Background: Oocytes are known to secrete factors that regulate the tertiary structure of cumulus cell-enclosed oocyte complexes (COCs).
Results: Intermedin (IMD) signaling promotes cumulus cell survival and cell contacts in COCs.
Conclusion: Endogenous IMD plays a critical role in coordinating COC development.
Significance: The study provided new insight into how the tertiary structure of COCs is maintained during folliculogenesis.
Keywords: Apoptosis, Bioinformatics, Cell Compartmentation, Cyclic AMP (cAMP), G Protein-coupled Receptors (GPCR), Ovary, Peptide Hormones, Adrenomedullin, CGRP
Abstract
Ovarian folliculogenesis has been studied as a model of hormonal regulation of development and differentiation, cell death, and cell-cell communication. In addition to gonadotropins from the pituitary and follicular paracrine factors, oocyte secreted factors have been shown to play critical roles in the regulation of follicular cell functions. Except for the well characterized BMP family proteins, including GDF9 and BMP15, oocytes are known to secrete oocyte secreted factors that are important for the regulation of cumulus cell survival and the maintenance of tertiary structure of cumulus cell-enclosed oocyte complexes (COCs). Based on genomic screening and studies of COCs cultured in vitro, we showed that intermedin (IMD)/adrenomedullin 2 (ADM2) is a novel oocyte-derived ligand important for the regulation of cell interactions in COCs that functions, in part, by suppressing cumulus cell apoptosis. Consistently, we showed that suppression of IMD/ADM2 signaling in growing rat ovaries in vivo leads to oocyte atresia and aberrant cell cycle progression in follicular cells. Together, our studies indicated that mammalian oocytes deploy a G protein-coupled receptor ligand to coordinate normal interactions of oocytes and cumulus cells and provided a better understanding of how the tertiary structure of a COC is maintained as follicles undergo exponential growth during the late stages of folliculogenesis.
Introduction
In mammals, ovarian folliculogenesis is regulated by gonadotropins from the anterior pituitary and a myriad of intraovarian factors that include BMP3 family factors, EGF family proteins, insulin-like growth factor family proteins, interleukins, and TNF family proteins among others (1–8). Under the control of gonadotropins and ovarian factors, subsets of primordial follicles periodically progress from primary to secondary to tertiary follicles. This process eventually leads to follicle rupture, cumulus expansion, and oocyte maturation (9–17). In addition, earlier work on cultured follicles has established that oocytes actively participate in the regulation of the surrounding cumulus cells, which are distinguished from steroid-producing mural granulosa cells in gene expression, protein synthesis, and physical interactions (16, 18–28).
The importance of oocytes in cumulus-cell-function regulation was first identified by studies showing that the removal of oocytes from cumulus cell-enclosed oocyte complexes (COCs) impairs various cumulus cell functions (8, 19, 24, 29–31). It was subsequently demonstrated that oocytes communicate with cumulus cells via both contact communication and paracrine oocyte-secreted factors (OSFs) (18, 19, 32–35). The actions of OSFs encompass many aspects of follicular cell functions, including the expression of cumulus cell markers, proliferation, apoptosis, luteinization, and cumulus expansion (18, 19, 36–43). Because oocyte-derived BMP family proteins, including growth differentiation factor 9 (GDF9) and bone morphogenetic factor 15 (BMP15), are capable of substituting OSFs in a variety of bioassays for evaluating OSF functions and because BMP family proteins (i.e. decapentaplegic family proteins in invertebrates) play important roles in ovarian follicle cell communications in various invertebrates (44, 45), these factors were considered de facto OSFs (19, 36–38, 41, 42, 46, 47). Despite this progress, the identities of the factors that are responsible for a number of OSF functions, including the regulation of cumulus cell survival, oocyte quality, and three-dimensional organization, have evaded researchers (19, 27, 48). Likewise, there is a complete lack of understanding about how continual association between oocytes and cumulus cells is maintained in the presence of stimulatory OSFs (i.e. GDF9 and BMP15) that mainly promote cell proliferation and cumulus expansion.
To explore oocyte-secreted ligands that contribute to the coordinated oocyte-cumulus cell communications, we used two intersecting criteria to identify candidate polypeptides. First, we generated a set of a priori candidate genes including known and novel secreted polypeptides (i.e. those that possess a signal peptide for secretion but lack a transmembrane domain) (49–52). Second, we conducted scans using the EST data base and the Gene Expression Omnibus data repository (51) to identify transcripts that show evidence of high representation in female gametes.
Based on genomic and biochemical analyses, herein we identified intermedin (IMD), also known as adrenomedullin 2 (ADM2), as an oocyte-derived ligand. IMD/ADM2 is a newly discovered hormone that belongs to a peptide family that includes calcitonin, calcitonin gene-related peptides (CGRPα and CGRPβ), amylin, and adrenomedullin (ADM) (53, 54). IMD/ADM2 has been shown to be expressed in the vasculature and a variety of tissues and signal through receptor complexes consisting of calcitonin receptor-like receptor (CLR) and one of the three receptor activity-modifying proteins (RAMP1, -2, and -3) (53–56). Reports have indicated that IMD/ADM2 acts as a pleiotropic hormone exhibiting anti-apoptosis and angiogenic effects in a variety of tissues, and the expression of IMD/ADM2 is regulated by estrogens and oxygen tension in select tissues (56–59). Based on these earlier studies, we hypothesized that oocytes may deploy IMD/ADM2 to regulate cumulus cell survival and oocyte-cumulus cell interactions. Based on studies of in vitro-cultured COCs and animal models in vivo, we report that IMD/ADM2 plays a direct role in regulating cumulus cell survival, cell-cell contacts in COCs, and the expression of cyclin D2 in companion somatic cells. Taken together, our studies suggest that oocytes deploy a G protein-coupled receptor ligand, in addition to BMP and FGF family proteins, to coordinate the interactions between oocytes and nursing cumulus cells during folliculogenesis.
EXPERIMENTAL PROCEDURES
Peptide Synthesis
Analogs of the IMD peptide (IMD, Ac-IMD(17–47), and Ac-IMD(17–47)/d-Arg-33 (Ac-VQNLSHRLWQLMGPAG(d-Arg)GQDSAPVDPSSPHSY-NH2)) were synthesized by the Stanford University Protein and Nucleic Acid Biotechnology Facility based on the solid-phase fluorenylmethoxycarbonyl protocol. The Ac-IMD(17–47)/d-Arg-33 peptide was acetylated at the N terminus and contained a d-amino acid substitution at Arg-33 (53). Peptides were analyzed by reverse-phase HPLC with Vydac C18 analytical columns and mass spectrometry using a MALDI-TOF Voyager-DE RP Workstation. Synthetic adrenomedullin, CGRPβ, and related peptides were obtained from AnaSpec, Inc. (San Jose, CA), Sigma-Aldrich, and American Peptide Company. Radiolabeled [125I]CGRP (2000 Ci/mmol) was purchased from Amersham Biosciences. Stocks of different hormones were prepared in phosphate-buffered saline (PBS) and diluted in culture medium.
