Abstract
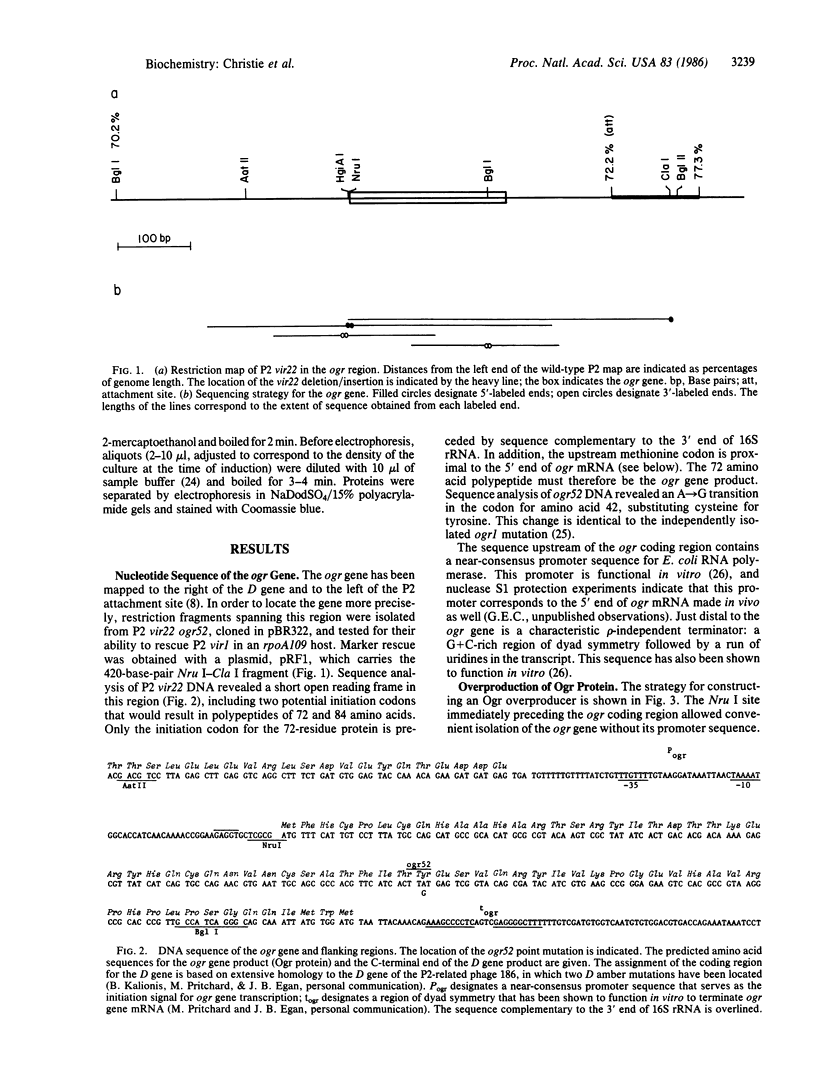
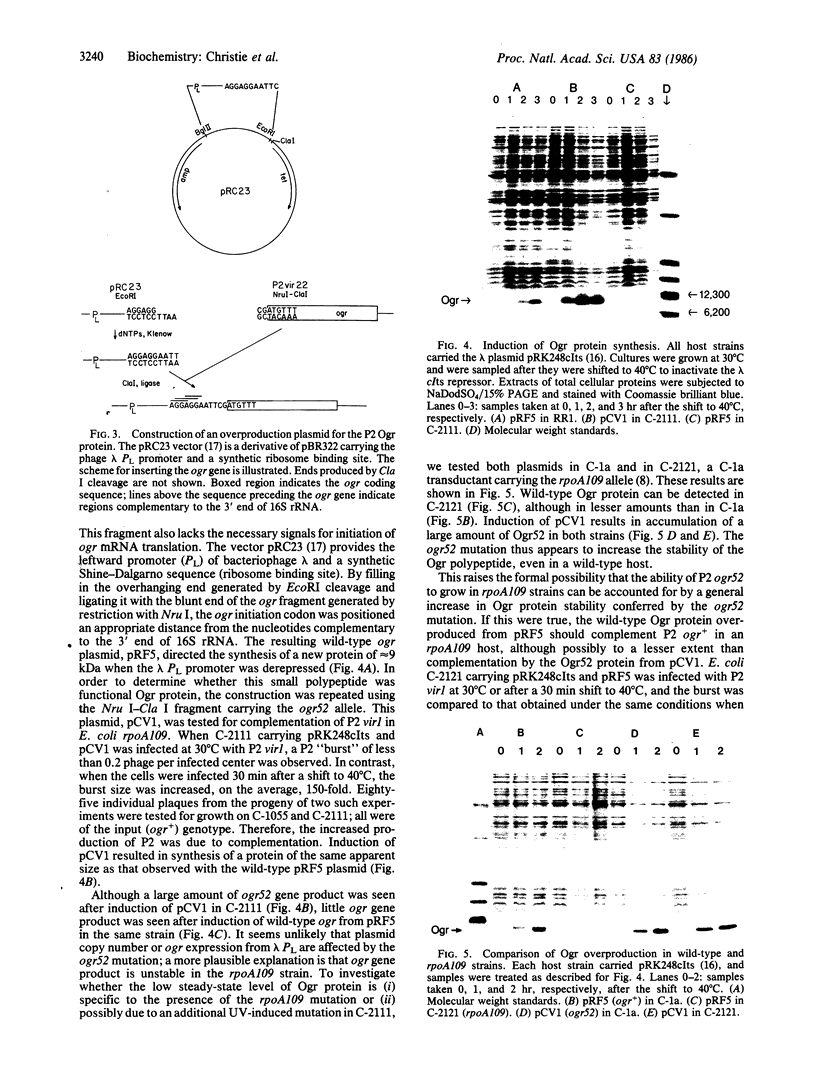
The ogr gene product of bacteriophage P2 is a positive regulatory factor required for P2 late-gene transcription. We have determined the nucleotide sequence of the ogr gene, which encodes a basic polypeptide of 72 amino acids. P2 growth is blocked by a host mutation, rpoA109, in the alpha subunit of DNA-dependent RNA polymerase. The ogr52 mutation, which allows P2 to grow in an rpoA109 strain, was shown to be a single nucleotide change, in the codon for residue 42, that changes tyrosine to cysteine. The predicted amino acid sequence of the Ogr protein does not show similarity to DNA-binding proteins that are known to affect promoter recognition, to sigma factors, or to other characterized transcriptional regulatory proteins. We have inserted the ogr gene into a plasmid under control of the leftward promoter and operator of bacteriophage lambda. Thermal induction of ogr gene expression in this plasmid results in overproduction of a small protein that has been shown by complementation to possess Ogr function.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett K. J., Marsh M. L., Calendar R. Interactions between a satellite bacteriophage and its helper. J Mol Biol. 1976 Sep 25;106(3):683–707. doi: 10.1016/0022-2836(76)90259-x. [DOI] [PubMed] [Google Scholar]
- Bernard H. U., Helinski D. R. Use of the lambda phage promoter PL to promote gene expression in hybrid plasmid cloning vehicles. Methods Enzymol. 1979;68:482–492. doi: 10.1016/0076-6879(79)68037-0. [DOI] [PubMed] [Google Scholar]
- Burton Z., Burgess R. R., Lin J., Moore D., Holder S., Gross C. A. The nucleotide sequence of the cloned rpoD gene for the RNA polymerase sigma subunit from E coli K12. Nucleic Acids Res. 1981 Jun 25;9(12):2889–2903. doi: 10.1093/nar/9.12.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattoraj D. K., Bertani G. Further physical characterization of deletion and substitution mutants affecting the control of lysogeny in bacteriophage P2. Mol Gen Genet. 1980 Apr;178(1):85–90. doi: 10.1007/BF00267216. [DOI] [PubMed] [Google Scholar]
