Abstract
This review describes the kinds of skeletal bone defects in bones which develop through enchondral ossification. It focuses on the biological reconstruction of those defects according to the two main subtypes, intercalary and osteoarticular. We list the causes of bone defects and outline the different types and configurations that result from them. We then review the currently available reconstructive options according to the patient’s age and describe the theoretical options as well. Finally, the history, surgical anatomy and clinical use of the free fibula flap will be reviewed. From our own clinical experience and review of the literature, we conclude that biological reconstruction is, in many ways, superior to alloplastic materials, especially in children, adolescents and young adults.
Keywords: Sarcoma, Free flap, Limb salvage, Biological reconstruction
Introduction
Discontinuity of an anatomical skeletal structure, whatever the cause, has many synonyms, among them, loss, gap, incontinuity, lack, impairment, deficiency, absence and defect [1–4]. We will use the word “defect” to discuss bone deficits of the skeletal parts which developed through enchondral ossification (excluding the skull and a few other bones, such as the clavicle, since they evolved through intramembranous ossification). The causes of bone defects are congenital, direct and indirect trauma, acute and, especially, chronic osteomyelitis, tumour resection, local bone disease, revision procedures in joint replacement, various operative complications (iatrogenic) and combinations of the above [5].
Bones and their defects are described by two dimensions, width and length (Figs. 1 and 2). Width defines three types of bone defects (Fig. 3): envelope defect (a shaved bone) that involves loss of the soft and periosteal tissue, partial defect that involves loss of all of the components in a part of the bone circumference, and complete defect that involves loss of all of the components of a whole bone circumference. Length defines five types of bone defects, of which three are common and two are rare (Fig. 4). The common ones include intercalary defect that involves the loss of a part of the diaphysis, extended intercalary defect that involves the loss of a part of the diaphysis and the whole metaphysis with or without the epiphysis, where only some subchondral bone and joint cartilage are left (the defect is actually through the epiphysis), and osteoarticular defect, which involves loss of a part of the bone and the adjacent joint (the defect is actually across the joint space). The two rare subtypes of bone defects are complete whole bone defect, which involves loss of a whole bone through joint spaces on both sides, and osteoarticular defect with a whole joint, which involves loss of a part of a bone and the whole adjacent joint, including the end of the bone of the other side (“extra-articular resection”).
Fig. 1.
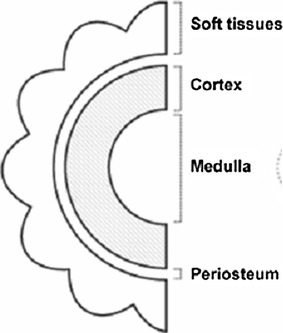
Bone width dimensions
Fig. 2.
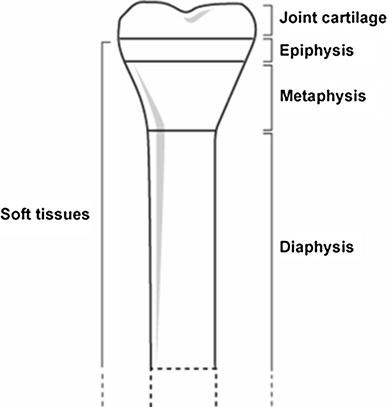
Bone length dimensions
Fig. 3.
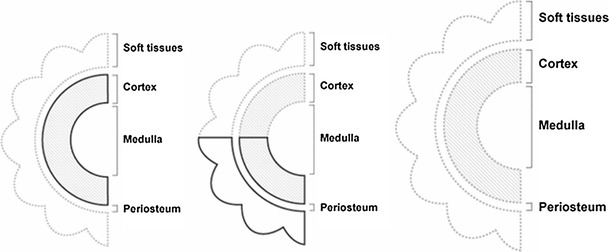
Width defects
Fig. 4.
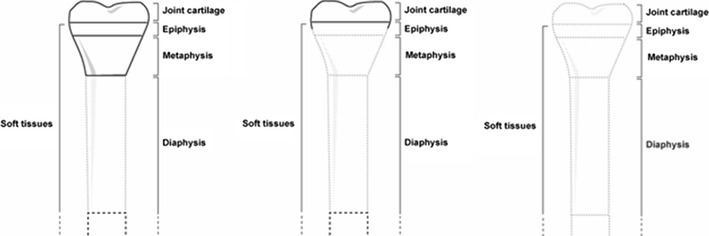
Length defects
In the longitudinal dimension, the size of the defect has critical mechanical significance. A long missing segment, especially in osteoarticular defects, is inherently problematic, since increased mechanical loads intensely affect the remaining short segment and it is more difficult to achieve mechanical stability in such a setup. In such a setting, it is more likely that bony healing will be impaired and will have a negative effect on functional outcome.
Most bone defects are combinations of the above, starting from the simpler and smaller ones, such as stress fractures, green-stick fractures or real transverse displaced fractures, up to complete whole bone defects and amputation.
