Background: The lack of understanding of the structure-function relation of PCSK9 hinders efforts to develop small molecule inhibitors.
Results: The prodomains of C-terminal domain deletion PCSK9 mutants enable secretion of prodomain deletion mutants.
Conclusion: An interaction between the prodomain and C-terminal domain regulates the secretion of PCSK9.
Significance: PCSK9 may be inhibited by disrupting the interaction between the prodomain and C-terminal domain.
Keywords: Intracellular Processing, Lipoprotein Receptor, Low Density Lipoprotein (LDL), Receptor Regulation, Secretion, PCSK9
Abstract
PCSK9 (proprotein convertase subtilisin/kexin type 9) has emerged as a novel therapeutic target for hypercholesterolemia due to its LDL receptor (LDLR)-reducing activity. Although its structure has been solved, the lack of a detailed understanding of the structure-function relation hinders efforts to develop small molecule inhibitors. In this study, we used mutagenesis and transfection approaches to investigate the roles of the prodomain (PD) and the C-terminal domain (CD) and its modules (CM1–3) in the secretion and function of PCSK9. Deletion of PD residues 31–40, 41–50, or 51–60 did not affect the self-cleavage, secretion, or LDLR-degrading activity of PCSK9, whereas deletion of residues 61–70 abolished all of these functions. Deletion of the entire CD protein did not impair PCSK9 self-cleavage or secretion but completely abolished LDLR-degrading activity. Deletion of any one or two of the CD modules did not affect self-cleavage but influenced secretion and LDLR-reducing activity. Furthermore, in cotransfection experiments, a secretion-defective PD deletion mutant (ΔPD) was efficiently secreted in the presence of CD deletion mutants. This was due to the transfer of PD from the cotransfected CD mutants to the ΔPD mutant. Finally, we found that a discrete CD protein fragment competed with full-length PCSK9 for binding to LDLR in vitro and attenuated PCSK9-mediated hypercholesterolemia in mice. These results show a previously unrecognized domain interaction as a critical determinant in PCSK9 secretion and function. This knowledge should fuel efforts to develop novel approaches to PCSK9 inhibition.
Introduction
PCSK9 (proprotein convertase subtilisin/kexin type 9) (1) is a modulator of the cellular LDL receptor (LDLR)3 (2, 3) and plasma cholesterol levels (4, 5). Gain-of-function mutations of PCSK9 cause hyperlipidemia and early-onset coronary heart disease (4, 6–9), whereas loss-of-function mutations result in low plasma cholesterol levels and protection against coronary heart disease without apparent negative consequences (5, 10–13). Therefore, inhibition of PCSK9 is being pursued as an approach to reduce plasma LDL cholesterol levels (14–19). Based on the crystal structure (20–22), removal of the PCSK9 signal peptide (residues 1–30) produces a secreted protein with three domains: 1) the N-terminal prodomain (PD; residues 31–152), which is autocleaved but remains associated with the catalytic domain; 2) the catalytic domain (CA; residues 153–425), which contains the proteolytic active site; and 3) the C-terminal domain (CD; residues 426–692), which consists of three modules, CM1 (residues 457–527), CM2 (residues 534–601), and CM3 (residues 608–692). However, knowledge of the PCSK9 high resolution structure has neither improved our understanding of the molecular mechanism of this protein nor informed structure-based drug design. It is important to note that the N-terminal 30 residues (residues 31–60) in PD and the C-terminal 10 residues in CD, as well as several other short regions between the domains or modules, are invisible in the crystal structure due to the lack of electron density. Also, the structure is based on the configuration in which PD is associated with CA. The structures of several other serine proteases have been solved in the mature form, where the prodomain is not part of the catalytically active protein (23–26). Finally, biochemical data show that the catalytic activity of CA is not required for the LDLR-reducing activity in cells (27, 28). This also makes PCSK9 different from other serine proteases (23–26), and thus, its mode of action cannot be deduced from in-family comparisons.
Little is known regarding how the three domains of PCSK9 coordinate intramolecular interactions and the main functions of the protein, including autoprocessing (self-cleavage of PD), secretion, and LDLR binding and degradation. Although it has been reported that the interaction between PCSK9 and LDLR is mediated mainly by the binding of CA of PCSK9 to the first EGF-like repeat (EGF-A) in the EGF homology domain of LDLR (29, 30), solid evidence suggests that other domains or regions of both PCSK9 and LDLR are also involved in PCSK9-mediated LDLR degradation. Importantly, a recent report by Yamamoto et al. (31) showed that CD of PCSK9 is also directly involved in LDLR binding. An intriguing feature of CD is its high content of histidine residues, located mainly in the second module (CM2). Although it has been speculated that these histidines contribute to the pH-dependent LDLR-binding and LDLR-degrading activities of PCSK9 (20, 22), there is no direct evidence either for or against it. The function of PD of PCSK9 is also elusive. It is a unique feature of PCSK9 that its PD is still associated with the rest of the protein when the protein is secreted. It is posited that, in the mature PCSK9 protein, PD blocks the catalytic site from contacting other potential substrates. Interestingly, the flexible N-terminal region of PD actually acts as an inhibitor of PCSK9 function (20, 22). A recent report attributed this inhibitory effect to the acidic residues (32). However, because this region is not visible in the x-ray crystal structure, it is unknown if it actually interacts with other regions of the protein.
To accelerate the translation of the opportunity provided by the discovery of PCSK9 into clinical benefit while bypassing the current limited understanding of the molecular mechanism of action, current drug development attempts are directed at reducing production of PCSK9 by antisense DNA (33) or RNA interference (34) technologies or at neutralizing circulating PCSK9 via antibodies (35–37). However, these therapeutic approaches are not the most desirable for chronic asymptomatic conditions such as hyperlipidemia. Therefore, further structure-function studies are needed to provide a more complete understanding of the molecular mechanisms of PCSK9 activity to rationally design small molecule inhibitors for PCSK9 targeting its autoprocessing, secretion, or LDLR-binding and LDLR-degrading functions. In this study, we focused on the functional relations of PD and CD; our data suggest that domain-domain interactions govern the secretion and function of PCSK9. This information should be useful in defining target sites in PCSK9 for small molecule inhibitors to block its secretion or otherwise inhibit the LDLR effect.
