Background: PPARα is a distinctive marker of the brown-versus-white fat phenotype.
Results: PPARα induces PGC-1α gene transcription in brown adipocytes through mechanisms involving PRDM16.
Conclusion: PPARα regulates brown fat thermogenesis via induction of PGC-1α and PRDM16 gene expression.
Significance: Activation of PGC-1α by PPARα provides a molecular mechanism for concerted induction of thermogenic genes (UCP1, mitochondrial genes, and lipid oxidation genes) in brown fat.
Keywords: Adipose Tissue, Cyclic AMP (cAMP), Differentiation, Obesity, Peroxisome Proliferator-activated Receptor (PPAR), Protein Kinase A (PKA), Transcription Coactivators, PGC-1, PRDM16, Brown Adipose Tissue
Abstract
Peroxisome proliferator activated receptor α (PPARα) is a distinctive marker of the brown fat phenotype that has been proposed to coordinate the transcriptional activation of genes for lipid oxidation and for thermogenic uncoupling protein 1 in brown adipose tissue. Here, we investigated the involvement of PPARα in the transcriptional control of the PPARγ coactivator (PGC)-1α gene. Treatment with PPARα agonists induced PGC-1α mRNA expression in brown fat in vivo and in primary brown adipocytes. This enhancement of PGC-1α transcription was mediated by PPARα binding to a PPAR-responsive element in the distal PGC-1α gene promoter. PGC-1α gene expression was decreased in PPARα-null brown fat, both under basal conditions and in response to thermogenic activation. Moreover, PPARα- and cAMP-mediated pathways interacted to control PGC-1α transcription. PRDM16 (PRD1-BF1-RIZ1 homologous domain-containing 16) promoted PPARα induction of PGC-1α gene transcription, especially under conditions in which protein kinase A pathways were activated. This enhancement was associated with the interaction of PRDM16 with the PGC-1α promoter at the PPARα-binding site. In addition, PPARα promoted the expression of the PRDM16 gene in brown adipocytes, and activation of PPARα in human white adipocytes led to the appearance of a brown adipocyte pattern of gene expression, including induction of PGC-1α and PRDM16. Collectively, these results suggest that PPARα acts as a key component of brown fat thermogenesis by coordinately regulating lipid catabolism and thermogenic gene expression via induction of PGC-1α and PRDM16.
Introduction
Mammals possess two specialized types of fat cells that serve opposite functions. White adipocytes store excess energy as triacylglycerols in large lipid droplets. When needed, this stored energy can be mobilized by activating lipolysis with the consequent release of free fatty acids into the circulation. In contrast, brown adipocytes oxidize endogenous triacylglycerols to generate heat (thermogenesis), a process made possible by brown fat-specific uncoupling protein (UCP)3 1 present in their abundant mitochondria (1). Thermogenesis in brown adipose tissue (BAT) is mainly controlled by norepinephrine, which is released from sympathetic terminals innervating the tissue in response to cold or dietary stimuli. Norepinephrine, through β-adrenergic receptors, leads to cAMP/protein kinase A (PKA)-mediated activation of lipolysis and thermogenic activity. The clear role of BAT in the defense against hypothermia and obesity described in rodent model studies is now being reevaluated in humans in light of the recent demonstration that considerable amounts of metabolically active BAT are present in adult humans (reviewed in Ref. 2).
Despite the differences in the functional and developmental characteristics of brown and white adipocytes, their terminal differentiation processes are mainly regulated by transcriptional factors in common: peroxisome proliferator-activated receptor γ (PPARγ/NR1C3) and CCAAT/enhancer binding proteins (C/EBPs) (3). In fact, both PPARγ and C/EBPα induce UCP1 gene transcription (4, 5), but UCP1 can still be induced in their absence (6, 7). Recently, comprehensible advances have been achieved on the identification of several transcriptional factors and coregulators that specifically promote embryonic development and acquisition of the BAT-specific gene expression profile, including UCP1, among them PRDM16 (PRD1-BF1-RIZ1 homologous domain-containing 16) and PGC-1α (PPARγ coactivator 1α) (reviewed in Ref. 8).
PGC-1α is a transcriptional coactivator involved in the control of energy metabolism that is highly expressed in BAT (9). PGC-1α is critical for the cAMP-dependent activation of BAT thermogenesis, playing a role in inducing UCP1 gene expression and enhancing overall mitochondrial oxidative activity (9). PGC-1α is not essential for BAT differentiation because it can be replaced by PGC-1β (10). However, ectopic expression of PGC-1α in white adipocytes induces acquisition of BAT features, including expression of mitochondrial and fatty acid-oxidation and thermogenic genes (9, 11). Therefore, increasing PGC-1α may be a plausible strategy for the treatment of obesity. In response to an adrenergic stimulus, PGC-1α gene expression is up-regulated by cAMP-mediated pathways in BAT. The underlying mechanism involves phosphorylation and binding of activating transcription factor 2 to a cAMP-responsive element (CRE) in the proximal PGC-1α promoter region (12). Furthermore, we previously reported that PPARγ is also a powerful inducer of PGC-1α gene transcription, acting through a PPAR-responsive element (PPRE) in the distal promoter region (13).
PRDM16, which is also highly expressed in BAT, has been identified as a transcriptional coactivator responsible for determining the BAT lineage (6, 14). When ectopically expressed in white adipocyte or myogenic precursor cells, PRDM16 induces the BAT-specific gene expression program (6, 14). Conversely, PRDM16 knockdown in brown adipocytes ablates brown-specific gene expression in association with induction of skeletal muscle-specific gene expression (14). PRDM16 is thus recognized as a critical determinant of the differentiation of the BAT lineage from myogenic progenitors during embryonic development, although it is unclear whether it plays a role in fully differentiated brown adipocytes. Recently, PRDM16 was reported to promote the induction of the thermogenic program in subcutaneous white adipose tissue (WAT) of rodents (15).
PPARα (NR1C1) plays an important role in the overall regulation of lipid metabolism. Many PPARα target genes are involved in cellular fatty acid uptake (e.g. lipoprotein lipase) and in mitochondrial and peroxisomal β-oxidation of fatty acids (16). Consistent with this function, PPARα is highly expressed in BAT (17), and actually, it is considered a distinctive marker of the BAT with respect to WAT phenotype (18). Moreover, PPARα regulates the expression of UCP1, the specific protein that enables brown adipocytes to perform thermogenesis (19). Here, we identify PPARα as a direct activator of PGC-1α and further demonstrate that the interaction of PPARα with PRDM16 and the cAMP-mediated pathways is necessary for full thermogenic activation of PGC-1α gene transcription in BAT.
EXPERIMENTAL PROCEDURES
Materials
Rosiglitazone was from Cayman Chemicals. Wy14,643 (pirinixic acid), bezafibrate, GW6471, GW501516, dibutyryl-cAMP, norepinephrine, and isobutyl-methyl-xanthine (IBMX) were obtained from Sigma. GW7647 was purchased from Tocris.
