Highlights
* The LPP was examined during a cognitive reappraisal task in 5- to 7-year-olds. * LPP amplitudes were larger for unpleasant versus neutral stimuli. * There were no significant effects of reappraisal on the LPP. * Larger LPPs to unpleasant versus neutral stimuli correlated with greater anxiety. * Young children may still be developing the ability to use cognitive reappraisal.
Keywords: Emotion regulation, Reappraisal, ERPs, Late positive potential, Children, Anxiety
Abstract
Cognitive emotion regulation strategies, such as reappraising the emotional meaning of events, are linked to positive adjustment and are disrupted in individuals showing emotional distress, like anxiety. The late positive potential (LPP) is sensitive to reappraisal: LPP amplitudes are reduced when unpleasant pictures are reappraised in a positive light, suggesting regulation of negative emotion. However, only one study has examined reappraisal in children using the LPP. The present study examined whether directed reappraisals reduce the LPP in a group of 5- to 7-year-olds, and correlate with individual differences in fear and anxiety. EEG was recorded from 32 typically developing children via 64 scalp electrodes during a directed reappraisal task. Mothers reported on child anxiety. Fearful behavior was observed. As predicted, LPP amplitudes were larger to unpleasant versus neutral pictures; counter to predictions, the LPP was not sensitive to reappraisal. The degree to which unpleasant versus neutral pictures elicited larger LPPs was correlated with greater anxiety and fear. Results suggest that reappraisal in young children is still developing, but that the LPP is sensitive to individual differences related to fear and anxiety. The utility of the LPP as a measure of cognitive emotion regulation and emotional processing biases in children is discussed.
1. Introduction
Emotion regulation involves the ability to change our experience of and attention to emotional information (Gross and Thompson, 2007). The study of emotion regulation in adults often focuses on the use of cognitive emotion regulation strategies (e.g., Moser et al., 2006, Moser et al., 2009, Hajcak et al., 2009, Hajcak and Nieuwenhuis, 2006, Krompinger et al., 2008). One such strategy, reappraisal, serves to change the emotional meaning and significance of an event or stimulus (Foti and Hajcak, 2008, Gross and John, 2003, Hajcak and Nieuwenhuis, 2006, Kalisch et al., 2006, Ochsner et al., 2002, Ochsner and Gross, 2005). For example, when viewing a picture of a man who is lying in a hospital bed, one could reappraise this picture in a more positive light by describing the man as a person who was sick, but is now on his way to making a full recovery. Difficulty in the ability to use reappraisal to modify the significance and meaning of unpleasant emotional material has been linked to problems with adjustment, such as mood disruptions (e.g., Gross and John, 2003). Other emotion regulation strategies, such as suppression, appear to be inferior to reappraisal because they are response-focused and thus occur after an emotional reaction has already occurred (Gross, 1998). Indeed, suppression compared to reappraisal is associated with increased cognitive and physiological “load”, impaired memory, and poorer adjustment (Gross and John, 2003, Richards and Gross, 2000, Gross and Levenson, 1993, Gross and Levenson, 1997).
Neuroscience studies with adults have identified neural mechanisms supporting the ability to reappraise. While reappraising, adults show increased activity in areas of the prefrontal cortex (PFC), including the dorsal lateral prefrontal cortex and dorsal anterior cingulate cortex; in contrast, subcortical regions associated with emotional arousal and emotional processing such as the amygdala are less active (Ochsner et al., 2002, Phan et al., 2005, Urry et al., 2006). This suggests that prefrontally mediated cognitive control resources are being recruited to down-regulate the experience of negative emotion. To date, the single fMRI study examining reappraisal in children (aged 5-10) shows similar patterns: when asked to reappraise sad stimuli, portions of the lateral and medial PFC were bilaterally activated in addition to the right ACC and right ventral lateral PFC activation (Levesque et al., 2004). These neuroimaging studies identify neural regions involved in emotion regulation with excellent spatial resolution; however, key emotion regulation processes may occur on the order of milliseconds and thus emerge more rapidly than can be detected via fMRI. Scalp-recorded event related potentials (ERPs) have excellent temporal resolution on the order of milliseconds, and thus are capable of capturing such rapid changes resulting from the use of cognitive emotion regulation strategies.
One ERP that is particularly well-suited for examining reappraisal is the late positive potential (LPP). The LPP emerges around 200-300 ms following stimulus onset and tends to be maximal at centro-parietal recording sites (e.g., Cuthbert et al., 2000, Foti and Hajcak, 2008, Hajcak and Nieuwenhuis, 2006). The LPP reflects increased processing of and facilitated attention to emotional stimuli, such that LPP amplitudes are larger to emotional versus neutral stimuli like pictures, faces, and words (Cuthbert et al., 2000, Schupp et al., 2000, Schupp et al., 2004, Herbert et al., 2008). Several studies have shown that the LPP is sensitive to emotion regulation strategies such as directed reappraisal (Foti and Hajcak, 2008, MacNamara et al., 2011) and directions to increase and decrease subjective emotional responses (Hajcak and Nieuwenhuis, 2006, Moser et al., 2006, Moser et al., 2009, Krompinger et al., 2008). Specifically, LPP amplitudes are reduced when adults are asked to reappraise an unpleasant stimulus in a more positive light compared to a negative appraisal (Foti and Hajcak, 2008), or when instructed to use cognitive strategies to decrease versus increase subjective emotional responses to pleasant (Krompinger et al., 2008) and unpleasant stimuli (e.g., Moser et al., 2006, Moser et al., 2009, Hajcak and Nieuwenhuis, 2006). These changes in the LPP, in adults, are correlated with reduced subjective arousal (Hajcak and Nieuwenhuis, 2006), suggesting the effective down-regulation of emotion, and are independent of factors that might increase cognitive load, such as task difficulty (Hajcak et al., 2006). A recent study also examined the LPP 30 min after a directed reappraisal task and found that LPP amplitudes were larger to reappraised stimuli versus those that were not reappraised (MacNamara et al., 2011). Therefore, the LPP is sensitive to changes in emotional processing that result from the use of cognitive emotion regulation strategies like reappraisal.
To date, however, only two published studies have examined the LPP in response to complex emotional pictures in children (Dennis and Hajcak, 2009, Hajcak and Dennis, 2009) and one study examined an LPP-like ERP component, the P400, in children, in response to emotional faces (Leppanen et al., 2007). In research from our lab, we confirmed that the LPP is sensitive to emotional stimuli in children: LPP amplitudes were larger to pleasant and unpleasant as compared to neutral stimuli in 5- to 10-year-old children (Hajcak and Dennis, 2009). In the other study (Dennis and Hajcak, 2009), we demonstrated that 5- to 10-year-old children were able to use directed reappraisal strategies to modulate the LPP, similar to effects found in adults: LPP amplitudes were reduced to unpleasant stimuli when reappraisal versus negative stories were provided. However, effects of reappraisal strategy emerged around 600–1000 ms, which is later than the timing documented in studies with adults (Foti and Hajcak, 2008, MacNamara et al., 2009). Moreover, reappraisal was not effective in modulating the LPP in younger girls in the sample (ages 5–6). In fact, younger girls in this study showed the opposite effect such that LPP amplitudes were larger that were reappraised versus those with negative stories. For the sample as a whole, LPP amplitudes were reduced in the reappraisal versus negative story condition (indicating effective down-regulation of the LPP via reappraisal) and were associated with fewer anxious/depressed symptoms while reappraisals (of unpleasant stimuli) were associated with more symptoms.
