Background: GIP is a gut hormone secreted in response to nutrient intake.
Results: GIP has an insulin-sensitizing effect on adipose via activation of the cAMP/PKA/CREB and p110β PI3K.
Conclusion: These data define a novel signal transduction pathway modulating insulin action in adipocytes.
Significance: Insulin-sensitizing activity points to a central role for GIP in coordinating intestinal nutrient sensing and regulation of metabolism.
Keywords: Adipose Tissue Metabolism, G Protein-coupled Receptors (GPCR), Glut4, Insulin, Phosphatidylinositol 3-Kinase, Insulin Sensitivity
Abstract
Gastric inhibitory peptide (GIP) is an incretin hormone secreted in response to food intake. The best known function of GIP is to enhance glucose-dependent insulin secretion from pancreatic β-cells. Extra-pancreatic effects of GIP primarily occur in adipose tissues. Here, we demonstrate that GIP increases insulin-dependent translocation of the Glut4 glucose transporter to the plasma membrane and exclusion of FoxO1 transcription factor from the nucleus in adipocytes, establishing that GIP has a general effect on insulin action in adipocytes. Stimulation of adipocytes with GIP alone has no effect on these processes. Using pharmacologic and molecular genetic approaches, we show that the effect of GIP on adipocyte insulin sensitivity requires activation of both the cAMP/protein kinase A/CREB signaling module and p110β phosphoinositol-3′ kinase, establishing a novel signal transduction pathway modulating insulin action in adipocytes. This insulin-sensitizing effect is specific for GIP because isoproterenol, which elevates adipocyte cAMP and activates PKA/CREB signaling, does not affect adipocyte insulin sensitivity. The insulin-sensitizing activity points to a more central role for GIP in intestinal regulation of peripheral tissue metabolism, an emerging feature of inter-organ communication in the control of metabolism.
Introduction
GIP2 and glucagon-like peptide-1 are incretin hormones secreted from the gastrointestinal tract into the circulation in response to nutrient intake (1, 2). The primary role of these hormones is to enhance glucose-stimulated insulin secretion from pancreatic β-cells (1, 2). Consequently, there is a greater increase in plasma insulin after an oral glucose administration as compared with the same amount of glucose given intravenously. This phenomenon has been termed as incretin effect and is estimated to account for 50–70% of the total insulin secretion after oral glucose administration (3). This is one of the key mechanisms by which the gut communicates with peripheral tissues in regulation of glucose homeostasis. The importance of this phenomenon in glucose homeostasis is highlighted by the fact that both GIP receptor-deficient and glucagon-like peptide-1 receptor-deficient mice show glucose intolerance after oral glucose loading (4, 5).
Several studies have demonstrated that in addition to the pancreas, the GIP receptor is expressed in other tissues, including adipose, where it regulates several aspects of lipid metabolism (6). GIP has been shown to augment insulin-induced lipogenesis, inhibit the lipolytic action of glucagon, and stimulate lipoprotein lipase activity (6–11). The effects of GIP on other aspects of adipose metabolism have not been as extensively examined. For example, there is controversy regarding the effect of GIP on adipocyte glucose metabolism (e.g. Refs. 5 and 12). Song et al. (12) reported that GIP acts as an insulin mimetic in 3T3-L1 adipocytes by activating PI3K, Akt, and promoting membrane translocation of glucose transporter-4 (Glut4), leading to an increase in glucose uptake. However, studies by other groups in 3T3-L1 adipocytes and in rat fat pad adipocytes showed that GIP did not possess any insulin mimetic property and did not increase glucose transport in the absence of insulin but increased insulin-stimulated glucose uptake (13, 14). Moreover, the mechanism by which GIP affects insulin action in adipocytes is not well understood.
Here, we establish that GIP increases the sensitivity of adipocytes to insulin without having insulin mimetic activities. We find that GIP augmentation of insulin sensitivity requires elevation of cAMP, activation of protein kinase A and functional cAMP-response element-binding protein (CREB), as well as activation of p110β PI3K activity. These data define a novel signal transduction pathway modulating insulin action in adipocytes and provide insight into the actions of GIP in adipose tissue.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
GIP (1–32) was purchased from Bachem, Inc. (Torrance, CA); formaldehyde, CPT-cAMP (8-(4-chlorophenyl-thio)adenosine 3′5′-cyclic monophosphate), and dimethyl sulfoxide were from Sigma-Aldrich; anti-HA antibodies were from Covance (Berkley, CA); anti-Akt, anti-phospho-Akt (Ser473 and Thr308), anti-phospho-CREB Ser133, anti-CREB antibodies, anti-perilipin A and anti-phospho-Ser/Thr PKA substrate antibodies were from Cell Signaling Technology (Beverly, MA); anti AS160 and anti-phospho AS160 from Millipore (Billerica, MA); Cy3-conjugated anti-mouse IgG and Cy5-conjugated anti-rabbit IgG were from Jackson ImmunoResearch Laboratories (West Grove, PA); secondary peroxidase-conjugated anti-rabbit IgG were from Pierce; H89 (2′,5′-dideoxyadenosine) and Akt inhibitor 1/2 (Akti) were from Calbiochem; TGX-221 was from Cayman Chemicals (Ann Arbor, Michigan), and siRNA oligonucleotides were from Invitrogen.
Plasmids and siRNA
FoxO1-GFP was a kind gift from Dr. Domenico Accili (Columbia University). Cells stably expressing shRNA for Rab10 have been described elsewhere (15). Akt2 silencing was achieved by using siRNA; non-targeting siRNA was used as a control as described (16). CREB vector set containing WT and dominant-negative forms (CREB133 and KCREB) were purchased from Clontech (Mountain View, CA).
Cell Culture and Electroporation
3T3-L1 fibroblasts were cultured, differentiated into adipocytes, and electroporated as described (17, 18). Experiments were performed 24 h after electroporation except for the siRNA experiments. For siRNA experiments, cells were electroporated together with HA-Glut4-GFP, and siRNA and experiments were performed 48 h after electroporation.
HA-Glut4-GFP Translocation Assay
HA-Glut4-GFP translocation was measured as described previously (18–20).
cAMP Assay
Cells were washed and incubated with serum-free medium for 2 h and then stimulated with 100 nm GIP and 0.2 nm insulin for 1 h at 37 °C in the presence and absence of 2,5-dideoxyadenosine. The medium was aspirated, and cells were lysed in 0.1 n HCl. Intracellular cAMP was measured using the direct immunoassay kit from Assay Designs (Plymouth Meeting, PA).
Nuclear Exclusion Assay for FoxO1
Adipocytes electroporated with FoxO1-GFP were incubated in serum-free medium for 8 h followed by incubation with insulin and GIP for 1 h. Cells were washed and fixed with 3.7% formaldehyde. Cells were imaged, and images were analyzed for the localization of GFP by manually counting the cells with nuclear or cytosolic GFP.
Glucose Uptake
Glucose transport activity in 3T3-L1 adipocytes was measured by the uptake of [3H]2-deoxyglucose as described previously (21).
Immunoprecipitation of Insulin Receptor
Cells were washed and lysed in 1× lysis buffer (Cell Signaling Technology) containing 20 mm Tris-HCl (pH 7.5), 150 mm NaCl, 1 mm Na2EDTA, 1 mm EGTA, 1% Triton, 2.5 mm sodium pyrophosphate, 1 mm β-glycerophosphate, 1 mm Na3VO4, 1 μg/ml leupeptin, and protease inhibitor mixture (Roche Applied Science). Insulin receptor-β was immunopurified from the cell lysates using anti-insulin receptor β antibodies (Santa Cruz Biotechnology).
