Background: Pimeloyl-CoA is an intermediate of the biotin pathway, but it is unknown how it is synthesized in fungi.
Results: Aspergillus nidulans mutants in β-oxidation are biotin-deficient, and the enzyme utilizing pimeloyl-CoA is targeted to the peroxisome.
Conclusion: Pimeloyl-CoA is synthesized in the peroxisome via the β-oxidation cycle in filamentous fungi.
Significance: This is the first implication of peroxisomal β-oxidation in biotin synthesis.
Keywords: β-Oxidation, Biotin, Fungi, Peroxisomes, Yeast Metabolism
Abstract
The first step in the synthesis of the bicyclic rings of d-biotin is mediated by 8-amino-7-oxononanoate (AON) synthase, which catalyzes the decarboxylative condensation of l-alanine and pimelate thioester. We found that the Aspergillus nidulans AON synthase, encoded by the bioF gene, is a peroxisomal enzyme with a type 1 peroxisomal targeting sequence (PTS1). Localization of AON to the peroxisome was essential for biotin synthesis because expression of a cytosolic AON variant or deletion of pexE, encoding the PTS1 receptor, rendered A. nidulans a biotin auxotroph. AON synthases with PTS1 are found throughout the fungal kingdom, in ascomycetes, basidiomycetes, and members of basal fungal lineages but not in representatives of the Saccharomyces species complex, including Saccharomyces cerevisiae. A. nidulans mutants defective in the peroxisomal acyl-CoA oxidase AoxA or the multifunctional protein FoxA showed a strong decrease in colonial growth rate in biotin-deficient medium, whereas partial growth recovery occurred with pimelic acid supplementation. These results indicate that pimeloyl-CoA is the in vivo substrate of AON synthase and that it is generated in the peroxisome via the β-oxidation cycle in A. nidulans and probably in a broad range of fungi. However, the β-oxidation cycle is not essential for biotin synthesis in S. cerevisiae or Escherichia coli. These results suggest that alternative pathways for synthesis of the pimelate intermediate exist in bacteria and eukaryotes and that Saccharomyces species use a pathway different from that used by the majority of fungi.
Introduction
d-Biotin (vitamin B7 or H) is a water-soluble vitamin that functions as a prosthetic group for many carboxylases, including acetyl-CoA carboxylase involved in fatty acid biosynthesis and pyruvate carboxylase involved in gluconeogenesis. Biotin is synthesized in plants, most bacteria, and fungi, but animal cells rely exclusively on an external supply of this vitamin. In addition to its important role as a cofactor for enzymes, biotin is known to play a role in the regulation of gene expression in mammalian cells (1).
The pathway of biotin synthesis from the precursor pimelate thioester (pimeloyl-CoA or pimeloyl-acyl carrier protein (ACP)3) relies on four enzymes, and it is highly conserved among prokaryotic and eukaryotic organisms that synthesize biotin (2, 3). Synthesis starts with the decarboxylative condensation of l-alanine and pimelate thioester to form 8-amino-7-oxononanoate (AON), which is catalyzed by AON synthase (EC 2.3.1.47). AON is then modified to 7,8-diaminononanoate (DAN) by DAN synthase (EC 2.6.1.62) using S-adenosyl-l-methionine as an NH2 donor. The enzyme dethiobiotin (DTB) synthase (EC 6.3.3.3) catalyzes the formation of an ureido ring via carboxylation to yield DTB. The final step of the pathway is mediated by biotin synthase (EC 2.8.1.6) and involves the introduction of sulfur to form a new ring, which yields d-biotin.