EXPERIMENTAL PROCEDURES
Materials and Reagents
HEK293T human embryonic kidney cells (CRL-1573) and HepG2 liver hepatocellular carcinoma cells (HB-8065) were purchased from American Type Culture Collection (Manassas, VA). DMEM was purchased from Invitrogen. FBS was purchased from Atlanta Biologicals (Norcross, GA). l-Glutamine, streptomycin, and penicillin were purchased from Mediatech (Herndon, VA). All tissue culture plasticware was purchased from Corning (Corning, NY). Rabbit polyclonal antibody to PCSK9 was obtained from Cayman Chemical (catalog no. 10007185; Ann Arbor, MI). Rabbit polyclonal antibody to polyhistidine (His6) was from eBioscience (catalog no. 14-6757; San Diego, CA). Rabbit anti-β-actin antibody and HRP-conjugated goat anti-rabbit IgG antibody were from Sigma-Aldrich. Chicken polyclonal antibody to LDLR and rabbit polyclonal antibody to chicken IgY (H & L, HRP) were purchased from Abcam (catalog nos. ab14056 and ab6753, respectively; Cambridge, MA).
Mutagenesis
Mutagenesis was carried out using the QuikChange II XL site-directed mutagenesis kit from Stratagene (La Jolla, CA). The general procedure was described previously (38). PCR primers were customer-synthesized by Invitrogen. Fourteen PCSK9 truncation mutants with deletions in different domains were generated (Fig. 1). The sequence of each mutant corresponding to full-length PCSK9 and the primers used for generation of the mutants are listed in supplemental Table 1. All of the DNA constructs except PD contained a His tag at the C terminus. We previously generated a pcDNA-PCSK9-GST construct in which a GST tag was in place of the His tag (38). One PCSK9 mutant with a single residue mutation (D70N) was also generated. All of the DNA constructs were confirmed by DNA sequencing.
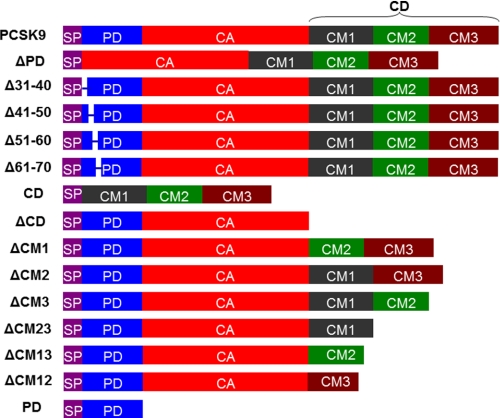
FIGURE 1.
Schematic drawing of PCSK9 and its truncation mutants used in this study. SP, signal peptide. Each construct except PD contained a His tag at the C terminus. For full-length PCSK9, another construct was also used in which the His tag at the C terminus was replaced with a GST tag.
Generation of Human LDLR DNA Construct
To generate the human LDLR ectodomain construct, we used human liver cDNA from Addgene (Cambridge, MA) as a template for PCR; a DNA fragment spanning the ectodomain (residues 1–699) was amplified using primers 5′-gttaagcttatggggccctg-3′ (forward) and 5′-gattctagacgggcaggcg-3′ (reverse). The PCR product was digested with HindIII and XbaI and ligated to pcDNA-PCSK9 DNA digested with the same restriction enzymes to generate the pcDNA-LDLR(1–699)-His construct. Next, a DNA sequence for GST was PCR-amplified using pcDNA-PCSK9-GST DNA as a template, digested with XbaI and PmeI, and inserted in place of the His tag in pcDNA-LDLR(1–699)-His to yield the pcDNA-LDLR(1–699)-GST DNA construct. The DNA construct was confirmed by DNA sequencing.
Transfection
HEK293T and HepG2 cells were cultured in DMEM with 10% FBS containing streptomycin (50 μg/ml), penicillin (50 units/ml), and l-glutamine (2 mm). To transfect HEK293T cells with one or two PCSK9 constructs, a ProFection® mammalian transfection system (Promega, Madison, WI) was used following the manufacturer's instructions and as described previously (38). HEK293T cells were transfected in 6-well plates; the medium was changed 8–16 h after transfection. The cells were further cultured for 16–24 h before the media and cell lysates were collected for Western blot analyses.
His·Bind®-Agarose Pulldown Assay
Transfected cells were lysed in PBS by mild sonication, and cell lysates were loaded onto a His·Bind-agarose column. The flow-through was saved for Western blotting. The agarose was washed with 20 column volumes of PBS containing 20 mm imidazole to eliminate nonspecific binding. The agarose-bound proteins were subjected to Western blotting.
Protein Purification
His-tagged full-length PCSK9 and mutants were purified using the His·Bind protein purification system (Invitrogen) as described previously (38). The LDLR ectodomain protein (LDLR(1–699) with a C-terminal GST tag, LDLR(1–699)-GST) from the culture medium of transfected HEK293T cells was purified using a glutathione-agarose column (Molecular Probes) similar to the method described previously for the purification of PCSK9-GST (38). The protein concentrations were determined by Lowry assays.
Western Blotting
Samples were loaded onto 10% SDS-polyacrylamide gels or 4–20% Tris/HEPES-SDS precast gels (Pierce) for electrophoresis. The size-separated proteins were then transferred to nitrocellulose membranes (Amersham Biosciences). The indicated primary antibodies and HRP-conjugated secondary antibodies were used to detect target proteins. Signal was detected using an ECL Plus kit (Amersham Biosciences). Quantification of the intensity of the bands was obtained using NIH ImageJ software.
Mice
Mice were purchased from The Jackson Laboratory (Bar Harbor, Maine) and housed at the Vanderbilt University Medical Center. All animal experiments were carried out in compliance with National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee of Vanderbilt University.