- Chattoraj D. K., Inman R. B. Position of two deletion mutations on the physical map of bacteriophage P2. J Mol Biol. 1972 May 28;66(3):423–434. doi: 10.1016/0022-2836(72)90424-x. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Christie G. E., Calendar R. Bacteriophage P2 late promoters. II. Comparison of the four late promoter sequences. J Mol Biol. 1985 Feb 5;181(3):373–382. doi: 10.1016/0022-2836(85)90226-8. [DOI] [PubMed] [Google Scholar]
- Christie G. E., Calendar R. Bacteriophage P2 late promoters. Transcription initiation sites for two late mRNAs. J Mol Biol. 1983 Jul 15;167(4):773–790. doi: 10.1016/s0022-2836(83)80110-7. [DOI] [PubMed] [Google Scholar]
- Costanzo M., Brzustowicz L., Hannett N., Pero J. Bacteriophage SPO1 genes 33 and 34. Location and primary structure of genes encoding regulatory subunits of Bacillus subtilis RNA polymerase. J Mol Biol. 1984 Dec 15;180(3):533–547. doi: 10.1016/0022-2836(84)90026-3. [DOI] [PubMed] [Google Scholar]
- Costanzo M., Hannett N., Brzustowicz L., Pero J. Bacteriophage SPO1 gene 27: location and nucleotide sequence. J Virol. 1983 Nov;48(2):555–560. doi: 10.1128/jvi.48.2.555-560.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo M., Pero J. Structure of a Bacillus subtilis bacteriophage SPO1 gene encoding RNA polymerase sigma factor. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1236–1240. doi: 10.1073/pnas.80.5.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowl R., Seamans C., Lomedico P., McAndrew S. Versatile expression vectors for high-level synthesis of cloned gene products in Escherichia coli. Gene. 1985;38(1-3):31–38. doi: 10.1016/0378-1119(85)90200-8. [DOI] [PubMed] [Google Scholar]
- Escarmís C., Salas M. Nucleotide sequence of the early genes 3 and 4 of bacteriophage phi 29. Nucleic Acids Res. 1982 Oct 11;10(19):5785–5798. doi: 10.1093/nar/10.19.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan J., Egan J. B. In vivo transcription studies of coliphage 186. J Virol. 1981 Jun;38(3):987–995. doi: 10.1128/jvi.38.3.987-995.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki H., Palm P., Zillig W., Calendar R., Sunshine M. Identification of a mutation within the structural gene for the a subunit of DNA-dependent RNA polymerase of E. coli. Mol Gen Genet. 1976 Apr 23;145(1):19–22. doi: 10.1007/BF00331552. [DOI] [PubMed] [Google Scholar]