Gel Electrophoresis and Immunoblotting
Separation of proteins by SDS-PAGE, immunoblotting with the indicated primary antibodies, and secondary HRP-conjugated antibodies using enhanced chemiluminescence detection and densitometry were performed as described (16).
Insulin Receptor Affinity Assay
3T3-L1 adipocytes were serum-starved for 2 h followed by treatment with or without 10 nm GIP for 30 min. Cells were washed twice with medium II (150 mm NaCl, 1 mm CaCl2, 5 mm KCl, 1 mm MgCl2, and 20 mm HEPES, pH 7.2) and incubated with different concentrations of 125I-insulin for 2 h. Cells were washed extensively with medium II and lysed in 1% Triton X-100, and cell-associated radioactivity was measured using a γ-counter. The radioactivity bound in the presence of excess unlabeled insulin (10 nm) was designated as nonspecific binding and subtracted from total binding to obtain specific binding.
Data Acquisition and Processing
Fluorescent images were collected on a DMIRB inverted microscope (Leica Microsystems, Deerfield, IL) using a 20× objective. Fluorescence quantifications were done using MetaMorph image processing software (Molecular Devices, Sunnyvale, CA) as described previously (18, 19, 22).
Statistical Analysis
Statistical significance was calculated by Student's t test.
RESULTS
GIP Potentiates Insulin Action in Adipocytes
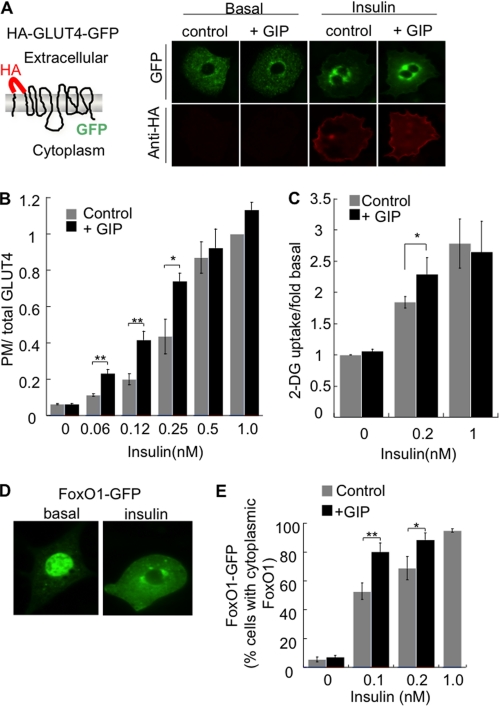
The insulin-stimulated glucose uptake into adipocytes is achieved by the translocation of intracellular Glut4 to the plasma membrane (23). We use a HA-Glut4-GFP reporter to quantify the effect of insulin on Glut4 distribution (Fig. 1A) (18, 20). To determine the effect of GIP on insulin-induced Glut4 translocation, adipocytes were incubated with insulin alone or in combination with 100 nm GIP for 1 h. Co-stimulation with 100 nm GIP induced a leftward shift in the insulin dose response, whereas incubation with GIP alone did not increase Glut4 in the plasma membrane (Fig. 1B). Consistent with the effect of GIP on the behavior of the HA-Glut4-GFP, GIP augmented the stimulatory effect of insulin on glucose uptake (Fig. 1C). These results, which are in agreement with past studies of the effects of GIP on glucose transport, extend those conclusions by demonstrating that the effect on glucose uptake is due to changes in the behavior of Glut4 (13, 14). Although GIP augmented insulin-stimulated Glut4 translocation, GIP did not alter the intracellular pattern of HA-Glut4-GFP in insulin-stimulated or unstimulated adipocytes (Fig. 1A).
FIGURE 1.
GIP enhances insulin action in 3T3-L1 adipocytes. A, schematic of the HA-Glut4-GFP reporter and epifluorescence microscopy images of HA-Glut4-GFP expressed in adipocytes. Cells were treated with 100 nm GIP for 1 h ± 0.2 nm insulin. B, quantification of HA-Glut4-GFP in the plasma membrane. Serum-starved adipocytes were incubated with the indicated concentrations of insulin ± 100 nm GIP for 60 min. The data are the plasma membrane (anti-HA fluorescence) to GFP fluorescence ratio (PM/total Glut4, plasma membrane to total Glut4). The data for each condition are normalized to the ratio in cells treated with 1 nm insulin. Each bar is the average of five experiments ± S.E. *, p < 0.05; **, p < 0.01. C, serum-starved adipocytes were incubated with indicated concentrations of insulin ± 100 nm GIP for 60 min. Glucose uptake was measured by using [3H]2-deoxyglucose. Each bar is the average of three experiments ± S.E. *, p < 0.05. D, transiently expressed localization of FoxO1-GFP in basal and adipocytes stimulated with 1 nm insulin. E, quantification of FoxO1 distribution. The data are the percent of cells in which FoxO1 is distributed to the cytoplasm (as illustrated in D). Each bar is an average of four experiments ± S.E. *, p < 0.05; **, p < 0.01.
Exclusion of the Forkhead transcription factor (FoxO1) from the nucleus is another key effect of insulin (24). In unstimulated adipocytes, FoxO1 is predominantly localized to the nucleus, where it functions to control gene expression. Insulin negatively regulates FoxO1 by inducing its redistribution from the nucleus to the cytosol (Fig. 1D). Insulin induced a dose-dependent exclusion of FoxO1 from the nucleus of adipocytes, an effect that was enhanced by GIP, whereas GIP stimulation alone had no effect on the FoxO1 distribution (Fig. 1E). The effect of GIP on insulin-stimulated glucose uptake, Glut4 translocation to the plasma membrane, and nuclear exclusion of FoxO1 in adipocytes demonstrate an insulin-sensitizing effect of GIP.
Dose- and Time-dependent GIP Enhancement of Insulin Action
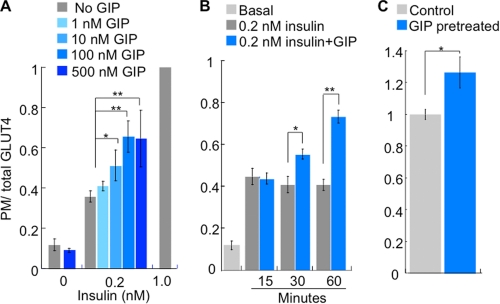
The effect of GIP on insulin action was dose-dependent, with the maximum effect observed at 100 nm and a half-maximal dose of ∼10 nm (Fig. 2A). Stimulation of adipocytes with 500 nm GIP alone did not induce Glut4 translocation, confirming that even at very high concentrations, GIP does not have insulin mimetic activities.
FIGURE 2.
