Abstract
Anergy is an important mechanism for the maintenance of peripheral tolerance and avoidance of autoimmunity. The up-regulation of E3 ubiqitin ligases, including GRAIL (gene related to anergy in lymphocytes), is a key event in the induction and preservation of anergy in T cells. However, the mechanisms of GRAIL-mediated anergy induction are still not completely understood. We examined which proteins serve as substrates for GRAIL in anergic T cells. Arp2/3-5 (actin-related protein 2/3 subunit 5) and coronin 1A were polyubiquitinated by GRAIL via Lys-48 and Lys-63 linkages. In anergic T cells and GRAIL-overexpressed T cells, the expression of Arp2/3-5 and coronin 1A was reduced. Furthermore, we demonstrated that GRAIL impaired lamellipodium formation and reduced the accumulation of F-actin at the immunological synapse. GRAIL functions via the ubiquitination and degradation of actin cytoskeleton-associated proteins, in particular Arp2/3-5 and coronin 1A. These data reveal that GRAIL regulates proteins involved in the actin cytoskeletal organization, thereby maintaining the unresponsive state of anergic T cells.
Keywords: Actin, Cytoskeleton, E3 Ubiquitin Ligase, Immunology, Ubiquitination, Arp2/3-5, GRAIL, T Cell Anergy, Coronin 1A, Immunological Synapse
Introduction
The regulation of T cell activation ensures efficient elimination of pathogens, as well as the maintenance of tolerance to self. Peripheral tolerance prevents the expansion of self-reactive T cells that escaped thymic selection, thus avoiding autoimmunity. T cell anergy is one form of peripheral tolerance that results in nonresponsiveness to antigen rechallenge following an initial partial activation; partial initial activation may result from the stimulation of T cell receptor (TCR)2 in the absence of co-stimulation or the stimulation of T cells with the calcium ionophore ionomycin (1, 2). The induction of T cell anergy is inhibited by the addition of cyclohexamide, suggesting that anergy induction requires new protein synthesis (3). Recent reports have demonstrated that the induction of E3 ubiquitin ligases, including CBL-b, Itch, Deltex-1, and GRAIL (gene related to anergy in lymphocytes), is required to induce and maintain T cell anergy (4–8). In particular, it is well known that Cbl and Cbl-b act as negative regulators of TCR or CD28 signal transduction cascade through their ability to ubiquitinate tyrosine kinases including Src family kinases such as Fyn and Lck; Syk family kinases such as ZAP-70, Syk, PKC-θ, phospholipase C-γ, and p85; and the regulatory subunit of PI3K (4, 5, 9–15).
GRAIL is a type I transmembrane E3 ligase identified as an early gene that promotes T cell anergy (8). The up-regulation of GRAIL was observed in anergic CD4 T cells after treatment with ionomycin in vitro (4). Overexpression of GRAIL in T cell hybridomas or in primary cells reduces IL-2 production as well as proliferation upon antigen stimulation. Naive T cells from GRAIL-deficient mice exhibit increased proliferation and cytokine expression upon activation compared with those from control mice and do not depend on co-stimulation for effector generation (16, 17). Moreover, GRAIL-deficient mice exhibit lymphocyte infiltration into the lung and kidney and exacerbation of experimental autoimmune encephalomyelitis, indicating an important role for GRAIL in preventing lymphoproliferative and autoimmune responses (17). Although several candidates for GRAIL targets have been reported, including membrane proteins such as CD40 ligand and cytosolic proteins such as Rho GDIs, the mechanisms of GRAIL-mediated anergy induction are still not completely understood (18–21).
T cell activation and function require a structured engagement of antigen-presenting cells. These cell contacts are characterized by prolonged contacts from stable junctions called immunological synapses (IS). Reorganization of the actin cytoskeleton plays an important role in IS formation and signaling. Treatment of T cells with the actin-destabilizing agent cytochalasin D inhibits TCR-mediated IL-2 gene transcription (22). The Arp2/3 (actin-related protein 2/3) complex has been reported to be essential for TCR-mediated cytoskeletal reorganization (23, 24), and Arp2/3 complex-mediated actin nucleation is required for the formation of an F-actin-rich lamellipod (22, 25, 26). Coronin 1A is preferentially expressed in hematopoietic cells and co-localizes with F-actin-rich membranes in activated T cells (27). Coronin 1A has been shown to bind the Arp2/3 complex and inhibit F-actin nucleation by freezing the Arp2/3 complex in its inactive conformation (28). Coronin 1A-deficient T cells exhibit reduced cytokine production, including of IL-2 and IFN-γ, and altered F-actin reorganization (29). Moreover, a nonsense mutation in coronin 1A was identified as a gene alteration associated with the Lmb3 locus, which plays a major role in modulating autoimmunity in Faslpr mice (30).