Statistical Analysis
GraphPad Prism 5 software was used to carry out statistical analyses.
RESULTS
Secretion and Function of PCSK9 Truncation Mutants
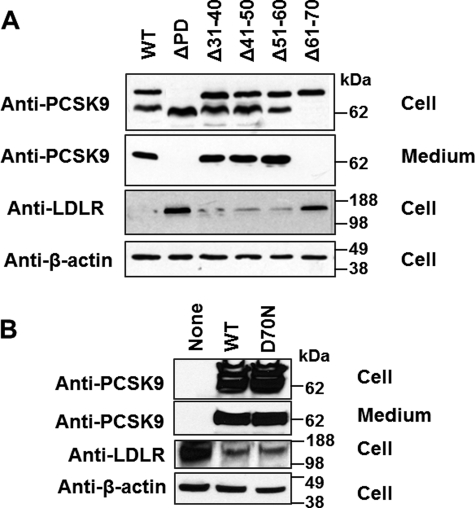
We transfected HEK293T cells using DNA constructs expressing PCSK9 or its mutants. First, we examined the effects of PD deletion on PCSK9 autoprocessing, secretion, and LDLR degradation. Fig. 2A shows that deletion of the entire PD protein abolished both the secretion and LDLR-reducing activity of PCSK9, consistent with previous reports (27, 28). Separate deletions of PD residues 31–40, 41–50, and 51–60 did not significantly affect PCSK9 processing, secretion, or function, except for a modest effect of Δ51–60 on autoprocessing. This contrasts with the stark effects of deletion of residues 61–70, which completely abolished autoprocessing, secretion, and LDLR degradation. Because Asp-70 was found to form a salt bridge with Arg-73 and to stabilize the PD structure (20), we sought to determine whether the effects of deletion of residues 61–70 are mainly due to the loss of the Asp-70–Arg-73 salt bridge. To this end, we compared WT PCSK9 and PCSK9(D70N), a mutant carrying an Asp-to-Asn substitution at position 70 that should abolish the Asp-70–Arg-73 salt bridge. However, Fig. 2B shows that this mutation did not affect PCSK9 function. We also generated a D70P mutant to insert a more dramatic alteration of the local structure. However, also the D70P mutant displayed no defect in self-processing and secretion (supplemental Fig. 1A).
FIGURE 2.
Autocleavage, secretion, and LDLR regulation of PCSK9 and its PD deletion mutants. HEK293T cells were transfected with full-length PCSK9, PD deletion mutants (A), or a single residue substitution mutant (D70N) (B). Western blot analyses were performed on the conditioned medium and cell lysates to examine the expression and secretion of PCSK9 constructs and cellular LDLR levels. In cell lysates, two main bands are shown for PCSK9: the upper band is pro-PCSK9, and the lower band is mature PCSK9 (without PD). The additional weak band represents the mature protein further cleaved by furin at Asn-218 (43).
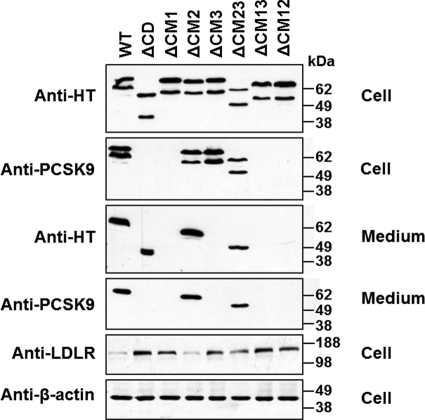
Next, we examined the role of CD in PCSK9 autoprocessing, secretion, and LDLR-degrading activity. Fig. 3 shows that deletion of the entire CD protein or any one or two of its modules did not affect autoprocessing of PCSK9. Whereas ΔCD, ΔCM2, and ΔCM23 were also efficiently secreted, secretion of ΔCM12 was drastically reduced, and that of ΔCM1, ΔCM3, and ΔCM13 was completely abolished. In agreement with previous reports (39, 40), we found that ΔCD did not mediate LDLR degradation. We used WT PCSK9 (Fig. 3, lane 1) and ΔCD (Fig. 3 lane 2) as positive and negative controls, respectively, to evaluate the LDLR-reducing activity of other CD deletion mutants. Quantitative analysis of Western blot results from three independent experiments revealed that the LDLR-reducing activity was normal for ΔCM2; drastically reduced (by 50%) for ΔCM23; and completely abolished for ΔCM1, ΔCM3, and ΔCM13 (Fig. 3 and supplemental Fig. 2A).
FIGURE 3.
Autocleavage, secretion, and LDLR regulation of PCSK9 and its CD deletion mutants. HEK293T cells were transfected with full-length PCSK9 or its CD deletion mutants. The autoprocessing and secretion of the PCSK9 proteins and cellular LDLR levels were analyzed by Western blotting. Quantitative analysis of the cellular LDLR levels is shown in supplemental Fig. 2A. HT, His tag.
