Abstract
Upon activation the human bradykinin B2 receptor (B2R) acts as guanine nucleotide exchange factor for the G proteins Gq/11 and Gi. Thereafter, it gets phosphorylated by G protein-coupled receptor kinases (GRKs) and recruits β-arrestins, which block further G protein activation and promote B2R internalization via clathrin-coated pits. As for most G protein-coupled receptors of family A, an intracellular helix 8 after transmembrane domain 7 is also predicted for the B2R. We show here that disruption of helix 8 in the B2R by either C-terminal truncation or just by mutation of a central amino acid (Lys-315) to a helix-breaking proline resulted in strong reduction of surface expression. Interestingly, this malfunction could be overcome by the addition of the membrane-permeable B2R antagonist JSM10292, suggesting that helix 8 has a general role for conformational stabilization that can be accounted for by an appropriate antagonist. Intriguingly, an intact helix 8, but not the C terminus with its phosphorylation sites, was indispensable for receptor sequestration and for interaction of the B2R with GRK2/3 and β-arrestin2 as shown by co-immunoprecipitation. Recruitment of β-arrestin1, however, required the presence of the C terminus. Taken together, our results demonstrate that helix 8 of the B2R plays a crucial role not only in efficient trafficking to the plasma membrane or the activation of G proteins but also for the interaction of the B2R with GRK2/3 and β-arrestins. Additional data obtained with chimera of B2R with other G protein-coupled receptors of family A suggest that helix 8 might have similar functions in other GPCRs as well.
Keywords: G Protein-coupled Receptors (GPCR), Inositol Phosphates, MAP Kinases (MAPKs), Receptor Endocytosis, Receptor Serine Threonine Kinase, Arrestin, Helix 8, JSM10292, Receptor Activation
Introduction
The human bradykinin B2 receptor (B2R)2 belongs to the family A (rhodopsin/β-adrenergic-like) of G protein-coupled receptors (GPCRs). The B2R is ubiquitously expressed in many cells and tissues, and its activation results in a variety of physiological effects that range from vasodilatation and increased vascular permeability to hyperalgesia and natriuresis (1). Recent studies with B2R knock-out mice also point to a protective role of the B2R in the process of aging and in diabetes (2). After extracellular binding of its endogenous agonists, of the nona-peptide bradykinin (BK), or of kallidin (Lys-BK), the B2R undergoes conformational changes that turn it into a guanine nucleotide exchange factor for the G proteins Gq/11 and Gi, thus leading to the activation of G protein-dependent signaling cascades. Among other events, this results in phosphatidylinositol hydrolysis and activation of MAPK pathways. As reported for many GPCRs, desensitization of the B2R comes along with phosphorylation of serine/threonine residues in its C terminus by G protein-coupled receptor kinases (GRKs) or second messenger kinases as well as recruitment of β-arrestins and ends with the sequestration of the receptor into intracellular compartments (1). Upon short term stimulation the receptor gets recycled to the plasma membrane, whereas long term stimulation leads to the down-regulation of the receptor (3–5). Currently, only limited information is available on the molecular regulation of the B2R interactions with the G proteins, GRKs, or arrestins. Thus, it is not clear whether the same or different domains of the B2R convey the active state of the receptor and are involved in coupling of the various signaling proteins.
As a typical GPCR of family A, the B2R contains the NPXXY sequence at the end of helix 7 and most likely the intracellular helix 8, linked at the N terminus to the NPXXY sequence by a four-amino acid spacer and anchored at its C terminus through palmitoylated cysteines to the inner leaflet of the plasma membrane. Helix 8 was first identified in the crystal structure of bovine rhodopsin (6) and later, with the exception of CXCR4, (7) also in the other resolved structures of GPCRs (8). It lies perpendicular to the seven-transmembrane domain bundle of GPCRs and has been reported to move significantly upon receptor activation (9, 10). Therefore, helix 8 is an excellent candidate for the agonist-induced recognition of and interaction with cytosolic binding partners and has been studied extensively in several GPCRs. Studies have not only shown the involvement of helix 8 in G protein activation (11–15) but also identified hydrophobic residues in helix 8 as essential for the surface expression of the GPCRs investigated (11, 16, 17). In addition, it has been published only recently that mutation of positively charged residues in helix 8 of the thyrotropin releasing hormone receptor prevented GRK-mediated receptor phosphorylation (18).
As demonstrated by our research group, mutation of Tyr7.53 (Ballesteros/Weinstein nomenclature (19)) to an alanine in the NPXXY sequence resulted in constitutive receptor phosphorylation and internalization (20). Homology modeling indicated that Tyr7.53 in the B2R exerts an aromatic stacking interaction with the highly conserved phenylalanine Phe7.60 in helix 8. Thus, we speculated that the properties of mutant Tyr7.53 are caused by a modified interaction with GRKs and arrestins involving helix 8. Further studies using chimera of the B2R with the non-sequestering bradykinin B1 receptor also suggested that helix 8 might be involved directly or indirectly in the interaction of the B2R with GRKs and β-arrestins (21).
Consequently, we investigated in the present study not only the function of helix 8 in the B2R with regard to surface expression and G protein activation but also assessed its role in the desensitization process involving the interaction with GRKs and β-arrestins. Our results clearly demonstrate that helix 8 is involved in all of the before-mentioned receptor-dependent processes, however, to a different degree and with different requirements for the receptor microenvironment comprising helix 8.
EXPERIMENTAL PROCEDURES
Materials
Flp-InTM TREx-293 (HEK 293) cells, and Opti-MEM I serum-free medium were obtained from Invitrogen. [2,3-Prolyl-3,4-3H]bradykinin (80 Ci/mmol) and myo-2-[3H]inositol (22 Ci/mmol) were from PerkinElmer Life Sciences. Vectors harboring the genes of GRK2–6 were kindly provided by Dr. A. de Blasi (University of Rome), and a vector comprising the gene of the human β2-adrenergic receptor was provided by Dr. M. J. Lohse (University of Würzburg). All other receptor sequences were obtained from the Missouri S&T cDNA Resource Center. Bradykinin was purchased from Bachem (Weil am Rhein, Germany). The B2R antagonist JSM10292 was a generous gift from the Jerini AG (Berlin, Germany). Roche Applied Science delivered FuGENE HD, complete mini EDTA-free protease inhibitor tablets, and the rat monoclonal anti-hemagglutinin (HA)-peroxidase high affinity antibody (3F10). EcoTransfect was purchased from OzBiosciences (Marseille, France). EZview red anti-HA affinity gel was bought from Sigma. Cell culture reagents were obtained from PAA Laboratories (Cölbe, Germany). Monoclonal mouse anti-β-arrestin1 antibody and polyclonal rabbit anti-β-arrestin2 antibody were from BD Transduction Laboratories and Millipore (Billerica, MA), respectively. Rabbit polyclonal anti-GRK3 antibody (sc-563; C-14) was from Santa Cruz Biotechnology, Inc. (Heidelberg, Germany). Two sets of siRNAs from Qiagen (Hilden, Germany) were used. The first set was predesigned HP-validated FlexiTube β-arrestin siRNAs: β-arrestin1 (catalog no. SI02776921, 5′-CGACGUUCUGCAAGGUCUATT-3′) and β-arrestin2 (catalog no. SI02776928, 5′-CGAACAAGAUGACCAGGUATT-3′). The second set was published siRNAs (22): β-arrestin1 (5′-AGCCUUCUGCGCGGAGAAUTT-3) and β-arrestin2 (5′-GGACCGCAAAGUGUUUGUGTT-3′). HiPerFect transfection reagent was acquired from Qiagen. The cross-linking agent dithiobis[succinimidylpropionate] (DSP) was purchased from Pierce.
Gene Mutagenesis and Expression of Constructs
Standard PCR techniques with appropriate primers were used to generate mutants of the B2R, of GRK3, and the receptor chimeras. Coding sequences of the B2R and the respective mutants started with the third encoded methionine (23). Receptor constructs, GRK3, β-arrestin1, and β-arrestin2 genes were all cloned into the HindIII and the XhoI sites of the pcDNA5/FRT/TO vector (Invitrogen). The receptor sequences were preceded at the N terminus by a single hemagglutinin tag (MGYPYDVPDYAGS) with the last two amino acids (Gly-Ser) of the tag being generated by the insertion of a BamHI site. For the N-terminal eYFP (enhanced yellow fluorescent protein) fusion constructs, the receptors were preceded by the signal sequence of the human frizzled receptor 4 followed by the coding sequence for the eYFP and the HA tag.