In the present study, we demonstrate that both Arp2/3 subunit 5 (Arp2/3-5), a component of the Arp2/3 complex, and coronin 1A serve as substrates for GRAIL. The expression of Arp2/3-5 and coronin 1A is reduced in anergic T cells and in T cells in which GRAIL is overexpressed. Retroviral-driven expression of Arp2/3-5 or coronin 1A in anergic ovalbumin (OVA)-specific T cells restores their proliferation upon antigen activation. The accumulation of F-actin, Arp2/3-5, and coronin 1A at the IS is decreased in anergic T cells as well as in T cells overexpressing GRAIL. Thus, our findings demonstrate that GRAIL maintains the anergic states of T cells by regulating IS formation via degradation of the actin cytoskeleton-associated proteins Arp2/3-5 and coronin 1A.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
We obtained ionomycin, polybrene, and OVA from Sigma-Aldrich, OVA peptide (OVA323–339)from TORAY Laboratory (Tokyo), lactacystin from Boston Biochem Inc., and recombinant IL-2 from Pepro Tech. We purchased antibodies (Abs) against Arp2/3-5 (C3), c-Myc (9E10), HA (F7), and GAPDH (6C5) from Santa Cruz Biotechnology, anti-Arp2/3-5 from Epitomics Inc. (Burlingame, CA), anti-coronin 1A from Everest Biotech Ltd. (Oxfordshire, UK), anti-CD28 Ab from BD Bioscience (San Jose, CA), and peroxidase-conjugated anti-rabbit IgG, anti-goat IgG, and anti-mouse IgG from DAKO-Japan (Tokyo). We obtained the pcDNA4-V5/His vector, pcDNA4-Myc/His vector, and SNARF-1 from Invitrogen and the pAcGFP1-N1 vector from Clontech Laboratories, Inc. HA-conjugated wild-type or mutated ubiquitin constructs were kind gifts from Dr. C. Akazawa at Tokyo Medical and Dental University. pAlter-MAX HA-Cbl-b was a kind gift from Dr. H. Band (University of Nebraska Medical Center).
Mice
DO11.10, OVA-specific TCR-transgenic mice were purchased from Jackson Laboratories. Seven-week-old female C57BL/6J mice were purchased from CLEA Laboratory Animal Corporation (Tokyo, Japan). The animals were maintained in specific pathogen-free conditions, and all care and use procedures were in accordance with institutional guidelines.
Cell Culture and Proliferation
DO11.10 splenocytes were cultured in complete DMEM (Invitrogen) supplemented with 0.05 mm 2-mercaptoethanol, 100 units/ml penicillin/streptomycin, and 10% FBS. Proliferative responses after 2 days of stimulation with plate-bound anti-CD3 (0.5 μg/ml) and anti-CD28 (1 μg/ml) Abs were determined by [3H]thymidine incorporation using a β-1205 counter (Pharmacia). To induce anergy in vitro, DO11.10 splenocytes incubated with 1 mg/ml OVA for 3 days were rested for 7–10 days and were then stimulated for 18 h with ionomycin (1 μg/ml) (3).
Constructs
GRAIL, Arp2/3-5, coronin 1A, RhoGDIα, RhoGDIβ, Lasp1, and RGS10 cDNAs from DO11.10 T cells in which anergy had been induced by ionomycin were amplified with following the specific PCR primers: GRAIL, 5′-CAGTGAATTCATGGGGCCGCCGCCCGGGATC-3′ and 5′-CAGTCTCGAGAGATTTAATCTCCCGAACAGCAGC-3′; Arp2/3-5, 5′-CATGGAATTCTCCGGGATGTCGAAGAACACGGTGTC-3′ and 5′-GATCGCGGCCGCCACGGTTTTCCTTGCAGTCA-3′; coronin1A, 5′-GATCGCGGCCGCCTACTTGGCCTGAACAGTCT-3′ and 5′-CAGTCTCGAGCTTGGCCTGAACAGTCTCCTC-3′; RhoGDIα, 5′-CATGGAATTCGTAAGCATGGCAGAACAGGAACCCAC-3′ and 5′-GATCGCGGCCGCGTCCTTCCACTCCTTTTTGA-3′; RhoGDIβ, 5′-CATGGGATCCATCAAGATGACGGAGAAGGATGCACA-3′ and 5′-GATCGCGGCCGTTCTGTCCAATCCTTCTTAA-3′; and RGS10, 5′-CAGTGGATCCATGTTCACCCGCGCCGTG-3′ and 5′-CAGTCTCGAGTGTGTTGTAAATTCTGGAGGCTCG-3′. SOD1 cDNA from brain was amplified with the following PCR primers: 5′-CAGTGAATTCATGGCGATGAAAGCGGTGTGC-3′ and 5′-CAGTCTCGAGCTGCGCAATCCCAATCACTCC-3′. PCR products were cloned into a pcDNA4 V5/His vector or pcDNA4 Myc/His vector. The H297N and H300N mutations in the RING domain of murine GRAIL were generated using a PCR site-directed mutagenesis kit (Stratagene, Santa Clara, CA). Deletion of the RING domain in murine GRAIL was generated using the following PCR primers: for the 5′-PCR product, CAGTGAATTCATGGGGCCGCCGCCCGGGATC and CAGTTTCGAATCTCCATCAGGGCCAATTTC; and for the 3′-PCR product, CAGTTTCGAAGTGTGACATTCTCAAAGCT and CAGTCTCGAGAGATTTAATCTCCCGAACAGCAGC. After these reactions, the DNAs were digested with BamHI and HpaI, and the fragments, which were WT-GRAIL-V5/His, H2N2-GRAIL-V5/His, ΔRF-GRAIL-V5/His, Arp2/3-5-Myc/His, coronin 1A-Myc/His, RhoGDIα-Myc/His or RhoGDIβ-Myc/His, were subcloned into a pMIG vector. After pcDNA4 WT-GRAIL-V5/His was digested with NheI and XhoI, the fragment was subcloned into pAcGFP N1 vector.
Retroviral Transductions and Proliferation of Transfected T Cells
HEK293T cells were transfected with a pMIG plasmid and pCLEco helper plasmid by calcium phosphate precipitation. Supernatants were collected 48 and 72 h later and filtered through 0.45-μm syringe filters (Millipore, MA). Activated DO11.10 CD4+T cells were resuspended in the collected supernatant (1 × 106 cells/ml) with recombinant IL-2 (50 units/ml) and polybrene (8 μg/ml) and were centrifuged at 2,500 rpm for 90 min. Transfected cells were expanded in complete DMEM with recombinant IL-2 for 48 h and were rested without IL-2. After treatment with ionomycin (0.3 μg/ml) for 18 h, the cells were stained with SNARF-1 (5 μm) for 15 min and were stimulated with plate-bound anti-CD3 and anti-CD28 Abs. Two days later, proliferation was analyzed using a FACSCalibur and the CELLQuest program (BD Biosciences).