GIP potentiation of insulin-stimulated Glut4 translocation is dose- and time-dependent. A, HA-Glut4-GFP plasma membrane (PM)-to-total distribution in adipocytes incubated for 60 min with the indicated amounts of insulin or GIP. The data are the plasma membrane (anti-HA fluorescence) to GFP fluorescence ratio. The data for each condition are normalized to the ratio in cells treated with 1 nm insulin. The bars are the mean ± S.E. (n = 3). *, p < 0.05; **, p < 0.01. B, HA-Glut4-GFP plasma membrane-to-total distribution in adipocytes incubated for 0.2 nm insulin ± 100 nm GIP for the indicated times. The bars are the mean ± S.E. (n = 4). *, p < 0.05; **, p < 0.01. C, HA-Glut4-GFP plasma membrane-to-total distribution in adipocytes pretreated with 100 nm GIP for 30 min followed by treatment with 0.2 nm insulin for 30 min. The bars are the mean ± S.E. (n = 3). *, p < 0.05.
In the experiments already discussed, we simultaneously treated adipocytes with GIP and insulin for 60 min. The full effect of insulin on Glut4 translocation plateaus within 15 min (Fig. 2B) (20, 25, 26). The effect of GIP, however, was slower. A 15-min co-stimulation with GIP had no effect on the Glut4 translocation induced by 0.2 nm insulin, whereas a 30-min co-incubation with GIP was about half as effective as the 60-min co-incubation (Fig. 2B). There was no further increase in the GIP insulin-sensitizing effect with incubations longer than 60 min (data not shown).
The difference in timing of the effects of insulin and GIP prompted us to investigate whether prestimulation of adipocytes with GIP would enhance the insulin response. Adipocytes were pretreated with 100 nm GIP for 30 min, washed to remove GIP and stimulated with insulin for 30 min without GIP. There was a significant increase in insulin-stimulated Glut4 translocation in adipocytes pretreated with GIP, demonstrating that GIP presensitizes cells to insulin (Fig. 2C).
GIP-augmented Insulin-stimulated Glut4 Translocation Requires Akt and Rab10
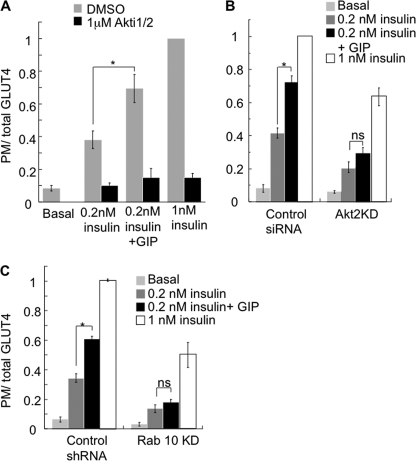
The conventional signaling pathway triggered by insulin involves tyrosine phosphorylation of insulin receptor substrate proteins and activation of class IA PI3K. This results in the generation of the critical second messenger PI 3,4,5-triphosphate, which triggers the phosphorylation and activation of Akt. Activation of Akt is a critical event in the process of insulin-stimulated Glut4 translocation to the plasma membrane (27). To determine whether activation of Akt is required for the effect of GIP on insulin sensitivity, we determined the effect of an Akt inhibitor (Akti1/2) on the insulin-sensitizing effects of GIP. Treatment of cells with Akti1/2 blunted insulin-stimulated Glut4 translocation and abolished the effect of GIP as well (Fig. 3A). Akti1/2 inhibits both Akt1 and Akt2 isoforms. It is known that activation of Akt2 is responsible mainly for insulin-stimulated Glut4 translocation in adipocytes (28–30). Therefore, we examined the effect of GIP on Glut4 translocation in adipocytes in which Akt2 was transiently knocked down. GIP did not potentiate insulin-stimulated Glut4 translocation in Akt2 knockdown cells substantiating our results from studies using Akt inhibitor (Fig. 3B). These data suggest that activation of AKT is required for GIP to enhance insulin-stimulated Glut4 translocation. (Fig. 3B).
FIGURE 3.
Akt activation is necessary for the effect of GIP on insulin action. A, HA- Glut4-GFP plasma membrane-to-total distribution in adipocytes incubated for 60 min with the indicated amounts of insulin, ± 100 nm GIP ± 1 μm Akti1/2 inhibitor. Cells were preincubated with 1 μm Akti1/2 or dimethyl sulfoxide (DMSO) for 30 min prior to stimulation with insulin and GIP. The data are the plasma membrane (anti-HA fluorescence) to GFP fluorescence ratio. The data are normalized to the ratio in cells treated with 1 nm insulin. The bars are the mean ± S.E. (n = 4). *, p < 0.01. B, HA-Glut4-GFP surface-to-total distribution in which cells were electroporated with a scrambled siRNA that does not target any known mouse gene (control) or siRNA targeting Akt2. Adipocytes were incubated for 60 min with the indicated amounts of insulin ± 100 nm GIP. The data are normalized to the ratio in control cells treated with 1 nm insulin. The bars are the mean ± S.E. (n = 3). *, p < 0.01. ns, not significant. C, HA-Glut4-GFP surface-to-total distribution in adipocytes in which Rab10 is stably knocked down by shRNA (Rab10 KD) and adipocytes stably expressing a scrambled shRNA that does not target any known mouse gene (control). Adipocytes were incubated for 60 min with the indicated amounts of insulin ± 100 nm GIP. The data are normalized to the ratio in control cells treated with 1 nm insulin. The bars are the mean ± S.E. (n = 3). *, p < 0.01.
The small GTPase, Rab10, is involved in insulin-stimulated Glut4 translocation. Akt2 phosphorylates and inactivates AS160/TBC1d4, a RabGAP of Rab10 (15). We next examined the effect of GIP on insulin-stimulated Glut4 translocation in adipocytes in which Rab10 had been knocked down. GIP did not enhance the effect of insulin in these cells (Fig. 3C). Thus, GIP potentiates insulin action on Glut4 translocation by influencing the canonical pathway involving Akt, AS160, and Rab10 rather than by a parallel pathway.
GIP Does Not Alter Insulin Receptor Affinity, Activity, or Activity of Akt
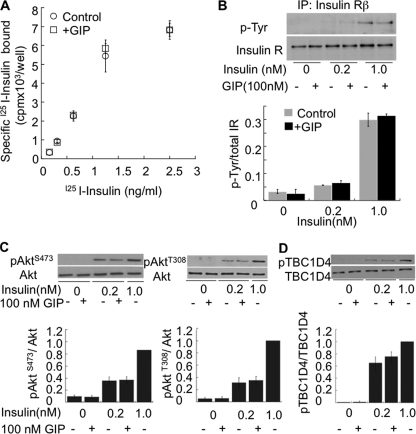
There are several possibilities by which GIP could enhance insulin action in adipocytes. It could alter the affinity of insulin receptor or act on one of the steps downstream of insulin receptor. Treatment of cells with GIP did not affect the affinity of insulin receptor for insulin and did not change the number of insulin receptors on the surface of adipocytes (Fig. 4A). Moreover, GIP did not change insulin-receptor autophosphorylation and did not alter the expression of insulin receptors (Fig. 4B). These results indicate that the effects of GIP are downstream of the insulin receptor.
FIGURE 4.
GIP does not change insulin-mediated phosphorylation of Akt and TBC1D4. A, insulin receptor binding assay was carried out as described under “Experimental Procedures,” and receptor affinity was determined by plotting bound versus free radioactivity. B, serum-starved adipocytes were treated with insulin and GIP for 30 min and insulin receptor (IR) β was immunopurified as described under “Experimental Procedures.” Immunoprecipitates were loaded onto a 10% SDS gel and blots were probed with p-Tyr antibodies (Santa Cruz Biotechnology) and insulin receptor β (Santa Cruz Biotechnology). The phosphorylation status of the insulin receptor was determined by measuring the ratio of p-Tyr to total insulin receptor (IR). Each bar represents average of two experiments ± S.D. C, Western blots of total cell extracts measuring p-Akt Ser473, p-Akt Thr308, and total Akt with the quantification underneath. The data are normalized to the p-Akt/Akt values in cells treated with 1nm insulin. The bars are the mean ± S.E. (n = 3). D, Western blots of total cell extracts measuring p-pTBC1D4 Thr642 and total TBC1D4. The bars are the mean ± S.E. (n = 3).
