Background: Many bacteria synthesize spermidine but lack orthologues of polyamine biosynthetic enzymes S-adenosylmethionine decarboxylase and spermidine synthase.
Results: An alternative spermidine biosynthetic pathway is essential in Campylobacter jejuni.
Conclusion: The alternative route via carboxyspermidine is the dominant pathway in the human gut microbiota and deep sea hydrothermal vents.
Significance: A multiplicity of polyamine biosynthetic pathways exist, providing novel targets for development of antimicrobial drugs.
Keywords: Biosynthesis, Campylobacter, Metabolism, Microbiology, Polyamines, Comparative Genomics, Evolution, Gut Microbiota
Abstract
The availability of fully sequenced bacterial genomes has revealed that many species known to synthesize the polyamine spermidine lack the spermidine biosynthetic enzymes S-adenosylmethionine decarboxylase and spermidine synthase. We found that such species possess orthologues of the sym-norspermidine biosynthetic enzymes carboxynorspermidine dehydrogenase and carboxynorspermidine decarboxylase. By deleting these genes in the food-borne pathogen Campylobacter jejuni, we found that the carboxynorspermidine decarboxylase orthologue is responsible for synthesizing spermidine and not sym-norspermidine in vivo. In polyamine auxotrophic gene deletion strains of C. jejuni, growth is highly compromised but can be restored by exogenous sym-homospermidine and to a lesser extent by sym-norspermidine. The alternative spermidine biosynthetic pathway is present in many bacterial phyla and is the dominant spermidine route in the human gut, stomach, and oral microbiomes, and it appears to have supplanted the S-adenosylmethionine decarboxylase/spermidine synthase pathway in the gut microbiota. Approximately half of the gut Firmicutes species appear to be polyamine auxotrophs, but all encode the potABCD spermidine/putrescine transporter. Orthologues encoding carboxyspermidine dehydrogenase and carboxyspermidine decarboxylase are found clustered with an array of diverse putrescine biosynthetic genes in different bacterial genomes, consistent with a role in spermidine, rather than sym-norspermidine biosynthesis. Due to the pervasiveness of ϵ-proteobacteria in deep sea hydrothermal vents and to the ubiquity of the alternative spermidine biosynthetic pathway in that phylum, the carboxyspermidine route is also dominant in deep sea hydrothermal vents. The carboxyspermidine pathway for polyamine biosynthesis is found in diverse human pathogens, and this alternative spermidine biosynthetic route presents an attractive target for developing novel antimicrobial compounds.
Introduction
Polyamines are primordial polycationic cellular metabolites that are found in almost all cells. The most ubiquitous polyamine, the triamine spermidine (Fig. 1A), is synthesized from the diamine putrescine (1,4-diaminobutane) by addition of an aminopropyl group donated by decarboxylated S-adenosylmethionine (Fig. 1B). The following two enzymes are known to be involved in spermidine biosynthesis from putrescine (Fig. 1B): S-adenosylmethionine decarboxylase (AdoMetDC)2 (1) and the aminopropyltransferase spermidine synthase (SpdSyn) (2). Both AdoMetDC and SpdSyn are found in almost all eukaryotes, most archaea, and in many bacterial phyla, including the species Escherichia coli (3), Bacillus subtilis (4), and Thermotoga maritima (5). However, some bacterial species use the triamine sym-homospermidine (Fig. 1A) rather than spermidine, synthesized by homospermidine synthase or a deoxyhypusine synthase-like enzyme (6). It is therefore surprising that analysis of complete bacterial genomes reveals that there are many bacterial species that are known to synthesize spermidine but do not possess AdoMetDC or SpdSyn orthologues, e.g. almost all ϵ-proteobacteria (7), Bacteroides, Prevotella, and Porphyromonas species within the Bacteroidetes phylum (8), species of Clostridium and Eubacterium, Ruminococcus, and Butyvibrio in the Firmicutes phylum (9), and members of the Deinococcus phylum (10), among others. Recently, we showed that an alternative sym-norspermidine (Fig. 1A) biosynthetic pathway synthesized sym-norspermidine from 1,3-diaminopropane in Vibrio cholerae, using aspartate β-semialdehyde, rather than decarboxylated S-adenosylmethionine as an aminopropyl group donor (11). The enzymes involved in the synthesis of sym-norspermidine by the alternative pathway are carboxynorspermidine dehydrogenase (CANSDH) and carboxynorspermidine decarboxylase (CANSDC) (11). We speculated that in the majority of bacterial species that encode orthologues of CANSDH and CANSDC, the alternative pathway was used mainly for the synthesis of spermidine from putrescine, rather than the synthesis of sym-norspermidine from 1,3-diaminopropane. This assumption was made because most species possessing CANSDH and CANSDC orthologues do not possess orthologues of the enzymes required to synthesize 1,3-diaminopropane (diaminobutyrate aminotransferase (DABA AT) and diaminobutyrate decarboxylase (DABA DC)) nor orthologues of AdoMetDC and SpdSyn (11). Here, we show that the food-borne pathogen Campylobacter jejuni, an ϵ-proteobacterium responsible for the majority of food poisoning cases in developed countries (12), synthesizes spermidine by the aspartate β-semialdehyde pathway. We also show that spermidine and carboxyspermidine decarboxylase (CASDC) are critical for growth of C. jejuni and that the function of spermidine in cell proliferation can be replaced by sym-homospermidine and to a lesser extent by sym-norspermidine. Orthologous genes potentially encoding carboxyspermidine dehydrogenase (CASDH) and CASDC are found in many bacterial phyla, including important human pathogens, and surprisingly, CASDH/CASDC constitute the dominant polyamine biosynthetic pathway in deep sea hydrothermal vents and in the human gut microbiota.
FIGURE 1.
Bacterial polyamines and decarboxylated S-adenosylmethionine pathway for spermidine biosynthesis. A, aminopropyl groups are shown in red; aminobutyl groups are shown in blue. B, biosynthesis of spermidine from putrescine by transfer of an aminopropyl group from decarboxylated S-adenosylmethionine.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Growth Conditions
All strains were derived from the sequenced C. jejuni strain 81116 (NCTC 11828) (13). Bacteria were grown at 37 °C under microaerobic conditions (10% CO2, 85% N2, 5% O2) on an orbital shaker (200 rpm) inside a MACS-MG-1000 controlled atmosphere workstation (DW Scientific). When required, kanamycin or chloramphenicol was added to the growth medium at 50 or 25 μg/ml, respectively. For growth assays, strains were grown overnight in Brucella broth (14) and washed twice in polyamine-deficient medium (Dulbecco's modified Eagle's medium, 3.7 g/liter sodium bicarbonate, 500 mg/liter aspartic acid, 500 mg/liter serine, 200 mg/liter sodium pyruvate, 100 mg/liter cysteine, 500 mg/liter glutamic acid, 100 mg/liter proline, 5 mg/liter ferric III chloride-6-hydrate, 10 mg/liter adenine sulfate (all components supplied by Sigma)). Cells were diluted to an initial A600 nm of 0.01 in polyamine-deficient medium ± polyamines or agmatine and incubated as described above.