Cell Culture and Transfection
For stable expression of the constructs in HEK 293 cells we used the Flp-In system from Invitrogen as described previously (24). For transient transfection cells were seeded into 24 wells (80% confluency) and transfected using 0.2 μg of DNA and 0.4 μl of EcoTransfect. Expression was induced with 0.5 μg/ml tetracycline 5–6 h after transfection and 16 h before the respective assay. For β-arrestin knockdown, monolayers (50% confluent) in 24 wells were treated with 125 ng of siRNA/1.5 μl of HiPerFect 72 h before the experiment.
Equilibrium Binding Experiments at 4 °C
The dissociation constant (Kd) was determined with [3H]BK as described previously (24). In brief, cells were incubated for 90 min on ice in incubation buffer (40 mm Pipes, 109 mm NaCl, 5 mm KCl, 0.1% glucose, 0.05% BSA, 2 mm CaCl2, 1 mm MgCl2, pH 7.4) containing increasing concentrations of up to 30 nm [3H]BK. Surface-bound [3H]BK (>95% of totally bound radioactivity) was dissociated by a 10-min incubation with 0.2 ml of an ice-cold dissociation solution (0.5 m NaCl, 0.2 m acetic acid, pH 2.7), transferred to a scintillation vial, and counted in a β-counter after the addition of scintillation fluid.
Determination of Inositol Phosphate (IP) Release
Phosphatidylinositol hydrolysis was measured as previously described (24). In brief, confluent cell monolayers (24 wells) were incubated overnight in 0.5 ml of complete medium containing 1 μCi [3H]inositol/ml. The cells were preincubated for 90 min on ice in incubation buffer supplemented with 50 mm LiCl with or without the addition of increasing concentrations of BK. Stimulation was started by placing the cells in a water bath at 37 °C. After 30 min IP accumulation was stopped by exchanging the medium for 1.5 ml of ice-cold 20 mm formic acid solution. After 1 h on ice and application of the supernatant to AG 1-X8 columns, total [3H]IP was determined as described before (24).
[3H]BK Internalization
Internalization of [3H]BK was determined as described recently (24). Cells on multiwell plates (24/48 wells) were incubated with the indicated [3H]BK concentration in incubation buffer for 60 min on ice to obtain equilibrium binding. [3H]BK internalization was started by placing the plates in a water bath at 37 °C for the indicated times and stopped by putting them back on ice. Surface-bound [3H]BK was dissociated by incubating the cells for 10 min with 0.2 m acetic acid, 0.5 m NaCl, pH 2.7. Thereafter, internalized [3H]BK was determined by lysing the cell monolayer with NaOH (0.3 m). [3H]BK in both samples was measured in a β-counter after the addition of scintillation fluid. Internalization was expressed as the amount of internalized [3H]BK in the percentage of the combined amounts of internalized and surface-bound [3H]BK.
For the determination of intrinsic receptor internalization properties (widely unaffected by high receptor expression and/or limited availability of endogenously expressed interaction partners) we used [3H]BK concentrations ≤1 nm (25). To detect endogenous factors that might limit the process (e.g. β-arrestins) we used 5 nm or higher concentrations as indicated.
Receptor Down-regulation
Monolayers (48-well) were incubated with or without 1 μm unlabeled BK in 0.5 ml of Opti-MEM I for the indicated times at 37 °C. Thereafter, plates were rinsed with ice-cold PBS and incubated on ice for 10 min with 0.2 ml of dissociation solution to remove all unlabeled extracellular ligand. The cells were washed again with ice-cold PBS, and specific binding was determined with 2 nm [3H]BK at 4 °C by subtracting nonspecific binding (determined in the presence of 5 μm unlabeled BK) from total surface binding.
Co-immunoprecipitation
Confluent monolayers of cells stably expressing HA-tagged receptor constructs on 10-cm dishes were transfected with 5 μg of plasmid harboring the respective constructs and 10 μl of EcoTransfect 36 h before co-immunoprecipitation. Cells were washed twice with PBS, warmed up to 37 °C in a water bath, and subsequently stimulated with 1 μm BK for the indicated times in 4.8 ml of PBS. The stimulation was stopped by the addition of 0.2 ml of 25 mm cross-linking agent (DSP, dissolved in DMSO) to obtain 1 mm final concentration. After incubation for 20 min at room temperature, cells were rinsed 3 times with quenching solution (50 mm Tris-HCl, pH 7.4) and solubilized in 1 ml of lysis buffer (10 mm Tris-HCl, 25 mm KCl, 150 mm NaCl, 0.1% Triton X-100, pH 7.4) including protease inhibitors for 15 min at 4 °C with gentle agitation. After centrifugation at 17000 × g for 15 min at 4 °C, 20 μl of the supernatant was mixed with an equal amount of 2× LDS sample buffer containing 0.2 m DTT and incubated for 10 min at 95 °C; the residual supernatant was added to 20 μl of EZview red anti-HA affinity gel and incubated for 1 h under gentle agitation at 4 °C. Thereafter, the anti-HA matrix was washed 3 times with ice-cold lysis buffer, then 30 μl of 1× LDS sample buffer containing 0.1 m DTT was added, and the immunocomplexes were dissociated at 95 °C for 10 min. Samples were separated on a 4–12% SDS-polyacrylamide gel and transferred to a 0.45-μm nitrocellulose membrane, which was blocked for 1 h at room temperature with milk powder dissolved in TBST (Tris-buffered saline, pH 7.5, 0.1% Tween 20). Subsequently the membrane was incubated overnight at 4 °C with anti-β-arrestin2 antibody (1:1000) or for 1 h at room temperature with anti-β-arrestin1- or anti-GRK3 antibody, each diluted 1:1000 in blocking buffer. Thereafter, the membrane was washed with TBST and incubated with HRP-linked goat anti-rabbit IgG diluted 1:2000 in blocking solution. For the recognition of the primary mouse anti-β-arrestin1 antibody, the peroxidase-labeled anti-mouse true blot secondary antibody (1:2000) (eBioscience, San Diego, CA) was used. Antibody binding was detected using ECL solution according to the instructions of the manufacturer.
Immunoblotting
Confluent monolayers in 6-well trays were washed 3 times with ice-cold PBS and solubilized in 300 μl of lysis buffer and cleared by centrifugation as described above. Aliquots of the supernatant were mixed with equal amounts of 2× LDS sample buffer (0.2 m DTT) and incubated for 10 min at 95 °C, and proteins were electrophoresed, electroblotted, and detected as described above. For deglycosylation, 5 μl of 10× deglycosylation buffer (PBS, 20 mm EDTA, 1% SDS, 5% Triton X-100, 10% 2-mercaptoethanol) was added to 45 μl of clear cell lysate and denatured at 80 °C for 10 min. After the addition of 1 unit of N-glycosidase F, the samples were incubated for 2 h at 37 °C without agitation and mixed with 1× LDS sample buffer (0.1 m DTT). HA-tagged receptors were visualized after 1 h of blocking in 5% milk powder at room temperature followed by 1 h of incubation with a monoclonal HRP-linked anti-HA high affinity antibody (1:2000) diluted in fresh blocking buffer.
Surface Biotinylation
Cells stably expressing receptor constructs were plated on 6 well and incubated for 3 h at 37 °C in Opti-MEM I containing ligand or vehicle. Subsequently, cells were washed with ice-cold PBS and incubated on ice for 30 min with a solution of 0.3 mg/ml thiol-cleavable sulfo-NHS-SS-biotin (Pierce) dissolved in PBS. After cell lysis, biotinylated receptors were precipitated and run on a SDS-PAGE as described above. Dissociation of precipitated receptors was performed in DTT-free Laemmli buffer to avoid cleavage of the biotin linker. After blotting, the receptors were detected with the Vectastain ABC Kit (Vector Laboratories, Burlingame, CA) according to the manufacturer's instructions.
ERK1/2 Phosphorylation
Cells stably expressing GRKs 2–6 were plated on 24 wells and transfected with the B2RwtH. After 12 h the culture medium was changed for DMEM containing reduced FCS (0.5%) and 0.5 μg/ml tetracycline to induce gene expression. Another 36 h later the cells were washed with ice-cold PBS and incubated on ice in 0.15-ml incubation buffer containing 1 μm BK. After 30 min, the plates were put in a 37 °C warm water bath for the indicated times. Subsequently the cells were solubilized in 0.2 ml of lysis buffer, and proteins were separated by SDS-PAGE as described above. After electroblotting and blocking with 5% milk powder in TBST, phospho-ERK1/2 and total-ERK1/2 were detected with phospho-p44/42 MAPK (E10) and a p44/42 MAP kinase (3A7) mouse monoclonal antibody (Cell Signaling, Frankfurt, Germany), respectively.