Following insulin receptor activation, Akt is phosphorylated at two residues (Ser473 and Thr308) crucial for activation (32, 33). Insulin-stimulated phosphorylation of Akt at Ser473 and Thr308 was not affected by GIP (Fig. 4C). To more directly investigate Akt activity, we investigated phosphorylation of one of its substrates, TBC1D4, which is involved in Glut4 translocation. Insulin stimulated the phosphorylation of TBC1D4 at Thr642; the major Akt phosphorylation site of TBC1D4 was not potentiated by GIP (Fig. 4D). Thus, the effect of GIP on insulin-stimulated Glut4 translocation is not a result of detectable changes in insulin receptor activation or subsequent Akt activity.
cAMP Is Required but Not Sufficient for Effect of GIP on Insulin Action
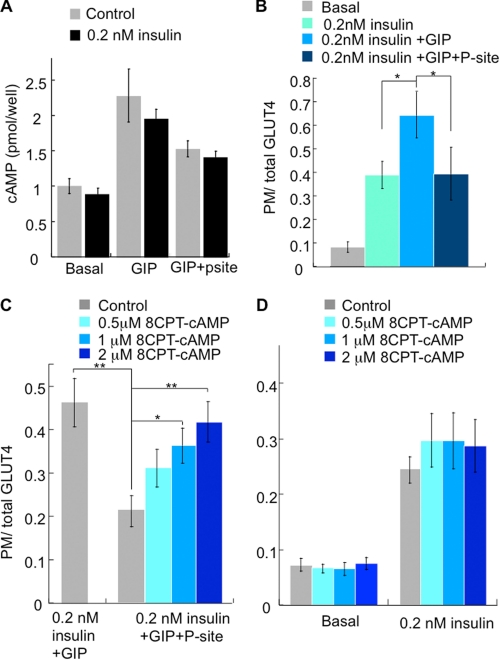
The GIP receptor is a class B G-protein coupled receptor that elevates cAMP and activates several different signal transduction modules (7, 34, 35). The potentiating effects of GIP on insulin secretion are thought to be mediated mainly by the cAMP/PKA signaling pathway in the β-cells (36, 37). However, some studies have suggested that GIP also exerts its effects in the β-cells in a cAMP-independent manner (38). In adipocytes, GIP-stimulated increase of cAMP that was not affected significantly by co-incubation with insulin but was inhibited by the transmembrane adenylate cyclase inhibitor 2′,5′-dideoxyadenosine (P-site) (Fig. 5A). The GIP insulin-sensitizing effect was abolished by P-site inhibition, demonstrating a requirement for elevated cAMP downstream of GIP receptor activation, whereas P-site inhibition of adenylate cyclase had no affect on insulin-stimulated Glut4 translocation (Fig. 5B).
FIGURE 5.
cAMP is required but not sufficient for the effect of GIP on insulin action. A, cAMP measurements in adipocytes. Cells were serum-starved for 2 h and incubated with insulin and GIP ± 2′,5′-dideoxyadenosine for 1 h. The bars are the mean ± S.E. (n = 3). B, HA-Glut4-GFP plasma membrane (PM)-to-total distribution in adipocytes treated ± insulin, ± GIP and ± 2′,5′-dideoxyadenosine (P-site) for 1 h. The data are normalized to the ratio in cells treated with 1 nm insulin. The bars are the mean ± S.E. (n = 3). *, p < 0.05. C, HA-Glut4-GFP plasma membrane-to-total distribution in adipocytes treated with insulin and GIP ± P-site supplemented with different concentrations of membrane permeant cAMP, CPT-cAMP. The data are normalized to the ratio in cells treated with 1 nm insulin. The bars are the mean ± S.E. (n = 6). *, p < 0.05; **, p < 0.01. D, HA-Glut4-GFP plasma membrane-to-total distribution in adipocytes supplemented with various concentrations of membrane permeant cAMP, CPT-cAMP. The data are normalized to the ratio in cells treated with 1 nm insulin. The bars are the mean ± S.E. (n = 6).
To further explore the requirement for cAMP in the GIP enhancement of insulin action, we used a membrane-permeant cAMP analog, 8-(4-chlorophenylthio)-adenosine-3′,5′-cyclic monophosphate (8-CPT-cAMP), which mimics some effects of elevated cAMP (39, 40). 8-CPT-cAMP, in a dose-dependent manner, restored the GIP enhancement of insulin-stimulated Glut4 translocation in adipocytes in which adenylate cyclase was inhibited by P-site, confirming a requirement for elevated cAMP (Fig. 5C). However, 8-CPT-cAMP alone did not affect insulin-stimulated Glut4 translocation, demonstrating that elevated cAMP is required but not sufficient for the effect of GIP on insulin action (Fig. 5D).
PKA Is Required for Effect of GIP on Insulin Action
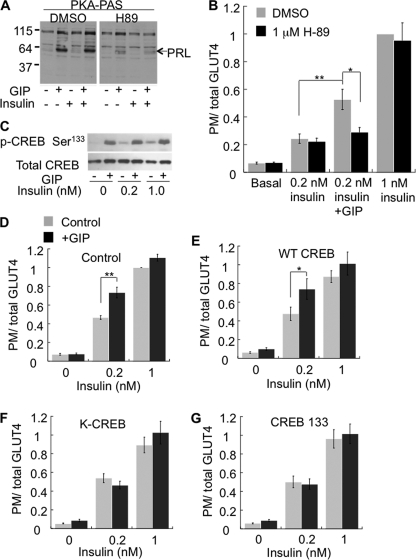
Protein kinase A (PKA), a serine/threonine kinase, is an effector of elevated cAMP signaling. GIP treatment induced PKA activation as indicated by increased phosphorylation of PKA substrates, the most prominent of which is perilipin A (Fig. 6A) (41). GIP-stimulated phosphorylation of perilipin A was not affected by insulin, consistent with GIP-induced elevation of cAMP being unaffected by insulin (Fig. 5A). The PKA inhibitor H89 (42) reduced GIP-stimulated phosphorylation of perilipin A (Fig. 6A) and abolished the effect of GIP on insulin-stimulated Glut4 translocation without affecting insulin-stimulated Glut4 translocation (Fig. 6B). These data demonstrate that PKA activation is required for GIP augmentation of insulin action.
FIGURE 6.