Quantification of Gene Transcripts
To analyze transcript expression, total RNA was extracted from germinal vesicle oocytes and cumulus cells using an RNeasy Mini kit (Qiagen, Valencia, CA). Aliquots of RNA were transcribed into first-strand cDNAs using Omniscript reverse transcriptase (Qiagen). TaqMan primers that were used for amplification of the rat IMD cDNAs included forward primer rIMDFW (5′-ATCACAGACCACAGAGTACTCGA-3′) and reverse primer rIMDRW (5′-TCCTGCTTGCTCACAAGTGGAG-3′). For the analysis of rat CLR and RAMP transcripts, TaqMan primers were designed based on NP_113833 (RAMP1), NM_031646 (RAMP2), NP_064485 (RAMP3), and NP_036849 (CLR). In all experiments, subcloned rat cDNAs were used as the copy number standard. The probes were labeled with the reporter fluorochrome 6-carboxyfluorescein at the 5′-end and the quencher fluorochrome 6-carboxy-tetramethyl-rhodamine at the 3′-end. Forward TaqMan primers used for amplification of the rat RAMP1, RAMP2, RAMP3, CLR, connexin 43, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNAs were rRAMP1FW (5′-CATCCTGCTCTAGCCTAGTTAG-3′), rRAMP2FW (5′-TCATCCTTGAGGCTCACCTGAT-3′), rRAMP3FW (5′-GCAGGCAAGGTCATCTGGAAG-3′), rCLRFW (5′-GCTCAGTCTTACAGACATGAAA-3′), rCX43FW (5′-TCTGAGTTTAACAGTCTTTTAGATTG-3′), and rGAPDHFW (5′-CCCATTCTTCCACCTTTGATGCTGG-3′), respectively. Reverse TaqMan primers included rRAMP1RW (5′-AGACCTGGCGCTTAGCGCTCTTT-3′), rRAMP2FW (5′-TTGGTGCAGCCTACCTTCTCCGATC-3′), rRAMP3RW (5′-TCATGGCAGATCGCCTAGAAGA-3′), rCLRRW (5′-TTCTTAAAGAAAGGAACACGAGG-3′), rCX43RW (5′-ACTGGTGTCTTTAGCATACAGTTCA-3′), and rGAPDHRW (5′-CATGTAGGCCATGAGGTCCACCACC-3′). The probes for IMD, RAMP1, RAMP2, RAMP3, CLR, connexin 43, and GAPDH were 5′-TGTGCCTAAAGAAAATCGGCAAAAG-3′, 5′-TGAAAGCAGATCTGCATTACACTAA-3′, 5′-CACAAGCGTAACGAGGAAAGGGATG-3′, 5′-AGCACATTCTCTGTTGGACATGAAG-3′, 5′-ACGGTTTGTCCAGAAACACTTTAAC-3′, 5′-CACTAAGACTTTGTTTGAAACACTA-3′, and 5′-ACAACTTTGTGAAGCTCATTTCCTG-3′, respectively. All quantitative PCR analyses were performed using the QuantiTect TaqMan probe PCR kit (Qiagen) with a Smart Cycler® system (Cepheid Inc., Sunnyvale, CA) or a LightCycler 2.0 system (Roche Applied Science). All experiments were repeated three or four times using independent samples and were normalized based on GAPDH expression as described earlier (58).
Collection of Granulosa Cells, Cumulus Cells, Oocytes, COCs, and Follicles
For the analysis of gene transcripts, immature 25-day-old female Sprague-Dawley rats were injected intraperitoneally with 15 IU of pregnant mare serum gonadotropin (PMSG) and human chorionic gonadotropin 48 h later. Batches of animals were sacrificed at 24 h after PMSG, 48 h after PMSG, or 3 h after human chorionic gonadotropin injection. After sacrifice, ovaries were removed, immediately placed in a warmed culture medium, and dissected under a dissecting microscope using fine needles. To obtain follicles of similar developmental stages, care was taken to select follicles with a size range. Tissues were snap-frozen and stored at −80 ºC before transcript analysis. For protein expression analysis, tissues were routinely fixed in Bouin's solution.
To obtain oocytes, cumulus cells, granulosa cells, and COCs, animals were sacrificed at 42–44 h after the PMSG treatment. Tertiary follicles were punctured with syringe needles to release COCs and granulosa cells. Intact COCs with at least three layers of cumulus cells were collected for the separation of cumulus cells and denuded oocytes. All oocyte samples were examined under the microscope to ensure that all oocytes had been stripped of somatic cells.
Receptor-binding Assay, cAMP, and Estradiol Production by Cultured Granulosa/Cumulus Cells
Granulosa/cumulus cells were obtained from ovaries of immature 21-day-old rats that had been implanted with diethylstilbestrol for 4 days by puncturing antral follicles with fine gauge needles; these cells were cultured in L15 medium with 0.1% BSA as described previously (60). For the receptor-binding assay, cells were resuspended in ice-cold binding buffer (20 mm Tris-HCl (pH 7.4), 2 mm MgCl2, and 0.1% BSA) in siliconized microcentrifuge tubes with 0.06 μg of [125I]CGRP and various concentrations of nonradioactive peptides. After a 1-h incubation at 37 ºC, the cell-associated radioactivity was determined using a γ-counter. To assess the effect of hormones on adenylate cyclase activation and estradiol production, aliquots of dispersed granulosa/cumulus cells were cultured in 24-well culture dishes (2 × 105 cells/well) in serum-free Macoy's 5A medium supplemented with 0.3% BSA, 100 mg/ml penicillin, and streptomycin and treated with IMD or FSH. In studies of cAMP production, media were supplemented with 250 μm 3-isobutyl-1-methylxanthine (Sigma-Aldrich) to inhibit phosphodiesterase activities. Following incubation, total cAMP production and estradiol contents in cultures were measured by specific radioimmunoassays (53).
Analysis of Cell Viability and Apoptosis
The viability of cells was determined using a tetrazolium salt-based cell proliferation kit (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay; Invitrogen) based on the ability of living cells to metabolize yellow tetrazolium salt to blue formazan crystals. Granulosa/cumulus cells were cultured for 24 h with different hormones before the addition of aliquots of tetrazolium salt solution. After overnight incubation, the absorption at 560 nm was determined. The Caspase-Glo® 3/7 luminescent assay (Promega Corp.) was used to measure caspase-3/7 activities. After incubation with the proluminescent caspase-3/7 DEVD-aminoluciferin substrate for 1 h, aliquots of cells were transferred to white wall 96-well culture plates, and the luminescence was measured with a luminometer.
To detect changes in mitochondrial membrane potential of cultured cumulus cells, COCs were cultured in serum-free M16 medium containing 0.3% BSA with or without hormones for 2 h. The JC-9 mitochondrial potential sensor (2.5 pg/ml; Molecular Probes) was added to the cultures and incubated for 10 min at 37 ºC in the dark. After washing twice with PBS, the staining was visualized with a fluorescence microscope. Emission filters of 485 and 535 nm were used to detect the green (JC-9 monomer) and orange (JC-9 aggregates) fluorescence, respectively.
Determination of Cell-Cell Contacts within COCs
Groups of 20 COCs were cultured in 4-well cell culture plates with 500 μl of M16 medium in the absence or presence of different hormones for 50 h at 37C under 5% CO2, 95% O2. All cultures were overlaid with mineral oil to avoid osmotic changes. The number of COCs that remain intact was scored at select time intervals, and select cultures were recorded using a Leica DFC300 FX digital camera and Image-Pro PLUS 5.0 software (Media Cybernetics, Inc., Silver Spring, MD) (58).
