Abstract
Hantaan virus (HTNV) is a member of the Hantavirus genus that causes human hemorrhagic fever with renal syndrome (HFRS) in humans. The CTL response seems to play a key role in control of viral infection, but only a few HTNV epitopes recognized by the CTLs have been reported. Herein, we screened a panel of overlapping peptides covering the HTNV nucleocapsid protein by ELISPOT assays for those that can elicit IFN-γ production in vitro. Three novel CD8+ CTL epitopes, N197-205 (RYRTAVCGL), N245-253 (KLLPDTAAV), and N258-266 (GPATNRDYL), were defined on the nucleocapsid protein and were found to be restricted by various HLA alleles including A11, A24, and B7. The epitopes were highly conserved among the reported HTNV strains and other hantanviruses, including Dobrava-Belgrade virus and Seoul virus, supporting their potential use in vaccine designs.
Introduction
Hantaan virus (HTNV), the prototypic member of the Hantavirus genus, is a rodent-borne pathogen that causes human hemorrhagic fever with renal syndrome (HFRS) endemic to Asia. Other hantaviruses that cause HFRS include Puumala virus (PUUV), Dobrava-Belgrade virus, and Seoul virus. In addition, Sin Nombre virus (SNV) can cause hantavirus pulmonary syndrome, another human disease caused by hantaviruses. The annual incidence of HFRS due to hantavirus infection is more than 100,000 cases, with a mortality rate of between 2% and 10%, most of which were reported in China.
The pathogenesis of HFRS caused by hantavirus infection is not well understood. In many other viral infections, virus-specific CTL responses have been shown to be involved in both clearance of virus and induction of immunopathology, which are mediated by different CD8+ T-cell populations (10,18). During the acute SNV infection, tetramer staining showed significantly higher frequencies of SNV-specific CD8+ T cells in patients with severe illness than in those with moderate disease (6), suggesting a role of virus-specific T cells in the pathology of the disease. However, our recent study showed that patients with milder disease, but not those with severe disease, displayed high frequencies of HTNV-specific IFN-γ–producing T cells (22). The features of the different CD8+ T-cell populations may be distinct. During acute PUUV infection, the kinetics of the PUUV-specific tetramer-positive T cells and the PUUV-specific T cells that produced IFN-γ in vitro were sharply different (19). CTL causes either pathology or virus control, possibly due to differences in viral proteins (5), HLA haplotypes (12), and altered efficiency of T-cell responses as a consequence of heterologous immunity (15), but only few CTL epitopes restricted by HLA-A1, A2.1, and B51 have been identified for HTNV (8,20), which limited the investigation of protective or pathologic immune responses against HTNV infection.
In this article, we screened a panel of overlapping peptides spanning the sequence of the HTNV nucleocapsid protein for those that can stimulate IFN-γ responses by ex vivo ELISPOT, and then determined the HLA context of the T-cell responses by in vitro ELISPOT. Our results identified relatively conserved epitopes which are important tools to explore for immunotherapy and analyze the immune response against HTNV.
Materials and Methods
Subjects
Two patients with acute HTNV infections were recruited in this study. The clinical diagnosis of HFRS was confirmed by detecting the presence of anti-HTNV IgM antibodies. PBMCs, collected during hospitalization and after discharge, were isolated by centrifugation of heparinized venous blood on Ficoll-Paque gradient and cryopreserved until use. CD4+ or CD8+ T cells were depleted or isolated from PBMCs by using Dynal CD4 or CD8 beads (Dynal, Oslo, Norway). EBV-transformed B-lymphoblastic cells (EBV-B cells) were generated from PBMCs of patients and maintained in RPMI 1640 (Sigma-Aldrich, St. Louis, MO) with FCS, L-glutamine, and antibiotics. All EBV-B cells were HLA typed using polymerase chain reaction-based Micro SSP™ HLA Class I and II DNA Typing Trays (One Lambda Inc., Canoga Park, CA). This study was approved by the Institutional Review Board of the Hospital and the University, and all subjects gave written, informed consent.
