Background: The aryl hydrocarbon receptor (AhR) suppresses lung inflammation and may protect against cigarette smoke-induced apoptosis.
Results: Genetic ablation of the AhR increases the sensitivity of lung cells to smoke-induced apoptosis by regulating antioxidant proteins.
Conclusion: The AhR regulates pulmonary cell survival.
Significance: The AhR control over lung cell survival may be why only some smokers develop chronic lung diseases such as chronic obstructive pulmonary disease.
Keywords: Cytochrome c, Heme Oxygenase, Lung, Mitochondrial Apoptosis, Superoxide Dismutase (SOD), Chronic Obstructive Pulmonary Disease, Emphysema
Abstract
Cigarette smoke is the primary risk factor for chronic obstructive pulmonary disease (COPD). Alterations in the balance between apoptosis and proliferation are involved in the etiology of COPD. Fibroblasts and epithelial cells are sensitive to the oxidative properties of cigarette smoke, and whose loss may precipitate the development of COPD. Fibroblasts express the aryl hydrocarbon receptor (AhR), a transcription factor that attenuates pulmonary inflammation and may also regulate apoptosis. We hypothesized the AhR would prevent apoptosis caused by cigarette smoke. Using genetically deleted in vitro AhR expression models and an established method of cigarette smoke exposure, we report that AhR expression regulates fibroblasts proliferation and prevents morphological features of apoptosis, including membrane blebbing and chromatin condensation caused by cigarette smoke extract (CSE). Absence of AhR expression results in cleavage of PARP, lamin, and caspase-3. Mitochondrial dysfunction, including cytochrome c release, was associated with loss of AhR expression, indicating activation of the intrinsic apoptotic cascade. Heightened sensitivity of AhR-deficient fibroblasts was not the result of alterations in GSH, Nrf2, or HO-1 expression. Instead, AhR−/− cells had significantly less MnSOD and CuZn-SOD expression, enzymes that protects against oxidative stress. The ability of the AhR to suppress apoptosis was not restricted to fibroblasts, as siRNA-mediated knockdown of the AhR in lung epithelial cells also increased sensitivity to smoke-induced apoptosis. Collectively, these results suggest that cigarette smoke induced loss of lung structural support (i.e. fibroblasts, epithelial cells) caused by aberrations in AhR expression may explain why some smokers develop lung diseases such as COPD.
Introduction
Exposure to tobacco smoke remains a leading cause of mortality and morbidity worldwide. Cigarette smoke causes more than 80% of cases of chronic obstructive pulmonary disease (COPD),2 a progressive airway disease characterized by chronic pulmonary and systemic inflammation and airspace enlargement (emphysema). A prevailing etiological hypothesis to explain the emphysematous component of COPD has been the protease-antiprotease imbalance where there is increased destruction of parenchymal tissue due to excess proteolytic enzymes such as matrix metalloproteinases (1–3) that are not counterbalanced by antiproteases. This hypothesis was sparked by the identification that, in some individuals, susceptibility to emphysema is associated with α1-antitrypsin deficiency (4), a heritable condition that accounts for less than 2% of all reported cases (5).
In addition, the gradual loss of alveolar wall structure associated with emphysema may also be precipitated by alterations in proliferation and apoptosis. Cigarette smoke is a potent oxidant (8), yielding an estimated 1 × 1017 oxidant molecules per puff. Oxidative stress caused by smoking can result in destruction of the alveolar wall due to apoptosis. Increased alveolar cell apoptosis is evident in the emphysematous lung (6–9) and may be coupled with insufficient proliferation (10). As proposed by Calabrese and colleagues (11), this apoptosis-proliferation imbalance suggests that excess apoptosis, coupled with reduced proliferation, causes loss of alveolar cells and eventual formation of emphysematous lesions. Many cells within the alveolar wall are sensitive to the toxic and apoptotic-inducing effects of cigarette smoke, including immune cells (12), endothelial (13, 14) and epithelial cells (15), and fibroblasts (16, 17). Both fibroblasts and epithelial cells rapidly undergo apoptosis after cigarette smoke exposure (16–18) and increase antioxidant defense proteins (19–22). Thus, coordinated loss of alveolar structural and repair cells, including alveolar epithelial cells and fibroblasts, coupled with chronic inflammation, are now considered central events during the development and progression of emphysema (23, 24) in susceptible individuals.
The signaling pathways that control inflammation and cell death, including nuclear factor-κB (NF-κB), have been targeted in an attempt to develop new strategies for intervention. Yet, pharmacological and experimental therapies aimed at slowing the progression and/or severity of COPD has largely been unsuccessful, and no existing treatment is able to reduce disease progression (25). We recently published that the aryl hydrocarbon receptor (AhR) is a novel attenuator of pulmonary inflammation caused by cigarette smoke (26). The AhR is a member of the basic helix-loop-helix Per-Arnt-Sim transcription factor family, and is well known to mediate the toxic and teratogenic effects of the manmade contaminant 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD, dioxin). After dioxin binds to the AhR, the AhR translocates to the nucleus and forms a heterodimer with the AhR nuclear transporter. This AhR·AhR nuclear transporter complex binds to DNA sequences termed the dioxin response element, initiating transcription of genes involved in detoxification. Many basic helix-loop-helix Per-Arnt-Sim members are involved in the adaptive response to cellular stress, including the AhR (27).
To investigate whether the AhR plays a role in cigarette smoke-induced pulmonary cell death, we utilized AhR-deficient in vitro models: primary pulmonary fibroblasts generated from AhR-deficient (AhR−/−) mice, AhR−/− mouse embryonic fibroblasts stably transfected with a doxycycline-regulated AhR expression vector (28), and mouse lung epithelial cells transiently transfected with siRNA for AhR. Here, we report for the first time that AhR expression is essential for regulating proliferation and preventing mitochondrial dysfunction and apoptotic cell death caused by cigarette smoke. We also show that this is not due to a generalized oxidative stress response, nor does it involve the transcription factor NF-E2-related factor 2 (Nrf2). Instead, the AhR regulates the expression of superoxide dismutases (SOD), cellular antioxidants that confer protection against lung injury (29, 30). These results, in concert with our recently published work (26), support the hypothesis that the AhR is a novel and central regulator of pathogenic processes implicated in COPD etiology and progression-inflammation and cell death.
EXPERIMENTAL PROCEDURES
Chemicals
All chemicals were purchased from Sigma unless otherwise indicated. 3,3′-Dihexyloxacarbocyanine iodide (DiOC6) and MitoTracker® Red CM-H2XRos were obtained from Molecular Probes (Eugene, OR). Actinomycin D was purchased from Biomol (Plymouth Meeting, PA). Recombinant mouse IL-1β was purchased from R&D Systems (Minneapolis, MN). Recombinant mouse CD40 ligand (CD40L) was generated as described (26).
Cell Culture
Primary Lung Fibroblasts
Primary lung fibroblasts were generated from AhR+/+, AhR heterozygous (AhR+/−), and AhR−/− C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) as described (31) and were maintained at 37 °C, incubated in humidified 5% CO2, 95% air, and cultured in minimum essential medium (MEM) supplemented with 2 mm glutamine (Invitrogen), 10% fetal bovine serum (FBS) (HyClone Labs, Logan, UT), and antibiotics/antimycotics (penicillin G, streptomycin, and amphotericin; Invitrogen). Unless otherwise indicated, fibroblasts were plated at a density of 10,000 cells/cm2 and experiments were conducted following 24 h in serum-free MEM. Lung fibroblasts from wild-type or heterozygous mice do not exhibit any difference in the ability to be activated by AhR ligands (26) and are used interchangeably as AhR-expressing cells.
Conditional AhR Knock-out Mouse Embryonic Fibroblasts (MEF)
The TET-OFF cell line, designated hereafter as Off*B6AhR MEFs (a kind gift of Dr. Alvaro Puga, University of Cincinnati), was generated and cultured as previously described (28, 32). Briefly, cells were cultured in MEM-α containing 10% FBS, 1% antibiotics/antimycotics, 26 mm NaHCO3, G-418 (600 μg/ml; Invitrogen), puromycin (3 μg/ml), and hygromycin B (400 μg/ml; Calbiochem, Gibbstown, NJ). Down-regulation of AhR expression was accomplished by the addition of 5 μg/ml of doxycycline (DOX).
Mouse Lung Epithelial (MLE) Cells
MLE-12 cells, a distal bronchiolar and alveolar epithelial cell line (ATCC, Manassas, VA) (33), were cultured in HITES medium (50:50, DMEM:Ham's F-12) supplemented with 2% FBS, 2 mm l-glutamine, 10 mm HEPES, 1:100 insulin/transferrin/selenium supplement (Invitrogen), and antibiotics/antimycotics.
AhR siRNA Knockdown Studies
MLE-12 cells were seeded at a density of 7.5 × 103 cells/cm2 and transiently transfected with 60 nm siRNA against AhR (Santa Cruz, catalogue number sc-29655) or nontargeting control siRNA (Santa Cruz, catalogue number sc-37007). Transfections were performed according to the manufacturer's instructions. Seven hours after the transfection, the cells were switched to MEM or HITES medium and treated with cigarette smoke extract (CSE) for 8–24 h. Verification of target knockdown was done by Western blot 48 h after transfection.
Preparation of Cigarette Smoke Extract
Research grade cigarettes with a filter were obtained from the Kentucky Tobacco Research Council (Lexington, KT) and CSE was generated as previously described (16, 19, 26, 34). An optical density of 0.65 (320 nm) was considered to represent 100% CSE (26). This CSE preparation was diluted to the appropriate concentration in serum-free MEM.
Histochemistry
To assess morphological changes consistent with apoptosis, fibroblasts and MLE-12 cells were seeded onto 8-well chamber slides (BD Biosciences) and left undisturbed for 24 h. Cells were then treated with 2% CSE or media alone (control) for 6 h. Hematoxylin & eosin (H&E) staining was performed according to standard histological protocols (16). Briefly, the cells were fixed in 4% paraformaldehyde for 10 min. Slides were then rinsed with water and stained with hematoxylin, rinsed with water, and then stained with eosin. Finally, cells were rinsed in water, coverslipped in Immu-mount (Shanndon, Pittsburgh, PA), and viewed using an Olympus BX51 microscope (New Hyde Park, NY).
Immunocytochemical Imaging
MitoTracker Red CM-H2XRos
Fibroblasts and MLE-12 cells were plated at a density of 1 × 104 cells/well, allowed to adhere overnight, and treated with 1 or 2% CSE for 6 h. Following treatment with CSE, MitoTracker Red (Molecular Probes) (0.5–1 μm) was added for an additional 30–45 min at 37 °C. Cells were then rinsed in PBS, 0.1% Tween 20 and coverslipped with Vectashield.