Western Blot Analysis
The cells were washed with PBS and lysed in 1% Nonidet P-40 lysis buffer (137 mm NaCl, 1% Nonidet P-40, 10% glycerol, 20 mm Tris, pH 7.5). After incubation for 10 min on ice, lysates were centrifuged at 13,200 rpm for 15 min at 4 °C, and supernatants were collected. After adjustment of protein concentrations using the Dc protein assay (Bio-Rad), the lysates were mixed with Laemmli's buffer (1.33% SDS, 10% glycerol, 2% 2-mercaptoethanol, 0.002% bromphenol blue, 83 mm Tris, pH 6.8) and were boiled for 5 min. Lysates (10–30 μg) were subjected to 10 or 12% SDS-PAGE and immobilized on nitrocellulose membranes. The membranes were blocked with 5% milk, PBS, 0.05% Tween for 1 h at room temperature. Proteins were detected with various Abs (mostly diluted at 1:1000) and horseradish peroxidase-coupled anti-rabbit, anti-mouse, or anti-goat IgG Abs (1:1000). The proteins were visualized using an enhanced chemiluminescence Western blot detection system (Amersham Biosciences).
Ubiquitination Assay
HEK293T cells were co-transfected with V5/His-tagged GRAIL, HA-tagged ubiquitin, and Myc/His-tagged substrate-containing expression vectors. Twenty-four hours later, the cells were incubated with 0.3 μm lactacystin for 12 h. The cells were lysed using 1% Nonidet P-40 lysis buffer containing protease inhibitors (Complete protease inhibitor mixture; Roche Applied Science) and were subjected to immunoprecipitation with anti-Myc Ab. Ubiquitination of substrates was analyzed by SDS-PAGE after blotting with anti-HA Ab.
Immunofluorescence Microscopy
To investigate co-localization of GRAIL and its substrates, HEK293T cells were co-transfected by calcium phosphate precipitation with the pAcGFP1-N1 vector containing GRAIL and pcDNA4-DsRed vector containing the substrate. Twenty-four hours later, the cells were incubated with lactacystin (0.3 μm) for 12 h and were fixed with MeOH for 15 min at 4 °C. To analyze T cell-B cell conjugation, A20 cells pulsed with 1 μg/ml OVA323–339 for 2 h at 37 °C were incubated at a ratio of 1:1 with transfected GFP+DO11.10 CD4+T cells sorted on a FACS Aria cell sorter (BD Biosciences) at 37 °C for 10 min. The cells were then plated on poly-l-lysine-coated slides for 15 min. To analyze lamellipodium formation, T cells overexpressing the control or indicated constructs were settled onto anti-CD3-coated coverslips for 5 min as described previously (26). The cells were fixed with 4% paraformaldehyde for 15 min at 4 °C and washed with PBS, 0.01% Tween 20. After blocking with PBS, 1% BSA for 1 h at room temperature, the cells were incubated with either anti-Arp2/3-5 (C3) or anti-coronin 1A Ab for 18 h at 4 °C. After washing, the cells were labeled with Cy5-conjugated anti-mouse IgG or anti-goat IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) for 2 h at room temperature. The slides were mounted with ProLong Gold antifade reagent (Invitrogen) with or without DAPI. Confocal images were acquired using FV1000-D (Olympus, Tokyo, Japan).
Statistical Analysis
Statistical differences between control and treatment groups were assessed with the Student's t test.
Additional Procedures
Information on semiquantitative RT-PCR and generation of shRNA is available in the supplemental materials.
RESULTS
Reduced Expression of Arp2/3-5 and Coronin 1A
E3 ubiquitin ligases including GRAIL are up-regulated in anergized T cells and play an important role in the induction of anergy (4, 8). To determine which proteins serve as substrates for GRAIL, we used two-dimensional difference gel electrophoresis to analyze proteins that were down-regulated in T cells in which anergy had been induced by ionomycin. Down-regulated proteins were identified by MALDI-TOF-MS and the nonredundant NCBI (NCBInr) database using MASCOT software (supplemental Table S1). Proteins related to cytoskeletal reorganization were the most frequently down-regulated proteins in anergic T cells. We decided to focus on actin-related proteins Arp2/3-5 and coronin 1A. We first confirmed that the expression levels of these proteins were reduced in T cells in ionomycin-induced anergy. We stimulated splenocytes of DO11.10 mice with OVA protein for 3 days and then rested them for 7 days. Anergy was induced by the addition of ionomycin for 18 h and the proliferative response upon the addition of anti-CD3 and anti-CD28 Abs detected by the incorporation of [3H]thymidine. The proliferative response was significantly suppressed in ionomycin-treated cells, confirming that anergy was properly induced (Fig. 1A). In these anergized cells, the protein expression of Arp2/3-5 and coronin 1A was reduced (Fig. 1B, lanes 2 and 4). To address the functional involvement of Arp2/3-5 and coronin 1A in T cell anergy, we examined whether overexpression of these proteins in DO11.10 CD4+ T cells enhanced their proliferative response upon stimulation. DO11.10 CD4+ T cells were transfected with Arp2/3-5 or coronin 1A. To analyze proliferation of transfected T cells by flow cytometry, the cells were treated with ionomycin and labeled with SNARF-1, which can monitor proliferating cells through dye dilution in a similar fashion to CFSE dilution assay. The number of proliferating cells upon stimulation (GFP+ SNARF-1− cells) was increased in Arp2/3-5 or coronin 1A-overexpressing cells compared with that of control cells (Fig. 1C). We also analyzed whether an anergy-like state was displayed by knockdown of Arp2/3-5 or coronin 1A. The percentage of proliferation increase upon the restimulation with anti-CD3/anti-CD28 was decreased in Arp2/3-5 shRNA-expressing T cells (8%) and in coronin 1A shRNA-expressing T cells (3%) compared with that in control shRNA-expressing cells (13%). These results indicate that the expression of Arp2/3-5 and coronin 1A is correlated with T cell responses and is reduced in anergic T cells.