Ablation of Ovarian IMD Signaling by Intrabursal Injection of a Specific IMD Antagonist in Vivo
To block ovarian IMD signaling in vivo, a specific IMD antagonist, Ac-IMD(17–47)/d-Arg-33, was injected into the periovarian sac (intrabursal) unilaterally (175 μg/bursa) as described previously (61). The reagent was administered under the bursa with a 29-gauge needle via the adjoining fat pad. The contralateral ovary in each animal was injected with PBS and served as the control. Animals were also injected with 12 IU of PMSG after surgery to stimulate ovarian follicle growth. Both ovaries were collected for analyses of histology and marker gene expression at 44 h after the PMSG injection. Five-micron-thick paraffin sections of fixed ovaries were immunostained with mouse monoclonal antibodies against cyclin D2 (Abcam Plc., AB3087). Antigen retrieval was performed in 0.01 m sodium citrate (Sigma-Aldrich) in a microwave for 10 min. After quenching with 3% H2O2, sections were washed in TBS and incubated with cyclin D2 antibodies at 4 ºC overnight. Nonspecific binding was blocked by incubating slides in TBS containing 0.1% Triton X-100 and 10% goat serum. An avidin-biotin peroxidase complex method was used to visualize the antigen antibody complex using Dako Liquid DAB Chromogen System. The signal intensity for cyclin D2 was captured with a Zeiss microscope system and quantified using HistoQuest Cell Analysis software (TissueGnostics GmbH; available on the World Wide Web).
Follicles were classified according to size and tertiary topology: primordial, with oocytes less than 25 μm in diameter and with few squamous granulosa cells; primary, oocytes covered with a single layer of cuboidal granulosa cells; secondary, oocytes with multiple layers of granulosa cells but without an antrum (100–140 μm); early antral, 140–200-μm follicles with an antrum; large antral, >200 μm in diameter and with a distinct cumulus cell layer surrounding the oocyte.
Statistical Analysis
Receptor activation and receptor-binding activity curves generated in nonlinear regression analyses (Prism 6.0; GraphPad Software) were evaluated relative to the samples without hormone treatment. In all cases, data are reported as the mean ± S.E. of assays in triplicate or quadruplicate. Statistically significant responses (p < 0.05) were determined for each stimulated response to the average nonspecific response from controls using analysis of variance or Student's t test. Correlation analysis was performed with the Spearman test.
RESULTS
Expression of IMD/ADM2 Transcripts in Rodent and Human Oocytes
Following the analysis of >1,000 genes known to encode ligands for human cell surface membrane receptors and secreted polypeptides without a described function (50–52, 62), we found that the transcript of IMD/ADM2 is highly represented in rodent oocyte and human germ cell EST libraries (supplemental Table 1). Among the eight mouse IMD/ADM2 ESTs, five were from either unfertilized oocyte or fertilized egg libraries. In the human EST data base, three of six IMD/ADM2 ESTs were derived from a germ cell teratoma cDNA library. Consistently, we found that IMD/ADM2 transcripts were detected in oocytes of nine published microarray studies in the Gene Expression Omnibus Profile database; seven analyzed mouse oocytes specifically, one used COC mRNAs, and one studied human oocytes (supplemental Fig. 1, data sets GDS 2300, 2387, 814, 1266, 1978, 3294, 3295, 3256, and GDS1677) (63–71). These studies showed that IMD/ADM2 transcripts were expressed in oocytes of follicles at primary, secondary, small antral, and large antral stages as well as in one-cell and two-cell embryos, but not in eight-cell embryos or blastocysts (Table 1). In contrast, transcripts of the closely related ADM and amylin genes were either below the detection limit or negligible in these microarray studies. In addition, a microarray study of granulosa cells showed that IMD/ADM2 transcript is absent in these somatic cells (supplemental Fig. 1, data set GDS3422) (72). The results of in silico analyses were intriguing because polypeptide hormones signaling through G protein-coupled receptors have not been shown to play a major role in the regulation of somatic cell functions by oocytes.
TABLE 1.
List of microarray studies of IMD/ADM2 expression in mouse oocytes and embryos as well as in human oocytes
| References | Developmental stages of oocytesa | ||||
|---|---|---|---|---|---|
| Su et al. (63) | Immature GV +++ | Mature metaphase II + | |||
| Potireddy et al. (64) | Oocyte + | One-cell embryo ++ | |||
| Zeng et al. (65) | Oocyte +++ | One-cell embryo +++ | Two-cell embryo + | Eight-cell embryo − | Blastocyst − |
| Pan et al. (66) | Primordial follicle − | Primary follicle ++ | Secondary follicle ++ | Small antral follicle ++ | Large antral follicle ++ |
| Ma et al. (67) | Control oocyte ++ | Basonuclin knockdown oocyte + | |||
| Hernandez-Gonzalez et al. (68) | Control COCs ++ | hCG-treated COCs − | |||
| Pan et al. (69) | Immature GV +++ | Metaphase II ++ | |||
| Wan et al. (70) | Immature GV ++ | ||||
| Kocabas et al. (71) | Human metaphase II oocytes ++ | ||||
a + to +++, present; −, absent or minimal.
IMD/ADM2 Signaling Pathway Is Present within Individual Follicles
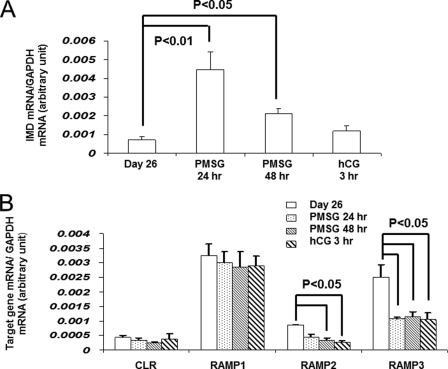
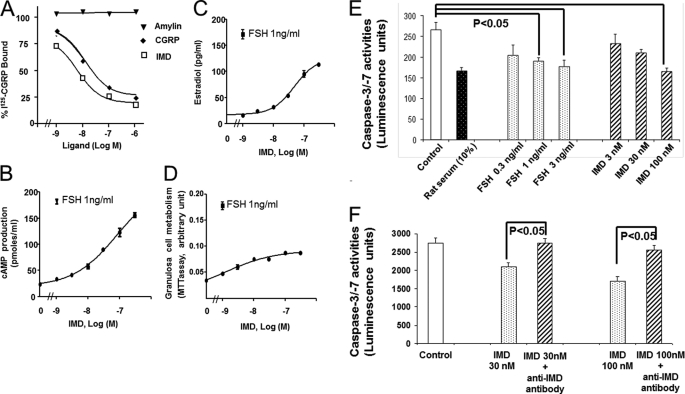
To support the in silico findings, we first investigated the expression of IMD in oocytes of follicles at select developmental stages. Quantitative PCR analysis showed that IMD/ADM2 transcripts are expressed in rat oocytes isolated from secondary and tertiary follicles and that PMSG treatments lead to significant increases of expression 24–48 h later (Fig. 1A, p < 0.05). In addition, quantitative PCR analyses showed that all components of the IMD/ADM2 receptor, including CLR and RAMP1, -2, and -3, are expressed in cumulus cells of immature rats (Fig. 1B), suggesting the presence of a paracrine IMD-CLR/RAMP signaling pathway in follicles. Consistent with this hypothesis, receptor-binding assays showed that IMD dose-dependently displaced the binding of an 125I-labeled CGRP tracer, which overlaps with IMD in receptor specificity, to granulosa/cumulus cells isolated from immature rats primed with diethylstilbestrol (Fig. 2A). In contrast, the distantly related amylin peptide was without effect on tracer binding. In addition, functional assays showed that IMD increases cAMP and estradiol production of cultured granulosa/cumulus cells in a dose-dependent manner (Fig. 2, B and C). These analyses collectively suggested that IMD/ADM2 represents an oocyte-derived ligand that could regulate surrounding cumulus cells during folliculogenesis.
FIGURE 1.