Hoechst Fluorescence
Hoechst stain is a cell-permeant dye that fluoresces upon binding to DNA. An increase in fluorescent intensity is associated with chromatin condensation, a characteristic feature of an apoptotic cell (35). Primary lung fibroblasts, Off*B6AhR MEFs, and MLE-12 cells were cultured on glass chamber slides and treated with control media or with 2% CSE for 6 h. Following this, cells were fixed in 4% paraformaldehyde, incubated with the Hoechst stain for 15 min, coverslipped, and viewed as described above. Viable and apoptotic cells were counted and results were quantitatively expressed as the percentage of apoptotic cells compared with the total number of cells.
Nrf2, HO-1, and Cytochrome c Detection
Fibroblasts were cultured on glass chamber slides and cytochemical detection of HO-1 and Nrf2 was performed as described (19). An anti-cytochrome c antibody (Cell Signaling Technologies, Beverly, MA) was used at 1:100. After secondary binding with biotinylated anti-rabbit antibody, cells were incubated with streptavidin (SA)-FITC, mounted in Immu-mount (Shandon), and photographed. Fluorescent images of nuclei are visualized by Hoechst. All photographs were taken at the same time with identical image settings.
Transient Transfection
AhR−/− fibroblasts were transiently transfected with a mouse AhR (mAhR) expression plasmid using Nucleofection (Basic Nucleofector Kit for Primary Fibroblasts, Amaxa Inc., Gaithersburg, MD) as previously described (26).
Western Blot
Fibroblasts were grown to confluence before being treated with CSE. In some experiments, fibroblasts were treated with recombinant mouse IL-1β or were pretreated with recombinant IFNγ prior to treatment with CD40L as described (26). Total cellular protein was prepared using 1% IGEPAL lysis buffer (19). Five μg of cellular proteins were fractionated on SDS-PAGE gels, electroblotted onto Immun-blot PVDF membrane (Bio-Rad). Antibodies against AhR (1:5000) (Biomol), Nrf2 (1:500; R&D Systems), HO-1 (1:5000; Stressgen Bioreagents, Victoria, BC, Canada), Cyp1B1 (1:500; Santa Cruz, Santa Cruz, CA), cleaved caspase-3, lamin A/C, cleaved PARP (1:1000; Cell Signaling Technologies), MnSOD (1:1000; R&D Systems), CuZn-SOD (1:1000; R&D Systems), Bax (1:1000; Santa Cruz), actin (1:10,000; Oncogene Research Products, San Diego, CA), or GAPDH (1:4000; Millipore, Billerica, MA) were used to assess changes in protein levels. In some experiments, equivalent numbers of Off*B6AhR MEFs were treated with or without DOX, followed by exposure to 2% CSE, and cytoplasmic and mitochondrial proteins were isolated using a commercially available kit (Qiagen, Toronto, ON). Densitometry was performed using Kodak One-dimensional Imaging Software (Eastman Kodak, Rochester, NY).
Viability
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay was performed to assess differences in viability between AhR-expressing and AhR−/− lung fibroblasts following cigarette smoke exposure. Equivalent numbers of fibroblasts were cultured in triplicate in flat bottom 96-well plates (Falcon, BD Biosciences). Cells were allowed to settle overnight. Fibroblasts were rinsed once in serum-free MEM and then treated with 140 μl/well of varying concentrations of CSE for 24 h (0.25–10%). Following this, 10 μl of 5 mg/ml of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide was added to each well for 4 h at 37 °C. The insoluble precipitate was dissolved by adding 200 μl of dimethyl sulfoxide to each well and read with a Bio-Rad microplate reader at 510 nm.
Proliferation
Proliferation was assessed by [3H]thymidine incorporation as previously described (36). Briefly, AhR+/− and AhR−/− fibroblasts were cultured in triplicate (5,000 cells/well in MEM containing 1% FBS), treated with increasing concentrations of CSE (0.25–5%) for 24 h, and pulsed with 1 μCi/well of [3H]thymidine. Plates were harvested with a Micromate 196 cell harvester (Packard Co., Meriden, CT) and incorporation of [3H]thymidine was determined with a Matrix 96 direct β counter (Packard).
Measurement of Intracellular GSH
AhR−/− and AhR+/+ fibroblasts were grown to confluence in 25-cm2 cell culture flasks and either treated with control media or with increasing percentages of CSE for 1, 3, 6, and 24 h. Measurements of intracellular GSH were performed as previously described (16, 37). Briefly, after treatments, monolayers of cells were washed with 2 ml of ice-cold PBS and scrapped into 300 μl of ice-cold extraction buffer (0.1% Triton X-100, 0.6% sulfosalicylic acid in 0.1 m phosphate buffer with 5 mm EDTA, pH 7.5). Cells were then sonicated, vortexed, and centrifuged. Determination of total intracellular levels of GSH was performed as originally described by Tietze (38) using dithiobis(2-nitrobenzoic acid)-GSSG/glutathione reductase recycling.
Mitochondrial Membrane Potential (ΔΨm)
Flow cytometric analysis of apoptosis included assessment of mitochondrial activity using DiOC6, a dye that strongly labels active mitochondria in living cells. Equivalent numbers of fibroblasts were treated with CSE for 6 h. Controls included incubation with serum-free media alone with and without DiOC6 (Molecular Probes). After treatment, DiOC6 was added at a final concentration of 40 nm for 15 min at 37 °C. Flow cytometric analysis was performed using a BD Biosciences FACSCalibur flow cytometer. A minimum of 10,000 events was acquired for each sample. Debris was gated out and analysis was performed only on the fibroblast population (16).
Caspase-3 Activity Assay
Equivalent numbers of fibroblasts were cultured in a 96-well clear-bottom plate, allowed to adhere overnight, and treated with control media, CSE, or actinomycin D for 6 h. Caspase-3 activity was measured using a commercially available fluorometric kit (Calbiochem).
Statistical Analysis
Statistical analysis was performed using JMP version 8.0 (SAS Institute, Cary, NC). An analysis of variance was used to assess differences between treatment groups of more than two. Results are expressed as the mean ± S.E. In all cases, a p value < 0.05 is considered statistically significant.
RESULTS
AhR−/− Lung Fibroblasts Are More Sensitive to CSE Compared with AhR+/+ Fibroblasts
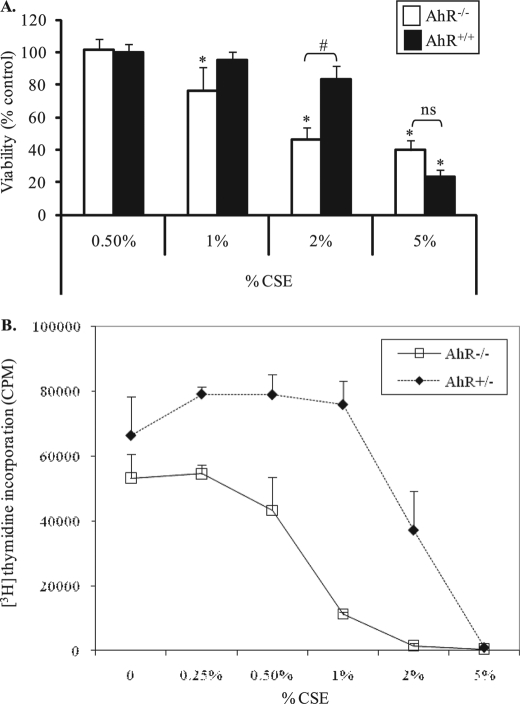
We published that cigarette smoke induces apoptosis in human lung fibroblasts (16) and hypothesize that the AhR promotes lung fibroblast survival when exposed to CSE, a widely used in vitro model of cigarette smoke exposure (16, 19, 26, 39). To determine the effect of CSE on cell viability, fibroblasts derived from AhR−/− and AhR-expressing mice were exposed to increasing percentages of CSE for 24 h and viability was assessed. Both AhR+/+ and AhR−/− lung fibroblasts exhibited a dose-dependent decrease in viability when exposed to CSE (Fig. 1A). In fibroblasts that express the AhR, viability was not significantly reduced at concentrations of CSE up to 2% (83 ± 8%). When AhR+/+ cells were exposed to 5% CSE, viability was significantly reduced (23% ± 4). However, AhR−/− lung fibroblasts were dramatically more sensitive to CSE, and viability significantly declined at much lower percentages of CSE. Compared with media-only, there was a significant reduction in viability in AhR−/− fibroblasts exposed to 1% CSE (76 ± 15%). The dramatic decline in viability in AhR−/− fibroblasts at 2% was also significantly different compared with AhR+/+ cells (Fig. 1A). Therefore, most experiments were conducted using 2% CSE, with key experiments also performed using 5% CSE, as these concentrations differently reduced viability between AhR+/+ and AhR−/− fibroblasts.
FIGURE 1.
AhR−/− fibroblasts exhibit a significant decline in viability and proliferation after exposure to CSE compared with lung fibroblasts that express the AhR. Equivalent numbers of fibroblasts were cultured in 96-well plates and exposed to increasing concentrations of CSE for 24 h. Viability was assessed by colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay and proliferation by [3H]thymidine incorporation. A, there was a statistically significant decrease in viability in AhR−/− fibroblasts when exposed to 1 (76 ± 15%) or 2% CSE (46 ± 8%). Neither 1 or 2% CSE significantly reduce viability of AhR-expressing cells (95 ± 5% and 83 ± 8%, respectively). There was no significant difference in viability between AhR−/− (40 ± 6%) and AhR+/+ (24 ± 4%) fibroblasts that were exposed to 5% CSE. These results were obtained from fibroblasts derived from two AhR−/− mice. Data for AhR+/+ cells represent combined results from AhR+/− and AhR+/+ fibroblasts. All samples were run in triplicate and the data are presented as the mean ± S.E. (n = 5 independent experiments). * indicates statistical significance compared with untreated controls (p < 0.05); #, indicates statistical significance between CSE-treated AhR−/− and AhR+/+ cells (p < 0.05); ns, no statistical significance. B, AhR−/− fibroblasts have a lower proliferative response compared with AhR+/− fibroblasts. Proliferation was dramatically lower when AhR−/− fibroblasts were exposed to 1% CSE (≈21% compared with untreated). In contrast, proliferation of AhR+/− lung fibroblasts was 114% (compared with untreated). Proliferation continued to decline with increasing percentages of CSE, where 5% CSE halted proliferation independent of AhR expression. Results are expressed as mean ± S.E.