This first study (Dennis and Hajcak, 2009) examining reappraisal and the LPP in children therefore suggests that children are able to effectively use reappraisal to modulate how they process unpleasant emotional stimuli as measured via the LPP, that effects may vary by age and gender, and that these changes in the LPP correlate with individual differences in mood and anxiety. Several methodological issues in the Dennis and Hajcak (2009) study, however, limited our ability to fully interpret results. For example, this previous study included a somewhat small sample size (N = 20) and lacked a neutral baseline condition. Additionally, the methodological parameters of Dennis and Hajcak (2009) were meant to bolster young children's ability to reappraise, a method that differs from the adult literature on reappraisal: reappraisal stories followed presentation of emotional pictures, whereas in the adult literature reappraisals always precede emotional stimuli given that reappraisal is conceptualized as an antecedent-focused strategy that occurs prior to an emotional reaction.
Thus, the goal of the present study was to add to the literature on reappraisal and the LPP in children and to clarify findings from Dennis and Hajcak (2009) by increasing the sample size, designing the reappraisal paradigm to mirror paradigms used in the adult literature, and focusing on younger age range of study (around ages 5–6) in which effects of reappraisal were unclear. This age period, when children are in kindergarten or first grade is a particularly important period in the development of cognitive emotion regulation. First, factors such as ongoing brain maturation, exposure to peers, and participation in structured education support more sophisticated cognitive and emotional abilities (Thompson and Goodman, 2010). Moreover, more complex information processing skills allow children at this age to generate emotion regulation strategies in more creative and independent ways (Garber et al., 1991, Gross and Thompson, 2007). The literature on the behavioral development of emotion regulation shows that clearly identifiable, effortful emotion regulation strategies can be detected in toddlers and preschoolers (Cole et al., 2004, Kopp, 1989); that children as young as 3 years identify effective behavioral and cognitive emotion regulation strategies, and that this understanding correlates with social skills (Dennis and Kelemen, 2009); and that by the end of early childhood, children are aware of how they use cognitive emotion regulation strategies (Davis et al., 2010). However, very few studies have used a neural measure to strengthen the inference that young children can effectively use cognitive strategies such as reappraisal to down-regulate emotion.
Thus, the present study focused on the question of whether the directed use of reappraisal results in the effective down-regulation of the LPP in young children around the time of kindergarten and first grade. We targeted typically developing children in order to identify normative patterns given the nascent stage of the research. To provide converging evidence that the LPP is capturing meaningful individual differences related to emotion, a second goal of the present study was to examine whether the LPP correlates with individual differences in fearful behavior, anxious mood, and temperamental fear. Previous studies suggest that the LPP might be particularly sensitive to fear- and anxiety-related characteristics. For example, Dennis and Hajcak (2009) showed that larger LPP amplitudes during directed reappraisal (i.e., during a condition in which LPP amplitudes should be reduced) were associated with greater anxious-depressed symptoms in children. In a study with adults, MacNamara and Hajcak (2009) demonstrated that biased processing of unpleasant versus neutral stimuli (i.e., larger LPP amplitudes to unpleasant versus neutral emotional pictures) during a spatial attention task was related to higher state anxiety in adults. To examine whether such associations would emerge in the present study, we included measures reflecting fearful and anxious traits (anxiety and temperamental fear) as well as states (observed fearful behavior).
We tested two hypotheses. First, LPP amplitudes will be reduced when reappraisals versus negative stories precede unpleasant stimuli. We will explore gender differences in the effects of condition given previous documentation of gender differences (Dennis and Hajcak, 2009). If directed reappraisals are not effective in reducing the LPP in this young age group, another potentially important LPP measure is of biased processing of unpleasant versus neutral stimuli (i.e., larger LPP amplitudes to unpleasant versus neutral emotional pictures; see MacNamara and Hajcak, 2009). Thus, the second exploratory hypothesis was that increased fear- and anxiety-related states and traits will be correlated with (a) reduced effective down-regulation of the LPP via reappraisal and (b) increased LPP amplitudes to unpleasant compared to neutral stimuli.
2. Methods
2.1. Participants
Thirty-four children (13 girls) between the ages of five and seven were recruited from in and around New York City. Of the 34 children that were recruited, two were excluded due to excessive blinks and movement artifacts. Therefore a sample of 32 children was used for analyses. Of the 32 children used in this sample, there were six children who were 5 years old, 24 children who were 6 years old and two children who had just turned 7 years old (M = 76.56, SD = 6.17, range = 60.00–84.00 in months). Race and ethnicity for the children were collected via maternal report and were as follows: Caucasian: 10, African American, 13, Hispanic/Latino: 4, Asian: 2, more than one race: 3. Participants were compensated $100 for their time and children were given astronaut ice-cream.
2.2. Stimulus materials
Stimuli were 30 unpleasant1 and 15 neutral2 pictures from the International Affective Picture System (IAPS; Lang et al., 2005). The unpleasant stimuli had a mean valence of 3.31 (SD = 0.73) and arousal of 5.78 (SD = 0.70). The neutral stimuli had a mean valence of 5.12 (SD = 0.68) and arousal of 2.81 (SD = 0.71). Although valence and arousal ratings both use a scale of one to nine, lower ratings for valence correspond to more unpleasant ratings, whereas lower ratings for arousal indicate less arousing stimuli.
Presentation software (Version 2, Neurobehavioral Systems, Inc., Albany, CA) was used to program the directed reappraisal task. All stimuli were presented using an IBM computer and 17″ monitor. Children were seated 65 cm from the computer monitor during the directed reappraisal task.
Analyses were conducted using PASW version 18 using general linear model and correlation software.
2.3. Procedures and measures
Prior to beginning the EEG portion of the visit, informed consent was obtained from the parent and child. All parents and children consented to participation. Children were informed they could take a break at any time.
Participants in this study are a part of a larger study examining emotional processing and emotion regulation in children. The 34 children that participated in the present study also completed a passive viewing task prior to the directed reappraisal task. All participants first completed the EEG portion of the visit (passive viewing task followed by the directed reappraisal task) and then completed the black box task and other observation-based tasks after a short break to clean up. The data from the passive viewing task are presented in a separate paper (Solomon et al., 2011). The entire visit lasted approximately 2.5 h.