Animals
Mice were cared for and used in accordance with European Community Council Directive 86/609/EEC, and animal protocols were approved by the Comitè Ètic d'Experimentació Animal of the University of Barcelona. For studies in Swiss neonates, pups were placed in a humidified, thermostatically controlled chamber at 28 °C and injected intraperitoneally 2 h after birth with Wy14,643 (50 μg/g body weight) or equivalent volumes of a 20% (v/v) dimethyl sulfoxide/saline solution. Pups were studied 15 h after treatment. Two-month-old female, 15-day lactating mice were also treated with a single intraperitoneal injection of Wy14,643 (50 μg/g body weight) or bezafibrate (100 μg/g body weight) in 50% dimethyl sulfoxide/saline. Controls were given equivalent volumes of the vehicle, and mice were studied 6 h after injections. Studies in PPARα-null (PPARα−/−) mice (The Jackson Laboratory, Bar Harbor, ME) and strain-matched wild-type (PPARα+/+) mice were performed to determine the effects of acute cold exposure. Two-month-old mice were acclimated to a thermoneutral environmental temperature (28 °C) for 1 week and then exposed to 4 °C for 4 or 24 h. In all experiments, animals were killed by decapitation, and interscapular BAT was dissected and frozen in liquid nitrogen. At least three different litters per experiment were analyzed.
Cell Culture
Primary cultures of brown adipocytes from mice were established and maintained as described previously (20). After culturing for 9 days, a time when 80–90% of cells had differentiated, cells were exposed to 10 μm rosiglitazone, 10 μm Wy14,643, 1 μm GW7647, 1 μm GW501516, 0.5 μm norepinephrine, or 1 mm dibutyryl-cAMP for 24 h (except where indicated otherwise) and then harvested. The effects of PPARα inhibition were determined using 10 μm GW6471. Mouse embryonic fibroblasts (MEFs) from PGC-1α−/− and PGC-1α+/+ mice were isolated and cultured as described previously (21). The SGBS human adipocyte cell line was cultured and differentiated as described previously (22).
RNA Isolation and Quantitative Real-time RT-PCR
RNA was extracted using the NucleoSpin RNAII kit (Macherey-Nagel, Düren, Germany). Quantitative real-time RT-PCR analysis of mRNA expression was performed as described previously (13). Assay-on-Demand probes for the following targets were used: mPGC-1α (Mm00447183), mPrdm16 (Mm00712556), hPGC-1α (Hs00173304), hPRDM16 (Hs00223161), UCP1 (Hs00222453), DIO2 (Hs00255341), COX4 (Hs00266371), UQCRC1 (Hs00163415), UCP3 (Hs00243297), LPL (Hs00173425), MCAD (Hs00163494), PPARγ (Hs00234592), FABP4 (Hs00609791), GLUT4 (Hs00168966), ADRβ3 (Hs00609046), and 18S rRNA (Hs99999901). Human cytochrome-c-oxidase subunit II mRNA was analyzed (Assay-by-Design, Applied Biosystems) using the primers 5′-AAA CCA CTT TCA CCG CTA CAC-3′ (forward) and 5′-GGA CGA TGG GCA TGA AAC TGT-3′ (reverse) and the FAM-labeled probe 5′-AAA TCT GTG GAG CAA ACC-3′. The relative amount of mRNA in each sample was normalized to that of the reference control 18 S rRNA using the comparative (2−ΔCT) method according to the manufacturer's instructions.
Plasmids and Transfection Assays
The plasmid −2553-PGC-1α-Luc (23) was a gift from Dr. B. Spiegelman. A mutated version of the −2553-PGC-1α-Luc plasmid (−2553-PPREmut-PGC-1α-Luc) containing substitutions at positions −2043/−2044 (AG to GT) and −2050/−2052 (AGG to GCT) in the putative PPRE, and a truncated version in which the proximal −146/−129 region containing the cAMP-responsive element (−2553-CREmut-PGC-1α-Luc) (13) was deleted, were also used. The plasmid UCP1-Luc contained a fragment of the 5′ non-coding region of the rat UCP1 gene from −4551 to +90 cloned into the pGL-3 basic vector. The plasmid ApoAII-PPRE-TK-Luc was a gift of Dr. L. Fajas (24). pSG5-PPARα (25), pSG5-PPARβ/δ (26), pSV-PGC-1α (9), SRα-PKA (27), and pcDNA3.1-PRDM16 (6) expression vectors have been described elsewhere. HIB-1B, CV-1, COS-7, and MEF cells were transiently transfected using FuGENE-6 (Roche). Transfection experiments were carried out as described previously (13).
ChIP Assays
ChIP assays in HIB-1B cells and brown adipocytes were performed as described previously (13). HIB-1B cells were transfected with the PPARα expression vector and exposed to 10 μm Wy 14,643 or 1 μm GW7647 as described above. Where indicated, cells were transfected with −2553-PGC-1α-Luc or −2553-PPREmut-PGC-1α-Luc and cotransfected with PRDM16 and PKA expression vectors or treated with 0.5 mm IBMX. Brown adipocytes were treated for 24 h with 1 μm GW7647or 1 mm dibutyryl-cAMP as indicated. The ChIP assay in BAT was performed as described (28). Chromatin samples were immunoprecipitated with 8 μg of anti-PPARα antibody (H98, Santa Cruz Biotechnology, Santa Cruz, CA), 3 μg of anti-PRDM16 antibody (EB 05579, Everest Biotech, UK), or an equal amount of an unrelated immunoglobulin (sc-9314, Santa Cruz Biotechnology). After phenol-chloroform extraction, immunoprecipitated chromatin DNA was used for PCR analysis. The primers used to amplify a 378-bp fragment encompassing the putative PPRE in the PGC-1α gene were 5′-GTA TCA GTT ACC ATC AGG-3′ (forward) and 5′-AAC AAG ATG GCC AAC AGC-3′ (reverse).
Adenovirus Transduction
Differentiated SGBS (day 12) adipocytes in DMEM/F12 medium were infected with adenoviral vectors driving human PPARα (AdCMV-hPPARα) (29) or AdCMV-GFP (control) at a multiplicity of infection of 400 for 4 h. Experiments were performed following an additional 48-hour incubation in fresh differentiation media. This treatment led to an efficiency of transduction of about 80% on the basis of an assessment of GFP fluorescence. Cells were treated for 24 h with the PPARα-selective ligand GW7647 (1 μm) or vehicle (dimethyl sulfoxide).
Western Blot Analysis
Protein extracts from brown adipocytes and BAT were prepared by homogenization in Nonidet P-40 lysis buffer (100 mm Tris HCl (pH 8.5), 1 mm EDTA, 1% Nonidet P-40, 0.5 mm dithiothreitol, 250 mm NaCl, 0.5 mm PMSF, 2.5 mm benzamidine, 10 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin). Proteins (40 μg/lane) were separated by 8% SDS-PAGE, transferred to Immobilon-P membranes (Millipore), and probed with an antibody against PRDM16 (ab106410, Abcam, UK). Incubation with an anti-α-tubulin antibody (clone DM-1A, Sigma) was performed to establish equal total protein loading.
Statistics
Student's t test was used for statistical analyses.
RESULTS
PPARα Activation Induces PGC-1α Gene Transcription in Brown Adipocytes
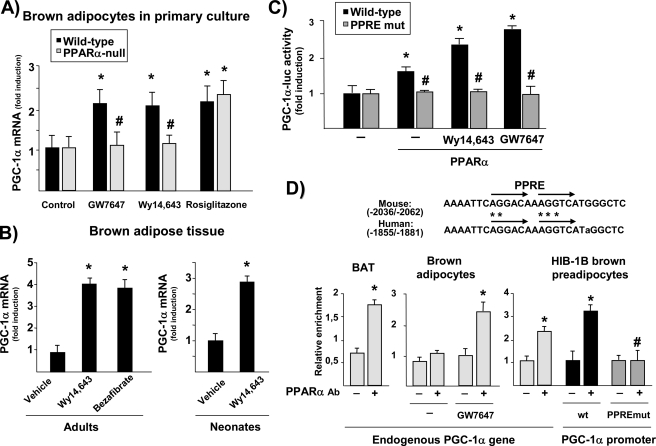
Treatment with the PPARα agonists GW7647 and Wy14,643 increased PGC-1α mRNA expression in mouse primary brown adipocytes differentiated in vitro (Fig. 1A). The specific PPARγ agonist rosiglitazone, which was previously reported to activate PGC-1α gene expression (13), had similar effects. We next established primary cultures of brown adipocytes from PPARα-null mice. Morphological differentiation was unchanged in PPARα-null brown adipocytes, as described elsewhere (30 and data not shown), as were basal levels of PGC-1α mRNA expression. However, PPARα agonist-induced increases in PGC-1α mRNA were totally suppressed in PPARα-null brown adipocytes, whereas the effect of rosiglitazone was essentially unchanged.