HPLC Analysis of Cellular Polyamines
Cells were harvested by centrifugation (2880 × g, 10 min) and washed twice in polyamine-deficient medium and then twice in phosphate-buffered saline. MOPS lysis buffer (100 mm MOPS, 50 mm NaCl, 20 mm MgCl2) at pH 8.0 was added at 5 μl per mg of cell fresh weight, and cells were subjected to three cycles of freeze/thawing. Trichloroacetic acid was added to a final concentration of 10%, and cells were incubated on ice for 5 min. After centrifugation (18,000 × g, 5 min, 4 °C), 5 μl of supernatant were derivatized using the AccQ-Fluor reagent kit for labeling amino acids (Waters). For normalization, 1,7-diaminoheptane was included as an internal standard. Labeled polyamines were separated by HPLC using a Luna 5-μm C18 (2) 100A column (250 × 4.6 mm; Phenomenex) with fluorescence detection (excitation 248 nm, emission 398 nm). Solvent A was 70 mm acetic acid, 25 mm triethylamine, pH 4.82; solvent B was 80% acetonitrile, 20% H2O (v/v); solvent C was methanol, and the gradient was run for 65 min at a flow rate of 1.2 ml/min with the following concentrations: t = 0 min, 100% A; t = 1 min, 78% A, 22% B; t = 27 min, 55% A, 39% B, 6% C; t = 27.5 min, 53% A, 33% B, 14% C; t = 34 min, 20% A, 10% B, 70% C; t = 37 min, 100% B; t = 58 min, 100% A.
Construction of Gene Deletion Mutants of C. jejuni 81116
Suicide plasmids were constructed for each gene knock-out. Each plasmid consisted of the DNA flanking the gene sequence to be knocked out, with an internal BamHI site into which a selectable marker kanamycin resistance cassette was located. The rest of the vector, pGEM®-T Easy (Promega), does not replicate in C. jejuni. The two regions flanking the insertion site were amplified using genomic DNA from 81116 as a template, and the ends of these were designed to overlap and incorporate a BamHI site. Primer “gene” disruption 1 was used with gene disruption 2 in PCR (each gene and the primer sequences are shown in supplemental Table S1). Primer gene disruption 3 was used with gene disruption 4. The PCR was performed with HotStart Taq polymerase (Qiagen) according to the manufacturer's instructions. Cycle parameters are as follows: 95 °C for 15 min; 95 °C for 30 s and 50 °C for 30 s, 72 °C for 2 min for 30 cycles, followed by 72 °C for 15 min. Each product was purified using QIAquick® PCR purification kit (Qiagen) according to the manufacturer's instructions. Products were then combined and diluted 1:20, and 1 μl was used as template for a further PCR using primer gene disruption 1 and primer gene disruption 4. This PCR used the same cycling parameters. The product was first purified using QIAquick® PCR purification kit (Qiagen) according to the manufacturer's instructions, before being ligated into pGEM®-T Easy and transformed into E. coli TOP10 competent cells (Invitrogen). Clones (white on X-Gal LB plates) were checked by sequencing using M13 universal forward and reverse primers and correct inserts retained. The resultant vectors have a unique BamHI site flanked by targeting DNA in the pGEM®-T Easy backbone.
To insert the selection marker, a BamHI-flanked kanamycin resistance cassette was ligated into each vector digested with BamHI and transformed into E. coli TOP10 competent cells (Invitrogen). Selection was performed on kanamycin −LB plates, and then plasmids were checked by sequencing using M13 universal forward and reverse primers and correct inserts retained. As these pGEM®-T Easy-based plasmids were unable to replicate in C. jejuni, they act as suicide vectors introducing the selectable marker into each gene locus by homologous recombination. The suicide plasmids were transformed into C. jejuni 81116 by electroporation and selected on Brucella solid medium containing kanamycin. Gene deletions were verified by retaining three independent clones, which were checked by isolating genomic DNA from each and then using PCR to assess the presence of the kanamycin resistance gene in the target locus using primers outside the disruption cassette and inside kanamycin resistance gene.
Complementation of CASDC Gene Deletion Mutant with the Cognate CASDC Gene
The CASDC gene was cloned under a constitutive promoter, the low level metK promoter, in a suicide vector that contained the flanking regions corresponding to the pseudogene cj0046 of C. jejuni 11168 CASDC gene deletion strain (ΔC8J_0715). The promoter-gene antibiotic selectable marker was inserted into the chromosome by homologous recombination to give a stable single copy of the gene (c_ΔC8J_0715).
Synthesis of sym-Homospermidine
sym-Homospermidine 1 was prepared as outlined in Scheme 1. 1,7-(bis)Cyano-N-benzyl-4-azaheptane (compound 3) was synthesized using a modification of the procedure of Covassin et al. (15). A 100-ml round bottom flask was charged with 17.97 g (130.2 mmol) of anhydrous potassium carbonate and 1.80 g (10.85 mmol) of potassium iodide in 40 ml of acetonitrile. A 4.69-g portion of N-benzylamine 2 (4.79 ml, 43.34 mmol) was then added, followed by the slow addition of 9.87 g (9.11 ml, 95.35 mmol) of 4-chlorobutyronitrile in 20 ml of acetonitrile. The reaction was refluxed for 16 h and was then cooled to room temperature and filtered, and the solvent was removed in vacuo. The yellow oily residue was chromatographed on silica gel (hexane/EtOAc 4:1) to afford 8.1 g of pure 3 as a pale yellow oil. The following procedures were used: 1H NMR (400 MHz, CDCl3,) δ 7.38–7.22 (m, 5H), 3.54 (s, 2H), 2.55 (t, J = 6.8 Hz, 4H), 2.36 (t, J = 6.8 Hz, 4H), and 1.79 (quintet, J = 6.8 Hz, 4H).
SCHEME 1.
Synthetic strategy for sym-homospermidine.
1,7-Diamino-5-azaheptane (Homospermidine), 4
A 3.0-g portion (12.39 mmol) of compound 3, 5.0 ml of concentrated ammonium hydroxide, and 5.0 ml of Raney nickel (50% in water) were placed in a hydrogenation bottle, followed by the cautious addition of 100 ml of methanol, and the mixture was hydrogenated (70 p.s.i.) (16, 17) with shaking for 64 h. The resulting mixture was filtered through a Celite plug to remove the catalyst, and the solvent was removed in vacuo to afford compound 4 as a pale yellow oil (3.10 g, quantitative yield). The product was of sufficient purity to be used in the next reaction without further purification. The following procedures were used: 1H NMR (400 MHz, CDCl3,) δ 7.35–7.15 (m, 5H), 3.53 (s, 2H), 2.65 (bs, 4H), 2.41 (s, 4H), 1.59 (bs, 4H), 1.47 (s, 8H).
1,7-Diamino-N5-benzyl-5-azaheptane, 1
A 2.95-g portion (11.82 mmol) of compound 4, 0.15 g of 20% Palladium C, and 0.15 g of 20% Pd(OH)2/C (18, 19) were placed in a hydrogenation bottle, followed by the cautious addition of 100 ml of methanol, and the mixture was hydrogenated (70 p.s.i.) with shaking for 24 h. The resulting mixture was filtered through a Celite plug to remove the catalyst, and the solvent was removed in vacuo to afford compound 1 as a clear oil. The oil was taken up in 3 ml of 12.1 m HCl, reconcentrated with a rotary evaporator, and then recrystallized (water/methanol) to yield 2.69 g (65.0%) of homospermidine hydrochloride 1 as a white crystalline solid. The following procedures were used: 1H NMR (400 MHz, CDCl3,) δ 2.66 (t, J = 6.8 Hz, 4H), 2.57 (t, J = 6.8 Hz, 4H), 1.58–1.38 (m, 13H).