2.3.1. Anxiety
All parents completed the Child Behavior Checklist (Achenbach and Rescorla, 2000, Achenbach and Rescorla, 2001), which measures symptoms of social, emotional and behavioral problems. This questionnaire consists of 100 items for 5 year old and 112 items for children ages 6 and older, from which parents report on their child's behavior using a three point Likert scale. DSM anxiety T-scores’ were computed for both ages separately (6–7 year olds: α = .74; 5 year olds: α = .36). Items that are included in the DSM anxiety T-score include “clings to adults or too dependent” or “doesn't want to go out of home”.
2.3.2. Temperamental fear
The Children's Behavior Questionnaire was administered to assess temperamental fear (CBQ; Rothbart et al., 2001). The CBQ is a 195 item questionnaire used to measure various facets of child temperament. All items on this questionnaire use a seven point Likert scale. The fear scale (α = .70) consists of 12 items which measures negative affect, feelings of uneasiness and worry in response to fear-inducing or novel situations.
2.3.3. Fearful behavior
Following the EEG portion of the visit, children completed the black box task taken from the laboratory temperament assessment battery (LabTAB; Goldsmith et al., 1993). This task is designed to measure inhibited and fearful behavior. In this task, children are presented with an opaque black box and told that there is something scary inside. They are then asked to place their hand in the box. The latency of the child placing their hand into the box was used as the measure of fearful behavior, with longer latencies indicating more fear.
2.4. Directed reappraisal
The directed reappraisal task occurred after EEG set up. Children were given verbal instructions about what the task entailed. The following was read to each child:
“For our next game we're going to see some pictures. Listen to the stories and think of the pictures so that they match the stories. Remember to stay still and look at the screen. Try to match the story to the picture. If you need to tell me anything, wait until the game is over to tell me.”
Children were reminded to not blink or move around too much during when viewing the pictures and listening to the stories. EEG was recorded continuously for the entire task.
There were 30 unpleasant stimuli, taken from the IAPS. Half of the stimuli were presented with a negative story prior to seeing the stimuli and half were presented with a reappraisal story. The negative and reappraisal stories were the same as those used in Dennis and Hajcak (2009). The type of story presented on the first trial was counterbalanced across participants.
Participants heard each story followed by a 500 ms delay prior to stimulus onset. Stimuli were then presented for 2000 ms with a 1500 ms inter-trial interval between each stimulus and the next story. After all 30 trials were presented participants were given a break and then continued to the second block. The second block presented all of the same stimulus/story pairings in order to achieve the greatest number of trials possible for the LPP.
Following the directed reappraisal portion of the experiment, participants then completed a neutral block. The neutral block always followed the directed reappraisal block. Within this neutral block, participants were given a neutral story prior to viewing a neutral picture (see Appendix A) which served as a neutral baseline condition. There was 500 ms delay between each story and the onset of the stimulus. Similar to the directed reappraisal block, stimuli were presented for 2000 ms with a 1500 ms inter-trial interval from the offset of the stimulus and onset of the next story.
2.5. EEG recording
Electroencephalography (EEG) was recorded from 64 Ag/AgCL scalp electrodes, using the ActiveTwo Biosemi System (Biosemi, Amsterdam, Netherlands) during the directed reappraisal task. The electrooculogram (EOG) generated from blinks and eye movements was recorded from four electrodes: Horizontal EOG was recorded from one electrode placed 1 cm to the left of the left eye and another placed 1 cm to the right of the right eye. Vertical EOG was recorded from one electrode placed 1 cm above the left eye and another placed below the left eye. As per Biosemi's design, during acquisition the ground electrode was formed using the Common Mode Sense active electrode and Driven Right Leg passive electrode.
2.6. EEG data reduction
EEG and EOG signals were digitized on a laboratory computer using ActiView software (BioSemi). The EEG was sampled at 512 Hz. Brain Vision Analyzer (Version 2.2, GmbH, Munich, Germany) was used to process the data offline and to generate the LPP. Data were band-pass filtered with cutoffs between .1 Hz3 and 30 Hz and referenced to the average of the left and right mastoids. EEG was corrected for blinks and artifacts using the method developed by Gratton et al. (1983). Artifact were identified using the following criteria: any data with voltage steps exceeding 75 μV, changes within a segment that were greater than 200 μV, amplitude differences greater than ±120 μV within a segment, and activity lower than .2 μV per 100 ms were considered artifacts and excluded from analyses.
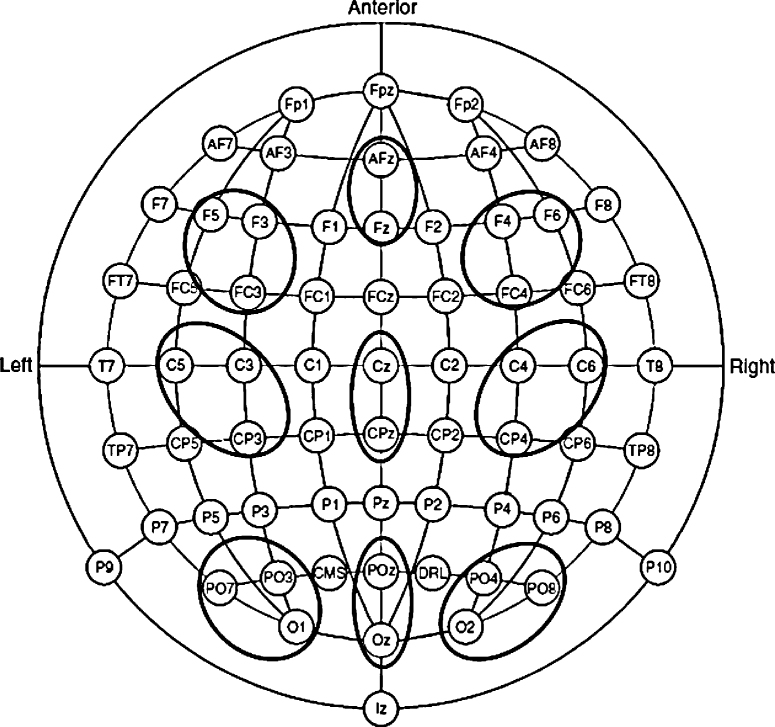
The EEG was segmented for each trial 400 ms before each picture and continuing for 2000 ms. The 400 ms window prior to the picture served as the baseline. Mean LPP amplitudes were computed within each of the following time windows based on visual inspection of the data: early (300–700 ms), middle (700–1200 ms) and late (1200–2000 ms). The LPP was averaged in three regions: posterior, central, and anterior. Each region included clusters in the right, midline, and left. Because there were no significant differences between these clusters, Mean LPP amplitudes were computed for each region averaging across the clusters (see Fig. 1), separately by window: posterior: PO4, PO8, O2, Oz, POz, PO3, PO7, and O1; central: C4, C6, CP6, Cz, CPz, C3, C5, and CP5; and anterior: FC4, F4, F6, Fpz, AFz, FC3, F3, and F5.
