstrate that the observed enhanced MC3R signaling capability depends directly on the basal activity of GHSR. In conclusion, we demonstrate a paradigm-shifting example of GPCR heterodimerization allowing for mutually opposite functional influence of two hypothalamic receptors controlling body weight. We found that the agonist-independent active conformation of one GPCR can determine the signaling modalities of another receptor in a heterodimer. Our discovery also implies that mutations within one of two interacting receptors might affect both receptors and different pathways simultaneously. These findings uncover mechanisms of important relevance for pharmacological targeting of GPCR in general and hypothalamic body weight regulation in particular.
Introduction
G-protein-coupled receptors (GPCRs)3 are involved into the modulation of almost any physiological process (1). These receptors are activated by a remarkable variety of different ligands, including ions, nucleotides, biogenic amines, small peptides, and large glycoprotein hormones, and they activate various signaling pathways (2). GPCRs are popular therapeutic targets, and >40% of approved drugs act on GPCRs (3).
Recently, it became evident that these receptors function in di- or oligomeric complexes. Among such receptor complexes, homodimeric (same GPCRs) or heterodimeric (different GPCRs) interactions between protomers can occur (4, 5). These types of structural and functional oligomer interactions are of great interest when they differ from well established functional characteristics of monomeric receptors (6). Relevant functional changes could be modified G-protein coupling, different ligand-receptor specificities, or altered ligand-induced receptor internalization (7, 8). GPCR oligomerization could be of particular interest for an improved molecular understanding of pathophysiological mechanisms also, as >35 GPCRs are known to be relevant for human diseases (9).
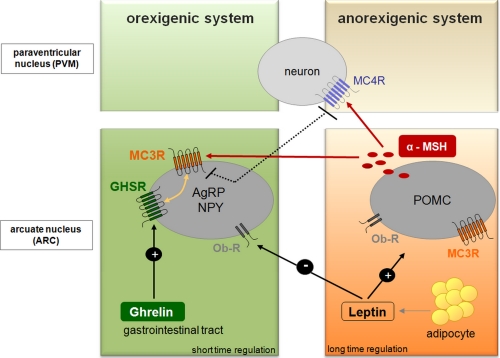
Obesity is one of the world's major health burdens of the 21st century. During the last 20 years, our understanding of mechanisms involved in body weight and appetite regulation has increased tremendously. A remarkable number of key components in hypothalamic body weight regulation turned out to be GPCRs such as the melanocortin-4 receptor (MC4R), the melanocortin-3 receptor (MC3R), and the growth hormone secretagogue receptor (GHSR; ghrelin receptor) (Fig. 1). Especially functional consequences of naturally occurring mutations in these receptors have demonstrated their physiological importance for maintenance of body weight (10, 11).
FIGURE 1.
Determinants of hypothalamic weight regulation. This schematic overview focuses on energy homeostasis and feeding centrally regulated in two subsets of neurons within the arcuate nucleus of the hypothalamus. The anorexigenic acting peptide leptin is secreted from adipocytes in proportion to body fat (long-time regulation). Stimulation of the anorexigenic system by leptin leads to satiety achieved through expression of POMC peptides like α-MSH, which acts on melanocortin receptors (MC3R and MC4R), whereas the appetite-activating (orexigenic) system is repressed. Neuropeptide Y (NPY) and agouti-related peptide (AgRP) expression by ghrelin results in appetite stimulation and the initiation of food intake. Ghrelin concentration in the blood fluctuates throughout the day and therefore permits short-time regulation of appetite and food intake. MC3R bridges between the regulatory networks of both feeding systems as well as the different time frames.
GHSR and MC3R are of specific interest because their hypothalamic activation induces food intake. GHSR is activated by stomach-derived Ser3-acetylated ghrelin (12). Ghrelin activation is promoted by ghrelin o-acyltransferase (EC 2.3.1.-) independent of dietary lipids (13, 14). Interestingly, ghrelin was recently found to be of major importance to survive starvation by modifying glucose homeostasis (15). Ghrelin o-acyltransferase knock-out mice lost the ability to stabilize glucose levels during starvation due to the lack of functional active ghrelin to stimulate growth hormone release (16). In contrast to the peripheral orexigenic action of the ghrelin o-acyltransferase/ghrelin/GHSR system, the orexigenic activation of MC3R is induced by pro-opiomelanocortin (POMC)-derived γ-melanocyte-stimulating hormone (MSH) produced in the hypothalamus (17). In addition, anticipatory food-seeking behaviors triggered by food restriction and circadian metabolic control by central circadian clock circuits have been reported to depend on MC3R (18).
In this study, we investigated the functional relevance of MC3R/GHSR heterodimerization using a multidisciplinary approach combining molecular biology, mutations with known loss-of-function impact, and advanced receptor pharmacology. We show that interaction of GHSR and MC3R leads to enhanced MC3R signaling efficacy (signaling surplus) while simultaneously causing a decreased GHSR signaling capacity. We further demonstrate that ligand-independent GHSR signaling activity plays a key role in this mutual and opposite receptor interrelation and is modulated by interaction with MC3R. This series of observations supports the importance of basal GHSR activity for body weight regulation (19) and for functional impact of GPCR dimerization and likely translates into the principal biology of other GPCRs characterized by a permanent basally active conformation.
EXPERIMENTAL PROCEDURES
Determination of MC3R and GHSR in the Arcuate Nucleus
In situ hybridization histochemistry was performed to map the hypothalamic expression of MC3R. Immunohistochemistry of β-galactosidase, the product of the lacZ gene driven by the GHSR promoter in GHSR knock-out mice (20), was used to visualize GHSR. GHSR knock-out mice were perfused and post-fixed with 4 °C diethyl pyrocarbonate-treated paraformaldehyde and sank in 30% diethyl pyrocarbonate-treated sucrose buffered with 0.1 m PBS. Brains were then cut with a cryostat into 25-μm slices and stored at −80 °C in antifreeze solution until processing for in situ hybridization. Sections were treated with 0.2 n HCl, 0.2% glycine buffer, and 0.1% Triton X-100 and rinsed with 0.1 m PBS between each step. Brain sections were then prehybridized for 30 min and hybridized in hybridization buffer (Sigma-Aldrich) containing 100 nm Locked nuclei acid-modified cDNA probes labeled with 6-carboxyfluorescein (forward, 5′-AGGAAGAAGTACATGGGAGAGT; and reverse, 5′-ATCTCCTTGAACGTGTTGCGCA) (Exiqon) for 8 h. After stringent washing with 2×, 0.5×, and 0.2× SSC and rinsing with 0.1 m PBS, sections were incubated with rabbit anti-fluorescein antibody (1:10,000; Invitrogen) and chicken anti-β-galactosidase antibody (1:200; Abcam) at 4 °C for 16 h. After rinsing with 0.1 m PBS, sections were incubated with both DyLight 488-conjugated goat anti-rabbit (1:200) and Cy3-conjugated donkey anti-chicken (1:300) secondary antibodies (Jackson ImmunoResearch Laboratories). After thorough rinsing, sections were mounted with Dabco® (Sigma-Aldrich) and visualized by confocal microscopy.