RESULTS
C. jejuni Synthesizes Spermidine by S-adenosylmethionine Decarboxylase- and Spermidine Synthase-independent Pathway
The ϵ-proteobacterium C. jejuni strain 81116 was grown in polyamine-deficient, defined minimal medium, and cells from the exponential and stationary phases were analyzed for polyamine content by HPLC (Fig. 2A). Spermidine was the only polyamine detected (0.87 ± 0.19 and 0.30 ± 0.11 nmol/mg fresh weight at exponential (A600 nm 0.2–0.3) and stationary (A600 nm 0.9–1.0) phase, respectively). There were no discernable orthologues of AdoMetDC and SpdSyn in any Campylobacter genome, but we noticed that orthologues of CANSDH (universally annotated as saccharopine dehydrogenase) and CANSDC were present in all of these genomes. No orthologues of DABA AT and DABA DC, which together are able to synthesize 1,3-diaminopropane, the precursor of sym-norspermidine, are present in Campylobacter genomes. It therefore seemed likely that the CANSDH- and CANSDC-encoding genes of the Campylobacter species actually encoded CASDH and carboxyspermidine decarboxylase (CASDC) enzymes. Orthologues encoding arginine decarboxylase (ADC), agmatine deiminase/amidohydrolase (AIH), and N-carbamoylputrescine amidohydrolase (NCPAH) are present in all Campylobacter genomes, and so we hypothesized that C. jejuni 81116 synthesizes spermidine by the pathway shown in Fig. 2B.
FIGURE 2.
Spermidine biosynthesis in C. jejuni. A, HPLC of polyamines from C. jejuni wild-type (81116) cells grown in polyamine-deficient medium. Polyamines were extracted from log phase (A600 nm 0.2 to 0.3) and stationary phase (A600 nm 0.9 to 1.0) cells. R, fluorescent labeling dye; Spd, spermidine; IS, internal standard (1,7-diaminoheptane). B, putative pathway for spermidine biosynthesis in C. jejuni. Candidate C. jejuni open reading frames encoding pathway enzymes are indicated. ADC, arginine decarboxylase; AIH, agmatine deiminase/iminohydrolase; NCPAH, N-carbamoylputrescine amidohydrolase; CASDH, carboxyspermidine dehydrogenase; CASDC, carboxyspermidine decarboxylase. The aminopropyl group transfer is shown in blue.
Alternative Spermidine Biosynthetic Pathway Is Present in C. jejuni
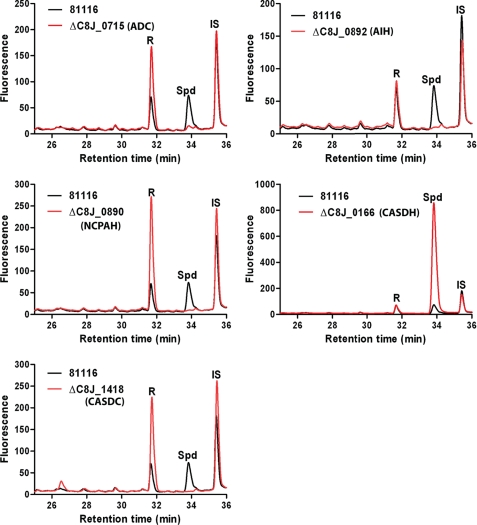
We constructed gene deletions in C. jejuni 81116 genes (Fig. 2B) putatively encoding ADC (ΔC8J_0715), AIH (ΔC8J_0892), NCPAH (ΔC8J_0890), CASDH (ΔC8J_0166), and CASDC (ΔC8J_1418). With each gene deletion, spermidine accumulation was abolished after growth in polyamine-deficient medium (Fig. 3) except for deletion of CASDH, which surprisingly resulted in a very large increase in the amount of spermidine. We were unable to separate a carboxyspermidine synthetic standard from putrescine by HPLC (Fig. 3). This is reminiscent of the difficulty of detecting carboxynorspermidine in the gene deletion of carboxynorspermidine decarboxylase in V. cholerae (11). However, we showed recently that the C. jejuni recombinant CASDC enzyme is active with both carboxynorspermidine and carboxyspermidine as substrates in vitro, producing sym-norspermidine and spermidine, respectively (20).
FIGURE 3.
HPLCs of polyamines from C. jejuni gene deletion mutants grown in polyamine-deficient medium. Cells were grown as described under “Experimental Procedures.” R, fluorescent labeling dye; Spd, spermidine; IS, internal standard (1,7-diaminoheptane).
Spermidine Is Critical for Growth of C. jejuni 81116
The ADC (ΔC8J_0715), CASDH (ΔC8J_0166), and CASDC (ΔC8J_1418) gene deletion strains were grown in polyamine-deficient liquid medium (Fig. 4A). Gene deletion strains for ADC and CASDC were severely compromised for growth, but the CASDH gene deletion strain grew normally. After 25 h of growth, the ADC gene deletion strain consistently grew slightly better than the CASDC knock-out mutant. When the ADC gene deletion strain was grown with 500 μm exogenous agmatine (the product of ADC), growth was restored more efficiently than with 500 μm spermidine in the medium. Spermidine at 500 μm restored the growth of the ADC and CASDC gene deletion strains to the same extent. Expression of a chromosomally integrated recombinant CASDC ORF expressed from a metK promoter in the CASDC gene deletion strain (c_ΔC8J_1418) completely restored growth of the CASDC gene deletion strain (Fig. 4A) and restored spermidine biosynthesis (Fig. 4B). The AIH (ΔC8J_0892) and NCPAH (ΔC8J_0890) gene deletion strains were severely compromised for growth in polyamine-deficient medium, but growth was mostly restored by addition of 500 μm spermidine (Fig. 4C).
FIGURE 4.
Cell growth in C. jejuni deletion mutants. Cells were grown in polyamine-deficient medium ± polyamines as described under “Experimental Procedures.” Data represent the means of triplicate cultures ± standard deviation. A, cell growth for wild-type (81116), ΔC8J_0715 (ADC), ΔC8J_0166 (CASDH) and ΔC8J_1418 (CASDC) deletion strains, and the genetically complemented strain c_ΔC8J_1418. a, wild-type parental strain; b, ΔC8J_1418 (CASDC); c, ΔC8J_1418 (CASDC) plus spermidine (Spd); d, ΔC8J_0715 (ADC); e, ΔC8J_0715 (ADC) plus agmatine (Agm); f, ΔC8J_0715 (ADC) plus spermidine; g, ΔC8J_0166 (CASDH); h, genetically complemented ΔC8J_1418 (CASDC), i.e. (c_ΔC8J_1418). Where added to the medium, spermidine and agmatine were at 500 μm final concentration. B, HPLCs of C. jejuni gene deletion strain ΔC8J_1418 (CASDC) and the same strain genetically complemented by expressing a chromosomally located copy of the CASDC-encoding ORF (c_ΔC8J_1418). C, a, C. jejuni 81116 wild-type parental strain; b, ΔC8J_0890 (NCPAH); c, ΔC8J_0890 plus 500 μm spermidine (Spd); d, ΔC8J_0892 (AIH); e, ΔC8J_0892 plus 500 μm spermidine. D, a, C. jejuni 81116 wild-type parental strain; b, ΔC8J_1418 (CASDC); c, ΔC8J_1418 plus 500 μm spermidine; d, ΔC8J_1418 plus 500 μm sym-norspermidine; e, ΔC8J_1418 plus 500 μm sym-homospermidine.