Fig. 1.
Electrode clusters used to quantify mean LPP amplitudes in posterior, central and anterior regions.
3. Results
3.1. Descriptive statistics
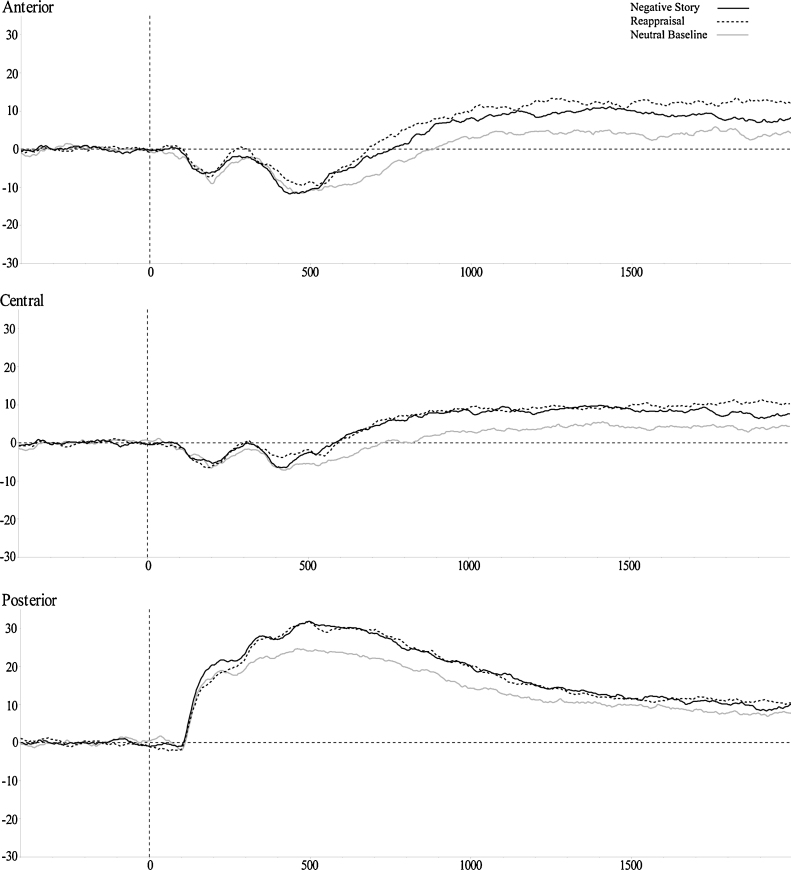
Fig. 2 presents the stimulus-locked ERPs at posterior, central, and anterior recording sites, separately. Mean LPP amplitudes for each condition, region and window are presented in Table 1. LPP amplitudes during the first and second presentations of the stimuli did not differ, and thus grand-average waveforms represent the average LPP across presentations. Descriptive statistics for anxiety, temperamental fear, and fearful behavior are presented in Table 2.
Fig. 2.
LPP amplitudes in each region and cluster for negative and reappraisal as well as the neutral baseline condition. LPP amplitudes were larger for negative and reappraisal versus the neutral baseline condition in all three regions. There were no significant differences between negative and reappraisal stories in any of the regions.
Table 1.
Mean LPP amplitudes for each region and time window.
| Condition |
|||
|---|---|---|---|
| Negative stories | Reappraisal | Neutral baseline | |
| Region | |||
| Anterior | |||
| Early | −6.01 (6.54) | −4.88 (6.39) | −8.48 (7.62) |
| Middle | 7.38 (7.32) | 7.79 (7.43) | 2.41 (6.63) |
| Late | 8.67 (7.47) | 11.41 (8.52) | 5.09 (6.47) |
| Central | |||
| Early | 0.04 (6.61) | 1.50 (5.77) | −3.79 (6.37) |
| Middle | 7.28 (6.88) | 8.22 (6.83) | 2.28 (5.43) |
| Late | 6.95 (7.65) | 9.55 (7.79) | 3.93 (4.82) |
| Posterior | |||
| Early | 22.00 (6.74) | 22.78 (7.99) | 15.62 (6.29) |
| Middle | 14.55 (8.21) | 15.05 (7.25) | 9.23 (5.70) |
| Late | 7.68 (8.24) | 8.85 (6.95) | 6.04 (5.53) |
Note: Standard deviations are in parentheses.
Table 2.
Descriptive statistics for maternal report of anxiety and temperamental fear, and observed fearful behavior.
| Anxiety | Temperamental fear | Observed fear | |
|---|---|---|---|
| Boys | 57.47 (7.36) | 4.60 (.96) | 2.04 (1.20) |
| Girls | 55.00 (6.48) | 4.58 (.86) | 1.54 (1.03) |
| Total | 56.62 (7.06) | 4.59 (.91) | 1.87 (1.15) |
| Range | 50.00–77.00 | 2.58–6.18 | 1.00–4.00 |
Note: Standard deviations are in parentheses. The range for each measure represents the range for the sample as a whole.
3.2. Effects of condition4
The first hypothesis was that LPP amplitudes would be reduced for unpleasant pictures following directed reappraisals versus negative stories. Given previous research (Dennis and Hajcak, 2009), we considered the possibility that in this age group (ages 5–7) effects of condition type would not be robust, but that a significant difference between emotional versus neutral stimuli (greater LPP amplitudes for unpleasant versus neutral pictures) would emerge. To examine this question, we conducted a 3 (condition: negative story, reappraisal, and neutral baseline) × 3 (window: early 300–700 ms, middle 700–1200 ms, and late 1200–2000 ms) × 2 (gender) repeated measures ANOVA, separately for each region (posterior, central, and anterior). Analyses were Greenhouse–Geisser corrected when analyses violated assumptions of sphericity.
3.2.1. Posterior region
The LPP was sensitive to condition, F(2,60) = 11.85, p < .001, η2 = .28, such that the negative stories, t(31) = 3.97, p < .001, and reappraisals, t(31) = 5.01, p < .001, generated larger LPP amplitudes than the neutral baseline condition. LPP amplitudes for the negative stories versus reappraisals, however, did not differ, t(31) = −.80, p = .42.
Effects of window, F(1.25,37.55) = 92.81, p = < .001, η2 = .75, show that the LPP was maximal in the early window as compared to the middle, t(31) = 7.85, p < .001, and late, t(31) = 11.20, p < .001, windows. Additionally, LPP amplitudes were larger in the middle versus late window, t(31) = 11.27, p < .001. Finally, the significant interaction between condition and window, F(2.80,84.25) = 4.36, p = .008, η2 = .12, showed that while the main effect of condition (LPP negative story and LPP reappraisal > LPP neutral baseline) was significant in the early and middle windows, all ps < .001, it did not reach significance in the late window. There were no significant gender effects.