Synthetic peptides
Seventy 15-mer peptides that overlapped by 9 amino acids and spanned the nucleocapsid protein of HTNV strain 76-118 (GenBank accession no. M14626) were purchased from CL Bio-Scientific (Xi'an, China).
In vitro presensitization with peptides
CD8+ T lymphocytes were separated from PBMCs by antibody-coated magnetic beads (Dynal) and seeded into 96-well plates at a concentration of 5×105 cells per well in RPMI medium 1640 supplemented with 10% human AB serum. As antigen-presenting cells, PBMCs depleted of CD8+ T cells were incubated overnight with 5 μmol/L peptide, and then washed, irradiated, and added to plates at a concentration of 1×106 cells per well. After 8 h, IL-2 (10 U/mL; Roche Molecular Biochemicals, Mannheim, Germany) and IL-7 (20 ng/mL; R&D Systems, Minneapolis, MN) were added to the culture wells, and this was repeated every 3–4 d thereafter. After 11 d of presensitization without additional restimulation, presensitized CD8+ T cells were tested by ELISPOT assay against target cells pulsed with peptides to detect IFN-γ production, as previously described (4).
Ex vivo and in vitro IFN-γ ELISPOT assays
For the screening of the CTL epitopes, 70 15-mer peptides were pooled in 10 mixtures, each containing 7 synthetic peptides. PBMCs (1×105 cells), or PBMCs depleted of CD4+ or CD8+ T cells (3×105 to 6×105) were stimulated overnight with peptides or peptide mixtures (20 μmol/L of each peptide) in 96-well plates (Multiscreen-IP; Millipore, Bedford, MA) precoated with anti-human IFN-γ antibody (1-D1K; Mabtech, Büro Deutschland, Germany), as recommended by the manufacturer. The wells were washed with PBS containing 0.05% Tween 20. Biotinylated anti-IFN-γ mAb (7-B6-1), streptavidin-conjugated alkaline phosphatase, and its substrate (Sigma-Aldrich) were used to develop the IFN-γ spots. Spots were counted using an automated ELISPOT reader (Champ II ELISPOT reader system; Sage Creation, Chaoyang, Beijing, China). The number of specific IFN-γ–secreting cells was calculated by subtracting the number of spots in unstimulated controls from that in the stimulated samples. Unstimulated wells never exceeded three spots per well. Stimulation with PHA (10 μg/mL; Sigma-Aldrich) induced vigorous responses in all samples and served as positive control. Wells were considered positive if a minimum of five spots were present in the well, and if they yielded values ≥2 times above background (21).
For the MHC class I restriction assays, partial MHC class I-matching EBV-B cells were pulsed with the indicated peptides, then washed extensively before being used as APC. Presensitized CD8+ T cells (2×104 to 5×104) were tested for IFN-γ production against peptide-loaded autologous or allogeneic EBV-B cells (5×104) by ELISPOT upon 11 d of in vitro presentization as described previously (4).
Results
Identification of HTNV-specific CTL epitopes
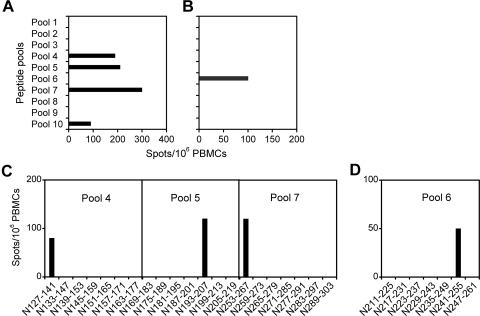
For the screening of HTNV epitopes, PBMCs of two patients with HFRS were tested by direct ex vivo ELISPOT assay using a panel of 15-mer peptides overlapping by 9 residues and spanning the HTNV nucleocapsid protein, pooled in 10 mixtures of 7 peptides each. Multiple IFN-γ responses were induced by the pools 4, 5, 7, and 10 in donor 1, whereas only a weak response against pool 6 was detected in donor 2 (Fig. 1A and B). To further determine the HTNV epitopes recognized, the peptide pools that were able to stimulate an ELISPOT response were then screened with individual component peptides. In donor 1, peptides N127-141, N193-207, and N253-267 were found to be responsible for the IFN-γ production induced by the peptide pools 4, 5, and 7, respectively, whereas N241-255 derived from pool 6 was the T-cell epitope that induced IFN-γ production in donor 2 (Fig. 1C and D). Due to the shortage of PBMC samples, pool 10, which induced a milder response in donor 1, was not analyzed further.