Cloning of WT MC3R and GHSR and Mutants for Functional Characterization
MC3R was cloned from genomic DNA, and GHSR cDNAs were purchased from the University of Missouri-Rolla cDNA Resource Center. All constructs were cloned into the eukaryotic expression vector pcDps (21) via KpnI/SpeI. For HA and FLAG tagging of MC3R and GHSR, epitope-coding primers were used. Mutant GHSR and MC3R were generated using standard mutagenesis techniques and WT GHSR-pcDps and WT MC3R-pcDps as templates.
Cell Culture and Transfection
COS-7 and HEK293 cells were cultured in DMEM (Biochrom, Berlin, Germany), and HEK293 cells were cultured in MEM (Biochrom), both supplemented with 10% fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin, and incubated at 37 °C in a humidified 7% CO2 incubator. For determination of total receptor expression, 1 × 106 COS-7 cells were seeded in 6-cm dishes and transfected with a total of 3 μg of DNA and 4 μl of Metafectene. Cell surface expression assays were performed in 48-well plates (5 × 104 cells/well), and cells were transfected with 0.25 μg of DNA/well and 1 μl Metafectene/well. cAMP assays and determination of inositol 1,4,5-trisphosphate (IP3) were performed in 48-well plates (5 × 104 cells/well). Cells were transfected with 80 ng of DNA/well and 0.93 μl of Metafectene/well. For binding studies, COS-7 cells (5 × 104 cells/well) were transfected with 0.25 μg of DNA and 0.4 μl of Metafectene/well.
Cell Surface Expression Studies
To investigate cell surface expression, receptors were N-terminally HA-tagged, and cell surface ELISAs were performed. GFP served as a negative control. Three days after transfection, cells were washed, paraformaldehyde-fixed, and probed with biotin-labeled anti-HA antibody (Roche Applied Science). Bound anti-HA antibody was detected by peroxidase-labeled streptavidin (Dianova, Hamburg, Germany) and a substrate/chromogen reaction (22).
Determination of Total Receptor Expression
Total receptor expression was determined with N-terminally HA-tagged and C-terminally FLAG-tagged receptors. Plasmids were transfected in 6-cm dishes. HA-tagged MC3R was used as a negative control. Forty-eight hours after transfection, cells were harvested and solubilized overnight. Lysates were incubated in anti-FLAG antibody (Sigma-Aldrich)-coated 96-well plates for 2 h. Detection of the HA epitope was performed as described (23).
Determination of Cell Surface-binding Properties
Forty-eight hours after transfection, cells were washed and incubated overnight in the presence of [125I-Nle4,d-Phe7]α-MSH (specific activity of 2000 Ci/mmol, 120,000 cpm/well; Amersham Biosciences) with increasing amounts of agonist [125I-Nle4,d-Phe7]α-MSH. After washing, specifically bound [125I-Nle4,d-Phe7]α-MSH was measured. Bmax values were calculated from displacement curves by the method of Cheng and Prusoff (24).
Investigation of Gs Activation and Gq/11 Activation after Ligand Challenge
Intracellular cAMP was investigated after transfection of COS-7 cells by a nonradioactive assay based on AlphaScreen technology (PerkinElmer Life Science). Thus, cells were seeded into 48-well plates and transfected on the next day. One day after transfection, the transfection mixture was replaced with medium. Stimulation of cells with agonists was performed 48 h after transfection. Cells were incubated in serum-free DMEM containing 1 mm 3-isobutyl-1-methylxanthine (Sigma-Aldrich) in the absence or presence of increasing concentrations of agonists for 45 min at 37 °C. The reactions were stopped by aspiration of the media, and cells were lysed in 50 μl of lysis buffer (see AlphaScreen manual) containing 1 mm 3-isobutyl-1-methylxanthine. From each well, 5 μl of lysate was transferred to a 384-well plate. Acceptor beads (in stimulation buffer) and donor beads were added according to the manufacturer's protocol. cAMP accumulation data were analyzed by the GraphPad Prism program.
For investigation of Gq/11 activation, we performed an IP3 reporter gene assay by cotransfecting HEK293 cells with plasmid DNA (MC3R or GHSR) and a reporter construct containing a response element and the firefly luciferase gene under the control of NFAT (nuclear factor of activated T-cells). Two days after transfection, cells were stimulated with ghrelin (Sigma-Aldrich) for 6 h and then lysed. Gq/11 activation is determined as luciferase activity in the luciferase reporter gene assay according to the manufacturer's instructions (Promega, Madison, WI).
Modeling of Structural MC3R and GHSR Conformations
Crystal structures in the inactive conformation serving for GPCR homology modeling have been published for several family A GPCR members such as rhodopsin, adenosine, and β-adrenergic receptors (reviewed in Refs. 25 and 26). For structural modeling of the human ghrelin receptor and MC3R, we used the inactive rhodopsin structure (Protein Data Bank code 2I35 (27)). Furthermore, we also designed a model of the active conformation of GHSR based on opsin, a GPCR crystal structure with features of an activated GPCR conformation (Protein Data Bank code 3CAP (28)). The principal modeling procedures were derived according to Kleinau et al. (29). Structure images were produced using PyMOL Version 1.03 software.