Although the pathway from pimelate thioester to biotin has been well studied, the source of the pimelate moiety remains enigmatic in most organisms, except Escherichia coli and Bacillus subtilis (2, 3). In E. coli, pimeloyl-ACP is synthesized via a modified fatty acid biosynthetic pathway encompassing the products of the bioC and bioH genes (4, 5). BioC has been proposed to transfer a methyl group from S-adenosyl-l-methionine to the ω-carboxyl group of malonyl-CoA, yielding a malonyl-CoA methyl ester, which is used as a primer for the fatty biosynthetic pathway to generate a pimeloyl-ACP methyl ester after two cycles of elongation (6). BioH could terminate chain elongation via cleavage of the methyl ester moiety to produce pimeloyl-ACP (6). A distinct pathway is found in B. subtilis where the genes bioI and bioW participate in the generation of the pimelate moiety. BioI is a cytochrome P450 enzyme that catalyzes the oxidative C–C cleavage of a fatty acyl-ACP precursor via a dihydroxyacid intermediate to generate pimeloyl-ACP (7, 8). BioW is a pimeloyl-CoA synthetase that enables the incorporation of exogenous pimelic acid into the biotin biosynthetic pathway of B. subtilis (9).
Fungal biotin synthesis was studied in Saccharomyces cerevisiae and more recently in the filamentous fungus Aspergillus nidulans (10–12). AON and biotin synthases are encoded by the BIO6 and BIO2 genes in S. cerevisiae and by the bioF and bioB genes in A. nidulans, respectively. In contrast, although DAN and DTB synthases are encoded by distinct genes in S. cerevisiae, namely BIO3 and BIO4, these enzymatic activities are combined on a single polypeptide encoded by the multifunctional gene bioDA in A. nidulans and in most fungi, plants, and Öomycota (10–12).
In this study, we aimed to provide insights into the synthesis of pimelate thioester in fungi. We observed that the A. nidulans AON synthase, encoded by the bioF gene (10), harbors a C-terminal tripeptide (ARL), which fits the consensus sequence for a type 1 peroxisomal targeting sequence (PTS1). Here, we show that BioF is a peroxisomal protein and that its correct localization is essential for biotin synthesis. Database mining revealed that AON synthases with a PTS1 are widespread in the fungal kingdom but absent from representatives of the Saccharomyces species complex. We also show that the A. nidulans aoxA and foxA genes, encoding an acyl-CoA oxidase and a multifunctional protein participating in the peroxisomal β-oxidation cycle, respectively, contribute to biotin biosynthesis. However, neither β-oxidation nor the import of proteins in peroxisomes is essential for biotin synthesis in S. cerevisiae. These results indicate that pimeloyl-CoA is generated via the peroxisomal β-oxidation cycle in A. nidulans and that AON synthesis also occurs in the peroxisomes of most Pezizomycotina and Basidiomycota species and representatives of basal fungal lineages but not in S. cerevisiae.
EXPERIMENTAL PROCEDURES
E. coli Strains and Media
Plasmids were routinely propagated in E. coli DH5α using standard procedures. Mutants with deletions in genes implicated in the aerobic and anaerobic β-oxidation cycles were previously generated in the framework of the Keio systematic deletion project (13). Mutants were obtained directly from the Keio collection or via the E. coli Genetic Stock Center at Yale University. All Keio collection mutants were in the background strain K12 BW25113 (JWC285) (13). The mutants included ΔfadB (JW3822), ΔfadE (JW5020), ΔfadA (JW5578), ΔfadJ (JW2338), ΔfadI (JW2339), ΔfadK (JW5910), and ΔbioD (JW0761). Biotin prototrophy was tested by growing strains aerobically on M9 medium supplemented with either 1 μg/ml avidin (Fordras S.A.) or 20 ng/ml d-biotin (AppliChem). For anaerobic growth, the same medium was supplemented with 25 mm KNO3, and bacteria were grown in an airtight jar with limited oxygen using the AnaeroGen system (Oxoid).
A. nidulans Strains, Media, and Transformation
Media used for A. nidulans growth were as described previously (14). Sources of carbon, nitrogen, and vitamins were added as appropriate to a minimal salt solution at pH 6.8. Preparation of A. nidulans protoplasts and transformation followed a standard protocol (15). Biotin-auxotrophic mutant biA1 (FGSC A26, Glasgow G051) and biotin-prototrophic strain FGSC A1145 (pyrG89 pyroA4 nkuA::argB riboB2) were obtained from the Fungal Genetic Stock Center. Biotin, avidin, and pimelic acid were added to media at 25 ng/ml, 1 μg/ml, and 100 μm, respectively.