Epifluorescence Microscopy
Cells stably expressing eYFP-receptor fusion proteins were seeded on glass coverslips. The cell culture medium was changed to Opti-MEM I medium before incubation with 1 μm BK or 1 μm JSM10292. Images were taken with a Olympus IX70 fluorescence microscope.
Data Analysis
All experiments were performed at least three times in duplicate or triplicate, and results are given as the mean ± S.E. unless otherwise indicated. Data analysis was carried out using GraphPad Prism for Macintosh, Version 4.0c (GraphPad Software, Inc.).
RESULTS
Helix 8 Requirement for High B2R Cell Surface Expression
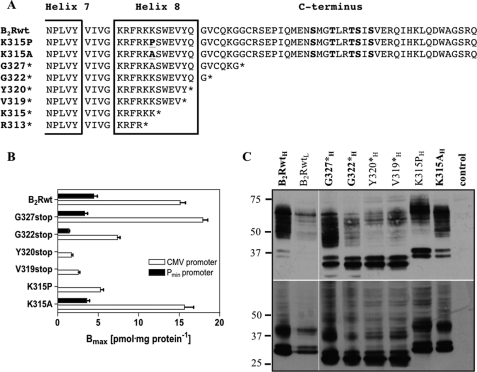
To investigate the importance of helix 8 for the function of the B2R, we generated several constructs with increased truncations of the C terminus up to the center of helix 8 (Fig. 1A). Moreover, to disturb the structure of helix 8 by a single point mutation, leaving the B2R C terminus otherwise unchanged, we mutated Lys-315, located in the center of helix 8, to proline (construct K315P), a known helix breaker (Fig. 1A). As a control that altered properties observed in K315P are not caused by Lys-315 being an important residue per se, e.g. as a target for ubiquitination or for the overall receptor structure, mutant K315A was generated as well.
FIGURE 1.
B2R constructs and their expression levels in HEK 293 cells. A, shown is a schematic representation of the C-terminal amino acid sequences of the human B2Rwt and the mutant receptor constructs. Alignment starts after the conserved NPXXY motif after transmembrane helix 7. The predicted helix 8 is framed, and the point mutations (K315P, K315A) are bold and underlined; the C-terminal serine/threonine phosphorylation sites are in bold. B, maximal numbers of binding sites were determined with 30 nm [3H]BK. Open columns, expression of constructs under the control of the CMV promoter; filled columns, expression of constructs under the weaker Pmin promoter. C, cells stably expressing HA-tagged B2Rwt and mutants (inferior H and L for high and low expression) were lysed and separated by SDS-PAGE followed by Western blotting and detection with a monoclonal HA-antibody (upper panel). Aliquots of the same lysates were incubated with 1 units of N-glycosidase F as described under “Experimental Procedures” but otherwise treated identically (lower panel). 5 μg protein was applied for constructs marked in bold, and 15 μg of protein was applied for the remaining constructs. Numbers on the left represent relative molecular masses (in kDa).
Employing the Flp-In system (Invitrogen), all N-terminally HA-tagged receptor constructs became stably integrated at an identical unique locus in the genome of the HEK 293 host cell line under the control of the strong cytomegalovirus (CMV) promoter. Construct G327* (G327stop), displayed with about 18 pmol·mg−1 protein−1 similar maximal surface receptor numbers (Bmax) as the wild-type B2R (B2Rwt) (Fig. 1B, Table 1). However, truncations of the C terminus proximal to Cys-324 resulted in progressively reduced surface expression down to 2–3 pmol·mg−1 protein−1. Lower surface receptor numbers were also observed for mutant K315P (5.3 pmol·mg−1 protein) but not for mutant K315A (∼16 pmol·mg−1 protein) (Fig. 1B; Table 1). These results demonstrate that the distal C terminus of the B2R is not required for high surface expression but that an intact helix 8 contributes to it as being reported for other GPCRs (11, 16, 17, 26). To be able to directly compare the properties of the different constructs without having to consider the potential influence of the highly different surface expression levels, we also generated cell lines, where the normally highly expressed constructs (B2Rwt, G327*, G322*, K315A) were expressed at distinctly lower levels under the control of the weaker Pmin promoter (Fig. 1B; Table 1, constructs were denoted with inferior L or H for low and high expression, respectively). These distinctly lower surface expression levels were similar to those determined for mutants, which exhibit low levels despite being under the control of the strong CMV promoter, i.e. constructs Y320*, V319*, and K315P.
TABLE 1.
[3H]BK binding data and BK-induced IP accumulation
Number of experiments are given in parentheses. ND, not determined. Significance was determined by one-way ANOVA using Dunnett's multiple comparison test. *, position of the stop codon in each truncated receptor.
| Receptor constructa | [3H]BK binding |
IP accumulation |
|||
|---|---|---|---|---|---|
| Bmaxb | Kd (4°) | Basalc | Maximal effectc | EC50d | |
| pmol/mg protein | nm | nm | |||
| B2RwtH | 15.1 ± 0.7 (6) | 2.90 ± 0.32 (19) | 2.02 ± 0.13 (10) | 12.55 ± 1.00 | 0.83 ± 0.22 (6) |
| B2RwtL | 4.5 ± 0.4 (6) | ||||
| K315PH | 5.3 ± 0.4 (6) | 6.61 ± 0.58 (5) | 1.78 ± 0.16 (4) | 8.28 ± 0.02 | 5.96 ± 0.73 (4)e |
| K315AH | 15.6 ± 1.1 (3) | 7.14 ± 1.35 (4) | 2.28 ± 0.17 (3) | 9.66 ± 1.52 | 0.28 ± 0.07 (3) |
| K315AL | 3.6 ± 0.4 (3) | ||||
| G327*H | 17.9 ± 0.7 (3) | 4.83 ± 0.84 (9) | 8.81 ± 1.60 (4) | 13.58 ± 1.22 | 0.03 ± 0.01 (5) |
| G327*L | 3.3 ± 0.4 (3) | ||||
| G322*H | 7.4 ± 0.3 (3) | 3.42 ± 0.73 (5) | ND | ND | ND |
| G322*L | 1.4 ± 0.1 (3) | ||||
| Y320*H | 1.7 ± 0.2 (6) | 2.63 ± 0.52 (9) | 1.61 ± 0.31 (6) | 11.08 ± 1.48 | 2.27 ± 0.95 (3) |
| V319*H | 2.6 ± 0.2 (6) | 2.99 ± 0.70 (7) | 1.64 ± 0.08 (7) | 7.75 ± 0.89 | 6.01 ± 1.26 (4)e |
| K315*H | 3.2 ± 0.4 (3) | 3.79 ± 0.22 (3) | 1.67 ± 0.16 (3) | 9.39 ± 1.08 | 10.63 ± 1.54 (4)e |
| R313*H | 2.7 ± 0.2 (3) | 6.51 ± 1.13 (3) | 1.78 ± 0.09 (3) | 3.98 ± 0.13 | ND |
a Receptor constructs were isogenically and stably expressed under the control of the strong CMV promoter or the weaker Pmin promoter. Expression levels are indicated with inferior H for high and L for low.
b Estimated with 30 nm [3H]BK on ice.
c Total IP accumulation after 30 min of incubation in buffer with inhibitors and 50 mm LiCl at 37 °C without (basal) and with (maximal effect) 1 μm BK, expressed as the -fold increase of initial total IP content (t = 0 min).
d Calculated from incubations in duplicates with 10−12–10−5 M BK for 30 min at 37 °C in the presence of 50 mm LiCl.
e p < 0.01.
Immunoblot analysis of the expressed constructs using the N-terminal HA tag revealed several bands between 45 and 75 kDa as well as prominent double bands below 40 kDa (Fig. 1C, upper panel). All truncations made proximal to Cys-324 (G322*, Y320*, Y319*) displayed a weaker pattern of bands between 40 and 75 kDa but very strong double bands. Pretreatment of protein lysates with N-glycosidase F resulted for all samples in the appearance of one additional major lower band and more intense double bands (Fig. 1C, lower panel), demonstrating that the different bands reflect different glycosylation states and that also the double bands represent intact receptor protein and not degradation products. These results point to a role of helix 8 in the B2R in stabilizing a proper conformation that allows for full glycosylation and efficient trafficking to the plasma membrane.