3.2.2. Central region
The LPP was sensitive to condition, F(2,60) = 8.75, p < .001, η2 = .22: LPP amplitudes were larger for the negative stories, t(31) = 2.86, p = .007, and reappraisals, t(31) = 4.86, p < .001, as compared to the neutral baseline condition. There were no significant differences between the negative stories and reappraisals, t(31) = −1.45, p = .15. LPP amplitudes differed between windows, F(1.24,37.46) = 49.44, p < .001, η2 = .62, such that the LPP was larger in the middle, t(31) = −9.64, p < .001, and late, t(31) = −7.43, p < .001, as compared to the early window. There were no differences between the middle and late windows, t(31) = −1.65, p = .10. There were no significant gender effects or interaction effects.
3.2.3. Anterior region
As seen in the posterior and central regions, the anterior region showed the same effect of condition, F(2,60) = 6.44, p = .002, η2 = .17: negative stories, t(31) = 2.47, p = .01, and reappraisals, t(31) = 4.35, p < .001, yielded larger LPP amplitudes than the neutral baseline. The negative stories and reappraisals did not differ, t(31) = −1.24, p = .22. Effects of window, F(1.54,46.26) = 190.00, p < .001, η2 = .86, showed that LPP amplitudes were larger in the late window versus the middle, t(31) = −4.01, p < .001, and early windows, t(31) = −15.76, p < .001. LPP amplitudes were also larger in the middle versus early window t(31) = −18.10, p < .001. There were no significant gender effects or interaction effects.
3.2.4. Summary
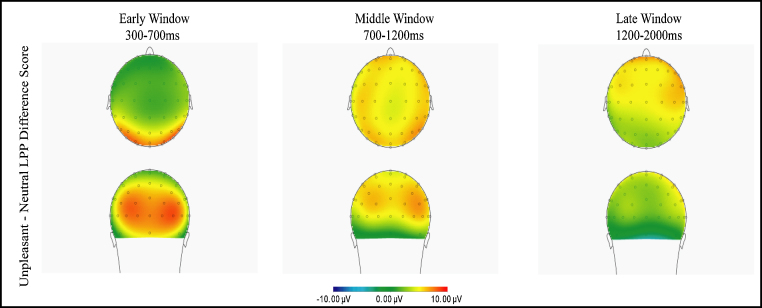
Across all three regions, negative stories and reappraisals generated larger LPP amplitudes as compared to the neutral baseline condition. There were no significant differences in LPP amplitudes for the negative stories versus reappraisals. The LPP appeared to be maximal in the posterior region in the early window, in the central region in the middle and late windows, and in the anterior region in the late window. Thus, emotional content, rather than reappraisal, modulated the LPP in this group of 5- to 7-year-olds. Fig. 3 shows the scalp topography for the unpleasant–neutral stimuli (LPP amplitudes for negative stories and reappraisals are averaged).
Fig. 3.
Scalp topography of the LPP (unpleasant LPP–neutral LPP), where the negative story and reappraisal conditions are averaged together to create an average unpleasant LPP. The left panel represents the early window, middle panel represents the middle window, and right panel represents the late window.
3.3. The LPP and individual differences
Next, because there were no effects of condition (negative stories versus reappraisals) on the LPP, we were interested in examining whether the differences between the two story conditions and the neutral baseline were associated with individual differences in fear- and anxiety-related states and traits. We predicted that the LPP difference scores (LPP negative stories–LPP neutral baseline and LPP reappraisal–LPP neutral baseline), indicating increased processing of negative versus neutral pictures, would be positively correlated with fear and anxiety in this normatively developing group of children. To test this hypothesis, we conducted correlations between LPP difference scores in the three region/windows in which LPP scores were maximal (the posterior region/early window, central region/middle window, and anterior region/late window) and three individual differences measures: anxiety, temperamental fear, and fearful behavior (see Table 3).
Table 3.
Correlations between LPP difference scores and individual differences in fear and anxiety.
| Difference score | Region/window | Anxiety (N = 32) | Temperamental fear (N = 31) | Fearful behavior (N = 32) |
|---|---|---|---|---|
| Negative stories–neutral baseline | Posterior/early | .34* | .11 | −.24 |
| Central/middle | .20 | .002 | −.05 | |
| Anterior/late | .08 | .12 | .24 | |
| Reappraisal–neutral baseline | Posterior/early | .39* | −.03 | −.29 |
| Central/middle | .13 | −.27 | .004 | |
| Anterior/late | .27 | .21 | .35* |
Note: Negative stories refer to the negative story condition whereas reappraisal refers to the reappraisal stories of unpleasant stimuli.
p < .05.
3.3.1. Anxiety5
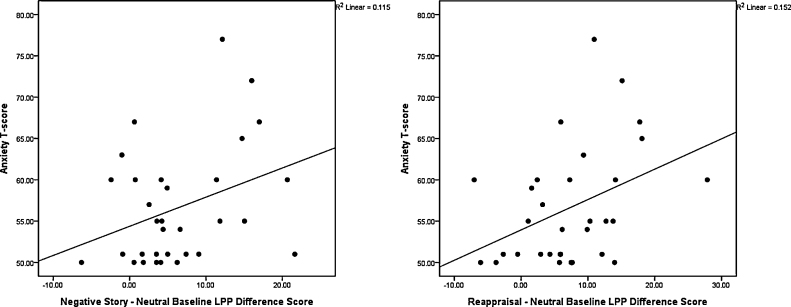
Larger LPP difference scores in the posterior region/early window for both difference scores (reappraisal–neutral baseline and negative stories–neutral baseline) were associated with higher anxiety (Fig. 4). No other significant correlations emerged.
Fig. 4.
Correlations between anxiety T-scores on the CBCL and LPP difference scores in the early window of the posterior region. A bias towards unpleasant stimuli (larger LPP amplitudes) was associated with greater anxiety T-scores.
3.3.2. Temperamental fear6
There were no significant correlations between the LPP and temperamental fear.
3.3.3. Fearful behavior7
One significant correlation emerged for fearful behavior: larger LPP difference scores in the anterior region/late window (reappraisal–neutral baseline) were associated with more fearful behavior (longer latencies to place one's hand into the black box). No other significant correlations emerged.
4. Discussion
The present study was only the second to examine the LPP in children during a cognitive emotion regulation task. Results suggest that children as young as 5 or 6 are still developing in their ability to use directed reappraisal to reduce the LPP in response to unpleasant stimuli. Despite this null finding, the magnitude of the LPP to unpleasant compared to neutral pictures – as a measure of biased or preferential processing of unpleasant pictures – was significantly correlated with anxiety and fearful behavior in this group of typically developing children. Taken together, these findings suggest that children in kindergarten and first grade are still developing their ability to use sophisticated cognitive emotion regulation strategies like reappraisal, and that the LPP is a sensitive measure of emotional processing tendencies that may reflect vulnerabilities for fear and anxiety.
