Background: The α2 isoform of Na,K-ATPase is unstable compared with α1 and α3.
Results: Mutations in TM8–10 strongly stabilize α2. A novel phospholipid antagonist selectively inactivates α2, and mutations in TM8–10 protect against inactivation.
Conclusion: A phosphatidylserine binding pocket within TM8–10 has been identified.
Significance: Mechanistic insights into α2 instability and a possible physiological role have been obtained.
Keywords: ATPases; Membrane Function; Na,K-ATPase; Phosphatidylserine; Protein Stability; Isoforms; α2
Abstract
The α2 isoform of Na,K-ATPase plays a crucial role in Ca2+ handling, muscle contraction, and inotropic effects of cardiac glycosides. Thus, structural, functional, and pharmacological comparisons of α1, α2, and α3 are of great interest. In Pichia pastoris membranes expressing human α1β1, α2β1, and α3β1 isoforms, or using the purified isoform proteins, α2 is most easily inactivated by heating and detergent (α2 ≫ α3 > α1). We have examined an hypothesis that instability of α2 is caused by weak interactions with phosphatidylserine, which stabilizes the protein. Three residues, unique to α2, in trans-membrane segments M8 (Ala-920), M9 (Leu-955), and M10 (Val-981) were replaced by equivalent residues in α1, singly or together. Judged by the sensitivity of the purified proteins to heat, detergent, “affinity” for phosphatidylserine, and stabilization by FXYD1, the triple mutant (A920V/L955F/V981P, called α2VFP) has stability properties close to α1, although single mutants have only modest or insignificant effects. Functional differences between α1 and α2 are unaffected in α2VFP. A compound, 6-pentyl-2-pyrone, isolated from the marine fungus Trichoderma gamsii is a novel probe of specific phospholipid-protein interactions. 6-Pentyl-2-pyrone inactivates the isoforms in the order α2 ≫ α3 > α1, and α2VFP and FXYD1 protect the isoforms. In native rat heart sarcolemma membranes, which contain α1, α2, and α3 isoforms, a component attributable to α2 is the least stable. The data provide clear evidence for a specific phosphatidylserine binding pocket between M8, M9, and M10 and confirm that the instability of α2 is due to suboptimal interactions with phosphatidylserine. In physiological conditions, the instability of α2 may be important for its cellular regulatory functions.
Introduction
The Na,K-ATPase or Na,K-pump is an integral membrane protein that actively transports Na+ and K+ ions across the plasma membrane of virtually all animal cells. The Na,K-pump maintains the electrochemical potential gradients of Na+ and K+ ions, required for regulation of cell volume, electrical excitability, nutrients and neurotransmitter uptake, intracellular pH, and particularly relevant to this paper, cellular Ca2+ handling (1, 2).
The Na,K-ATPase is a heterodimer composed of the catalytic α subunit that couples ATP hydrolysis and ion transport and the β subunit that stabilizes the enzyme and permits trafficking to the cytoplasmic membrane (3, 4). The Na,K-ATPase usually also contains an auxiliary subunit of the FXYD protein family (5–7). Recently, the structures of pig kidney Na,K-ATPase at 3.5 Å (8) and shark rectal gland Na,K-ATPase at 2.4 Å resolution (9) have been determined. These structures resolve most of the features of α and β subunits and FXYD subunits. In addition, lower resolution structures of the Na,K-ATPase with bound ouabain have become available (10, 11).
Na,K-ATPase has four isoforms of the α subunit (α1–4) and three isoforms of the β subunit (β1–3), expressed and regulated in a tissue- and development-specific fashion, together with one of seven different mammalian FXYD regulatory proteins (1–7, 12). α1 is almost ubiquitously distributed, whereas α2 is expressed strongly in muscle (skeletal, smooth, and cardiac) and astrocytes, α3 primarily in nerve cells, and α4 only in spermatozoa. Human heart expresses α1, α2, and α3 isoforms and β1 (13). In cardiac myocytes, α2 is concentrated in the T-tubules adjacent to the SR,3 whereas α1 is more evenly distributed in T-tubules and SR (14). In smooth muscle and brain astrocytes, α1 is uniformly distributed but α2 and α3 show a punctated distribution, overlying the SR (15). α2 carries a large fraction of the Na,K-pump current in T-tubules of rat and mouse cardiac myocytes (16) and preferentially modulates Ca2+ transients and SR Ca2+ release in mouse cardiac myocytes compared with α1 (17). Also, α2 is the major regulator of cardiac contractility in rat cardiomyocytes (18). In particular, α2 is functionally coupled to the Na/Ca exchanger and regulates Ca2+ handling without changing the [Na]i (19). Compared with α1, α2 shows a higher K0.5 Ko (20–22) and steeper voltage dependence (18, 20), properties that allow a rapid change in rate with change in Ko and membrane potential. This fits well with a specific regulatory role of α2 in muscle Ca2+ handling. Similarly, there is good evidence that α2 plays an important role in contractility of vascular smooth muscle and control of blood pressure and regulation of Ca2+ handling (23, 24). Experiments with mice engineered to have ouabain-sensitive α1 and -insensitive α2 isoforms (α1S/Sα2R/R) or ouabain-insensitive α1 and α2 (α1R/Rα2R/R), instead of the wild-type with ouabain-insensitive α1 and ouabain-sensitive α2 (α1R/Rα2S/S), have shown that α2 plays a predominant role in cardiac glycoside-induced positive inotropy (17, 25) and also provide strong evidence for endogenous mammalian cardiac glycosides (25, 26). α2 also plays the major role in cardiac glycoside-induced positive inotropy in rat cardiomyocytes (18). Recent observations that human α2 shows a moderate selectivity over α1 for some digitalis glycosides, such as digoxin, support the notion that cardiotonic effects of digoxin in humans are exerted via α2 (27).
Familial hemiplegic migraine is a rare but severe autosomal dominant subtype of migraine with aura (28). About 25% of familial hemiplegic migraine cases (type 2) are attributed to mutations to α2 (29, 30). α2 is expressed in brain astrocytes, and most FHM2 α2 mutations cause functional defects in active Na+ and K+ transport, and compromised clearing of extracellular K+ or glutamate, with a tendency to cortical spreading depression (31).
In view of the above and much more information available in the literature, there is obviously a great interest in comparing the structural, functional, and pharmacological differences of the α1, α2, and α3 isoforms. We have described extensively the Pichia pastoris expression system for Na,K-ATPase isoforms and purification of α1β1, α2β1, and α3β1 complexes (21, 27, 32–34), as well as reconstitution, in vitro, of αβFXYD complexes using purified FXYD proteins (21, 35). A fundamental condition for maintenance of Na,K-ATPase activity of all the purified detergent-soluble αβ or αβFXYD complexes is the presence of an acidic phospholipid, especially phosphatidylserine (33, 34). A requirement of detergent-soluble native Na,K-ATPase for an acidic phospholipid has been known for many years (36). Recent work shows that phosphatidylserine undergoes a specific interaction with the protein and protects against thermally mediated or detergent-mediated inactivation (33–35). Together with the phosphatidylserine, cholesterol also provides strong additional stabilization (34). One indication of a specific interaction of the phosphatidylserine with the protein is the structural selectivity of both headgroups and fatty acyl chains. Of the many phospholipids tested, 1-stearoyl-2-oleoyl-sn-glycero-3-phospho-l-serine (SOPS) is optimal (34). More recently, the FXYD proteins (FXYD1, FXYD2, and FXYD4) have been shown to strongly stabilize the αβ complexes (21) by a mechanism involving amplification of the SOPS-protein interaction. This confirmed the specific nature of the SOPS-protein interaction and suggested two possible locations for an SOPS molecule, close to the FXYD protein, but no definitive answer on the location of an SOPS binding pocket was obtained (35).
In experiments to express and purify the α2 isoform of Na,K-ATPase, we came across a serious problem of instability (21). This problem has been noted previously in attempts to express α2 in Saccharomyces cerevisiae and is the cause of a very low expression level (37). This low expression is likely to compromise experiments to analyze cardiac glycoside binding properties of α2 in comparison with α1 and α3 (38). In the P. pastoris system, expression of α2 was significantly raised by reducing the growth temperature of the yeast to 20 °C and selecting for high copy number clones. Sufficiently stable purified α2β1 protein for biochemical work was obtained by washing and eluting complexes in the presence of SOPS/cholesterol, and the α2β1 complex could be further stabilized by FXYD1 (21). Nevertheless, the α2β1 or α2β1FXYD1 complexes are still much more sensitive to thermally mediated and detergent-mediated inactivation than the α1β1 or α1β1FXYD1 complexes. Importantly, it was observed that the α2β1 complex binds SOPS less well than α1β1, and as discussed (21), this feature might be the key to explaining the increased thermally mediated and detergent-mediated inactivation of α2.
This study addresses two questions, namely the origin of instability of α2 and the location of a specific SOPS binding pocket. We have exchanged residues in trans-membrane segments of α2, which could be responsible for the weaker interaction with SOPS, with the equivalent residues in α1. The result is that a stabilized α2β1 complex and a clear-cut answer on the location of an SOPS binding pocket have been obtained.