Reduced G Protein Activation of Helix 8 Mutants
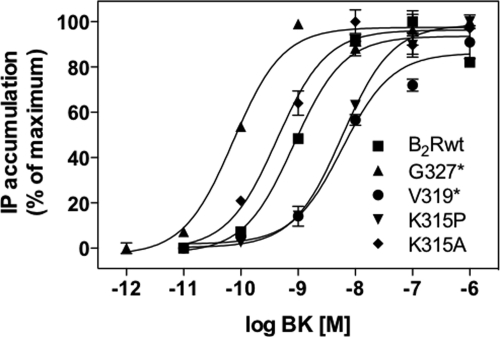
To determine the participation of helix 8 in G protein activation, we measured the accumulation of total IPs in the respective cell lines after stimulation with increasing concentrations of BK for 30 min at 37 °C. Mutant G327*, lacking the whole C terminus, exhibited an almost 30-fold lower EC50 value than B2Rwt and also elevated levels (∼2–3-fold as compared with B2Rwt) of basal IP accumulation (Fig. 2; Table 1).
FIGURE 2.
Dose-dependent IP release. HEK 293 cells stably expressing B2Rwt or the mutants were stimulated with the indicated concentrations of BK for 30 min at 37 °C. Total inositol phosphate accumulations are shown as representative sigmoidal dose-response curves, normalized for maximal response (100%).
In contrast, all mutants with a defective helix 8 (including K315P) displayed ∼3–8-fold lower G protein-coupling efficiencies, whereas the maximal responses were comparable for all constructs (Fig. 2; Table 1). Mutant K315A behaved very much like B2Rwt, demonstrating that a positive charge in this position is not required for G protein activation. Together, all mutants showed robust phosphatidylinositol hydrolysis, indicating that the overall receptor structure is largely unaffected by the mutations. But helix 8 must directly or indirectly participate in the G protein activation of the B2R as the EC50 values of the helix 8-defective mutants clearly differed from that of B2Rwt.
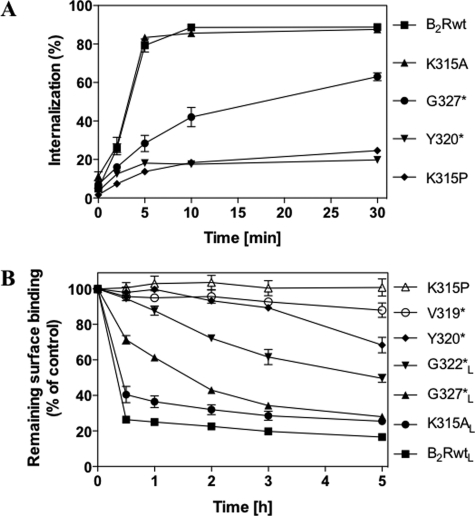
Requirement of an Intact Helix 8 for B2R-mediated [3H]BK Internalization
After stimulation with BK, the B2R becomes rapidly sequestered to compartments within the cell. To investigate the role of helix 8 for receptor sequestration, we examined the capability of the receptor constructs to internalize [3H]BK. As a consequence of the lacking C terminus, construct G327* displayed strongly reduced internalization of 3[H]BK as compared with the B2Rwt (Fig. 3A). Mutants with a disturbed helix 8 were not capable of internalizing [3H]BK at all, whereas construct K315A behaved like B2Rwt. These results show that lack of the receptor C terminus with the phosphorylation sites only reduces rapid receptor sequestration, whereas a defective helix 8 prevents it almost completely as best demonstrated with K315P, a construct still comprising the complete C terminus with all phosphorylation sites (Fig. 3A).
FIGURE 3.
[3H]BK internalization and long term down-regulation. A, HEK 293 cells stably expressing B2Rwt and the shown constructs were preincubated with 1 nm [3H]BK for 60 min on ice and subsequently warmed up to 37 °C to start internalization. At the indicated times surface-bound and internalized [3H]BK were determined as described under “Experimental Procedures.” Internalization is given as a percentage of total bound [3H]BK (surface plus internalized [3H]BK). B, HEK 293 cells expressing B2Rwt or mutants were incubated in the presence of 1 μm BK at 37 °C for the indicated times. Subsequently cells were treated with NaCl/acetic acid solution to remove all unlabeled agonist. Remaining surface binding was determined with 2 nm [3H]BK at 4 °C and is given in a percentage of control (not treated with BK). Constructs denoted with an inferior L (for low expression) were expressed under the control of the Pmin promoter.
Requirement of an Intact Helix 8 for Sequestration upon Long Term Stimulation
We next investigated how the mutations affect the outcome of long term stimulation by treating the cells with a saturating concentration of BK (1 μm) for up to 5 h at 37 °C. Remaining surface binding was determined with 2 nm 3[H]BK at 4 °C after removal of all unlabeled BK with NaCl/acetic acid solution. As receptor sequestration is considerably slowed down per se by high expression levels of constructs (25), we used the stable cell lines expressing receptor genes under the control of the weaker Pmin promoter when available. Cell surface binding was reduced by more than 80% after 30 min for the B2RwtL and remained at that level for at least 5 h (Fig. 3B). Even though receptor numbers decreased strikingly slower for G327*L than for B2RwtL, they reached a similarly low level after 5 h of BK treatment. The degree of receptor sequestration was progressively reduced with shortening the C terminus and was completely prevented in mutation V319*, indicating that an intact helix 8 is crucial for the sequestration of the B2R. This notion was strengthened by the result that mutant K315P also showed no reduction of surface binding within the 5-h time frame of stimulation with BK, whereas K315AL behaved like B2RwtL.
Epifluorescence Microscopy with N-terminally eYFP-tagged Constructs
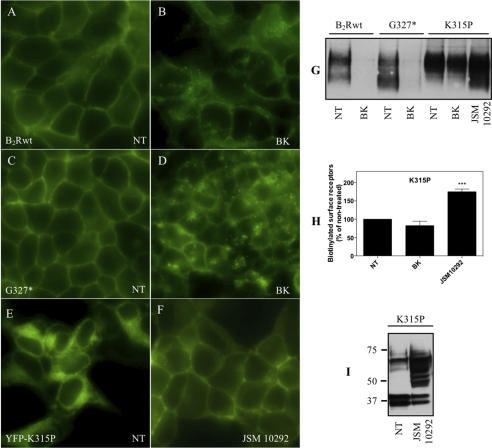
To visualize the localization of selected receptor variants without affecting the function of the C terminus, we generated fusion proteins with eYFP joined to their N terminus. To allow for proper extracellular expression, the eYFP sequence was preceded by the signal sequence of the human frizzled 4 receptor as otherwise the fusion proteins were trapped inside the cell. The integrity of the fusion constructs was confirmed by Western blot analysis (not shown). Epifluorescence microscopy revealed a strongly dispersed intracellular localization of eYFP-V319* (not shown here) and of eYFP-K315P (Fig. 4E), whereas eYFP-B2Rwt (Fig. 4A) and eYFP-G327* (Fig. 4C) were almost exclusively located at the plasma membrane. In these latter two cell lines, a strong redistribution of the receptors from the plasma membrane to intracellular vesicles after BK treatment became obvious, which proceeded more rapidly for B2Rwt (shown after 30 min, Fig. 4B) than for G327* (shown after 120 min, Fig. 4D), in accordance with the sequestration data given in Fig. 3B. The fusion constructs of V319* and K315P did not display any recognizable redistribution even upon prolonged stimulation (up to 5 h; not shown here). However, when these two constructs were treated for 3 h with the small molecule B2R antagonist JSM10292 (27),3 they were found predominantly at the cell surface (shown here only for eYFP-K315P (Fig. 4F).
FIGURE 4.
Cellular distribution of B2Rwt, G327*, and K315P. A–F, HEK 293 cells stably expressing eYFP-B2Rwt or eYFP-G327* were incubated at 37 °C in the absence (NT) or presence of 1 μm BK for 30 min (eYFP-B2Rwt) or 120 min (eYFP-G327*). Mutant eYFP-K315P was treated 3 h with the small molecule antagonist JSM10292. The eYFP fusion constructs were visualized by fluorescence microscopy. G, surface expression levels of receptor proteins were determined with a membrane-impermeable biotinylated linker before (NT) and after 3 h of treatment of BK or JSM10292 as described under “Experimental Procedures.” H, shown is quantification of biotin-reactive bands using Image J software. Receptor density is given as the mean ± S.E. of four different experiments in percentage of the non-treated control. Significance was determined by one-way ANOVA using Bonferroni's multiple comparison test. ***, p < 0.001. I, shown is a representative immunoblot of K315P (∼15 μg protein) treated without or with JSM10292 for 16 h.