RESULTS
Coexpression of MC3R and GHSR in Hypothalamic Neurons
We demonstrated previously that cotransfection of COS-7 or HEK293 cells with equal amounts of MC3R and GHSR induces the formation of the following pairs of receptor dimers (30): MC3R homodimers, GHSR homodimers, and MC3R/GHSR heterodimers. This finding, in combination with the physiological relevance of both receptors for appetite regulation, directed us to question the potential functional importance of GHSR and MC3R heterodimerization.
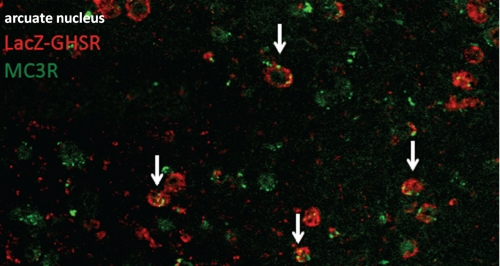
The in vivo combination of in situ hybridization with targeted mouse mutagenesis and immunohistochemistry indicated that the vast majority of GHSR-expressing neurons in the arcuate nucleus also express MC3R, but reciprocally, not all MC3R-expressing neurons express GHSR (Fig. 2). This is consistent with the assumption that MC3R is expressed on POMC as well as on agouti-related peptide/neuropeptide Y (GHSR) neurons (31) (Fig. 1).
FIGURE 2.
Co-localization of MC3R and GHSR in vivo. lacZ-immunoreactive cells (representing GHSR) were labeled with Cy3 (red), and cells expressing MC3R mRNA were labeled with Cy2 (green). Arrows indicate examples of neurons expressing both GHSR and MC3R. Some of the MC3R-expressing neurons did not express GHSR, whereas most of the GHSR-expressing neurons were MC3R-positive.
Opposite Effects on Signaling Capacities in MC3R/GHSR Heterodimers
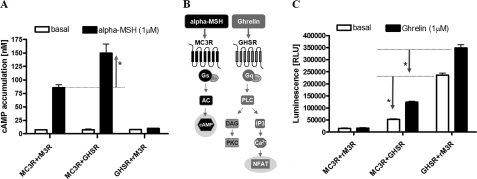
GHSR couples to the Gq/11/phospholipase Cβ system, whereas stimulation of MC3R results in activation of the Gs/adenylyl cyclase system (Fig. 3B). To compare data from cotransfection, constant levels of cotransfected DNA were used. The rat muscarinic 3 receptor (rM3R) signals via the Gq protein coupling system like GHSR, but dimerization of rM3R with MC4R or MC3R could not be observed (23). Therefore, we used cotransfection with rM3R to ensure equal amounts of transfected plasmid DNA.
FIGURE 3.
Effects of MC3R/GHSR heterodimerization on protomeric signaling pathways. Assays were carried out 48 h after transient transfection with equal amounts of MC3R and GHSR or with rM3R as transfection compensation in COS-7 cells (cAMP) and HEK293 cells (IP3 via reporter gene assay). A, stimulation of the MC3R/GHSR heterodimer with 1 μm α-MSH resulted in a significant increase in cAMP accumulation by a factor of 2 (p < 0.001) compared with the MC3R homodimer. Assays were performed 48 h after cotransfection of COS-7 cells. Data are the means ± S.E. of three independent experiments performed in triplicates. B, illustration of the protomeric signaling transduction pathways. MC3R couples to the Gs/adenylyl cyclase (AC) system, whereas GHSR signals via Gq/11 proteins. PLC, phospholipase C; DAG, diacylglycerol; PKC, protein kinase C. C, using the NFAT-luciferase reporter system, a reduction in basal (*, p < 0.001) as well as agonist-induced signaling (*, p < 0.001) of GHSR coexpressed with MC3R after incubation with 1 μm ghrelin was observed. Statistical analysis was performed by one-way analysis of variance and Tukey's tests. RLU, relative light units.
Stimulation of COS-7 cells coexpressing MC3R and GHSR with α-MSH resulted in a 2-fold higher increase (Table 1) in Gs-induced cAMP accumulation (signaling cAMP surplus) compared with MC3R in the absence of GHSR (Fig. 3A). Using luciferase reporter gene assays, we quantified changes in intracellular Ca2+ levels caused by GHSR signaling in the presence or absence of its ligand. Cotransfection of MC3R and GHSR in HEK293 cells for determination of Gq activation revealed diminished basal as well as impaired ghrelin-induced signaling of the MC3R/GHSR heterodimer. Both basal and MSH-induced signaling were reduced to ∼60% compared with the signaling properties of GHSR alone (Fig. 3C and Table 1).
TABLE 1.
Summary of functional characterization of MC3R, GHSR, and MC3R/GHSR coexpression
Functional in vitro assays were performed in transiently transfected COS-7 or HEK293 cells to investigate Gs-mediated cAMP accumulation and Gq/11-mediated IP3 activation via a Ca2+-dependent reporter gene assay. To ensure equal concentrations of transfected DNA, we cotransfected MC3R and GHSR with rM3R when examining the functional characteristics of both receptors. We have demonstrated previously that rM3R is able to interact neither with MC3R nor with GHSR and does not influence the signaling capability (30). All data indicate -fold increases in the MC3R + rM3R basal activity of 3.77 ± 2.34 for cAMP and 51,670 ± 28,495 for IP3, which was set as 1. Co-stimulation of α-MSH and ghrelin was performed in equimolar concentrations. EC50 values were calculated using the computer program GraphPad Prism. Data are means ± S.D. of four experiments performed in triplicates for cAMP accumulation (with the exception of two experiments performed for ghrelin stimulation) and of six experiments performed in sextuplicates for Gq/11 activation.