EXPERIMENTAL PROCEDURES
Materials
Escherichia coli XL-1 blue strain was used for propagation and preparation of plasmid constructs. Yeast Lytic Enzyme from ICN Biomedicals Inc. (catalog no. 152270) was used for transformation of P. pastoris protease-deficient strain SMD1165 (his4, prb1). DDM (catalog no. D310) and C12E8 (25% w/w, catalog no. O330) were purchased from Anatrace. Synthetic SOPS (sodium salt) was obtained from Avanti Polar Lipids and stored as a chloroform solution. BD Talon metal affinity resin (catalog no. 635503) was obtained from Clontech. Cholesterol and ouabain (O3125) were obtained from Sigma. [3H]Ouabain was obtained from PerkinElmer Life Sciences. 6-Pentyl-2-pyrone (6-amyl-α-pyrone) was obtained from Sigma (W396608). All other materials were of analytical grade.
Construction of Plasmids for Expression of Human α1 and α2 and Mutated α2 and α3 with Human β1
The P. pastoris pHIL-D2 expression vectors containing cDNAs encoding human α1, α2, and α3 (Swiss-Prot accession numbers P05023, P050993, and P13637, respectively) and human His10β1 (Swiss-Prot accession number P05026) were constructed as described previously (21, 27). Site-directed mutagenesis was done on α2 by overlap extension using PCR, as described previously (39).
Expression in P. pastoris and Purification of Na,K-ATPase Isoforms and Mutants
pHIL-D2 vector, containing Na,K-ATPase genes, was linearized by NotI digestion and used to transform spheroplasts of P. pastoris SMD1165, and His+Muts transformants were selected as described previously (32). Membrane preparations were made, and clones were screened for optimal protein expression by Western blotting using the anti-KETYY antibody. Large scale growth of isoforms was done in Bellco Spinner FlasksTM in 10-liter volumes of growth medium, as described previously (21, 27). Expression of the Na,K-ATPase was induced by adding 0.5% methanol daily for 4 days or 42 hours (α2 mutants) at 20 °C. Cells were collected, washed, and broken with glass beads, and membranes were prepared as described previously (21, 34). Procedures for solubilization of membranes in DDM followed by purification of the Na,K-ATPase enzymes have been described in detail (21, 27, 33, 34). All isoform complexes were eluted from BD Talon beads in a solution containing 250 mm imidazole, 100 mm NaCl, 20 mm Tricine-HCl, pH 7.4, 0.1 mg/ml C12E8, 0.08 mg/ml SOPS, 0.01 mg/ml cholesterol, and 25% glycerol. The proteins were stored in aliquots in −80 °C. In the experiment of Fig. 4, α1β1, α2β1, and α2VFP complexes were purified and eluted with different concentrations of SOPS (from 0 to 0.1 mg/ml). Protein complexes were analyzed by SDS-PAGE, and immunoblots were probed with anti-KETYY (anti-all α isoforms).
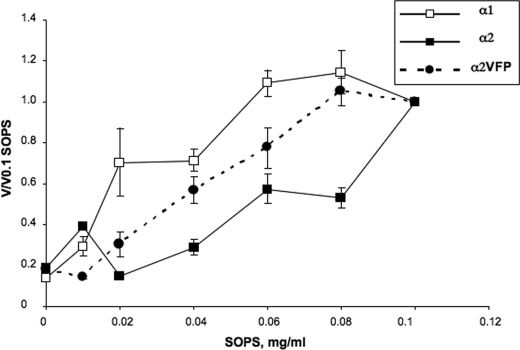
FIGURE 4.
Dependence of Na,K-ATPase activity of α1β1, α2β1, and α2VFPβ1 on SOPS concentration. α1β1, α2β1, and α2VFPβ1 isoform complexes were eluted with buffer containing different concentrations of SOPS (from 0 to 0.1 mg/ml). Complexes were diluted to 0.12 μg/μl and incubated for 17 min at 37 °C, prior to measurement of Na,K-ATPase activity. The v/v 0.1 represents the activity at each SOPS concentration after inactivation compared with the activity at 0.1 mg/ml SOPS. The data represent average ± S.E. of three separate experiments. The observed specific activities (μmol/min/mg), in the range of 0 to 0.1 mg/ml SOPS, were 0.5–8.4 for α1β1, 3.7–12.8 for α2β1, and 3.5–9.8, for α2VFPβ1.
Ouabain binding to yeast membranes using [3H]ouabain was done as described in Ref. 40. For thermal inactivation experiments, yeast membranes were incubated at 45 °C for indicated times, and aliquots of 200 μg were moved to ice-cold tubes, before measurement of ouabain binding.
Na,K-ATPase Activity of Purified Isoform Complexes and Mutants, Thermal Detergent-mediated and 6-Pentyl-2-pyrone-mediated Inactivation
Purified enzymes were diluted with elution buffer to 0.05–0.15 μg/μl and then preincubated 10 min at 30 °C. The standard reaction medium consisted of 400 μl of 130 mm NaCl, 20 mm KCl, 3 mm MgCl2, 25 mm histidine, pH 7.4, 1 mm EGTA, 1 mm ATP with added lipids 0.005 mg/ml C12E8, 0.01 mg/ml SOPS, 0.001 mg/ml cholesterol. The reaction was started by addition of enzymes (0.1–0.2 μg). Phosphate release was measured over 2, 4, and 6 min at 37 °C, using a malachite green dye to detect the phosphate (Pi Color Lock, Innova Biosciences). Na,K-ATPase activity was calculated from the slope of the time course by linear regression analysis (r >0.99). For measurements of K0.5 Na+ and K0.5 K+, the Na+ concentration was varied with 150 mm (Na+ + choline) and constant 20 mm KCl, or K+ was varied at constant 130 mm Na+, respectively (21).
For thermal inactivation experiments, the different isoform complexes were diluted with the elution buffer to equal concentrations, 0.1–0.15 mg/ml, and then incubated at 37 °C for the indicated times. Alternatively, C12E8 or 6-pentyl-2-pyrone was added to the enzymes at the indicated concentrations and incubated for 5 min at 37 °C. Aliquots of 0.02–0.1 μg were removed at the indicated times to ice-cold tubes containing the standard reaction medium (−ATP). The percent of control was calculated from specific activities obtained before and after heating or in the absence or presence of detergent.
Expression of FXYD1 in E. coli, Purification of FXYD1, and Reconstitution of αβFXYD1 Complexes
Human FXYD1 was cloned into the pET28-TevH E. coli expression vector as described previously (35). Protein expression of FXYD1 was performed in CD41 E. coli cells. Cells were grown in LB medium containing kanamycin (50 μg/ml) overnight at 37 °C and then diluted 1:1000 in to 200 ml of medium and grown overnight. The next day, the culture was diluted into a 10-liter fermentor to an A600 of 0.05 and then grown again at 37 °C to an A600 0.8. Induction was performed overnight with 0.5 mm IPTG, with gradual reduction of temperature to 20 °C, and the cell density reached an A600 of 5.6. The cells were harvested by centrifugation and washed twice with lysis buffer containing 50 mm Tricine/Tris, pH 7.4, 250 mm NaCl, 5 mm MgCl2, 1 mm EDTA, 10 μg/ml DNase, and 0.1 mm of PMSF. The cells were disrupted by three passages through a high pressure emulsifier (Emulsiflex C5, AVESTIN). The lysate was harvested at 200,000 × g for 90 min at 4 °C. The pellet was resuspended in solubilization buffer containing 50 mm Tricine/Tris, pH 7.4, 250 mm NaCl, 10% glycerol, 4 mg/ml of n-dodecyl β-maltoside (final DDM/protein ratio was 2:1) and 8 m urea, followed by homogenization (glass-Teflon) at 25 °C. The solution was diluted 2-fold with a buffer containing 50 mm Tricine/Tris, pH 7.4, 250 mm NaCl, 10% glycerol, and 20 mm imidazole and centrifuged at 200,000 × g for 90 min at 4 °C. The supernatant was incubated for 1 h with BD Talon beads (Co2+-chelate) at a cells/bead ratio of 1:4 (w/w). The beads were washed three times with a buffer containing 50 mm Tricine/Tris, pH 7.4, 250 mm NaCl, 10% glycerol, 2 mg/ml DDM, 4 m urea, and 40 mm imidazole. The FXYD1 was eluted with a 1-bead volume of buffer containing 50 mm Tricine/Tris, pH 7.4, 500 mm NaCl, 10% glycerol, 250 mm imidazole, 4 m urea, and 0.1 mg/ml C12E8. Urea was removed from purified FXYD1 by dialysis in gradual steps, using a buffer containing 50 mm Tricine/Tris, pH 7.4, 500 mm NaCl, 10% glycerol, and 3, 2, and 1 m urea, and finally a buffer without urea. After dialysis, the protein concentration was 0.15 mg/ml, and 0.1 mg/ml C12E8, 0.08 mg/ml SOPS, 0.01 mg/ml cholesterol, and glycerol to 25% were then added. The protein was separated into aliquots and stored at −80 °C. For reconstitution of αβFXYD complexes, pure FXYD1 was added to the αβ complex at a molar ratio of 2.5:1–30:1 (FXYD/ Na,K-ATPase) and incubated 4 h on ice (35).
Extraction and Purification of 6-Pentyl-2-pyrone
The fungus Trichoderma gamsii (strain NF-26) was isolated from two Mediterranean sponges Axinella polypoides and Axinella verrucosa (41). The fungus was cultured for 3 weeks in potato dextrose broth (4.8 liters), and the medium was extracted with (3× 3 liters) of ethyl acetate to yield 1.4 g of crude extract. The crude extract was separated on a Sephadex LH-20 column eluted with MeOH/CHCl3 1:1 to yield a semi-pure compound that was re-purified on the same column eluted with MeOH/CHCl3/petroleum ether 1:1:2 to afford a pure 6-pentyl-2-pyrone (447 mg, 31.5% of crude extract). The pure compound was identified by analysis of its spectroscopic data (IR, NMR, and MS). 6-Pentyl-2-pyrone is a known flavor and fragrance agent previously isolated from Trichoderma viridie, Trichoderma koningii, and Trichoderma harzianum (42).