Another aspect that needs to be addressed when analysing a bone defect is whether it is real, virtual or potential. Real bone defects are the ones listed above. A virtual bone defect denotes a situation in which a bony segment can be seen to exist on imaging studies but it is functionally insufficient to support the necessary mechanical loads. Examples of “ghost” bones are those that are heavily irradiated, have severe active fibrous dysplasia or contain a brown tumour or avascular necrosis of a metaepiphysis (such as the neck and head of the femur). In many cases, these virtual bone defects will be treated as real ones, but they can, or even should, often be treated by eliminating the systemic cause of the defect or augmenting or bridging the defective area. A “radiological lytic bone lesion” is another way to describe a bone defect. Its extensions and dimensions will determine its classification into one of the above definitions.
Most, if not all, bone defects can be determined by imaging studies, including plain radiographs, different types of bone and soft tissue isotope scans, computed tomography and magnetic resonance imaging. Their final size and configuration, however, are still sometimes determined during surgery. Advances in imaging studies and their availability enables a comprehensive preoperative evaluation of a bone defect, thus, reducing the discrepancy between preoperative planning and intraoperative reality. With the aid of modern imaging studies, reconstruction can be planned and performed almost precisely according to the preoperative plan.
The decision of how to reconstruct a bone defect must take the following factors into consideration: age of the patient, cause of the defect, need for pre- or postoperative adjuvant treatments, size and anatomical location of the defect, general health condition of the patient, the patient’s lifestyle, functional expectations, social-cultural issues, cost of the procedure, the availability of a donor site, and whether there will be an allograft or an artificial device. Soft tissue coverage and reconstruction with skin grafts, local flaps (fasciocutaneous and muscle flaps) or free microvascularised soft tissue flaps are major factors when considering the reconstructive options for all types of bone defects from any cause: they will not be addressed in this review.
Age plays a major role in decision-making and is divided into two categories for this purpose: from 0 up to 18–20 years and above this range. The main difference between the groups is the diminishing potential of regeneration/remodelling of the skeleton with time. General health conditions, cost and availability are issues related to socioeconomic status worldwide. Chemotherapy and/or radiotherapy before or after surgery obviously influence the choice of the reconstructive procedure.
Reconstructive options and techniques
There are diverse options available in our armamentarium to fill the various types of bone gaps. The first option is to rely solely on spontaneous fibrous scar and callus formation for wound and bony healing. The second option is to use autogenous bone. Autogenous bone can be transferred in several ways: as a free microvascularised bone flap, a locally vascularised bone pedicled flap (i.e. ipsilateral fibula to tibia, clavicle for humerus [Winkelman’s procedure] including or excluding the acromioclavicular joint or the proximal radioulnar joint [PRUJ, plasty for elbow joint]) [6–8], non-vascularised bone (whole bone, such as a fibula or a rib, cortical bone only, such as a tibial strut, cancellous bone graft only or cortico-cancellus, such as in the iliac crest graft) [9]. Small-to-medium bone gaps of up to 10 cm can be bridged using distraction osteogenesis techniques (local bone transports) [9].
The third reconstruction option is the use of an allograft supplied from bone banks (intercalary or ostechondral, fresh or frozen). The conventional way for allograft reconstruction is as non-vascularised bone graft [9]. A promising reconstructive option is the use of a free microvascularised allograft. This can be termed as “composite tissue allotransplantation” and is indicated only in rare situations. This technique allows the replacement of any part of the skeletal system with an identical biological piece, but two unsolved problems prevent its popular use: the need for life-long immunosuppression and chronic rejection. Life-long immunosuppression can lead to infections, drug toxicity and neoplasia, and chronic rejection may cause a gradual functional decline and eventual allograft loss [10, 11]. Another so-called biological reconstruction option is the use of rotationplasty, which is really a type of amputation with functional enhancement (this review does not deal with amputations).
Non-biological reconstructive options are synthetic bone substitutes which are improvised implants composed of hardware and bone cement and endoprostheses. The final reconstructive solution can be derived from the various combinations cited above. Every option used in a reconstructive approach has to be fixed within the gap: the techniques used for fixation are beyond the scope of this review.
Reconstructive solutions
The reconstructive solutions to some of the defined bone defects are well accepted, well established and need no further discussion, whereas others are diverse and controversial. The available solutions can be classified according to the width or length of the defect. The first “width” type is the envelope defect, which usually needs no reconstruction. In a partial envelope defect, the preferred reconstructive option will almost always be some kind of autogenous bone graft, usually a non-vascularised or locally vascularised bone if possible. A complete bone defect will be discussed below, under intercalary defect.
There are a number of reconstructive solutions classified according to the “length” of the defect. In the case of an intercalary defect, the preferred reconstructive option will almost always be some kind of an allograft with or without a non-vascularised autogenous bone graft, unless the patient is a small child (i.e. <10 years of age). Extended intercalary and, especially, osteoarticular defects are the most prevalent and problematic bone gaps, most notably among young people. It is here that the lack of consensus on the one hand and innovation on the other play key roles. There are no reconstructive options in the rare situation of complete whole bone and osteoarticular defect with a whole joint, and the only solution is the use of endoprostheses or arthrodesis techniques (or even amputation). If the defect is too large to be approximated by the local bone edges, even arthrodesis will sometimes require a reconstructive solution.