These results were confirmed by cell surface biotinylation followed by immunoprecipitation. Whereas the number of biotinylated cell surface receptors was strongly reduced for B2Rwt and mutant G327* after a 3-h incubation with BK, no change was observed for mutant K315P (Fig. 4H). However, for the latter mutant a significant increase was observed upon incubation with JSM10292 for 3 h (Fig. 4, G and H). A 16-h incubation of mutant K315P with this antagonist resulted even in a change to an immunoblot pattern that resembled that of the B2Rwt (Fig. 4I). These results suggest that the said B2R antagonist can correct the trafficking to the plasma membrane, hampered by the disturbed helix 8.
Together these results confirm that rapid receptor internalization is dependent on the presence of the C terminus. Yet slower sequestration can occur in a C terminus-independent way as well. For both processes, however, an intact helix 8 is crucial.
C-terminal-independent B2R Internalization Mediated by β-Arrestin2
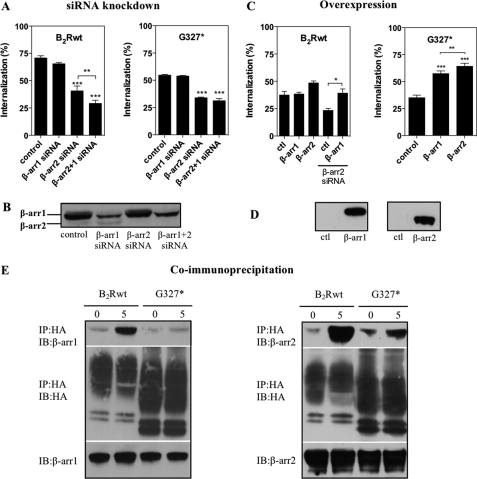
The ubiquitously expressed β-arrestins1 and -2 have been shown to target GPCRs to clathrin-coated pits through their interaction with clathrin and the adapter protein AP-2, thereby promoting receptor internalization (28, 29). Furthermore, Simaan et al. (30) have reported that in COS7 cells β-arrestin2 mediates B2R internalization. To investigate whether C-terminal-dependent and -independent receptor endocytosis have different requirements for β-arrestins, we used siRNA technology to reduce the endogenous levels of β-arrestin1 and -2 in HEK 293 cells (Fig. 5B).
FIGURE 5.
Subtype-specific role of β-arrestin2 for the internalization of B2Rwt and G327*. A, HEK 293 cells stably expressing B2Rwt or G327* were transfected with the indicated siRNAs. 72 h later internalization was determined with 5 nm [3H]BK (B2Rwt) or 1 nm [3H]BK (G327*) after 5 min (B2Rwt) or 15 min (B2Rwt) as described under “Experimental Procedures.” B, shown is a representative immunoblot of endogenous β-arrestin levels in HEK 293 cells silenced with the indicated siRNAs and probed with a monoclonal β-arrestin1 antibody recognizing both subtypes. C, HEK 293 cells stably expressing β-arrestin1 or β-arrestin2 were transfected with B2Rwt or G327*, and internalization was determined after 48 h with 5 nm [3H]BK (B2Rwt) or 1 nm [3H]BK (G327*) after 5 min (B2Rwt) or 15 min (G327*) at 37 °C. Where indicated, cells were pretreated with β-arrestin2 siRNA. D, shown is a representative immunoblot of cell lysates using ∼2 μg of protein of cells overexpressing β-arrestin1 and -2. Statistical analysis was done with one-way ANOVA using Bonferroni's multiple comparison test. ***, p < 0.001, **, p < 0.01; *, p < 0.05. ctl, control. E, HEK 293 cells stably expressing HA-tagged B2RwtH or G327*H were transiently transfected with β-arrestin1 (left) or β-arrestin2 (right) and stimulated (or not) with 1 μm BK for 5 min at 37 °C. After cross-linking with DSP and cell lysis, the protein complexes were precipitated with anti-HA-matrix. Precipitates were separated by reducing SDS-PAGE, transferred onto nitrocellulose membranes, and probed for β-arrestin1 (left) or β-arrestin2 (right) (top panels). Blots were stripped and re-probed to confirm receptor expression (middle panels), and lysates were tested for β-arrestin expression (bottom panels). IP, immunoprecipitation; IB, immunoblot.
Single knockdown of β-arrestin1 had no significant impact on B2Rwt internalization (Fig. 5A). Knockdown of β-arrestin2 reduced receptor endocytosis by 40%, which could be further significantly decreased by an additional β-arrestin1 knockdown, indicating a partly compensatory effect of β-arrestin1 in the case of diminished β-arrestin2 levels (Fig. 5A). The latter effect did not turn significant with a second set of β-arrestin siRNAs (supplemental Fig. S2A). Comparing the two different sets of siRNAs for their ability to reduce overexpressed β-arrestin1 and -2 levels, the first set proved to be much more efficient than the second (supplemental Fig. S2B). This might explain why the additional effect of β-arrestin1 was not observed with the second siRNA set, as it can apparently be observed only with a highly efficient β-arrestin2 siRNA.
Receptor internalization of stably transfected G327* in HEK 293 cells was monitored using 1 nm [3H]BK for 15 min, as higher concentrations made receptor endocytosis hardly detectable for that mutant. Only knockdown of β-arrestin2, but not of β-arrestin1, significantly reduced internalization of G327* (Fig. 5A). Simultaneous knockdown of both β-arrestin isoforms could not further lower receptor internalization, indicating a subtype-specific role of β-arrestin2 for the C terminus-independent internalization.
We next examined [3H]BK internalization of transiently transfected B2Rwt in HEK 293 cells, which stably overexpressed β-arrestin1 or β-arrestin2 (Fig. 5D). Only minor enhancing effects of β-arrestin2 but not of β-arrestin1 overexpression on [3H]BK internalization of B2Rwt were observed (Fig. 5C). However, under certain circumstances β-arrestin1 can also play a role in this process as β-arrestin1 overexpression was able to rescue internalization that had been reduced by siRNA knockdown of endogenous β-arrestin2 (Fig. 5C). In contrast, overexpression of both β-arrestin subtypes strongly enhanced ligand-induced internalization of G327*, with β-arrestin2 exhibiting a significantly larger impact (Fig. 5C).
To directly assess the interaction of β-arrestins with receptor constructs, we performed co-immunoprecipitation with the chemical cross-linker DSP using cells stably overexpressing B2RwtH or G327*H. Each cell line was transiently transfected with either β-arrestin1 or β-arrestin2. Both arrestins could be co-immunoprecipitated with the B2Rwt after 5 min of stimulation (Fig. 5E). Mutant G327* still bound β-arrestin2, but not β-arrestin1, upon BK treatment, albeit obviously to a lower extent than the B2Rwt.
These results suggest that internalization of G327* and B2Rwt is largely mediated by the action of β-arrestin2, although β-arrestin1 is able to functionally compensate in part for β-arrestin2 under certain circumstances (e.g. such as overexpression of β-arrestin1 or after β-arrestin2 knockdown). Furthermore, the C terminus of the B2R is less important for the interaction with β-arrestin2 than for coupling of β-arrestin1.
Regulation of B2R-induced ERK1/2 Activation by GRK2 and GRK3
GRKs phosphorylate almost exclusively agonist-activated receptors, and consequently there must be molecular determinants of the receptor that signal the agonist-bound status to the GRKs. We hypothesized that helix 8 might function as such a signaling determinant. Overexpression of the ubiquitously expressed GRKs 2–6 produced a different phosphorylation pattern of the B2R after stimulation (31); however, so far it was not investigated whether this also results in different signaling outcomes. Therefore, we first determined in our study how overexpression of GRKs 2–6 in HEK 293 cells (supplemental Fig. S1A) affects ERK1/2 activation by the B2R. Of all here-examined GRKs, only overexpression of GRK2 and -3 significantly reduced the ERK1/2 signal during the determination period of 30 min (supplemental Fig. S1, B and C).