FIGURE 1.
PPARα activation enhances PGC-1α gene expression in brown adipocytes. A, differentiated brown adipocytes from either wild-type or PPARα-null mice were treated for 24 h with specific agonists of PPARα (1 μm GW7647, 10 μm Wy14,643) or PPARγ (10 μm rosiglitazone) on day 9 of culture. Data are presented as fold induction relative to values in untreated cells from wild-type mice. *, p < 0.05 between untreated cells for each genetic background for each treatment condition; #, p < 0.05 between wild-type and PPARα-null cells for each treatment condition. B, PGC-1α mRNA levels were determined in BAT from adult lactating mice (left panel) or neonates (right panel) after a single injection of Wy14,643 (50 μg/g body weight) or bezafibrate (100 μg/g body weight). Bars indicate the mean ± S.E. of 5–9 mice per group. *, p < 0.05 versus vehicle-injected mice. C, transient transfection experiments were performed in HIB-1B cells with either the wild-type 2kbPGC-1α-luciferase construct or a PPRE-mutated construct (PPREmut) that contains the point mutations in the PPRE sequence denoted by asterisks in D. Where indicated, cells were cotransfected with a PPARα expression vector and exposed to 10 μm Wy14,643 or 1 μm GW7647. Results are presented as mean ± S.E. of at least three independent experiments done in triplicate. *, p < 0.05 versus untreated controls; #, p < 0.05 for comparisons of the mutated construct with the wild-type construct under equivalent cotransfection or treatment conditions. D, sequence of the PPAR response element in the mouse and human PGC-1α gene promoters. Arrows indicate one-base spaced direct repeat alignment (top panel). ChIP analysis of PPARα binding to the endogenous PGC-1α gene in BAT, brown adipocytes, and HIB-1B cells or to the transfected wild-type or PPRE-mutated PGC-1α promoter in HIB-1B cells (always in the presence of a PPARα expression vector and 10 μm Wy14,643) (bottom panel). Data are expressed as mean ± S.E. of the fold induction in relative intensity of the amplified PCR product from three independent experiments. *, p < 0.05 versus controls; #, p < 0.05 for comparisons between the WT and PPRE-mutated constructs.
The effects of acute treatment of mice with the PPARα-specific agonist Wy14,643 were studied both in adult and neonatal mice. For adult studies, lactating dams were used, as previous studies had indicated that sensitivity to PPARα is enhanced in physiological states involving low levels of circulating free fatty acids (19). Wy14,643 significantly increased PGC-1α gene expression in BAT in vivo in both physiological contexts (Fig. 1B). The PPAR-panagonist bezafibrate induced PGC-1α expression to a similar extent. These results demonstrate that PPARα agonists acutely regulate the PGC-1α gene both in vivo and in differentiated primary brown adipocytes.
We next performed transient transfection experiments in the brown adipocyte-derived HIB-1B cell line using a plasmid containing 2 kb of the mouse PGC-1α gene promoter region fused to the luciferase reporter gene. Cotransfection with PPARα, and particularly further addition of the PPARα agonists Wy14,643 or GW7647, increased PGC-1α promoter activity (Fig. 1C). Conversely, responsiveness to PPARα was abolished in a mutated construct (mutPPRE-PGC-1α-luc) in which the PPRE, which had previously been shown to mediate sensitivity to PPARγ (13), was mutated (Fig. 1D, asterisks). Notably, this PPRE did not behave as a PPARβ/δ-responsive element in this brown adipocyte context (108 ± 18% activity of the mutPPRE-PGC-1α-luc with respect to the wild-type PGC-1α-luc construct in the presence of cotransfected PPARβ/δ expression vector plus the PPARβ/δ-specific ligand GW501516).
To assess whether PPARα binds specifically to the endogenous PGC-1α gene, we performed ChIP assays in brown fat and primary brown adipocytes (Fig. 1D, left panel). Incubation with an anti-PPARα antibody significantly enriched the specific PGC-1α-promoter PCR product, thus indicating that endogenous PPARα protein does indeed bind to the endogenous PGC-1α gene in brown fat. When analyzed in primary brown adipocytes, we found that activation of the endogenous PPARα led to a significant enhancement of its recruitment to the endogenous PGC-1α gene (Fig. 1D, center panel). Moreover, ChIP was also performed in HIB-1B cells transfected with the 2kb-PGC-1α or mutPPRE-PGC-1α promoter constructs. Results showed that enrichment was impaired when the construct in which the PPRE had been mutated was transfected (Fig. 1D, right panel). These results confirm that PPARα binds and activates the PPRE element in the distal region of the PGC-1α gene promoter in brown adipocytes.
Interaction between PPARα- and cAMP-mediated Pathways in the Control of PGC-1α Gene Transcription
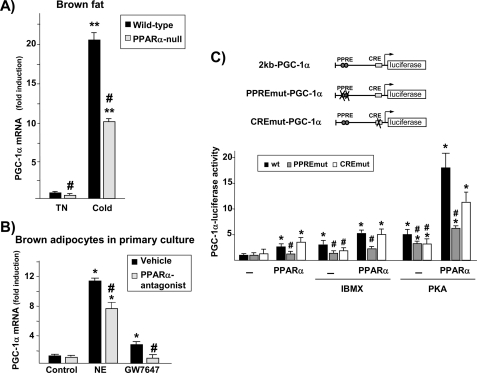
To further study the role of PPARα in the control of the PGC-1α gene, we analyzed the response of the PGC-1α gene to acute cold induction in PPARα-null mice. Basal expression of PGC-1α mRNA was significantly decreased in BAT from PPARα-null mice reared at a thermoneutral temperature compared with that in wild-type animals (Fig. 2A). Exposure of mice to cold (4 °C for 24 h) caused a significant increase in PGC-1α mRNA levels in wild-type BAT, as reported previously (9). This increase was significantly blunted in the PPARα-null mice, suggesting that PPARα is involved in the noradrenergic regulatory pathway in BAT in response to thermogenic activation.
FIGURE 2.