Reconstructive options can be divided into two groups from “an articular point of view” (±epiphysis): joint-preserving and joint-abolishing procedures comprising all types of resection arthrodesis techniques with the different available materials. These are not simple joint closures in most, cases since there is longitudinal bone loss on one side.
The reconstruction of intercalary bone defects and joint-preserving procedures can be divided into four groups (Table 1):
Vascularised autograft
Allografts
Non-biological reconstruction endoprostheses (NBRE)
Combined reconstruction (Com-R)
Table 1.
Susceptibility and durability of materials available for filling bone gaps
| Vascularised autograft | Allograft | NBRE | Com-R | |||||
|---|---|---|---|---|---|---|---|---|
| FMV or LV | NV | Allograft + FMV | FMV–OA | NV–OA | Endoprostheses | Allograft + endoprostheses | ||
| Susceptibility according to age in years | 0–5 | +++++ | +++ | ++++ | – | – | −+ | – |
| ↓ | ++++ | ++ | +++ | −+ | −+ | + | + | |
| +++ | + | ++ | + | ++ | +++ | +++ | ||
| ++ | + | ++ | + | ++ | ++++ | ++++ | ||
| >70 | + | −+ | + | – | −+ | +++++ | ++ | |
| Durability in years | For life | + | −+ | + | ? | – | – | – |
| 5–20 | / | / | / | ? | + | + | + | |
| >20 | / | / | / | ? | ? | / | / | |
FMV free microvascularised, LV locally vascularised, NV non-vascularised, OA osteoarticular, NBRE non-biological reconstruction endoprostheses, Com-R combined reconstruction
Of the many currently available autogenous grafts (scapula, rib, iliac crest, tibial strut and more), in the vascularised autografts group (Table 1), the fibula flap appears to be the superior and most flexible option, especially for the most prevalent bone defects, i.e. extended intercalary and osteoarticular defects in children, adolescents and young adults.
The history, surgical anatomy and clinical use of the fibula flap
The fibula is almost a completely dispensable bone, since it is a rudiment throughout evolution from quadrupedal ancient ancestors. It does not contribute significantly to weight-bearing or strength of the leg. The fibula flap was first described by Taylor et al. in 1975 [12]. The inclusion of a skin island for monitoring flap viability was described by Yoshimura et al. in 1983 [13] and the fibula osteoseptocutaneous flap by Wei et al. in 1986 [14]. The use of a folded fibula flap to create a structure twice as strong without the need to perform additional vascular anastomosis was described by Jones et al. [15], introducing the term “double-barrel fibula flap” in 1988. Tang reported the successful use of the fibula head for joint reconstruction in 16 patients [16].
The head of the fibula is an almost perfect replica of the distal radius, it is quite close in shape to the olecranon process and it can become the head of the humerus or even the femoral head. Since its process of ossification is the last to occur in the skeleton, its epiphysis remains open almost until the age of 18 years [17]. The unique blood supply of the fibula shaft and head with its multiple alternatives makes it a particularly valuable flap for the microvascular reconstructive surgeon [18]. It can be quite variable and, if the viability of the epiphysis and its growth plate is needed, angiography or computed tomography angiography (CTA) is recommended to evaluate its blood supply.
The fibula is especially suitable for donating material for filling bone defects. For one, it can be harvested from above the ankle syndesmosis up to the proximal tibiofibular joint (inclusively). Then, it contains almost an entire shaft of about 25–30 cm, a metaphysis, an epiphysis and a joint surface. It can be taken with soft tissue, including muscle, subcutaneous tissue and skin island (Fig. 5) [14]. Moreover, it can be combined with an allograft or some other types of autografts whenever there is a need for mechanical stability and augmentation (usually in the femur and occasionally in the proximal tibia). It is also the best currently available solution for virtual bone defects, as mentioned above (osteoradionecrosis, fibrous dysplasia, avascular necrosis of hip). The fibula is the only TBR solution for regaining a living epiphysis in children [19–21].
Fig. 5.
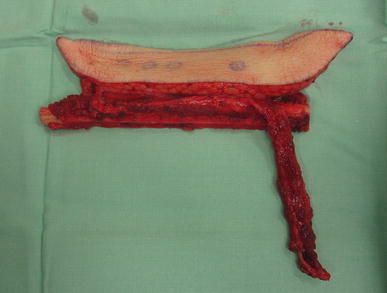
The free fibula flap, including its skin island and the vascular pedicle
There are some rare and unusual problems and complications of the fibula donor site after fibula flap harvest [22, 23]:
Lateral instability of the knee joint—almost unheard of in children and in cases in which it is well reconstructed
Peroneal palsy—extremely rare and usually transient
Distal instability of the ankle joint—almost unheard of (a syndesmotic screw should be used if the excision is close to the syndesmosis)
Cosmetic problems—only when skin island is harvested and the donor site needs a skin graft for closure
First toe problem after harvesting the flexor hallucis longus muscle, when indicated
Development of growth defects of the tibia—rare and not really problematic
Persistent mild peroneal muscle weakness
The specific complications of the fibula in the recipient site include:
From 10 to 20% of “osteopaenic” fractures within the first 6 months—these almost always unite without intervention (non-weight-bearing [NWB]) after vascularised autograft reconstruction with fibula is for 4–6 to 9–12 months)
About 5% of non-union at one junction (meaning 95% union)—this is because of the nature of the host bone and soft tissue and mechanical instability due to poor fixation
Spontaneous resorption of the flap, especially in the upper NWB limb—rare
The rate of infections—when performed for reasons other than osteomyelitis, this is surprisingly very low (<5%), even when dealing with immunosuppressed patients and despite very long operations (6–12 h) and wide exposure.