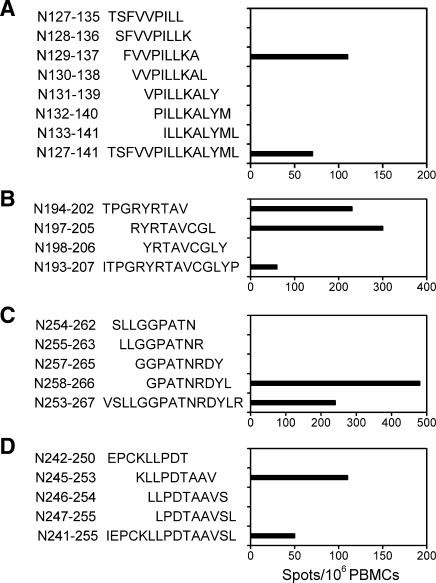
FIG. 1.
IFN-γ production by direct ex vivo ELISPOT assay. PBMCs of donor 1 (A and C), and donor 2 (B and D) were stimulated overnight with peptide pools (A and B), or individual peptides (C and D) from the panel of overlapping peptides covering the HTNV nucleocapsid protein.
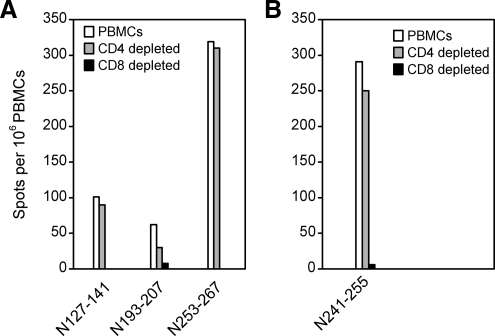
Depletion of CD4+ or CD8+ T cells from PBMCs prior to ELISPOT assay indicated that the responding cells against the four peptides were primarily CD8+ T cells (Fig. 2). We then prepared nonamer peptides from the sequences with CTL epitopes nested inside, which were predicted by computer analysis to bind HLA-I alleles based on the anchor motifs using the BIMAS (13) and SYFPEITHI (14) databases. The peptides were synthesized and tested in the similar ELISPOT assays. We found that the nonamer N129-137 (FVVPILLKA) within N127-141, N194-202 (TPGRYRTAV), and N197-205 (RYRTAVCGL) within N193-207, N258-266 (GPATNRDYL) within N253-267, and N245-253 (KLLPDTAAV) within N241-255 were the nonamer CTL epitopes that can simulate strong IFN-γ responses (Fig. 3).
FIG. 2.
CD8+ CTL responses in HFRS patients. PBMCs from donor 1 (A) and 2 (B) were depleted of CD4+ or CD8+ T cells, and then stimulated overnight with the indicated peptides. IFN-γ production was determined by direct ex vivo ELISPOT.
FIG. 3.
The nonamer CTL epitopes nested in 15-mer peptides. Direct ex vivo ELISPOT assay was performed using nonamer peptides derived from N127-141 (A), N193-207 (B), and N253-267 (C) in donor 1, and N241-255 (D) in donor 2.