Expression of IMD/ADM2, CLR, and RAMP transcripts in follicle compartments. A, expression of IMD/ADM2 transcripts in oocytes of secondary and tertiary follicles from immature 26-day-old rats and those primed with PMSG for 1 or 2 days or PMSG for 2 days followed by a 3-h human chorionic gonadotropin treatment. B, expression of CLR, RAMP1, RAMP2, and RAMP3 transcripts in cumulus cells of immature female rats with or without gonadotropin treatments. Similar results were obtained in three separate experiments. Error bars, S.E.
FIGURE 2.
IMD/ADM2 increased survival of granulosa/cumulus cells cultured in vitro. A, competitive receptor-binding analysis using isolated granulosa/cumulus cells from immature 26-day-old rats primed with diethylstilbestrol (DES) for 4 days. Each data point represents the mean ± S.E. of triplicate samples. B and C, dose-dependent stimulation of cAMP (B) and estradiol (C) production by IMD and FSH in isolated granulosa/cumulus cells. Cell cultures were treated with different hormones in a serum-free condition for 16 h. Each data point represents the mean ± S.E. (error bars) of quadruplicate samples. D, dose-dependent stimulation of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) conversion by IMD and FSH in cultured granulosa/cumulus cells. E, dose-dependent inhibition of granulosa/cumulus cell caspase-3 and -7 activities by IMD and FSH. The activities of caspases were determined using a Caspase-Glo 3/7 luminescent assay (Promega) at 24 h after culture. Cultures treated with 10% rat serum were used as positive controls. F, the anti-apoptosis effect of IMD (represented by an inhibition of caspase-3 and -7 activities) was neutralized by coincubation with an anti-IMD antibody (n = 4). *, significantly different from controls (p < 0.05). Similar results were obtained in three separate experiments.
IMD/ADM2 Enhances Cumulus Cell Survival
Because IMD exhibited an anti-apoptosis effect on various tissues (56) and because cumulus cells partially depend on OSFs for survival (19), IMD could represent a novel survival factor for cumulus cells. Indeed, IMD increased the capability of granulosa/cumulus cells to metabolize 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Fig. 2D) and to suppress the activation of executioner caspases (caspase-3 and -7; Fig. 2E, p < 0.01) in a dose-dependent manner. Compared with controls, IMD treatments led to a >35% reduction of caspase-3/7 activities. The IMD action is specific because co-incubation with an affinity-purified anti-IMD antibody blunted the anti-apoptosis effect of IMD (Fig. 2F, p < 0.01). However, the anti-apoptosis effect of IMD appeared to be much smaller than that of FSH.
IMD/ADM2 Plays a Critical Role in the Maintenance of Normal Oocyte-Cumulus Cell Interactions
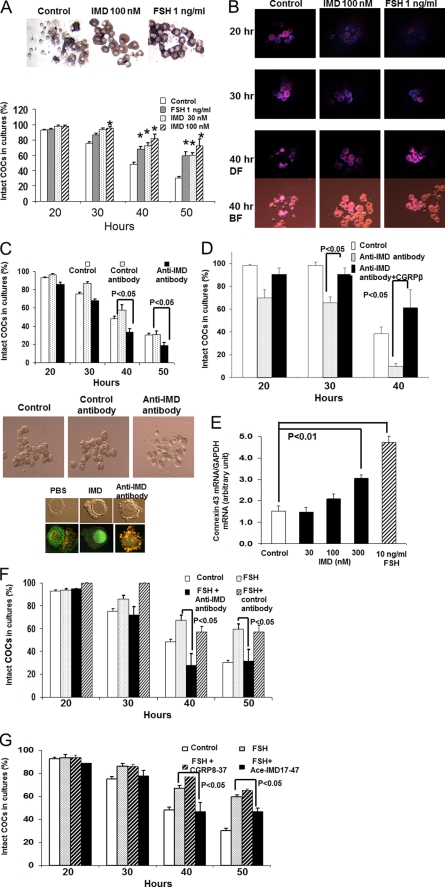
Because the effect of IMD appeared to be less robust as compared with that of FSH in multiple bioassays using granulosa/cumulus cells, it is possible that IMD only acts on a select population of follicular cells. To specifically study the effect of IMD on cells that are most pertinent to a regulation by oocytes, we next analyzed the ability of IMD to maintain the three-dimensional structure of cultured COCs. In these studies, we scored the total number of COCs that retained at least two or three layers of cumulus cells (referred to as “intact COCs” in the rest of this work) at 20, 30, 40, and 50 h after culture in the absence or presence of different hormones.
Under the serum-free culture condition, cumulus cells in the control group started to detach from COCs at 20 h after culture, and the percentage of intact COCs decreased from >90% at 20 h after culture to 75.2 ± 3.4, 49.1 ± 2.2, and 30.5 ± 2.3% at 30, 40, and 50 h, respectively (Fig. 3A, top left and bottom; see also the movie in supplemental Fig. 2A). By contrast, at 40 h after culture, 67.4 ± 5.2 and 81.7 ± 5.6% of oocytes retained layers of cumulus cells in the FSH and IMD treatment groups, respectively (Fig. 3A, lower panel, p < 0.05; see also movies in supplemental Fig. 2, B and C).
FIGURE 3.
IMD/ADM2 signaling is important for cumulus cell survival and normal cell-cell contacts in COCs. A, treatments of FSH or IMD reduced the detachment of cumulus cells from COCs cultured in vitro. The images were taken at 40 h after culture (top). The percentage of intact COCs was significantly increased by IMD or FSH at 40–50 h after culture (bottom, n = 4). *, significantly different from controls (p < 0.05). Similar results were obtained in five separate experiments. B, detection of cumulus-cell apoptosis by staining with propidium iodide (red fluorescence, 562–588 nm) and Hoechst 33342 (blue fluorescence, 461 nm). The staining of dead cells by propidium iodide was extensive in control COCs at 20 h after culture (top left). The staining in IMD-treated (top middle) or FSH-treated (top right) COCs was sparse at this time. At 40 h after culture, most oocytes in the control group were stripped of cumulus cells (bottom left panels; DF, dark field; BF, bright field). C, ablation of IMD signaling in COCs by an anti-IMD antibody led to accelerated detachment of cumulus cells from oocytes. The percentage of intact COCs was significantly reduced by the anti-IMD antibody treatment at 40 h after culture (bottom, n = 4). By contrast, the percentage of intact COCs in cultures was not affected by a control antibody. Staining with a mitochondria membrane potential sensor, JC-9, verified that COCs treated with the anti-IMD antibody have a perturbed membrane potential (orange-red fluorescence, top) at 2 h after culture, whereas COCs in the control group or cultures with 100 nm IMD exhibited normal mitochondrial membrane potentials (green fluorescence, top). D, treatment with a high dose of CGRPβ (300 nm) prevented the anti-IMD antibody-induced detachment of cumulus cells from COCs. E, IMD and FSH stimulated connexin 43 transcript expression in cumulus cells at 24 h after culture (p < 0.01). F, cotreatment with an anti-IMD antibody blocked the protective effect of FSH on the loss of intact COCs in cultures. G, cotreatment with an IMD antagonist (Ac-IMD(17–47)), but not with a CGRP antagonist (CGRP(8–37)), significantly reduced the protective effect of FSH on the loss of intact COCs in cultures. *, significantly different from controls (p < 0.05). Similar results were obtained in three separate experiments. Error bars, S.E.
Staining of COCs with propidium iodide showed that many cumulus cells in control COCs had died after 20 h of culture, whereas cell death was sparse in IMD- or FSH-treated COCs (Fig. 3B). In IMD- and FSH-treated COCs, dead cells became prevalent only after 40 h of culture (Fig. 3B). By this time, most oocytes in the control group had become completely denuded.