| cAMP |
IP3 |
|||
|---|---|---|---|---|
| Basal | Stimulated (1 μm) | Basal | Stimulated (1 μm) | |
| -fold MC3R + rM3R basal | -fold MC3R + rM3R basal | |||
| α-MSH | ||||
| MC3R + rM3R | 1.00 ± 0.24 | 16.7 ± 3.68 | 1.00 ± 0.17 | 1.36 ± 0.31 |
| MC3R + GHSR | 1.67 ± 0.64 | 32.5 ± 7.98 | 2.80 ± 0.71 | 1.93 ± 0.78 |
| GHSR + rM3R | 2.63 ± 1.53 | 6.21 ± 2.66 | 4.62 ± 1.64 | 4.92 ± 1.31 |
| Ghrelin | ||||
| MC3R + rM3R | 1.00 ± 0.53 | 0.73 ± 0.43 | 1.00 ± 0.17 | 1.30 ± 0.33 |
| MC3R + GHSR | 1.93 ± 1.14 | 1.68 ± 1.02 | 2.80 ± 0.71 | 4.32 ± 1.09 |
| GHSR + rM3R | 0.59 ± 0.26 | 2.20 ± 0.35 | 4.62 ± 1.64 | 6.15 ± 2.00 |
| α-MSH + ghrelin | ||||
| MC3R + rM3R | 1.00 ± 0.19 | 21.3 ± 5.87 | 1.00 ± 0.17 | 1.87 ± 0.55 |
| MC3R + GHSR | 1.44 ± 0.85 | 34.6 ± 8.04 | 2.80 ± 0.71 | 2.91 ± 1.05 |
| GHSR + rM3R | 1.37 ± 0.42 | 5.67 ± 3.14 | 4.62 ± 1.64 | 6.38 ± 1.54 |
Co-stimulation of α-MSH and ghrelin did not change signaling properties profoundly; however, the cAMP response after co-stimulation of α-MSH and ghrelin was further enhanced (Table 1). In summary, our study indicates that MC3R/GHSR coexpression and heterodimerization lead to a mutual and opposite effect on their respective signaling capacities.
Basal Activity of GHSR Is Related to α-MSH-induced MC3R Hyperstimulation in Heterodimers
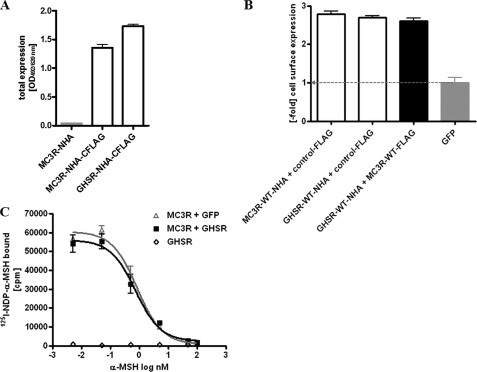
Because of these findings, we were eager to clarify the molecular reasons for the observed increased capacity of MC3R to induce cAMP accumulation. Three potential mechanisms were under consideration. (i) Up-regulation of MC3R cell surface expression by heterodimerization with GHSR should cause an increased signaling capacity of MC3R in response to α-MSH. The maximal ligand-binding capacity of GPCR depends on the number of receptors expressed on the cell surface. By total receptor expression (Fig. 4A), cell surface expression (Fig. 4B), and ligand binding studies (Fig. 4C), we determined that MC3R expression was not increased in the MC3R/GHSR heterodimer. In conclusion, the MSH-induced signaling surplus in cAMP formation is not due to an increase in MC3R expression.
FIGURE 4.
Expression and binding studies of the MC3R/GHSR heterodimer. A, total receptor expression was determined with N-terminally HA (NHA) and C-terminally FLAG-tagged (CFLAG) constructs. N-terminally HA-tagged MC3R served as a negative control. Three days after transfection, cells were lysed, and total receptor expression was determined as described under “Experimental Procedures.” B, for determination of cell surface expression, COS-7 cells were cotransfected with equal amounts of plasmid DNA coding for N-terminally HA-tagged MC3R or GHSR and the C-terminally FLAG-tagged control (thyrotropin receptor). ELISA measurements were carried out with intact COS-7 cells in 48-well plates as described under “Experimental Procedures.” Values are given as -fold over GFP expression. C, to investigate the binding properties of MC3R and MC3R/GHSR, COS-7 cells were transiently transfected, and after 48 h, [125I-Nle4,d-Phe7]α-MSH (125-NDP-α-MSH) displacement binding was performed as described under “Experimental Procedures.” Comparison of the binding properties of MC3R coexpressed with GHSR or with GFP to keep DNA amounts equal implicated no changes in ligand binding between MC3R and MC3R/GHSR. GHSRs were used as a negative control. Displacement with unlabeled α-MSH resulted in IC50 values of 53 nm for the MC3R homodimer and 39 nm for the MC3R/GHSR heterodimer. IC50 values were obtained using GraphPad Prism software.
(ii) Hyperstimulation of the Gs pathway might be induced by GHSR or other factors and not by α-MSH-induced MC3R activation. If this holds true, then GHSR might mediate cAMP accumulation in the absence of functional MC3R. To test this hypothesis, we cotransfected a complete loss-of-function mutant of MC3R, I183N (32), together with GHSR and measured α-MSH-induced cAMP accumulation (Fig. 5A). The MC3R mutant was still able to bind α-MSH, although with a reduced affinity, and to form dimers with wild-type MC3R and GHSR (supplemental “Materials and Methods” and Fig. S1). However, coupling of the MC3R mutant to its intracellular effectors was greatly diminished (33). In other words, the complete loss of MC3R signaling by introduction of the I183N mutation prevented the observed surplus in cAMP formation in cells cotransfected with MC3R and GHSR (Fig. 5A). We therefore conclude that the α-MSH-induced cAMP surplus is dependent on MC3R activation and is not due to G-protein-independent regulation of adenylyl cyclase (for example, by Ca2+ or calmodulin (34)).
FIGURE 5.
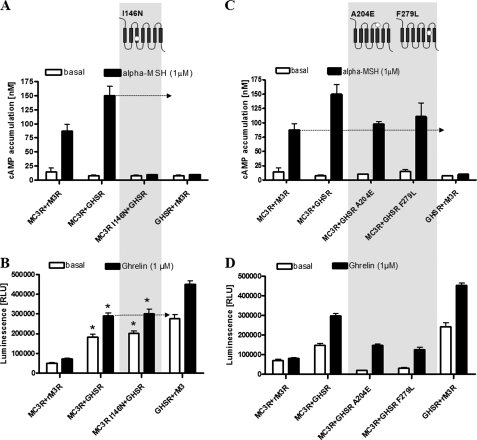
Functional investigation of pathogenic mutations in MC3R and GHSR. Investigations of functional properties were performed 48 h after transient transfection in COS-7 cells for determination of cAMP accumulation (A and C) and in HEK293 cells for investigation of Gq/11 activation (B and D) as described under “Experimental Procedures.” A, MC3R loss-of-function mutant I183N coexpressed with GHSR completely blocked cAMP surplus formation in response to α-MSH stimulation. B, reduction of basal and ligand-induced signaling of GHSR coexpressed with MC3R I183N was detectable (*, p > 0.05) as seen for wild-type MC3R (*, p > 0.05). RLU, relative light units. C, cotransfection of MC3R with GHSR mutant A204E as well as F279L completely abolished the cAMP surplus. D, cotransfection with MC3R did not influence the signaling properties of GHSR mutants. In the case of cAMP assays, the means ± S.E. of three independent experiments performed in triplicates are shown. Data based on the NFAT-luciferase reporter system are means ± S.E. of one representative experiment performed in sextuplicates (see also Table 1). Statistical analysis was performed by one-way analysis of variance and Tukey's tests.