- Geisselsoder J., Mandel M., Calendar R., Chattoraj D. K. In vivo transcription patterns of temperate coliphage P2. J Mol Biol. 1973 Jul 5;77(3):405–415. doi: 10.1016/0022-2836(73)90447-6. [DOI] [PubMed] [Google Scholar]
- Gram H., Rüger W. Genes 55, alpha gt, 47 and 46 of bacteriophage T4: the genomic organization as deduced by sequence analysis. EMBO J. 1985 Jan;4(1):257–264. doi: 10.1002/j.1460-2075.1985.tb02344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking S. M., Egan J. B. Genetic characterization of twelve P2-186 hybrid bacteriophages. Mol Gen Genet. 1982;187(1):174–176. doi: 10.1007/BF00384403. [DOI] [PubMed] [Google Scholar]
- Kahn M., Ow D., Sauer B., Rabinowitz A., Calendar R. Genetic analysis of bacteriophage P4 using P4-plasmid ColE1 hybrids. Mol Gen Genet. 1980 Feb;177(3):399–412. doi: 10.1007/BF00271478. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landick R., Vaughn V., Lau E. T., VanBogelen R. A., Erickson J. W., Neidhardt F. C. Nucleotide sequence of the heat shock regulatory gene of E. coli suggests its protein product may be a transcription factor. Cell. 1984 Aug;38(1):175–182. doi: 10.1016/0092-8674(84)90538-5. [DOI] [PubMed] [Google Scholar]
- Lengyel J. A., Calendar R. Control of bacteriophage P2 protein and DNA synthesis. Virology. 1974 Feb;57(2):305–313. doi: 10.1016/0042-6822(74)90170-6. [DOI] [PubMed] [Google Scholar]
- Lin C. S. Nucleotide sequence of the essential region of bacteriophage P4. Nucleic Acids Res. 1984 Nov 26;12(22):8667–8684. doi: 10.1093/nar/12.22.8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl G. On the control of transcription in bacteriophage P2. Virology. 1971 Dec;46(3):620–633. doi: 10.1016/0042-6822(71)90065-1. [DOI] [PubMed] [Google Scholar]
- Lindqvist B. H. Expression of phage transcription in P2 lysogens infected with helper-dependent coliphage P4. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2752–2755. doi: 10.1073/pnas.71.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungquist E., Kockum K., Bertani L. E. DNA sequences of the repressor gene and operator region of bacteriophage P2. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3988–3992. doi: 10.1073/pnas.81.13.