FIGURE 1.
Arp2/3-5 and coronin 1A are down-regulated in T cells anergized by ionomycin. A and B, splenocytes derived from DO11.10 mice were stimulated with OVA for 3 days and rested for 7–10 days. The rested T cells were then treated with or without ionomycin for 18 h and restimulated with plate-bound anti-CD3 and soluble anti-CD28. A, proliferation was assessed by [3H]thymidine uptake for 48 h. The mean c.p.m. of triplicate wells ± S.E. is shown (n = 9). *, p = 0.0000033 versus control. B, cells were lysed and analyzed by immunoblotting after 1-hour activation with plate-bound anti-CD3 and soluble anti-CD28. Each protein level analyzed by ImageJ software was normalized to the corresponding GAPDH level and is expressed as relative quantity to that of untreated control. C, DO11.10 CD4+ T cells were transfected with vector control (GFP alone), Arp2/3-5, or coronin 1A. Forty-eight hours later, the transfected cells were treated with ionomycin for 18 h and were labeled with SNARF-1. The cells were restimulated with plate-bound anti-CD3 and soluble anti-CD28. Forty-eight hours later, proliferation was analyzed by FACS.
GRAIL Polyubiquitinates Arp2/3-5 and Coronin 1A
We next examined whether Arp2/3-5 and coronin 1A serve as substrates for GRAIL. Myc-tagged Arp2/3-5, coronin 1A or other candidate substrate proteins were transiently co-expressed with V5-tagged GRAIL and HA-tagged ubiquitin (Ub) in HEK293T cells. Twenty-four hours after transfection, the cells were treated with the proteasome inhibitor lactacystin for 12 h, and then lysates were prepared and immunoprecipitated with an anti-Myc Ab. SDS-PAGE followed by immunoblotting with anti-HA revealed a polyubiquitinated laddering pattern of Arp2/3-5 and coronin 1A in the presence of GRAIL (Fig. 2A, lanes 6 and 10). As Rho GDP dissociation inhibitors (RhoGDI) α and β were previously reported as substrates of GRAIL, we confirmed that these two proteins were polyubiquitinated as well (Fig. 2A, lanes 8 and 4). On the other hand, Lasp1 (LIM and SH3 protein 1), RGS10 (regulator of G-protein signaling 10), and SOD1 (superoxide dismutase 1), which were identified as proteins with reduced expression in anergized T cells by the two-dimensional difference gel electrophoresis analysis, were not ubiquitinated in the presence of GRAIL (Fig. 2A, lanes 2, 12, and 14). These results indicate that Arp2/3-5 and coronin 1A are selectively polyubiquitinated by GRAIL. Histidine to asparagine substitution in the RING finger domain (H2N2) or deletion of the RING finger domain (ΔRF) of GRAIL (Fig. 2B) reportedly inactivates GRAIL. These mutant forms of GRAIL abrogated the ability of GRAIL to ubiquitinate Arp2/3-5 and coronin 1A as well as RhoGDIα and β (Fig. 2C). Recent evidence suggests that Cbl-b, which is E3 ligase as well as GRAIL, is important for induction of T cell anergy. We also analyzed whether Arp2/3-5 and coronin 1A are substrates of Cbl-b. However, Arp2/3-5 and coronin 1A are not ubiquitinated by Cbl-b (supplemental Fig. S1). These data indicate that GRAIL but not Cbl-b E3 ligase selectively ubiquitinates Arp2/3-5 and coronin 1A.
FIGURE 2.
Arp2/3-5 and coronin 1A are ubiquitinated by GRAIL. A and C, HEK293T cells were transiently transfected with the indicated constructs and were treated with lactacystin for 12 h before lysis. Ubiquitination of the indicated proteins was detected by immunoprecipitation (IP) with anti-Myc Ab, followed by anti-HA immunoblotting (IB). The membrane was stripped and reprobed with anti-Myc Ab. B, schematic structures of the WT-, H2N2-, and ΔRF-GRAIL proteins.
GRAIL Co-localizes with Arp2/3-5 and Coronin 1A
To address the interaction of Arp2/3-5 and coronin 1A with GRAIL, we examined the co-localization of these proteins. We transiently expressed GFP-tagged GRAIL together with HA-tagged ubiquitin and DsRed-tagged Arp2/3-5, coronin 1A, or RhoDGIα/β. After treatment with lactacystin, the localization of GRAIL and its substrates was analyzed by confocal microscopy. Indeed, Arp2/3-5 (Fig. 3A), coronin 1A (Fig. 3B), and RhoDGIα and β (Fig. 3, C and D) all co-localized with GRAIL. The substrates were localized together with GRAIL in contrast to the diffuse localization of GFP and substrate proteins in the cells transfected with GFP control vector and substrate proteins, indicating the co-localization of GRAIL and Arp2/3-5 or coronin 1A. These findings suggest that Arp2/3-5 and coronin 1A interact with GRAIL.
FIGURE 3.
GRAIL co-localizes with Arp2/3-5 and coronin 1A. HEK293T cells were transiently transfected with constructs expressing GFP-tagged GRAIL, DsRed-tagged substrates (Arp2/3-5, A; coronin1A, B; RhoGDIα, C, and RhoGDIβ, D), and HA-ubiquitin and were treated with lactacystin for 12 h before being fixed. Co-localization with GFP-GRAIL was analyzed by confocal microscopy.