The AhR Regulates Lung Fibroblast Proliferation
The AhR may also regulate cell proliferation (28, 32). Therefore, we hypothesized that primary pulmonary fibroblasts exposed to cigarette smoke would exhibit alterations in proliferation based on AhR expression. To test this, lung fibroblasts from AhR−/− and AhR+/− mice were left untreated or were treated with increasing percentages of CSE and proliferation was measured by [3H]thymidine incorporation. Basal proliferation was less in AhR−/− cells (∼80%) when compared with the proliferative capacity of AhR-expressing fibroblasts (0% CSE, Fig. 1B). CSE dose dependently reduced proliferation in both AhR−/− and AhR+/− fibroblasts. In AhR+/− fibroblasts, proliferation was reduced by 2% CSE exposure, but not lower concentrations of CSE (Fig. 1B, dotted line). Although there was a slight increase in proliferation with concentrations of CSE up to 1% in AhR+/− cells (Fig. 1B, dotted line), the proliferation of AhR-null fibroblasts in the presence of 1% CSE decreased by ∼80% (Fig. 1B, solid line).
CSE Induces Morphological Changes Characteristic of Apoptosis in AhR−/− Fibroblasts
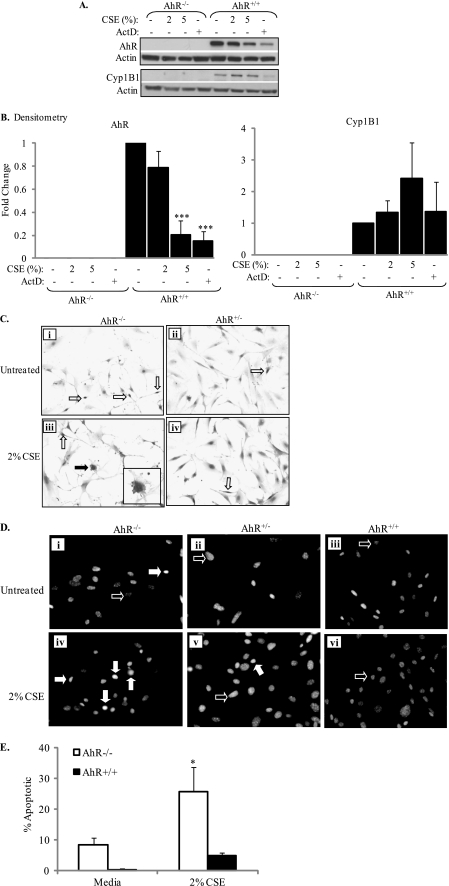
To test our hypothesis that the AhR regulates apoptosis in response to cigarette smoke, we next confirmed that the AhR−/− pulmonary fibroblasts used in this study were devoid of AhR expression. Densitometry performed on Western blots (Fig. 2A) revealed that there is no AhR protein expression in lung fibroblasts derived from AhR−/− mice. In addition, AhR−/− fibroblasts also lack cytochrome P4501B1 (Cyp1B1) protein expression, an AhR-regulated gene (40). Exposure of AhR+/+ fibroblasts to CSE significantly decreased AhR expression, concomitant with a slight increase in Cyp1B1 expression (Fig. 2, A and B). Actinomycin D (ActD), an RNA synthesis inhibitor and inducer of apoptosis (41), also decreased AhR protein expression.
FIGURE 2.
CSE induces morphological changes characteristic of apoptosis in AhR−/− fibroblasts. A, AhR and Cyp1B1 expression in lung fibroblasts: AhR−/− and AhR+/+ pulmonary fibroblasts were exposed to CSE or actinomycin D (ActD; 5 μg/ml) and cell lysates were harvested for Western blot. A representative Western blot is shown. B, there was no AhR or Cyp1B1 protein in cells derived from AhR−/− mice. Exposure to 5% CSE and ActD significantly decreased AhR protein levels in wild-type cells (***, p < 0.0001 compared with media only). There was a slight but not statistically significant increase in Cyp1B1 expression only in AhR+/+ pulmonary fibroblasts. C, AhR−/− and AhR+/− fibroblasts were cultured on glass chamber slides and exposed to 2% CSE for 6 h and H&E staining was performed. Lung fibroblasts generated from AhR−/− (i) and AhR+/− (ii) mice display typical fibroblastic morphology. Upon exposure to 2% CSE, AhR+/− fibroblasts retain this morphology (iv); nuclear condensation is observed in a few cells (iv, open arrow). AhR−/− fibroblasts exposed to 2% CSE exhibit morphological signs characteristic of apoptosis, including membrane blebbing (iii, closed arrow) and nuclear condensation (iii, open arrow). Inset, enlargement of the cell depicting membrane blebbing. Magnification, ×20; inset depicting membrane blebbing, ×60. D, in pulmonary fibroblasts that are treated with control media (Untreated), most of the cells have nuclei that are oval, with minimal fluorescence (i–iii, open arrows), regardless of AhR expression. There was an increase in the number of cells exhibiting chromatin condensation (closed arrows) in fibroblasts that lack the AhR (iv) compared with those that express the AhR (v and vi) upon exposure to 2% CSE. Magnification, ×40. E, based on Hoechst fluorescence, there was a significant increase in the percentage of apoptotic cells when AhR−/− cells were exposed to 2% CSE (*, p < 0.05 compared with both media only and CSE-exposed wild-type cells). Results are expressed as mean ± S.E. of 2–4 independent experiments.
To then determine whether these AhR−/− fibroblasts are more sensitive to CSE-induced apoptosis, fibroblasts from AhR−/− and AhR-expressing mice were exposed to 2% CSE for 6 h and H&E staining was used to assess morphological parameters characteristic of apoptosis, such as nuclear condensation and membrane blebbing (42, 43). Cells derived from the lungs of both AhR−/− and AhR+/− mice exhibit typical fibroblastic morphology (elongate, spindle-shaped cells) (Fig. 2C, i and ii). Treatment with 2% CSE for 6 h did not dramatically alter the morphology of fibroblasts that express the AhR (Fig. 2C, compare ii with iv). In contrast, lung fibroblasts that are deficient in AhR expression are dramatically more sensitive to CSE. Here, treatment with 2% CSE for 6 h results in fibroblasts that exhibited apoptotic characteristics, including nuclear condensation (open arrows) and membrane blebbing (closed arrows). Note that more untreated AhR−/− fibroblasts exhibit nuclear condensation compared with AhR+/− fibroblasts (Fig. 2C, open arrows).
Chromatin condensation is a characteristic of cells undergoing apoptosis, resulting in nuclei that have compact and simple shapes (44). Increased fluorescence of the DNA dye Hoechst is indicative of chromatin condensation (35). Therefore, we examined the ability of CSE to induce nuclear condensation in fibroblasts from AhR+/+, AhR+/−, and AhR−/− mice. The majority of fibroblasts that were treated with control media had nuclei that were spherical to ovoid in shape (Fig. 2D, open arrows). AhR-deficient fibroblasts that were treated with 2% CSE had an increase in the number of cells exhibiting nuclear condensation (Fig. 2D, iv, closed arrows), as evidenced by increased fluorescence. Few CSE-treated AhR-expressing fibroblasts exhibited nuclear condensation (Fig. 2D, v and vi). The increase in apoptotic cells in CSE-exposed AhR−/− fibroblasts was significant compared with media only and CSE-exposed wild-type cells (Fig. 2E).
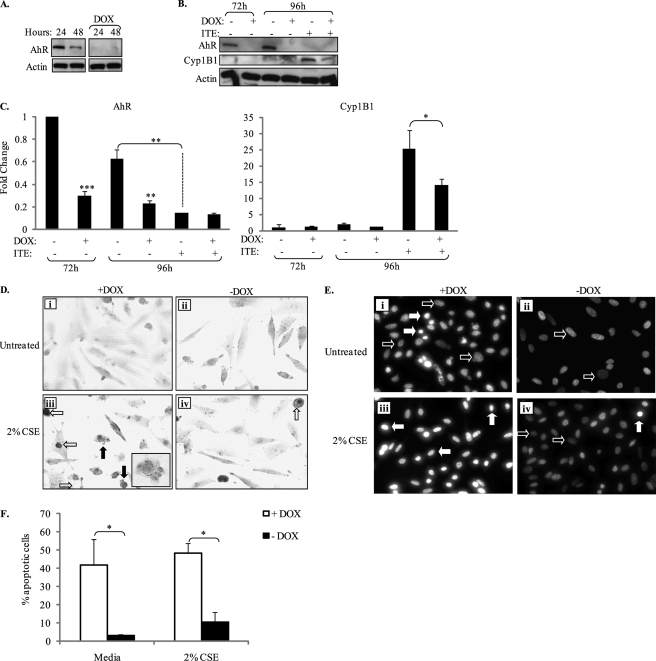
To eliminate the possibility that by virtue of being derived from different mice, the enhanced sensitivity of AhR-null pulmonary fibroblasts was not the result of clonal variability, we utilized MEFs derived from AhR−/− mice (designated Off*B6AhR) stably transfected with the TET-regulated AhR expression vector, where treatment with DOX attenuates AhR expression and function (28, 32). The use of these cells, whereby AhR expression is governed by the presence of DOX, rules out the possibility that our results are due to genetic differences between clonally unrelated cell lines. Therefore, we replicated key pieces of data utilizing these cells. We first confirmed that treatment of Off*B6AhR MEFs with DOX virtually eliminated AhR expression (Fig. 3, A and B). In addition, exposure of Off*B6AhR MEFs (−DOX) with the AhR ligand 2-(1′-H-indolo-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE) (26, 45, 46) significantly increased Cyp1B1 expression and decreased AhR expression (Fig. 3, B and C), consistent with AhR activation. Elimination of AhR expression (+DOX) significantly reduced Cyp1B1 protein expression elicited by ITE (Fig. 3, B and C). There was residual ITE-induced Cyp1B1 expression in Off*B6AhR cells treated with DOX (no AhR) that likely reflects an AhR-independent induction (47, 48).
FIGURE 3.
Elimination of AhR expression in Off*B6AhR MEFs results in increased basal- and CSE-induced cell death. A, attenuation of AhR expression in Off*B6AhR MEFs by DOX: Off*B6AhR MEFs were cultured in MEMα containing 1% FBS in the presence or absence of DOX (5 μg/ml) and whole cell lysates were collected for Western blot analysis of AhR expression. Exposure of Off*B6AhR MEFs to DOX through 48 h results in a near complete loss of AhR expression. All samples were run on the same gel. Actin was used as a loading control. B, attenuation of AhR expression by DOX reduces ITE-induced Cyp1B1 expression. In a separate experiment, Off*B6AhR MEFs were cultured with or without DOX for 72 or 96 h. Some cells were treated with ITE (1 μm) for 24 h. Representative Western blot for AhR and Cyp1B1 is shown. C, densitometry: in the continued presence of DOX, AhR expression was significantly decreased through 96 h (***, p < 0.0001 and **, p < 0.001 compared with no DOX at the respective time point). Treatment of MEFs (−DOX) with ITE significantly reduced AhR expression (**, p < 0.001). There was significantly less ITE-induced Cyp1B1 expression in +DOX Off*B6AhR MEFs compared with −DOX MEFs (*, p < 0.05). D, treatment with DOX (to eliminate AhR expression) in conjunction with 2% CSE (i and iii) resulted in membrane blebbing (iii, closed arrows). Few MEFs express the AhR (−DOX) and were exposed to 2% CSE exhibited morphological changes consistent with apoptosis (iv). Inset, enlargement of cell depicting membrane blebbing. Magnification, ×40; inset depicting membrane blebbing, ×60. E, chromatin condensation: Off*B6AhR MEF were cultured with or without DOX for 24 h and then treated with 2% CSE for 6 h. Off*B6AhR MEFs were treated with DOX (+DOX) to eliminate AhR expression, but did not receive any CSE (i), had a greater number of cells with condensed nuclei (closed arrows) compared with Off*B6AhR that express the AhR (ii). Nuclei from viable cells are indicated by open arrows. Few Off*B6AhR cells not treated with DOX exhibited condensed nuclei (iv, closed arrow). F, quantitative assessment of condensed nuclei revealed there was significantly more apoptosis in Off*B6AhR MEFs exposed to DOX (*, p < 0.05 compared with −DOX). Results are presented as mean ± S.E.; n = 2–4 independent experiments.