Activation of PKA and CREB are required for the GIP effect on insulin action. A, Western blot of total cell extracts of adipocytes for PKA phospho-serine/threonine substrate antibody (PKA PAS). Cells pretreated with 1 μm H89 for 30 min followed by incubation with insulin and GIP. The arrow indicates migration of perilipin (PRL ∼ 57 kDa), the major PKA substrate in adipocytes. B, HA-Glut4-GFP plasma membrane (PM)-to-total distribution in adipocytes pretreated with 1 μm H89 followed by incubation with insulin and GIP for 1 h. The data are normalized to the ratio in cells treated with 1 nm insulin. The bars are averages of three or more experiments ± S.E. *, p < 0.05; **, p < 0.01. C, Western blot of total cell extracts of adipocytes probed for p-CREB Ser133 and total CREB. D–G, HA-Glut4-GFP plasma membrane-to-total distribution in adipocytes expressing no ectopic CREB (D), WT-CREB (E), KCREB (F), or CREB 133 (G). The bars are averages of four experiments ± S.E. *, p < 0.05; **, p < 0.01. DMSO, dimethyl sulfoxide.
One of the key downstream targets of PKA is the transcription factor CREB. GIP treatment led to an increase in phosphorylation of CREB, and co-stimulation with insulin did not affect GIP-induced CREB phosphorylation (Fig. 6C). To functionally determine whether CREB is required for the effect of GIP on insulin-stimulated Glut4 translocation, we transiently overexpressed either wild type (WT) or dominant-inhibitory CREBs, KCREB, or CREB 133 (43, 44). All three CREB constructs were transiently overexpressed to about four times that of endogenous CREB expression (supplemental Fig. S1). Overexpression of WT CREB did not affect the GIP-insulin-sensitizing effect and did not affect the amount of Glut4 in the plasma membrane of unstimulated adipocytes (Fig. 6, D and E). However, overexpression of either KCREB or CREB 133 abolished the effect of GIP on insulin-stimulated Glut4 translocation, demonstrating that the insulin-sensitizing effect of GIP requires functional CREB (Fig. 6, F and G).
p110β PI3K Is Required for Effect of GIP on Insulin Action
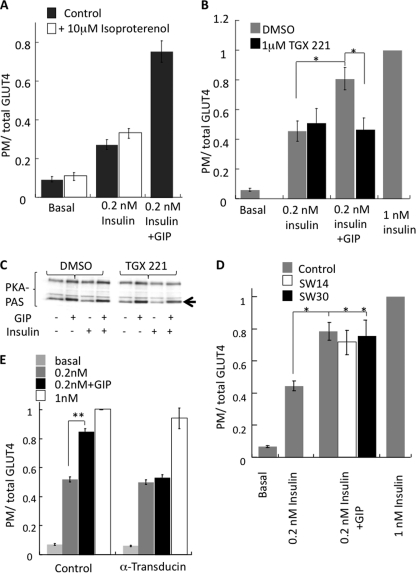
Our observation that elevation of cytosolic cAMP with 8-CPT-cAMP can overcome the adenylate cyclase inhibition yet not promote insulin sensitivity (Fig. 5) indicates that additional information downstream of GIP receptor activation is required to modulate insulin sensitivity. Isoproterenol, a synthetic catecholamine, which has been shown to activate cAMP/PKA signaling pathway in adipocytes did not promote insulin sensitivity (Fig. 7A), providing further evidence for additional signaling downstream of GIPR. In our experimental setup, isoproterenol was able to activate adipocyte PKA similar to GIP, and this activation was unaffected by the presence of insulin (supplemental Fig. S1).
FIGURE 7.
p110β activation is necessary for GIP effect on insulin action. A, HA-Glut4-GFP plasma membrane-to-total distribution in adipocytes treated with insulin, GIP, and isoproterenol, as noted, for 60 min. The data are normalized to the ratio in cells treated with 1 nm insulin. The bars are averages ± S.E. (n = 3). *, p < 0.05. B, HA-Glut4-GFP plasma membrane-to-total distribution in adipocytes treated with insulin and GIP ± 1 μm TGX-221, a PI3K p110β inhibitor. The data are normalized to the ratio in cells treated with 1 nm insulin. The bars are averages ± S.E. (n = 3). *, p < 0.05. C, Western blot of total cell extracts of adipocytes for PKA phospho-serine/threonine substrate antibody (PKA PAS). The arrow indicates migration of perilipin, the major PKA substrate in adipocytes. Cells were pretreated with 1 μm TGX-221 followed by incubation with insulin and GIP for 30 min. D, HA-Glut4-GFP plasma membrane-to-total distribution in adipocytes treated with insulin and GIP ± 1 μm SW-14 (PI3K p110δ/γ) or 1 μm SW-30 (PI3K p110δ) inhibitor. Cells were pretreated with inhibitors for 30 min before incubation with insulin and GIP for 1 h. The data are normalized to the ratio in cells treated with 1 nm insulin. The bars are averages ± S.E. (n = 3). *, p < 0.05. E, HA-Glut4-GFP plasma membrane-to-total distribution in adipocytes co-electroporated with or without α-transducin. The bars are averages ± S.E. (n = 3). **, p < 0.01.
The Gα subunit of trimeric G proteins, via modulation of cAMP levels, is the principle transducer of G protein-coupled receptor signaling. However, the Gβγ subunits can also transmit signals. For example, the p110β isoform of PI3K is activated by Gβγ subunits (45–47). The exact functions of p110β have not been elucidated, although recent evidence suggests that it is involved in cell growth, metabolism, and tumorigenesis (48–51).
We next investigated whether p110β is required for the effects of GIP on insulin sensitivity. Past studies have shown that p110β is not required for insulin-stimulated glucose transport and that it is through the activation of p110α that insulin signals to Akt (52). To determine whether p110β is required for the GIP enhancement of insulin action, we used TGX-221, a specific inhibitor of p110β (53). The effect of GIP on insulin-stimulated Glut4 translocation was abolished by TGX-221, whereas insulin-stimulated Glut4 translocation was unaffected (Fig. 7B). Inhibition of p110β with TGX-221 did not affect GIP stimulation of PKA, demonstrating the inhibition of the GIP effect was not due to effects on PKA (Fig. 7C). Inhibitors of other isoforms of PI3K (SW14 (p110δ) or SW30 (p110γ)) did not inhibit the GIP effect of insulin-stimulated Glut4 translocation (Fig. 7D). Furthermore, expression of the Gβγ sequestrant, α-transducin (54), abolished the effect of GIP on insulin-stimulated Glut4 translocation (Fig. 7E). These data support the hypothesis that p110β activation via Gβγ is necessary for the GIP effect. Thus, both the cAMP/PKA/CREB signaling module and p110β activity are required for the GIP enhancement of insulin-stimulated Glut4 translocation.
DISCUSSION
GIP is well recognized for the incretin effects on pancreatic β-cells, and the bulk of the studies have focused on this particular aspect of its functions (55). Several groups have studied the role of GIP on metabolic regulation in adipocytes (7). Most of those studies have focused on lipid metabolism, and GIP has been shown to enhance insulin action on fatty acid synthesis and incorporation into lipids (6, 56). We have evaluated the effect of GIP on Glut4 translocation and FoxO1 nuclear exclusion, two key functions of insulin action in adipocytes. Our results show that GIP potentiates insulin sensitivity in a dose- and time-dependent manner. We did not observe any affect of GIP stimulation by itself on Glut4 translocation, glucose uptake, FoxO1 nuclear exclusion, or Akt activation, strongly arguing that GIP is a sensitizer to insulin rather than insulin mimetic.