Trans-Acting Effects of CD Deletion Mutants on PD-deficient PCSK9
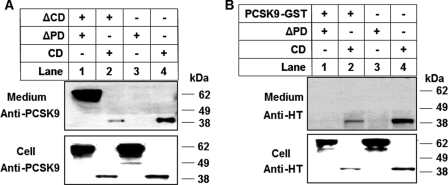
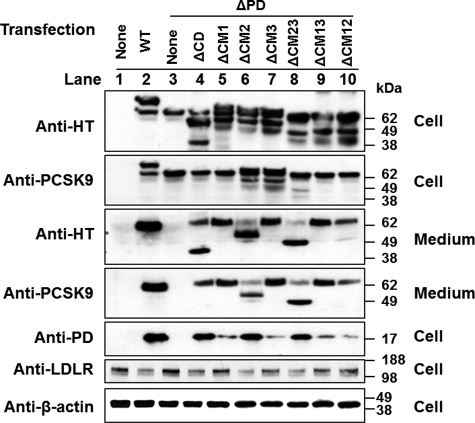
It was reported previously that cotransfection of cells with a PCSK9 construct containing only PD and a construct missing PD caused secretion of a mature and fully functional chimeric PCSK9 protein (27, 28). To investigate the trans-acting effects of different domains on PCSK9 secretion, we first coexpressed either ΔCD or full-length PCSK9 with either ΔPD or the CD fragment. In this experiment, we placed a GST tag on full-length PCSK9 so that this protein would not be detectable by the anti-His tag antibody. Also, the commercially available anti-PCSK9 antibody (Cayman Chemical), which is raised against a CD-derived peptide within CM1 (residues 490–502), does not detect ΔCD. We used these two antibodies to detect the secretion of ΔPD and CD. It must be noted that ΔCD was not detected by the anti-PCSK9 antibody (Fig. 4A) and that PCSK9-GST was not detected by the anti-His tag antibody (Fig. 4B). Interestingly, Fig. 4 shows that ΔCD (A, lane 1), but not full-length PCSK9 (B, lane 1), enabled ΔPD secretion. Neither ΔCD nor full-length PCSK9 promoted the secretion of the CD protein fragment but rather decreased its concentration both in the cells and in the medium (Fig. 4, A and B, lanes 2 versus lanes 4), possibly due to the competition between similar proteins in the secretory pathway. We next cotransfected HEK293T cells with ΔPD and each of the various CD deletion mutants. The Western blots of cell lysate using the anti-His tag antibody showed that each of the PCSK9 mutants was expressed in the cells at comparable levels (Fig. 5). It must be noted that the sizes of non-processed ΔCM23, ΔCM13, and ΔCM12 are similar to the size of ΔPD, and therefore, these bands overlap. Fig. 5 shows that coexpression of ΔPD did not change the secretion of the mutants with deletion in CD: ΔCD, ΔCM2, and ΔCM23 were secreted normally, whereas only trace amounts of ΔCM12 were secreted, and no secretion occurred for ΔCM1, ΔCM3, and ΔCM13 (summarized in supplemental Table 2). However, coexpression of any of the CD deletion mutants, including the non-secreted ones, caused the secretion of ΔPD (Fig. 5, lanes 4–10 versus lane 3), suggesting that PD of secretion-defective PCSK9 trans-acted on PD-deficient PCSK9. Interestingly, the various CD deletion mutants influenced secretion of ΔPD differently, in the following order (from the smallest to largest effect): ΔCM2 = ΔCM23 < ΔCD = ΔCM12 < ΔCM1 = ΔCM3 = ΔCM13 (Fig. 5). This order is opposite that of the secretory efficiency of these mutants when expressed alone (Fig. 3) or coexpressed with ΔPD (Fig. 5), as summarized in supplemental Table 2. In addition, PD appeared in the medium with secreted ΔPD, even when the cotransfection was done with non-secreted CD deletion mutants. This indicates that PD of CD deletion mutants was transferred to the ΔPD protein and caused its secretion. Quantitative analysis of the Western blot results from three independent experiments showed that, compared with either non-transfected or ΔPD-transfected cells, the cells cotransfected with ΔPD and any of the CD deletion mutants displayed significantly lower cellular LDLR levels (supplemental Fig. 2B), suggesting that the secreted chimeric PD-ΔPD protein is functional.
FIGURE 4.
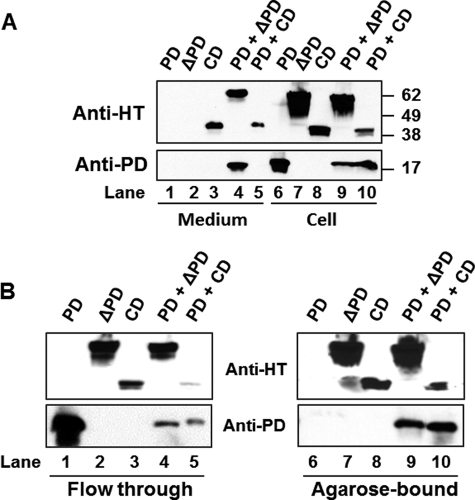
Domain trans-acting effects on PCSK9 secretion. A, HEK293T cells were cotransfected with ΔCD and ΔPD or CD. Protein was detected with the anti-PCSK9 antibody against a region of PCSK9 CM1 (therefore, ΔCD is invisible). B, HEK293T cells were cotransfected with PCSK9-GST and ΔPD or CD. Protein was detected with the anti-His tag (HT) antibody, which recognizes the His tag at the C terminus of ΔPD and CD (therefore, PCSK9-GST is invisible).
FIGURE 5.
Trans-Acting effects of CD deletion mutants on ΔPD secretion. HEK293T cells were cotransfected with ΔPD and PCSK9 mutants with deletions in CD. Protein was detected with anti-His tag (HT) and anti-PCSK9 antibodies for PCSK9 and its mutants in cell lysates and in the conditioned medium, with the anti-LDLR antibody for LDLR levels in the total cell lysates, and with the anti-β-actin antibody as a protein loading control. Quantitative analysis of the cellular LDLR levels is shown in supplemental Fig. 2B.
C679X is a naturally occurring PCSK9 mutant with normal self-processing but defective secretion. This mutation results in loss of function and decreased plasma LDL cholesterol levels (11, 12, 41). To establish whether the secretory defect of C679X is due to impaired PD-CD interaction, we set out to determine whether its PD can trans-act on and induce secretion of our ΔPD construct. When HEK293T cells were cotransfected with ΔPD and C679X, we did observe secretion of ΔPD, enabled by PD of mutant C679X (supplemental Fig. 1B).
To examine the PD-CD interaction further, we transfected HEK293T cells with the discrete PD protein (without a His tag) along with ΔPD or CD. As shown in Fig. 6A, PD was not secreted from cells transfected with PD alone (lane 1), but it was secreted with ΔPD (lane 4) in PD- and ΔPD-cotransfected cells. However, PD was not secreted with CD (lane 5), even though CD was secreted in either CD only- or CD- and PD-cotransfected cells. To examine whether PD interacts with the discrete CD protein in the cells, we performed His·Bind-agarose pulldown experiments to pull down His-tagged ΔPD or CD and to detect whether PD was also pulled down. Fig. 6B shows that whereas no PD was pulled down from cells transfected with PD alone, PD was pulled down from cells cotransfected with PD and either ΔPD or CD. These data clearly support the notion that PD directly associates with CD as such or as part of ΔPD.