We then analyzed whether or not ablation of AhR expression in MEFs would result in morphological alterations indicative of apoptosis when provoked with CSE. Here, cells not treated with DOX (i.e. those expressing the AhR) exhibited typical morphology (i.e. elongate cells that are spindle-shaped) (Fig. 3D). Exposure to 2% CSE for 6 h resulted in a few cells with condensed nuclei (Fig. 3D, iv, open arrow). Similar to that observed in the AhR-deficient fibroblasts (compare with Fig. 2C), fibroblasts exposed to DOX (i.e. no AhR) followed by exposure to 2% CSE resulted in dramatic morphological alteration, including extensive membrane blebbing (Fig. 3, D, iii, closed arrows). We also utilized the Off*B6AhR MEFs to assess chromatin condensation. Here, deletion of AhR expression by DOX resulted in a significant increase in basal (Fig. 3, E, i, closed arrows, and F) and CSE-induced chromatin condensation (Fig. 3E, iii, closed arrows). Off*B6AhR MEFs, which express the AhR exhibited only a modest change in chromatin condensation (Fig. 3, E, iv, closed arrow, and F). Collectively, these data provide strong evidence that AhR expression confers protection against cigarette smoke-induced cell death.
AhR−/− Pulmonary Fibroblasts Have Increased Caspase Activation and Apoptotic Protein Cleavage in Response to Cigarette Smoke
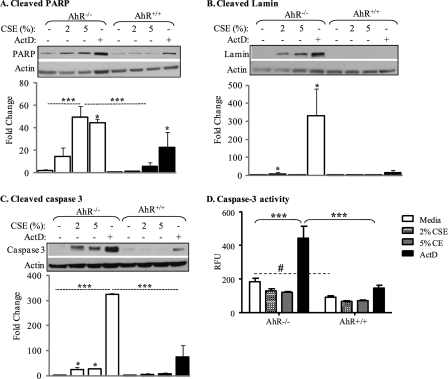
Changes in cellular morphology associated with apoptosis (Figs. 2 and 3) are the result of caspase activation and subsequent degradation of proteins involved in cellular assembly and repair, such as lamin A/C and poly(ADP-ribose)polymerase (PARP) (49, 50). Densitometric analysis revealed that in CSE- and ActD-exposed AhR−/− fibroblasts, there was a significant increase in both cleaved PARP and the small fragment of lamin A/C (Fig. 4, A and B). There was approximately a 60-fold increase in the level of cleaved PARP when AhR−/− fibroblasts were exposed to 5% CSE or ActD, respectively (Fig. 4A). In AhR-expressing cells, only ActD increased the amount of cleaved PARP (∼20-fold). It is interesting to note that basal amounts of cleaved PARP were considerably less in media-treated AhR+/+ fibroblasts (∼30% compared with AhR−/− cells). Cleavage of lamin A/C was also significantly increased in AhR−/− fibroblasts (Fig. 4B). In contrast, there was no significant indication of cleaved lamin in AhR+/+ fibroblasts.
FIGURE 4.
AhR−/− pulmonary fibroblasts have increased apoptotic protein cleavage in response to cigarette smoke. Pulmonary fibroblasts from AhR−/− and AhR+/+ mice were exposed to CSE or ActD for 24 h and the level of apoptotic proteins were assessed by Western blot analysis. Caspase-3 activity was determined using a fluorometric assay. Graphical representation (densitometry) is based on relative protein expression normalized to actin (n = 2–4 independent experiments and corresponding Western blots). The expression in the media-treated AhR+/+ fibroblasts was set to a value of 1 and fold-change represents comparison to this group. A, cleaved PARP: there was a significant increase in the level of cleaved PARP when AhR−/− fibroblasts were exposed to 5% CSE as well as ActD (***, p < 0.0001 and *, p < 0.05, compared with media control). The increase in AhR−/− fibroblasts exposed to 5% CSE was significantly higher than AhR+/+ fibroblasts. There was a statistically significant increase in cleaved PARP when AhR+/+ fibroblasts were treated with ActD. B, cleaved lamin: there was a significant increase in cleaved lamin A/C when AhR−/−, but not AhR+/+, fibroblasts were treated with CSE (*, p < 0.05 compared with media control). ActD also significantly increased cleaved lamin A/C in AhR−/− cells. C, cleaved caspase-3: CSE significantly increased cleaved caspase-3 only in the AhR−/− pulmonary fibroblasts. ActD increased cleavage of caspase-3 in both AhR−/− and AhR+/+ pulmonary fibroblasts, with the increase in AhR−/− cells being significantly higher (***, p < 0.0001). D, detection of caspase-3 activity: AhR−/− fibroblasts have significantly higher basal caspase-3 activity compared with AhR+/+ fibroblasts (#, p < 0.05). ActD significantly increased caspase-3 activity in AhR−/− (***, p < 0.001). This increase in caspase-3 activity in the AhR−/− fibroblasts was significantly higher compared with AhR-expressing fibroblasts.
Next, we used Western blot, and an antibody that detects only the cleaved fragment of caspase-3, to assess caspase-3 activity in pulmonary fibroblasts. Activation of caspase-3 results in catalytic processing, yielding smaller molecular weight fragments (51). Here, densitometric analysis indicated that cigarette smoke (CSE-2 and 5%) significantly increased cleaved caspase-3 only in the AhR−/− fibroblasts (Fig. 4C). ActD also yielded the greatest significant increase in cleaved caspase-3 in the AhR−/− cells, compared with AhR+/+ fibroblasts. Finally, we used an in vitro fluorescent enzyme activity assay that uses a fluorescent, selective caspase-3 substrate (DEVD-AFC); cleavage of this substrate by caspase-3 yields a shift in fluorescence. We treated AhR−/− and AhR+/+ fibroblasts with media only, CSE (2 and 5%), or ActD for 6 h. Media-only treated AhR−/− fibroblasts had significantly more basal caspase-3 activity compared with AhR+/+ fibroblasts (Fig. 4D). There was a significant increase in caspase-3 activity when AhR−/− fibroblasts were exposed to ActD, which was also significantly higher compared with AhR+/+ fibroblasts. There was no significant increase in caspase-3 activity in either AhR−/− and AhR+/+ cells exposed to CSE (Fig. 4D). Taken together, these data support the concept that the AhR is a regulator of apoptosis in pulmonary fibroblasts.
Genetic Deficiency of the AhR Potentiates Cigarette Smoke-induced Mitochondrial Dysfunction in Pulmonary Fibroblasts
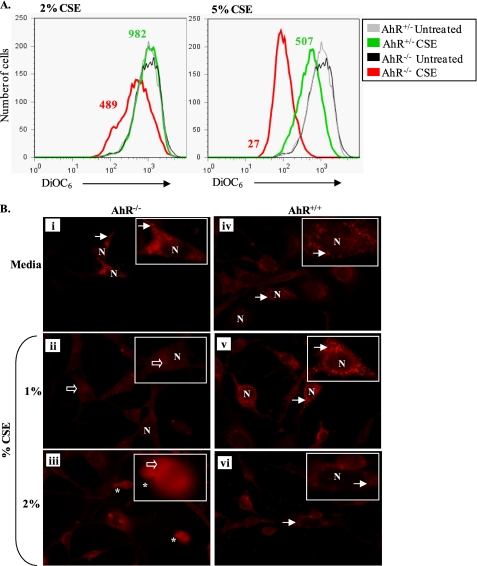
Induction of apoptosis by the intrinsic pathway results from an intracellular cascade of events leading to mitochondrial membrane permeabilization and subsequent loss of mitochondrial membrane potential (ΔΨm) (52). We therefore tested for differential alterations in ΔΨm between AhR−/− and AhR+/+ mouse lung fibroblasts exposed to CSE using DiOC6, a fluorescent dye that strongly labels intact mitochondria; a decrease in ΔΨm is identified by reduced DiOC6 incorporation (16). Although AhR expression did not influence resting ΔΨm (mean fluorescence intensity 942 versus 971 for AhR+/− and AhR−/−, respectively), treatment with 2 and 5% CSE (Fig. 5A) resulted in a dramatic decline in ΔΨm. Treatment with 2% CSE decreased DiOC6 incorporation by ∼50% (mean fluorescence intensity = 982 for untreated versus 489 for 2% CSE) in AhR−/− fibroblasts. In contrast, 2% CSE had negligible effects on lung fibroblasts that express the AhR (Fig. 5A, left histograms). Alterations in ΔΨm were more dramatic when fibroblasts were treated with 5% CSE. Here, AhR-deficient fibroblasts exhibited a robust decline in ΔΨm compared with AhR+/+ cells (Fig. 5A, right histograms).
FIGURE 5.
AhR expression prevents mitochondrial dysfunction caused by CSE. A, flow cytometric measurement of ΔΨm: lung fibroblasts from AhR−/− and AhR+/− mice were exposed to 2 or 5% CSE for 6 h and ΔΨm was assessed by flow cytometric analysis of DiOC6 incorporation. Basal ΔΨm was similar between the fibroblast strains. Treatment with 2% CSE caused a larger decrease in DiOC6 incorporation in AhR−/− fibroblasts compared with AhR+/− fibroblasts (mean fluorescence intensity (MFI) = 489 versus 982, red and green histograms, respectively). Treatment with 5% CSE resulted in decreased ΔΨm in both AhR−/− and AhR+/− fibroblasts, as measured by DiOC6 incorporation. Fluorescence intensity was dramatically lower in the AhR-null fibroblasts (MFI = 27) when compared with lung fibroblasts that express the AhR (MFI = 507). B, MitoTracker Red CM-H2XRos: AhR−/− and AhR+/+ fibroblasts were exposed to CSE for 6 h and ΔΨm was assessed via fluorescence microscopy with the mitochondrion-selective dye MitoTracker Red. Media-exposed pulmonary fibroblasts AhR−/− (i) or AhR+/+ (iv) exhibited punctuate cytoplasmic fluorescence (arrows). No fluorescence was observed in the nuclei (N). This same cytoplasmic distribution was observed in AhR+/+ fibroblasts treated with 1 or 2% CSE (v and vi, respectively). In contrast, exposure of AhR−/− fibroblasts to 1% CSE resulted in diffuse cytoplasmic fluorescence (ii, open arrows). Morphological alterations indicative of apoptosis were also evident (asterisk) in the 2% CSE-treated AhR−/− pulmonary fibroblasts (iii). Magnification, ×40 (inset is a digital enlargement of a single cell to illustrate the subcellular localization).