sym-Norspermidine, Spermidine, and sym-Homospermidine Support Growth of C. jejuni
We assessed the relative ability of different triamines (Fig. 1A) at 500 μm to restore growth of the CASDC gene deletion strain (ΔC8J_1418). Spermidine and sym-homospermidine restored growth with similar efficiencies; however, sym-norspermidine was less effective (Fig. 4D). When the accumulation of each exogenously supplied triamine in the CASDC gene deletion strain was assessed by HPLC, sym-norspermidine and sym-homospermidine were found to have accumulated to similar levels, but spermidine was much less abundant in the cells (supplemental Fig. S1), suggesting that sym-norspermidine and sym-homospermidine need to be present at higher levels to replace the function of spermidine. We investigated whether sym-norspermidine would outcompete spermidine for uptake as detected by growth restoration. However, equal amounts of sym-norspermidine and spermidine in the growth medium restored growth of the CASDC gene deletion strain to the level of the spermidine-only rescue (supplemental Fig. S2). In this growth experiment, spermidine and sym-norspermidine accumulated in the CASDC gene deletion strain cells to similar levels when present in the growth medium individually or when mixed (supplemental Fig. S3A). The growth restoration by sym-norspermidine was surprising to us, so we assessed whether the sym-norspermidine stock solutions might have contained small amounts of spermidine. If any spermidine was present in the sym-norspermidine stock solution, it was at levels less than 0.1% of the sym-norspermidine content (supplemental Fig. S3B).
The effect of spermidine and sym-norspermidine dosage (500, 750, and 1000 μm) on growth restoration of the CASDC gene deletion strain was examined. Growth restoration was concentration-dependent, although 750 and 1000 μm spermidine were equally effective at almost completely restoring growth. This effect was not observed with the less effective sym-norspermidine, and 1000 μm sym-norspermidine was more effective than 750 μm sym-norspermidine, although 1000 μm sym-norspermidine was markedly less effective than 500 μm spermidine at growth restoration (Fig. 5A). The relative efficiencies of different diamines for growth restoration of the C. jejuni 81116 ADC gene deletion strain (ΔC8J_0715) were determined. Putrescine, 1,3-diaminopropane, and cadaverine were equally ineffective at restoring growth (Fig. 5B), and HPLC analysis indicated a complete lack of accumulation of these diamines in the cells (results not shown).
FIGURE 5.
Growth restoration of C. jejuni polyamine auxotrophic strains by exogenous polyamines. Cells were grown in polyamine-deficient medium ± polyamines as described under “Experimental Procedures.” A, dose response of cell growth in ΔC8J_1418 (CASDC) gene deletion mutant in polyamine-deficient medium supplemented with spermidine or sym-norspermidine. a, C. jejuni wild-type parental strain (81116); b, ΔC8J_1418 (CASDC); c, ΔC8J_1418 plus 500 μm spermidine; d, ΔC8J_1418 plus 750 μm spermidine; e, ΔC8J_1418 1000 μm spermidine; f, ΔC8J_1418 plus 500 μm sym-norspermidine; g, ΔC8J_1418 plus 750 μm sym-norspermidine; h, ΔC8J_1418 plus 1000 μm sym-norspermidine. B, dose response of cell growth in ΔC8J_0715 (ADC) gene deletion mutant in polyamine-deficient medium supplemented with polyamines. a, C. jejuni wild-type parental strain (81116); b, ΔC8J_0715; c, ΔC8J_0715 plus 500 μm agmatine; d, ΔC8J_0715 plus 500 μm putrescine; e, ΔC8J_0715 plus 500 μm cadaverine; f, ΔC8J_0715 plus 500 μm 1,3-diaminopropane; g, ΔC8J_0715 plus 500 μm spermidine; h, ΔC8J_0715 plus 500 μm sym-norspermidine.
CASDH and CASDC Are Found in Diverse Bacterial Phyla
Orthologues of both CASDH and CASDC are pervasive in the Firmicutes, Bacteroidetes, and in the α-, γ-, δ-, and ϵ-proteobacteria, although infrequent in the β-proteobacteria (Table 1). The alternative pathway is also found in the lesser known (and lesser sequenced) phyla, including Deinococcus-Thermi, Verrucomicrobia, Spirochaetes, Deferribacteres, Fusobacteria, Lentisphaerae, Elusimicrobia, Chrysiogenetes, and Fibrobacteres. However, it is rare in the cyanobacteria and archaea, and although some distant homologues of CASDH are found in many actinobacteria, there are no CASDC orthologues, and thus the pathway appears to be absent from the actinobacteria. Only two ϵ-proteobacterial genomes possess an AdoMetDC orthologue, Nitratiruptor sp. SB155-2 (YP_00156990) and Caminibacter medialanticus TB-2 (ZP_01872533), and so the CASDH/CASDC pathway is almost the only pathway for spermidine biosynthesis in this phylum. A number of important human pathogens possess the CASDH/CASDC pathway, including Streptococcus pneumoniae, Bordetella pertussis, Pasteurella multocida, Bartonella species, Brucella species, Moraxella catarrhalis, V. cholerae, Helicobacter pylori, Bacteroides fragilis, Porphyromonas gingivalis, the food-borne pathogen C. jejuni and the botulism bacterium Clostridium botulinum.
TABLE 1.
Number of genomes encoding both CASDH and CASDC in bacterial phyla
| Phylum | Genomes |
|---|---|
| g-Proteobacteria | 66 |
| Firmicutes | 60 |
| α-Proteobacteria | 51 |
| ϵ-Proteobacteria | 45 |
| Bacteroidetes | 38 |
| δ-Proteobacteria | 23 |
| Deinococcus-thermi | 5 |
| β-Proteobacteria | 4 |
| Verrucomicrobia | 4 |
| Spirochaetes | 4 |
| Deferribacteres | 3 |
| Fusobacteria | 2 |
| Cyanobacteria | 1 |
| Lentisphaerae | 1 |
| Elusimicrobia | 1 |
| Chrysiogenetes | 1 |
| Fibrobacteres | 1 |
| Archaea | 1 |
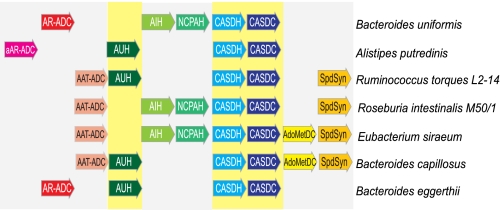
CASDH and CASDC Are Often Found Clustered with Putrescine Biosynthetic Genes
Analysis of bacterial genomes indicates that CASDH- and CASDC-encoding ORFs are frequently localized to clusters of putrescine biosynthetic genes, including alanine racemase- and aspartate aminotransferase-fold arginine decarboxylases together with agmatine ureohydrolase, or agmatine deiminase, and N-carbamoylputrescine amidohydrolase (Fig. 6). Less frequently, alanine racemase- and aspartate aminotransferase-fold ornithine decarboxylase orthologues are found clustered with CASDH and CASDC. Usually CASDH is positioned immediately upstream of CASDC; however, the situation is reversed in the β-proteobacterium B. pertussis Tohoma 1 and uncultured Termite Groups 1 Rs-D17. Uniquely in Magnetococcus sp. MC-1, the CASDH and CASDC pair are arranged divergently and must be transcribed from an intervening divergent promoter. In some Firmicutes species, both CASDH/CASDC and AdoMetDC/SpdSyn pairs can be found in the same cluster. In the gut firmicute species Ruminococcus gnavus and Clostridium leptum, an identical gene cluster is present containing an aspartate aminotransferase-fold arginine decarboxylase, spermidine synthase, agmatine ureohydrolase, CASDH, and CASDC (Fig. 6). However, C. leptum has retained an AdoMetDC immediately upstream of the cluster, whereas R. gnavus has lost AdoMetDC from its genome (Fig. 6). Other gut firmicutes such as the opportunistic pathogen Clostridium difficile appear to possess only the AdoMetDC/SpdSyn pathway for spermidine biosynthesis, with no orthologues of CASDH and CASDC detectable in the genome. The pattern of presence and absence of the AdoMetDC/SpdSyn and CASDH/CASDC pathways in the Firmicutes as a whole suggests that the CASDH/CASDC pathway is enriched in the gut microbiota and is in the process of supplanting the AdoMetDC/SpdSyn pathway.