Promotion of Phosphorylation-independent Internalization by GRK3
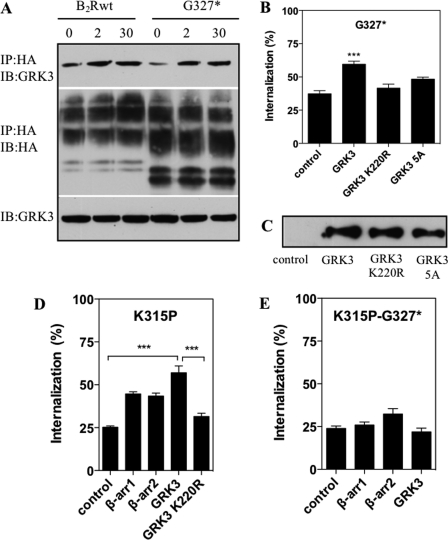
As GRK3 displayed the strongest effect, we chose this kinase to investigate the importance of helix 8 for the interaction of the B2R with GRKs. Stimulation with BK resulted after 2 min in a stable interaction of GRK3 with the B2Rwt as well as with G327*, which lasted for at least 30 min (Fig. 6A). Hence, GRK3 interaction can occur independently of the C terminus, its substrate, raising the question of whether there are other functions of GRKs besides the mere phosphorylation of the receptor C terminus. Overexpression of GRK2 or -3 resulted in minor, but significant increases in the internalization of the B2Rwt (not shown). However, also the internalization of G327* (lacking the C terminus with all the phosphorylation sites) was significantly increased by overexpression of GRK3 (Fig. 6B).
FIGURE 6.
C terminus-independent GRK interaction and rescue of K315P internalization. A, HEK 293 cells stably expressing HA-tagged B2Rwt or G327* were transiently transfected with GRK3 and stimulated (or not) with 1 μm BK for 2 or 30 min at 37 °C. After cross-linking with DSP and cell lysis, the protein complexes were precipitated with anti-HA matrix. Precipitates were separated by reducing SDS-PAGE, transferred onto nitrocellulose membranes, and probed for GRK3 (top panel). Blots were stripped and re-probed to confirm receptor expression (middle panel), and lysates were probed for GRK expression (bottom panel). IP, immunoprecipitation; IB, immunoblot. B, cells stably expressing GRK3wt, GRK3-5A, or GRK3-K220R were transiently transfected with G327*, and internalization was monitored with 1 nm [3H]BK for 15 min at 37 °C. Significance was determined by one-way ANOVA using Bonferroni's multiple comparison test. ***, p < 0.001. C, a representative immunoblot of cell lysates using ∼2 μg protein of cells (over)expressing GRK3wt, GRK3-K220R, and GRK3-5A is shown. D and E, HEK 293 cells stably (over)expressing the indicated β-arrestin subtypes or GRK3 constructs were transiently transfected with either K315P or K315P-G327*. Internalization was monitored for 15 min at 37 °C with 1 nm 3H. Significance was determined by one-way ANOVA using Bonferroni's multiple comparison test. ***, p < 0.001
It has been shown that GRKs contain consensus clathrin-binding motifs through which they are able to promote receptor internalization (32, 33). To discriminate whether clathrin binding and/or kinase activity promotes internalization of G327*, we generated a GRK3 variant with a group mutation of the clathrin binding motif to alanines (498LLDCD502 → 498AAAAA502; GRK3-5A) in addition to a kinase activity-deficient mutant GRK3-K220R (Fig. 6C). Overexpression of GRK3-5A or GRK3-K220R did not increase significantly the internalization of G327* (Fig. 6B). Thus, the capability of GRK3 to promote B2R endocytosis independently of the C-terminal phosphorylation sites still requires its kinase activity and the simultaneous presence of the clathrin binding motif.
Requirement of the Receptor C Terminus for Rescue of K315P Internalization by GRK3 and β-Arrestin2
Next, we investigated whether overexpression of GRK3 and β-arrestin1 or -2 would be able to rescue the internalization of mutant K315P, which had a disturbed helix 8 but otherwise an intact C terminus. Overexpression of GRK3 resulted in a strong increase of ligand-induced internalization of K315P more than 2-fold, whereas that of the kinase-dead mutant GRK3-K220R did not, indicating the requirement of phosphorylation for the internalization process (Fig. 6D). Internalization of K315P could also be rescued by β-arrestin overexpression independently of the subtype, albeit to a smaller extent as compared with GRK3 (Fig. 6D). In contrast, in HEK 293 cells expressing the receptor mutant K315P-G327* (which combined the defective helix 8 with the lack of the distal C terminus) neither overexpression of β-arrestins nor GRK3 could significantly re-induce internalization (Fig. 6E), suggesting that the rescue by GRK3 or β-arrestins is strongly dependent on the presence of the intact C terminus or an intact helix 8.
Requirement of a Specific Microenvironment for Proper Function of Helix 8
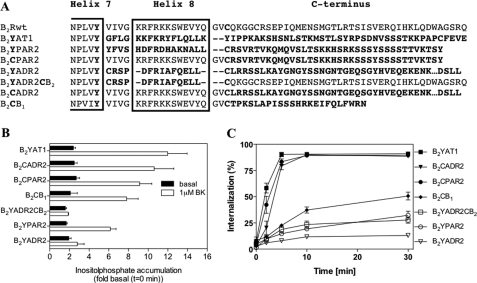
Finally, to test whether C-terminal sequences and/or helices 8 of different GPCRs of family A are functionally transferable to the B2R, we generated various chimeras of the B2R with the C termini/helices 8 of the protease-activated receptor-2, the β2-adrenergic receptor, the angiotensin II type 1 receptor, and the B1 bradykinin receptor. Except B1 bradykinin receptor, all these receptors become phosphorylated and internalized after agonist stimulation (34–37).
All chimeric constructs (Fig. 7A) were robustly expressed on the cell surface (∼5–10 pmol·mg−1protein−1) as determined by radioligand binding assays with [3H]BK. The chimeras with transfer of the C termini distal to helix 8 (B2CPAR2, B2CADR2, B2CB1) showed strong phosphatidylinositol hydrolysis (Fig. 7B). For the other chimeras with transfer of helix 8 either with or without the distal C terminus phosphatidylinositol hydrolysis apparently depended on the source of helix 8. When helix 8 was derived from another G protein Gq/11-coupled GPCR (protease-activated receptor-2, angiotensin II type 1 receptor), the chimeras displayed robust signaling, whereas there was no signaling when helix 8 was taken from the β2ADR (constructs B2YADR2, B2YADR2CB2) that couples to G protein Gs.
FIGURE 7.
Internalization and signaling of chimeric constructs. A, alignment of the C-terminal residues of the human B2Rwt swapped with sequences of various other GPCRs (marked in bold). Sequences were exchanged directly after the NPXXY motif of transmembrane helix 7 or after the proximal Cys-324, respectively. The predicted helix 8 is framed. B, inositol phosphate release of B2Rwt and chimeras, stably expressed in HEK 293 cells, was determined with 1 μm BK as described under “Experimental Procedures.” The results are presented as -fold increase over the IP content of identically treated control cells that had remained on ice. C, internalization of B2Rwt and chimeras stably expressed in HEK 293 cells was determined as described under “Experimental Procedures.”
Determination of receptor internalization showed a fast uptake of [3H]BK in all chimeras with C terminus exchanges distal of helix 8 (Fig. 7C). Solely, when the C terminus was derived from the non-internalizing B1 bradykinin receptor (construct B2CB1), a quite slow and low uptake was observed. When helix 8 was substituted in addition, only the angiotensin II type 1 receptor chimera was able to function like the B2Rwt, whereas the other chimeras displayed hardly any [3H]BK up-take at all.
Taken together, these results indicate that G protein activation might require only a helix 8 of a “donor” receptor that couples to the same G protein, whereas with regard to receptor internalization the structural requirements are much more specific for a helix 8 to function properly in a different receptor environment. In contrast, the C terminus distal to helix 8 can apparently function to a great extent independently from the receptor core in the internalization process.
DISCUSSION
Helix 8 and Surface Expression
The existence of an intracellularly located 8th helix has been predicted for some GPCRs (for review, see Ref. 38) and then confirmed with the resolution of the first structure of a GPCR, the inactive rhodopsin (6). All the following structures also displayed this helix 8 (8) with the exception of CXCR4 (7). One reason for this high conservation seems to be an important role of helix 8 in stabilizing the receptor in a conformation that supports trafficking to the plasma membrane. In this respect it has been reported for several GPCRs that truncation of the C terminus up into the region of helix 8 results in a strong loss of receptor surface expression (11, 16, 17, 26). Our results with the receptor chimeras and in particular with the helix 8 point mutant K315P of the B2R suggest that for the presumably stabilizing effect of helix 8, its specific amino acid composition is less important than maintaining its overall helical structure; all our chimeric constructs with complete transfers of helix 8 from one receptor to another were robustly expressed, but disturbance of helix 8 in the B2R (point mutant K315P) resulted in much lower surface binding than observed for the B2RwtH accompanied by strong intracellular localization (Fig. 4E).