We also assessed mitochondrial dysfunction using MitoTracker Red, a mitochondrion selective dye that accumulates in the mitochondria in a membrane potential-dependent manner (53); the use of MitoTracker Red as an indicator of changes in ΔΨm has been described (54). In media-treated AhR+/+ fibroblasts, staining was evident as punctuate cytoplasmic fluorescence, indicative of selective uptake of MitoTracker Red by healthy and active mitochondria. A similar punctuate cytoplasmic pattern was observed in AhR-deficient fibroblasts (Fig. 5B, media, i and iv, arrows). Exposure to 1% CSE did not alter this staining pattern in fibroblasts that express the AhR (AhR+/+, panel v). However, in AhR−/− fibroblasts, this concentration of CSE resulted in a dramatic loss of punctuate staining and more diffuse cytoplasmic staining (Fig. 5B, panel ii, open arrows), indicative of reduced uptake of MitoTracker Red. Upon exposure to 2% CSE, the fluorescence of the MitoTracker Red became more diffuse in AhR−/− fibroblasts, and indicators of apoptosis (i.e. membrane blebbing) were also evident (Fig. 5B, panel iii, asterisk). In AhR+/+ fibroblasts, there was some diffuse staining, although the punctuate pattern remained in many cells (Fig. 5B, panel vi, arrows).
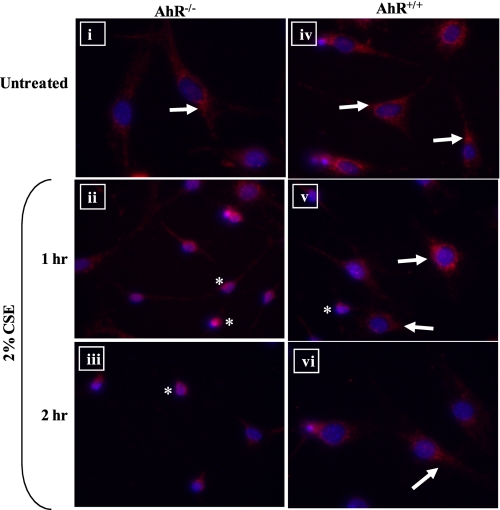
Cytochrome c release from the mitochondria and translocation to the nucleus is a hallmark of early events that initiates the intrinsic apoptotic cascade (55). Therefore, pulmonary fibroblasts from AhR−/− or AhR+/+ mice were treated with CSE, and cytochrome c release was determined by immunofluorescence. In fibroblasts that were treated with control media only, the fluorescence was punctuate, indicative of mitochondrial distribution (Fig. 6, panels i and iv, arrows). Upon exposure to 2% CSE, the cellular distribution of cytochrome c changed dramatically only in the AhR−/− cells where there was a rapid and pronounced alteration in the subcellular distribution of cytochrome c to the nucleus (Fig. 6, panels ii and iii, asterisk). In AhR+/+ fibroblasts, cytochrome c remained restricted to the cytoplasm, regardless of CSE treatment (Fig. 6, compare panel iv with panels v and vi, arrows). Collectively, these data highlight that mitochondrial dysfunction plays a key role in the enhanced apoptotic response of AhR−/− fibroblasts to cigarette smoke.
FIGURE 6.
Cigarette smoke causes cytochrome c release in AhR−/− pulmonary fibroblasts. Pulmonary fibroblasts were treated with control MEM (Untreated) or 2% CSE for 1 or 2 h and the intracellular distribution of cytochrome c was determined by immunofluorescence. i and iv, in both AhR−/− and AhR+/+ pulmonary fibroblasts cultured in control media (Untreated), cytochrome c was restricted to the cytoplasm, as evidenced by punctuate fluorescence (arrows). In AhR−/− cells treated with 2% CSE for 1 (ii) or 2 h (iii), there was an increase in the immunolabeling for cytochrome c within the nucleus (asterisk). In AhR+/+ fibroblasts treated with 2% CSE (v and vi), cytochrome c remained in the cytoplasm of most cells (arrows), with a few cells exhibiting nuclear cytochrome c (asterisk). Magnification, ×40.
The AhR Does Not Regulate Elements of the Antioxidant Response in Primary Fibroblasts
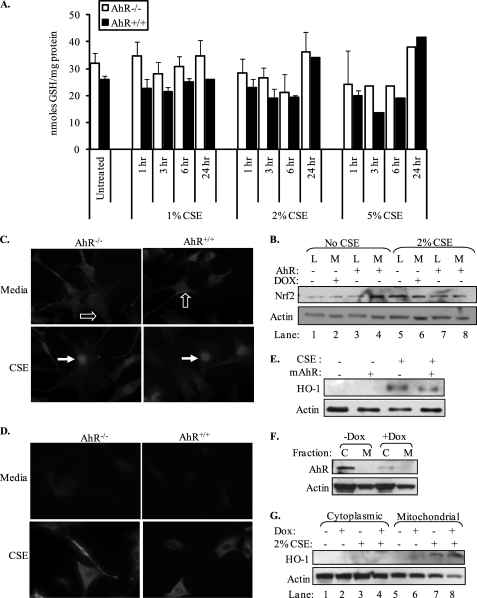
We also assessed whether or not the AhR regulates intracellular GSH levels, the most abundant nonprotein thiol in the lung. Here, the concentration of GSH from media-treated (untreated) cells was not significantly different between AhR−/− and AhR+/+ fibroblasts (32 ± 4 and 26 ± 1 nmol of GSH/mg of protein, respectively) (Fig. 7A). Although exposure to higher concentrations of CSE (2 and 5%) resulted in a slight decline in GSH levels at 3 and 6 h post-exposure, there was no significant difference in intracellular GSH levels between AhR−/− and AhR+/+ fibroblasts (Fig. 7A).
FIGURE 7.
The aryl hydrocarbon receptor is not involved in the regulation of key elements of the antioxidant response. A, pulmonary fibroblasts from AhR−/− and AhR+/+ mice were exposed to media (Untreated) or to increasing concentrations of CSE for 1, 3, 6, or 24 h and intracellular GSH levels were measured. Basal GSH was not significantly different between AhR−/− (open bars) and AhR+/+ (closed bars) fibroblasts. Treatment with increasing concentrations of CSE resulted in a dose-dependent decline in intracellular GSH, with the largest decrease occurring when fibroblasts were exposed to 5% CSE for 3 or 6 h. There was no significant difference in GSH between AhR−/− and AhR+/+ fibroblasts exposed to 1, 2, or 5% CSE. Results are expressed as mean ± S.E. and values represent duplicate samples of 2–6 independent experiments. B, Nrf2 expression was similar between AhR−/− and AhR+/+ fibroblasts. Pulmonary fibroblasts (lanes 1, 3, 5, and 7) and Off*B6AhR MEFs (lanes 2, 4, 6, and 8; ± DOX) were left untreated (No CSE) or exposed to 2% CSE for 24 h and Nrf2 expression was analyzed by Western blot. Basal Nrf2 expression was similar regardless of AhR expression. Exposure to 2% CSE slightly increased Nrf2 expression in all cells examined. L, lung fibroblasts; M, Off*B6AhR MEFs. A representative Western blot is shown. C, translocation of Nrf2 to the nucleus in response to 2% CSE is independent of AhR expression. Nrf2 localization in untreated AhR−/− and AhR+/+ pulmonary fibroblasts was predominantly cytoplasmic (open arrows). Exposure to 2% CSE resulted in a pronounced translocation of Nrf2 to the nucleus (closed arrows). D, CSE increases HO-1 expression in AhR−/− and AhR+/+ fibroblasts. Fibroblasts that were exposed to media alone expressed little HO-1. Treatment with CSE dramatically increased HO-1 expression, predominantly in the cytoplasm. E, CSE-induced HO-1 occurred independent of AhR expression. Transient transfection of a mouse AhR expression plasmid (mAhR) into AhR−/− fibroblasts did not dramatically affect either basal or CSE-induced HO-1 expression. F, cytoplasmic AhR expression in Off*B6AhR MEFs is attenuated by treatment with DOX. Off*B6AhR MEFs were untreated or treated with DOX and cytoplasmic and mitochondrial proteins were isolated. AhR was undetectable in the mitochondrial fraction (M), confirming the purity of the mitochondrial isolation. AhR expression was repressed in the cytoplasmic (C) fraction upon treatment of Off*B6AhR MEFs with DOX. G, mitochondrial HO-1 protein expression is independent of AhR expression. Off*B6AhR MEFs (+/−DOX) were treated with 2% CSE and cytoplasmic and mitochondrial proteins were isolated. CSE increased HO-1 expression only in the mitochondria. There was little difference in the relative expression of CSE-induced HO-1 between Off*B6AhR MEFs that express the AhR (−DOX; lane 7) or where AhR expression is attenuated (+DOX; lane 8).
Next, we examined the expression and nuclear localization of Nrf2, a transcription factor known to regulate antioxidant defense in response to cigarette smoke (19). Expression of Nrf2 was not different between AhR−/− and AhR+/+ fibroblasts. In either lung fibroblasts or Off*B6AhR MEFs, which express the AhR or not, Nrf2 was expressed at approximately equivalent levels (Fig. 7B). Exposure to 2% CSE yielded a slight increase in Nrf2 expression that was not dramatically different between the cell types (lung fibroblast or Off*B6AhR MEF) (Fig. 7B). Nrf2 expression was largely cytoplasmic in both AhR−/− and AhR+/+ lung fibroblasts cultured in control media (Fig. 7C, open arrows). Following treatment with 2% CSE, there was a pronounced translocation of Nrf2 to the nucleus (Fig. 7C, closed arrows). There was no perceptible difference in nuclear Nrf2 between AhR−/− and AhR+/+ fibroblasts (Fig. 7C, closed arrows).