HTNV epitopes are presented by MHC class I to antiviral CD8+ T cells
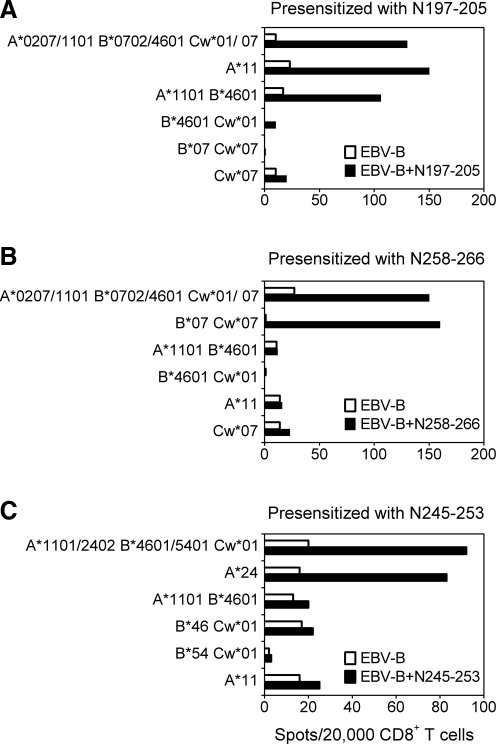
Peptides N197-205 and N258-266 elicited the strongest CTL activity among the various peptides tested in donor 1, while N245-253 was the only CTL inducer in donor 2. To identify their specific CTLs more closely, CD8+ T cells were purified from PBMCs and characterized with regard to MHC restriction. To avoid the high background, the general problem with ELISPOT upon three rounds of in vitro stimulation, an approach which was developed to monitor the anti-tumor T-cell responses and involves the in vitro presensitization of purified CD8+ T cells, was used without further modification (4). CD8+ and CD8− cell populations were prepared from PBMCs, and the purified CD8+ T cells were presensitized with autologous irradiated CD8− cells pulsed with HTNV epitopes. After 11 days of in vitro sensitization without additional restimulation, CD8+ T cells were mixed with EBV-B cells pulsed with the peptides and tested for IFN-γ production in ELISPOT assays. Pulsing N197-205 on EBV-B cells sharing one or more HLA-I alleles with donor 1 demonstrated that the CD8+ T cells recognized the epitope in the context of HLA-A11 (Fig. 4A), Whereas the N258-266 presensitized CD8+ T cells specifically produced IFN-γ upon stimulation with the N258-266 peptide in the context of HLA-B7 (Fig. 4B). In donor 2, pulsing N245-253 on EBV-B cells sharing one or more HLA-I alleles with donor 2 demonstrated that the CD8+ T cells recognize the N245-253 epitope in the context of HLA-A24 (Fig. 4C). Due to the shortage of PBMC samples, HLA restriction has not been analyzed for the recognition of N127-141 peptide.
FIG. 4.
HLA restriction usage for presentation of peptides to CD8+ T cells specific for N197-205, N258-266, and N245-253. ELISPOT assay with CD8+ T-cell effectors from donor 1 presensitized with N197-205 (A) and N258-266 (B), and CD8+ T-cell effectors from donor 2 presensitized with N245-253 (C), were tested on day 11 against partially histocompatible EBV-B cells alone or pulsed with the peptides. The HLA class I alleles shared between effectors and targets are indicated. One representative experiment of two is shown.
Viral sequence homology of the epitopes
We next examined sequence variability among the reported HTNV strains to assess conservation and divergence for these newly discovered peptide epitopes (Table 1). Of the epitopes identified, the N245-253 and N258-266 epitopes demonstrated 90% (36 of 40 sequences) and 87.5% (35 of 40) homology among the reported HTNV sequences, respectively. N197-205 was the least conserved, with 20% (8 of 40) encoding the epitope sequence we identified in the 76-118 strain, and with 80% (32 sequences) displaying a conservative valine to isoleucine substitution at position 6. The sequences of N197-205 and N258-266 also are conserved among the other hantaviruses. The N197-205 epitope is conserved in reported Seoul virus strains (23 of 23 sequences) with a conservative tyrosine to phenylalanine change at position 2, and less conserved in Dobrava-Belgrade virus, with a conservative tyrosine to phenylalanine change at position 2 and a selective valine to isoleucine change at position 6. The N258-266 epitope in Dobrava-Belgrade virus strains (13 of 13 sequences) differs from the HTNV epitope by only one amino acid, with a conservative alanine to serine substitution at position 3.