FIGURE 6.
Intracellular association of PD and CD. A, HEK293T cells were cotransfected with PD and ΔPD or CD. Cellular and secreted proteins were detected by Western blotting. B, cell lysates were loaded onto His·Bind-agarose to pull down His-tagged ΔPD or CD. The flow-through and agarose-bound proteins were detected by Western blotting using the anti-His tag (HT) or anti-PD antibody.
Function of Discrete PCSK9 CD Protein
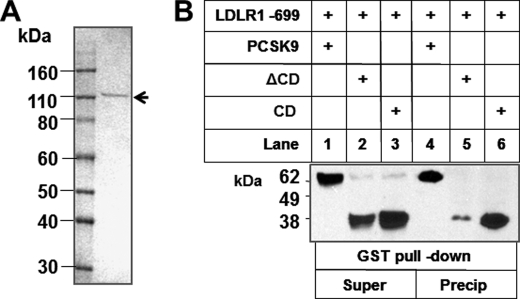
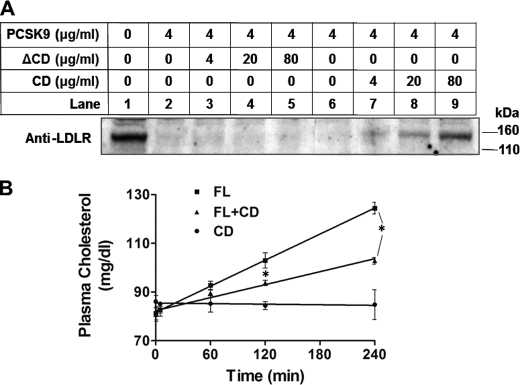
We purified full-length PCSK9, ΔCD, and CD from transfected HEK293T cell-conditioned medium as described previously (38) and also purified a GST-tagged LDLR(1–699) protein (Fig. 7A). We co-incubated LDLR(1–699) with WT PCSK9 (positive control), ΔCD, and CD protein in PBS for 1 h and pulled down LDLR(1–699) using glutathione-agarose. Proteins in the supernatant or pulled down by agarose were detected by Western blotting using the anti-His tag antibody. Although both ΔCD and CD bound to LDLR, CD had a significantly stronger affinity (Fig. 7B). To investigate further whether the discrete CD protein can compete with PCSK9 for LDLR binding, we added increasing amounts of CD or ΔCD protein together with full-length PCSK9 to HepG2 cell culture medium (DMEM with 10% FBS). After a 24-h incubation, cell membrane fractions were prepared as described (38) and analyzed for LDLR levels. Fig. 8A shows that the LDLR effect exerted by PCSK9 was attenuated by the CD fragment but not by the ΔCD protein. Next, we examined whether CD can inhibit PCSK9 function in vivo. We injected full-length PCSK9 (20 μg), CD protein (40 μg), or both into the retro-orbital venous plexus of C57BL/6 mice fasted overnight. Blood samples were taken at base line and 10 min, 1 h, 2 h, and 4 h after injection. Fig. 8B shows that injection of full-length PCSK9 increased plasma cholesterol levels at 1, 2, and 4 h by 14.0, 26.6, and 52.9%, respectively. CD protein alone did not affect plasma cholesterol levels but drastically influenced the effect of full-length PCSK9, with plasma cholesterol increases at 1, 2, and 4 h reduced to 11.1, 16.3, and 27.5%, respectively, which are significantly less than those produced by unopposed full-length PCSK9.
FIGURE 7.
Discrete PCSK9 CD protein binds to LDLR. A, SDS-PAGE of purified GST-tagged LDLR(1–699). B, LDLR(1–699) was co-incubated with WT PCSK9 (positive control), ΔCD, and CD protein (5 μg/ml) in PBS (pH 7.4) for 1 h, and LDLR(1–699) was pulled down using glutathione-agarose. Proteins in the supernatant (Super) or pulled down by agarose (Precip) were detected by Western blotting using the anti-His tag antibody.
FIGURE 8.
Discrete CD protein inhibits PCSK9 function in vitro and in vivo. A, HepG2 cells were treated with purified PCSK9 and increasing amounts of ΔCD or CD. Cell membrane LDLR levels were measured by Western blotting. B, full-length PCSK9 (FL; 20 μg/mouse) and the discrete CD protein (40 μg/mouse) separately or in combination were injected into C57BL/6 mice (n = 3 for each group). Plasma cholesterol levels were followed for 4 h. *, p < 0.01.
DISCUSSION
The aim of this study was to examine the role of PD and CD and its modules on the main functions of PCSK9, including autoprocessing, secretion, and LDLR-degrading activity. Our findings suggest that 1) residues 61–70 are critical for the autocatalytic removal of PD, 2) different modules in CD have different effects on PCSK9 secretion, 3) a previously unrecognized interaction between PD and CD governs PCSK9 secretion, and 4) the discrete CD protein inhibits the LDLR-binding and LDLR-degrading activities of full-length PCSK9 both in vitro and in vivo.