FIGURE 6.
Polyamine-related gene clusters containing CASDH and CASDC orthologues in bacterial genomes. Protein accession numbers are shown below the first and last ORFs. Bacterial phyla are listed in parentheses. AUH, agmatine ureohydrolase; AAT, aspartate aminotransferase-fold; AR, alanine racemase-fold; hypo, hypothetical protein.
CASDH/CASDC Is the Dominant Polyamine Biosynthetic Pathway in Human Gut Microbiota
As CASDH and CASDC are pervasive in the Bacteroidetes and Firmicutes phyla, we analyzed the polyamine biosynthetic pathways in the 55 most ubiquitous and abundant species in the human gut microbiota described by Qin et al. (21). In Fig. 7 it can be seen that not a single species possesses AdoMetDC and SpdSyn only, and the AdoMetDC/SpdSyn pathway is found in only five species, all of which contain the CASDH/CASDC pathway. There are 15 Firmicutes species and 22 Bacteroidetes species that possess the CASDH/CASDC pathway, and 1 Bacteroidetes species and 15 Firmicutes species are putatively auxotrophic for polyamine biosynthesis (Fig. 7). Each of the gut polyamine putative auxotrophic species nevertheless possesses the spermidine-preferential ABC cassette uptake transporter (22) encoded by the potABCD genes (Table 2) except for the actinobacterial species Collinsella aerofaciens. In this transport system, PotA is an energy-generating ATPase; PotB and PotC each have six transmembrane-spanning segments and form a channel for spermidine and putrescine, and PotD is a periplasmic spermidine-binding protein. In each of the polyamine auxotrophic species, the potABCD genes are clustered into an operon, and for five species, the potC and potD genes are fused (Table 2). This is an unusual fusion since potC encodes one of the two transmembrane channel proteins, and potD encodes the substrate-binding protein. The potC-potD fusion is also found in some of the gut firmicute polyamine prototrophs, but this fusion appears to be limited to the Firmicutes.
FIGURE 7.
Spermidine biosynthetic pathways in the human gut microbiota. Protein accession numbers are given for CASDH, CASDC, AdoMetDC, and SpdSyn ORFs in the 55 most abundant, ubiquitous bacterial species in the human gut (21). Firmicutes species (F) are shown in pink and Bacteroidetes species in green (B). Absent ORFs are not colored.
TABLE 2.
Prominent human gut bacterial putative polyamine auxotrophs possessing the spermidine-specific PotABCD transporter
Protein accession numbers are followed by the amino acid size in parentheses.
| Species | PotA | PotB | PotC | PotD |
|---|---|---|---|---|
| Dorea longicatena DSM 13814 | ZP_01996030 (525) | ZP_01996029 (278) | ZP_01996028 (630) PotC+D fusion | |
| Ruminococcus bromii L2–63 | CBL16182 (474) | CBL16183 (263) | CBL16184 (268) | CBL16185 (362) |
| Clostridium sp. SS2/1 | ZP_02439016 (465) | ZP_02439015 (261) | ZP_02439014 (267) | ZP_02439013 (475) |
| Dorea formicigenerans ATCC 27755 | ZP_02233769 (359) | ZP_02233770 (275) | ZP_02233771 (576) PotC+D fusion | |
| Coprococcus comes ATCC 27758 | ZP_03800103 (347) | ZP_03800102 (278) | ZP_03800101 (627) PotC+D fusion | |
| Eubacterium ventriosum ATCC 27560 | ZP_02026516 (347) | ZP_02026515 (275) | ZP_02026514 (625) PotC+D fusion | |
| Streptococcus thermophilus LMD-9 | YP_820847 (384) | YP_820846 (264) | YP_820845 (259) | YP_820844 (355) |
| Holdemania filiformis DSM 12042 | ZP_03634130 (439) | ZP_03634132 (262) | ZP_03634133 (267) | ZP_03634134 (360) |
| Coprococcus eutactus ATCC 27759 | ZP_02205354 (482) | ZP_02205353 (266) | ZP_02205352 (264) | ZP_02205351 (572) |
| P. johnsonii DSM 18315 | ZP_03476052 (468) | ZP_03476053 (266) | ZP_03476054 (262) | ZP_03476055 (443) |
| Clostridium sp. L2–50 | ZP_02075935 (378) | ZP_02075936 (288) | ZP_02075937 (266) | ZP_02075938 (354) |
| Anaerotruncus colihominis DSM 17241 | ZP_02444651 (359) | ZP_02444650 (283) | ZP_02444649 (268) | ZP_02444648 (343) |
| ZP_02442817 (349) | ZP_02442816 (270) | ZP_02442815 (270) | ZP_02442814 (413) | |
| ZP_02441016 (350) | ZP_02441018 (293) | ZP_02441017 (274) | ZP_02441019 (386) | |
| Clostridium asparagiforme DSM 15981 | ZP_03762568 (358) | ZP_03762569 (278) | ZP_03762570 (265) | ZP_03762571 (374) |
| Enterococcus faecalis TX0104 | ZP_03949715 (378) | ZP_03949716 (268) | ZP_03949717 (278) | ZP_03949718 (357) |
| ZP_03948530 (346) | ZP_03948528 (278) | ZP_03948529 (259) | ZP_03948531 (354) | |
| Clostridium scindens ATCC 35704 | ZP_02430529 (357) | ZP_02430530 (276) | ZP_02430531 (628) PotC+D fusion | |
Intriguingly, of the 15 Firmicutes species that possess the CASDH/CASDC pathway, 11 possess SpdSyn but not AdoMetDC. Analysis of putrescine biosynthetic pathways indicate that within the list of 55 gut species (23), Firmicutes species use the aspartate aminotransferase-fold ADC, and Bacteroidetes use the alanine racemase-fold ADC. When the polyamine biosynthetic pathway as a whole is assessed in these 55 species, seven different biosynthetic pathway configurations can be discerned (Fig. 8). The first part of the pathway consists of two separate submodules: one of three forms of ADC to form agmatine from arginine, and either AUH or AIH/NCPAH to form putrescine from agmatine. All pathways have a second module of CASDH/CASDC and may have an additional module of AdoMetDC and SpdSyn or only SpdSyn.
FIGURE 8.
Polyamine biosynthetic pathway configurations in human gut microbiota species. aAR-ADC, ancestral alanine racemase-fold biosynthetic arginine decarboxylase (23); AR-ADC, alanine racemase-fold biosynthetic arginine decarboxylase (23); biosynthetic aspartate aminotransferase-fold arginine decarboxylase (23); AUH, agmatine ureohydrolase.