Early loss of vascular supply with increased of delayed or non-union
The use of a fibula flap for extended intercalary defects is well described in the literature and has proven to be superior to non-biologic and non-vascularised biological reconstructions [1, 4, 24]. The free fibula flap is versatile and can be used in three major types of reconstructive combinations for extended intercalary defects:
Vascularised fibula flap combined with an allograft (Capanna et al.’s method [1])
Vascularised fibula flap as a sole bony replacement
Vascularised double-barrel fibula
The decision on which type of reconstructive method to use is based on the anticipated mechanical load that the reconstructed bone will bear, the length of the defect, the age of the patient and the presence of infection. A detailed surgical algorithm for intercalary bone defect reconstruction was presented by our group in 2004 [4].
Review of the actual clinical use of the fibula for osteoarticular bone defects
A review of the published data together with our experience, leading to a total of about 60 fibula flaps as a joint-preserving procedure in osteoarticular bone defects, reveals the following (Table 2):
Proximal humerus and shoulder joint (glenohumeral joint) procedures are often performed with good functional results (depending more on the soft tissues than on the bone itself), and the fibular head often becomes a humeral head, depending on the age of the patient [16, 25–28].
Distal humerus or proximal ulna and elbow joint (humeroulnar joint) procedures have been carried out about ten times or more (two by us) only during the last few years and, so, the follow-up time is short. There is now a real new distal humeral condyle (the fibular head) which articulates with the olecranon fossa or vice versa (Fig. 6) [29–32].
Distal radius and wrist joint procedures have been performed many times with good functional joints. This is the oldest and most prevalent operation that uses the fibula for these types of bone defects (Fig. 7) [4, 16, 26, 33, 34].
Proximal femur and hip joint procedures were performed five times by Manfrini et al.’s group [35] and once by us [4]. The first such case was treated in 1997 and involved a 4-year-old girl whose fibular head has since (after 8 years of follow-up) been completely transformed into a living femoral head and epiphysis. The other surgeries were carried out during 1999 up to 2007 (Fig. 8) [36, 37].
Distal femur and knee joint, proximal tibia and knee joint, distal tibia and ankle joint (f, g and h in Table 2) have not been performed but can be done according to the schemes shown in Fig. 9. They evolve from the transformation of the knee and ankle joints from shallow unstable joints to ball-and-socket type joints (Fig. 9).
One distal fibula and ankle joint (tibiotalar joint) procedure was performed by replacing the lateral malleolus with a proximal fibula [36].
Table 2.
The actual osteoarticular bone defects
| Upper limb | a. Proximal humerus + shoulder joint (glenohumeral joint) b. Distal humerus + elbow joint (humeroulnar joint) c. Proximal ulna + elbow joint (humeroulnar joint) d. Distal radius + wrist joint |
| Lower limb | e. Proximal femur + hip joint f. Distal femur + knee joint g. Proximal tibia + knee joint h. Distal tibia + ankle joint (tibiotalar joint) i. Distal fibula + ankle joint (tibiotalar joint) |
* The proximal radio-humeral joint, the distal ulnar-carpal joint and the proximal tibiofibular joint: no need for reconstruction at all
Fig. 6.
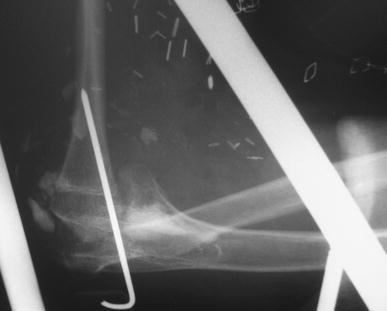
Distal humerus reconstruction. The head of the fibula creates a new distal humeral condyle, which articulates with the olecranon fossa
Fig. 7.

Distal radius reconstruction
Fig. 8.
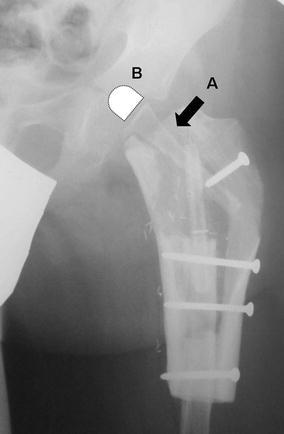
Proximal femur reconstruction, hip joint procedure. A—the fibula flap, B—illustration of the cartilaginous head of the fibula
Fig. 9.