Table 1.
Conservation of HTNV CTL Epitopes Among Hantaviruses
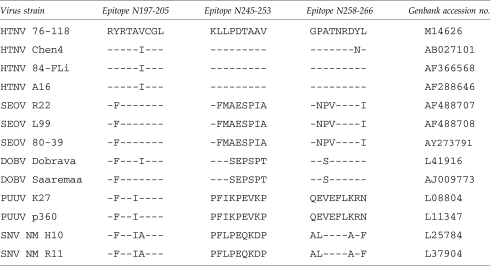 |
SEOV, Seoul virus; DOBV, Dobrava-Belgrade virus; PUUV, Puumala virus; SNV, Sin Nombre virus.
Discussion
Despite the long history of HTNV epidemics in humans as an important public health threat, our knowledge of the human immune response directed against this virus is very limited. Over the past 10 y, the definition of peptide epitopes recognized by T lymphocytes has provided the basis for detailed studies of T-cell immunity to many viral infections; however, only a few CTL epitopes have been identified for HTNV (8, 20), which limited the development of a new hantavirus vaccine and the analysis of the immune response against HTNV.
In this study, we focused our investigations on the HTNV nucleocapsid protein, which has been shown to induce efficient protection against virus challenge in animal models (1,2,9), and stronger IFN-γ–producing T-cell responses in patients with milder HFRS than in those with severe disease (22), because we were unable to collect enough blood samples from patients to study the T-cell responses to peptide antigens spanning the entire genome. We have analyzed the nucleocapsid-specific T-cell response in two HTNV-infected donors. Five CD8+ CTL epitopes on nucleocapsid protein could elicit IFN-γ responses in this study (Fig. 3). The CTL response in the two donors was directed to different epitopes on the HTNV nucleocapsid protein, and was restricted by a variety of HLA alleles, thus demonstrating a diverse HTNV-specific T-cell repertoire. T cell epitopes identified in our study were different from those identified in previous publications (8,20,23,24), possibly due to the mixed HLA backgrounds in each of the small cohorts. Another probable explanation is that the antiviral T-cell response is a dynamic process, with low or absent ELISPOT responses at hospital admission, reaching their peaks during a follow-up period of several weeks, that then wane over time (11,19). The identification of HTNV-specific CD8+ T-cell epitopes provides important tools for future studies of the kinetics and magnitude of the virus-specific CTL response during acute HFRS and the potential role of T-cell responses in the pathogenesis of disease.
The presensitized CTLs that recognized the N258-266 (GPATNRDYL) of the nucleocapsid protein were restricted by HLA-B7 (Fig. 4). This peptide fits the B7 binding motif with a proline at position 2 and a leucine at position 9 (16). However, the other two nonamers, N197-205 and 245-253, were restricted by HLA-A11 and A24, respectively, and fit neither the HLA-A11 binding motif (3) nor the HLA-A24 binding motif (17). Interestingly, the two HLA alleles, HLA-A11 and A24, are very common among populations in Asia (7,25), where HTNV is endemic. Moreover, the epitopes were highly conserved among the different hantanviruses, although the cross-reactivity was not determined in this study. The epitopes are therefore interesting tools to explore for immunotherapy.
To identify the CTL epitopes, 15-mer overlapping peptides covering the HTNV nucleocapsid protein, and subsequently, together with the nonamer peptides nested in the 15-mer peptides were screened for those that can elicit IFN-γ responses by ex vivo ELISPOT. Interestingly, the nonamer epitopes might induce antigen-specific CD8+ T-cell responses more efficiently than the longer, 15-mer peptides containing the nonamers (Fig. 3), perhaps because the nonamers can bind directly to the professional APCs in peripheral blood. Therefore, the magnitude of the antiviral T-cell responses, particularly of the CTL responses, may be underestimated using a set of overlapping 15-mer peptides only. This may be of great importance in the future studies of HTNV-specific CTL responses.