PPARα- and cAMP-mediated pathways interact to control PGC-1α gene expression in brown adipocytes. A, wild-type and PPARα-null mice were acclimated to a thermoneutral temperature (TN, 28 °C) and then exposed to 4 °C ambient temperature for 24 h (Cold) or maintained at 28 °C. Data are presented as fold induction of PGC-1α mRNA levels relative to values in BAT from wild-type mice at 28 °C. **, p < 0.01 for differences because of cold exposure for each genetic background; #, p < 0.05 for differences between wild-type and PPARα-null mice under each temperature condition. B, differentiated brown adipocytes in culture were treated with 1 μm GW7647 for 24 h or with 0.5 μm norepinephrine (NE) for 3 h in the presence or absence of the PPARα antagonist GW7647 (10 μm). Data are presented as mean ± S.E. of three independent experiments done in triplicate. *, p < 0.05 versus controls; #, p < 0.05 for differences because of the PPARα antagonist. C, HIB-1B cells were transfected with the 2kbPGC-1α-luciferase construct (WT), the PPRE-mutated construct (PPREmut), or the CRE-mutated plasmid (CREmut). Where indicated, cells were cotransfected with an expression vector for the constitutively active form of PKA or were exposed to 0.5 mm IBMX in the presence or absence of the PPARα expression vector plus 1 μm GW7647. Results are presented as mean ± S.E. of the fold change in promoter activity relative to the untreated, non-cotransfected WT. *, p < 0.05 versus controls; #, p < 0.05 for comparisons of mutated constructs with the WT construct under equivalent cotransfection or treatment conditions.
We next used the specific PPARα antagonist GW6471 to analyze whether an active PPARα-dependent regulatory pathway is required for effective noradrenergic induction of the PGC-1α gene in primary cultures of brown adipocytes (Fig. 2B). This drug not only suppressed the action of the PPARα agonist GW7647, it also significantly reduced the action of norepinephrine, indicating the existence of cross-talk between the PPARα-dependent and the noradrenergic pathways that regulate PGC-1α gene expression.
This PPARα/noradrenergic cross-talk was further analyzed at the transcriptional level (Fig. 2C). The increase in cAMP levels caused by the addition of IBMX led to a significant induction of PGC-1α promoter activity, an increase that was comparable with that achieved via PPARα activation. The degree of induction was even greater following cotransfection with an expression vector driving the catalytic subunit of PKA, and when cotransfected together with PPARα plus its ligand, a robust interaction was detected. Mutation of a previously reported CRE in the proximal region of the PGC-1α gene promoter (31) significantly impaired cAMP- and PKA-dependent induction but had no effect on sensitivity to activation by PPARα. In contrast, the PPRE-mutant form of the PGC-1α promoter (13) showed a significantly diminished capacity to respond to both PPARα- and cAMP/PKA-dependent activation. Taken together, these results indicate that PPARα and cAMP-mediated pathways interact to regulate PGC-1α gene transcription, a regulatory mechanism that requires the PPRE.
Involvement of PRDM16 in PPARα-mediated Regulation of PGC-1α Gene Transcription
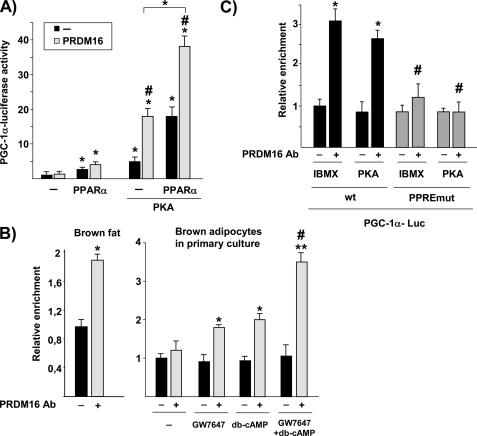
The requirement of an intact PPARα signaling pathway for cAMP responsiveness of PGC-1α gene expression led us to analyze whether other transcriptional regulators could also be involved. We first analyzed PGC-1α itself. In contrast to what has been reported for PPARγ-dependent activation of PGC-1α (13), where PGC-1α serves as a coactivator of its own gene, there was no evidence for a PPARα-dependent autoinduction mechanism (2.6 ± 0.4-fold induction by cotransfection of PPARα versus 2.0 ± 0.3-fold induction by cotransfection of PPARα and PGC-1α). We next analyzed PRDM16, which has been reported as another PPARγ-coactivating protein involved in determining BAT fate (14). Cotransfection of a PRDM16 expression vector did not stimulate basal PGC-1α promoter activity and tended to activate PPARα induction of PGC-1α gene transcription, although this last effect did not reach statistical significance (p < 0.061). Furthermore, this effect was significantly potentiated when the PKA pathway was also activated (Fig. 3A).
FIGURE 3.
PRDM16 potently enhances the capacity of PPARα to induce PGC-1α gene transcription, especially when the PKA pathway is activated. A, HIB-1B cells were transiently transfected with the 2kbPGC-1α-luciferase construct. Where indicated, cells were cotransfected with an expression vector for PPARα (plus 1 μm GW7647 treatment), PKA and/or PRDM16. Results are presented as mean ± S.E. of the fold change in promoter activity relative to non-cotransfected, untreated cells expressing the 2kbPGC-1α-luciferase construct. *, p < 0.05 for the effects of PPARα or PKA; #, p < 0.05 for the effect of PRDM16 under each condition. B, ChIP analysis of PRDM16 binding to the endogenous PGC-1α gene in BAT and brown adipocytes in primary culture. When indicated, brown adipocytes were treated for 24 h with 1 μm GW7647, 1 mm db-cAMP, or both. Data are expressed as the mean ± S.E. of the fold induction in relative intensity of the amplified PCR product from three independent experiments. *, p < 0.05; **, p < 0.01 versus controls; #, p < 0.05 versus GW7647 or db-cAMP treatment alone. C, ChIP analysis of PRDM16 binding to the PGC-1α gene promoter. HIB-1B cells were transfected with the WT or PPREmut version of the 2kbPGC-1α-luciferase vector and treated with 0.5 mm IBMX or cotransfected with a PKA expression vector, as indicated (always in the presence of the PPARα expression vector plus 1 μm GW7647). Data are expressed as the mean ± S.E. of the fold induction in relative intensity of the amplified PCR product from three independent experiments. *, p < 0.05 versus controls; #, p < 0.05 for comparisons between WT and PPRE-mutated constructs.
To assess whether PRDM16 binds to the PGC-1α gene promoter, we performed ChIP experiments. Results indicated a significant recruitment of endogenous PRDM16 protein to the endogenous PGC-1α gene in brown adipose tissue (Fig. 3B, left panel). Treatment with either GW7647 or dibutyryl-cAMP significantly increased the recruitment of endogenous PRDM16 to the endogenous PGC-1α gene in primary brown adipocytes. This recruitment was maximal when both compounds were added (Fig. 3B, right panel). We next performed ChIP assays in HIB-1B cells cotransfected with either 2kb-PGC-1α or mutPPRE-PGC-1α promoter constructs together with expression vectors for PPARα and PRDM16 in the presence of either IBMX or a PKA stimulus. Results indicated that PRDM16 binding to the PGC-1α gene promoter occurs at the PPRE site (Fig. 3C). Given the recent demonstration of a direct physical interaction between PRDM16 and PPARα (14), it is likely that the recruitment of PRDM16 to the PGC-1α promoter involves PRDM16 binding to PPARα. Although it has been reported that PRDM16 and PPARα interact in a non-ligand-dependent manner in coimmunoprecipitation assays (14), the binding of PRDM16 to the endogenous PGC-1α gene is induced by addition of the PPARα-ligand in brown adipocytes (Fig. 3B).