Preparation of Rat Heart Sarcolemma Microsomes
Sarcolemma microsomes were prepared as described previously for preparation of kidney plasma membranes (43). Briefly, left ventricular rat cardiac tissue was homogenized in ISE buffer (25 mm imidazole, pH 7.3, 1 mm EDTA, and 250 mm sucrose). The homogenate was centrifuged at 3500 × g for 20 min. The supernatant from this centrifugation was centrifuged again at 6,000 × g, and the resulting supernatant was centrifuged at 48,000 × g for 1 h. The resulting pellets were homogenized in ISE buffer and represent the microsomal fraction. Microsomes were further purified by layering on a discontinuous sucrose density gradient (from 8.5 to 60%) and centrifuged for 3 h at 100,000 × g. Bands of interest were aspirated, washed, suspended in ISE buffer, and kept at −80 °C. The Na,K-ATPase-rich fraction was further purified by incubation with SDS after titration to estimate the optimum SDS concentration producing maximum stimulation of ouabain-inhibitable ATP hydrolysis (44). Following several washings, the membranes were suspended in ISE buffer and kept at −80 °C. The ouabain-inhibited Na,K-ATPase activity ranged between 50 and 100 μmol·h−1·mg−1 at 37 °C. Full details will be published elsewhere.4 Immunoblots of the sarcolemma membranes were probed with the following isoform-specific antibodies: α1 monoclonal 464.6 (6H) (GeneTex); α2 polyclonal antibody raised synthetic peptide against 432–445 amino acids, (Upstate Cell Signaling Solutions 07-674), and α3 monoclonal raised against cardiac microsomes (Santa Cruz Biotechnology, sc −58631). The blots were calibrated using purified human α1β1, α2β1, and α3β1 0.1–2 μg per lane.
Thermally Mediated and Detergent-mediated Inactivation of Na,K-ATPase Activity of Rat Heart Sarcolemma
Rat sarcolemma membranes were diluted with buffer containing 10 mm Mops/Tris, pH 7.2, and 25% glycerol to 0.34 mg/ml. Aliquot (∼0.25 μg) was diluted into 500 μl of the standard reaction medium with varying concentrations of ouabain (from 0 to 5 mm) and incubated for 30 min at 37 °C. For thermally mediated or detergent-mediated inactivation experiments, the sarcolemma membranes were diluted to 0.34 mg/ml and either incubated at 45 °C for the indicated times or 200 μm C12E8 was added, and the suspension was incubated at 37 °C for the indicated times. Aliquots of ∼0.25 μg were then added to the standard reaction medium containing 1 mm ATP and 0, 30 μm, or 5 mm of ouabain and incubated 30 min at 37 °C. Na,K-ATPase activity for α2 + α3 was calculated as (activitycontrol − activity30 μM ouabain), and activity of α1 was calculated as (activity30 μM ouabain − activity5 mM ouabain).
RESULTS
Instability of α2
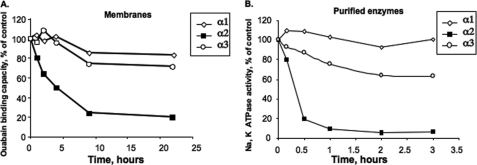
As described in the Introduction, when human α1 and α2 isoforms were expressed in P. pastoris and purified, it was found that α2 is much less stable than α1, an effect attributed to suboptimal interaction with SOPS (21). As a first step to explore this finding, we have compared the thermal inactivation of three human isoform complexes, α1β1, α2β1, and α3β1. This was done in measurements of both ouabain binding to the intact yeast membranes (Fig. 1A) and Na,K-ATPase activity of the purified isoform proteins (Fig. 1B). In both types of assay, the pattern is similar, namely the α2 isoform is thermally inactivated much more quickly compared with α3 and α1. It is also obvious that the detergent-soluble purified isoforms are all inactivated more quickly than the membrane-bound proteins. Nevertheless, the rank order of thermal inactivation, α2 ≫ α3 > α1, is the same for both membrane-bound and detergent-soluble proteins. Thus, either ouabain binding on membranes or Na,K-ATPase activity of the purified proteins can be used to assess loss of active pump units upon heating, i.e. thermal stability.
FIGURE 1.
Thermal inactivation of α1β1, α2β1, and α3β1 in P. pastoris membranes and purified isoform complexes. A, membranes expressing α1β1, α2β1, and α3β1 were heated at 45 °C for the indicated times and then ouabain binding was measured. B, purified complexes α1β1, α2β1, and α3β1 were diluted to the same concentration and heated at 37 °C for the indicated times, prior to measurement of Na,K-ATPase activity. These data represent the average of two experiments. The ouabain binding capacities in A were α1β1 25–30 pmol/mg protein, α2β1 20–25 pmol/mg protein, and α3β1 20 pmol/mg protein, and Na,K-ATPase activities in B were in the range α1β1 12–15 μmol of Pi/mg/min, α2β1 6–8 μmol of Pi/mg/min, and α3β1 ≈10 μmol of Pi/mg/min, respectively.
It is worth pointing out that the yeasts expressing α1, α2, and α3, used in Fig. 1, were all grown at 20 °C, which is optimal for expression of α2, whereas α1 is expressed optimally at 25 °C (21). It turns out that the growth temperature affects both expression levels and stability of α2 (see supplemental Fig. 1). Thus, comparisons of stability of ouabain binding to isoforms, such as in Fig. 1, must be done with membranes prepared from cells grown at the same temperature.
Design and Expression of Stabilizing Mutants of α2
Knowing that (a) the order of thermal stability is α1 > α3 ≫ α2, (b) that α2 binds SOPS less well than α1 (21), and (c) that the protein is destabilized by a displacement of bound phosphatidylserine by the detergent (34, 35), one could propose that instability of α2 is associated with suboptimal binding of SOPS to specific trans-membrane residues, leading to easy displacement of the SOPS by the detergent. The test of this hypothesis is to identify residues in trans-membrane segments that are unique to α2, mutate them to the equivalent residues in α1, singly or together, with the prediction that the changes should stabilize the altered α2 isoform. Inspection of human α1, α2, and α3 sequences reveals that there are only a few candidate residues unique to α2, including four in M8, M9, and M10, one in L9–10, and one each in M2 and M4. These are collected in Table 1, together with the mutants prepared and the names used for this paper. Apart from these residues, the trans-membrane segments of α1, α2, and α3 are fully conserved, except for a few differences that are not unique to α2 and are therefore considered irrelevant to the differential instability of α2. The mutations studied here have focused on the residues located in TM8, TM9, and TM10, as detailed in Table 1, because they are all located in a pocket, which could bind a phospholipid.5 This concept was proposed previously (21).
TABLE 1.
Mutants in trans-membrane segments of α2
Numbering for human α2 was without the initiator methionine.
| Residues unique to α2 in trans-membrane segments | Sequence location | Mutants prepared, α2→α1 | Mutant name |
|---|---|---|---|
| TM8: α2,Ala-920; α1/α3Val | α1 FVSIVVV | A920V | α2V |
| α2 FASIVVV | |||
| α3 FVSIVVV | |||
| TM9: α2,Leu-955; α1/α3,Phe | α1 LFEET | L955F | α2F |
| α2 LLEET | A920V,L955F | α2VF | |
| α3 LFEET | |||
| TM10:α2,Val-981; α1/α3, Pro | α1 KPTWWF | V981P | α2P |
| α2 KVTWWF | A920V,L955F,V981P | α2VFP | |
| TM10:α2,Ile-994; α1/α3, Val | α3 KPSWWF | ||
| L-9–10:α2,Tyr-1006; α1,Arg:α3,Asn | V981P,I994V,Y1006R | α2PVR |
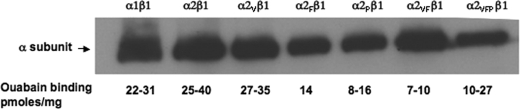
Fig. 2 presents a Western blot and ouabain binding capacities of the yeast membranes expressing the wild-type proteins α1β1, α2β1, and all the mutants α2Fβ1, α2Vβ1,α2Pβ1, α2VFβ1, and α2VFPβ1. The triple mutant α2PVRβ1 was expressed only at very low levels and was not studied further. The wild-type α1β1, α2β1, and all the mutants were purified as described under “Experimental Procedures” and our recent papers (21, 27). A representative purification of α1β1, α2β1, α2Fβ1, and α2VFPβ1 is shown in supplemental Fig. 2, and purification of the other mutants α2Vβ1, α2Pβ1, and α2VFβ1 was essentially identical. The specific Na,K-ATPase activities of the different purified proteins were as follows: α1, 15–25; α2, 10–15; α2V, 10–15; α2F, 12–16; α2P, 8–9; α2VF, 16; α2VFP, 10–12 μmol/min/mg.
FIGURE 2.
Expression of α1β1, α2β1, and mutant α2β1 complexes. Top, immunoblots probed with anti-KETYY (anti-α). 2 μg of protein was loaded in each lane. Bottom, ouabain binding capacity of α1β1, α2β1, and mutant proteins.