In summary, we have identified several CTL epitopes that were conserved among hantaviruses. But unfortunately, because of the difficulty to obtain sufficient PBMCs, the T-cell responses to the HTNV peptides have not been well analyzed. Further understanding of the protective or immunopathologic mechanisms mediated by the CTLs may provide insights into potential therapeutic interventions.
Acknowledgments
We thank the volunteers who generously participated in this study. This study was supported by the National Natural Science Foundation of China (grant 30471625).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Dargeviciute A. Brus Sjolander K. Sasnauskas K. Kruger DH. Meisel H. Ulrich R. Lundkvist A. Yeast-expressed Puumala hantavirus nucleocapsid protein induces protection in a bank vole model. Vaccine. 2002;20:3523–3231. doi: 10.1016/s0264-410x(02)00341-9. [DOI] [PubMed] [Google Scholar]
- 2.de Carvalho Nicacio C. Gonzalez Della Valle M. Padula P. Bjorling E. Plyusnin A. Lundkvist A. Cross-protection against challenge with Puumala virus after immunization with nucleocapsid proteins from different hantaviruses. J Virol. 2002;76:6669–66677. doi: 10.1128/JVI.76.13.6669-6677.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan P. Doytchinova IA. Flower DR. HLA-A3 supermotif defined by quantitative structure-activity relationship analysis. Protein Eng. 2003;16:11–18. doi: 10.1093/proeng/gzg005. [DOI] [PubMed] [Google Scholar]
- 4.Jager E. Nagata Y. Gnjatic S, et al. Monitoring CD8 T cell responses to NY-ESO-1: correlation of humoral and cellular immune responses. Proc Natl Acad Sci USA. 2000;97:4760–4765. doi: 10.1073/pnas.97.9.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiepiela P. Ngumbela K. Thobakgale C, et al. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med. 2007;13:46–53. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- 6.Kilpatrick ED. Terajima M. Koster FT. Catalina MD. Cruz J. Ennis FA. Role of specific CD8+ T cells in the severity of a fulminant zoonotic viral hemorrhagic fever, hantavirus pulmonary syndrome. J Immunol. 2004;172:3297–3304. doi: 10.4049/jimmunol.172.5.3297. [DOI] [PubMed] [Google Scholar]
- 7.Lee KW. Oh DH. Lee C. Yang SY. Allelic and haplotypic diversity of HLA-A, -B, -C, -DRB1, and -DQB1 genes in the Korean population. Tissue Antigens. 2005;65:437–447. doi: 10.1111/j.1399-0039.2005.00386.x. [DOI] [PubMed] [Google Scholar]
- 8.Lee KY. Chun E. Kim NY. Seong BL. Characterization of HLA-A2.1-restricted epitopes, conserved in both Hantaan and Sin Nombre viruses, in Hantaan virus-infected patients. J Gen Virol. 2002;83:1131–1136. doi: 10.1099/0022-1317-83-5-1131. [DOI] [PubMed] [Google Scholar]
- 9.Maes P. Clement J. Cauwe B. Bonnet V. Keyaerts E, Robert A, and Van Ranst M: Truncated recombinant puumala virus nucleocapsid proteins protect mice against challenge in vivo. Viral Immunol. 2008;21:49–60. doi: 10.1089/vim.2007.0059. [DOI] [PubMed] [Google Scholar]
- 10.Maini MK. Boni C. Lee CK, et al. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med. 2000;191:1269–1280. doi: 10.1084/jem.191.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mongkolsapaya J. Dejnirattisai W. Xu XN, et al. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat Med. 2003;9:921–927. doi: 10.1038/nm887. [DOI] [PubMed] [Google Scholar]