GRAIL Ubiquitinates Arp2/3-5 and Coronin 1A via Lys-63 and Lys-48
GRAIL has been reported to form polyubiquitin chains through lysine 63, resulting in proteolysis-independent functional modulation of Rho GDIs. However, when CD151 is the substrate, polyubiquitin chains are formed through lysine 48, which leads to protein degradation (18). We therefore assessed whether GRAIL ubiquitinates Arp2/3-5 and coronin 1A through Lys-63 and Lys-48. A similar polyubiquitinated ladder pattern of Arp2/3-5 was observed in the presence of WT Ub or Ub containing a lysine to arginine substitution at residue 29 (K29R) (Fig. 4A, lanes 4 and 6). In contrast, Ub conjugation of Arp2/3-5 was barely detected in the presence of Ub containing a lysine to arginine substitution at residue 48 (K48R) or at residue 63 (K63R) (Fig. 4A, lanes 8 and 10). Similarly, Ub conjugation of coronin 1A was observed in the presence of WT or K29R Ub (Fig. 4B, lanes 4 and 6) but was much lower when K48R or K63R Ub was used (Fig. 4B, lanes 8 and 10). These data reveal that Arp2/3-5 and coronin 1A were modified by Lys-48 and Lys-63 mixed linkage ubiquitin chains. To address the effect of GRAIL on the protein levels of Arp2/3-5 and coronin 1A, we overexpressed GRAIL and its enzymatically inactive mutant, H2N2-GRAIL or ΔRF-GRAIL, in DO11.10 CD4+ T cells and determined Arp2/3-5 and coronin 1A expression by immunoblotting with specific Abs. Both Arp2/3-5 and coronin 1A were reduced when GRAIL, but not the enzymatically inactive forms of GRAIL, was overexpressed (Fig. 4C). These results indicate that GRAIL polyubiquitinates Arp2/3-5 and coronin 1A through Lys-48 and Lys-63 and eventually leads them to be degraded.
FIGURE 4.
Arp2/3-5 and coronin 1A are polyubiquitinated though Lys-48 and/or Lys-63 ubiquitin linkages and are down-regulated by catalytically active GRAIL. A and B, HEK293T cells were transiently transfected with the indicated vectors and were treated with lactacystin for 12 h before lysis. Arp2/3-5 (A) and coronin 1A (B) were immunoprecipitated (IP) with anti-Myc Ab followed by immunoblotting (IB) with anti-HA Ab. The membrane was stripped and reprobed with anti-Myc Ab. C, CD4+ T cells were transfected with vector control (GFP alone) or WT-, H2N2-, or ΔRF-GRAIL expression constructs. Forty-eight hours later, the transfected cells (GFP+ cells) were sorted using a FACS Aria cell sorter. Sorted cells were rested for 2 days and were subjected to immunoblot analysis with anti-coronin 1A or Arp2/3-5 Ab.
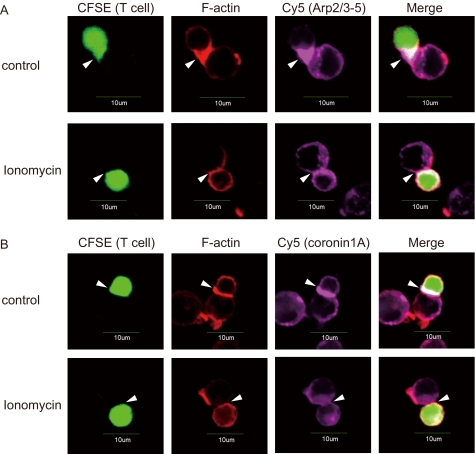
Less Arp2/3-5 and Coronin 1A Localize at the IS in Anergy
To investigate the role of Arp2/3-5 and coronin 1A in anergic T cells, we next examined the accumulation of F-actin, Arp2/3-5, and coronin 1A at the IS using confocal microscopy. As described previously, F-actin and Arp2/3-5 were recruited to the IS formed between DO11.10 CD4+ T cells and OVA323–339 peptide-pulsed A20 B cells (Fig. 5, A and B, top panels). In contrast, the accumulation of F-actin and the recruitment of Arp2/3-5 to the IS were reduced in ionomycin-treated DO11.10 CD4+ T cells compared with those in control cells (Fig. 5A, bottom panel). Similarly, the recruitment of coronin 1A to the IS in ionomycin-treated DO11.10 CD4+ T cells was reduced compared with that in nontreated DO11.10 CD4+ T cells (Fig. 5B, bottom panel). These data demonstrate that the accumulation of Arp2/3-5 and coronin 1A together with F-actin at the IS is impaired in anergic T cells.
FIGURE 5.
The accumulation of Arp2/3-5, coronin 1A, and F-actin at the IS is reduced in anergic T cells. A and B, OVA-stimulated DO11.10 splenocytes were rested for 7–10 days. Rested T cells were stained with CFSE, treated with or without ionomycin for 18 h, incubated with OVA323–339-pulsed A20 cells, and co-stained with rhodamine-phalloidin (red) to visualize F-actin and either anti-Arp2/3-5 Ab (A) or anti-coronin 1A Ab (purple) (B). The arrowheads indicate IS.