Finally, we examined the expression of HO-1, a cytoprotective, Nrf2-regulated enzyme that can be induced by cigarette smoke in lung fibroblasts (19). Basal HO-1 expression was low in both AhR−/− and AhR+/+ pulmonary fibroblasts (Fig. 7D). Exposure to CSE for 24 h yielded a dramatic increase in HO-1 expression that was comparable between AhR−/− and AhR+/+ fibroblasts (Fig. 7D). Re-expression of the AhR in AhR−/− lung fibroblasts using an expression plasmid for mAhR (26) also had no effect on the ability of CSE to increase HO-1 protein expression (Fig. 7E). HO-1 localizes to the mitochondria following CSE exposure (21). Therefore, utilizing Off*B6AhR MEFs that were exposed to DOX to decrease AhR levels, we generated cytoplasmic and mitochondrial protein and assessed HO-1 expression. AhR expression was exclusively cytoplasmic (C) (Fig. 7F), which was dramatically attenuated following treatment with DOX. Treatment of Off*B6AhR MEFs with 2% CSE resulted in an increase in mitochondrial HO-1 expression, regardless of whether DOX was present or not (Fig. 7G). Collectively, these data suggest that key components of the antioxidant defense system are not controlled by the AhR and cannot account for the increased sensitivity of the AhR−/− pulmonary fibroblasts to cigarette smoke.
Genetic Ablation of the AhR Dysregulates SOD Expression
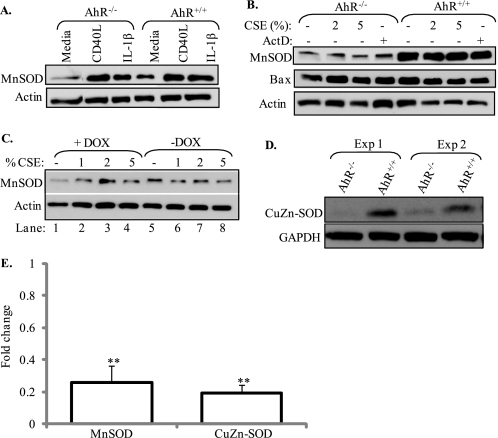
Copper-zinc SOD (CuZn) and manganese SOD (MnSOD) are cellular proteins that convert superoxide anions to hydrogen peroxide and water, and protect cells against oxidative damage. MnSOD is a mitochondrial enzyme whose expression is induced by several cytokines, including IL-1β and members of the tumor necrosis factor (TNF) family (56). Therefore, we first examined whether IL-1β or CD40L (a TNF family member) could regulate MnSOD in pulmonary fibroblasts. When pulmonary fibroblasts derived from AhR−/− and AhR+/+ mice were exposed to either IL-β or CD40L, there was an increase in MnSOD expression (Fig. 8A). There was no apparent difference in the induction of MnSOD between AhR−/− and AhR+/+ fibroblasts by either IL-1β or CD40L. Neither CSE (2% or 5%) actinomycin D increased MnSOD expression (Fig. 8B). It was evident, however, that AhR−/− fibroblasts expressed less basal MnSOD compared with AhR+/+ cells (compare media-only; Fig. 8, A and B). These results were verified using DOX-treated Off*B6AhR MEFs, whereby ablation of the AhR by DOX resulted in less MnSOD protein compared with AhR-expressing Off*B6AhR MEFs (−DOX) (Fig. 8C); treatment with DOX attenuated MnSOD expression by ∼80% (Fig. 8C, compare lane 1 to lane 5), confirming that AhR regulates SOD protein expression. The AhR did not regulate the expression of the pro-apoptotic protein Bax (Fig. 8B). There was also less basal CuZn-SOD in AhR-deficient fibroblasts (Fig. 8D). Densitometry on Western blots revealed that there was significantly less basal MnSOD and CuZn-SOD in AhR−/− fibroblasts (Fig. 8E). Here, AhR−/− fibroblasts expressed only 25% of MnSOD and 20% of CuZn-SOD protein compared with AhR+/+ fibroblasts. These results suggest that the AhR may confer protection in the lung via the regulation of critical antioxidant enzymes.
FIGURE 8.
AhR-dependent regulation of basal SOD expression. Primary lung fibroblasts were treated with CD40L (1:100), IL-1β (10 ng/ml), CSE, or actinomycin D (ActD) for 24 h and MnSOD protein expression was determined. A, treatment with CD40L or IL-1β increased MnSOD expression in both AhR−/− and AhR+/+ fibroblasts. B, pulmonary fibroblasts from AhR−/− mice have decreased expression of MnSOD (compare with A). There was no change in MnSOD protein expression following treatment with CSE or ActD for 24 h. Bax levels were similar and not altered by CSE exposure or AhR expression. C, attenuation of AhR expression by doxycycline (+DOX) results in less basal MnSOD protein expression. D, basal CuZn-SOD was also less in the AhR−/− fibroblast. E, densitometry revealed that there was significantly less MnSOD and CuZn-SOD expression in the AhR−/− fibroblasts compared with AhR+/+ fibroblasts (**, p < 0.01). MnSOD expression in AhR+/+ fibroblasts was arbitrarily set to one and the results shown are for AhR−/− fibroblasts. Results are expressed as the mean ± S.E.; n = 3–5 separate experiments and corresponding Western blots.
siRNA Knockdown of the AhR Increases Susceptibility of Lung Epithelial Cells to Cigarette Smoke-induced Apoptosis
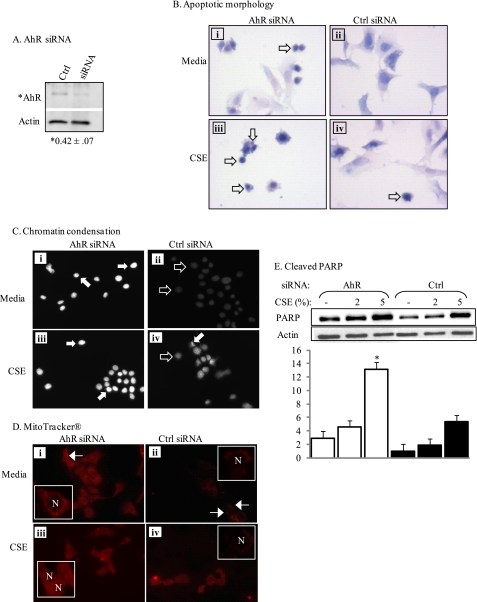
Epithelial cells are a direct target for smoke-induced damage, rapidly undergoing apoptosis after cigarette smoke exposure (16–18). To determine whether the AhR also promotes epithelial cell survival, we transiently transfected siRNA for AhR into MLE-12 cells, a distal bronchiolar-alveolar epithelial cell line (33). After confirming successful reduction of AhR expression (Fig. 9A), apoptosis was assessed as described above. Knockdown of the AhR in the absence of smoke increased morphological parameters of apoptosis, including nuclear condensation. Following exposure to 2% CSE, there was a further increase in apoptotic markers (Fig. 9, B and C, compare with Figs. 2 and 3). AhR-knockdown epithelial cells also exhibited mitochondrial dysfunction upon CSE exposure, as assessed by cytoplasmic retention of MitoTracker Red (Fig. 9D, compare with Fig. 5). Finally, reducing AhR levels in lung epithelial cells also resulted in a significant increase in the levels of cleaved PARP (Fig. 9E). Taken together, our data highlight the universality of the AhR as an important and novel promoter of pulmonary structural cell survival.
FIGURE 9.
siRNA-mediated knockdown of the AhR increases susceptibility to smoke-induced apoptosis in mouse lung epithelial cells. MLE-12 cells were transiently transfected with siRNA against AhR or control siRNA (Ctrl), exposed to 2% CSE, and apoptosis was determined. A, transfection of MLE-12 cells with AhR siRNA for 48 h reduced AhR levels. Densitometric analysis revealed that AhR levels were reduced to 42% compared with Ctrl (*, p < 0.05; n = 4). B, knockdown of AhR increases apoptosis, AhR siRNA- or Ctrl-transfected MLE-12 cells were incubated with media or 2% CSE and H&E staining was performed. MLE-12 cells transfected with Ctrl siRNA exhibited few morphological indicators of apoptosis, with or without CSE exposure (compare ii and iv). MLE-12 cells with AhR knockdown (AhR siRNA) exposed to media had condensed nuclei (open arrows). Exposure to CSE further increased nuclear condensation (open arrows). C, chromatin condensation, MLE-12 cells transfected with AhR-siRNA and maintained in medium had increased chromatin condensation (white arrows, i) compared with cells receiving the Ctrl siRNA (black arrows, ii). There was a further increase in chromatin condensation in both the siRNA- (iii) and Ctrl-transfected cells (iv) exposed to 2% CSE, with the increase being more dramatic in the AhR knockdown cells. D, MitoTracker Red, AhR siRNA MLE-12 cells (i) exposed to medium exhibited slightly diffuse staining compared with the punctuate fluorescence of Ctrl siRNA-transfected epithelial cells (ii, arrows). Note that nuclei (N) in the Ctrl siRNA-transfected cells exhibited negligible fluorescence. Exposure to 2% CSE resulted in dramatic cytoplasmic distribution of the MitoTracker, where the fluorescence was very diffuse; this was especially evident in the AhR knockdown cells (iii) compared with Ctrl (iv). Magnification, ×40 (insets represent digital enlargements of representative cells). E, cleaved PARP- siRNA-mediated knockdown of the AhR significantly increased CSE-induced PARP cleavage. Results are representative of 2–3 independent experiments.
DISCUSSION
COPD is a heterogeneous disease that worldwide claims the lives of ∼3 million people annually (57). Despite the global burden of COPD, there are no new pharmacological therapies that prevent disease progression. COPD is notoriously resistant to conventional anti-inflammatory treatment, including corticosteroids, which fail to reduce the progression or mortality of COPD (58), accentuating the need for new therapeutic targets that have the capacity to regulate the multifaceted pathologies that exist in obstructive airway disease. Recent evidence from our lab highlights the role of the AhR in attenuating pulmonary inflammation caused by cigarette smoke (26). The results presented in our current study now reveal that the AhR is critical in preventing excessive cell death caused by cigarette smoke.
Excessive apoptosis contributes to the gradual loss of alveolar wall structure and eventual formation of emphysematous lesions (23). Markers of apoptosis are evident in human emphysema, and apoptotic cells include epithelial cells and fibroblasts (6, 8, 59). In our study, using genetically altered in vitro cell culture models, we have demonstrated that when challenged with cigarette smoke, AhR expression reduced cellular features consistent with apoptosis, including morphological changes and the cleavage of apoptotic proteins (Figs. 2–4 and 9). The AhR also maintained mitochondrial function (Figs. 5, 6, and 9). AhR−/− cells also have significantly higher basal caspase-3 activity, which was significantly increased by ActD (Fig. 4D). However, there was no increase in caspase-3 activity in response to CSE, despite the appearance of cleaved caspase-3 and a robust apoptotic response. CSE can reduce caspase-3 activity due to a direct effect on the active protein, without affecting the processing of procaspase-3 or impending apoptosis (60). Here, the threshold level of caspase-3 activity needed to induce apoptosis is very low, and only complete inhibition of caspase-3 suppresses apoptosis (60). Although our results do not exclude the involvement of upstream caspases (i.e. caspase-9) in smoke-induced apoptosis, our findings suggest that higher basal levels of caspase-3 activity in AhR−/− fibroblasts are sufficient to drive the apoptotic response due to cigarette smoke.