DISCUSSION
An alternative pathway for spermidine biosynthesis, i.e. one that does not use AdoMetDC and SpdSyn, was first proposed by Tait (24) 35 years ago. He proposed aspartate β-semialdehyde as the source of the aminopropyl group for spermidine biosynthesis from putrescine and suggested a carboxyspermidine intermediate in the α-proteobacterial species Paracoccus denitrificans and Rhodobacter sphaeroides. Previously, we demonstrated that the genes encoding CANSDH and CANSDC were essential for sym-norspermidine biosynthesis in V. cholerae (11). The 1,3-diaminopropane precursor of sym-norspermidine was synthesized by a fusion protein comprised of DABA AT and DABA DC. However, because there is relatively little overlap between genomes possessing DABA AT and DABA DC orthologues with those containing CANSDH and CANSDC orthologues (except for the Vibrionales and a limited number of other species), we tentatively suggested that the carboxypolyamine pathway was used primarily for spermidine biosynthesis in other species (11). We obtained an x-ray crystal structure of the C. jejuni CANSDC orthologue and determined that it has a relatively equal preference for carboxynorspermidine and carboxyspermidine, in contrast to the V. cholerae CANSDC, which has a marked preference for carboxynorspermidine (20). Here, we have shown that the C. jejuni enzyme is a CASDC and not a CANSDC in vivo and is essential for spermidine biosynthesis. Only spermidine is present in C. jejuni, and there is no pathway for 1,3-diaminopropane biosynthesis, and none is detectable by HPLC. Consequently, sym-norspermidine is not synthesized in the C. jejuni cells.
In contrast to V. cholerae, where deletion of the gene encoding CANSDH leads to complete elimination of sym-norspermidine biosynthesis (11), deletion of the C. jejuni CASDH does not abolish spermidine accumulation. Instead, it paradoxically produces a very large increase in spermidine accumulation. This unexpected result of deletion of the CASDH-encoding gene suggests that a metabolic bypass is activated by the removal of CASDH and that CASDH is a rate-limiting enzyme regulating spermidine levels. Overexpression of CANSDH in V. cholerae caused a large accumulation of spermidine, which is normally undetectable in these cells, suggesting that the excess CANSDH had depleted its main substrate 1,3-diaminopropane and as a result then converted putrescine to spermidine (11). The diversity of mechanisms for bypassing metabolic blocks has been revealed by a recent study of an E. coli mutant lacking 4-phosphoerythronate dehydrogenase, which is required for biosynthesis of the key enzyme cofactor pyridoxal 5′-phosphate. A multicopy suppressor strategy identified seven different genes in three serendipitous pathways that could restore pyridoxal 5′-phosphate synthesis (25). Even in polyamine biosynthesis, there is a precedent for bypassing metabolic blocks. The Pseudomonas aeruginosa PAO1 N-carbamoylputrescine amidohydrolase gene deletion produced a leaky biosynthetic phenotype, and it was consequently found that acetylputrescine amidohydrolase, which has a very similar substrate to N-carbamoylputrescine (26), is induced in the gene deletion mutant and allows bypass of the metabolic block. The CANSDH/CASDH enzyme is related to homospermidine synthase, lysine 6-dehydrogenase, saccharopine dehydrogenase, and aspartate dehydrogenase (6). There may be a dehydrogenase in C. jejuni that can serendipitously substitute the activity of the missing CASDH. Aspartate β-semialdehyde is able to nonenzymatically form a Schiff base with carboxynorspermidine (27), which may facilitate a biosynthetic bypass. The evidence that supports a role for CASDH homologues in the carboxyspermidine pathway is as follows: deletion of the CASDH-encoding gene causes a very large increase in spermidine, which at least suggests involvement in polyamine metabolism; CASDH is found adjacent to CASDC in several ϵ-proteobacterial lineages; CASDH is found adjacent to putrescine biosynthetic genes; CASDH is closely related to CANSDH of V. cholerae, which is able to synthesize carboxyspermidine as well as carboxynorspermidine.
A gene described as a CANSDC was deleted in the gastric pathogen H. pylori 26695 as part of a systematic screen for genes essential for growth in this strain (28). The CANSDC orthologue was essential for growth as determined by a colony growth assay. However, H. pylori contains spermidine as its only polyamine (29), and as no orthologue of AdoMetDC is present, the H. pylori CANSDC must be considered a CASDC. There is no AdoMetDC orthologue in H. pylori; however, a divergent SpdSyn-like gene is present, which was shown by Chalker et al. (28) to be inessential for colony growth.
Spermidine Can Be Replaced by sym-Homospermidine for Growth Restoration of C. jejuni Spermidine Auxotrophs
Depletion of polyamines in C. jejuni, whether by deletion of the ADC- or CASDC-encoding genes, results in a very slow growth rate, underlining the critical role of spermidine in cell proliferation. However, sym-homospermidine, which is longer than spermidine and is symmetrical, is able to replace the function of spermidine for growth restoration. In contrast, sym-norspermidine, also symmetrical but shorter than spermidine, is much less effective for growth restoration of C. jejuni spermidine auxotrophs. Previously, N1-aminopropylcadaverine, was found to restore growth of E. coli spermidine (N1-aminopropylputrescine) auxotrophs as effectively as spermidine itself (30). It should be noted that sym-homospermidine accumulated to higher levels than spermidine in the C. jejuni spermidine auxotrophs, indicating that a higher concentration of sym-homospermidine was required to achieve the same effect as a lower level of spermidine. There was also a higher accumulation of sym-norspermidine compared with spermidine in the C. jejuni spermidine auxotrophs, further underscoring the lower effectiveness of sym-norspermidine for replacement of spermidine function in growth. The ability of sym-homospermidine to replace spermidine in C. jejuni, and of N1-aminopropylcadaverine to replace spermidine in E. coli, indicates that the exact structure of the polyamine is not critical. Indeed, in most species in the bacterial phyla Chlorobi and Chloroflexi, in filamentous Cyanobacteria, and in many α-proteobacteria, sym-homospermidine is the only polyamine present (6). Although sym-homospermidine and N1-aminopropylcadaverine are one carbon longer than spermidine, they are flexible linear chains and can bend, whereas sym-norspermidine, which is one carbon shorter than spermidine, is unlikely to stretch.
Deep Sea Hydrothermal Vent Communities and the Human Gastrointestinal Tract Are Enriched for the Carboxyspermidine Pathway
The CASDH/CASDC pathway is present in many bacterial phyla, but it is notably absent in the Chloroflexi, Chlorobi, almost all of the cyanobacteria, acidobacteria, Planctomycetes, and most prominently in the actinobacteria. These observations are based entirely on sequence similarity analyses and not gene annotations. Almost all CASDH orthologues are annotated erroneously as saccharopine dehydrogenase. In phyla such as the Chloroflexi, Chlorobi, and the filamentous cyanobacteria, sym-homospermidine is the main polyamine. The ubiquity of the CASDH/CASDC pathway in the ϵ-proteobacteria means that it is the dominant spermidine biosynthetic pathway in deep sea hydrothermal vent communities, as a consequence of the prominence of the ϵ-proteobacteria in that environment (31). Other phyla in deep sea hydrothermal vents possess CASDH/CASDC orthologues, including the Deferribacteres species Deferribacter desulfuricans SSM1 (32), which also possess the AdoMetDC/SpdSyn pathway and accumulates spermidine but not sym-norspermidine (18), and in δ-proteobacteria such as Geobacter sulfurreducens and Desulfovibrio vulgaris. The alternative carboxyspermidine biosynthetic pathway is therefore pervasive in deep sea hydrothermal vent communities.