GRAIL Inhibits Arp2/3 and Coronin 1A Accumulation at the IS
To address the contribution of GRAIL to IS formation, we overexpressed GRAIL, ΔRF-GRAIL, or a control vector in DO11.10 CD4+ T cells and analyzed the accumulation of Arp2/3-5, coronin 1A, and F-actin at the IS. First, the expression of Arp2/3-5 and coronin 1A was reduced in T cells (GFP-positive cells) in which GRAIL was overexpressed compared with expression levels in control cells (Fig. 6, A and B, compare top and middle panels). The accumulation of both Arp2/3-5 and coronin 1A together with F-actin was reduced in DO11.10 CD4+ T cells overexpressing GRAIL compared with that in control vector-transfected T cells (Fig. 6, A and B, compare top and middle panels). On the other hand, the accumulation of Arp2/3-5, coronin 1A, and F-actin at the IS in DO11.10 CD4+ T cells overexpressing ΔRF-GRAIL was similar to that in controls (Fig. 6, A and B, bottom panels). We also examined whether the formation of IS occurred in ionomycin-treated T cells in which GRAIL was down-regulated by GRAIL shRNA-encoding retroviral infection. Coincident with the results for GRAIL-overexpressing experiments, both Arp2/3–5 and coronin 1A together with F-actin fully accumulated at the IS in ionomycin-treated GRAIL knockdown DO11.10 CD4+ T cells compared with that in ionomycin-treated control T cells (anergic T cells) (supplemental Fig. S2). These results indicated that GRAIL regulates the recruitment of Arp2/3-5 and coronin 1A into the IS and the subsequent accumulation of F-actin at the site of the IS.
FIGURE 6.
GRAIL inhibits the accumulation of Arp2/3-5, coronin 1A, and F-actin at the IS. A and B, DO11.10 CD4+ T cells were transfected with vector control (GFP alone) or WT- or ΔRF-GRAIL expression constructs (green). Each population was incubated with OVA323–339-pulsed A20 cells and co-stained with rhodamine-phalloidin (red) and either anti-Arp2/3-5 (A) or anti-coronin 1A (purple) (B). The arrowheads indicate IS.
GRAIL Inhibits Lamellipodium Formation
Because Arp2/3 has been reported to be essential for the formation of lamellipodia at the IS, we next examined the effect of GRAIL on lamellipodium formation. Because the spreading of T cells on anti-TCR-coated coverslips requires the formation of stable actin structures and the generation of lamellipodia, we first analyzed whether T cells could spread onto anti-CD3-coated coverslips under anergic conditions. Control DO11.10 CD4+ T cells spread onto anti-TCR-coated coverslips and formed round lamellipodial interfaces containing F-actin-rich structures (Fig. 7A). In contrast, DO11.10 CD4+ T cells in which anergy had been induced by ionomycin barely formed lamellipodia (Fig. 7A, bottom panels). We next analyzed the lamellipodium formation in CD4+ T cells overexpressing GRAIL. Lamellipodia were not efficiently formed on anti-CD3-coated coverslips when GRAIL was overexpressed in DO11.10 CD4+ T cells (Fig. 7B, middle panels). In contrast, lamellipodia were efficiently formed at the IS when a catalytically inactive mutant GRAIL (ΔRF) was overexpressed in DO11.10 CD4+ T cells (Fig. 7B, bottom panels). These data demonstrate that GRAIL inhibits lamellipodium formation at the IS.
FIGURE 7.
GRAIL inhibits lamellipodium formation during TCR stimulation. A, OVA-stimulated DO11.10 splenocytes were rested for 7–10 days and stained with CFSE. The cells were treated with or without ionomycin for 18 h. The cells were stimulated with plate-bound anti-CD3 mAb and stained with rhodamine-phalloidin (red) to visualize F-actin. B, DO11.10 CD4+ T cells were transfected with a control vector (GFP alone) or WT- or ΔRF-GRAIL expression vectors. The cells were stimulated with coated anti-CD3 mAb and stained with rhodamine-phalloidin (red). The arrowheads indicate lamellipodium formation.
DISCUSSION
In this study, we demonstrate that Arp2/3-5 and coronin 1A are down-regulated in anergic T cells as well as in T cells that overexpress GRAIL. Arp2/3-5 and coronin 1A co-localize with GRAIL and are ubiquitinated by GRAIL but not by Cbl-b via Lys-48 and Lys-63 linkage. Furthermore, the accumulation of Arp2/3-5 and coronin 1A together with F-actin is reduced at the IS in anergic T cells or in T cells that overexpress GRAIL. Coincident with the results for GRAIL-overexpressing experiments, IS formation in ionomycin-treated anergic T cells occurred by knockdown of GRAIL. Finally, we showed that overexpression of GRAIL suppresses lamellipodium formation at the IS.