In addition, we noted that, unlike with the IMD treatment, the outer layer of cumulus cells of COCs treated with FSH, which can stimulate cumulus expansion in vitro at pharmacological doses (e.g. >100 ng/ml) (19, 73, 74), tended to become loose and detached from COCs by 30–40 h after culture (Fig. 3, A (top) and B (bottom right)).
To investigate whether endogenous IMD is critical for normal cell-cell contacts in COCs, we cultured COCs with or without an affinity-purified anti-IMD antibody. Interestingly, we found that treatments with the anti-IMD antibody, but not an affinity-purified control antibody (i.e. an anti-urocortin II antibody), significantly reduced the number of intact COCs as compared with the control group (Fig. 3C, p < 0.05, top and middle), suggesting that the sequestration of endogenous IMD fastens the disintegration of the tertiary structure of COCs.
Furthermore, we traced early signs of cell death in cultured COCs with a dual emission mitochondrial potential-sensitive dye, JC-9 (3,3′-dimethyl-β-naphthoxazolium iodide). As expected, we found that many cumulus cells that had been treated with the anti-IMD antibody exhibited intense orange-red fluorescence, which is indicative of a loss of mitochondrial membrane potential (Fig. 3C, bottom right). By contrast, most cumulus cells in control COCs exhibited intense green fluorescence, an indication of mitochondria membrane integrity (Fig. 3C, bottom left and middle). To analyze whether the blockage effect of the anti-IMD antibody was specific to the CLR-RAMP receptor signaling, we cultured COCs with the anti-IMD antibody together with a high dosage of a CLR/RAMP1 receptor agonist, CGRPβ peptide. As shown in Fig. 3D, cotreatment with CGRPβ reversed the action of the anti-IMD antibody, suggesting that the anti-IMD antibody targets endogenous IMD specifically (Fig. 3D).
Because the action of IMD is pertinent to cell-cell contacts and because gap junctions play a critical role in cell-cell contacts of mammalian cumulus cells, we also analyzed the expression of connexin 43, which was tightly regulated by gonadotropins, in cultured cumulus/granulosa cells (25, 26, 75). Consistent with the hypothesis that IMD could act by promoting the gap junctional network, quantitative PCR analyses showed that IMD treatments result in a dose-dependent increase of connexin 43 expression, similar to that found in cells treated with FSH (Fig. 3E, p < 0.01).
In COCs, IMD/ADM2 Functions Downstream of FSH Action
Because FSH plays a central role in ovarian folliculogenesis, it is possible that IMD represents a downstream effector of the FSH-mediated follicle development. Consistent with this hypothesis, we found that co-incubation of an anti-IMD antibody, but not a control antibody, significantly reduced the percentage of intact COCs in cultures with FSH (Fig. 3F, p < 0.05). Likewise, cotreatment with an IMD antagonist (Ac-IMD(17–47), 300 nm) significantly reduced the percentage of intact COC in the presence of FSH (Fig. 3G, p < 0.05). By contrast, treatments with a CLR/RAMP1-specific antagonist (CGRP(8–37)) had a negligible effect on levels of intact COCs in cultures. Thus, a most parsimonious explanation for these results is that IMD acts downstream of FSH to promote oocyte-cumulus cell interactions.
IMD/ADM2 Signaling Regulates Cell Cycle Progression in Granulosa/Cumulus Cells in Vivo
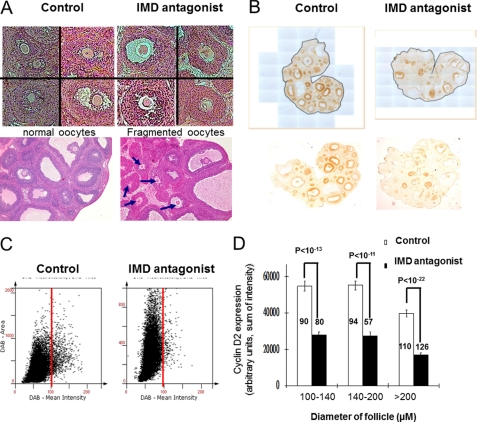
To explore the role of IMD signaling in vivo, we treated growing rat ovaries with intrabursal injections of an IMD antagonist (Ac-IMD(17–47)/d-Arg-33). Whereas the antagonist had a negligible effect on the ovarian weight at 2 days after a PMSG injection (240.1 ± 4.9 mg in the IMD antagonist group versus 246.2 ± 1.7 mg in the control group; n = 4), we found that many oocytes in secondary and early tertiary follicles of antagonist-treated ovaries were fragmented and that many oocytes in large antral follicles were devoid of surrounding cumulus cells, indicative of oocyte atresia (Fig. 4A). By contrast, most oocytes in contralateral ovaries injected with PBS appeared normal.
FIGURE 4.
Blockage of IMD/ADM2 signaling in vivo led to aberrant oocyte development and reduced cyclin D2 expression in growing follicles. A, intrabursal injection of an IMD antagonist led to increased incidences of fragmented or shrunk oocytes in secondary and tertiary follicles (right panels). By contrast, follicles of contralateral ovaries injected with PBS exhibited normal morphology (left panels). Fragmented oocytes in large antral follicles of an IMD antagonist-treated ovary are indicated by arrows (bottom right). Similar results were obtained in three separate experiments. B, ovaries that had received the intrabursal injection of PBS exhibited robust cyclin D2 expression in granulosa and cumulus cells of most growing follicles (brown stain, left). The intensity of immunoreactive cyclin D2 staining in contralateral ovaries that had received the IMD antagonist was restricted to a fraction of follicles (right). Images of representative ovaries include original photographs and the digitized versions for HistoQuest analysis (with a light blue background). C, scattergrams of staining intensity of whole ovaries that had received PBS (top) or the IMD antagonist injection (bottom). Results are displayed in dot plots, with the intensity of dots on the x axis and the DAB staining area on the y axis. Analyses of time-matched sections based on the HistoQuest software showed that the IMD antagonist treatment reduced the overall staining by more than 32%. The cut-off values for background staining were chosen manually using the forward/backward gating tool of the HistoQuest software. D, tissue cytometric analysis of staining in individual follicles of time-matched sections. Intrabursal injections of an IMD antagonist down-regulated cyclin D2 expression in secondary, small antral, and large antral follicles (p < 10−11). Eleven representative sections from 3–4 independent ovaries were analyzed in each treatment group. A total of 170 secondary, 151 early antral, and 236 large antral follicles were scanned individually and analyzed. Data are represented as the mean ± S.E. (error bars). The number of follicles analyzed in each data point is shown within or above the bar graph.
Because cyclin D2 is a rate-limiting activator of cell cycle progression in growing follicles and inducible by unknown OSFs (19, 76), we then analyzed the expression of cyclin D2 in ovaries that had received intrabursal injections. As expected, we found a robust cyclin D2 expression in granulosa/cumulus cells of control ovaries (Fig. 4B, left). By contrast, cyclin D2 expression was sparse in contralateral ovaries treated with the antagonist (Fig. 4B, right). Analysis of staining intensities with HistoQuest software showed that IMD antagonist treatments resulted in ∼32% reduction of cyclin D2 staining in antagonist-treated ovaries when compared with contralateral control ovaries. The difference was reflected in scattergrams of the staining area versus the mean intensity of ovarian sections (Fig. 4C). In addition, quantification of the staining in individual follicles with a diameter over 100 μm showed that the IMD antagonist reduced cyclin D2 expression by >45% in secondary (100–140 μm), early antral (140–200 μm), and large antral (>200 μm) follicles (Fig. 4D).