Stability, SOPS “Affinity,” and FXYD1 Interactions of α2 Mutants
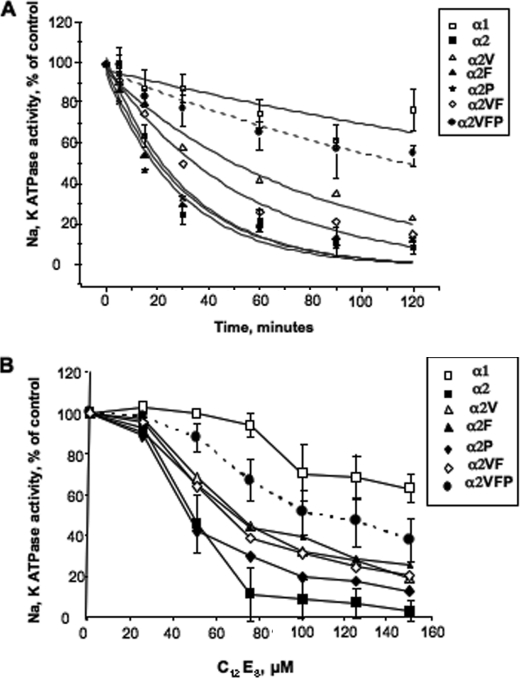
Thermal stability of the purified α1, α2 and α2F, α2V,α2P, α2VF, and α2VFP mutants was compared at 37 °C (Fig. 3A). Values of half-times calculated from fits to exponential decay curves are given in the legend. It is striking that α2 is much less stable than α1, whereas the triple mutant α2VFP was much more stable than α2 and only slightly less stable than α1. The stability of single mutant α2V and double mutant α2VF was also greater than that of α2 but lower than the α2VFP mutant, whereas the stability of the single mutants α2F and α2P was similar to that of α2. Inactivation by excess detergent, C12E8, showed essentially the same features (Fig. 3B) except that, in this case, the single mutants α2F and α2P were also a little more resistant to C12E8 than α2 itself. The most striking feature in both types of experiments is that there is a cooperative effect of the three mutations in stabilizing the α2, whereas single mutations have smaller or only minor effects (see “Discussion” and Fig. 11 for a possible interpretation).
FIGURE 3.
Thermally mediated and detergent-mediated inactivation of purified α1β1, α2β1, and mutant α2β1 complexes. A, purified complexes α1β1, α2β1, α2Vβ1, α2Fβ1, α2Pβ1, α2VFβ1, and α2VFPβ1 were diluted to the same concentration (0.1 mg/ml) and incubated at 37 °C for the indicated times prior to measurement of Na,K-ATPase activity. The data points represent the average ± S.E. of three different experiments. (For clarity of viewing, error bars are shown only for α1β1, α2β1, and α2VFPβ1.) The data were fitted to exponential decay curves, v/v0 = e−kt + c. The following half-times in minutes (±S.E.) were calculated from the fitted rate constants, k: α1, 215 ± 60; α2VFP, 127 ± 16; α2V, 54.4 ± 4.5; α2VF, 35.8 ± 3; α2F, 21.8 ± 3.5; α2P, 20.4 ± 3.5; and α2, 21.5 ± 3.7. The values of the constant c lie between 0.96 and 0.99. B, purified complexes α1β1, α2β1, α2Vβ1, α2Fβ1, α2Pβ1, α2VFβ1, and α2VFPβ1 were diluted to the same concentration (0.1 mg/ml) and incubated for 5 min at 37 °C with C12E8 added at the indicated concentration, prior to measurement of Na,K-ATPase activity. The data points represent the average ± S.E. of three different experiments. (For clarity of viewing, error bars are shown only for α1β1, α2β1 and α2VFPβ1.)
FIGURE 11.
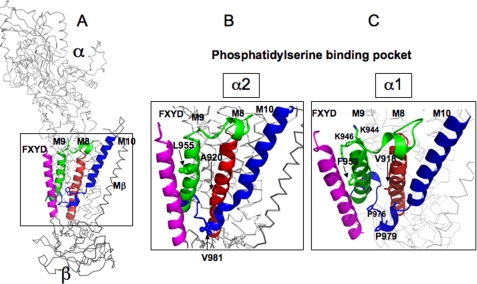
Phosphatidylserine binding pocket between M8, M9, and M10 of the α subunit. A, overview. B, detail with residues unique to α2: Leu-955, Ala-920, and Val-981. C, detail with equivalent residues of α1: Phe-953, Val-918, and Pro-979 plus other residues of interest. The models were prepared by PyMOL and are based on the structure Protein Data Bank code 3KDP (pig α1β1FXYD2) (A and C) or the structure with Phe-953, Val-918, and Pro-979 replaced by Leu-955, Ala-920, and Val-981 (B).
Instability of α2 compared with α1 is associated with a lower “apparent affinity” for the SOPS (∼4-fold) (21). Accordingly, the stabilizing effect of the α2VFP mutant could be predicted to increase the apparent affinity for SOPS compared with α2. This feature was shown clearly in a series of experiments that examined the specific Na,K-ATPase activities of several preparations of α1, α2, and α2VFP at increasing concentrations of SOPS (Fig. 4). Because the difference between α2 and α1 is itself relatively small, it was necessary to measure the effect of increasing SOPS concentrations in at least three separate experiments, with each point representing the average value of (v/v) 0.1 SOPS ± S.E. The important point is that the curve for α2VFP clearly lies above that for α2 and below that for α1. The other mutants were not examined in this type of experiment, because it would be very difficult to detect the partial effects of α2V and α2VF.
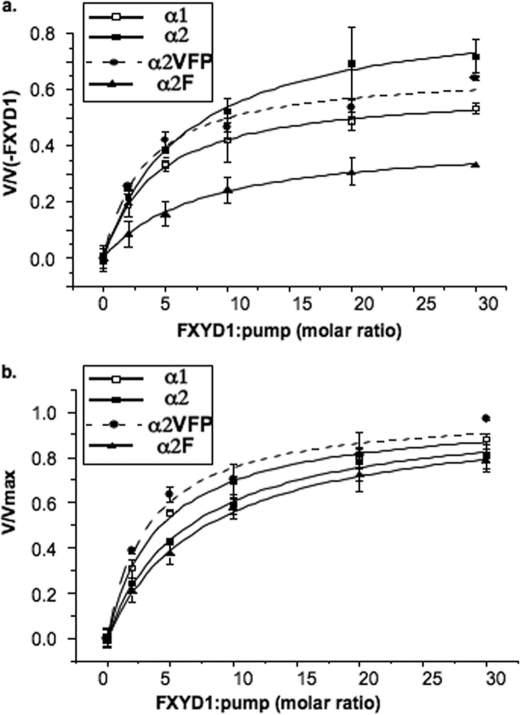
Another aspect of stabilization concerns the effects of FXYD1 on α1, α2, and the mutants (Fig. 5 and Table 2). As described recently, purified FXYD1 and other FXYD proteins, expressed in E. coli, stabilize the α1β1 complexes by amplifying the protein-SOPS interaction (35). Both α1β1 and α2β1 were also found previously to be stabilized by unpurified FXYD1 expressed in P. pastoris (21). Thus, mutants of α2 that are predicted to affect protein-SOPS interaction could also affect the protein-FXYD1 interaction. The stabilizing effect of FXYD1 was assessed by reconstituting the purified α1β1, α2β1, and α2VFPβ1 and α2Fβ1 complexes with purified FXYD1 in solution, to yield the αβFXYD1 complexes, see “Experimental Procedures.” The purified FXYD1 was added to the purified αβ complexes at increasing molar ratios, and after 4 h on ice, the complexes were incubated for 5 min at 37 °C in the presence of 500 μm C12E8, and Na,K-ATPase activity was then measured. FXYD1 protected against the C12E8, and from the hyperbolic curves in Fig. 5a the maximal degree of protection (vmax/v(−FXYD1)), and the K0.5 for FXYD1 were obtained by fitting the data as described in the legend to Table 2, and are collected together in Table 2. The normalized curves in Fig. 5b allow easy visualization of the different apparent affinities of the FXYD1. In these conditions there are two significant effects that distinguish between α1, α2, and α2VFP. First, the apparent affinity of α1 for FXYD1 is higher than for α2 and that for α2VFP is similar to α1. Second, the degree of stabilization of α2 by FXYD1 is significantly higher than for α1 and again α2VFP is closer to α1. The latter feature may arise because the initial degree of inactivation of α2 is greater than for either α1 or α2VFP, but in any case, the experiments show that the interaction of FXYD1 with α2VFP is more similar to that of α1 than α2. We have also looked at the effects of FXYD1 on the single mutant α2F because, in the molecular structures, the phenylalanine residue makes close contact with the FXYD protein, and it might be thought that it would affect the FXYD1 interaction on its own. In fact, the data of Fig. 5 show that the affinity of the α2F mutant for FXYD1 is similar or lower than that of α2, and the degree of protection by FXYD1 against the detergent is significantly lower than that of α1, α2, or the triple mutant α2VFP. In other words, the difference between α2VFP and α2 results from a collaborative effect of the three mutations, as found also in the experiments without FXYD1. The absolute thermal stability of the purified αβFXYD1 complexes, measured at 37 and 45 °C, also shows clearly that the order is α1β1FXYD1 > α2VFPβ1FXYD1 > α2β1FXYD1 (data not shown).
FIGURE 5.