PRDM16 Coactivates PPARα- and PKA-dependent Induction of UCP1 Gene Transcription
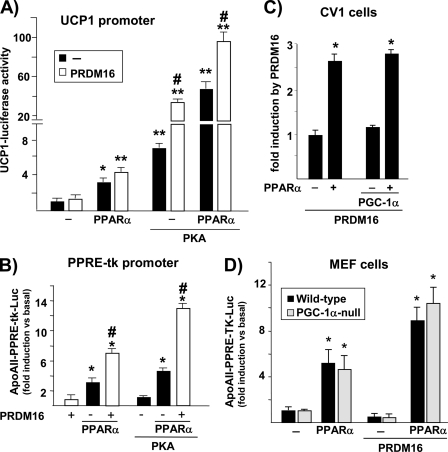
We next studied whether UCP1, another key thermogenesis gene that shares with PGC-1α the property of dual regulation by PPARα and PKA, was also a direct target of PRDM16 coactivation. As shown in Fig. 4A, the transcriptional activity of a luciferase reporter construct driven by a 4.5-kb upstream region of the rat Ucp1 gene was induced by cotransfected PPARα or PKA, as reported previously (19). An additional increase in luciferase activity was observed upon cotransfection of PPARα and PKA together. Cotransfection of PRDM16 did not affect basal UCP1 promoter activity but significantly enhanced its response to PKA (Fig. 4A). Moreover, PRDM16 tended to increase PPARα responsiveness of the UCP1 promoter (p < 0.057) and, when the PKA pathway was also activated, maximal effects of PRDM16 were observed.
FIGURE 4.
PRDM16 coactivates PPARα on PPARα-driven promoters, and this effect does not require PGC-1α. Relative luciferase activity of transiently transfected promoter-luciferase reporter constructs in response to cotransfection of PPARα, PRDM16, and PKA expression vectors in HIB-1B cells. *, p < 0.05; **, p < 0.01 for differences because of PPARα or PKA; #, p < 0.05 for differences because of PRDM16 under each condition. A, UCP1 promoter construct. B, construct in which the PPRE element of the ApoAII gene is placed upstream of the basal thymidine kinase (TK) promoter driving the luciferase reporter (PPRE-tk promoter). Also shown is an analysis of PRDM16 coactivation of PPARα in cells lacking PGC-1α. CV-1 cells (C) and MEF cells (D) were transiently transfected with the ApoAII-PPRE-tk-luciferase construct. Where indicated, the expression vectors for PPARα, PGC-1α, and/or PRDM16 were cotransfected. *, p < 0.05 for differences because of PPARα).
PRDM16 Coactivates a Consensus PPARα-driven Promoter, an Effect That Is Favored by PKA but Does Not Require PGC-1α
To further analyze the involvement of PRDM16 in the regulation of gene transcription by PPARα, we transiently transfected HIB-1B brown adipocytes with a promoter-reporter construct in which expression of the luciferase reporter gene is driven by a consensus PPARα-responsive promoter. PRDM16 alone had no effect, but it did significantly coactivate PPARα-mediated induction of PPRE-dependent promoter activity (Fig. 4B). Cotransfection of an expression vector for PKA alone did not affect PPRE promoter activity but potently enhanced PRDM16/PPARα coactivation. These results indicate that PRDM16 coactivation of PPARα in brown adipocytes is just dependent on the presence of a PPARα-responsive element and enhanced when PKA pathways are activated.
PRDM16 has been reported to coactivate and bind PGC-1α (6). Because PGC-1α is highly expressed in HIB-1B brown adipocytes, involvement of PGC-1α in the molecular mechanism responsible for PRDM16 coactivation of PPARα could not be ruled out. Therefore, we analyzed whether PRDM16 coactivation of PPARα occurs in cells lacking PGC-1α. For this purpose, we performed transient transfection experiments of the consensus PPRE promoter construct using CV-1 cells. As shown in Fig. 4C, the extent of induction of PPARα-dependent transcriptional activity by PRDM16 was similar with and without transfection of a PGC-1α expression vector. The same results were obtained using COS-7 cells (not shown). Transient transfection experiments were also performed using MEFs obtained from wild-type and PGC-1α-null mice. PPARα-mediated induction and its coactivation by PRDM16 were unaltered in PGC-1α-null MEFs (Fig. 4D), indicating that PGC-1α is not required for PRDM16/PPARα regulation of consensus PPRE promoter activity.
PRDM16 Gene Expression Is Controlled by PPARα and by Noradrenergic, cAMP-mediated Mechanisms in Brown Adipocytes
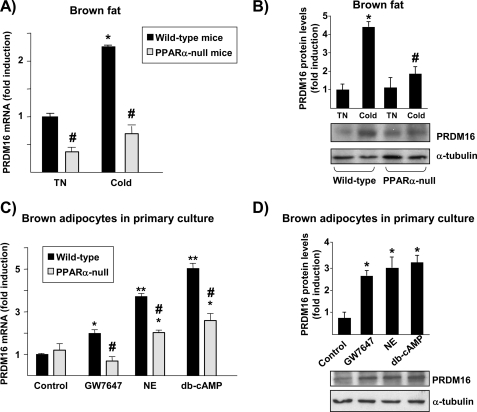
We next analyzed whether PRDM16 expression is affected by PPARα and/or noradrenergic regulation. Acute cold exposure of mice (4 °C for 24 h) significantly induced PRDM16 mRNA expression in BAT (Fig. 5A), although this effect was not observed at short times of cold exposure (0.82 ± 0.20 fold-change after 4 h at 4 °C versus controls), in agreement with previous reports (6). In PPARα-null mice, PRDM16 mRNA levels were lower under basal conditions, and their response to cold was greatly diminished (Fig. 5A).
FIGURE 5.
Effects of PPARα and noradrenergic activation on PRDM16 gene expression in brown adipocytes. PRDM16 mRNA (A) or protein (B) levels in BAT from PPARα-null and wild-type mice maintained at a thermoneutral temperature (TN, 28 °C) or exposed to 4 °C ambient temperature for 24 h (Cold). Bars indicate the mean ± S.E. of 6–7 mice per group. *, p < 0.05 for differences because of cold exposure for each genetic background; #, p < 0.05 for differences between wild-type and PPARα-null mice under each temperature condition. Protein data are expressed as the ratio of the densitometric intensity of the immunoreactive signal of PRDM16 protein normalized by the immunoreactive signal of α-tubulin. C and D, differentiated brown adipocytes in culture from wild-type or PPARα-null mice were treated for 24 h with 1 μm GW7647, 0.5 μm norepinephrine (NE), or 1 mm dibutyryl-cAMP (db-cAMP). Data on PRDM16 mRNA (C) or protein (D) are mean ± S.E. of three independent experiments done in triplicate. *, p < 0.05; **, p < 0.01 for differences because of treatment condition in each genetic background; #, p < 0.05 for differences between wild-type and PPARα-null cells under each treatment condition.
Exposure of differentiated brown adipocytes to the PPARα-specific activator GW7647 resulted in a significant increase in PRDM16 mRNA levels (Fig. 5B). Norepinephrine and cAMP also significantly increased PRDM16 mRNA expression. Basal levels of PRDM16 mRNA were unaltered in PPARα-null brown adipocytes, whereas the effects of GW7647 were blocked. Induction by norepinephrine and cAMP, although not completely impaired, was significantly reduced in brown adipocytes lacking PPARα, providing further support for cross-talk between PPARα-dependent and noradrenergic regulation of PRDM16 gene expression.