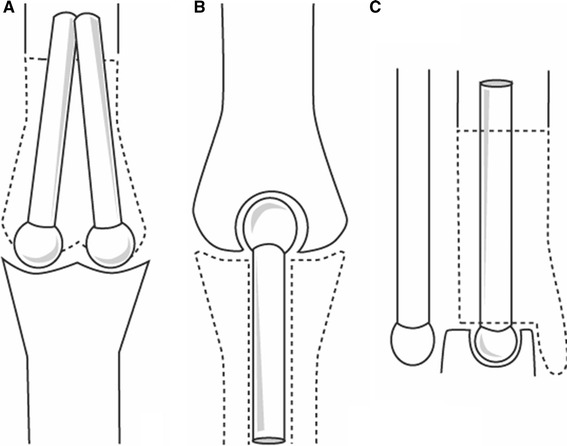
Schematic illustrations proposed for the reconstruction of the distal femur (knee joint), proximal tibia (knee joint) and distal tibia (ankle joint)
Discussion
When dealing with bone defects, it is important to have a clear understanding and to use precise nomenclature of the actual bone defect, since the reconstructive solution must be individually tailored. An ideal reconstruction should be stable, durable, allow growth when needed and provide an adequate range of joint motion.
When an intercalary bone resection for a diaphyseal tumour or a resection arthrodesis is performed, the reconstructive options are massive allografts, Ilizarov’s techniques (bone transport), non-vascularised bone graft or free microvascularised bone autografts [3, 9, 34, 38].
Despite technical improvements in bone bonding and in the methods of fixation, allograft reconstruction still has numerous complications. The overall complication rate exceeds 50%, and the complications include infection, fractures, non-union, joint instability and joint degeneration [3, 39]. The Ilizarov technique can be used solely for small bone defects (<10 cm), it is time-consuming and it needs the patient’s cooperation [34]. In intercalary resections, the use of the vascularised fibula flap as an isolated osseous flap might be insufficient. Different body sites have different stress loads to carry, which is another factor that depends on the age of the patient and on the individual’s physical status. As such, in order to achieve initial strength in the early period, the free fibula flap needs to be combined with an allograft (Capanna et al.’s method) or augmented by a double-barrel fibula [1, 4].
The fibula is a narrow and weaker bone than the original resected long bone, especially in the femur, but it is sufficient in many cases that involve the humerus, radius and even the tibia. In order to functionally replace these bones, a long period of NWB is needed, during which the osteotomy sites will unite and, so, more time will have to elapse in order for the fibula to hypertrophy through a process of pressure transport, microfractures and callus formation [40]. To achieve strength in the early period, until the fibula is capable of taking the entire physical load, Capanna et al. advised combining the free fibula flap with an allograft. Another advantage of Capanna et al.’s method is a low incidence of non-union and graft fracture [1].
According to the literature, endoprosthetic reconstruction is the mainstay of limb salvage when the location of the bone tumour is periarticular [41–44]. This type of reconstruction allows a functional reconstruction by preserving joint movement. There are, however, disadvantages, including infection, prosthesis loosening, the need to change prostheses in a growing child, a limitation to their function and lifestyle, and, perhaps the most significant disadvantage, limited durability, especially when performed in paediatric populations and in young adults [45, 46].
To achieve an ideal reconstruction, the resected bone and joint should be replaced with an identical vascularised bone. This is essential, especially when reconstructing partial or total joint losses. One of the promising methods for achieving this in the future is to use a composite tissue allotransplant. The vascularised allograft can provide a true biological reconstruction with a perfect anatomical shape, including the articular surface. To date, however, unsolved issues prevent its common use in clinical practice, including the need for life-long immunosuppression and the inability to prevent chronic rejection [10]. A second promising method is by tissue engineering, although this technique is still far from being established [47].
There have been growing numbers of reports on the use of vascularised autogenous tissue for periarticular reconstruction. The fibula is suitable for a hemiarthroplasty procedure since it can be harvested together with the proximal articular surface [16, 21, 48]. Taylor et al. described the importance of the anterior tibial vessels as the vascular pedicle when the fibula flap includes the fibular head [49]. Mizumoto used the lateral genicular artery as the pedicle for the fibula head, while others used the peroneal vessels and reported having sufficient blood supply to the fibular head [16, 21].
We favour a true biological reconstruction rather than the use of an endoprosthesis or allograft. Our aim is to save the joint and the growth plate, if possible, in children. The various reconstructive options that the vascularised fibula flap provides enable the fulfilment of many of those goals. As noted earlier, the patient’s age is a key factor. The younger the patient, the greater will be the plastic ability of the fibular head to grow, remodel and transform into the “new joint”. Cases of the shoulder joint and hip joint are good examples, and the same holds true for hypertrophy of any other segment of the fibula.