DISCUSSION
In mammals, the transition from a secondary follicle to an antral follicle during ovarian folliculogenesis is signaled by the differentiation of cumulus cells, the development of a COC, and the formation of a fluid-filled antrum. In the present study, we showed that IMD represents a novel oocyte-derived ligand that acts downstream of the FSH-stimulated follicle development and that the action of IMD in COCs is particularly pertinent to the maintenance of normal tertiary structure of COCs.
Overall, at least three lines of evidence support the hypothesis that oocyte-derived IMD plays a regulatory role in COCs. First, IMD was expressed in oocytes, and IMD prevented cumulus cell apoptosis. Second, the blockage of IMD signaling in COCs fastens the disintegration of the tertiary structure of COCs in the presence or absence of FSH in vitro. Third, intrabursal injections of an IMD antagonist led to oocyte atresia and a reduction of follicular cyclin D2 expression. Because cumulus cell differentiation and the COC structure are not decimated in animals deficient for GDF9 and/or BMP15 (36), it is possible that oocyte-derived IMD plays a unique role in the regulation of oocyte-cumulus cell interaction. In addition, our study may explain why cumulus cells are characteristically less susceptible to apoptosis as compared with mural granulosa cells (19).
Furthermore, our findings have several implications for how a COC maintains its biochemical and physical identities during folliculogenesis. First, earlier studies have demonstrated the presence of OSFs that are specifically important for the maintenance of the three-dimensional organization of COCs but without an effect on cumulus expansion (27). Although the factor(s) responsible for the maintenance of three-dimensional organization of cumulus cells remains to be studied, our study suggests that IMD could be part of the signaling cascade that maintains a cohesive COC during folliculogenesis. Accordingly, we speculate that the development of COCs could be regulated by both stimulatory and counteractive factors (Fig. 5). The BMP family of OSFs (i.e. GDF9 and BMP15) mainly play stimulatory roles geared toward follicle growth and final maturation by promoting proliferation and differentiation of cumulus cells as well as promoting cumulus expansion or mucification (19, 24, 29). By contrast, IMD promotes sustained interactions between the oocyte and companion cells and provides a “magnet-like” or “stationary” signal to cumulus cells prior to the luteinizing hormone-mediated cumulus expansion. This model is consistent with the finding that IMD is more effective in maintaining a “cohered COC structure” as compared with FSH in vitro (Fig. 3A). It is conceivable that, in the absence of a factor, such as IMD, the proexpansion BMP family proteins could trigger premature cumulus expansion and abnormal oocyte development as found in mice deficient for pentraxin 3 (78). Moreover, we speculate that the reduction of IMD expression in oocytes following luteinizing hormone receptor activation (Fig. 1A) may provide a signal to reduce gap junction communication to facilitate cumulus expansion after the luteinizing hormone surge (25, 26, 28, 29, 75, 79–81).
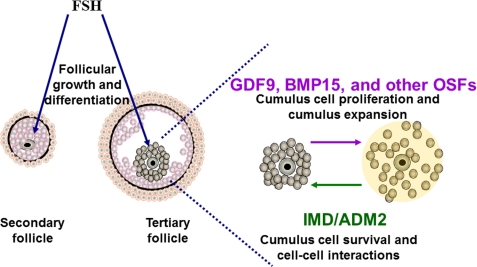
FIGURE 5.
Schematic representation of the role of IMD/ADM2 signaling in oocyte-cumulus cell interactions. The endocrine FSH from the pituitary stimulates follicular growth by acting on granulosa and cumulus cells. In parallel, GDF9, BMP15, and other OSFs from oocytes coordinate the folliculogenesis and final maturation process by stimulating cell proliferation and cumulus expansion. By contrast, the IMD/ADM2 signaling from oocytes may provide a stationary signal to maintain cumulus cell survival and cell-cell contacts in COCs prior to cumulus cell expansion.
Second, our in vitro data implied that FSH promotes oocyte-cumulus cell interactions indirectly by stimulating cumulus cells, which, in turn, may signal oocytes to secrete IMD. Although it is not clear how FSH promotes IMD signaling in COCs, we speculate that estrogens might play a role in this process because the IMD/ADM2 gene promoter contains estrogen response element sequences, and the expression of IMD is, at least in the pituitary, estrogen-dependent (58). In addition, it is worth noting that IMD/ADM2 promoter contains multiple hypoxia response elements for interacting with HIF-1, and the expression of IMD in select tissues increases under hypoxic conditions (59). Because the follicular oxygen concentration is inversely correlated with follicle diameter, and COC microenvironment becomes hypoxic as it becomes more distant from the vasculature when the follicle grows in size (82), oxygen tension may play a role in fine-tuning the expression of IMD in COCs. Likewise, we speculate the antiparallel gradients of IMD and oxygen tension surrounding oocytes may constitute part of the mechanism that allows cumulus cells to differentiate differently from mural granulosa cells that are distant from the oocyte.
Finally, it is important to note that future studies of transgenic mice with deficiency of IMD/ADM2 are needed to gain a better understanding of the exact role of IMD signaling in folliculogenesis. Because mice deficient for CLR (77) or IMD/ADM24 are embryonically lethal, a conditional knockdown approach would be necessary to dissect the intricate communication network between oocyte and cumulus cells as well as for improving our understanding of human reproduction.
Supplementary Material
Acknowledgments
We thank Yi Wei for technical assistance. We thank Drs. Aaron J. W. Hsueh and Renee A. Reijo Pera (Stanford University) for encouragement. C.L.C. further expresses gratitude for the encouragement of Drs. Hong Yuan Huang and Ying Ming Lai (Chang Gung Memorial Hospital, Taiwan).
This work was supported, in whole or in part, by National Institutes of Health Grant DK70652 (to S. Y. T. H.). This work was also supported by Avon Foundation Research Award 02-2009-054 (to S. Y. T. H.), New Century Health Care Promotion Foundation, Taiwan, Grant NCHCPF-94001 (to C. L. C.), and Chang Gung Memorial Hospital Grant CMRPG34002 (to C. L. C.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1 and Figs. 1 and 2.
Y. Takei, personal communication.
- BMP
- bone morphogenetic protein
- COC
- cumulus cell-enclosed oocyte complex
- OSF
- oocyte-secreted factor
- EST
- expressed sequence tag
- IMD
- intermedin
- ADM
- adrenomedullin
- CLR
- calcitonin receptor-like receptor
- PMSG
- pregnant mare serum gonadotropin
- RAMP
- receptor activity-modifying protein.