Another ecological niche that has assimilated the CASDH/CASDC pathway is the human gastrointestinal tract. Of the two dominant bacterial phyla within the human large intestine, the Bacteroidetes species universally possess the CASDH/CASDC route as their only polyamine biosynthetic pathway, although Parabacteroides johnsonii has become a polyamine auxotroph due to degradation of its CASDH-/CASDC-encoding genes. Horizontal transfer almost certainly explains the presence of AdoMetDC/SpdSyn genes in Bacteroides capillosus, so that two physically clustered spermidine biosynthetic pathways are present in the genome of this Bacteroidetes species. Half of the Firmicutes species among the 55 most abundant bacterial species in the human gut (21) appear to be polyamine auxotrophs, but all possess orthologues of the potABCD spermidine transporter genes. This is a common occurrence in bacteria with reduced genomes, such as in intracellular pathogens, where biosynthetic pathways are lost, and so the bacterium becomes dependent on uptake. In the intracellular pathogen Mycoplasma genitalium, the polyamine biosynthetic pathway has been lost, but all components of the potABC (no potD orthologue) spermidine transporter are essential (33). The other half possesses the CASDH/CASDC pathway but also has a SpdSyn orthologue; however, there are no Firmicutes species that possess an AdoMetDC orthologue but not SpdSyn. The gut firmicute Butyvibrio crossotus possesses CASDH, CASDC, and SpdSyn orthologues and has been shown previously to accumulate spermidine as its only polyamine (18). An active AdoMetDC in the absence of an aminopropyltransferase like SpdSyn would lead to an accumulation of decarboxylated S-adenosylmethionine, a potent inhibitor of methyltransferases (34). The CASDH/CASDC pathway appears to have replaced the AdoMetDC/SpdSyn pathway in many gut Firmicutes species, with the SpdSyn remaining as a remnant, although it is possible that the SpdSyn orthologue has acquired a new function. There is only one actinobacterial species among the 55 most ubiquitous, abundant human gut bacterial species, but Collinsella aerofaciens does not possess any recognizable polyamine biosynthetic genes and has been shown previously to lack polyamine biosynthesis (18), i.e. it is a polyamine auxotroph. It also lacks orthologues of the potABCD genes encoding the spermidine-preferential transporter, and therefore it is formally possible that C. aerofaciens may not require polyamines for growth. Verrucomicrobia are a relatively small component of the human gut microbiome; however, it is notable that the gut verrucomicrobium Akkermansia mucinophila also possesses the CASDH/CASDC pathway.
The bacterial AdoMetDC/SpdSyn pathway was first described in E. coli (3), and this is the only polyamine biosynthetic pathway in the Enterobacteria, which entirely lack the CASDH/CASDC route. It is therefore surprising that the AdoMetDC/SpdSyn pathway is only a minor polyamine biosynthetic route in the human gastrointestinal tract. However, it is known now that the proteobacteria, which include E. coli, make up less than 3% of the gut microbiota (35). Not only is the CASDH/CASDC pathway dominant in the gut (Bacteroidetes and Firmicutes) and stomach (Helicobacter), the oral microbiome is composed of a large contingent of Bacteroidetes species that also contain the CASDH/CASDC route as the only polyamine biosynthetic pathway, e.g. Prevotella, Bacteroides, and Porphyromonas species, which have been shown to accumulate spermidine as the sole polyamine (8). Taking into account the number of bacterial cells in the human gastrointestinal tract, the CASDH/CASDC pathway is likely to be present in more cells within the human supra-organism than the AdoMetDC/SpdSyn pathway.
The AdoMetDC/SpdSyn route for spermidine biosynthesis is widely distributed in bacteria, archaea, and eukaryotes. In contrast, the CASDH/CASDC route is absent from the archaea, except for a clear case of horizontal gene transfer in the euryarchaeote Methanoplanus petrolearius DSM 11571, and is absent in eukaryotes. Probably the CASDH/CASDC route evolved after the AdoMetDC/SpdSyn pathway. It is clear that CASDC has evolved from other polyamine-related basic amino acid decarboxylases (36), and CASDH has evolved from an enzyme family that includes homospermidine synthase, lysine 6-dehydrogenase, saccharopine dehydrogenase, aspartate dehydrogenase, and homoserine dehydrogenase (6). The intriguing question is why the CASDH/CASDC pathway is supplanting AdoMetDC/SpdSyn in the gut Firmicutes species. The co-substrate of CASDH is aspartate β-semialdehyde, and the cofactor is NADPH (27), and CASDC requires the cofactor pyridoxal 5′-phosphate (20, 37). The AdoMetDC/SpdSyn pathway does not require exogenous cofactors but does require S-adenosylmethionine as a co-substrate. Not only is S-adenosylmethionine a relatively energetically expensive molecule being made from ATP and methionine, but the co-product of SpdSyn (methylthioadenosine) must be salvaged to recover methionine. The necessity for salvage may confer a fitness cost in a fast growing environment like the human gut, which is one of the most densely populated biomes known. It is notable that species using the CASDH/CASDC pathway are frequently found in anaerobic environments.
Because prominent human pathogens such as H. pylori, P. gingivalis, S. pneumoniae, Brucella species, B. pertussis, P. multocida, Bartonella species, M. catarrhalis, V. cholerae, B. fragilis, and food-associated bacteria such as the opportunistic pathogen C. jejuni and the deadly C. botulinum possess the CASDH/CASDC pathway, a new potential target for the development of antimicrobial compounds has been revealed by our findings. Most of these pathogens also use the arginine route, rather than the ornithine route for putrescine biosynthesis, so the entire polyamine biosynthetic pathway in these bacterial species differs from the human host, and therefore presents a novel target for exploration of antimicrobial drug development.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants 7RO1 CA149095 (to P. M. W.) and R01 AI034432 (to M. A. P.). This work was also supported by a Biotechnology and Biological Sciences Research Council (United Kingdom) CSG Grant, Institute Development Fellowship BB/E024467/1, University of Texas Southwestern Medical Center grant (to A. J. M.), and the Welch Foundation Grant I-1257 (to M. A. P.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and Figs. S1–S3.
- AdoMetDC
- S-adenosylmethionine decarboxylase
- ADC
- arginine decarboxylase
- AIH
- agmatine deiminase/iminohydrolase
- CANSDC
- carboxynorspermidine decarboxylase
- CANSDH
- carboxynorspermidine dehydrogenase
- CASDC
- carboxyspermidine decarboxylase
- CASDH
- carboxyspermidine dehydrogenase
- DABA AT
- diaminobutyrate aminotransferase
- DABA DC
- diaminobutyrate decarboxylase
- NCPAH
- N-carbamoylputrescine amidohydrolase
- SpdSyn
- spermidine synthase.