Although the toxicological aspects of AhR signaling are not in doubt, there is ample new evidence to indicate a physiological role for the AhR independent of its response to dioxin. Ligand-independent functions associated with AhR expression include apoptosis and proliferation (32, 61). Our data demonstrate that in addition to apoptosis, AhR expression regulates the proliferative capacity of pulmonary cells (Fig. 1). Here, AhR−/− fibroblasts proliferated less, particularly when exposed to CSE, compared with AhR+/+ fibroblasts. As an imbalance in both apoptosis and proliferation has been proposed as a contributing factor in the development of emphysema (11), AhR deficiency may hasten airspace enlargement in an animal model. Our results support the concept that the AhR is a previously undiscovered regulator of both apoptosis and proliferation in the lung, which has important implications for the development of COPD. It is intriguing to speculate that smokers' who develop COPD have lower expression of the AhR, and are unable to prevent the loss of alveolar cells nor maintain a correct proliferative balance in the lung, ultimately skewing this apoptosis/proliferation ratio, and inciting the development of emphysema.
The ability of the AhR to control proliferation and apoptosis was unrelated to a generalized defect in the antioxidant response, as neither GSH nor HO-1 expression was altered as a consequence of AhR expression (Fig. 7). These were surprising results, as activation of the AhR induces rat hepatic HO-1 (62). Also intriguing was the lack of association between AhR expression and Nrf2 activation. Nrf2 is an oxidant-sensitive transcription factor that is activated by cigarette smoke (19) to increase the expression of antioxidant proteins, including HO-1 and GSH (63). Moreover, there is a strong reciprocal association between AhR and Nrf2 pathways, including direct regulation of Nrf2 by the AhR (64). However, we were unable to find a difference in the expression or nuclear translocation of Nrf2 (Fig. 7). This discrepancy could be the result of cell-specific differences in Nrf2 in those studies compared with ours, as has been previously reported (19). Another key difference is the use of TCDD to activate the AhR in the aforementioned studies. TCDD is an environmental chemical and the most potent AhR ligand known. Activation of the AhR by TCDD is associated with numerous pathologies, including tumor promotion and teratogenesis. Although the endogenous ligand of the AhR is unclear, the recently reported AhR ligand ITE, isolated from the lung (46), exhibits potent AhR agonist activity, but does not elicit the same toxic response as TCDD (45). This suggests that activation of the AhR by TCDD may not be reflective of the physiological response of the AhR to an endogenous ligand, and hence explain the lack of differences in Nrf2 observed in our study.
We found a strong association between AhR expression and the levels of basal MnSOD and CuZn-SOD (Fig. 8). MnSOD is a principal antioxidant within the mitochondria, and is responsible for degrading superoxide (O2˙̄) into H2O2. Cigarette smoke is a potent oxidant, containing high concentrations of ROS as well as stable free radicals capable of generating O2˙̄ within the pulmonary alveoli (65). MnSOD is induced by inflammatory cytokines, including IL-1 and members of the TNFα family, and is also increased or repressed to match ROS conditions. In our study, basal MnSOD was significantly less in fibroblasts lacking AhR expression (Fig. 8). Likewise, there was less basal CuZn-SOD in AhR−/− fibroblasts.
Although the mechanism by which the AhR regulates SOD expression is unclear, it is unlikely to be via modulation of NF-κB. Although NF-κB is thought to be a dominant transcription factor involved in regulating MnSOD expression (66), several lines of evidence preclude its involvement. First, there was no obvious difference in the ability of IL-1β, a potent inducer of NF-κB (20), to increase MnSOD expression between AhR−/− and AhR+/+ fibroblasts (Fig. 8A). In addition, we have published that there is also no difference in NF-κB (p65) signaling with regards to AhR expression (26). Here, AhR−/− and AhR+/+ fibroblasts did not show differences in p65 translocation in response to CSE or IL-1β. It is possible that interaction of the AhR with other transcription factors, such as activator protein-1, or the ability of the AhR to manipulate post-transcriptional control of MnSOD expression, are involved in its regulation.
The functional significance of lower basal SOD in lung fibroblasts may extend beyond its involvement in promoting oxidative stress and mitochondrial dysfunction. MnSOD is also important in fibroblast proliferation. Increased expression of MnSOD boosts fibroblast proliferation (67), and it could be inferred that the higher MnSOD in the AhR-expressing fibroblasts may be one reason for the higher proliferative capacity observed (Fig. 1). MnSOD is also postulated to function as a tumor suppressor, and many cancer cells express lower levels of MnSOD compared with their normal counterparts (68). Along these same lines, it has also been shown that boosting MnSOD protects against tumor formation in many experimental cancer models (69, 70). Thus, attenuation of MnSOD caused by low AhR expression, in addition to the lack of inflammatory control (26), may further favor the formation of a malignant phenotype, and develop into lung cancer when provoked by chronic cigarette smoke.
Using a combination of genetic, biochemical, and cellular approaches, we have demonstrated that the AhR is crucial in regulating apoptosis, proliferation, and mitochondrial dysfunction caused by cigarette smoke. We also show that the AhR controls SOD expression, enzymes that protect against oxidative stress and apoptosis (71–73). Until recently, the AhR has been regarded as a toxicology receptor, with little information on its physiological function. These data, combined with our recent publications on the AhR and regulation of pulmonary inflammation (26, 74), solidifies an important role for this receptor in modulating lung physiology. A further understanding of the signaling pathways governing activation of the AhR, independent of its ability to bind manmade environmental chemicals, has the potential to lead to new treatment options for those afflicted with airway diseases associated with inflammation and apoptosis, particularly COPD and lung cancer.
Acknowledgment
We acknowledge Dr. Alvaro Puga (University of Cincinnati) for kindly providing the Off*B6AhR fibroblasts.
This work was supported, in whole or in part, by National Institutes of Health Grants HL075432, HL088325, HL04492, and ES01247, an American Thoracic Society Research Grant, the Department of Medicine at McGill University, and the Research Institute of the McGill University Health Centre.
- COPD
- chronic obstructive pulmonary disease
- AhR
- aryl hydrocarbon receptor
- CSE
- cigarette smoke extract
- TCDD (dioxin)
- 2,3,7,8-tetrachlorodibenzo-p-dioxin
- DOX
- doxycycline
- HO-1
- heme oxygenase-1
- ITE
- 2-(1′-H-indolo-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester
- Nrf2
- NF-E2-related factor 2
- PARP
- poly(ADP-ribose) polymerase
- SOD
- superoxide dismutase
- DiOC6
- 3,3′-dihexyloxacarbocyanine iodide
- MEM
- minimal essential medium
- MLE
- mouse lung epithelial
- ROS
- reactive oxygen species
- mAhR
- mouse AhR
- MEF
- mouse embryonic fibroblast.
REFERENCES
- 1. Finlay G. A., O'Driscoll L. R., Russell K. J., D'Arcy E. M., Masterson J. B., FitzGerald M. X., O'Connor C. M. (1997) Am. J. Respir. Crit. Care Med. 156, 240–247 [DOI] [PubMed] [Google Scholar]
- 2. Demedts I. K., Morel-Montero A., Lebecque S., Pacheco Y., Cataldo D., Joos G. F., Pauwels R. A., Brusselle G. G. (2006) Thorax 61, 196–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ohnishi K., Takagi M., Kurokawa Y., Satomi S., Konttinen Y. T. (1998) Lab. Invest. 78, 1077–1087 [PubMed] [Google Scholar]
- 4. Abboud R. T., Vimalanathan S. (2008) Int. J. Tuberc. Lung Dis. 12, 361–367 [PubMed] [Google Scholar]
- 5. Sampsonas F., Karkoulias K., Kaparianos A., Spiropoulos K. (2006) Curr. Med. Chem. 13, 2857–2873 [DOI] [PubMed] [Google Scholar]
- 6. Segura-Valdez L., Pardo A., Gaxiola M., Uhal B. D., Becerril C., Selman M. (2000) Chest 117, 684–694 [DOI] [PubMed] [Google Scholar]
- 7. Kasahara Y., Tuder R. M., Cool C. D., Lynch D. A., Flores S. C., Voelkel N. F. (2001) Am. J. Respir. Crit. Care Med. 163, 737–744 [DOI] [PubMed] [Google Scholar]
- 8. Imai K., Mercer B. A., Schulman L. L., Sonett J. R., D'Armiento J. M. (2005) Eur. Respir. J. 25, 250–258 [DOI] [PubMed] [Google Scholar]
- 9. Hodge S., Hodge G., Holmes M., Reynolds P. N. (2005) Eur. Respir. J. 25, 447–454 [DOI] [PubMed] [Google Scholar]
- 10. Aoshiba K., Nagai A. (2009) Proc. Am. Thorac. Soc. 6, 596–601 [DOI] [PubMed] [Google Scholar]
- 11. Calabrese F., Giacometti C., Beghe B., Rea F., Loy M., Zuin R., Marulli G., Baraldo S., Saetta M., Valente M. (2005) Respir. Res. 6, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zavitz C. C., Gaschler G. J., Robbins C. S., Botelho F. M., Cox P. G., Stampfli M. R. (2008) Cell. Immunol. 253, 38–44 [DOI] [PubMed] [Google Scholar]