S. cerevisiae Strains, Media, Constructs, and Transformation
S. cerevisiae strain BY4742 (MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0) and mutants pox1Δ0 (YGL205w::kanMX4), fox2Δ0 (YKR009c::kanMX4), pot1Δ0 (YIL160c::kanMX4), pex5Δ0 (YDR244W::kanMX4), and pex7Δ0 (YDR142C::kanMX4) were all derived from BY4742 and obtained from EUROSCARF. Biotin-prototrophic strain A364a was obtained from the National Collection of Yeast Cultures.
A genomic region encompassing the syntenic BIO6 and BIO1 genes was amplified by PCR using genomic DNA from strain A364a and oligonucleotides Bio1–6-F and Bio1–6-R (sequences for all oligonucleotides are provided in supplemental Table S1). The PCR fragment was then cloned into the SacI-XhoI sites of vector p415-GPD. The resulting p415/BIO1-BIO6 plasmid was introduced into various S. cerevisiae mutant strains using the lithium acetate procedure before selection for growth on leucine-deficient medium containing 0.67% yeast nitrogen base without amino acids (Difco), 0.5% ammonium sulfate, 2% glucose, and 0.69 g/liter leucine dropout supplement (Clontech). Biotin prototrophy was tested by growing strains on a similar biotin-deficient medium with 1 μg/ml avidin or supplemented with 20 ng/ml biotin. The growth rates of parental strain BY4742 and β-oxidation mutants pox1Δ0 and fox2Δ0 containing the p415/BIO1-BIO6 plasmid were assessed by measuring the absorbance at 600 nm (0.6-cm light path) of cells growing on leucine- and biotin-deficient medium supplemented with 1 μg/ml avidin.
Complementation of E. coli bioF-deficient Mutants with GFP-bioF Chimeric Variants
The biotin-auxotrophic E. coli mutant bioF103 was obtained from the E. coli Genetic Resource Center at Yale University (4, 16). A coding sequence without a stop codon for a version of GFP was PCR-amplified from plasmid pAN52-1-GFP (17) using oligonucleotides GFP-NcoI and GFP-KpnI, and the resulting fragment was cloned into the NcoI-KpnI sites of the bacterial expression vector pTRC99a (18) to give pTRC/GFP. For in-frame fusion of GFP to the N-terminal end of BioF, the full-length coding sequence (i.e. from start to stop codon) of A. nidulans bioF was amplified from a functional cDNA clone (10) using oligonucleotides bioF-FL-KpnI and bioF-FL-BamHI. The resulting fragment was cloned into the KpnI-BamHI sites of pTRC/GFP to produce pTRC/GFP-BioF-FL. A variant construct lacking the codons for the last three amino acids of BioF was similarly constructed using oligonucleotides bioF-FL-KpnI and bioF-TR-BamHI to yield clone pTRC/GFP-BioF-TR. The E. coli mutant bioF103 strain was transformed with pTRC/GFP-BioF-FL or pTRC/GFP-BioF-TR, and cells were plated on minimal M9 medium with 100 μg/ml ampicillin and 1 mm isopropyl β-d-1-thiogalactopyranoside with or without 0.1 μg/ml biotin.
Creation of A. nidulans Deletion Mutants
Gene deletions were performed using a Multisite Gateway system (Invitrogen). The gene coding for the Aspergillus fumigatus GTP cyclohydrolase (riboB) was amplified from plasmid pTN2 (19) and cloned into plasmid pENTR/D-TOPO (Invitrogen). Two 500-bp fragments located upstream and downstream of the bioF, aoxA, aoxB, pexE, and foxA coding regions were amplified from A1145 genomic DNA and cloned around A. fumigatus riboB using the Multisite Gateway three-fragment vector construction kit (Invitrogen) to yield the final plasmids riboB-ΔbioF, riboB-ΔaoxA, riboB-ΔaoxB, riboB-ΔpexE, and riboB-ΔfoxA, respectively. Plasmids were transformed into A1145 and selected for riboflavin prototrophy on plates containing osmostable minimal medium with 1% glucose, 10 mm diammonium tartrate, and appropriate supplements. For the ΔpexE mutant, the auxotrophy test medium contained 1 m sorbitol to promote sporulation (20).