REFERENCES
- 1. Zachow R. J., Tash J. S., Terranova P. F. (1993) Endocrinology 133, 2269–2276 [DOI] [PubMed] [Google Scholar]
- 2. De A., Park J. I., Kawamura K., Chen R., Klein C., Rauch R., Mulders S. M., Sollewijn Gelpke M. D., Hsueh A. J. (2006) Mol. Endocrinol. 20, 2528–2538 [DOI] [PubMed] [Google Scholar]
- 3. Park J. Y., Su Y. Q., Ariga M., Law E., Jin S. L., Conti M. (2004) Science 303, 682–684 [DOI] [PubMed] [Google Scholar]
- 4. Hernandez E. R., Hurwitz A., Payne D. W., Dharmarajan A. M., Purchio A. F., Adashi E. Y. (1990) Endocrinology 127, 2804–2811 [DOI] [PubMed] [Google Scholar]
- 5. Neulen J., Raczek S., Pogorzelski M., Grunwald K., Yeo T. K., Dvorak H. F., Weich H. A., Breckwoldt M. (1998) Mol. Hum. Reprod 4, 203–206 [DOI] [PubMed] [Google Scholar]
- 6. Shimasaki S., Zachow R. J., Li D., Kim H., Iemura S., Ueno N., Sampath K., Chang R. J., Erickson G. F. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 7282–7287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Adashi E. Y., Resnick C. E., D'Ercole A. J., Svoboda M. E., Van Wyk J. J. (1985) Endocr. Rev. 6, 400–420 [DOI] [PubMed] [Google Scholar]
- 8. Richards J. S., Russell D. L., Ochsner S., Espey L. L. (2002) Annu. Rev. Physiol. 64, 69–92 [DOI] [PubMed] [Google Scholar]
- 9. Hsueh A. J., Eisenhauer K., Chun S. Y., Hsu S. Y., Billig H. (1996) Recent Prog. Horm. Res. 51, 433–455; discussion 455–436 [PubMed] [Google Scholar]
- 10. Richards J. S. (1994) Endocr. Rev. 15, 725–751 [DOI] [PubMed] [Google Scholar]
- 11. Hillensjö T., Dekel N., Ahrén K. (1976) Acta. Physiol. Scand. 96, 558–568 [DOI] [PubMed] [Google Scholar]
- 12. Tsafriri A., Lindner H. R., Zor U., Lamprecht S. A. (1972) J. Reprod. Fertil. 31, 39–50 [DOI] [PubMed] [Google Scholar]
- 13. Gwatkin R. B., Andersen O. F. (1976) Life Sci. 19, 527–536 [DOI] [PubMed] [Google Scholar]
- 14. Moor R. M., Trounson A. O. (1977) J. Reprod. Fertil. 49, 101–109 [DOI] [PubMed] [Google Scholar]
- 15. Thibault C. (1977) J. Reprod. Fertil. 51, 1–15 [DOI] [PubMed] [Google Scholar]
- 16. Thibault C., Gerard M., Menezo Y. (1975) J. Reprod. Fertil. 45, 605–610 [DOI] [PubMed] [Google Scholar]
- 17. Miller W. A., Jagiello G. (1973) Fertil. Steril. 24, 609–617 [PubMed] [Google Scholar]
- 18. Eppig J. J., Wigglesworth K., Pendola F. L. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 2890–2894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gilchrist R. B., Lane M., Thompson J. G. (2008) Hum. Reprod. Update 14, 159–177 [DOI] [PubMed] [Google Scholar]
- 20. Richards J. S. (2005) Mol. Cell. Endocrinol. 234, 75–79 [DOI] [PubMed] [Google Scholar]
- 21. Thibault C. (1973) in Regulation of Mammalian Reproduction (Crozier S. J., Corfman P., Condliffe C. C. eds) pp. 231–240, Thomas, Springfield, IL [Google Scholar]
- 22. Bachvarova R., Baran M. M., Tejblum A. (1980) J. Exp. Zool 211, 159–169 [DOI] [PubMed] [Google Scholar]
- 23. Canipari R. (2000) Hum. Reprod. Update 6, 279–289 [DOI] [PubMed] [Google Scholar]
- 24. Eppig J. J. (2001) Reproduction 122, 829–838 [DOI] [PubMed] [Google Scholar]
- 25. Juneja S. C., Barr K. J., Enders G. C., Kidder G. M. (1999) Biol. Reprod. 60, 1263–1270 [DOI] [PubMed] [Google Scholar]
- 26. Simon A. M., Goodenough D. A., Li E., Paul D. L. (1997) Nature 385, 525–529 [DOI] [PubMed] [Google Scholar]
- 27. Vanderhyden B. C., Caron P. J., Buccione R., Eppig J. J. (1990) Dev. Biol. 140, 307–317 [DOI] [PubMed] [Google Scholar]
- 28. Wiesen J. F., Midgley A. R., Jr. (1993) Endocrinology 133, 741–746 [DOI] [PubMed] [Google Scholar]
- 29. Buccione R., Schroeder A. C., Eppig J. J. (1990) Biol. Reprod. 43, 543–547 [DOI] [PubMed] [Google Scholar]
- 30. Salustri A., Yanagishita M., Hascall V. C. (1990) Dev. Biol. 138, 26–32 [DOI] [PubMed] [Google Scholar]
- 31. Buccione R., Vanderhyden B. C., Caron P. J., Eppig J. J. (1990) Dev. Biol. 138, 16–25 [DOI] [PubMed] [Google Scholar]
- 32. Byskov A. G., Yding Andersen C., Hossaini A., Guoliang X. (1997) Mol. Reprod Dev 46, 296–305 [DOI] [PubMed] [Google Scholar]
- 33. Tsafriri A., Chun S. Y., Zhang R., Hsueh A. J., Conti M. (1996) Dev. Biol. 178, 393–402 [DOI] [PubMed] [Google Scholar]
- 34. Diaz F. J., Wigglesworth K., Eppig J. J. (2007) Dev. Biol. 305, 300–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Matzuk M. M., Burns K. H., Viveiros M. M., Eppig J. J. (2002) Science 296, 2178–2180 [DOI] [PubMed] [Google Scholar]
- 36. Sugiura K., Su Y. Q., Diaz F. J., Pangas S. A., Sharma S., Wigglesworth K., O'Brien M. J., Matzuk M. M., Shimasaki S., Eppig J. J. (2007) Development 134, 2593–2603 [DOI] [PubMed] [Google Scholar]
- 37. Pangas S. A., Matzuk M. M. (2005) Biol. Reprod. 73, 582–585 [DOI] [PubMed] [Google Scholar]
- 38. Su Y. Q., Wu X., O'Brien M. J., Pendola F. L., Denegre J. N., Matzuk M. M., Eppig J. J. (2004) Dev. Biol. 276, 64–73 [DOI] [PubMed] [Google Scholar]
- 39. McKenzie L. J., Pangas S. A., Carson S. A., Kovanci E., Cisneros P., Buster J. E., Amato P., Matzuk M. M. (2004) Hum. Reprod. 19, 2869–2874 [DOI] [PubMed] [Google Scholar]
- 40. Dong J., Albertini D. F., Nishimori K., Kumar T. R., Lu N., Matzuk M. M. (1996) Nature 383, 531–535 [DOI] [PubMed] [Google Scholar]
- 41. Yoshino O., McMahon H. E., Sharma S., Shimasaki S. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 10678–10683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yan C., Wang P., DeMayo J., DeMayo F. J., Elvin J. A., Carino C., Prasad S. V., Skinner S. S., Dunbar B. S., Dube J. L., Celeste A. J., Matzuk M. M. (2001) Mol. Endocrinol. 15, 854–866 [DOI] [PubMed] [Google Scholar]
- 43. Sugiura K., Pendola F. L., Eppig J. J. (2005) Dev. Biol. 279, 20–30 [DOI] [PubMed] [Google Scholar]
- 44. Akiyama-Oda Y., Oda H. (2003) Development 130, 1735–1747 [DOI] [PubMed] [Google Scholar]
- 45. Shravage B. V., Altmann G., Technau M., Roth S. (2007) Development 134, 2261–2271 [DOI] [PubMed] [Google Scholar]
- 46. Chang H., Brown C. W., Matzuk M. M. (2002) Endocr. Rev. 23, 787–823 [DOI] [PubMed] [Google Scholar]
- 47. Elvin J. A., Clark A. T., Wang P., Wolfman N. M., Matzuk M. M. (1999) Mol. Endocrinol. 13, 1035–1048 [DOI] [PubMed] [Google Scholar]