CD40 ligand, CD151, CD83, and RhoGDI have been reported to be candidate substrates of GRAIL; however, the mechanism of GRAIL-mediated anergy induction is not yet fully understood (18–21). In fact, the expression of CD40 ligand was not up-regulated, and the down-regulation of CD3 was impaired in GRAIL-deficient mice. Because GRAIL is the only membrane protein among E3 ligases up-regulated in anergic T cells, it is reasonable that membrane proteins such as CD151 or CD83 are regulated by GRAIL. In this study, we confirmed that cytosolic proteins such as RhoGDIs serve as substrates for GRAIL. Furthermore, we identified Arp2/3-5 and coronin 1A as novel substrates for GRAIL. Interestingly, these proteins as well as RhoGDIs are reportedly involved in the regulation of cytoskeletal organization. Although ubiquitination of target proteins was almost completely lost when either K63R or K48R mutant ubiquitin was used, it remains unclear whether Arp2/3-5 and coronin 1A are ubiquitinated via Lys-48, Lys-63, or both sites. To address this issue, characterization of ubiquitin chain using MALDI-TOF-MS or mutants in which Lys-48 or Lys-63 is the only lysine residue that can mediate the ubiquitin chain formation will be needed for future studies
The immunological synapse is important in sustained signaling and delivery of a subset of effector cytokines by CD4+ T cells (25, 29, 31, 32). Although the precise contribution of actin cytoskeletal remodeling to T cell signaling and biologic function is not completely understood, both anergic T cells and T cells overexpressing GRAIL have been reported to form unstable immunologic synapses (4, 38). Actin nucleation in T cells is induced by the WAVE2 complex (33) and the actin-nucleation-promoting factor WASPs, which are required to promote and stabilize interactions between T cells and APC in vitro and TCR clustering on artificial surfaces. WASPs bind to actin monomers, whereas the acidic stretch associates with the Arp2/3-5 complex (23, 34), a seven-subunit complex that has intrinsic actin-nucleating activity and is essential for polarization of F-actin at the IS (25, 35). In addition, co-localization of WASPs and the Arp2/3-5 complex at the interface between anti-CD3-coated beads and Jurkat T cells suggests that these cytoskeletal components are essential for the dynamics of the actin cytoskeleton and for T cell function (24). Arp2/3-5 is essential for the formation of a stable synapse by creating lamellipodia (25). Consistent with these findings, overexpression of GRAIL reduced the protein expression of Arp2/3-5 and impaired lamellipodium formation. These results suggest that proteins related to cytoskeletal reorganization at the IS are cytosolic targets for GRAIL.
An earlier study of coronin 1A knock-out mice reported that coronin 1A has an Arp2/3-5-dependent inhibitory effect on F-actin formation and concluded that coronin 1A is indispensable for TCR signaling (27, 29). In the present study, overexpression of coronin 1A restored the proliferative response. These findings suggest that coronin 1A participates in modulating T cell signaling and thereby contributes to the maintenance of anergy. In anergic T cells and in T cells overexpressing GRAIL, F-actin accumulation at the IS was decreased, although the expression of coronin 1A was reduced in contrast to previous studies. This may be because GRAIL regulates not only coronin 1A but also the Arp2/3-5 complex as well as RhoGDIs, which are important in the regulation of the accumulation of F-actin.
Anergic T cells have been reported to exhibit initial interaction, but implementation of T cell anergy results in reduced binding of LFA-1 to its ligand ICAM-1 (4). This process is mediated through degradation of PKC-θ and phospholipase C-γ by Cbl-b. A recent report demonstrated that overexpression of GRAIL impairs LFA-1 polarization at the IS (37). Stimulation through the TCR was shown to result in WAVE2-Arp2/3-5-dependent F-actin nucleation and the formation of a complex containing WAVE2, Arp2/3-5, vinculin, and talin (33). Moreover, TCR stimulation induces integrin clustering through the recruitment of vinculin and talin (33). Therefore, our study might link the unstable immunological synapse formation and impaired LFA-1 polarization at the IS in anergic T cells. Thus, whereas Cbl-b leads to unstable immunological synapse through degradation of tyrosine kinase, GRAIL leads to the phenotype of synapse disorganization via degradation of proteins involved in the actin cytoskeletal organization. In summary, we provide evidence that GRAIL regulates cytoskeletal reorganization to keep cells unresponsive to further antigen stimulation through the ubiquitination and down-regulation of the Arp2/3-5 complex and coronin 1A.
Supplementary Material
This work was supported by Grant-in-Aid for Scientific Research B: 7210 (to S. M.) and Grant-in-Aid for Young Scientists B: 20790093 (to D. I.) from the Japan Society for the Promotion of Science, and Health and Labor Sciences Research Grants for Research on Intractable Diseases from the Ministry of Health, Labor and Welfare of Japan.

The on-line version of this article (available at http://www.jbc.org) contains supplemental text, Table S1, and Figs. S1 and S2.
- TCR
- T cell receptor
- IS
- immunological synapse(s)
- OVA
- ovalbumin
- Ab
- antibody
- Ub
- ubiquitin.
REFERENCES
- 1. Schwartz R. H. (2003) Annu. Rev. Immunol. 21, 305–334 [DOI] [PubMed] [Google Scholar]
- 2. Walker L. S., Abbas A. K. (2002) Nat. Rev. Immunol. 2, 11–19 [DOI] [PubMed] [Google Scholar]
- 3. Quill H., Schwartz R. H. (1987) J. Immunol. 138, 3704–3712 [PubMed] [Google Scholar]