The effects of directed reappraisal on the LPP are robust in adults (e.g., Foti and Hajcak, 2008, MacNamara et al., 2011). In one study, we also documented effects of reappraisal on the LPP in children aged 5–10, although effects were sensitive to age and gender (Dennis and Hajcak, 2009). The finding in the present study that reappraisal did not modulate the LPP suggests that the use of reappraisal is still a developing ability in 5- to 7-year-olds. Given neuroimaging literature documenting that regions of the prefrontal cortex are activated when using emotion regulation strategies (e.g., Ochsner et al., 2002, Urry et al., 2006), and given the ongoing development of these prefrontal cortical regions during childhood and adolescence (Casey et al., 2000, Segalowitz and Davies, 2004), it is likely that the ability to effectively use cognitive emotion regulation strategies is unstable and still developing in early childhood. It will be important for future research to chart the development of cognitive emotion regulation in relation to neural and cognitive development in order to identify sensitive or critical periods in the link between key developmental attainments and emotion regulatory abilities.
Another important goal of the present study was to examine the time course and scalp topography of the LPP in children. Consistent with our previous study (Dennis and Hajcak, 2009), the LPP emerged around 300 ms post stimulus and showed predictable topographical changes such in posterior regions, early portions of the LPP were maximal (300–700 ms), whereas in central and anterior regions, the LPP was maximal in middle to late windows of the LPP (700–1200 and 1200–2000 ms, respectively). This suggests that over the time course of the LPP, the earliest time window may primarily reflect initial detection and visual processing operations, whereas at later time windows, a range of other more elaborated processes may underlie the LPP (i.e., prefrontally mediated working memory and cognitive control functions). The time course of the LPP is also important to track in order to identify when emotion regulation modulates the LPP, whether the timing of effects differ for distinct cognitive strategies (Thiruchselvam et al., 2011), and whether effects differ across developmental periods (Dennis and Hajcak, 2009).
Although the LPP was not sensitive to reappraisal in this group of children, we examined the possibility that increased processing of unpleasant versus neutral pictures might reflect preferential processing of unpleasant stimuli relating to individual differences in anxiety and fear (Dennis and Hajcak, 2009, MacNamara and Hajcak, 2009). As predicted, we found that larger LPPs to unpleasant compared to neutral stimuli in posterior regions (early window) were correlated with greater anxiety. There were no associations between the LPP and temperamental fear, and the association between the LPP and fearful behavior was not robust (it failed to reach significance when 5- and 7-year-olds were removed). These patterns of correlation suggest that increased initial capture of attention (the early window) by unpleasant stimuli may reflect an emotional processing bias linked to anxiety-related problems. This finding is consistent with studies with adults (MacNamara and Hajcak, 2009, Mocaiber et al., 2009) showing that the LPP can serve as a sensitive measure of negativity biases, such as anxiety-related attentional biases towards threat (MacNamara and Hajcak, 2009). These early results suggest a future program of research with clinical populations in which one could examine relations between the LPP, behavioral measures of attentional biases, and the emergence of anxiety symptoms. Such research would be able to test the hypothesis the LPP is a viable biomarker for emotional processing biases that create risk for affective disorders.
Results are also relevant to an ongoing debate concerning whether anxiety is characterized by vigilance for threat and/or avoidance of threat (Matthews and Mackintosh, 1998). The present study shows that links between the LPP and anxiety symptoms were only significant for the early processing window, and that increased rather than decreased LPP amplitudes were correlated with greater symptoms of anxiety and fearful behavior. This would suggest that from a neurophysiological standpoint, the initial capture of attention, rather than avoidance, is correlated with the experience of anxiety and internalizing problems. We cannot draw conclusions about whether avoidance occurs at later stages of processing (beyond the 2000 ms stimulus duration).
Extending previous findings, such as the study by MacNamara and Hajcak (2009) which demonstrated that greater LPP amplitudes to unpleasant versus neutral stimuli were associated with greater state anxiety, results from the current study document that the LPP is also sensitive to trait-like individual differences in anxiety and internalizing problems. Whether the LPP reflects affective or behavioral states and traits may depend in part of the context of measurement. While the present study measured the LPP in an emotion regulation context, MacNamara and Hajcak (2009) measured the LPP during a decision making task with emotional stimuli. By measuring the LPP in an explicit regulatory context – the reappraisal task – the present study may have measured changes in affective processing that are directly relevant to the types of emotion dysregulatory symptoms that characterize internalizing problems like anxiety. Future research should directly compare the predictive utility of the LPP measured in distinct contexts in relation to processing biases and affective disruptions.
As this was only the second study to examine the LPP in the context of cognitive emotion regulation in children, it is important to note a few key differences between this study and the previous study (Dennis and Hajcak, 2009). One important methodological difference is that in Dennis and Hajcak (2009) the stimulus was presented for 2000 ms, followed by the story, and then the stimulus was presented again. In the present study the story was presented first, followed by the stimulus. The design of the present study is consistent with the five-stage model of emotion regulation (Gross, 1998, Gross and Thompson, 2007), in which reappraisal is conceived as an antecedent-focused strategy – that is, appraisal is employed before an emotional response is experienced. Dennis and Hajcak (2009) may have captured response-focused regulatory attempts as well, since the picture was shown before the directed reappraisal.
It is important to consider alternative explanations for why children in the present study did not show modulation of the LPP via reappraisal. One possibility is that children had difficulty holding the reappraisal in working memory, rather than them failing to perform the reappraisal. That is, if children were not able to remember the story once the picture appeared on the screen, they could not have successfully reappraised the pictures. An important future direction is to identify developmentally sensitive reappraisal paradigms that differ from the adult paradigm, similar to the methodological approach in Dennis and Hajcak (2009), in order bootstrap children's developing cognitive capabilities and to more closely mirror the ways in which reappraisal might be used in daily life with the support of caregivers, peers, and teachers.
Limitations of the present study include the lack of self-report data on children's mood. Thus, we are missing an important source of information on children's emotional experiences during the reappraisal task as well as on changes in children's subjective emotional arousal attributable to reappraisal. Also, although the stimuli used in this study have been normatively rated, participants in the present study did not subjectively rate the stimuli. Piloting showed that 5- to 6-year-olds had difficulty using the self-assessment manikin or SAM rating system to rate their experience of the valence and arousal properties of the stimuli. Thus, we cannot conclude confidently that the unpleasant pictures were perceived as such by the children – although a previous study from our lab provides evidence these specific stimuli do (cf., Hajcak and Dennis, 2009). Future studies should concurrently examine other independent measures of arousal to determine the impact of reappraisal, such as the galvanic skin response (GSR) and electromyography (EMG).