The N-terminal portion of PD (residues 31–60) is flexible in the x-ray crystal structure (20–22). Human genetic studies have suggested that the residues in this region modulate PCSK9 function (6, 13, 42). Experimental data further showed that deletion of residues 31–53 significantly increases the affinity of PCSK9 for LDLR (30) and that the acidic residues in this region contribute to the effect (32). Our results show that mutants with deletion of a stretch of 10 residues in region 30–60 (Δ31–40, Δ41–50, and Δ51–60) had preserved autoprocessing and secretion and displayed normal effects in reducing cellular LDLR levels. It has been recently reported that Δ33–40, Δ41–45, Δ48–50, and Δ31–53 have an enhanced ability to inhibit cellular LDL internalization compared with WT PCSK9 (32). In our study, we examined only cellular LDLR levels and did not measure LDL internalization. Nevertheless, we found that deletion of residues 61–70 completely abolished PCSK9 autoprocessing. It was reported that deletion of Ser-47–Lys-69 nearly blocks secretion but only moderately reduces self-processing (20). Comparison of our data of Δ61–70 with the studies of the Δ47–69 mutant suggests that Asp-70 is critical for the autocleavage activity. In the x-ray structure, residues 61–74 form an extra strand β0 (Thr-61–Lys-69), followed by a helical turn (Asp-70–Leu-74), stabilized by salt bridges (Arg-73–Glu-145 and Arg-73–Asp-70) (20); therefore, Asp-70 may be important either because of the salt bridge Arg-73–Asp-70 or because it is an integral component of the helical turn. The fact that the D70N mutant, which disallows the salt bridge Arg-73–Asp-70, did not affect PCSK9 self-processing and secretion suggests that the structural integrity of the helical turn starting with Asp-70 is critical for PCSK9 self-cleavage and secretion. Interestingly, the D70P mutant also did not impair PCSK9 self-processing and secretion. This suggests that 70PPWRL74 can preserve the 310 helical turn structure of the original 70DPWRL74 sequence.
It is known that deletion of the entire CD protein produces a truncated protein that is cleaved and secreted, suggesting that CD is not required for PCSK9 secretion (39). However, it is also known that the C679X mutant, a natural loss-of-function mutant causing low cholesterol in humans (12, 41), is not secreted by cells even though the autocleavage of PD is preserved (38). These paradoxical facts suggest a complex role for CD in PCSK9 secretion. A possibility is that when CD is part of the proprotein, it must meet certain sequence or structure requirements to be compatible for secretion. Our data are in favor of this possibility. Among the three single module deletion mutants of PCSK9, only ΔCM2 was secreted. In the x-ray crystal structure, both CM1 and CM3 directly contact CA, whereas CM2 does not (20). We speculate that when either CM1 or CM3 is missing, CD loses its structural integrity and does not contact CA properly; therefore, the resulting proteins are not compatible with efficient secretion. However, this speculation is complicated by the findings using dual module deletion mutants. Among the three dual module deletion mutants, only ΔCM23 was efficiently secreted and maintained substantial LDLR-degrading activity. It is noteworthy that CM1 allowed efficient secretion of PCSK9 both alone and when paired with CM3 but not with CM2. We propose that, in the ΔCM3 mutant, where CM1 pairs with CM2, the structurally less stable CM2 (in which strand β4 is not stabilized by a disulfide bond and is thus disordered) distorts the structure of CM1 or the interaction of CM1 with the rest of the protein including CA, therefore impairing protein secretion. It should also be pointed out that secretion is required (but not sufficient) for PCSK9 CD deletion mutants to acquire LDLR-reducing activity. Our data show that whereas ΔCD did not mediate LDLR degradation, this effect was normal in ΔCM2 and reduced by 50% in ΔCM23, suggesting that CM1 is required for the LDLR-reducing activity of secreted PCSK9. Additionally, ΔCM2 maintained almost the full capacity to mediate LDLR degradation. CM2 contains several histidine residues thought to play a critical role in the pH-dependent PCSK9-LDLR interaction and is considered as a potential target for drug design (20, 22). However, our finding that deletion of CM2 did not impair any of PCSK9 functions suggests that the histidine residues in CM2 are not critical for function. Asn-533, part of CM2 and the only glycosylation site in PCSK9, has been found not to be involved in the LDLR-reducing function of PCSK9 (11); our data support this conclusion.
Elegant studies have shown previously that coexpressed PD trans-acts on PD-deficient PCSK9 and enables the latter to be secreted (27, 28). We clearly confirmed these results in this study. Furthermore, our results show that PD in ΔCD, but not that in full-length PCSK9, also allowed secretion of a PD-free PCSK9 protein. These results are unexpected, as PD in full-length PCSK9 and ΔCD should be associated with their own intramolecular CA. A likely explanation is that an interaction between PD and CD is essential for PCSK9 secretion. We speculate that a trans-acting interaction between PD in ΔCD and CD in ΔPD enables chimeric PD-ΔPD protein secretion. Because PD in full-length PCSK9 associates with its own CD, it is not available to interact with CD in ΔPD and thus cannot trans-activate the secretion of ΔPD. The findings obtained upon cotransfection of CD module deletion mutants with ΔPD provide further insight into the potential PD-CD interaction. All of the single or dual module deletion mutants increased ΔPD secretion to some extent. More strikingly, even mutants (ΔCM1, ΔCM3, and ΔCM13) that were not secreted were able to lend their PD to the ΔPD protein and cause its secretion. Furthermore, the ability of these mutants to enable ΔPD secretion was inversely correlated to their own ability to be secreted (ΔCM2 = ΔCM23 < ΔCM12 < ΔCM1 = ΔCM3 = ΔCM13). C679X, a secretion-defective mutant, enabled the secretion of ΔPD. This suggests that the defective secretion of C679X is due to an impaired intramolecular PD-CD interaction. In our cotransfection experiments, the discrete PD protein associated with and helped in the secretion of ΔPD. More interestingly, the discrete PD protein also associated with the discrete CD protein, although it did not affect the already efficient secretion of this domain construct. On the basis of these data, we propose the following. 1) There is an intra- and intermolecular interaction between PD and CD in PCSK9 along the secretory pathway. Early in this pathway, this interaction is more important than the previously reported PD-CA interaction and places PCSK9 in the appropriate secretion route, whereas at the later stage, the PD-CA interaction may allow the protein to adopt the final conformation for secretion. 2) All three modules of CD are involved in the interaction with PD, in the order CM1 > CM3 > CM2. 3) When CM1 and/or CM3 is missing, CD does not interact with PD efficiently to ensure secretion. 4) When the CD module deletion mutants are coexpressed with ΔPD, these mutants lend their PD to the coexpressed protein early in the secretion route, leading to the assembly of a secretion-competent chimeric protein. However, if the intramolecular PD-CD interaction is still relatively strong, as in the case of ΔCM2, only a small amount of PD will be acquired by ΔPD, causing only minimal secretion. These assumptions should be confirmed by in vivo protein imaging techniques. A detailed understanding of the nature of the PD-CD interaction may guide the design of small molecules to inhibit the secretion and function of PCSK9.