- 4. Heissmeyer V., Macián F., Im S. H., Varma R., Feske S., Venuprasad K., Gu H., Liu Y. C., Dustin M. L., Rao A. (2004) Nat. Immunol. 5, 255–265 [DOI] [PubMed] [Google Scholar]
- 5. Jeon M. S., Atfield A., Venuprasad K., Krawczyk C., Sarao R., Elly C., Yang C., Arya S., Bachmaier K., Su L., Bouchard D., Jones R., Gronski M., Ohashi P., Wada T., Bloom D., Fathman C. G., Liu Y. C., Penninger J. M. (2004) Immunity 21, 167–177 [DOI] [PubMed] [Google Scholar]
- 6. Venuprasad K., Elly C., Gao M., Salek-Ardakani S., Harada Y., Luo J. L., Yang C., Croft M., Inoue K., Karin M., Liu Y. C. (2006) J. Clin. Invest. 116, 1117–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hsiao H. W., Liu W. H., Wang C. J., Lo Y. H., Wu Y. H., Jiang S. T., Lai M. Z. (2009) Immunity 31, 72–83 [DOI] [PubMed] [Google Scholar]
- 8. Anandasabapathy N., Ford G. S., Bloom D., Holness C., Paragas V., Seroogy C., Skrenta H., Hollenhorst M., Fathman C. G., Soares L. (2003) Immunity 18, 535–547 [DOI] [PubMed] [Google Scholar]
- 9. Andoniou C. E., Lill N. L., Thien C. B., Lupher M. L., Jr., Ota S., Bowtell D. D., Scaife R. M., Langdon W. Y., Band H. (2000) Mol. Cell Biol. 20, 851–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rao N., Miyake S., Reddi A. L., Douillard P., Ghosh A. K., Dodge I. L., Zhou P., Fernandes N. D., Band H. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 3794–3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lupher M. L., Jr., Rao N., Lill N. L., Andoniou C. E., Miyake S., Clark E. A., Druker B., Band H. (1998) J. Biol. Chem. 273, 35273–35281 [DOI] [PubMed] [Google Scholar]
- 12. Lupher M. L., Jr., Songyang Z., Shoelson S., Cantley L. C., Band H. (1997) J. Biol. Chem. 272, 33140–33144 [DOI] [PubMed] [Google Scholar]
- 13. Duan L., Reddi A. L., Ghosh A., Dimri M., Band H. (2004) Immunity 21, 7–17 [DOI] [PubMed] [Google Scholar]
- 14. Fang D., Liu Y. C. (2001) Nat. Immunol. 2, 870–875 [DOI] [PubMed] [Google Scholar]
- 15. Liu Y. C. (2004) Annu. Rev. Immunol. 22, 81–127 [DOI] [PubMed] [Google Scholar]
- 16. Kriegel M. A., Rathinam C., Flavell R. A. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 16770–16775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nurieva R. I., Zheng S., Jin W., Chung Y., Zhang Y., Martinez G. J., Reynolds J. M., Wang S. L., Lin X., Sun S. C., Lozano G., Dong C. (2010) Immunity 32, 670–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lineberry N. B., Su L. L., Lin J. T., Coffey G. P., Seroogy C. M., Fathman C. G. (2008) J. Immunol. 181, 1622–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lineberry N., Su L., Soares L., Fathman C. G. (2008) J. Biol. Chem. 283, 28497–28505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Su L. L., Iwai H., Lin J. T., Fathman C. G. (2009) J. Immunol. 183, 438–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Su L., Lineberry N., Huh Y., Soares L., Fathman C. G. (2006) J. Immunol. 177, 7559–7566 [DOI] [PubMed] [Google Scholar]
- 22. Holsinger L. J., Graef I. A., Swat W., Chi T., Bautista D. M., Davidson L., Lewis R. S., Alt F. W., Crabtree G. R. (1998) Curr. Biol. 8, 563–572 [DOI] [PubMed] [Google Scholar]
- 23. Machesky L. M., Insall R. H. (1998) Curr. Biol. 8, 1347–1356 [DOI] [PubMed] [Google Scholar]
- 24. Krause M., Sechi A. S., Konradt M., Monner D., Gertler F. B., Wehland J. (2000) J. Cell Biol. 149, 181–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gomez T. S., Kumar K., Medeiros R. B., Shimizu Y., Leibson P. J., Billadeau D. D. (2007) Immunity 26, 177–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nolz J. C., Gomez T. S., Zhu P., Li S., Medeiros R. B., Shimizu Y., Burkhardt J. K., Freedman B. D., Billadeau D. D. (2006) Curr. Biol. 16, 24–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Föger N., Rangell L., Danilenko D. M., Chan A. C. (2006) Science 313, 839–842 [DOI] [PubMed] [Google Scholar]
- 28. Rodal A. A., Sokolova O., Robins D. B., Daugherty K. M., Hippenmeyer S., Riezman H., Grigorieff N., Goode B. L. (2005) Nat. Struct. Mol. Biol. 12, 26–31 [DOI] [PubMed] [Google Scholar]
- 29. Mugnier B., Nal B., Verthuy C., Boyer C., Lam D., Chasson L., Nieoullon V., Chazal G., Guo X. J., He H. T., Rueff-Juy D., Alcover A., Ferrier P. (2008) PLos One. 3, e3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haraldsson M. K., Louis-Dit-Sully C. A., Lawson B. R., Sternik G., Santiago-Raber M. L., Gascoigne N. R., Theofilopoulos A. N., Kono D. H. (2008) Immunity. 28, 40–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bachmaier K., Krawczyk C., Kozieradzki I., Kong Y. Y., Sasaki T., Oliveira-dos-Santos A., Mariathasan S., Bouchard D., Wakeham A., Itie A., Le J., Ohashi P. S., Sarosi I., Nishina H., Lipkowitz S., Penninger J. M. (2000) Nature. 403, 211–216 [DOI] [PubMed] [Google Scholar]
- 32. Fang D., Wang H. Y., Fang N., Altman Y., Elly C., Liu Y. C. (2001) J. Biol. Chem. 276, 4872–4878 [DOI] [PubMed] [Google Scholar]
- 33. Nolz J. C., Medeiros R. B., Mitchell J. S., Zhu P., Freedman B. D., Shimizu Y., Billadeau D. D. (2007) Mol. Cell Biol. 27, 5986–6000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miki H., Miura K., Takenawa T. (1996) EMBO J. 15, 5326–5335 [PMC free article] [PubMed] [Google Scholar]
- 35. Goley E. D., Welch M. D. (2006) Nat. Rev. Mol. Cell Biol. 7, 713–726 [DOI] [PubMed] [Google Scholar]
- 36. Deleted in proof.
- 37. Schartner J. M., Simonson W. T., Wernimont S. A., Nettenstrom L. M., Huttenlocher A., Seroogy C. M. (2009) J. Biol. Chem. 284, 34674–34681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mueller P., Massner J., Jayachandran R., Combaluzier B., Albrecht I., Gatfield J., Blum C., Ceredig R., Rodewald H. R., Rolink A. G., Pieters J. (2008) Nat. Immunol. 9, 424–431 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.