(iii) The basally active conformation of GHSR increases the capacity of MC3R for Gs activation and cAMP accumulation after MSH binding. To test this hypothesis, we investigated the impact of basally active GHSR using naturally occurring GHSR mutations with diminished basal signaling activity as well as the inverse agonist substance P, which has been reported to suppress ligand-independent basal activity (35). We used two naturally occurring GHSR mutations that lead to either partial or complete lack of basal ligand-independent activity: A204E and F279L (supplemental Fig. S2) (36, 37). Such mutations can be termed silencing mutations, inverse agonistic mutations, or constitutively inactivating mutations. We assume that both mutations are not located directly in the potential dimerization interface (Fig. 6A). Both mutant receptors were able to homodimerize with wild-type GHSR and to heterodimerize with MC3R (supplemental “Materials and Methods” and Fig. S3). After cotransfection of MC3R with these GHSR mutants, α-MSH challenge demonstrated a reduced or completely abolished cAMP signaling surplus down to the level of MC3R without interactions with GHSR. This observation indicated that the MC3R-related signaling surplus depended entirely on the basal GHSR activity (Fig. 5C).
FIGURE 6.
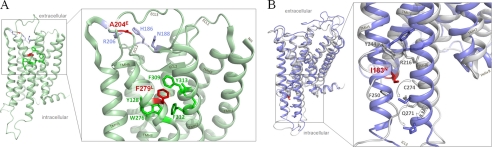
Pathogenic MC3R and GHSR loss-of-function mutations are not directly located at potential dimer interfaces. We explored structural MC3R and GHSR models to gain insights into the location and potential involvement of the pathogenic variations used in this study in direct dimer interactions. A, the GHSR mutations leading to a loss of ligand-independent basal signaling activity are localized in ECL2 (A204E) and in transmembrane helix TMH6 (F279L). The aromatic wild-type amino acid Phe279 (red) is tightly embedded in a cluster of aromatic residues (green) between TMH6 and TMH7. Although the leucine side chain is hydrophobic like phenylalanine, this non-aromatic substitution leads to a hindrance of aromatic cluster interactions, which prevents signal transduction-related movement of TMH6. In mutant A204E (ECL2), the introduction of a large negatively charged hydrophilic side chain instead of the small hydrophobic alanine leads to new interaction(s) within particular residues within ECL2 (blue sticks). In consequence, ECL2 properties important for receptor signaling regulation such as structural flexibility, spatial adjustment, and interactions with residues at the transmembrane helices are modified. B, two different homology models of human MC3R based on either inactive (gray backbone) or active (lilac backbone) GPCR crystal structure conformation are shown superimposed. The hydrophobic isoleucine at position 183 at the intracellular site of TMH3 (red stick) points directly between helices 5 and 6 and is flanked by Phe250 (TMH5) and Cys274 (TMH6). The I183N side chain substitution likely leads to a new hydrophilic interaction with Gln271 at TMH6, which locks both TMH3 and TMH6 and prevents helical movement (arrow) necessary for G-protein activation.
Interestingly, we showed that the signaling properties of GHSR mutants coexpressed with MC3R did not differ from those of the GHSR mutants expressed alone (Fig. 5D and supplemental Fig. S2). Moreover, the complete loss-of-function MC3R variant I183N (Fig. 6B) did not increase the inhibitory effect of MC3R on the basal GHSR signaling activity (Fig. 5B).
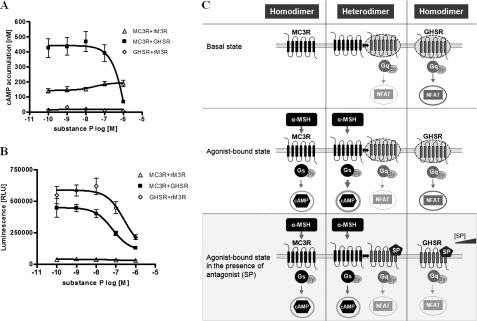
These results point to a fundamental role of the basally active conformation and signaling of GHSR for functionalities of the MC3R/GHSR heterodimer. This finding is further confirmed by experiments using a GHSR inverse agonist, substance P (Fig. 7C). In a concentration-dependent manner, substance P reduced the maximal MC3R cAMP surplus (obtained after challenge with 1000 nm α-MSH) (Fig. 7A). We independently confirmed that substance P acts as an inverse agonist of GHSR (Fig. 7B).
FIGURE 7.
Substance P, an inverse agonist of GHSR, blocks cAMP surplus of MC3R/GHSR. HEK293 cells were transfected, and assays were performed as described under “Experimental Procedures.” A, determination of cAMP accumulation after stimulation with constant amounts of 1 μm α-MSH and co-stimulation with substance P in a dose-response manner (0.1–1 μm). The α-MSH-induced cAMP surplus of MC3R/GHSR was blocked by increasing amounts of substance P to a level comparable with MC3R expressed alone. The means ± S.E. of three independent transfection experiments performed in triplicates are shown. B, testing the efficiency of substance P on Gq/11 signaling after stimulation in a concentration-dependent manner (0.1 nm to 1 μm). RLU, relative light units. C, schematic illustration of the functional effects of substance P (SP) on the signaling properties of MC3R, GHSR, and the MC3R/GHSR heterodimer. NF, nuclear factor; NFAT, nuclear factor of activated T-cells.