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Pritchard M., Egan J. B. Control of gene expression in P2-related coliphages: the in vitro transcription pattern of coliphage 186. EMBO J. 1985 Dec 16;4(13A):3599–3604. doi: 10.1002/j.1460-2075.1985.tb04123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R. L., West R. W., Heyneker H. L., Bolivar F., Boyer H. W. Characterizing wild-type and mutant promoters of the tetracycline resistance gene in pBR313. Nucleic Acids Res. 1979 Jul 25;6(10):3267–3287. doi: 10.1093/nar/6.10.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki I., Bertani G. Growth abnormalities in Hfr derivatives of Escherichia coli strain C. J Gen Microbiol. 1965 Sep;40(3):365–376. doi: 10.1099/00221287-40-3-365. [DOI] [PubMed] [Google Scholar]
- Sauer B., Calendar R., Ljungquist E., Six E., Sunshine M. G. Interaction of satellite phage P4 with phage 186 helper. Virology. 1982 Jan 30;116(2):523–534. doi: 10.1016/0042-6822(82)90145-3. [DOI] [PubMed] [Google Scholar]
- Sironi G. Mutants of Escherichia coli unable to be lysogenized by the temperate bacteriophage P2. Virology. 1969 Feb;37(2):163–176. doi: 10.1016/0042-6822(69)90196-2. [DOI] [PubMed] [Google Scholar]
- Six E. W. The helper dependence of satellite bacteriophage P4: which gene functions of bacteriophage P2 are needed by P4? Virology. 1975 Sep;67(1):249–263. doi: 10.1016/0042-6822(75)90422-5. [DOI] [PubMed] [Google Scholar]
- Souza L., Calendar R., Six E. W., Lindqvist B. H. A transactivation mutant of satellite phage P4. Virology. 1977 Aug;81(1):81–90. doi: 10.1016/0042-6822(77)90060-5. [DOI] [PubMed] [Google Scholar]
- Sunshine M. G., Sauer B. A bacterial mutation blocking P2 phage late gene expression. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2770–2774. doi: 10.1073/pnas.72.7.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunshine M. G., Thorn M., Gibbs W., Calendar R., Kelly B. P2 phage amber mutants: characterization by use of a polarity suppressor. Virology. 1971 Dec;46(3):691–702. doi: 10.1016/0042-6822(71)90071-7. [DOI] [PubMed] [Google Scholar]
- Takeda Y., Ohlendorf D. H., Anderson W. F., Matthews B. W. DNA-binding proteins. Science. 1983 Sep 9;221(4615):1020–1026. doi: 10.1126/science.6308768. [DOI] [PubMed] [Google Scholar]
- Wiman M., Bertani G., Kelly B., Sasaki I. Genetic map of Escherichia coli strain C. Mol Gen Genet. 1970;107(1):1–31. doi: 10.1007/BF00433220. [DOI] [PubMed] [Google Scholar]