Localization of BioF to the Peroxisome and Complementation of the ΔbioF Mutant
The GFP-bioF fragments were excised from plasmids pTRC/GFP-BioF-FL and pTRC/GFP-BioF-TR (see above) with NcoI-BamHI and cloned into the same restriction sites of plasmid pTMH44.2 (21), which placed the fusion proteins under the control of the A. nidulans gpdA promoter and trpC terminator. The promoter-GFP-bioF-terminator expression cassette was excised by EcoRI-XbaI and cloned into the EcoRI-SpeI restriction sites of vector pMT1612, which contains the bar gene from Streptomyces hygroscopicus, conferring resistance to the herbicide glufosinate (dl-phosphinothricin) (19). For localization studies of BioF, plasmids pMT1612/GFP-BioF-FL and pMT1612/GFP-BioF-TR were separately cotransformed with a 10-fold molar excess of plasmid pDsRed-SKL (22) into protoplasts of a ΔbioF deletion mutant (see above). For complementation of biotin auxotrophy, plasmids pMT1612/GFP-BioF-FL and pMT1612/GFP-BioF-TR were transformed into protoplasts of the ΔbioF strain. Transformed protoplasts were selected on agar-solidified medium containing 0.25 mg/ml of DL-phosphinothricin (Duchefa Biochemie) and 25 ng/ml d-biotin. Primary phosphinothricin-resistant clones were first passed over medium containing phosphinothricin and biotin, followed by two passages over medium with biotin and no phosphinothricin, before being retested for phosphinothricin resistance. Stable transformants were subsequently grown on phosphinothricin-free medium supplemented with biotin.
Mycelia were grown in liquid minimal medium and transferred to a glass slide for microscopic examination. Microscopy was performed using a Zeiss LSM 700 confocal microscope with a C-Apochromat 63×/1.20 objective. Excitation of the samples was performed at 488 and 555 nm for chimeric proteins fused to GFP or DsRed, respectively. Fluorescent GFP emission was collected between 490 and 534 nm using a combination of a band pass filter (490–555 nm) and a dichroic beam splitter (534 nm). A long-pass filter (560 nm) was used for DsRed. The pinhole was adjusted to achieve a depth of field of 1 μm.
RESULTS
Biotin Synthesis Requires AON Synthase (BioF) Localization in the Peroxisome
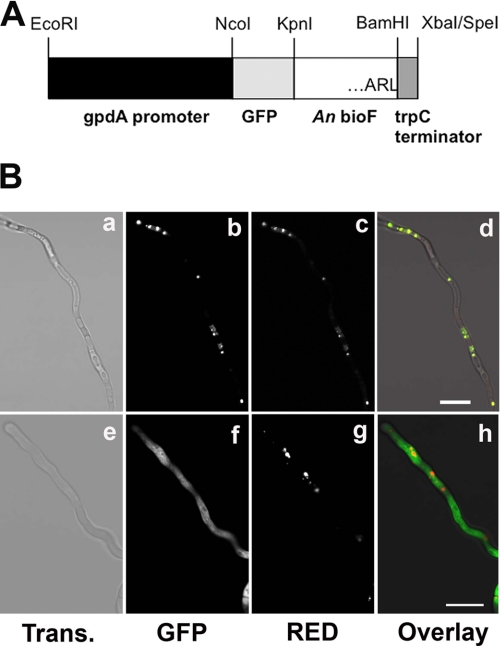
The protein sequence of A. nidulans BioF ends with the C-terminal tripeptide ARL (10). This sequence fits the consensus sequence (S/A/C)(K/R/H)(L/M) for PTS1 (23). To determine the localization of BioF experimentally, the coding sequence of GFP was fused in-frame to the 5′-extremity of bioF, leaving the putative C-terminal PTS1 intact. This chimeric gene was cloned between the constitutive glyceraldehyde-3-phosphate dehydrogenase promoter (gpdA) and the trpC terminator, creating plasmid GFP-BioF-FL (Fig. 1A). A truncated construct was also made that lacked the codons for the last three amino acids of the BioF protein, resulting in plasmid GFP-BioF-TR. Both constructs were separately cotransformed with the pDsRed-SKL plasmid into A. nidulans (22). The DsRed-SKL construct encodes Discosoma sp. red fluorescent protein (DsRed) modified at the C-terminal end by the addition of the PTS1 tripeptide SKL, allowing its import into the peroxisome (22, 24, 25). Confocal microscopy of transformant hyphae showed that the full-length GFP-BioF protein was present in discrete foci that were also labeled with the peroxisomal pDsRed-SKL reporter (Fig. 1B). In contrast, expression of the truncated GFP-BioF protein lacking the C-terminal tripeptide ARL produced a diffuse green fluorescence that did not match the peroxisomal DsRed localization (Fig. 1B). Thus, we concluded that BioF is a peroxisomal enzyme in A. nidulans and that the terminal tripeptide ARL is an indispensable component of a functional PTS1.