No single procedure has an advantage of life-time durability, a factor that should determine which method will emerge as the gold standard. The only exception is an arthrodesis technique, whose consequence is the permanent loss of a functional joint. It would appear obvious that the potential of the fibula as a TBR for the joints of adults (>25 years of age) is dramatically diminished compared to younger patients but, curiously, no one has actually tested this notion: why should a fibular head not become a functional humeral head in a 30- or 40-year-old patient through mobilisation in time, even if the radiological picture will never be perfect as in a child?
Conclusions
Although biological reconstruction is technically a more demanding procedure, needs longer healing and rehabilitation time, and it has the additional morbidity of the donor site, it gives a superior long-term result compared to all alloplastic materials, especially in children, adolescents and young adults, except maybe in some situations when allografts can be used.
Contributor Information
Arik Zaretski, Phone: +972-3-6973320, FAX: +972-3-6973712, Email: arik.zaretski@gmail.com.
Isaac Meller, Phone: +972-3-6974688, FAX: +972-3-6974690, Email: imortonc@tasmc.health.gov.il.
References
- 1.Capanna R, Bufalini C, Campanacci M. A new technique for reconstructions of large metadiaphyseal bone defects. A combined graft (Allograft Shell plus Vascularized Fibula) Orthop Traumatol. 1993;2(3):159–177. doi: 10.1007/BF02620523. [DOI] [Google Scholar]
- 2.Heller L, Levin LS. Lower extremity microsurgical reconstruction. Plast Reconstr Surg. 2001;108(4):1029–1041. doi: 10.1097/00006534-200109150-00036. [DOI] [PubMed] [Google Scholar]
- 3.Mankin HJ, Fogelson FS, Thrasher AZ, Jaffer F. Massive resection and allograft transplantation in the treatment of malignant bone tumors. N Engl J Med. 1976;294(23):1247–1255. doi: 10.1056/NEJM197606032942301. [DOI] [PubMed] [Google Scholar]
- 4.Zaretski A, Amir A, Meller I, Leshem D, Kollender Y, Barnea Y, et al. Free fibula long bone reconstruction in orthopedic oncology: a surgical algorithm for reconstructive options. Plast Reconstr Surg. 2004;113(7):1989–2000. doi: 10.1097/01.PRS.0000122213.82011.C5. [DOI] [PubMed] [Google Scholar]
- 5.Lew DP, Waldvogel FA. Osteomyelitis. Lancet. 2004;364(9431):369–379. doi: 10.1016/S0140-6736(04)16727-5. [DOI] [PubMed] [Google Scholar]
- 6.Amin SN, Ebeid WA (2002) Shoulder reconstruction after tumor resection by pedicled scapular crest graft. Clin Orthop Relat Res (397):133–142 [DOI] [PubMed]
- 7.Ebeid W, Amin S, Abdelmegid A, Refaat Y, Ghoneimy A. Reconstruction of distal tibial defects following resection of malignant tumours by pedicled vascularised fibular grafts. Acta Orthop Belg. 2007;73(3):354–359. [PubMed] [Google Scholar]
- 8.Winkelmann WW. Clavicula pro humero—a new surgical method for malignant tumors of the proximal humerus. Z Orthop Ihre Grenzgeb. 1992;130(3):197–201. doi: 10.1055/s-2008-1040138. [DOI] [PubMed] [Google Scholar]
- 9.Malawer M, Sugarbaker PH. Musculoskeletal cancer surgery: treatment of sarcoma and allied diseases. Dordrecht, Boston, London: Kluwer Academic Publishers; 2001. [Google Scholar]
- 10.Hettiaratchy S, Randolph MA, Petit F, Lee WP, Butler PE. Composite tissue allotransplantation—a new era in plastic surgery? Br J Plast Surg. 2004;57(5):381–391. doi: 10.1016/j.bjps.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Siemionow M, Bozkurt M, Kulahci Y. Current status of composite tissue allotransplantation. Handchir Mikrochir Plast Chir. 2007;39(3):145–155. doi: 10.1055/s-2007-965233. [DOI] [PubMed] [Google Scholar]
- 12.Taylor GI, Miller GD, Ham FJ. The free vascularized bone graft. A clinical extension of microvascular techniques. Plast Reconstr Surg. 1975;55(5):533–544. doi: 10.1097/00006534-197505000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Yoshimura M, Shimamura K, Iwai Y, Yamauchi S, Ueno T. Free vascularized fibular transplant. A new method for monitoring circulation of the grafted fibula. J Bone Joint Surg Am. 1983;65(9):1295–1301. [PubMed] [Google Scholar]