Protection of α1β1, α2β1, α2VFPβ1, and α2Fβ1 by FXYD1 against detergent-mediated inactivation. α1β1, α2β1, α2VFPβ1, and α2Fβ1 isoform complexes were diluted to 0.11 μg/μl and incubated on ice for 4 h with FXYD1 at 0–30:1 molar ratio (FXYD1/Na,K-ATPase). C12E8 0.5 mm was added, and after incubation for 5 min at 37 °C, Na,K-ATPase was measured. Rates of ATP hydrolysis at different (FXYD1/Na,K-ATPase) ratios were fitted to the function v = ((Vmax·X)/(X + K0.5)) + V(−FXYD1). X-(FXYD1/Na,K-ATPase) and V(−FXYD1) is the rate in the absence of FXYD1. The upper plot represents v/v(−FXYD1) versus (FXYD1/Na,K-ATPase), and the lower plot normalized function represents v/Vmax versus (FXYD1/Na,K-ATPase).
TABLE 2.
Stabilizing effects of FXYD1 on α1β1, α2β1, α2VFPβ1, and α2Fβ1
K0.5 FXYD1 was obtained by fitting rates of ATP hydrolysis at different (FXYD1/Na,K-ATPase) ratios to the function v = ((Vmax·X)/(X + K0.5)) + V(−FXYD1). X is (FXYD1/Na,K-ATPase) and V(−FXYD1) is the rate in the absence of FXYD1. The degree of protection is calculated as the ratio Vmax/V(−FXYD1) ±S.E. S.E. was calculated as √((Vmax/V(−FXYD1)·S.E.v0)2 + S.E.Vmax2)/V(−FXYD1). The data represent the average ±S.E. of three or four separate experiments.
| α1β1 | α2β1 | α2VFPβ1 | α2Fβ1 | |
|---|---|---|---|---|
| K0.5 FXYD1 ± S.E. | 4.2 ± 0.34 | 6.5 ± 0.78 | 3.5 ± 1 | 7.8 ± 0.74 |
| (molar ratio FXYD1/αβ) | n = 4 | n = 4 (pα2:α1 = 0.035) | n = 3 | n = 3 (pα2:α1 = 0.005) |
| Vmax/V(−FXYD1) ± S.E. | 4.05 ± 0.26 | 8.81 ± 1.47 (pα2:α1 = 0.02) | 5.03 ± 0.07 | 1.79 ± 0.53 (pα2:α1 = 0.009) |
A Phospholipid Antagonist
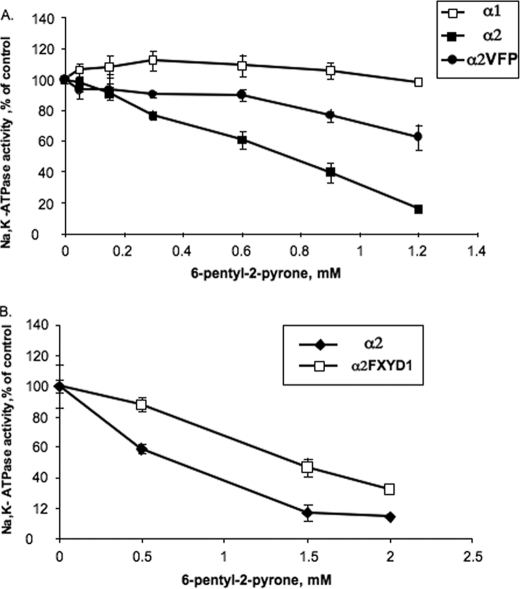
A novel way to study protein-lipid interactions and the mechanistic origin of instability of α2 is described in Figs. 6 and 7. In a search for natural compounds with selectivity for α2 compared with α1 and α3 isoforms, we have screened organic solvent extracts from marine organisms (see “Experimental Procedures”). One extract (from a fungus, Trichoderma gamsii strain NF-26) was found to selectively inactivate ouabain binding to P. pastoris membranes expressing α2 by comparison with membranes expressing α1 and α3 (Fig. 6). This extract was subjected to a series of purification steps, and eventually, the active principle was isolated and found to be a hydrophobic aromatic δ-lactone with the structure shown in Fig. 6. The compound, 6-pentyl-2-pyrone (also known as 6-amyl-α-pyrone), is well known and has been used as an artificial flavor in the food industry. The selectivity sequence for inactivating the Na,K-pump isoforms seen in Fig. 6 α2 > α3 > α1 suggested that it might act as an antagonist of the protein-SOPS interaction. To test this hypothesis, we have looked at inactivation of the purified α1β1, α2β1, and α2VFPβ1 complexes, by commercially available pure 6-pentyl-2-pyrone. The data in Fig. 7A show clearly that 6-pentyl-2-pyrone, at concentrations in the millimolar range, strongly inactivates Na,K-ATPase activity of α2β1, and α1β1 is insensitive while the triple mutant α2VFP is partially inactivated. The protection of α2VFP against this compound is similar to the effects observed for this mutant in thermally mediated and detergent-mediated inactivation experiments and dependence of activity on SOPS concentrations (seen in Figs. 3 and 4). Therefore, the conclusion is that the compound interferes with the specific SOPS-protein interaction, within the M8, M9, M10 pocket, and preferentially inactivates α2 because the SOPS-α2 interaction is weaker than the SOPS-α3 and SOPS-α1 interaction. In other experiments, it was also found that FXYD1 significantly protects against the 6-pentyl-2-pyrone (Fig. 7B). Because the FXYD1 stabilizes the SOPS in its binding pocket (35), these data also indicate that 6-pentyl-2-pyrone acts as an antagonist of the SOPS in the binding pocket.
FIGURE 6.
A, inactivation of α1β1, α2β1, and α3β1 in P. pastoris membranes by an extract from T. gamsii (strain NF-26). See the “Experimental Procedures.” B, structure of 6-pentyl-2-pyrone.
FIGURE 7.
Inactivation of purified α1β1, α2β1 α3β1, and α2VFPβ1 complexes by 6-pentyl-2-pyrone. FXYD1 protects α2 against 6-pentyl-2-pyrone. The enzymes were incubated with 6-pentyl-2-pyrone as indicated under “Experimental Procedures,” and the Na,K-ATPase activity was then measured.
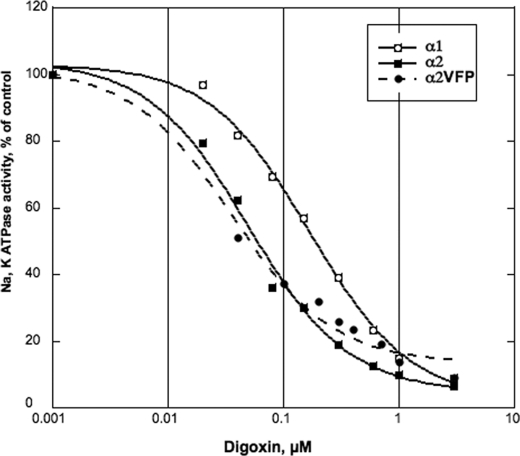
Fig. 8 and Table 3 together collect the functional properties of the α2VFP mutant in comparison with α1 and α2 and show that the triple mutant behaves almost exactly like α2. This is particularly striking in the case of inhibition of Na,K-ATPase activity by digoxin, which displays a 3–4-fold preference for α2/α1, as reported recently (27), and an almost identical preference for α2VFP/α1. Similarly, small but significant differences in K0.5 K+ and K0.5 Na+ between α2 and α1, which have also been described previously (20–22), are preserved between α2VFP and α1. In short, the large differences in stability of α2 and α2VFP, described above, are not paralleled by any detectable differences in functional properties. This is consistent with localized and highly specific effects of the mutations on phospholipid-protein interactions predicted above.
FIGURE 8.
Inhibition of Na,K-ATPase activity α1β1, α2β1, and α2VFPβ1 by digoxin. α1β1, α2β1, and α2VFPβ1 were incubated at 25 °C for 30 min for activation. The enzyme (≈0.08–0.2 μg of protein) was added to 400 μl of the reaction medium containing the following: 1 mm ATP, NaCl; 130 mm, KCl; 5 mm, MgCl2; 3 mm, histidine; 25 mm, pH 7.4, EGTA; 1 mm, SOPS 0.01 mg/ml; cholesterol 0.001 mg/ml; C12E8 0.005 mg/ml; the indicated concentrations of digoxin, and were incubated for 1 h at 37 °C. Na,K-ATPase activity was measured as described under “Experimental Procedures.”
TABLE 3.