REFERENCES
- 1. Pegg A. E. (2009) Essays Biochem. 46, 25–45 [DOI] [PubMed] [Google Scholar]
- 2. Wu H., Min J., Ikeguchi Y., Zeng H., Dong A., Loppnau P., Pegg A. E., Plotnikov A. N. (2007) Biochemistry 46, 8331–8339 [DOI] [PubMed] [Google Scholar]
- 3. Tabor C. W., Tabor H. (1976) Annu. Rev. Biochem. 45, 285–306 [DOI] [PubMed] [Google Scholar]
- 4. Sekowska A., Coppée J. Y., Le Caer J. P., Martin-Verstraete I., Danchin A. (2000) Mol. Microbiol. 36, 1135–1147 [DOI] [PubMed] [Google Scholar]
- 5. Toms A. V., Kinsland C., McCloskey D. E., Pegg A. E., Ealick S. E. (2004) J. Biol. Chem. 279, 33837–33846 [DOI] [PubMed] [Google Scholar]
- 6. Shaw F. L., Elliott K. A., Kinch L. N., Fuell C., Phillips M. A., Michael A. J. (2010) J. Biol. Chem. 285, 14711–14723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hamana K. (2004) Microbiol. Culture Collections 20, 3–8 [Google Scholar]
- 8. Hosoya R., Hamana K. (2004) J. Gen. Appl. Microbiol. 50, 255–260 [DOI] [PubMed] [Google Scholar]
- 9. Hamana K. (1999) Microbiol. Cult. Collect. 15, 9–28 [Google Scholar]
- 10. Hamana K. (1994) J. Gen. Appl. Microbiol. 40, 181–195 [Google Scholar]
- 11. Lee J., Sperandio V., Frantz D. E., Longgood J., Camilli A., Phillips M. A., Michael A. J. (2009) J. Biol. Chem. 284, 9899–9907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gilbreath J. J., Cody W. L., Merrell D. S., Hendrixson D. R. (2011) Microbiol. Mol. Biol. Rev. 75, 84–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pearson B. M., Gaskin D. J., Segers R. P., Wells J. M., Nuijten P. J., van Vliet A. H. (2007) J. Bacteriol. 189, 8402–8403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang W. L., Luechtefeld N. W., Reller L. B., Blaser M. J. (1980) J. Clin. Microbiol. 12, 479–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Covassin L., Desjardins M., Soulet D., Charest-Gaudreault R., Audette M., Poulin R. (2003) Bioorg. Med. Chem. Lett. 13, 3267–3271 [DOI] [PubMed] [Google Scholar]
- 16. Bellevue F. H., Boahbedason M., Wu R., Woster P. M., Casero R. A., Jr., Rattendi D., Lane S., Bacchi C. J. (1996) Bioorg. Med. Chem. Lett. 6, 2765–2770 [Google Scholar]
- 17. Zou Y., Wu Z., Sirisoma N., Woster P. M., Casero R. A., Jr., Weiss L. M., Rattendi D., Lane S., Bacchi C. J. (2001) Bioorg. Med. Chem. Lett. 11, 1613–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hosoya R., Yokoyama Y., Hamana K., Itoh T. (2006) Microbiol. Cult. Collect. 22, 21–33 [Google Scholar]
- 19. Li Y., Manicham G., Ghoshal A., Subramaniam P. (2006) Synthet. Commun. 36, 925–928 [Google Scholar]
- 20. Deng X., Lee J., Michael A. J., Tomchick D. R., Goldsmith E. J., Phillips M. A. (2010) J. Biol. Chem. 285, 25708–25719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qin J., Li R., Raes J., Arumugam M., Burgdorf K. S., Manichanh C., Nielsen T., Pons N., Levenez F., Yamada T., Mende D. R., Li J., Xu J., Li S., Li D., Cao J., Wang B., Liang H., Zheng H., Xie Y., Tap J., Lepage P., Bertalan M., Batto J. M., Hansen T., Le Paslier D., Linneberg A., Nielsen H. B., Pelletier E., Renault P., Sicheritz-Ponten T., Turner K., Zhu H., Yu C., Li S., Jian M., Zhou Y., Li Y., Zhang X., Li S., Qin N., Yang H., Wang J., Brunak S., Doré J., Guarner F., Kristiansen K., Pedersen O., Parkhill J., Weissenbach J., Bork P., Ehrlich S. D., Wang J. (2010) Nature 464, 59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Igarashi K., Kashiwagi K. (2010) Plant Physiol. Biochem. 48, 506–512 [DOI] [PubMed] [Google Scholar]
- 23. Burrell M., Hanfrey C. C., Murray E. J., Stanley-Wall N. R., Michael A. J. (2010) J. Biol. Chem. 285, 39224–39238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tait G. H. (1976) Biochem. Soc. Trans. 4, 610–612 [DOI] [PubMed] [Google Scholar]
- 25. Kim J., Kershner J. P., Novikov Y., Shoemaker R. K., Copley S. D. (2010) Mol. Syst. Biol. 6, 436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chou H. T., Kwon D. H., Hegazy M., Lu C. D. (2008) J. Bacteriol. 190, 1966–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nakao H., Shinoda S., Yamamoto S. (1991) J. Gen. Microbiol. 137, 1737–1742 [DOI] [PubMed] [Google Scholar]
- 28. Chalker A. F., Minehart H. W., Hughes N. J., Koretke K. K., Lonetto M. A., Brinkman K. K., Warren P. V., Lupas A., Stanhope M. J., Brown J. R., Hoffman P. S. (2001) J. Bacteriol. 183, 1259–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hamana K., Mariko T. (1998) Microbiol. Cult. Coll. 14, 1–14 [Google Scholar]
- 30. Linderoth N., Morris D. R. (1983) Biochem. Biophys. Res. Commun. 117, 616–622 [DOI] [PubMed] [Google Scholar]
- 31. Huber J. A., Mark Welch D. B., Morrison H. G., Huse S. M., Neal P. R., Butterfield D. A., Sogin M. L. (2007) Science 318, 97–100 [DOI] [PubMed] [Google Scholar]
- 32. Takaki Y., Shimamura S., Nakagawa S., Fukuhara Y., Horikawa H., Ankai A., Harada T., Hosoyama A., Oguchi A., Fukui S., Fujita N., Takami H., Takai K. (2010) DNA Res. 17, 123–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hutchison C. A., Peterson S. N., Gill S. R., Cline R. T., White O., Fraser C. M., Smith H. O., Venter J. C. (1999) Science 286, 2165–2169 [DOI] [PubMed] [Google Scholar]
- 34. Frostesjö L., Holm I., Grahn B., Page A. W., Bestor T. H., Heby O. (1997) J. Biol. Chem. 272, 4359–4366 [DOI] [PubMed] [Google Scholar]
- 35. Arumugam M., Raes J., Pelletier E., Le Paslier D., Yamada T., Mende D. R., Fernandes G. R., Tap J., Bruls T., Batto J. M., Bertalan M., Borruel N., Casellas F., Fernandez L., Gautier L., Hansen T., Hattori M., Hayashi T., Kleerebezem M., Kurokawa K., Leclerc M., Levenez F., Manichanh C., Nielsen H. B., Nielsen T., Pons N., Poulain J., Qin J., Sicheritz-Ponten T., Tims S., Torrents D., Ugarte E., Zoetendal E. G., Wang J., Guarner F., Pedersen O., de Vos W. M., Brunak S., Doré J., Antolín M., Artiguenave F., Blottiere H. M., Almeida M., Brechot C., Cara C., Chervaux C., Cultrone A., Delorme C., Denariaz G., Dervyn R., Foerstner K. U., Friss C., van de Guchte M., Guedon E., Haimet F., Huber W., van Hylckama-Vlieg J., Jamet A., Juste C., Kaci G., Knol J., Lakhdari O., Layec S., Le Roux K., Maguin E., Mérieux A., Melo Minardi R., M'rini C., Muller J., Oozeer R., Parkhill J., Renault P., Rescigno M., Sanchez N., Sunagawa S., Torrejon A., Turner K., Vandemeulebrouck G., Varela E., Winogradsky Y., Zeller G., Weissenbach J., Ehrlich S. D., Bork P. (2011) Nature 473, 174–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee J., Michael A. J., Martynowski D., Goldsmith E. J., Phillips M. A. (2007) J. Biol. Chem. 282, 27115–27125 [DOI] [PubMed] [Google Scholar]
- 37. Yamamoto S., Sugahara T., Tougou K., Shinoda S. (1994) Microbiology 140, 3117–3124 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.