Activation of PPARα in Human White Adipocytes Leads to Activation of the Brown Adipocyte-specific Pattern of Gene Expression, including Induction of PGC-1α and PRDM16
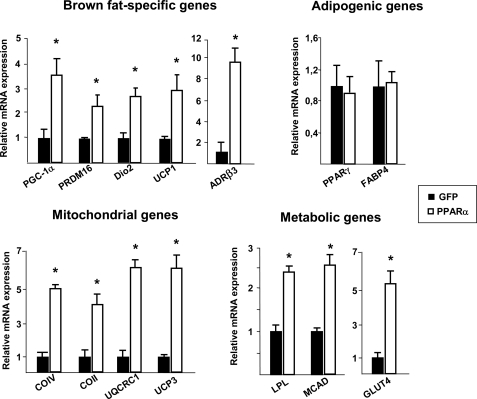
To further analyze the role of PPARα in the control of PGC-1α and PRDM16 gene expression, we investigated whether increasing PPARα expression in white adipocytes could confer on these cells the capacity to activate the brown-fat specific gene expression pattern. Adenoviral transduction of human SGBS white adipocytes with PPARα increased PPARα mRNA levels by approximately 30-fold compared with those in basal conditions. These high levels are comparable with those of endogenous PPARα mRNA levels in mouse BAT. In the presence of GW7647, PPARα expression led to a significant induction of PGC-1α and PRDM16 mRNA expression in human SGBS white adipocytes (Fig. 6). Furthermore, expression of other key BAT-selective genes, such as UCP1 and type 2 deiodinase (DIO2), were also induced, as was expression of the β3-adrenergic receptor (ADRβ3), the main β-adrenergic receptor involved in the regulation of thermogenesis. PPARα activation in SGBS white adipocytes also increased the mRNA levels for several mitochondrial proteins that are known to be enriched in brown relative to white adipocytes. These include UCP3; ubiquinol-cytochrome c reductase core protein 1 (UQCRC1), a component of mitochondrial respiratory complex III; cytochrome c oxidase-2 (COII) and −4 (COIV), subunits of mitochondrial respiratory complex IV; and medium-chain acyl-coenzyme A dehydrogenase (MCAD), a metabolic protein involved in lipid catabolism. PPARα activation also induced gene expression of lipoprotein lipase (LDL) and glucose transporter type 4 (GLUT4), which are involved in lipid and glucose uptake, respectively. In contrast, expression of the adipogenic genes PPARγ and fatty acid binding protein 4 (FABP4) remained unchanged. This confirmed that the action of PPARα on adipocyte gene expression favors the acquisition of BAT-like features through induction of PGC-1α and PRDM16, master regulators of the brown adipocyte-specific phenotype.
FIGURE 6.
Effects of PPARα on gene expression in human white adipocytes. Human SGBS white adipocytes differentiated in culture were transduced with adenoviral vectors driving PPARα or GFP as a control and were then exposed to 1 μm GW7647. Shown is the analysis of the transcript levels of the indicated genes by real-time RT-PCR. Bars represent the mean ± S.E. from three independent experiments done in duplicate. Results are expressed as fold induction with respect to values in control GFP adipocytes. *, p < 0.05 versus control.
DISCUSSION
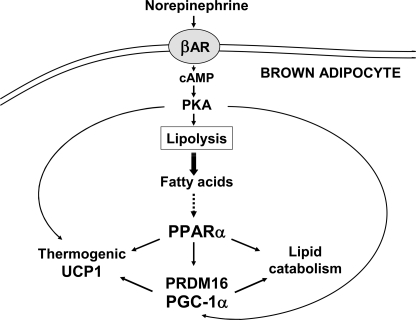
PPARα plays an important role in the overall regulation of energy metabolism, mainly by controlling genes involved in lipid metabolism. Furthermore, PPARα constitutes a specific marker of mature brown adipocytes, where together with PGC-1α, it regulates key components of the thermogenic function, such as UCP1 (18, 19). In this study, we identified PGC-1α as a direct target of PPARα transcriptional regulation in BAT. PPARα activators regulated the expression of the PGC-1α gene in brown adipocytes through a PPARα-responsive element in the distal promoter region. This PPRE binds both PPARα (these results) and PPARγ (13) and behaves as a promiscuous responsive site to activation by either PPARα or PPARγ, but not PPARδ. Transcriptional control by PPAR subtypes through a unique PPRE has been reported previously for mammalian genes (16). This mechanism provides tissue-specific regulation and/or specific responsiveness to metabolic challenges in a particular cell-type and probably relies on the interaction of PPARs with tissue-specific coregulators, such as PRDM16 in brown adipocytes. Dual PPARα and PPARγ (but not PPARδ) regulation has been reported for the UCP1 gene (4, 19) and probably reflects the regulatory dynamics of thermogenic gene expression in BAT, namely PPARγ-dependent induction of thermogenic genes in association with brown adipocyte differentiation, and coordination between PPARα-induced fatty acid oxidation and thermogenesis in fully differentiated brown adipocytes (18). Both PGC-1α and UCP1 genes also share a common transcriptional regulatory mechanism involving PPAR- and cAMP-responsive elements located in distal and proximal promoter regions, respectively (4, 12, 13, 19, 32). Moreover, the present results provide evidence for a robust interaction between these two pathways in the regulation of UCP1 and PGC-1α genes that is mediated at the molecular level by PPARα and PRDM16, as discussed below and schematized in Fig. 7.
FIGURE 7.
Schematic representation of the regulation of brown fat thermogenesis by PPARα. The schematic summarizes the role of PPARα in the control of brown fat thermogenesis through coordinate regulation of lipid catabolism and thermogenic gene expression via induction of PGC-1α and PRDM16.
Although PPARγ is essential for fat cell differentiation and lipid storage in both WAT and BAT, PPARα is specifically induced in the terminal steps of brown adipocyte differentiation (17) as well as in brown-like adipocytes (“brite” adipocytes) appearing in WAT after cold exposure of mice (33). Cold exposure induces the expression and nuclear translocation of PPARα in rodent BAT (34), and genetic analyses have revealed that PPARα is involved in PGC-1α and UCP1 gene induction in mice (35). The role of PPARα in brown adipocytes may be related to its capacity to respond to lipid ligands originating from lipolysis after noradrenergic activation of brown adipocytes. The activation of PGC-1α gene expression by PPARα may provide a mechanism for concerted induction of thermogenic genes (UCP1, fatty acid oxidation genes, mitochondrial genes) in response to the enhanced intracellular availability of fatty acids sensed by PPARα. PPARα-null mice, despite being resistant to cold and having overtly normal BAT morphology (35, 36, 30), show thermogenesis-related disturbances in response to cold stress, such as a marked suppression of BAT growth concurrent with a prominent decrease in fatty acid oxidative and thermogenic activities (37, 38, 18). Consistent with our current findings of a role for PPARα in the control of PGC-1α gene transcription, we observed that PGC-1α gene expression is decreased in PPARα-null BAT both under basal conditions and in response to acute thermogenic activation.
We found that PPARα- and cAMP-mediated pathways strongly interacted in the control of PGC-1α gene transcription. This dynamic was greatly potentiated by PRDM16, which we found binds to the PGC-1α gene promoter at the PPARα-binding site. Because the action of PRDM16 regulating brown-fat-specific gene expression occurs independently from binding to DNA (6) and PRDM16 can bind PPARα (14), we propose that PRDM16 coactivation activity upon the PGC-1α gene relies on direct PRDM16 interaction with PPARα bound to the PPRE. PRDM16 has been identified as the key molecular switch that determines the development of brown adipocytes during embryonic development (6) and the appearance of brown adipocytes in WAT depots in adult mice (15). It has been suggested that the ability of PRDM16 to stimulate a BAT phenotype is due, in part, to its association with PGC-1 coactivators and PPARγ (6, 14). These results further indicate that the interaction of PPARα with PRDM16 is required for the full thermogenic activation of PGC-1α gene transcription in BAT. Hence, we propose a novel role for PRDM16 in brown adipocytes. By interacting with PPARα, PRDM16 coordinately regulates the expression of genes required for active thermogenesis through induction of the PGC-1α gene.