Functional properties of α1β1, α2β1, and α2VFPβ1
K0.5 K+ and K0.5 Na+ values were obtained by measuring Na,K-ATPase activity at varying [K+] or [Na+], as described under “Experimental Procedures,” and fitting the data to the function v = V0 + Vmax·Xn/(Kn + Xn), where Kn = K0.5 K+ or K0.5 Na+. For different digoxin concentrations in Fig. 8, the percent inhibition vCG/v0 was calculated, and Ki digoxin was obtained by fitting the data to the function vCG/v0 = Ki/([CG] + Ki) + c, where CG indicates cardiac glycoside. The constant c represents a small fraction of Na,K-ATPase activity that is not inhibited (<5%).
| Isoform complex | K0.5K+ ± S.E. | K0.5Na+ ± S.E. | Ki digoxin ± S.E. |
|---|---|---|---|
| mm | mm | nm | |
| α1β1 | 1.56 ± 0.12 | 7.07 ± 0.49 | 170.3 ± 18.9 |
| α2β1 | 2.33 ± 0.28 | 9.75 ± 0.46 | 50.1 ± 6.7 |
| α2VFPβ1 | 2.46 ± 0.3 | 9.79 ± 1.44 | 36.3 ± 7.3 |
Stability of α2 in Rat Heart Membranes
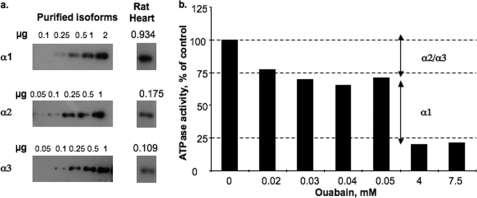
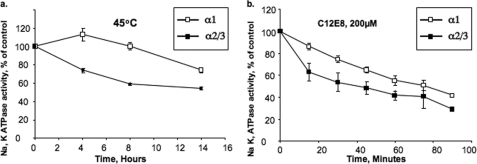
The instability of α2 expressed in the yeast membrane and in the purified α2β1 complexes raises a question whether α2 expressed in a native membrane shows the same stability characteristics. To address this question, we have utilized a partially purified preparation of Na,K-ATPase prepared from rat heart sarcolemma. The specific Na,K-ATPase activity was ∼50 μmol/mg/h. Fig. 9a assessed the isoform content of these membranes using α1-, α2-, and α3-specific antibodies in immunoblots, calibrated with known amounts of the purified human α1β1, α2β1, and α3β1 complexes. This showed that the heart enzyme contains all three isoforms in the following fractions: α1, 0.77; α2, 0.14; and α3, 0.089. In Na,K-ATPase assays, the activity of the rodent α1, with very low affinity for ouabain, can be easily distinguished from the activity of α2 and α3, which have high affinity for ouabain. Fig. 9b shows that about 30% of the ATPase activity was inhibited at ouabain concentrations, 30–50 μm, representing the activity of α2 plus α3, whereas a further 50% was inhibited at 4–7.5 mm ouabain, representing the activity of α1. Overall about 80% of the activity was inhibited at 5 mm ouabain. In the experiments of Fig. 10, the membranes were either heated at 45 °C for several hours or incubated over 90 min with 200 μm C12E8, as described under “Experimental Procedures,” and ATP hydrolysis was then measured without ouabain, with 30 μm ouabain, or with 5 mm ouabain. The activity of α2 + α3 was measured as the control activity − activity at 30 μm ouabain and that of α1 by activity at 30 μm activity − activity at 5 mm ouabain. Evidently, a significant proportion of the α2 plus α3 was inactivated more rapidly compared with α1. The differences are significant and quite reproducible, but are smaller than observed between α1 and α2 in the yeast membranes. This is not surprising because the proportion of α2/α3 is about 60:40 (Fig. 9a), and a significant fraction of the activity inhibited at 30 μm ouabain is contributed by α3, which is closer in stability to α1 than to α2 (see Fig. 1). Another possible factor is that a small percentage of α1 may also be inhibited by 30 μm ouabain, because the reported Ki value for inhibition of the all α1 rat kidney Na,K-ATPase is reported to be 100–150 μm (45). Nevertheless, the finding that a significant fraction of activity, attributed to α2 + α3, is more sensitive to thermal inactivation and detergent than activity ascribed solely to α1 provides a strong indication that the α2 in the rat heart membranes is a less stable protein than α1.
FIGURE 9.
Isoform content and Na,K-ATPase activity of α2/α3 versus α1 isoforms in rat heart sarcolemma membranes. a, immunoblots using isoform-specific antibodies of α1, α2, and α3 to determine isoform content of rat heart sarcolemma membranes. Calibration of the signals used was 0.05–2 μg of purified human α1β1, α2β1, and α3β1 complexes. The calculated amounts of the isoforms are given in micrograms. b, ATPase activity of rat heart sarcolemma membranes measured in the standard reaction medium containing 0, 30 μm, or 5 mm ouabain. Na,K-ATPase attributable to α2 + α3 and α1 were calculated from control activity − activity at 30 μm and activity at 30 μm − activity at 5 mm ouabain, respectively. The data represent one of several similar experiments.
FIGURE 10.
Thermally mediated and detergent-mediated inactivation of Na,K-ATPase isoforms in rat heart sarcolemma membranes. Rat heart sarcolemma membranes were heated at 45 °C (a) or incubated at 37 °C with 200 μm C12E8 (b) for the indicated times, and Na,K-ATPase activities of α2 + α3 or α1 isoforms were then measured as described in Fig. 9. The data represent the average ± S.E. of three separate experiments.
DISCUSSION
Binding Pocket for Phosphatidylserine, the Origin of Instability of α2
Fig. 11 highlights trans-membrane segments M8, M9, and M10 of the α subunit and the FXYD protein in the structure of Na,K-ATPase, with the detail depicting the residues in α2 (Fig. 11B), which have been mutated to the equivalent residues of α1 and other residues of interest (Fig. 11C). The mutations, α2A920V in M8, L955F in M9, and V981P at the end of M10, were predicted, singly or together, to strengthen the interaction of α2 with SOPS and to stabilize the protein. Our findings indeed provide a clear indication that 1) the SOPS binds specifically within a pocket created by M8, M9, and M10, in proximity to the FXYD protein; 2) a weak interaction of SOPS in this pocket is the source of the instability of α2, compared with α1 and α3; and 3) the triple mutation α2VFP strongly stabilizes the protein. The detailed evidence is presented below.
Evidence for the SOPS Binding Pocket
The triple mutant α2VFP is strongly protected against thermally mediated and detergent-mediated inactivation, with stability closer to α1 than to α2 (Figs. 3 and 4). Similarly, the “affinity “ of α2VFP for SOPS lies between that of α1 and α2. These features are consistent with the prediction that the residues (Ala-920, Leu-955, and Val-981) facing the lipid bilayer in M8, M9, and M10 are responsible for the weak interaction of α2 with SOPS, and instability of α2 compared with α1 (or α3). In contrast to the triple mutant, effects of the single mutants, where tested on stability, were less pronounced or even insignificant. The implication is that there are cooperative effects of the triple mutant in binding SOPS, as could be imagined for binding of the head group and two fatty acyl chains. Note in Fig. 11 that there are also two lysine residues, Lys-944 and Lys-946, at the entrance to M9 and membrane-water interface that are well placed to interact with the negatively charged phosphate and serine carboxylate of the headgroup. These conclusions do not exclude the possibility that there are additional specific phospholipid-binding sites. However, they do suggest that SOPS binding in the pocket between M8, M9, and M10 is of major importance for stabilizing the protein.
Fig. 11 also depicts a second proline residue, Pro-978, in the loop between M9 and M10, adjacent to Pro-981. An α2 P978L mutation has been reported in FHM2. The interest of this mutant is that the protein is expressed in mammalian cells at 28 °C but not at 37 °C, indicating that it is unstable and is degraded at 37 °C (47). By contrast, the many other α2 FHM2 mutants are expressed at 37 °C but inactivate pump function in various ways (30, 48, 49). The location of the P978L, and its property of destabilizing the protein, fits very well with the other evidence presented here for the specific SOPS-FXYD-α stabilizing interactions.
The finding that α2VFP shows stability properties closer to α1 but functional and inhibition properties identical to α2 (Fig. 8 and Table 3) indicates that the mutations have a strong local effect but no general effects on the protein folding. This is entirely compatible with the notion of a selective SOPS binding pocket. These features also suggest that the stabilized α2VFP mutant could become an important experimental tool, e.g. for structural work or development of α2-selective inhibitors (27).
It is a significant observation that the order of stability of the isoforms in the intact yeast membrane is the same as for the purified detergent-soluble proteins, i.e. α1 > α3 > α2 (Fig. 1). By contrast, the thermal stability of all three isoforms in the intact membrane is much higher than that of the purified proteins, due presumably to protection by the more ordered phospholipid bilayer, compared with the mobile mixed detergent-protein-lipid micelle. Thus, the relative stabilities of the isoforms, α1 > α3 > α2, are not explained by the different environments of the bilayer versus micelles. With the understanding obtained from the purified proteins, it is natural to propose that the instability of α2 in the intact membranes arises from suboptimal specific interactions with phosphatidylserine, which are easily disrupted by heat or detergents, leading to irreversible inactivation of the protein. Similarly, the lower stability of α2 compared with the α1 in the rat heart membrane (Fig. 10) might also reflect different specific phosphatidylserine-protein interactions.