- 14.Wei FC, Chen HC, Chuang CC, Noordhoff MS. Fibular osteoseptocutaneous flap: anatomic study and clinical application. Plast Reconstr Surg. 1986;78(2):191–200. doi: 10.1097/00006534-198608000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Jones NF, Swartz WM, Mears DC, Jupiter JB, Grossman A. The “double barrel” free vascularized fibular bone graft. Plast Reconstr Surg. 1988;81(3):378–385. doi: 10.1097/00006534-198803000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Tang CH. Reconstruction of the bones and joints of the upper extremity by vascularized free fibular graft: report of 46 cases. J Reconstr Microsurg. 1992;8(4):285–292. doi: 10.1055/s-2007-1006709. [DOI] [PubMed] [Google Scholar]
- 17.Cardoso HF. Epiphyseal union at the innominate and lower limb in a modern Portuguese skeletal sample, and age estimation in adolescent and young adult male and female skeletons. Am J Phys Anthropol. 2008;135(2):161–170. doi: 10.1002/ajpa.20717. [DOI] [PubMed] [Google Scholar]
- 18.Zhu YL, Xu YQ, Yang J, Li J, Lan XF. An anatomic study of vascularized fibular grafts. Chin J Traumatol. 2008;11(5):279–282. doi: 10.1016/S1008-1275(08)60056-5. [DOI] [PubMed] [Google Scholar]
- 19.Innocenti M, Delcroix L, Manfrini M, Ceruso M, Capanna R. Vascularized proximal fibular epiphyseal transfer for distal radial reconstruction. J Bone Joint Surg Am. 2004;86-A(7):1504–1511. doi: 10.2106/00004623-200407000-00021. [DOI] [PubMed] [Google Scholar]
- 20.Pho RW, Patterson MH, Kour AK, Kumar VP. Free vascularised epiphyseal transplantation in upper extremity reconstruction. J Hand Surg Br. 1988;13(4):440–447. doi: 10.1016/0266-7681(88)90175-1. [DOI] [PubMed] [Google Scholar]
- 21.Tsai TM, Ludwig L, Tonkin M (1986) Vascularized fibular epiphyseal transfer. A clinical study. Clin Orthop Relat Res (210):228–234 [PubMed]
- 22.Bodde EW, de Visser E, Duysens JE, Hartman EH. Donor-site morbidity after free vascularized autogenous fibular transfer: subjective and quantitative analyses. Plast Reconstr Surg. 2003;111(7):2237–2242. doi: 10.1097/01.PRS.0000060086.99242.F1. [DOI] [PubMed] [Google Scholar]
- 23.Garrett A, Ducic Y, Athre RS, Motley T, Carpenter B. Evaluation of fibula free flap donor site morbidity. Am J Otolaryngol. 2006;27(1):29–32. doi: 10.1016/j.amjoto.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 24.O’Brien BM, Gumley GJ, Dooley BJ, Pribaz JJ. Folded free vascularized fibula transfer. Plast Reconstr Surg. 1988;82(2):311–318. doi: 10.1097/00006534-198808000-00017. [DOI] [PubMed] [Google Scholar]
- 25.Ad-El DD, Paizer A, Pidhortz C. Bipedicled vascularized fibula flap for proximal humerus defect in a child. Plast Reconstr Surg. 2001;107(1):155–157. doi: 10.1097/00006534-200101000-00024. [DOI] [PubMed] [Google Scholar]
- 26.Gao YH, Ketch LL, Eladoumikdachi F, Netscher DT. Upper limb salvage with microvascular bone transfer for major long-bone segmental tumor resections. Ann Plast Surg. 2001;47(3):240–246. doi: 10.1097/00000637-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Hirayama T, Suematsu N, Inoue K, Baitoh C, Takemitsu Y. Free vascularised bone grafts in reconstruction of the upper extremity. J Hand Surg Br. 1985;10(2):169–175. doi: 10.1016/0266-7681(85)90008-7. [DOI] [PubMed] [Google Scholar]
- 28.Shiraishi H, Fujii S, Kobayashi R. Function of the upper extremity and ability to perform housework following reconstructive surgery of the shoulder joint using a free vascularized fibula bone graft: a case report. J Hand Ther. 2002;15(3):274–281. doi: 10.1016/S0894-1130(02)70011-2. [DOI] [PubMed] [Google Scholar]
- 29.Alnot JY, Ismael F, Wodecki P. Reconstruction of the proximal quarter of the ulna after post-traumatic destruction. 3 case reports. Rev Chir Orthop Reparatrice Appar Mot. 1999;85(5):497–502. [PubMed] [Google Scholar]
- 30.Gianoutsos MP, Marsden FW, McCarthy SW, Lee KK. Ulnar adamantinoma: en bloc excision and fibular osteoseptocutaneous free flap reconstruction. J Hand Surg Am. 1994;19(3):495–499. doi: 10.1016/0363-5023(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 31.Helm RH, Pho RW, Tonkin MA. The sequelae of osteomyelitis of the proximal ulna occurring in early childhood. J Hand Surg Br. 1992;17(2):232–234. doi: 10.1016/0266-7681(92)90098-M. [DOI] [PubMed] [Google Scholar]
- 32.Kimura K, Tatezaki S, Ishii T, Yonemoto T, Shigehara T, Takenouchi T. Hemiarthroplasty of the elbow with a vascularized fibular graft after excision of Ewing’s sarcoma of the proximal ulna: a case report. Jpn J Clin Oncol. 2002;32(10):430–434. doi: 10.1093/jjco/hyf088. [DOI] [PubMed] [Google Scholar]