Conceptually, the insulin-sensitizing effect of GIP on adipocytes is similar to the sensitizing effects of GIP on glucose-stimulated insulin secretion, with both effects contributing to a more efficient disposal of nutrients in the postprandial state. Moreover, in view of the importance of adipose as an endocrine tissue in the regulation of whole body metabolism (57, 58), GIP, by setting the tone of insulin response of adipocytes, would affect whole body metabolism. The insulin-sensitizing activity we describe points to a more central role for GIP in coordinating intestinal nutrient sensing to the control peripheral tissue metabolism, an emerging central feature of interorgan communication in the control of metabolism.
Our results that GIP modulates insulin sensitivity of 3T3-L1 adipocytes are in agreement with earlier reports of the effect of GIP on glucose transport in adipocytes (13, 14). Our results are also in agreement with a recent study in human subjects in which authors used a prolonged high insulin high glucose clamp technique with plasma glucose and insulin concentrations similar to those found after ingestion of a carbohydrate-rich meal (59). They found that during the high insulin high glucose clamp experiment in combination with GIP, glucose uptake in adipose increased significantly compared with the high insulin high glucose clamp experiment without GIP. Interestingly, in the absence of insulin, GIP did not have any effect on adipose glucose uptake substantiating our finding that GIP potentiates insulin action without having any insulin mimetic activity.
There are two possible mechanisms by which, GIP could modulate insulin-stimulated Glut4 translocation. It could either signal through an independent pathway or influence the canonical insulin pathway to enhance insulin-stimulated Glut4 translocation. As GIP does not have any effect on glucose uptake, Glut4 translocation and nuclear exclusion of FoxO1 in the absence of insulin; it is unlikely that GIP is operating via an independent pathway. We found that inhibition of Akt by an inhibitor or by siRNA abolished the effect of GIP on Glut4 translocation. Additionally, GIP did not have any effect in Rab10 knockdown cells. Rab10 is a key molecule, and its activation is vital for insulin-stimulated Glut4 translocation. Taken together, these data indicate that activation of conventional insulin signaling pathway is required for GIP effect on Glut4 translocation. However, despite GIP affecting two distinct insulin-controlled Akt-dependent processes, we were unable to detect an effect of GIP on either insulin-stimulated Akt activity as measured by phosphorylation of TBC1D4 or insulin-stimulated Akt phosphorylation on Thr308 or Ser473 (surrogate measures of Akt activation). A number of Akt binding proteins have been shown to play a role in Akt signaling, possibly via control of the subcellular localization of Akt (e.g. Refs. 16, 60, 61). Perhaps GIP modulates Akt activity via alterations in localization or association with accessory proteins, mechanisms that would not be detected in total cell extract phosphorylation assays. It is also possible that the Akt and TBC1D4 phosphorylation assays are not of sufficient sensitivity to detect the changes induced by GIP. Regardless, the functional assessments we have used, FoxO1 nuclear exclusion and Glut4 translocation, clearly demonstrate that these Akt-dependent activities downstream of insulin receptor are indeed affected by co-stimulation with GIP.
Our finding that elevated levels of cAMP by GIP are required to enhance insulin action appears to conflict with the notion that hormones, which activate adenylate cyclase and elevate cAMP levels have counter-regulatory effects on insulin-stimulated glucose transport (62–65). However, using rat adipocytes, Kuroda et al. (66) found that inhibition of insulin-stimulated glucose uptake by isoproterenol, glucagon, or adrenocorticotropic hormone was independent of cAMP formation. Inhibition of insulin-stimulated glucose transport by forskolin in rat adipose tissues has also been shown to be independent of its ability to activate adenylate cyclase and elevate cAMP levels (67).
Other hormones that activate adenylate cylase and induce formation of cAMP do not replicate the effect of GIP on insulin sensitivity in adipocytes. For instance, the counter-regulatory hormones, which have the opposing effects of insulin on adipose metabolism, signal in adipocytes through elevated cAMP (62, 63, 65, 68). Our data suggest that both activation of p110β and elevated cAMP contribute to the specific insulin-sensitizing action of GIP. However, we cannot rule out that other mechanisms contribute to the specific effect of GIP. For example, subcellular compartmentalization is an important component of cAMP signaling and there are examples of stimuli-specific responses being mediated through the cAMP common second messenger (69). Future studies are required to better define the molecular mechanism. Regardless, here we reveal a novel-signaling pathway to modulate insulin sensitivity in adipocytes.
In our experimental system, GIP- and isoproterenol-induced PKA activation was unaffected by insulin (supplemental Fig. S1). This is surprising as one of the key metabolic actions of insulin is the inhibition of lipolytic activity in fat cells (70, 71). Activation of the adipocyte cAMP phosphodiesterase by insulin is believed to be the mechanism by which, insulin reduces cellular cAMP and inhibits PKA phosphorylation downstream of β-adrenergic receptor signaling (71). The possible explanation is that in our experimental setup, we use comparatively lower amounts of insulin simultaneously with GIP/isoproterenol with out any preincubation (72).
The requirement of CREB implies that new transcription/translation is needed for insulin-sensitizing effect of GIP. This is also reflected in the timing of the GIP effect, which is slower (∼30–60 min) compared with the effect of insulin (<15 min) on Glut4 translocation.
It has recently been reported that hyperactivated CREB in mature adipocytes promotes whole body insulin resistance and correspondingly, that the loss of CREB activity in adipose improves whole body insulin sensitivity (73). How do we reconcile our findings that GIP activation of CREB promotes insulin sensitivity with those results? Although, without further study, we cannot answer this question, the effects we describe for activation of CREB are acute cell autonomous changes that would be coordinated via increased circulating GIP levels, with the postprandial state, which differs from the chronic activation of CREB that would occur in obesity (73).
In summary, we have demonstrated a novel phenomenon by which GIP, a gut hormone potentiates insulin sensitivity of adipocytes through the activation of cAMP/protein kinase A/CREB signaling module and p110β phosphoinositol-3′ kinase. Past studies have shown that GIP has a significant role in metabolic control (74), and our results advance the understanding of GIP biology by establishing an insulin-sensitizing role in adipocytes. GIP serum levels and signaling are altered in obesity and type 2 diabetes mellitus (31, 75), and our study suggests that normalizing GIP function might contribute to improvements in metabolism through effects on insulin sensitivity in addition to effects on glucose-stimulated insulin secretion and the control of lipolysis.
Supplementary Material
Acknowledgments
We thank members of the McGraw laboratory for helpful discussions and suggestions and Jennifer Wen and David Iaea for expert technical assistance. We are grateful to Dr. Xin-Yun Huang (Department of Physiology and Biophysics, Weill Cornell Medical College) for providing the α-transducin construct.
This work was supported, in whole or in part, by National Institutes of Health Grants DK52852 and DK69982 (to T. E. M.). This work was also supported by the Biomedical Research Program funds at Weill Cornell Medical College, a program funded by Qatar Foundation.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- GIP
- gastric inhibitory peptide
- PKA
- protein kinase A
- Akti
- Akt inhibitor
- 8-CPT-cAMP
- 8-(4-chlorophenylthio)-adenosine-3′,5′-cyclic monophosphate.