Finally, we have shown that CD of PCSK9 binds to LDLR and that a discrete CD fragment competes with full-length PCSK9 for LDLR binding both in vitro and in vivo. Our results are in agreement with recent findings reported by Yamamoto et al. (31) showing that CD is involved in the PCSK9-LDLR interaction on the cell surface.
In conclusion, we have shown 1) a critical role of residues 61–70 in PCSK9 autoprocessing, 2) an interaction between PD and CD crucial to PCSK9 secretion and function, and 3) an inhibitory effect of isolated CD on PCSK9-induced LDLR degradation. Our results provide a rationale for development of mimetics of PCSK9 CD protein as PCSK9 inhibitors to diminish the LDLR-degrading activity of PCSK9.
Supplementary Material
Acknowledgment
We thank Dr. Jay Horton (University of Texas Southwestern Medical Center) for kindly sharing the anti-PCSK9 PD antibody.
This work was supported, in whole or in part, by National Institutes of Health Grants HL106845 (to S. F.) and HL106325 (to D. F.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2 and Tables 1 and 2.
- LDLR
- LDL receptor
- PD
- prodomain
- CA
- catalytic domain
- CD
- C-terminal domain
- CM
- C-terminal domain module.
REFERENCES
- 1. Seidah N. G., Benjannet S., Wickham L., Marcinkiewicz J., Jasmin S. B., Stifani S., Basak A., Prat A., Chretien M. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 928–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benjannet S., Rhainds D., Essalmani R., Mayne J., Wickham L., Jin W., Asselin M. C., Hamelin J., Varret M., Allard D., Trillard M., Abifadel M., Tebon A., Attie A. D., Rader D. J., Boileau C., Brissette L., Chrétien M., Prat A., Seidah N. G. (2004) J. Biol. Chem. 279, 48865–48875 [DOI] [PubMed] [Google Scholar]
- 3. Horton J. D., Cohen J. C., Hobbs H. H. (2007) Trends Biochem. Sci. 32, 71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kotowski I. K., Pertsemlidis A., Luke A., Cooper R. S., Vega G. L., Cohen J. C., Hobbs H. H. (2006) Am. J. Hum. Genet. 78, 410–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cohen J. C., Boerwinkle E., Mosley T. H., Jr., Hobbs H. H. (2006) N. Engl. J. Med. 354, 1264–1272 [DOI] [PubMed] [Google Scholar]
- 6. Cameron J., Holla Ø. L., Ranheim T., Kulseth M. A., Berge K. E., Leren T. P. (2006) Hum. Mol. Genet. 15, 1551–1558 [DOI] [PubMed] [Google Scholar]
- 7. Maxwell K. N., Breslow J. L. (2005) Curr. Opin. Lipidol. 16, 167–172 [DOI] [PubMed] [Google Scholar]
- 8. Abifadel M., Varret M., Rabès J. P., Allard D., Ouguerram K., Devillers M., Cruaud C., Benjannet S., Wickham L., Erlich D., Derré A., Villéger L., Farnier M., Beucler I., Bruckert E., Chambaz J., Chanu B., Lecerf J. M., Luc G., Moulin P., Weissenbach J., Prat A., Krempf M., Junien C., Seidah N. G., Boileau C. (2003) Nat. Genet. 34, 154–156 [DOI] [PubMed] [Google Scholar]
- 9. Miyake Y., Kimura R., Kokubo Y., Okayama A., Tomoike H., Yamamura T., Miyata T. (2008) Atherosclerosis 196, 29–36 [DOI] [PubMed] [Google Scholar]
- 10. Rashid S., Curtis D. E., Garuti R., Anderson N. N., Bashmakov Y., Ho Y. K., Hammer R. E., Moon Y. A., Horton J. D. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 5374–5379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao Z., Tuakli-Wosornu Y., Lagace T. A., Kinch L., Grishin N. V., Horton J. D., Cohen J. C., Hobbs H. H. (2006) Am. J. Hum. Genet. 79, 514–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cohen J., Pertsemlidis A., Kotowski I. K., Graham R., Garcia C. K., Hobbs H. H. (2005) Nat. Genet. 37, 161–165 [DOI] [PubMed] [Google Scholar]
- 13. Abifadel M., Rabès J. P., Devillers M., Munnich A., Erlich D., Junien C., Varret M., Boileau C. (2009) Hum. Mutat. 30, 520–529 [DOI] [PubMed] [Google Scholar]
- 14. Brown M. S., Goldstein J. L. (2006) Science 311, 1721–1723 [DOI] [PubMed] [Google Scholar]
- 15. Fazio S., Linton M. F. (2007) Curr. Opin. Lipidol. 18, 387–388 [DOI] [PubMed] [Google Scholar]
- 16. Alborn W. E., Cao G., Careskey H. E., Qian Y. W., Subramaniam D. R., Davies J., Conner E. M., Konrad R. J. (2007) Clin. Chem. 53, 1814–1819 [DOI] [PubMed] [Google Scholar]
- 17. Seidah N. G. (2009) Expert Opin. Ther. Targets 13, 19–28 [DOI] [PubMed] [Google Scholar]
- 18. Mousavi S. A., Berge K. E., Leren T. P. (2009) J. Intern. Med 266, 507–519 [DOI] [PubMed] [Google Scholar]
- 19. Lilly S. M., Rader D. J. (2007) Curr. Opin. Lipidol. 18, 650–655 [DOI] [PubMed] [Google Scholar]
- 20. Cunningham D., Danley D. E., Geoghegan K. F., Griffor M. C., Hawkins J. L., Subashi T. A., Varghese A. H., Ammirati M. J., Culp J. S., Hoth L. R., Mansour M. N., McGrath K. M., Seddon A. P., Shenolikar S., Stutzman-Engwall K. J., Warren L. C., Xia D., Qiu X. (2007) Nat. Struct. Mol. Biol. 14, 413–419 [DOI] [PubMed] [Google Scholar]