DISCUSSION
Heterodimer Formation between GHSR and MC3R Leads to Mutual Signaling Interference
We found that a direct interplay between both receptors, GHSR and MC3R, leads to mutually opposite effects on their signaling capacities (Fig. 3). In the heterodimer, an increased capacity for signaling of MC3R to stimulate cAMP after ligand treatment was observed, but, in contrast, the capacity of GHSR to stimulate inositol phosphate accumulation in the basal and ligand-induced states was diminished. Remarkably, in our approach, we found that partial inactivation of one receptor was caused just by interaction with a second receptor. In other words, we observed an opposite modification of signaling potentials caused by physical interactions of two different receptors. We are aware of the fact that the described findings were obtained from in vitro studies using overexpressing systems. However, low amounts of transfected DNA (see “Experimental Procedure”) and the circumstance that rM3R is not able to interact with MC3R and GHSR (30) support the fact that the influence of signaling properties of MC3R and GHSR is not due to non-physiological high expression levels.
The known mechanisms of GPCR interaction, such as cross-talk of signaling pathways or cross-activation by one ligand, are likely not relevant in the models studied here. Our findings differ from several studies regarding other GPCR dimers, especially concerning the molecular reasons for particular functional dimer properties. Enhanced signaling after ligand binding, as we found here for MC3R, has been reported for heterodimers between thromboxane receptor isoforms (38). In contrast to our results, however, this effect was related to specificities of ligands, and investigated receptors were of the same group, sharing identical G-protein preferences. Inhibitory effects in dimer constellations were reported for the GPR50/MT1 melatonin receptor heterodimer (7), but those were restricted to ligand binding. This was not the case in our study, where effects are caused by constitutive activity of GHSR. Only one published study reported that a GPCR heterodimer (serotonin-2A receptor and glutamate-2 receptor) up-regulated signaling at one receptor with simultaneous down-regulation at the other receptor (39). However, in contrast to our findings, ligand binding of the receptors was modified in a reciprocal way.
GHSR Basal Activity Is a Determinant for Signaling Regulation in Heterodimers with MC3R and Can Be Modified by Pathogenic Mutations
Coexpression of MC3R and mutants of GHSRs (naturally occurring mutations silencing the basal (constitutive) signaling activity) revealed that α-MSH-induced MC3R hyperstimulation observed for cAMP (surplus) is dependent on the basal activity of GHSR (Fig. 3). This finding points to a fundamental role of a basally active GHSR conformation for signaling regulation in the heterodimer. It has been described that the basally activated state of GHSR represents a signaling set point in energy balance regulation (10). Basal activity was found to be of importance for other GPCRs and in a pathophysiological context also (40–42). However, the molecular underpinnings for the mutually opposite influence on signaling capacities between MC3R and GHSR described here remain unknown. We presume that the discovered effects might be caused by specific modalities of the direct but multilayered structural interplay between GHSR and MC3R. Several studies of GPCR support such relevant structure-function relationships within GPCR dimers. (i) For homodimers of dopamine receptors, it has been shown that the interface between the protomers at transmembrane helix 4 determines receptor activation (43). (ii) Activation of the dimeric metabotropic glutamate receptor is related to helical intersubunit rearrangements (44) proven by helix exchanges. (iii) Conformational changes between α2A-adrenergic and μ-opioid receptor heterodimers have been reported whereby the propagated conformational change from one receptor caused inactivation of the second receptor (45). (iv) Studies on homodimeric GPCR have shown that after agonist-mediated activation in the presence of G-proteins, only G-protein coupling at one protomer might occur, which leads to asymmetric conformations of the receptors (46), indicating that G-protein coupling prevents symmetric functioning of the dimer. Considering points i–iv for the MC3R/GHSR dimer with the mutual and opposite signaling effects on each other reported here, a structural interplay is to be assumed, where the receptor interfaces on one hand mediate constraints on GHSR and on the other hand improve signaling at MC3R.
Our study emphasizes the important role of GPCR heterodimerization for signaling regulation by two major findings. 1) The heterodimeric organization of two GPCRs with preferences for different G-proteins can modulate mutually and oppositely the signaling capacities of both receptors. 2) An agonist-independent basal signaling activity of one receptor molecule can determine the functional signaling modalities of the second GPCR in a dimer. Such importance of basal signaling activity was also reported recently for TAAR1 (trace amine associated receptor 1) and the dopamine-2 receptor (47). It was found that the acute application of an inverse agonist for TAAR1 increases the potency of dopamine at neuronal dopamine-2 receptors.
Implications for Physiology and Drug Development
Our findings offer new implications for the current established model of hypothalamic body weight regulation. The basal activity of GHSR was thought to be one key determinant for this orexigenic pathway (48), in which the activation of Ca2+/calmodulin-induced CREB (cAMP-responsive element-binding protein) enhancement may play an important role. Here, we have provided evidence that GHSR interacts with MC3R, which results in hyperstimulation of the α-MSH-induced cAMP signaling pathway and could potentially further increase CREB formation. So far, MC3R is thought to be an inhibitory autocrine regulated receptor located on POMC neurons (31). Peripheral application of a selective MC3R agonist does indeed stimulate feeding (49). Our study adds an additional role for MC3R as a potential regulatory receptor on agouti-related peptide/neuropeptide Y neurons.
To circumvent leptin resistance in obese patients, the activation of anorexigenic pathways downstream of the leptin receptor, e.g. MC4R, represents an attractive strategy: activation of MC4R by POMC-derived α- and β-MSH results in a reduction of food intake and an increase in energy expenditure (50). Unfortunately, the drugs developed so far are only effective in mice, and these drugs have failed to offer a therapeutic window in humans that would make them suitable for clinical use (51). One has to keep in mind that the interplay of all GPCRs on a neuron of interest has to be considered to estimate the effect of potential drug-like molecules for treatment. For example, female MC3R knock-out mice demonstrate a reduced response to ghrelin (52), indicating that more components are probably involved in this homeostatic system. Alternative therapeutic strategies focus on the inactivation of leptin-independent pathways such as ghrelin and its receptor (53, 54).