Effects of FXYD1
The effects of FXYD1 seen in Fig. 5 and Table 2 fit in well with the other recently described stabilizing effects of FXYD proteins. As mentioned, FXYD1 expressed in P. pastoris was found to stabilize both α1β1 and α2β1 (21), and FXYD1, FXYD2 and FXYD4, expressed in E. coli and then purified, all stabilized α1β1 with the order FXYD1 > FXYD2 > FXYD4 (35). The mechanism of stabilization appears similar for all the FXYD proteins and was worked out in greatest detail for FXYD1. It involves amplification of the specific SOPS-protein interaction as detected by a higher affinity for SOPS, protection against thermally mediated and detergent-mediated inactivation and detergent-SOPS competition, and also protection against inactivating effects of a specific phosphatidylserine decarboxylase. The FXYD proteins were suggested to interact with both the SOPS and the α subunit to stabilize the proteins. Two possible locations of the SOPS were proposed, either between M8, M9, and M10 or between M2, M6, and M9, in proximity to the trans-membrane segment of the FXYD protein (35). In the present series of experiments, the stabilizing effects of FXYD1 were characterized by the following: (a) the lower apparent affinity for FXYD1 and higher maximal degree of stabilization for α2 compared with α1; (b) behavior of α2VFP almost identical to α1, and (c) affinity for FXYD1 of α2F similar to α2 and a lower maximal degree of stabilization. It is remarkable that the α2VFP triple mutant largely mimics α1 in the binding and stabilization properties of FXYD1, whereas the single mutant α2F binds FXYD1 like α2 and is even less stabilized. This behavior is similar to the stabilizing effects of triple versus single mutants, without the FXYD1, and strongly supports the concept that the FXYD1 interacts with the SOPS in the pocket between M8, M9, and M10, stabilizing the SOPS-protein interaction. The affinity of FXYD1 for α2 may be lower than for α1 because α2 binds SOPS less well. Previously, co-immunoprecipitation experiments suggested that FXYD1 associates less well with α2 than with α1 (21, 46). Thus, the present reconstitution experiments confirm that conclusion directly and show that the mechanism involves the phosphatidylserine-protein interaction.
Phospholipid Antagonist
The inference that the 6-pentyl-2-pyrone behaves as a “phospholipid antagonist” is based on the following observations: (a) this compound inactivates the isoforms in both the intact membrane and purified proteins with the order α2 > α3 > α1, similar to the effect of heating or excess detergent; (b) both the triple mutant α2VFP and reconstitution of the αβFXYD1 complex protect the protein against the 6-pentyl-2-pyrone, similar, again, to their effects against heating and excess detergent (Figs. 6 and 7). Because both the α2VFP triple mutant and FXYD1 stabilize the specific SOPS-protein interaction, protection against inactivation by 6-pentyl-2-pyrone shows that the 6-pentyl-2-pyrone antagonizes the SOPS binding and provides independent confirmation for the specific SOPS binding pocket. Obviously, phospholipid antagonists such as 6-pentyl-2-pyrone, or similar compounds yet to be discovered, may become useful tools to analyze specific phospholipid-protein interactions in Na,K-ATPase or other membrane proteins.
Mechanism of Thermal Inactivation of Na,K-ATPase
All the findings discussed above support the idea that thermally or detergent-mediated inactivation of either detergent-soluble or membrane-bound Na,K-ATPase is triggered by removal or displacement of specifically bound phosphatidylserine. It may be followed by irreversible disruption of protein subunit interactions. Thermal inactivation of renal Na,K-ATPase is known to be associated with irreversible changes in native topology of M8, M9, and M10, causing exposure of the cytoplasmic L8–9 loop or the C terminus of the α subunit to the extracellular surface or loss of the FXYD protein (50–52). This C-terminal region of the α subunit interacts strongly with both the β subunit and FXYD proteins (8, 9), and the α-β, α-FXYD, and β-FXYD protein-protein interactions must be crucial in maintaining the structure and folding of the proteins. The trans-membrane segment of the FXYD protein is in contact with numerous residues in αM9, and the conserved FXYDY motif interacts with both α and β subunits. Thus, one could propose that thermal inactivation involves first disruption of the SOPS-FXYD-α interaction, followed by the loss of the FXYD-αβ protein-protein contacts, namely weakening of αM9 anchoring in the membrane, destabilization of the M8-M10 topology, release of the FXYD protein, loosening of α-β links, and then general protein unfolding. In another recent example, the shark rectal gland Na,K-ATPase has been shown to be much more thermally labile than the pig kidney Na,K-ATPase, both α1β1 isoforms, and thermal inactivation is well correlated with protein unfolding (53). Because both membrane-bound and detergent-soluble proteins show this species-specific difference, it cannot be explained by a difference in lipid bilayer versus micellar environment. However, the findings do not exclude a difference between shark and renal enzymes in specific protein-phospholipid interaction. In fact, several of the residues that differ between shark and pig α1 are found in the SOPS binding pocket (data not shown).
Specific Lipid Binding Pockets in Other Pumps
The evidence for a specific binding pocket for SOPS on Na,K-ATPase fits well with observations of specifically bound phospholipids in various membrane proteins, including other pumps (54–57). Type P4 ATPases (flippases) that transport phosphatidylserine and phosphatidylethanolamine from exoplasmic to cytoplasmic leaflet of the bilayer, by definition, must bind the phospholipid in a specific fashion (58).
Rapid Turnover and Instability of α2 in Vivo?
In addition to instability of α2, heterologously expressed in yeast, and α2 in rat heart membranes (Fig. 10), a number of other observations in the literature indicate rapid turnover and probably instability of α2 in physiological and pathophysiological situations. For example, rat skeletal muscle cells, in culture, express α1 (and β1 and β2) but not α2, whereas the native muscle expresses both α1 and α2 (and β1 and β2) (59). Similarly, it has been found that in skeletal muscles of K+-deprived rats, α2 and β2 proteins, but not α1 and β1, are strongly suppressed (60). An interesting point is that in both these situations, mRNA levels of both α1 and α2 are maintained. Thus, the α2-protein level must be regulated post-transcriptionally by either (or both) reduced synthesis and trafficking or by increased degradation. Another example, concerns mice deficient in FXYD1, which showed increased cardiac mass and ejection fraction and also lowered Na,K-ATPase activity in isolated cardiac membranes. The latter was due, apparently, to a lower catalytic turnover rate and also to reduced α subunit expression (61). Significantly, although the level of α1 was reduced by about 20%, α2 was reduced to a much larger degree, about 60%. Because FXYD1 strongly stabilizes both α1 and α2, the latter finding would be consistent with the notion that α2 is intrinsically less stable than α1, and therefore, deletion of FXYD1 is more destabilizing for α2 than for α1 and causes α2 to be more readily degraded. A final example of a selective suppression of one isoform concerns isolated myocytes from some heart failure models, primarily rat, which show a large decrease in expression of α2 but only a small decrease in α1 and appropriately decreased α2-mediated pump current (13, 62).
In the situations just mentioned, α2 undergoes rapid turnover, compared with α1, either by decreased synthesis/trafficking or increased degradation or both mechanisms. Of course, a destabilized or unfolded protein would be consistent with increased degradation or, in other words, instability of α2 in vivo may serve to allow rapid turnover of the protein. As mentioned above, many studies have shown that α2 is intimately involved in regulation of intracellular Ca2+ homeostasis or Ca2+ transients in cardiac myocytes. Rapid turnover or instability of α2 fits very well with the other indications for a primary Ca2+ regulatory function of α2. This property should allow rapid changes in the α2 expression levels to fit the physiological situation (by as yet unknown signaling mechanisms). In contrast, the α1 isoform, which is more evenly distributed on the membrane surface, compared with α2, is the general function gene, responsible for maintenance of steady-state Na+ and K+ gradients. This role does not require the ability to undergo such rapid changes and would be best served by a more stable protein.
Supplementary Material
This work was supported in part by a grant from the Minerva Foundation (to S. J. D. K.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
Y. Mahmmoud, manuscript in preparation.
The solitary residues in M2 and M4 that are unique to α2 are not in a potential pocket and were not considered further.
- SR
- sarcoplasmic reticulum
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine
- SOPS
- 1-stearoyl-2-oleoyl-sn-glycero-3-phosphor-l-serine
- DDM
- n-dodecyl-β-maltoside.