REFERENCES
- 1. Dupre J., Ross S. A., Watson D., Brown J. C. (1973) J. Clin. Endocrinol. Metab. 37, 826–828 [DOI] [PubMed] [Google Scholar]
- 2. Ross S. A., Dupre J. (1978) Diabetes 27, 327–333 [DOI] [PubMed] [Google Scholar]
- 3. Drucker D. J. (2006) Cell Metab. 3, 153–165 [DOI] [PubMed] [Google Scholar]
- 4. Preitner F., Ibberson M., Franklin I., Binnert C., Pende M., Gjinovci A., Hansotia T., Drucker D. J., Wollheim C., Burcelin R., Thorens B. (2004) J. Clin. Invest. 113, 635–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miyawaki K., Yamada Y., Yano H., Niwa H., Ban N., Ihara Y., Kubota A., Fujimoto S., Kajikawa M., Kuroe A., Tsuda K., Hashimoto H., Yamashita T., Jomori T., Tashiro F., Miyazaki J., Seino Y. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 14843–14847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yip R. G., Wolfe M. M. (2000) Life Sci. 66, 91–103 [DOI] [PubMed] [Google Scholar]
- 7. Usdin T. B., Mezey E., Button D. C., Brownstein M. J., Bonner T. I. (1993) Endocrinology 133, 2861–2870 [DOI] [PubMed] [Google Scholar]
- 8. Yip R. G., Boylan M. O., Kieffer T. J., Wolfe M. M. (1998) Endocrinology 139, 4004–4007 [DOI] [PubMed] [Google Scholar]
- 9. Kim S. J., Nian C., McIntosh C. H. (2010) J. Lipid Res. 51, 3145–3157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Widenmaier S. B., Kim S. J., Yang G. K., De Los Reyes T., Nian C., Asadi A., Seino Y., Kieffer T. J., Kwok Y. N., McIntosh C. H. (2010) PLoS One 5, e9590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim S. J., Nian C., McIntosh C. H. (2007) J. Biol. Chem. 282, 34139–34147 [DOI] [PubMed] [Google Scholar]
- 12. Song D. H., Getty-Kaushik L., Tseng E., Simon J., Corkey B. E., Wolfe M. M. (2007) Gastroenterology 133, 1796–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miyawaki K., Yamada Y., Ban N., Ihara Y., Tsukiyama K., Zhou H., Fujimoto S., Oku A., Tsuda K., Toyokuni S., Hiai H., Mizunoya W., Fushiki T., Holst J. J., Makino M., Tashita A., Kobara Y., Tsubamoto Y., Jinnouchi T., Jomori T., Seino Y. (2002) Nat. Med. 8, 738–742 [DOI] [PubMed] [Google Scholar]
- 14. Starich G. H., Bar R. S., Mazzaferri E. L. (1985) Am. J. Physiol. 249, E603–607 [DOI] [PubMed] [Google Scholar]
- 15. Sano H., Eguez L., Teruel M. N., Fukuda M., Chuang T. D., Chavez J. A., Lienhard G. E., McGraw T. E. (2007) Cell Metab. 5, 293–303 [DOI] [PubMed] [Google Scholar]
- 16. Gonzalez E., McGraw T. E. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 7004–7009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karylowski O., Zeigerer A., Cohen A., McGraw T. E. (2004) Mol. Biol. Cell 15, 870–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zeigerer A., Lampson M. A., Karylowski O., Sabatini D. D., Adesnik M., Ren M., McGraw T. E. (2002) Mol. Biol. Cell 13, 2421–2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lampson M. A., Racz A., Cushman S. W., McGraw T. E. (2000) J. Cell Sci. 113, 4065–4076 [DOI] [PubMed] [Google Scholar]
- 20. Martin O. J., Lee A., McGraw T. E. (2006) J. Biol. Chem. 281, 484–490 [DOI] [PubMed] [Google Scholar]
- 21. Gibbs E. M., Lienhard G. E., Gould G. W. (1988) Biochemistry 27, 6681–6685 [DOI] [PubMed] [Google Scholar]
- 22. Lampson M. A., Schmoranzer J., Zeigerer A., Simon S. M., McGraw T. E. (2001) Mol. Biol. Cell 12, 3489–3501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang S., Czech M. P. (2007) Cell Metab. 5, 237–252 [DOI] [PubMed] [Google Scholar]
- 24. Brunet A., Bonni A., Zigmond M. J., Lin M. Z., Juo P., Hu L. S., Anderson M. J., Arden K. C., Blenis J., Greenberg M. E. (1999) Cell 96, 857–868 [DOI] [PubMed] [Google Scholar]
- 25. Eguez L., Lee A., Chavez J. A., Miinea C. P., Kane S., Lienhard G. E., McGraw T. E. (2005) Cell Metab. 2, 263–272 [DOI] [PubMed] [Google Scholar]
- 26. Govers R., Coster A. C., James D. E. (2004) Mol. Cell. Biol. 24, 6456–6466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Potti A., Mukherjee S., Petersen R., Dressman H. K., Bild A., Koontz J., Kratzke R., Watson M. A., Kelley M., Ginsburg G. S., West M., Harpole D. H., Jr., Nevins J. R. (2006) N. Engl. J. Med. 355, 570–580 [DOI] [PubMed] [Google Scholar]
- 28. Bae S. S., Cho H., Mu J., Birnbaum M. J. (2003) J. Biol. Chem. 278, 49530–49536 [DOI] [PubMed] [Google Scholar]
- 29. Jiang Z. Y., Zhou Q. L., Coleman K. A., Chouinard M., Boese Q., Czech M. P. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 7569–7574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gonzalez E., McGraw T. E. (2006) Mol. Biol. Cell 17, 4484–4493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meier J. J., Hücking K., Holst J. J., Deacon C. F., Schmiegel W. H., Nauck M. A. (2001) Diabetes 50, 2497–2504 [DOI] [PubMed] [Google Scholar]
- 32. Alessi D. R., Andjelkovic M., Caudwell B., Cron P., Morrice N., Cohen P., Hemmings B. A. (1996) EMBO J. 15, 6541–6551 [PMC free article] [PubMed] [Google Scholar]
- 33. Welsh G. I., Hers I., Berwick D. C., Dell G., Wherlock M., Birkin R., Leney S., Tavaré J. M. (2005) Biochem. Soc Trans 33, 346–349 [DOI] [PubMed] [Google Scholar]
- 34. Ehses J. A., Casilla V. R., Doty T., Pospisilik J. A., Winter K. D., Demuth H. U., Pederson R. A., McIntosh C. H. (2003) Endocrinology 144, 4433–4445 [DOI] [PubMed] [Google Scholar]
- 35. Trümper A., Trümper K., Trusheim H., Arnold R., Göke B., Hörsch D. (2001) Mol. Endocrinol. 15, 1559–1570 [DOI] [PubMed] [Google Scholar]
- 36. Zawalich W. S., Rasmussen H. (1990) Mol. Cell. Endocrinol. 70, 119–137 [DOI] [PubMed] [Google Scholar]
- 37. Jones P. M., Persaud S. J. (1998) Endocr. Rev. 19, 429–461 [DOI] [PubMed] [Google Scholar]
- 38. Lu M., Wheeler M. B., Leng X. H., Boyd A. E., 3rd (1993) Endocrinology 132, 94–100 [DOI] [PubMed] [Google Scholar]
- 39. Rhoads J. M., Argenzio R. A., Chen W., Graves L. M., Licato L. L., Blikslager A. T., Smith J., Gatzy J., Brenner D. A. (2000) Gastroenterology 118, 90–100 [DOI] [PubMed] [Google Scholar]