FIGURE 1.
Localization of A. nidulans BioF protein. A, the bioF open reading frame was fused to GFP, and the chimeric gene was expressed from the constitutive gpdA promoter. The BioF C-terminal amino acids are ARL. Construct GFP-BioF-FL contains full-length bioF fused to GFP (as shown in A), whereas construct GFP-BioF-TR contains bioF lacking the codons for the last three C-terminal amino acids (ARL) fused to GFP. An, A. nidulans. B, vegetative hyphae of stable transformants expressing a DsRed-PTS1 peroxisomal marker and either GFP-BioF-FL (panels a–d) or GFP-BioF-TR (panels e–h) were analyzed by confocal microscopy. Fluorescence was acquired in the green channel (GFP; panels b and f) and red channel (panels c and g). Light transmission (Trans.) images (panels a and e) and the superimposition of green and red fluorescence (panels d and h) are shown. Scale bars = 20 μm.
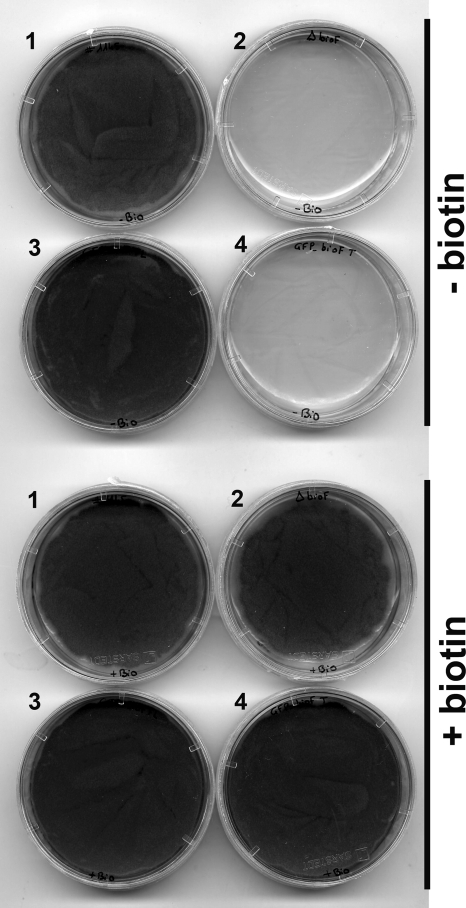
Deletion of the bioF gene by homologous recombination led to biotin auxotrophy with no hyphal growth when the mutant was grown on biotin-deficient medium, whereas growth and sporulation were normal on biotin-supplemented medium (Fig. 2). To determine whether BioF localization in the peroxisome is essential for biotin biosynthesis, the GFP-BioF-FL and GFP-BioF-TR constructs were introduced into a ΔbioF mutant. The peroxisomal GFP-BioF-FL variant restored biotin prototrophy to the ΔbioF mutant, whereas the cytosolic GFP-BioF-TR variant failed to complement the mutant phenotype (Fig. 2). To verify that the truncated GFP-BioF-TR variant retained AON synthase activity, both GFP-BioF-FL and GFP-BioF-TR variants were expressed in the E. coli biotin-auxotrophic mutant bioF103. Fig. 3 shows that both GFP-BioF-FL and GFP-BioF-TR variants complemented the bioF103 mutant phenotype, indicating that both are active in E. coli. These results show that AON synthase must be localized in the peroxisome to contribute to biotin synthesis in A. nidulans.