Results of the present study attempt to fill an important gap in our understanding of neural processes related to cognitive emotion regulation in young children. We found that children in kindergarten and first grade do not show expected changes in the LPP via use of directed reappraisal. Thus, these young children may not have the neural maturity to effectively use reappraisal strategies to down regulate affective and attentional processes measured via the LPP. Future research, however, should explore the methodological boundary conditions under which children successfully or unsuccessfully use reappraisal. Future research will examine how children change in their ability to use reappraisal over the course of development. Future directions also include determining whether there is a sensitive period during which children start to effectively use reappraisal in adult-like ways to change how they process and experience emotional information. The LPP is emerging as a useful tool for examining cognitive emotion regulation strategies in normative groups; future studies should systematically examine whether anxiety and mood problems are associated with distinctive patterns of neurophysiological change associated with the use reappraisal and other cognitive emotion regulation strategies.
Acknowledgments
This research was supported in part by grants from the National Institutes of Mental Health (NIMH) grant 5K01MH075764 and National Institute of General Medical Sciences Grant 5S06GM060654 awarded to T.D. This research was also made possible by Grant RR03037 form the National Center for Research Resources (NCRR), a component of the National Institutes of Health.
Footnotes
Unpleasant stimuli used in the directed reappraisal task: 1050, 1120, 1201, 1300, 1321, 1930, 2120, 2130, 2688, 2780, 2810, 2900, 3022, 3230, 3280, 5970, 6190, 6300, 6370, 7380, 9050, 9250, 9421, 9470, 9480, 9490, 9582, 9594, 9600, and 9611.
Neutral stimuli used in the directed reappraisal task: 5740, 5820, 7000, 7002, 7004, 7009, 7010, 7041, 7090, 7100, 7140, 7150, 7224, 7595, and 7950.
When data were instead filtered with a high-pass filter of 0.01, results did not differ from those reported in this manuscript using a high-pass filter of 0.1.
When 5- and 7-year olds are removed from analyses, all effects of condition on the LPP remained the same.
Correlations were also conducted without the 5- and 7-year-olds: all associations between the LPP and anxiety remained significant.
Correlations were conducted without the 5- and 7-year-olds: higher fearfulness was associated with larger LPP difference scores in the anterior region/late window, r = .40, p = .04.
Correlations were conducted without the 5- and 7-year-olds. The correlation between the LPP anterior region/late window reappraisal–neutral baseline and fearful behavior just missed significance r = .34, p = .075.
Appendix A.
| Picture | Neutral Baseline Stories |
|---|---|
| 5740 | This is a leaf from a maple tree. |
| 5820 | Fish live in this river. |
| 7000 | This rolling pin is used to roll dough. |
| 7002 | This is a wet towel that fell on the floor. |
| 7004 | This spoon has pictures of geese on it. |
| 7009 | This mug was just used to drink water. |
| 7010 | This woven basket is for holding bread. |
| 7041 | This farmer uses these baskets to hold apples after they are picked. |
| 7090 | This old black book has many stories in it. |
| 7100 | The grass is growing up around the fire hydrant. |
| 7140 | This bus takes people from one city to another. |
| 7150 | This umbrella is drying off after the rain. |
| 7224 | These filing cabinets are full of important papers. |
| 7595 | People are going to work in these cars. |
| 7950 | There are many tissues in this box. |
References
- Achenbach T.M., Rescorla L.A. University of Vermont, Research Center for Children, Youth, and Families; Burlington: 2001. Manual for the ASEBA School-age Forms Profiles: Child Behavior Checklist for Ages 6–18, Teacher's Report Form, Youth Self-report, an Integrated System of Multi-informant Assessment. [Google Scholar]
- Achenbach T.M., Rescorla L.A. University of Vermont, Research Center for Children, Youth, and Families; Burlington, VT: 2000. Manual for the ASEBA Preschool Forms Profiles. [Google Scholar]
- Casey B.J., Giedd J.N., Thomas K.M. Structural and functional brain development and its relation to cognitive development. Biol. Psychol. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Cole P.M., Martin S.E., Dennis T.A. Emotion regulation as a scientific construct: challenges and directions for child development research. Child Dev. 2004;75:317–333. doi: 10.1111/j.1467-8624.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- Cuthbert B.N., Schupp H.T., Bradley M.M., Birbaumer N., Lang P.J. Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biol. Psychol. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Davis E.L., Levine L.J., Lench H.C., Quas J.A. Metacognitive emotion regulation: children's awareness that changing thoughts and goals can alleviate negative emotion. Emotion. 2010;10:498–510. doi: 10.1037/a0018428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis T.A., Hajcak G. The late positive potential: a neurophysiological marker for emotion regulation in children. J. Child Psychol. Psychiatry. 2009;50(11):1373–1383. doi: 10.1111/j.1469-7610.2009.02168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis T.A., Kelemen D.A. Preschool children's views on emotion regulation: functional associations and implications for social-emotional adjustment. Int. J. Behav. Dev. 2009;33(3):243–252. doi: 10.1177/0165025408098024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D., Hajcak G. Deconstructing reappraisal: descriptions preceding arousing pictures modulate the subsequent neural response. J. Cogn. Neurosci. 2008;20(6):977–988. doi: 10.1162/jocn.2008.20066. [DOI] [PubMed] [Google Scholar]
- Garber J., Braafladt N. The regulation of sad affect: an information-processing perspective. In: Garber J., Dodge K.A., editors. The Development of Emotion Regulation and Dysregulation. Cambridge University Press; New York: 1991. pp. 208–240. [Google Scholar]
- Gratton G., Coles M.G.H., Donchin E. A new method for off-line removal of ocular artifacts. Electroencephalogr. Clin. Neurophysiol. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Goldsmith H.H., Reilly J., Lemery K.S., Longley S., Prescott A. Department of Psychology, University of Wisconsin-Madison; 1993. Preschool Laboratory Temperament Assessment Battery (PS Lab-TAB; Version 1.0). Technical Report. [Google Scholar]
- Gross J. The emerging field of emotion regulation: an integrative review. Rev. Gen. Psychol. 1998;2:271–299. [Google Scholar]
- Gross J.J., John O.P. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J. Pers. Soc. Psychol. 2003;85(2):348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Gross J.J., Levenson R.W. Hiding feelings: the acute effects of inhibiting negative and positive emotion. J. Abnorm. Psychol. 1997;106(1):95–103. doi: 10.1037//0021-843x.106.1.95. [DOI] [PubMed] [Google Scholar]