- 13. Csiszar A., Labinskyy N., Podlutsky A., Kaminski P. M., Wolin M. S., Zhang C., Mukhopadhyay P., Pacher P., Hu F., de Cabo R., Ballabh P., Ungvari Z. (2008) Am. J. Physiol. Heart Circ. Physiol. 294, H2721–2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Damico R., Simms T., Kim B. S., Tekeste Z., Amankwan H., Damarla M., Hassoun P. M. (2011) Am. J. Respir. Cell Mol. Biol. 44, 323–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pace E., Ferraro M., Siena L., Melis M., Montalbano A. M., Johnson M., Bonsignore M. R., Bonsignore G., Gjomarkaj M. (2008) Immunology 124, 401–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baglole C. J., Bushinsky S. M., Garcia T. M., Kode A., Rahman I., Sime P. J., Phipps R. P. (2006) Am. J. Physiol. Lung Cell Mol. Physiol. 291, L19–29 [DOI] [PubMed] [Google Scholar]
- 17. Carnevali S., Petruzzelli S., Longoni B., Vanacore R., Barale R., Cipollini M., Scatena F., Paggiaro P., Celi A., Giuntini C. (2003) Am. J. Physiol. Lung Cell Mol. Physiol. 284, L955–963 [DOI] [PubMed] [Google Scholar]
- 18. Tagawa Y., Hiramatsu N., Kasai A., Hayakawa K., Okamura M., Yao J., Kitamura M. (2008) Free Radic. Biol. Med. 45, 50–59 [DOI] [PubMed] [Google Scholar]
- 19. Baglole C. J., Sime P. J., Phipps R. P. (2008) Am. J. Physiol. Lung Cell Mol. Physiol. 295, L624–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martey C. A., Pollock S. J., Turner C. K., O'Reilly K. M., Baglole C. J., Phipps R. P., Sime P. J. (2004) Am. J. Physiol. Lung Cell Mol. Physiol. 287, L981–991 [DOI] [PubMed] [Google Scholar]
- 21. Slebos D. J., Ryter S. W., van der Toorn M., Liu F., Guo F., Baty C. J., Karlsson J. M., Watkins S. C., Kim H. P., Wang X., Lee J. S., Postma D. S., Kauffman H. F., Choi A. M. (2007) Am. J. Respir. Cell Mol. Biol. 36, 409–417 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22. Mulligan R. M., Atkinson C., Vertegel A. A., Reukov V., Schlosser R. J. (2009) Am. J. Rhinol. Allergy 23, e1–4 [DOI] [PubMed] [Google Scholar]
- 23. Demedts I. K., Demoor T., Bracke K. R., Joos G. F., Brusselle G. G. (2006) Respir. Res. 7, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park J. W., Ryter S. W., Choi A. M. (2007) COPD 4, 347–353 [DOI] [PubMed] [Google Scholar]
- 25. Barnes P. J., Stockley R. A. (2005) Eur. Respir. J. 25, 1084–1106 [DOI] [PubMed] [Google Scholar]
- 26. Baglole C. J., Maggirwar S. B., Gasiewicz T. A., Thatcher T. H., Phipps R. P., Sime P. J. (2008) J. Biol. Chem. 283, 28944–28957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Furness S. G., Lees M. J., Whitelaw M. L. (2007) FEBS Lett. 581, 3616–3625 [DOI] [PubMed] [Google Scholar]
- 28. Chang X., Fan Y., Karyala S., Schwemberger S., Tomlinson C. R., Sartor M. A., Puga A. (2007) Mol. Cell. Biol. 27, 6127–6139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nagata K., Iwasaki Y., Yamada T., Yuba T., Kono K., Hosogi S., Ohsugi S., Kuwahara H., Marunaka Y. (2007) Respir. Med. 101, 800–807 [DOI] [PubMed] [Google Scholar]
- 30. Joseph A., Li Y., Koo H. C., Davis J. M., Pollack S., Kazzaz J. A. (2008) Free Radic. Biol. Med. 45, 1143–1149 [DOI] [PubMed] [Google Scholar]
- 31. Baglole C. J., Reddy S. Y., Pollock S. J., Feldon S. E., Sime P. J., Smith T. J., Phipps R. P. (2005) Methods Mol. Med. 117, 115–127 [DOI] [PubMed] [Google Scholar]
- 32. Marlowe J. L., Fan Y., Chang X., Peng L., Knudsen E. S., Xia Y., Puga A. (2008) Mol. Biol. Cell 19, 3263–3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wikenheiser K. A., Vorbroker D. K., Rice W. R., Clark J. C., Bachurski C. J., Oie H. K., Whitsett J. A. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 11029–11033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carp H., Janoff A. (1978) Am. Rev. Respir. Dis. 118, 617–621 [DOI] [PubMed] [Google Scholar]
- 35. Zhivotosky B., Orrenius S. (2001) Curr. Protoc. Cell Biol. 18.3.1–18.2.23 [DOI] [PubMed] [Google Scholar]
- 36. Padilla J., Leung E., Phipps R. P. (2002) Clin. Immunol. 103, 22–33 [DOI] [PubMed] [Google Scholar]
- 37. Rahman I., Kode A., Biswas S. K. (2006) Nat. Protoc. 1, 3159–3165 [DOI] [PubMed] [Google Scholar]
- 38. Tietze F. (1969) Anal. Biochem. 27, 502–522 [DOI] [PubMed] [Google Scholar]
- 39. Shapiro S. D. (2004) Am. J. Respir. Cell Mol. Biol. 31, 481–482 [DOI] [PubMed] [Google Scholar]
- 40. Eltom S. E., Larsen M. C., Jefcoate C. R. (1998) Carcinogenesis 19, 1437–1444 [DOI] [PubMed] [Google Scholar]
- 41. Kleeff J., Kornmann M., Sawhney H., Korc M. (2000) Int. J. Cancer 86, 399–407 [DOI] [PubMed] [Google Scholar]
- 42. Vermes I., Haanen C., Reutelingsperger C. (2000) J. Immunol. Methods 243, 167–190 [DOI] [PubMed] [Google Scholar]
- 43. Loo D. T., Rillema J. R. (1998) Methods Cell Biol. 57, 251–264 [DOI] [PubMed] [Google Scholar]
- 44. Leist M., Jäättelä M. (2001) Nat. Rev. Mol. Cell Biol. 2, 589–598 [DOI] [PubMed] [Google Scholar]
- 45. Henry E. C., Bemis J. C., Henry O., Kende A. S., Gasiewicz T. A. (2006) Arch. Biochem. Biophys. 450, 67–77 [DOI] [PubMed] [Google Scholar]
- 46. Song J., Clagett-Dame M., Peterson R. E., Hahn M. E., Westler W. M., Sicinski R. R., DeLuca H. F. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 14694–14699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lehmann G. M., Xi X., Kulkarni A. A., Olsen K. C., Pollock S. J., Baglole C. J., Gupta S., Casey A. E., Huxlin K. R., Sime P. J., Feldon S. E., Phipps R. P. (2011) Am. J. Pathol. 178, 1556–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ryu D. Y., Hodgson E. (1999) J. Biochem. Mol. Toxicol. 13, 249–251 [DOI] [PubMed] [Google Scholar]
- 49. Rao L., Perez D., White E. (1996) J. Cell Biol. 135, 1441–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Oliver F. J., de la Rubia G., Rolli V., Ruiz-Ruiz M. C., de Murcia G., Murcia J. M. (1998) J. Biol. Chem. 273, 33533–33539 [DOI] [PubMed] [Google Scholar]
- 51. Nicholson D. W., Ali A., Thornberry N. A., Vaillancourt J. P., Ding C. K., Gallant M., Gareau Y., Griffin P. R., Labelle M., Lazebnik Y. A., et al. (1995) Nature 376, 37–43 [DOI] [PubMed] [Google Scholar]
- 52. Kroemer G., Galluzzi L., Brenner C. (2007) Physiol. Rev. 87, 99–163 [DOI] [PubMed] [Google Scholar]
- 53. Peraza M. A., Cromey D. W., Carolus B., Carter D. E., Gandolfi A. J. (2006) J. Appl. Toxicol. 26, 356–367 [DOI] [PubMed] [Google Scholar]
- 54. Pendergrass W., Wolf N., Poot M. (2004) Cytometry A 61, 162–169 [DOI] [PubMed] [Google Scholar]
- 55. Scovassi A. I., Soldani C., Veneroni P., Bottone M. G., Pellicciari C. (2009) Ann. N.Y. Acad. Sci. 1171, 12–17 [DOI] [PubMed] [Google Scholar]
- 56. Rogers R. J., Monnier J. M., Nick H. S. (2001) J. Biol. Chem. 276, 20419–20427 [DOI] [PubMed] [Google Scholar]
- 57. Sin D. D., Vestbo J. (2009) Proc. Am. Thorac. Soc. 6, 543–545 [DOI] [PubMed] [Google Scholar]
- 58. To Y., Ito K., Kizawa Y., Failla M., Ito M., Kusama T., Elliott W. M., Hogg J. C., Adcock I. M., Barnes P. J. (2010) Am. J. Respir. Crit. Care Med. 182, 897–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yokohori N., Aoshiba K., Nagai A. (2004) Chest 125, 626–632 [DOI] [PubMed] [Google Scholar]
- 60. Stringer K. A., Tobias M., O'Neill H. C., Franklin C. C. (2007) Am. J. Physiol. Lung Cell Mol. Physiol. 292, L1572–1579 [DOI] [PubMed] [Google Scholar]
- 61. Shimba S., Komiyama K., Moro I., Tezuka M. (2002) J. Biochem. 132, 795–802 [DOI] [PubMed] [Google Scholar]
- 62. Niittynen M., Tuomisto J. T., Pohjanvirta R. (2008) Toxicology 250, 132–142 [DOI] [PubMed] [Google Scholar]
- 63. Emmert S. W., Desai D., Amin S., Richie J. P., Jr. (2010) Bioorg. Med. Chem. Lett. 20, 2675–2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Karaflou M., Lambrinoudaki I., Christodoulakos G. (2008) Mini Rev. Med. Chem. 8, 912–918 [DOI] [PubMed] [Google Scholar]
- 65. Valavanidis A., Vlachogianni T., Fiotakis K. (2009) Int. J. Environ. Res. Public Health 6, 445–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kinnula V. L., Crapo J. D. (2003) Am. J. Respir. Crit. Care Med. 167, 1600–1619 [DOI] [PubMed] [Google Scholar]
- 67. Sarsour E. H., Agarwal M., Pandita T. K., Oberley L. W., Goswami P. C. (2005) J. Biol. Chem. 280, 18033–18041 [DOI] [PubMed] [Google Scholar]
- 68. Huang Y., He T., Domann F. E. (1999) DNA Cell Biol. 18, 643–652 [DOI] [PubMed] [Google Scholar]
- 69. Weydert C., Roling B., Liu J., Hinkhouse M. M., Ritchie J. M., Oberley L. W., Cullen J. J. (2003) Mol. Cancer Ther 2, 361–369 [PubMed] [Google Scholar]
- 70. Ridnour L. A., Oberley T. D., Oberley L. W. (2004) Antioxid. Redox. Signal. 6, 501–512 [DOI] [PubMed] [Google Scholar]
- 71. Batini-Haberle I., Rebouças J. S., Spasojevi I. (2010) Antioxid. Redox Signal. 13, 877–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Petrache I., Medler T. R., Richter A. T., Kamocki K., Chukwueke U., Zhen L., Gu Y., Adamowicz J., Schweitzer K. S., Hubbard W. C., Berdyshev E. V., Lungarella G., Tuder R. M. (2008) Am. J. Physiol. Lung Cell Mol. Physiol. 295, L44–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tanaka K. I., Tanaka Y., Miyazaki Y., Namba T., Sato K., Aoshiba K., Azuma A., Mizushima T. (2011) J. Pharmacol. Exp. Ther. 338, 810–818 [DOI] [PubMed] [Google Scholar]
- 74. Thatcher T. H., Maggirwar S. B., Baglole C. J., Lakatos H. F., Gasiewicz T. A., Phipps R. P., Sime P. J. (2007) Am. J. Pathol. 170, 855–864 [DOI] [PMC free article] [PubMed] [Google Scholar]