FIGURE 2.
Complementation of A. nidulans bioF deletion mutant with GFP-bioF chimeric constructs. A. nidulans biotin-prototrophic strain FGSC A1145 (dish 1), the ΔbioF mutant (dish 2), and the ΔbioF mutant transformed with either the GFP-BioF-FL (dish 3) or GFP-BioF-TR (dish 4) construct were grown on minimal medium deficient in biotin and supplemented with avidin (upper) or supplemented with biotin (lower).
FIGURE 3.
Complementation of E. coli biotin-auxotrophic mutant bioF103 with GFP-bioF constructs. The E. coli mutant bioF103 strain was transformed with a full-length A. nidulans bioF cDNA cloned in vector pTRC99A (sector 1), empty vector pTRC99A (sector 2), and either the GFP-BioF-FL (sector 3) or GFP-BioF-TR (sector 4) chimeric construct, both in pTRC99A. The medium in the left dish was biotin-deficient and contained avidin, whereas that in the right dish was supplemented with biotin.
Peroxisomal β-Oxidation Enzymes Contribute to Biotin Synthesis in A. nidulans
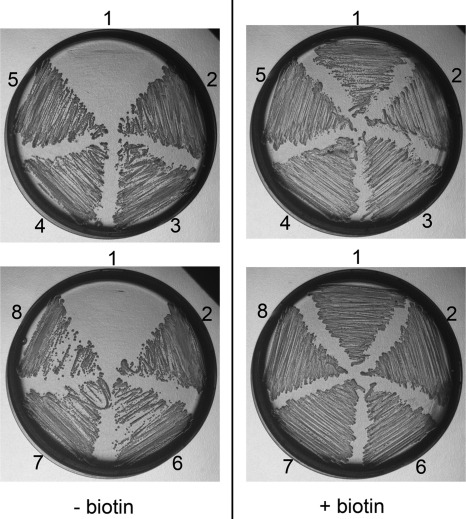
The A. nidulans pexE gene (locus AN10215) is the ortholog of S. cerevisiae and mammalian PEX5, which encodes the PTS1 receptor, essential for the import of proteins with a PTS1 into peroxisomes (26). A. nidulans ΔpexE mutants are biotin-auxotrophic (Fig. 4) (26), and this phenotype could be explained by a defect in the import of BioF from the cytosol into the peroxisome. However, the localization of the AON synthase enzyme to the peroxisome indicated that its substrates, pimeloyl-CoA and l-alanine, must also be present in the peroxisome. Because the peroxisome hosts the fatty acid β-oxidation cycle, the contribution of this pathway to biotin synthesis was examined. The first committed step of β-oxidation is mediated by acyl-CoA oxidase, whereas the second and third steps are mediated by the multifunctional protein with enoyl-CoA hydratase and 3-hydroxyacyl-CoA dehydrogenase activities. Two genes are known in A. nidulans that encode proteins with similarity to the S. cerevisiae acyl-CoA oxidase Pox1p (called aoxA and aoxB; loci AN6752 and AN6765, respectively), whereas another gene encodes a functional homolog of the S. cerevisiae multifunctional protein Fox2p (called foxA; locus AN7111) (27, 28). Comparison of the growth phenotypes of various mutants was performed by point inoculation of spores on minimal medium supplemented with biotin or without biotin and supplemented with avidin (to bind any contaminating biotin). Colonial growth of the biotin-prototrophic reference strain was clearly stimulated by the addition of biotin, but vigorous outgrowth still occurred on medium without biotin and with avidin (Fig. 4, upper and lower rows). Growth of the ΔaoxA and ΔpexE mutants was strongly inhibited in the absence of biotin, as was growth of the ΔbioF and biA1 mutants. The latter lacked the DAN synthase domain of the BioDA bifunctional protein (10). The ΔaoxB mutant was biotin-prototrophic, and, like the reference strain, it grew on biotin-deficient medium. The ΔfoxA mutant showed a clear reduction in colonial growth rate on biotin-deficient medium relative to the control strain, but it grew slightly faster than the ΔaoxA strain. The addition of pimelic acid to the biotin-deficient medium resulted in a significant increase in the colonial growth rate of mutants ΔaoxA and ΔfoxA but not of mutants ΔbioF, ΔpexE, biA1 (Fig. 4, middle row). These results indicate that enzymes of the peroxisomal β-oxidation cycle contribute to the synthesis of pimeloyl-CoA, which is essential for biotin production.