- Gross J.J., Levenson R.W. Emotional suppression: physiology, self-report, and expressive behavior. J. Pers. Soc. Psychol. 1993;64(6):970–986. doi: 10.1037//0022-3514.64.6.970. [DOI] [PubMed] [Google Scholar]
- Gross J.J., Thompson R.A. Emotion regulation: conceptual foundations. In: Gross J.J., editor. Handbook of Emotion Regulation. Guilford Press; New York: 2007. [Google Scholar]
- Hajcak G., Dennis T.A. Brain potentials during affective picture viewing in children. Biol. Psychol. 2009;80:333–338. doi: 10.1016/j.biopsycho.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G., Dunning J.P., Foti D. Motivated and controlled attention to emotion: time-course of the late positive potential. Int. J. Clin. Neuropsychol. 2009;120:505–510. doi: 10.1016/j.clinph.2008.11.028. [DOI] [PubMed] [Google Scholar]
- Hajcak G., Moser J., Simons R. Attending to affect: appraisal strategies modulate the electrocortical response to arousing pictures. Emotion. 2006;6(3):517–522. doi: 10.1037/1528-3542.6.3.517. [DOI] [PubMed] [Google Scholar]
- Hajcak G., Nieuwenhuis S. Reappraisal modulates the electrocortical response to unpleasant pictures. Cogn. Affect. Behav. Neurosci. 2006;6(4):291–297. doi: 10.3758/cabn.6.4.291. [DOI] [PubMed] [Google Scholar]
- Herbert C., Junghofer M., Kissler J. Event related potentials to emotional adjectives during reading. Psychophysiology. 2008;45(3):487–498. doi: 10.1111/j.1469-8986.2007.00638.x. [DOI] [PubMed] [Google Scholar]
- Kalisch R., Wiech K., Herrmann K., Dolan R.J. Neural correlates of self-distraction from anxiety and a process model of cognitive emotion regulation. J. Cogn. Neurosci. 2006;18(8):1266–1276. doi: 10.1162/jocn.2006.18.8.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp C.B. Emotional distress and control in young children. In: Eisenberg N., Fabes R.A., editors. Emotion and Its Regulation in Early Development. Jossey-Bass; San Francisco: 1989. pp. 41–56. [Google Scholar]
- Krompinger J.W., Moser J.S., Simons R.F. Modulations of the electrophysiological response to pleasant stimuli by cognitive reappraisal. Emotion. 2008;8(1):132–137. doi: 10.1037/1528-3542.8.1.132. [DOI] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. The Center for Research in Psychophysiology, University of Florida; 2005. International Affective Picture System (IAPS): Instruction Manual and Affective Ratings. Technical Report A-6. [Google Scholar]
- Leppanen J.M., Moulson M.C., Vogel-Farley V.K., Nelson C.A. An ERP study of emotional face processing in the adult and infant brain. Child Dev. 2007;78(1):232–245. doi: 10.1111/j.1467-8624.2007.00994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque J., Joanette Y., Mensour B., Beaudoin G., Leroux J.M., Bourgouin P., Beauregard M. Neural basis of emotional self-regulation in childhood. Neuroscience. 2004;129:361–369. doi: 10.1016/j.neuroscience.2004.07.032. [DOI] [PubMed] [Google Scholar]
- MacNamara A., Foti D., Hajcak G. Tell me about it: neural activity elicited by emotional stimuli and preceding descriptions. Emotion. 2009;9(4):531–543. doi: 10.1037/a0016251. [DOI] [PubMed] [Google Scholar]
- MacNamara A., Hajcak G. Anxiety and spatial attention moderate the electrocortical response to aversive pictures. Neuropsychologia. 2009;47:2975–2980. doi: 10.1016/j.neuropsychologia.2009.06.026. [DOI] [PubMed] [Google Scholar]
- MacNamara A., Ochsner K.N., Hajcak G. Previously reappraised: the lasting effect of description type on picture-elicited electrocortical activity. Soc. Cogn. Affect. Neurosci. 2011;6:348–358. doi: 10.1093/scan/nsq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews A., Mackintosh B. A cognitive model of selective processing in anxiety. Cogn. Ther. Res. 1998;22(6):539–560. [Google Scholar]
- Mocaiber I., Pereira M.G., Erthal F.S., Figueira I., Machado-Pinheiro W., Cagy M., Volchan E., de Oliveira L. Regulation of negative emotions in high trait anxious individuals. An ERP study. Psychol. Neurosci. 2009;2(2):211–217. [Google Scholar]
- Moser J.S., Hajcak G., Bukay E., Simons R.F. Intentional modulation of emotional responding to unpleasant pictures: an ERP study. Psychophysiology. 2006;43:292–296. doi: 10.1111/j.1469-8986.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- Moser J.S., Krompinger J.W., Dietz J., Simons R.F. Electrophysioloigcal correlates of decreasing and increasing emotional responses to unpleasant pictures. Psychophysiology. 2009;46:17–27. doi: 10.1111/j.1469-8986.2008.00721.x. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Bunge S.A., Gross J.J., Gabrieli J.D.E. Rethinking feelings: an fMRI study of the cognitive regulation of emotion. J. Cogn. Neurosci. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. The cognitive control of emotion. Trends Cogn. Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Phan K.L., Fitzgerald D.A., Nathan P.J., Moore G.J., Uhde T.W., Tancer M.E. Neural substrates of voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol. Psychiatry. 2005;57:210–221. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Richards J.M., Gross J.J. Emotion regulation and memory: the cognitive costs of keeping one's cool. J. Pers. Soc. Psychol. 2000;79(3):410–424. doi: 10.1037//0022-3514.79.3.410. [DOI] [PubMed] [Google Scholar]
- Rothbart M.K., Ahadi S.A., Hershey K.L., Fisher P. Investigations of temperament at 3-7 years: the Children's Behavior Questionnaire. Child Dev. 2001;72:1394–1408. doi: 10.1111/1467-8624.00355. [DOI] [PubMed] [Google Scholar]
- Schupp H.T., Cuthbert B.N., Bradley M.B., Cacioppo J.T., Ito T., Lang P.J. Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257–261. [PubMed] [Google Scholar]
- Schupp H.T., Junghofer M., Weike A.I., Hamm A.O. The selective processing of briefly presented affective pictures: an ERP analysis. Psychophysiology. 2004;41:441–449. doi: 10.1111/j.1469-8986.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- Segalowitz S.J., Davies P.L. Charting the maturation of the frontal lobe: an electrophysiological strategy. Brain Cogn. 2004;55:116–133. doi: 10.1016/S0278-2626(03)00283-5. [DOI] [PubMed] [Google Scholar]
- Solomon B., DeCicco J.M., Dennis T.A. Emotional picture processing in children: an ERP study. Dev. Cogn. Neurosci. 2011;2(1):110–119. doi: 10.1016/j.dcn.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiruchselvam R., Blechert J., Scheppes G., Rydstrom A., Gross J.J. The temporal dynamics of emotion regulation: an EEG study of distraction and reappraisal. Biol. Psychol, Bio Psychol. 2011;87:84–92. doi: 10.1016/j.biopsycho.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Thompson R.A., Goodman M. Development of emotion regulation: more than meets the eye. In: Kring A.M., Sloan D.M., editors. Emotion Regulation and Psychopathology: A Transdiagnostic Approach to Etiology and Treatment. Guilford Press; New York: 2010. pp. 38–58. [Google Scholar]
- Urry H.L., van Reekum C.M., Johnstone T., Kalin N.H., Thurow M.E., Schaefer H.S., Jackson C.A., Frye C.J., Greischar L.L., Alexander A.L. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J. Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]