- 12.Neumann-Haefelin C. McKiernan S. Ward S, et al. Dominant influence of an HLA-B27 restricted CD8+ T cell response in mediating HCV clearance and evolution. Hepatology. 2006;43:563–572. doi: 10.1002/hep.21049. [DOI] [PubMed] [Google Scholar]
- 13.Parker KC. Bednarek MA. Coligan JE. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J Immunol. 1994;152:163–175. [PubMed] [Google Scholar]
- 14.Rammensee H. Bachmann J. Emmerich NP. Bachor OA. Stevanovic S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics. 1999;50:213–29. doi: 10.1007/s002510050595. [DOI] [PubMed] [Google Scholar]
- 15.Selin LK. Wlodarczyk MF. Kraft AR. Nie S. Kenney LL. Puzone R. Celada f. Heterologous immunity: immunopathology, autoimmunity and protection during viral infections. Autoimmunity. 2011;44:328–347. doi: 10.3109/08916934.2011.523277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sidney J. Southwood S. del Guercio MF. Grey HM. Chesnut RW. Kubo RT. Sette A. Specificity and degeneracy in peptide binding to HLA-B7-like class I molecules. J Immunol. 1996;157:3480–3490. [PubMed] [Google Scholar]
- 17.Sidney J. Southwood S. Sette A. Classification of A1- and A24-supertype molecules by analysis of their MHC-peptide binding repertoires. Immunogenetics. 2005;57:393–408. doi: 10.1007/s00251-005-0004-2. [DOI] [PubMed] [Google Scholar]
- 18.Thimme R. Oldach D. Chang KM. Steiger C. Ray SC. Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194:1395–1406. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuuminen T. Kekalainen E. Makela S, et al. Human CD8+ T cell memory generation in Puumala hantavirus infection occurs after the acute phase and is associated with boosting of EBV-specific CD8+ memory T cells. J Immunol. 2007;179:1988–1995. doi: 10.4049/jimmunol.179.3.1988. [DOI] [PubMed] [Google Scholar]
- 20.Van Epps HL. Schmaljohn CS. Ennis FA. Human memory cytotoxic T-lymphocyte (CTL) responses to Hantaan virus infection: identification of virus-specific and cross-reactive CD8(+) CTL epitopes on nucleocapsid protein. J Virol. 1999;73:5301–5308. doi: 10.1128/jvi.73.7.5301-5308.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Epps HL. Terajima M. Mustonen J. Arstila TP. Corey EA. Vaheri A. Ennis FA. Long-lived memory T lymphocyte responses after hantavirus infection. J Exp Med. 2002;196:579–588. doi: 10.1084/jem.20011255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang M. Wang J. Zhu Y. Xu Z. Yang K. Yang A. Jin B. Cellular immune response to hantaan virus nucleocapsid protein in the acute phase of hemorrhagic fever with renal syndrome: correlation with disease severity. J Infect Dis. 2009;199:188–195. doi: 10.1086/595834. [DOI] [PubMed] [Google Scholar]
- 23.Wang M. Wang J. Zhu Y. Xu Z. Yang K. Yang A. Jin B. Cellular immune response to hantaan virus nucleocapsid protein in the acute phase of hemorrhagic fever with renal syndrome: correlation with disease severity. J Infect Dis. 2009;199:188–195. doi: 10.1086/595834. [DOI] [PubMed] [Google Scholar]
- 24.Wang PZ. Huang CX. Zhang Y, et al. Analysis of the immune response to Hantaan virus nucleocapsid protein C-terminal-specific CD8(+) T cells in patients with hemorrhagic fever with renal syndrome. Viral Immunol. 2009;22:253–260. doi: 10.1089/vim.2008.0097. [DOI] [PubMed] [Google Scholar]
- 25.Yang G. Deng YJ. Hu SN, et al. HLA-A, -B, and -DRB1 polymorphism defined by sequence-based typing of the Han population in Northern China. Tissue Antigens. 2006;67:146–152. doi: 10.1111/j.1399-0039.2006.00529.x. [DOI] [PubMed] [Google Scholar]