- 21. Hampton E. N., Knuth M. W., Li J., Harris J. L., Lesley S. A., Spraggon G. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 14604–14609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Piper D. E., Jackson S., Liu Q., Romanow W. G., Shetterly S., Thibault S. T., Shan B., Walker N. P. (2007) Structure 15, 545–552 [DOI] [PubMed] [Google Scholar]
- 23. Betzel C., Gourinath S., Kumar P., Kaur P., Perbandt M., Eschenburg S., Singh T. P. (2001) Biochemistry 40, 3080–3088 [DOI] [PubMed] [Google Scholar]
- 24. Wright C. S., Alden R. A., Kraut J. (1969) Nature 221, 235–242 [DOI] [PubMed] [Google Scholar]
- 25. McPhalen C. A., James M. N. (1988) Biochemistry 27, 6582–6598 [PubMed] [Google Scholar]
- 26. Henrich S., Cameron A., Bourenkov G. P., Kiefersauer R., Huber R., Lindberg I., Bode W., Than M. E. (2003) Nat. Struct. Biol. 10, 520–526 [DOI] [PubMed] [Google Scholar]
- 27. McNutt M. C., Lagace T. A., Horton J. D. (2007) J. Biol. Chem. 282, 20799–20803 [DOI] [PubMed] [Google Scholar]
- 28. Li J., Tumanut C., Gavigan J. A., Huang W. J., Hampton E. N., Tumanut R., Suen K. F., Trauger J. W., Spraggon G., Lesley S. A., Liau G., Yowe D., Harris J. L. (2007) Biochem. J. 406, 203–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang D. W., Lagace T. A., Garuti R., Zhao Z., McDonald M., Horton J. D., Cohen J. C., Hobbs H. H. (2007) J. Biol. Chem. 282, 18602–18612 [DOI] [PubMed] [Google Scholar]
- 30. Kwon H. J., Lagace T. A., McNutt M. C., Horton J. D., Deisenhofer J. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 1820–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yamamoto T., Lu C., Ryan R. O. (2011) J. Biol. Chem. 286, 5464–5470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Holla Ø. L., Laerdahl J. K., Strøm T. B., Tveten K., Cameron J., Berge K. E., Leren T. P. (2011) Biochem. Biophys. Res. Commun. 406, 234–238 [DOI] [PubMed] [Google Scholar]
- 33. Graham M. J., Lemonidis K. M., Whipple C. P., Subramaniam A., Monia B. P., Crooke S. T., Crooke R. M. (2007) J. Lipid Res. 48, 763–767 [DOI] [PubMed] [Google Scholar]
- 34. Frank-Kamenetsky M., Grefhorst A., Anderson N. N., Racie T. S., Bramlage B., Akinc A., Butler D., Charisse K., Dorkin R., Fan Y., Gamba-Vitalo C., Hadwiger P., Jayaraman M., John M., Jayaprakash K. N., Maier M., Nechev L., Rajeev K. G., Read T., Röhl I., Soutschek J., Tan P., Wong J., Wang G., Zimmermann T., de Fougerolles A., Vornlocher H. P., Langer R., Anderson D. G., Manoharan M., Koteliansky V., Horton J. D., Fitzgerald K. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 11915–11920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chan J. C., Piper D. E., Cao Q., Liu D., King C., Wang W., Tang J., Liu Q., Higbee J., Xia Z., Di Y., Shetterly S., Arimura Z., Salomonis H., Romanow W. G., Thibault S. T., Zhang R., Cao P., Yang X. P., Yu T., Lu M., Retter M. W., Kwon G., Henne K., Pan O., Tsai M. M., Fuchslocher B., Yang E., Zhou L., Lee K. J., Daris M., Sheng J., Wang Y., Shen W. D., Yeh W. C., Emery M., Walker N. P., Shan B., Schwarz M., Jackson S. M. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 9820–9825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Duff C. J., Scott M. J., Kirby I. T., Hutchinson S. E., Martin S. L., Hooper N. M. (2009) Biochem. J. 419, 577–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ni Y. G., Condra J. H., Orsatti L., Shen X., Di Marco S., Pandit S., Bottomley M. J., Ruggeri L., Cummings R. T., Cubbon R. M., Santoro J. C., Ehrhardt A., Lewis D., Fisher T. S., Ha S., Njimoluh L., Wood D. D., Hammond H. A., Wisniewski D., Volpari C., Noto A., Lo Surdo P., Hubbard B., Carfí A., Sitlani A.(2010) J. Biol. Chem. 285, 12882–12891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fan D., Yancey P. G., Qiu S., Ding L., Weeber E. J., Linton M. F., Fazio S. (2008) Biochemistry 47, 1631–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang D. W., Garuti R., Tang W. J., Cohen J. C., Hobbs H. H. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 13045–13050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nassoury N., Blasiole D. A., Tebon Oler A., Benjannet S., Hamelin J., Poupon V., McPherson P. S., Attie A. D., Prat A., Seidah N. G. (2007) Traffic 8, 718–732 [DOI] [PubMed] [Google Scholar]
- 41. Hooper A. J., Marais A. D., Tanyanyiwa D. M., Burnett J. R. (2007) Atherosclerosis 193, 445–448 [DOI] [PubMed] [Google Scholar]
- 42. Berge K. E., Ose L., Leren T. P. (2006) Arterioscler. Thromb. Vasc. Biol. 26, 1094–1100 [DOI] [PubMed] [Google Scholar]
- 43. Benjannet S., Rhainds D., Hamelin J., Nassoury N., Seidah N. G. (2006) J. Biol. Chem. 281, 30561–30572 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.