The results presented here also reveal implications for potential drug-induced side effects. To ensure effectiveness even under changing disease conditions, detailed knowledge not only on the targeted protomer but also on possible interaction partners of GPCR in the targeted tissue is important and may represent a new area of high potential. For example, in the case of asthma treatment, β2-adrenergic receptor agonists are used for bronchodilatation; however, because of frequently occurring infections, the concentration of prostaglandins in the lung increases and activates their receptors. Interestingly, the β2-adrenergic receptor can interact with the prostaglandin receptor. Activation of the prostaglandin receptors uncouple β2-adrenergic receptors from their signaling pathway, which could theoretically interfere with the activity of conventional asthma treatment (55, 56). As an example for the complexity of interference by receptor/receptor interactions, it was shown that drugs targeting the metabotropic glutamate receptor compete with hallucinogenic agonists of the serotonin 5-hydroxytryptamine type 2A receptors and vice versa (39). In conclusion, efficient development of specific and safe drug candidates targeting particular GPCRs might require considering the possibility of functionally relevant receptor heterodimerization.
Supplementary Material
This work was supported by Deutsche Forschungsgemeinschaft Grants BI 893/2-1 and KL2334/2-1 and Graduate School 1208 Hormonal Regulation of Energy Metabolism, Body Weight and Growth, TP1, and Bundesministerium für Bildung und Forschung NGFN-Plus Grant 01GS825.

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Materials and Methods,” Figs. S1–S3, and additional references.
- GPCR
- G-protein-coupled receptor
- MC4R
- melanocortin-4 receptor
- MC3R
- melanocortin-3 receptor
- GHSR
- growth hormone secretagogue receptor (ghrelin receptor)
- POMC
- pro-opiomelanocortin
- MSH
- melanocyte-stimulating hormone
- IP3
- inositol 1,4,5-trisphosphate
- rM3R
- rat muscarinic 3 receptor.
REFERENCES
- 1. Rozenfeld R., Devi L. A. (2011) Biochem. J. 433, 11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kristiansen K. (2004) Pharmacol. Ther. 103, 21–80 [DOI] [PubMed] [Google Scholar]
- 3. Hopkins A. L., Groom C. R. (2002) Nat. Rev. Drug Discov. 1, 727–730 [DOI] [PubMed] [Google Scholar]
- 4. Bouvier M. (2001) Nat. Rev. Neurosci. 2, 274–286 [DOI] [PubMed] [Google Scholar]
- 5. George S. R., O'Dowd B. F., Lee S. P. (2002) Nat. Rev. Drug Discov. 1, 808–820 [DOI] [PubMed] [Google Scholar]
- 6. Rosenbaum D. M., Rasmussen S. G., Kobilka B. K. (2009) Nature 459, 356–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Levoye A., Dam J., Ayoub M. A., Guillaume J. L., Couturier C., Delagrange P., Jockers R. (2006) EMBO J. 25, 3012–3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lohse M. J. (2010) Curr. Opin. Pharmacol. 10, 53–58 [DOI] [PubMed] [Google Scholar]
- 9. Schöneberg T., Schulz A., Biebermann H., Hermsdorf T., Römpler H., Sangkuhl K. (2004) Pharmacol. Ther. 104, 173–206 [DOI] [PubMed] [Google Scholar]
- 10. Holst B., Schwartz T. W. (2006) J. Clin. Invest. 116, 637–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tao Y. X. (2010) Endocr. Rev. 31, 506–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tschöp M., Smiley D. L., Heiman M. L. (2000) Nature 407, 908–913 [DOI] [PubMed] [Google Scholar]
- 13. Kirchner H., Gutierrez J. A., Solenberg P. J., Pfluger P. T., Czyzyk T. A., Willency J. A., Schürmann A., Joost H. G., Jandacek R. J., Hale J. E., Heiman M. L., Tschöp M. H. (2009) Nat. Med. 15, 741–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Romero A., Kirchner H., Heppner K., Pfluger P. T., Tschöp M. H., Nogueiras R. (2010) Eur. J. Endocrinol. 163, 1–8 [DOI] [PubMed] [Google Scholar]
- 15. Goldstein J. L., Zhao T. J., Li R. L., Sherbet D. P., Liang G., Brown M. S. (2011) Cold Spring Harb. Symp. Quant. Biol., in press [DOI] [PubMed] [Google Scholar]
- 16. Zhao T. J., Liang G., Li R. L., Xie X., Sleeman M. W., Murphy A. J., Valenzuela D. M., Yancopoulos G. D., Goldstein J. L., Brown M. S. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 7467–7472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marks D. L., Hruby V., Brookhart G., Cone R. D. (2006) Peptides 27, 259–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sutton G. M., Perez-Tilve D., Nogueiras R., Fang J., Kim J. K., Cone R. D., Gimble J. M., Tschöp M. H., Butler A. A. (2008) J. Neurosci. 28, 12946–12955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Petersen P. S., Woldbye D. P., Madsen A. N., Egerod K. L., Jin C., Lang M., Rasmussen M., Beck-Sickinger A. G., Holst B. (2009) Endocrinology 150, 4920–4930 [DOI] [PubMed] [Google Scholar]
- 20. Diano S., Farr S. A., Benoit S. C., McNay E. C., da Silva I., Horvath B., Gaskin F. S., Nonaka N., Jaeger L. B., Banks W. A., Morley J. E., Pinto S., Sherwin R. S., Xu L., Yamada K. A., Sleeman M. W., Tschöp M. H., Horvath T. L. (2006) Nat. Neurosci. 9, 381–388 [DOI] [PubMed] [Google Scholar]
- 21. Okayama H., Berg P. (1983) Mol. Cell. Biol. 3, 280–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tarnow P., Rediger A., Brumm H., Ambrugger P., Rettenbacher E., Widhalm K., Hinney A., Kleinau G., Schaefer M., Hebebrand J., Krause G., Grüters A., Biebermann H. (2008) Obes. Facts 1, 155–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Elsner A., Tarnow P., Schaefer M., Ambrugger P., Krude H., Grüters A., Biebermann H. (2006) Peptides 27, 372–379 [DOI] [PubMed] [Google Scholar]