- 48. Gilchrist R. B., Ritter L. J., Myllymaa S., Kaivo-Oja N., Dragovic R. A., Hickey T. E., Ritvos O., Mottershead D. G. (2006) J. Cell Sci. 119, 3811–3821 [DOI] [PubMed] [Google Scholar]
- 49. Emanuelsson O., Brunak S., von Heijne G., Nielsen H. (2007) Nat. Protoc. 2, 953–971 [DOI] [PubMed] [Google Scholar]
- 50. Grimmond S. M., Miranda K. C., Yuan Z., Davis M. J., Hume D. A., Yagi K., Tominaga N., Bono H., Hayashizaki Y., Okazaki Y., Teasdale R. D. (2003) Genome Res. 13, 1350–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Clark H. F., Gurney A. L., Abaya E., Baker K., Baldwin D., Brush J., Chen J., Chow B., Chui C., Crowley C., Currell B., Deuel B., Dowd P., Eaton D., Foster J., Grimaldi C., Gu Q., Hass P. E., Heldens S., Huang A., Kim H. S., Klimowski L., Jin Y., Johnson S., Lee J., Lewis L., Liao D., Mark M., Robbie E., Sanchez C., Schoenfeld J., Seshagiri S., Simmons L., Singh J., Smith V., Stinson J., Vagts A., Vandlen R., Watanabe C., Wieand D., Woods K., Xie M. H., Yansura D., Yi S., Yu G., Yuan J., Zhang M., Zhang Z., Goddard A., Wood W. I., Godowski P., Gray A. (2003) Genome Res. 13, 2265–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ben-Shlomo I., Yu Hsu S., Rauch R., Kowalski H. W., Hsueh A. J. (2003) Sci. STKE 2003, RE9. [DOI] [PubMed] [Google Scholar]
- 53. Roh J., Chang C. L., Bhalla A., Klein C., Hsu S. Y. (2004) J. Biol. Chem. 279, 7264–7274 [DOI] [PubMed] [Google Scholar]
- 54. Takei Y., Inoue K., Ogoshi M., Kawahara T., Bannai H., Miyano S. (2004) FEBS Lett. 556, 53–58 [DOI] [PubMed] [Google Scholar]
- 55. McLatchie L. M., Fraser N. J., Main M. J., Wise A., Brown J., Thompson N., Solari R., Lee M. G., Foord S. M. (1998) Nature 393, 333–339 [DOI] [PubMed] [Google Scholar]
- 56. Bell D., McDermott B. J. (2008) Br. J. Pharmacol. 153, Suppl. 1, S247–S262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Smith R. S., Jr., Gao L., Bledsoe G., Chao L., Chao J. (2009) Am. J. Physiol. Heart Circ. Physiol. 297, H1040–H1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lin Chang C., Roh J., Park J. I., Klein C., Cushman N., Haberberger R. V., Hsu S. Y. (2005) Mol. Endocrinol. 19, 2824–2838 [DOI] [PubMed] [Google Scholar]
- 59. Pfeil U., Aslam M., Paddenberg R., Quanz K., Chang C. L., Park J. I., Gries B., Rafiq A., Faulhammer P., Goldenberg A., Papadakis T., Noll T., Hsu S. Y., Weissmann N., Kummer W. (2009) Am. J. Physiol. Lung Cell Mol. Physiol. 297, L837–L845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. McGee E., Spears N., Minami S., Hsu S. Y., Chun S. Y., Billig H., Hsueh A. J. (1997) Endocrinology 138, 2417–2424 [DOI] [PubMed] [Google Scholar]
- 61. Roberts A. E., Arbogast L. K., Friedman C. I., Cohn D. E., Kaumaya P. T., Danforth D. R. (2007) Biol. Reprod. 76, 218–223 [DOI] [PubMed] [Google Scholar]
- 62. Chang C. L., Cai J. J., Lo C., Amigo J., Park J. I., Hsu S. Y. (2011) Genome Res. 21, 21–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Su Y. Q., Sugiura K., Woo Y., Wigglesworth K., Kamdar S., Affourtit J., Eppig J. J. (2007) Dev. Biol. 302, 104–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Potireddy S., Vassena R., Patel B. G., Latham K. E. (2006) Dev. Biol. 298, 155–166 [DOI] [PubMed] [Google Scholar]
- 65. Zeng F., Baldwin D. A., Schultz R. M. (2004) Dev. Biol. 272, 483–496 [DOI] [PubMed] [Google Scholar]
- 66. Pan H., O'brien M. J., Wigglesworth K., Eppig J. J., Schultz R. M. (2005) Dev. Biol. 286, 493–506 [DOI] [PubMed] [Google Scholar]
- 67. Ma J., Zeng F., Schultz R. M., Tseng H. (2006) Development 133, 2053–2062 [DOI] [PubMed] [Google Scholar]
- 68. Hernandez-Gonzalez I., Gonzalez-Robayna I., Shimada M., Wayne C. M., Ochsner S. A., White L., Richards J. S. (2006) Mol. Endocrinol. 20, 1300–1321 [DOI] [PubMed] [Google Scholar]
- 69. Pan H., Ma P., Zhu W., Schultz R. M. (2008) Dev. Biol. 316, 397–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wan L. B., Pan H., Hannenhalli S., Cheng Y., Ma J., Fedoriw A., Lobanenkov V., Latham K. E., Schultz R. M., Bartolomei M. S. (2008) Development 135, 2729–2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kocabas A. M., Crosby J., Ross P. J., Otu H. H., Beyhan Z., Can H., Tam W. L., Rosa G. J., Halgren R. G., Lim B., Fernandez E., Cibelli J. B. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 14027–14032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pangas S. A., Jorgez C. J., Tran M., Agno J., Li X., Brown C. W., Kumar T. R., Matzuk M. M. (2007) Mol. Endocrinol. 21, 2458–2471 [DOI] [PubMed] [Google Scholar]
- 73. Diaz F. J., O'Brien M. J., Wigglesworth K., Eppig J. J. (2006) Dev. Biol. 299, 91–104 [DOI] [PubMed] [Google Scholar]
- 74. Salustri A., Yanagishita M., Hascall V. C. (1989) J. Biol. Chem. 264, 13840–13847 [PubMed] [Google Scholar]
- 75. Gilula N. B., Epstein M. L., Beers W. H. (1978) J. Cell Biol. 78, 58–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sicinski P., Donaher J. L., Geng Y., Parker S. B., Gardner H., Park M. Y., Robker R. L., Richards J. S., McGinnis L. K., Biggers J. D., Eppig J. J., Bronson R. T., Elledge S. J., Weinberg R. A. (1996) Nature 384, 470–474 [DOI] [PubMed] [Google Scholar]
- 77. Dackor R. T., Fritz-Six K., Dunworth W. P., Gibbons C. L., Smithies O., Caron K. M. (2006) Mol. Cell. Biol. 26, 2511–2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Varani S., Elvin J. A., Yan C., DeMayo J., DeMayo F. J., Horton H. F., Byrne M. C., Matzuk M. M. (2002) Mol. Endocrinol. 16, 1154–1167 [DOI] [PubMed] [Google Scholar]
- 79. Sela-Abramovich S., Edry I., Galiani D., Nevo N., Dekel N. (2006) Endocrinology 147, 2280–2286 [DOI] [PubMed] [Google Scholar]
- 80. Herlands R. L., Schultz R. M. (1984) J. Exp Zool. 229, 317–325 [DOI] [PubMed] [Google Scholar]
- 81. Bruzzone R., White T. W., Paul D. L. (1996) Eur. J. Biochem. 238, 1–27 [DOI] [PubMed] [Google Scholar]
- 82. Fischer B., Künzel W., Kleinstein J., Gips H. (1992) Eur. J. Obstet. Gynecol. Reprod. Biol. 43, 39–43 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.