As published recently, for the leukotriene B4 type 2 receptor an intact helix 8 is required for the receptor to pass the quality control process in the endoplasmic reticulum (17). In line with this report, Western blot analysis of our constructs showed that most of the newly synthesized helix 8-defective receptor proteins are apparently insufficiently glycosylated and trapped inside the cell. The observation that the helix 8-truncated construct V319* as well as construct K315P reached the surface when a membrane-permeable antagonist was added (Fig. 4F) suggests that the receptor molecule in the absence of a functional helix 8 can be stabilized by other means, e.g. an appropriate antagonist. A similar pharmacological chaperone effect was also reported for helix 8-defective mutants of the leukotriene B4 type 2 receptor (17) and for the muscarinic M1 receptor (14), implying a potential therapeutic approach to rescue respective defective receptors.
Helix 8 and G Protein Activation
Structural data and experimental approaches suggested that after binding an agonist on the extracellular site, helix 8 might be one of the key elements for transferring the information into the cell through becoming accessible for recruitment and activation of intracellular signaling proteins (9, 39, 40). There is now substantial experimental evidence for the involvement of helix 8 (11–13, 41–44) in the process of G protein activation, e.g. for several GPCRs it has been reported that truncation or deletion of helix 8 results in reduced activation (11–13, 44). Moreover, earlier studies have demonstrated that peptides derived from the helix 8 of rhodopsin (41) or the angiotensin II receptor (42, 43) can directly interact with the respective G proteins. It has also recently been reported that the modified C terminus of the G protein subunit Gq can be cross-linked to several helix 8 residues of the muscarinic M3 receptor (45). Pointing in the same direction of a direct involvement of helix 8 in the interaction with the G proteins, all our B2R mutants with a disturbed helix 8 displayed a distinct increase in the EC50 values for phosphatidylinositol hydrolysis as compared with the B2Rwt.
Helix 8 and B2R-GRK Interaction
Whereas the publications so far have focused almost exclusively on a role of helix 8 in receptor expression or in G protein activation, it was just recently reported that helix 8 might also play a role in receptor phosphorylation and, therefore, in the interaction with GRKs (18). Mutation of basic residues in the helix 8 of the thyrotropin releasing hormone receptor resulted in reduced receptor internalization and phosphorylation that could be rescued partly by overexpression of GRK2 or -3 and fully by overexpression of GRK5 or -6. We have shown here that disturbance of helix 8 in the B2R by truncation or mutation of a central residue to a proline (construct K315P) strongly diminished ligand-induced receptor internalization. For K315P, this effect could in part be overcome through overexpression of GRK3 (or GRK2, data not shown here) but not of the kinase-inactive variant GRK3-K220R (or of GRK5 or GRK6; data not shown). Therefore, we suggest that helix 8 of the B2R participates directly or indirectly in specific high affinity binding of GRK3 (or GRK2). Accordingly, low affinity binding due to a defective helix 8 can be compensated for by GRK3 overexpression, which presumably results in normal phosphorylation of serine/threonine residues in the B2R C terminus if present, thus rescuing receptor internalization. In agreement with this proposal, rescue by GRK3 was not possible for construct K315P-G327*, having a defective helix 8 and lacking the C terminus (Fig. 6E). On the other hand, the reduced internalization observed for the B2R truncation G327*, caused by the absence of the receptor C terminus with its phosphorylation sites, could also be partly rescued by overexpression of GRK3 but only when GRK3 kinase activity was left functional and when the GRK3 clathrin binding site was preserved. Together with the data that mutant G327* ligand-dependently binds GRK3 (Fig. 6A), our results support the idea that receptor-bound GRK3 may also phosphorylation-independently mediate directly internalization via clathrin-coated pits.
Helix 8 and Arrestin Interaction
Our results show that the presence of an intact helix 8 alone without the respective C terminus is sufficient for the B2R to associate with β-arrestin2, but not with β-arrestin1, and to generate robust internalization, although to a distinctly lesser extent than observed for the B2Rwt. The combination of a disturbed helix 8 with C-terminal truncation (mutant K315P-G327*) completely abrogates receptor endocytosis and prevents even any rescue attempts by overexpression of β-arrestins. These data indicate that helix 8 is crucial to preferably recruit β-arrestin2 to the activated receptor and that preceding phosphorylation of the B2R C terminus might ensure high affinity β-arrestin2 binding but is not absolutely required for a productive interaction. In contrast, β-arrestin1 is likewise strictly dependent on the B2R C terminus, as it does not associate notably with construct G327*, at least not under the conditions applied (Fig. 5E). However, it may substitute for β-arrestin2 in the sequestration of the B2R and even of G327* when β-arrestin2 levels are markedly reduced (siRNA knockdown) or with β-arrestin1 being strongly overexpressed (Fig. 5C). Our observed subtype-specific differences are in good agreement with a report by Zhang et al. (46, 47), who have previously shown that the phosphorylation-independent internalization of the δ-opioid receptor is regulated via β-arrestin2, but not via β-arrestin 1, which exclusively promotes the phosphorylation-dependent internalization.
Conclusions
Our data support the following models for the activation of the B2R. Subsequent to an agonist-induced change in the overall receptor conformation, helix 8 becomes accessible as an interaction site not only for G proteins but also for GRK2/3 and for β-arrestin2, yet less for β-arrestin1. With regard to receptor internalization of the B2R, helix 8 is apparently more important than the remaining C terminus with its phosphorylation sites, as ligand-induced receptor internalization was still functional in the absence of the C terminus but completely abrogated when helix 8 was disturbed by a proline point mutation. Alternatively, helix 8 does not function as a direct interaction site for G proteins, GRKs, or arrestins but indirectly as a key player in stabilizing receptor conformations that result in these interactions after binding of an agonist.
Finally, recent reports have shown that helix 8 can serve as an allosteric binding site for small molecule compounds (48, 49). Given the fact that the various functions of GPCRs are differentially affected by manipulations of helix 8, as demonstrated by our group and other researchers, targeting helix 8 as an allosteric regulatory binding site appears to be an interesting approach in the quest for more specific GPCR-related drugs.
Supplementary Material
Acknowledgments
The expert technical assistance of C. Seidl and H. Grondinger is gratefully acknowledged.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
A. Faussner, S. Schüssler, J. Feierler, M. Bermudez, J. Pfeifer, K. Schnatbaum, T. Tradler, M. Jochum, G. Wolber, and C. Gibson, submitted for publication.
- B2R
- bradykinin B2 receptor
- BK
- bradykinin
- GPCR
- G protein-coupled receptor
- GRK
- G protein-coupled receptor kinase
- Pmin
- minimal promoter
- DSP
- dithiobis[succinimidylpropionate]
- IP
- inositol phosphate
- PAR2
- protease-activated receptor-2
- β2ADR
- β2-adrenergic receptor
- eYFP
- enhanced yellow fluorescent protein
- ANOVA
- analysis of variance
- LDS
- lithium dodecyl sulfate.