- 33.Minami A, Kato H, Iwasaki N. Vascularized fibular graft after excision of giant-cell tumor of the distal radius: wrist arthroplasty versus partial wrist arthrodesis. Plast Reconstr Surg. 2002;110(1):112–117. doi: 10.1097/00006534-200207000-00020. [DOI] [PubMed] [Google Scholar]
- 34.Campanacci DA, Capanna R, Ceruso M, Angeloni R, Innocenti M, Lauri G, et al. Indications for combined grafts (allografts + vascularized fibula) after intercalary resections for bone tumor. In: Czitrom AA, Winkler H, et al., editors. Orthopaedic allograft surgery. Wien: Springer; 1996. pp. 149–155. [Google Scholar]
- 35.Manfrini M, Innocenti M, Ceruso M, Mercuri M. Original biological reconstruction of the hip in a 4-year-old girl. Lancet. 2003;361(9352):140–142. doi: 10.1016/S0140-6736(03)12192-7. [DOI] [PubMed] [Google Scholar]
- 36.Rajacic N, Dashti H. Reconstruction of the lateral malleolus using a reverse-flow vascularized fibular head: a case report. Microsurgery. 1996;17(3):158–161. doi: 10.1002/(SICI)1098-2752(1996)17:3<158::AID-MICR12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 37.Taddei F, Viceconti M, Manfrini M, Toni A. Mechanical strength of a femoral reconstruction in paediatric oncology: a finite element study. Proc Inst Mech Eng H. 2003;217(2):111–119. doi: 10.1243/09544110360579321. [DOI] [PubMed] [Google Scholar]
- 38.Parrish FF. Allograft replacement of all or part of the end of a long bone following excision of a tumor. J Bone Joint Surg Am. 1973;55(1):1–22. [PubMed] [Google Scholar]
- 39.Gebhardt MC, Flugstad DI, Springfield DS, Mankin HJ (1991) The use of bone allografts for limb salvage in high-grade extremity osteosarcoma. Clin Orthop Relat Res (270):181–196 [PubMed]
- 40.Ceruso M, Falcone C, Innocenti M, Delcroix L, Capanna R, Manfrini M. Skeletal reconstruction with a free vascularized fibula graft associated to bone allograft after resection of malignant bone tumor of limbs. Handchir Mikrochir Plast Chir. 2001;33(4):277–282. doi: 10.1055/s-2001-16597. [DOI] [PubMed] [Google Scholar]
- 41.Capanna R, Morris HG, Campanacci D, Del Ben M, Campanacci M. Modular uncemented prosthetic reconstruction after resection of tumours of the distal femur. J Bone Joint Surg Br. 1994;76(2):178–186. [PubMed] [Google Scholar]
- 42.Chao EY, Sim FH. Modular prosthetic system for segmental bone and joint replacement after tumor resection. Orthopedics. 1985;8(5):641–651. doi: 10.3928/0147-7447-19850501-17. [DOI] [PubMed] [Google Scholar]
- 43.Muschler GF, Ihara K, Lane JM, Healey JH, Levine MJ, Otis JC, et al. A custom distal femoral prosthesis for reconstruction of large defects following wide excision for sarcoma: results and prognostic factors. Orthopedics. 1995;18(6):527–538. doi: 10.3928/0147-7447-19950601-04. [DOI] [PubMed] [Google Scholar]
- 44.Unwin PS, Cannon SR, Grimer RJ, Kemp HB, Sneath RS, Walker PS. Aseptic loosening in cemented custom-made prosthetic replacements for bone tumours of the lower limb. J Bone Joint Surg Br. 1996;78(1):5–13. [PubMed] [Google Scholar]
- 45.Myers GJ, Abudu AT, Carter SR, Tillman RM, Grimer RJ. The long-term results of endoprosthetic replacement of the proximal tibia for bone tumours. J Bone Joint Surg Br. 2007;89(12):1632–1637. doi: 10.1302/0301-620X.89B12.19481. [DOI] [PubMed] [Google Scholar]
- 46.Myers GJ, Abudu AT, Carter SR, Tillman RM, Grimer RJ. Endoprosthetic replacement of the distal femur for bone tumours: long-term results. J Bone Joint Surg Br. 2007;89(4):521–526. doi: 10.1302/0301-620X.89B4.18631. [DOI] [PubMed] [Google Scholar]
- 47.Sterodimas A, De Faria J, Correa WE, Pitanguy I. Tissue engineering in plastic surgery: an up-to-date review of the current literature. Ann Plast Surg. 2009;62(1):97–103. doi: 10.1097/SAP.0b013e3181788ec9. [DOI] [PubMed] [Google Scholar]
- 48.Gerwin M, Weiland AJ. Vascularized bone grafts to the upper extremity. Indications and technique. Hand Clin. 1992;8(3):509–523. [PubMed] [Google Scholar]
- 49.Taylor GI, Wilson KR, Rees MD, Corlett RJ, Cole WG. The anterior tibial vessels and their role in epiphyseal and diaphyseal transfer of the fibula: experimental study and clinical applications. Br J Plast Surg. 1988;41(5):451–469. doi: 10.1016/0007-1226(88)90001-X. [DOI] [PubMed] [Google Scholar]