FIGURE 4.
A. nidulans mutants in the peroxisomal β-oxidation cycle require biotin for growth. Spores (103) from a control (CTL) biotin-prototrophic strain (FGSC A1145) and from biA1, ΔbioF, ΔaoxA, ΔaoxB, ΔpexE, and ΔfoxA mutants were point-inoculated individually in wells of a 24-well microtiter plate containing either minimal medium supplemented with biotin (lower row) or biotin-deficient minimal medium supplemented with avidin (upper row) or with avidin and pimelic acid (middle row) and incubated for 1 day at 37 °C and for 2 days at 28 °C. As reported previously (26), the ΔpexE mutant showed reduced sporulation compared with all other strains, even when grown on medium containing biotin, explaining the paler appearance of the ΔpexE colony. However, the mycelial growth rate of the ΔpexE mutant (colony diameter and mycelial density) is comparable with that of the wild-type control strain on biotin-supplemented medium.
BioF Orthologs throughout the Fungal Kingdom Contain a Peroxisomal Targeting Sequence
To determine whether the peroxisomal localization of BioF is a general feature of fungi or specific to Aspergilli, bioF ortholog genes were mined with TBLASTN using the A. nidulans BioF protein as the query, whereas gene models and proteins were manually deduced. A bioF gene encoding an AON synthase with a PTS1-like sequence was present in 97 fungal species, including all but 12 of the sequenced Pezizomycotina, all Basidiomycota, all Mucoromycotina, both Chytridiomycota, Allomyces macrogynus (Blastocladiomycota), and Capsaspora owczarzaki (Ichthyosporea class; Fungi/Metazoa incertae sedis) (supplemental data and Fig. S1). In most cases (Table 1), the C-terminal tripeptide fits with the canonical PTS1 sequence (S/A/C)(K/R/H)(L/M) or a more relaxed consensus that is still recognized by a PTS1 predictor algorithm (IMP Bioinformatics Group) (29).
TABLE 1.
Fungal BioF proteins with a consensus PTS1
Complete BioF protein sequences are given in FASTA format in the supplemental data. A phylogenetic tree of fungal BioF is presented in supplemental Fig. S1.
| PTS1 | Species | Classificationa |
|---|---|---|
| ARL | Aspergillus aculeatus, A. carbonarius, A. oryzae, A. flavus, A. terreus, A. niger, A. clavatus, A. fumigatus, A. nidulans | E |
| Coccidioides posadasii, C. immitis | ||
| Neosartorya fischeri | ||
| Uncinocarpus reesii | ||
| Alternaria brassicicola | D | |
| Cochliobolus heterostrophus | ||
| Leptosphaeria maculans | ||
| Mycosphaerella fijiensis, M. graminicola, | ||
| Phaeosphaeria nodorum | ||
| Pyrenophora teres f. teres, P. tritici-repentis | ||
| Geomyces destructans, G. pannorum | L | |
| Fusarium graminearum (Gibberella zeae), | S | |
| Sporotrichum thermophile (Thielavia heterothallica) | ||
| Coniophora puteana | A | |
| Dichomitus squalens | ||
| Fomitiporia mediterranea | ||
| Stereum hirsutum | ||
| Rhodotorula graminis | Pu | |
| FRL | Ajellomyces dermatitidis | E |
| PRL | Dothistroma septosporum (Mycosphaerella pini) | D |
| SRL | Talaromyces stipitatus | E |
| Epichloe festucae | S | |
| Glomerella graminicola | ||
| Nectria haematococca | ||
| Podospora anserina | ||
| Trichoderma reesei | ||
| D. squalens | A | |
| Schizophyllum commune | ||
| TRL | Septoria musiva (Mycosphaerella populorum) | D |
| AKL | Arthroderma benhamiae, A. gypseum, A. otae | E |
| Penicillium marneffei | ||