REFERENCES
- 1. Leeb-Lundberg L. M., Marceau F., Müller-Esterl W., Pettibone D. J., Zuraw B. L. (2005) Pharmacol. Rev. 57, 27–77 [DOI] [PubMed] [Google Scholar]
- 2. Kakoki M., Kizer C. M., Yi X., Takahashi N., Kim H. S., Bagnell C. R., Edgell C. J., Maeda N., Jennette J. C., Smithies O. (2006) J. Clin. Invest. 116, 1302–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blaukat A., Micke P., Kalatskaya I., Faussner A., Müller-Esterl W. (2003) Am. J. Physiol. Heart Circ. Physiol. 284, H1909–H1916 [DOI] [PubMed] [Google Scholar]
- 4. Kalatskaya I., Schüssler S., Seidl C., Jochum M., Faussner A. (2006) Biol. Chem. 387, 603–610 [DOI] [PubMed] [Google Scholar]
- 5. Faussner A., Bathon J. M., Proud D. (1999) Immunopharmacology 45, 13–20 [DOI] [PubMed] [Google Scholar]
- 6. Palczewski K., Kumasaka T., Hori T., Behnke C. A., Motoshima H., Fox B. A., Le Trong I., Teller D. C., Okada T., Stenkamp R. E., Yamamoto M., Miyano M. (2000) Science 289, 739–745 [DOI] [PubMed] [Google Scholar]
- 7. Wu B., Chien E. Y., Mol C. D., Fenalti G., Liu W., Katritch V., Abagyan R., Brooun A., Wells P., Bi F. C., Hamel D. J., Kuhn P., Handel T. M., Cherezov V., Stevens R. C. (2010) Science 330, 1066–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tebben A. J., Schnur D. M. (2011) Methods Mol. Biol. 672, 359–386 [DOI] [PubMed] [Google Scholar]
- 9. Wess J., Han S. J., Kim S. K., Jacobson K. A., Li J. H. (2008) Trends Pharmacol. Sci. 29, 616–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shi L., Simpson M. M., Ballesteros J. A., Javitch J. A. (2001) Biochemistry 40, 12339–12348 [DOI] [PubMed] [Google Scholar]
- 11. Ahn K. H., Nishiyama A., Mierke D. F., Kendall D. A. (2010) Biochemistry 49, 502–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Delos Santos N. M., Gardner L. A., White S. W., Bahouth S. W. (2006) J. Biol. Chem. 281, 12896–12907 [DOI] [PubMed] [Google Scholar]
- 13. Swift S., Leger A. J., Talavera J., Zhang L., Bohm A., Kuliopulos A. (2006) J. Biol. Chem. 281, 4109–4116 [DOI] [PubMed] [Google Scholar]
- 14. Kaye R. G., Saldanha J. W., Lu Z. L., Hulme E. C. (2011) Mol. Pharmacol. 79, 701–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Okuno T., Ago H., Terawaki K., Miyano M., Shimizu T., Yokomizo T. (2003) J. Biol. Chem. 278, 41500–41509 [DOI] [PubMed] [Google Scholar]
- 16. Thielen A., Oueslati M., Hermosilla R., Krause G., Oksche A., Rosenthal W., Schülein R. (2005) FEBS Lett. 579, 5227–5235 [DOI] [PubMed] [Google Scholar]
- 17. Yasuda D., Okuno T., Yokomizo T., Hori T., Hirota N., Hashidate T., Miyano M., Shimizu T., Nakamura M. (2009) FASEB J. 23, 1470–1481 [DOI] [PubMed] [Google Scholar]
- 18. Gehret A. U., Jones B. W., Tran P. N., Cook L. B., Greuber E. K., Hinkle P. M. (2010) Mol. Pharmacol. 77, 288–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ballesteros J. A., Weinstein H. (1995) Methods Neurosci. 25, 366–428 [Google Scholar]
- 20. Kalatskaya I., Schüssler S., Blaukat A., Müller-Esterl W., Jochum M., Proud D., Faussner A. (2004) J. Biol. Chem. 279, 31268–31276 [DOI] [PubMed] [Google Scholar]
- 21. Faussner A., Bauer A., Kalatskaya I., Schüssler S., Seidl C., Proud D., Jochum M. (2005) FEBS J 272, 129–140 [DOI] [PubMed] [Google Scholar]
- 22. Ahn S., Nelson C. D., Garrison T. R., Miller W. E., Lefkowitz R. J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 1740–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hess J. F., Borkowski J. A., Young G. S., Strader C. D., Ransom R. W. (1992) Biochem. Biophys. Res. Commun. 184, 260–268 [DOI] [PubMed] [Google Scholar]
- 24. Faussner A., Wennerberg G., Schüssler S., Feierler J., Seidl C., Jochum M., Proud D. (2009) FEBS J. 276, 3491–3503 [DOI] [PubMed] [Google Scholar]
- 25. Faussner A., Bauer A., Kalatskaya I., Jochum M., Fritz H. (2003) Am. J. Physiol. Heart Circ. Physiol. 284, H1892–H1898 [DOI] [PubMed] [Google Scholar]
- 26. Tetsuka M., Saito Y., Imai K., Doi H., Maruyama K. (2004) Endocrinology 145, 3712–3723 [DOI] [PubMed] [Google Scholar]
- 27. Gibson C., Schnatbaum K., Pfeifer J. R., Locardi E., Paschke M., Reimer U., Richter U., Scharn D., Faussner A., Tradler T. (2009) J. Med. Chem. 52, 4370–4379 [DOI] [PubMed] [Google Scholar]
- 28. Ferguson S. S. (2001) Pharmacol. Rev. 53, 1–24 [PubMed] [Google Scholar]
- 29. Claing A., Laporte S. A., Caron M. G., Lefkowitz R. J. (2002) Prog. Neurobiol. 66, 61–79 [DOI] [PubMed] [Google Scholar]
- 30. Simaan M., Bédard-Goulet S., Fessart D., Gratton J. P., Laporte S. A. (2005) Cell. Signal. 17, 1074–1083 [DOI] [PubMed] [Google Scholar]
- 31. Blaukat A., Pizard A., Breit A., Wernstedt C., Alhenc-Gelas F., Muller-Esterl W., Dikic I. (2001) J. Biol. Chem. 276, 40431–40440 [DOI] [PubMed] [Google Scholar]
- 32. Mangmool S., Haga T., Kobayashi H., Kim K. M., Nakata H., Nishida M., Kurose H. (2006) J. Biol. Chem. 281, 31940–31949 [DOI] [PubMed] [Google Scholar]
- 33. Shiina T., Arai K., Tanabe S., Yoshida N., Haga T., Nagao T., Kurose H. (2001) J. Biol. Chem. 276, 33019–33026 [DOI] [PubMed] [Google Scholar]
- 34. Qian H., Pipolo L., Thomas W. G. (2001) Mol. Endocrinol 15, 1706–1719 [DOI] [PubMed] [Google Scholar]
- 35. Ricks T. K., Trejo J. (2009) J. Biol. Chem. 284, 34444–34457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Krasel C., Zabel U., Lorenz K., Reiner S., Al-Sabah S., Lohse M. J. (2008) J. Biol. Chem. 283, 31840–31848 [DOI] [PubMed] [Google Scholar]
- 37. Faussner A., Proud D., Towns M., Bathon J. M. (1998) J. Biol. Chem. 273, 2617–2623 [DOI] [PubMed] [Google Scholar]
- 38. Huynh J., Thomas W. G., Aguilar M. I., Pattenden L. K. (2009) Mol. Cell. Endocrinol. 302, 118–127 [DOI] [PubMed] [Google Scholar]
- 39. Altenbach C., Cai K., Klein-Seetharaman J., Khorana H. G., Hubbell W. L. (2001) Biochemistry 40, 15483–15492 [DOI] [PubMed] [Google Scholar]
- 40. Altenbach C., Klein-Seetharaman J., Cai K., Khorana H. G., Hubbell W. L. (2001) Biochemistry 40, 15493–15500 [DOI] [PubMed] [Google Scholar]
- 41. Ernst O. P., Meyer C. K., Marin E. P., Henklein P., Fu W. Y., Sakmar T. P., Hofmann K. P. (2000) J. Biol. Chem. 275, 1937–1943 [DOI] [PubMed] [Google Scholar]
- 42. Sano T., Ohyama K., Yamano Y., Nakagomi Y., Nakazawa S., Kikyo M., Shirai H., Blank J. S., Exton J. H., Inagami T. (1997) J. Biol. Chem. 272, 23631–23636 [DOI] [PubMed] [Google Scholar]
- 43. Kai H., Alexander R. W., Ushio-Fukai M., Lyons P. R., Akers M., Griendling K. K. (1998) Biochem. J. 332, 781–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Okuno T., Yokomizo T., Hori T., Miyano M., Shimizu T. (2005) J. Biol. Chem. 280, 32049–32052 [DOI] [PubMed] [Google Scholar]
- 45. Hu J., Wang Y., Zhang X., Lloyd J. R., Li J. H., Karpiak J., Costanzi S., Wess J. (2010) Nat. Chem. Biol. 6, 541–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang X., Wang F., Chen X., Li J., Xiang B., Zhang Y. Q., Li B. M., Ma L. (2005) J. Neurochem. 95, 169–178 [DOI] [PubMed] [Google Scholar]
- 47. Zhang X., Wang F., Chen X., Chen Y., Ma L. (2008) J. Neurochem 106, 781–792 [DOI] [PubMed] [Google Scholar]
- 48. Dowal L., Sim D. S., Dilks J. R., Blair P., Beaudry S., Denker B. M., Koukos G., Kuliopulos A., Flaumenhaft R. (2011) Proc. Natl. Acad. Sci. U.S.A. 108, 2951–2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Andrews G., Jones C., Wreggett K. A. (2008) Mol. Pharmacol. 73, 855–867 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.