- 40. Rydel R. E., Greene L. A. (1988) Proc. Natl. Acad. Sci. U.S.A. 85, 1257–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Greenberg A. S., Egan J. J., Wek S. A., Garty N. B., Blanchette-Mackie E. J., Londos C. (1991) J. Biol. Chem. 266, 11341–11346 [PubMed] [Google Scholar]
- 42. Chijiwa T., Mishima A., Hagiwara M., Sano M., Hayashi K., Inoue T., Naito K., Toshioka T., Hidaka H. (1990) J. Biol. Chem. 265, 5267–5272 [PubMed] [Google Scholar]
- 43. Walton K. M., Rehfuss R. P., Chrivia J. C., Lochner J. E., Goodman R. H. (1992) Mol. Endocrinol. 6, 647–655 [DOI] [PubMed] [Google Scholar]
- 44. Gonzalez G. A., Montminy M. R. (1989) Cell 59, 675–680 [DOI] [PubMed] [Google Scholar]
- 45. Maier U., Babich A., Nürnberg B. (1999) J. Biol. Chem. 274, 29311–29317 [DOI] [PubMed] [Google Scholar]
- 46. Guillermet-Guibert J., Bjorklof K., Salpekar A., Gonella C., Ramadani F., Bilancio A., Meek S., Smith A. J., Okkenhaug K., Vanhaesebroeck B. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 8292–8297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kurosu H., Maehama T., Okada T., Yamamoto T., Hoshino S., Fukui Y., Ui M., Hazeki O., Katada T. (1997) J. Biol. Chem. 272, 24252–24256 [DOI] [PubMed] [Google Scholar]
- 48. Jia S., Liu Z., Zhang S., Liu P., Zhang L., Lee S. H., Zhang J., Signoretti S., Loda M., Roberts T. M., Zhao J. J. (2008) Nature 454, 776–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhao J. J., Liu Z., Wang L., Shin E., Loda M. F., Roberts T. M. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 18443–18448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ciraolo E., Iezzi M., Marone R., Marengo S., Curcio C., Costa C., Azzolino O., Gonella C., Rubinetto C., Wu H., Dastrù W., Martin E. L., Silengo L., Altruda F., Turco E., Lanzetti L., Musiani P., Rückle T., Rommel C., Backer J. M., Forni G., Wymann M. P., Hirsch E. (2008) Sci. Signal 1, ra3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Graupera M., Guillermet-Guibert J., Foukas L. C., Phng L. K., Cain R. J., Salpekar A., Pearce W., Meek S., Millan J., Cutillas P. R., Smith A. J., Ridley A. J., Ruhrberg C., Gerhardt H., Vanhaesebroeck B. (2008) Nature 453, 662–666 [DOI] [PubMed] [Google Scholar]
- 52. Knight Z. A., Gonzalez B., Feldman M. E., Zunder E. R., Goldenberg D. D., Williams O., Loewith R., Stokoe D., Balla A., Toth B., Balla T., Weiss W. A., Williams R. L., Shokat K. M. (2006) Cell 125, 733–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jackson S. P., Schoenwaelder S. M., Goncalves I., Nesbitt W. S., Yap C. L., Wright C. E., Kenche V., Anderson K. E., Dopheide S. M., Yuan Y., Sturgeon S. A., Prabaharan H., Thompson P. E., Smith G. D., Shepherd P. R., Daniele N., Kulkarni S., Abbott B., Saylik D., Jones C., Lu L., Giuliano S., Hughan S. C., Angus J. A., Robertson A. D., Salem H. H. (2005) Nat. Med. 11, 507–514 [DOI] [PubMed] [Google Scholar]
- 54. Federman A. D., Conklin B. R., Schrader K. A., Reed R. R., Bourne H. R. (1992) Nature 356, 159–161 [DOI] [PubMed] [Google Scholar]
- 55. McIntosh C. H., Widenmaier S., Kim S. J. (2009) Vitam. Horm. 80, 409–471 [DOI] [PubMed] [Google Scholar]
- 56. Kim S. J., Nian C., McIntosh C. H. (2007) J. Biol. Chem. 282, 8557–8567 [DOI] [PubMed] [Google Scholar]
- 57. Galic S., Oakhill J. S., Steinberg G. R. (2010) Mol. Cell. Endocrinol. 316, 129–139 [DOI] [PubMed] [Google Scholar]
- 58. Herman M. A., Kahn B. B. (2006) J. Clin. Invest. 116, 1767–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Asmar M., Tangaa W., Madsbad S., Hare K., Astrup A., Flint A., Bülow J., Holst J. J. (2010) Am. J. Physiol. Endocrinol. Metab. 298, E614–621 [DOI] [PubMed] [Google Scholar]
- 60. Schenck A., Goto-Silva L., Collinet C., Rhinn M., Giner A., Habermann B., Brand M., Zerial M. (2008) Cell 133, 486–497 [DOI] [PubMed] [Google Scholar]
- 61. Ding J., Du K. (2009) Mol. Cell. Biol. 29, 1459–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Harber M. P., Schenk S., Barkan A. L., Horowitz J. F. (2005) Am. J. Physiol. Endocrinol. Metab. 289, E306–312 [DOI] [PubMed] [Google Scholar]
- 63. Shanahan M. F., Edwards B. M., Ruoho A. E. (1986) Biochim. Biophys. Acta 887, 121–129 [DOI] [PubMed] [Google Scholar]
- 64. Young D. A., Wallberg-Henriksson H., Cranshaw J., Chen M., Holloszy J. O. (1985) Am. J. Physiol. 248, C406–C409 [DOI] [PubMed] [Google Scholar]
- 65. Chang E., Donkin S. S., Teegarden D. (2009) Mol. Cell. Endocrinol. 307, 77–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kuroda M., Honnor R. C., Cushman S. W., Londos C., Simpson I. A. (1987) J. Biol. Chem. 262, 245–253 [PubMed] [Google Scholar]
- 67. Joost H. G., Steinfelder H. J. (1987) Mol. Pharmacol. 31, 279–283 [PubMed] [Google Scholar]
- 68. Regen D. M., Young D. A., Davis W. W., Jack J., Jr., Park C. R. (1964) J. Biol. Chem. 239, 381–384 [PubMed] [Google Scholar]
- 69. Smith F. D., Scott J. D. (2006) Eur. J. Cell Biol. 85, 585–592 [DOI] [PubMed] [Google Scholar]
- 70. Rahn T., Ridderstråle M., Tornqvist H., Manganiello V., Fredrikson G., Belfrage P., Degerman E. (1994) FEBS Lett. 350, 314–318 [DOI] [PubMed] [Google Scholar]
- 71. Elks M. L., Manganiello V. C. (1985) Endocrinology 116, 2119–2121 [DOI] [PubMed] [Google Scholar]
- 72. Choi S. M., Tucker D. F., Gross D. N., Easton R. M., DiPilato L. M., Dean A. S., Monks B. R., Birnbaum M. J. (2010) Mol. Cell. Biol. 30, 5009–5020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Qi L., Saberi M., Zmuda E., Wang Y., Altarejos J., Zhang X., Dentin R., Hedrick S., Bandyopadhyay G., Hai T., Olefsky J., Montminy M. (2009) Cell Metab. 9, 277–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Irwin N., Flatt P. R. (2009) Best Pract. Res. Clin. Endocrinol. Metab. 23, 499–512 [DOI] [PubMed] [Google Scholar]
- 75. Nauck M. A., Heimesaat M. M., Orskov C., Holst J. J., Ebert R., Creutzfeldt W. (1993) J. Clin. Invest. 91, 301–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.