- 24. Cheng Y., Prusoff W. H. (1973) Biochem. Pharmacol. 22, 3099–3108 [DOI] [PubMed] [Google Scholar]
- 25. Worth C. L., Kleinau G., Krause G. (2009) PLoS ONE 4, e7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yarnitzky T., Levit A., Niv M. Y. (2010) Curr. Opin. Drug Discov. Devel. 13, 317–325 [PubMed] [Google Scholar]
- 27. Salom D., Lodowski D. T., Stenkamp R. E., Le Trong I., Golczak M., Jastrzebska B., Harris T., Ballesteros J. A., Palczewski K. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 16123–16128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Park J. H., Scheerer P., Hofmann K. P., Choe H. W., Ernst O. P. (2008) Nature 454, 183–187 [DOI] [PubMed] [Google Scholar]
- 29. Kleinau G., Hoyer I., Kreuchwig A., Haas A. K., Rutz C., Furkert J., Worth C. L., Krause G., Schülein R. (2011) J. Biol. Chem. 286, 25859–25871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rediger A., Tarnow P., Bickenbach A., Schaefer M., Krude H., Gruters A., Biebermann H. (2009) Obes. Facts 2, 80–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cowley M. A., Smart J. L., Rubinstein M., Cerdán M. G., Diano S., Horvath T. L., Cone R. D., Low M. J. (2001) Nature 411, 480–484 [DOI] [PubMed] [Google Scholar]
- 32. Tao Y. X., Segaloff D. L. (2004) J. Clin. Endocrinol. Metab. 89, 3936–3942 [DOI] [PubMed] [Google Scholar]
- 33. Rached M., Buronfosse A., Begeot M., Penhoat A. (2004) Biochim. Biophys. Acta 1689, 229–234 [DOI] [PubMed] [Google Scholar]
- 34. Sunahara R. K., Taussig R. (2002) Mol. Interv. 2, 168–184 [DOI] [PubMed] [Google Scholar]
- 35. Holst B., Cygankiewicz A., Jensen T. H., Ankersen M., Schwartz T. W. (2003) Mol. Endocrinol. 17, 2201–2210 [DOI] [PubMed] [Google Scholar]
- 36. Pantel J., Legendre M., Cabrol S., Hilal L., Hajaji Y., Morisset S., Nivot S., Vie-Luton M. P., Grouselle D., de Kerdanet M., Kadiri A., Epelbaum J., Le Bouc Y., Amselem S. (2006) J. Clin. Invest. 116, 760–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang H. J., Geller F., Dempfle A., Schäuble N., Friedel S., Lichtner P., Fontenla-Horro F., Wudy S., Hagemann S., Gortner L., Huse K., Remschmidt H., Bettecken T., Meitinger T., Schäfer H., Hebebrand J., Hinney A. (2004) J. Clin. Endocrinol. Metab. 89, 157–162 [DOI] [PubMed] [Google Scholar]
- 38. Wilson S. J., McGinley K., Huang A. J., Smyth E. M. (2007) Biochem. Biophys. Res. Commun. 352, 397–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. González-Maeso J., Ang R. L., Yuen T., Chan P., Weisstaub N. V., López-Giménez J. F., Zhou M., Okawa Y., Callado L. F., Milligan G., Gingrich J. A., Filizola M., Meana J. J., Sealfon S. C. (2008) Nature 452, 93–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kleinau G., Jaeschke H., Mueller S., Worth C. L., Paschke R., Krause G. (2008) Cell. Mol. Life Sci. 65, 3664–3676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Piscione F., Iaccarino G., Galasso G., Cipolletta E., Rao M. A., Brevetti G., Piccolo R., Trimarco B., Chiariello M. (2008) J. Am. Coll. Cardiol. 52, 1381–1388 [DOI] [PubMed] [Google Scholar]
- 42. Srinivasan S., Lubrano-Berthelier C., Govaerts C., Picard F., Santiago P., Conklin B. R., Vaisse C. (2004) J. Clin. Invest. 114, 1158–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guo W., Shi L., Filizola M., Weinstein H., Javitch J. A. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 17495–17500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brock C., Oueslati N., Soler S., Boudier L., Rondard P., Pin J. P. (2007) J. Biol. Chem. 282, 33000–33008 [DOI] [PubMed] [Google Scholar]
- 45. Vilardaga J. P., Nikolaev V. O., Lorenz K., Ferrandon S., Zhuang Z., Lohse M. J. (2008) Nat. Chem. Biol. 4, 126–131 [DOI] [PubMed] [Google Scholar]
- 46. Damian M., Martin A., Mesnier D., Pin J. P., Banères J. L. (2006) EMBO J. 25, 5693–5702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bradaia A., Trube G., Stalder H., Norcross R. D., Ozmen L., Wettstein J. G., Pinard A., Buchy D., Gassmann M., Hoener M. C., Bettler B. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 20081–20086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Holst B., Schwartz T. W. (2004) Trends Pharmacol. Sci. 25, 113–117 [DOI] [PubMed] [Google Scholar]
- 49. Cai M., Mayorov A. V., Cabello C., Stankova M., Trivedi D., Hruby V. J. (2005) J. Med. Chem. 48, 1839–1848 [DOI] [PubMed] [Google Scholar]
- 50. Cone R. D. (2005) Nat. Neurosci. 8, 571–578 [DOI] [PubMed] [Google Scholar]
- 51. Krishna R., Gumbiner B., Stevens C., Musser B., Mallick M., Suryawanshi S., Maganti L., Zhu H., Han T. H., Scherer L., Simpson B., Cosgrove D., Gottesdiener K., Amatruda J., Rolls B. J., Blundell J., Bray G. A., Fujioka K., Heymsfield S. B., Wagner J. A., Herman G. A. (2009) Clin. Pharmacol. Ther. 86, 659–666 [DOI] [PubMed] [Google Scholar]
- 52. Shaw A. M., Irani B. G., Moore M. C., Haskell-Luevano C., Millard W. J. (2005) Peptides 26, 1720–1727 [DOI] [PubMed] [Google Scholar]
- 53. Chollet C., Meyer K., Beck-Sickinger A. G. (2009) J. Pept. Sci. 15, 711–730 [DOI] [PubMed] [Google Scholar]
- 54. Schellekens H., Dinan T. G., Cryan J. F. (2010) Neuropharmacology 58, 2–16 [DOI] [PubMed] [Google Scholar]
- 55. Barnes P. J. (2006) J. Clin. Invest. 116, 1210–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McGraw D. W., Mihlbachler K. A., Schwarb M. R., Rahman F. F., Small K. M., Almoosa K. F., Liggett S. B. (2006) J. Clin. Invest. 116, 1400–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.