REFERENCES
- 1. Bers D. M. (2002) Nature 415, 198–205 [DOI] [PubMed] [Google Scholar]
- 2. Bers D. M., Despa S. (2009) IUBMB Life 61, 215–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jorgensen P. L., Hakansson K. O., Karlish S. J. (2003) Annu. Rev. Physiol. 65, 817–849 [DOI] [PubMed] [Google Scholar]
- 4. Kaplan J. H. (2002) Annu. Rev. Biochem. 71, 511–535 [DOI] [PubMed] [Google Scholar]
- 5. Garty H., Karlish S. J. (2006) Annu. Rev. Physiol. 68, 431–459 [DOI] [PubMed] [Google Scholar]
- 6. Geering K. (2006) Am. J. Physiol. Renal Physiol. 290, F241–F250 [DOI] [PubMed] [Google Scholar]
- 7. Sweadner K. J., Rael E. (2000) Genomics 68, 41–56 [DOI] [PubMed] [Google Scholar]
- 8. Morth J. P., Pedersen B. P., Toustrup-Jensen M. S., Sørensen T. L., Petersen J., Andersen J. P., Vilsen B., Nissen P. (2007) Nature 450, 1043–1049 [DOI] [PubMed] [Google Scholar]
- 9. Shinoda T., Ogawa H., Cornelius F., Toyoshima C. (2009) Nature 459, 446–450 [DOI] [PubMed] [Google Scholar]
- 10. Ogawa H., Shinoda T., Cornelius F., Toyoshima C. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 13742–13747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yatime L., Laursen M., Morth J. P., Esmann M., Nissen P., Fedosova N. U. (2011) J. Struct. Biol. 174, 296–306 [DOI] [PubMed] [Google Scholar]
- 12. Blanco G., Mercer R. W. (1998) Am. J. Physiol. 275, F633–F650 [DOI] [PubMed] [Google Scholar]
- 13. Sweadner K. J., Herrera V. L., Amato S., Moellmann A., Gibbons D. K., Repke K. R. (1994) Circ. Res. 74, 669–678 [DOI] [PubMed] [Google Scholar]
- 14. Berry R. G., Despa S., Fuller W., Bers D. M., Shattock M. J. (2007) Cardiovasc. Res. 73, 92–100 [DOI] [PubMed] [Google Scholar]
- 15. Juhaszova M., Blaustein M. P. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 1800–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Despa S., Bers D. M. (2007) Am. J. Physiol. Cell Physiol. 293, C321–C327 [DOI] [PubMed] [Google Scholar]
- 17. Despa S., Wu Y., Lingrel J. B., Stefani E., Bers D. M. (2010) Biophys. J. 98, 201–202a [Google Scholar]
- 18. Swift F., Tovsrud N., Enger U. H., Sjaastad I., Sejersted O. M. (2007) Cardiovasc. Res. 75, 109–117 [DOI] [PubMed] [Google Scholar]
- 19. Swift F., Tovsrud N., Sjaastad I., Sejersted O. M., Niggli E., Egger M. (2010) Cell Calcium 48, 54–60 [DOI] [PubMed] [Google Scholar]
- 20. Crambert G., Hasler U., Beggah A. T., Yu C., Modyanov N. N., Horisberger J. D., Lelièvre L., Geering K. (2000) J. Biol. Chem. 275, 1976–1986 [DOI] [PubMed] [Google Scholar]
- 21. Lifshitz Y., Petrovich E., Haviv H., Goldshleger R., Tal D. M., Garty H., Karlish S. J. (2007) Biochemistry 46, 14937–14950 [DOI] [PubMed] [Google Scholar]
- 22. Han F., Tucker A. L., Lingrel J. B., Despa S., Bers D. M. (2009) Am. J. Physiol. Cell Physiol. 297, C699–C705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Blaustein M. P., Zhang J., Chen L., Hamilton B. P. (2006) Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R514–R523 [DOI] [PubMed] [Google Scholar]
- 24. Zhang J., Lee M. Y., Cavalli M., Chen L., Berra-Romani R., Balke C. W., Bianchi G., Ferrari P., Hamlyn J. M., Iwamoto T., Lingrel J. B., Matteson D. R., Wier W. G., Blaustein M. P. (2005) J. Physiol. 569, 243–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dostanic-Larson I., Lorenz J. N., Van Huysse J. W., Neumann J. C., Moseley A. E., Lingrel J. B. (2006) Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R524–R528 [DOI] [PubMed] [Google Scholar]
- 26. Lingrel J. B. (2010) Annu. Rev. Physiol. 72, 395–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Katz A., Lifshitz Y., Bab-Dinitz E., Kapri-Pardes E., Goldshleger R., Tal D. M., Karlish S. J. (2010) J. Biol. Chem. 285, 19582–19592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pietrobon D. (2007) Neurotherapeutics 4, 274–284 [DOI] [PubMed] [Google Scholar]
- 29. De Fusco M., Marconi R., Silvestri L., Atorino L., Rampoldi L., Morgante L., Ballabio A., Aridon P., Casari G. (2003) Nat. Genet. 33, 192–196 [DOI] [PubMed] [Google Scholar]
- 30. Segall L., Mezzetti A., Scanzano R., Gargus J. J., Purisima E., Blostein R. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 11106–11111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Leo L., Gherardini L., Barone V., De Fusco M., Pietrobon D., Pizzorusso T., Casari G. (2011) PLoS Genet. 7, e1002129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Strugatsky D., Gottschalk K. E., Goldshleger R., Bibi E., Karlish S. J. (2003) J. Biol. Chem. 278, 46064–46073 [DOI] [PubMed] [Google Scholar]
- 33. Cohen E., Goldshleger R., Shainskaya A., Tal D. M., Ebel C., le Maire M., Karlish S. J. (2005) J. Biol. Chem. 280, 16610–16618 [DOI] [PubMed] [Google Scholar]
- 34. Haviv H., Cohen E., Lifshitz Y., Tal D. M., Goldshleger R., Karlish S. J. (2007) Biochemistry 46, 12855–12867 [DOI] [PubMed] [Google Scholar]
- 35. Mishra N. K., Peleg Y., Cirri E., Belogus T., Lifshitz Y., Voelker D. R., Apell H. J., Garty H., Karlish S. J. (2011) J. Biol. Chem. 286, 9699–9712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hayashi Y., Mimura K., Matsui H., Takagi T. (1988) Prog. Clin. Biol. Res. 268A, 205–210 [PubMed] [Google Scholar]
- 37. Müller-Ehmsen J., Juvvadi P., Thompson C. B., Tumyan L., Croyle M., Lingrel J. B., Schwinger R. H., McDonough A. A., Farley R. A. (2001) Am. J. Physiol. Cell Physiol. 281, C1355–C1364 [DOI] [PubMed] [Google Scholar]
- 38. Hauck C., Potter T., Bartz M., Wittwer T., Wahlers T., Mehlhorn U., Scheiner-Bobis G., McDonough A. A., Bloch W., Schwinger R. H., Müller-Ehmsen J. (2009) Eur. J. Pharmacol. 622, 7–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. (1989) Gene 77, 51–59 [DOI] [PubMed] [Google Scholar]
- 40. Pedersen P. A., Rasmussen J. H., Jørgensen P. L. (1996) Biochemistry 35, 16085–16093 [DOI] [PubMed] [Google Scholar]
- 41. Paz Z., Komon-Zelazowaska M., Druzhinina I. S., Aveskamp M. M., Schniderman A., Aluma Y., Carmeli S., Ilan M., Yarden O. (2010) Fungal Divers 42, 17–26 [Google Scholar]
- 42. Rubio M. B., Hermosa R., Reino J. L., Collado I. G., Monte E. (2009) Fungal Genet. Biol. 46, 17–27 [DOI] [PubMed] [Google Scholar]
- 43. Klodos I., Esmann M., Post R. L. (2002) Kidney Int. 62, 2097–2100 [DOI] [PubMed] [Google Scholar]
- 44. Jorgensen P. L. (1974) Biochim. Biophys. Acta 356, 36–52 [DOI] [PubMed] [Google Scholar]
- 45. Ferrandi M., Molinari I., Barassi P., Minotti E., Bianchi G., Ferrari P. (2004) J. Biol. Chem. 279, 33306–33314 [DOI] [PubMed] [Google Scholar]
- 46. Crambert G., Fuzesi M., Garty H., Karlish S., Geering K. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 11476–11481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tavraz N. N., Dürr K. L., Koenderink J. B., Freilinger T., Bamberg E., Dichgans M., Friedrich T. (2009) Channels 3, 82–87 [DOI] [PubMed] [Google Scholar]
- 48. Morth J. P., Poulsen H., Toustrup-Jensen M. S., Schack V. R., Egebjerg J., Andersen J. P., Vilsen B., Nissen P. (2009) Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 217–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tavraz N. N., Friedrich T., Dürr K. L., Koenderink J. B., Bamberg E., Freilinger T., Dichgans M. (2008) J. Biol. Chem. 283, 31097–31106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Goldshleger R., Tal D. M., Karlish S. J. (1995) Biochemistry 34, 8668–8679 [DOI] [PubMed] [Google Scholar]
- 51. Arystarkhova E., Gibbons D. L., Sweadner K. J. (1995) J. Biol. Chem. 270, 8785–8796 [DOI] [PubMed] [Google Scholar]
- 52. Donnet C., Arystarkhova E., Sweadner K. J. (2001) J. Biol. Chem. 276, 7357–7365 [DOI] [PubMed] [Google Scholar]
- 53. Miles A. J., Wallace B. A., Esmann M. (2011) Biochim. Biophys. Acta 1808, 2573–2580 [DOI] [PubMed] [Google Scholar]
- 54. Lee A. G. (2003) Biochim. Biophys. Acta 1612, 1–40 [DOI] [PubMed] [Google Scholar]
- 55. Obara K., Miyashita N., Xu C., Toyoshima I., Sugita Y., Inesi G., Toyoshima C. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 14489–14496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Niggli V., Adunyah E. S., Carafoli E. (1981) J. Biol. Chem. 256, 8588–8592 [PubMed] [Google Scholar]
- 57. Mangialavori I., Giraldo A. M., Buslje C. M., Gomes M. F., Caride A. J., Rossi J. P. (2009) J. Biol. Chem. 284, 4823–4828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tanaka K., Fujimura-Kamada K., Yamamoto T. (2011) J. Biochem. 149, 131–143 [DOI] [PubMed] [Google Scholar]
- 59. Sharabani-Yosef O., Bak A., Langzam L., Lui Z., Nir U., Braiman L., Sweadner K. J., Sampson S. R. (1999) J. Cell. Physiol. 180, 236–244 [DOI] [PubMed] [Google Scholar]
- 60. McDonough A. A., Thompson C. B., Youn J. H. (2002) Am. J. Physiol. Renal Physiol. 282, F967–F974 [DOI] [PubMed] [Google Scholar]
- 61. Jia L. G., Donnet C., Bogaev R. C., Blatt R. J., McKinney C. E., Day K. H., Berr S. S., Jones L. R., Moorman J. R., Sweadner K. J., Tucker A. L. (2005) Am. J. Physiol. Heart Circ. Physiol. 288, H1982–H1988 [DOI] [PubMed] [Google Scholar]
- 62. Swift F., Birkeland J. A., Tovsrud N., Enger U. H., Aronsen J. M., Louch W. E., Sjaastad I., Sejersted O. M. (2008) Cardiovasc. Res. 78, 71–78 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.