We also demonstrate for the first time that PRDM16 gene expression is induced by both norepinephrine and PPARα activation in brown adipocytes. Furthermore, an active PPARα-dependent pathway is required for maximal adrenergic stimulation of PRDM16 expression, both in BAT in vivo and in cultured brown adipocytes. This PPARα- and cAMP-mediated regulation of PRDM16 gene expression is expected to contribute to the cross-talk between PPARα- and cAMP-dependent signaling pathways and further suggests that PRDM16 is linked to activation of BAT thermogenesis. In addition, it has been reported that overexpression of PRDM16 induces PPARα gene expression (6). Thus, a feed-forward loop of PPARα and PRDM16 gene regulation may provide a way to maintain high levels of both PPARα and PRDM16 in thermogenically active BAT. Accordingly, PRDM16 may play a pivotal role not only in early differentiation processes of the brown adipose lineage but also in specific metabolic regulation in relation to thermogenesis in the already differentiated and functional brown adipocyte.
Whether PPARα-dependent pathways affect energy expenditure in adult humans remains to be determined. Recently, several laboratories have reported the presence of discrete, metabolically active BAT depots in healthy adult humans that can be activated by cold exposure and blocked using β-adrenergic antagonists (39–41). Fibrates are synthetic ligands of PPARα that are currently used as hypolipidemic drugs. It is possible that increased fatty acid uptake and subsequent oxidation by BAT via activation of PPARα contribute to the hypolipidemic effect of fibrates. On the other hand, the expression of PPARα in human WAT is low but has been reported to be negatively correlated with body mass index (42). According to our current findings in human SGBS white adipocytes, one aspect of the biological effects of PPARα activation would be to promote a BAT-like phenotype in WAT depots, acting through the induction of PGC-1α and PRDM16 gene expression. This is consistent with previous observations that PPARα agonists induce the expression of genes involved in lipid oxidation and mitochondrial biogenesis in human white adipocytes in vitro (43, 44). Those parameters are also induced by pharmacological activation of the cAMP/PKA pathway, which also increases PPARα expression in human white adipocytes (43). In rodents, chronic cold exposure and β-adrenergic stimulation induce the appearance of brown-like adipocytes, now referred to as brite adipocytes (33), in WAT depots (45–47). PPARα, which is up-regulated by β3-adrenergic stimulation, may be involved in this process because PPARα-null mice exhibit impaired induction of lipid oxidation and mitochondrial biogenesis in WAT in response to β3-adrenergic agonist treatment (48).
In mice, BAT has been described as having antiobesity and antidiabetic effects (49, 50). In adult humans, the possibility of activating BAT metabolism by stimulating specific BAT depots and/or by facilitating conversion of WAT into BAT could be a promising approach for treating obesity and related metabolic disorders. Unfortunately, many candidate agonists of the β3-adrenergic receptor have failed in human clinical trials, although these drugs have been efficacious in rodent models of obesity (51, 52). Because this failure has been related to the low number of β3-adrenergic receptors in human adipose tissue, the observation that PPARα induces β3-adrenergic receptor expression in human white adipocytes, reported here, suggests that combined treatment with β-adrenergic and PPARα-specific agonists might have the potential to promote energy expenditure in adipose depots.
In summary, we have established that PPARα is an important actor in the control of BAT thermogenic activity via induction of PGC-1α and PRDM16, key players in the acquisition of the thermogenic competence of brown adipocytes.
Acknowledgments
We thank Drs. B. Spiegelman, B. Staels, L. Fajas, S. Green, and M. Muramatsu for kindly supplying plasmids and adenoviral vectors. We also thank Dr. B. Spiegelman for PGC-1α-null mice and Dr. M. Wabitsch for SGBS cells.
This research was supported by Instituto de Salud Carlos III Grant PI081715 and Ministerio de Ciencia e Innovación Grants SAF2008-01896, SAF2009-07599, and CSD 2007-00020), Spain. The CNIC is supported by the Spanish Ministry of Science and Innovation and the Pro-CNIC Foundation. CIBER Fisiopatología de la Obesidad y Nutrición is an initiative of the Instituto de Salud Carlos III, Spain.
- UCP1
- uncoupling protein 1
- BAT
- brown adipose tissue(s)
- PKA
- protein kinase A
- PPARα
- peroxisome proliferator-activated receptor α
- PRDM16
- PRD1-BF1-RIZ1 homologous domain-containing 16
- PGC-1α
- PPARγ-coactivator-1α
- CRE
- cAMP response element
- PPRE
- PPAR-responsive element
- WAT
- white adipose tissue(s)
- IBMX
- isobutyl-methyl-xanthine
- MEF
- mouse embryonic fibroblast.
REFERENCES
- 1. Cannon B., Nedergaard J. (2004) Physiol. Rev. 84, 277–359 [DOI] [PubMed] [Google Scholar]
- 2. Enerbäck S. (2010) Cell Metab. 11, 248–252 [DOI] [PubMed] [Google Scholar]
- 3. Farmer S. R. (2006) Cell Metab. 4, 263–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sears I. B., MacGinnitie M. A., Kovacs L. G., Graves R. A. (1996) Mol. Cell. Biol. 16, 3410–3419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yubero P., Manchado C., Cassard-Doulcier A. M., Mampel T., Viñas O., Iglesias R., Giralt M., Villarroya F. (1994) Biochem. Biophys. Res. Commun. 198, 653–659 [DOI] [PubMed] [Google Scholar]
- 6. Seale P., Kajimura S., Yang W., Chin S., Rohas L. M., Uldry M., Tavernier G., Langin D., Spiegelman B. M. (2007) Cell Metab. 6, 38–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carmona M. C., Iglesias R., Obregón M. J., Darlington G. J., Villarroya F., Giralt M. (2002) J. Biol. Chem. 277, 21489–21498 [DOI] [PubMed] [Google Scholar]
- 8. Kajimura S., Seale P., Spiegelman B. M. (2010) Cell Metab. 11, 257–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Puigserver P., Wu Z., Park C. W., Graves R., Wright M., Spiegelman B. M. (1998) Cell 92, 829–839 [DOI] [PubMed] [Google Scholar]
- 10. Uldry M., Yang W., St-Pierre J., Lin J., Seale P., Spiegelman B. M. (2006) Cell Metab. 3, 333–341 [DOI] [PubMed] [Google Scholar]
- 11. Tiraby C., Tavernier G., Lefort C., Larrouy D., Bouillaud F., Ricquier D., Langin D. (2003) J. Biol. Chem. 278, 33370–33376 [DOI] [PubMed] [Google Scholar]
- 12. Cao W., Daniel K. W., Robidoux J., Puigserver P., Medvedev A. V., Bai X., Floering L. M., Spiegelman B. M., Collins S. (2004) Mol. Cell. Biol. 24, 3057–3067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hondares E., Mora O., Yubero P., Rodriguez de la Concepción M., Iglesias R., Giralt M., Villarroya F. (2006) Endocrinology 147, 2829–2838 [DOI] [PubMed] [Google Scholar]
- 14. Seale P., Bjork B., Yang W., Kajimura S., Chin S., Kuang S., Scimè A., Devarakonda S., Conroe H. M., Erdjument-Bromage H., Tempst P., Rudnicki M. A., Beier D. R., Spiegelman B. M. (2008) Nature 454, 961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seale P., Conroe H. M., Estall J., Kajimura S., Frontini A., Ishibashi J., Cohen P., Cinti S., Spiegelman B. M. (2011) J. Clin. Invest. 121, 96–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mandard S., Müller M., Kersten S. (2004) Cell. Mol. Life Sci. 61, 393–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Valmaseda A., Carmona M. C., Barberá M. J., Viñas O., Mampel T., Iglesias R., Villarroya F., Giralt M. (1999) Mol. Cell. Endocrinol. 154, 101–109 [DOI] [PubMed] [Google Scholar]
- 18. Villarroya F., Iglesias R., Giralt M. (2007) PPAR Res. 2007, 74364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barbera M. J., Schluter A., Pedraza N., Iglesias R., Villarroya F., Giralt M. (2001) J. Biol. Chem. 276, 1486–1493 [DOI] [PubMed] [Google Scholar]
- 20. Carmona M. C., Hondares E., Rodríguez de la Concepción M. L., Rodríguez-Sureda V., Peinado-Onsurbe J., Poli V., Iglesias R., Villarroya F., Giralt M. (2005) Biochem. J. 389, 47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Olmos Y., Valle I., Borniquel S., Tierrez A., Soria E., Lamas S., Monsalve M. (2009) J. Biol. Chem. 284, 14476–14484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wabitsch M., Brenner R. E., Melzner I., Braun M., Möller P., Heinze E., Debatin K. M., Hauner H. (2001) Int. J. Obes. Relat. Metab. Disord. 25, 8–15 [DOI] [PubMed] [Google Scholar]
- 23. Handschin C., Rhee J., Lin J., Tarr P. T., Spiegelman B. M. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 7111–7116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fajas L., Egler V., Reiter R., Hansen J., Kristiansen K., Debril M. B., Miard S., Auwerx J. (2002) Dev. Cell 3, 903–910 [DOI] [PubMed] [Google Scholar]
- 25. Issemann I., Green S. (1990) Nature 347, 645–650 [DOI] [PubMed] [Google Scholar]
- 26. Hondares E., Pineda-Torra I., Iglesias R., Staels B., Villarroya F., Giralt M. (2007) Biochem. Biophys. Res. Commun. 354, 1021–1027 [DOI] [PubMed] [Google Scholar]
- 27. Muramatsu M., Kaibuchi K., Arai K. (1989) Mol. Cell. Biol. 9, 831–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Villena J. A., Hock M. B., Chang W. Y., Barcas J. E., Giguère V., Kralli A. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 1418–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barbier O., Villeneuve L., Bocher V., Fontaine C., Torra I. P., Duhem C., Kosykh V., Fruchart J. C., Guillemette C., Staels B. (2003) J. Biol. Chem. 278, 13975–13983 [DOI] [PubMed] [Google Scholar]
- 30. Petrovic N., Shabalina I. G., Timmons J. A., Cannon B., Nedergaard J. (2008) Am. J. Physiol. Endocrinol. Metab. 295, E287–96 [DOI] [PubMed] [Google Scholar]
- 31. Herzig S., Long F., Jhala U. S., Hedrick S., Quinn R., Bauer A., Rudolph D., Schutz G., Yoon C., Puigserver P., Spiegelman B., Montminy M. (2001) Nature 413, 179–183 [DOI] [PubMed] [Google Scholar]
- 32. Yubero P., Barberá M. J., Alvarez R., Viñas O., Mampel T., Iglesias R., Villarroya F., Giralt M. (1998) Mol. Endocrinol. 12, 1023–1037 [DOI] [PubMed] [Google Scholar]
- 33. Petrovic N., Walden T. B, Shabalina I. G., Timmons J. A., Cannon B., Nedergaard J. (2010) J. Biol. Chem. 285, 7153–7164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rim J. S., Xue B., Gawronska-Kozak B., Kozak L. P. (2004) J. Biol. Chem. 279, 25916–25926 [DOI] [PubMed] [Google Scholar]
- 35. Xue B., Coulter A., Rim J. S., Koza R. A., Kozak L. P. (2005) Mol. Cell. Biol. 25, 8311–8322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kersten S., Seydoux J., Peters J. M., Gonzalez F. J., Desvergne B., Wahli W. (1999) J. Clin. Invest. 103, 1489–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tong Y., Hara A., Komatsu M., Tanaka N., Kamijo Y., Gonzalez F. J., Aoyama T. (2005) Biochem. Biophys. Res. Commun. 336, 76–83 [DOI] [PubMed] [Google Scholar]
- 38. Komatsu M., Tong Y., Li Y., Nakajima T., Li G., Hu R., Sugiyama E., Kamijo Y., Tanaka N., Hara A., Aoyama T. (2010) Genes Cells 15, 91–100 [DOI] [PubMed] [Google Scholar]
- 39. van Marken Lichtenbelt W. D., Vanhommerig J. W., Smulders N. M., Drossaerts J. M., Kemerink G. J., Bouvy N. D., Schrauwen P., Teule G. J. (2009) N. Engl. J. Med. 360, 1500–1508 [DOI] [PubMed] [Google Scholar]
- 40. Cypess A. M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A. B., Kuo F. C., Palmer E. L., Tseng Y. H., Doria A., Kolodny G. M., Kahn C. R. (2009) N. Engl. J. Med. 360, 1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Virtanen K. A., Lidell M. E., Orava J., Heglind M., Westergren R., Niemi T., Taittonen M., Laine J., Savisto N. J., Enerbäck S., Nuutila P. (2009) N. Engl. J. Med. 360, 1518–1525 [DOI] [PubMed] [Google Scholar]
- 42. MacLaren R., Cui W., Simard S., Cianflone K. (2008) J. Lipid Res. 49, 308–323 [DOI] [PubMed] [Google Scholar]
- 43. Bogacka I., Ukropcova B., McNeil M., Gimble J. M., Smith S. R. (2005) J. Clin. Endocrinol. Metab. 90, 6650–6656 [DOI] [PubMed] [Google Scholar]
- 44. Ribet C., Montastier E., Valle C., Bezaire V., Mazzucotelli A., Mairal A., Viguerie N., Langin D. (2010) Endocrinology 151, 123–133 [DOI] [PubMed] [Google Scholar]
- 45. Young P., Arch J. R., Ashwell M. (1984) FEBS Lett. 167, 10–14 [DOI] [PubMed] [Google Scholar]
- 46. Guerra C., Koza R. A., Yamashita H., Walsh K, Kozak L. P. (1998) J. Clin. Invest. 102, 412–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Granneman J. G., Li P., Zhu Z., Lu Y. (2005) Am. J. Physiol. Endocrinol. Metab. 289, E608–16 [DOI] [PubMed] [Google Scholar]
- 48. Li P., Zhu Z., Lu Y., Granneman J. G. (2005) Am. J. Physiol. Endocrinol. Metab. 289, E617–26 [DOI] [PubMed] [Google Scholar]
- 49. Lowell B. B., S-Susulic V., Hamann A., Lawitts J. A, Himms-Hagen J., Boyer B. B., Kozak L. P., Flier J. S. (1993) Nature 366, 740–742 [DOI] [PubMed] [Google Scholar]
- 50. Guerra C., Navarro P., Valverde A. M., Arribas M., Brüning J., Kozak L. P., Kahn C. R., Benito M. (2001) J. Clin. Invest. 108, 1205–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Arch J. R. (2002) Eur. J. Pharmacol. 440, 99–107 [DOI] [PubMed] [Google Scholar]
- 52. Larsen T. M., Toubro S., van Baak M. A., Gottesdiener K. M., Larson P., Saris W. H., Astrup A. (2002) Am. J. Clin. Nutr. 76, 780–788 [DOI] [PubMed] [Google Scholar]