| Trichophyton verrucosum, T. rubrum, T. tonsurans, T. equinum | ||
| Botryotinia fuckeliana (Botrytis cinerea) | L | |
| Sclerotinia sclerotiorum | ||
| Fusarium oxysporum f. sp. lycopersici | S | |
| Gibberella moniliformis | ||
| Ceriporiopsis subvermispora | A | |
| C. puteana | ||
| Cryptococcus neoformans var. neoformans, C. gattii | ||
| D. squalens | ||
| F. mediterranea | ||
| Fomitopsis pinicola | ||
| Postia pacenta | ||
| Punctularia strigosozonata | ||
| Serpula lacrymans | ||
| Tremella mesenterica Fries | ||
| Trametes versicolor | ||
| Wolfiporia cocos | ||
| R. hodotorula graminis | Pu | |
| Sporobolomyces roseus | ||
| Mucor circinelloides | M | |
| Rhizopus oryzae | ||
| Batrachochytrium dendrobatidis | C | |
| Allomyces macrogynus | B | |
| CKL | Paracoccidioides brasiliensis | E |
| HKL | Postia pacenta | A |
| NKL | Cryphonectria parasitica | S |
| PKL | Phycomyces blakesleeanus | M |
| Spizellomyces punctatus | C | |
| SKL | Metarhizium anisopliae, M. acridum | S |
| Trichoderma atroviride, T. virens | ||
| Coprinopsis cinerea | A | |
| Heterobasidion annosum | ||
| Phanerochaete chrysosporium | ||
| Laccaria bicolor | ||
| Pleurotus ostreatus | ||
| Ustilago maydis | U | |
| Wallemia sebi | W | |
| Mortierella verticillata | M | |
| TKL | S. hirsutum | A |
| WKL | Penicillium chrysogenum | E |
| AKI | Chaetomium globosum | S |
| ARM | Aspergillus flavus, A.oryzae | E |
| AHL | Verticillium dahliae, V. albo-atrum | S |
| SHL | Puccinia graminis f. sp. tritici | Pu |
| ASL | Malassezia globosa | U |
| SSL | Capsaspora owczarzaki | I |
| AVL | Erysiphe pisi | L |
a Classification of fungi is abbreviated as follows. For Dikarya: D, Dithiodeomycetes class (subphylum Pezizomycotina of the Ascomycota); E, Eurotiomycetes class (subphylum Pezizomycotina of the Ascomycota); L, Leotiomycetes class (subphylum Pezizomycotina of the Ascomycota); S, Sordariomycetes class (subphylum Pezizomycotina of the Ascomycota); A, Agaricomycotina subphylum (Basidiomycota); Pu, Pucciniomycotina subphylum (Basidiomycota); U, Ustilaginomycotina subphylum (Basidiomycota); and W, Wallemiomycetes class (Basidiomycota incertae sedis). For basal fungal lineages: B, Blastocladiomycota (phylum); C, Chytridiomycota (phylum); M, Mucoromycotina (subphylum; Fungi incertae sedis); and I, Ichthyosporea (class; Fungi/Metazoa incertae sedis).
Interestingly, the absence of a bioF gene in all Microsporidia (a phylum of unicellular parasites of animals) and virtually all Saccharomycotina and Taphrinomycotina coincided with the absence of a eukaryotic bifunctional bioDA gene in these fungi (10). This was also true for 10 of the Pezizomycotina lacking bioF (supplemental data). In contrast, several species of Dikaryota have several BioF paralogs, where some contain a conventional PTS1, whereas others lack a PTS1-like sequence but instead have a C-terminal extension relative to the peroxisomal BioF (supplemental data). Unlike most other species in the Aspergillus genus, A. nidulans has only one bioF gene.
Biotin Synthesis Does Not Involve the Peroxisome or β-Oxidation in S. cerevisiae
We tested E. coli mutants with deletions in genes involved in the β-oxidation cycle under aerobic (ΔfadA, ΔfadB, and ΔfadE) and anaerobic (ΔfadK, ΔfadJ, and ΔfadI) conditions and found that all are biotin prototrophs, whereas the ΔbioD mutant is a biotin auxotroph (Fig. 5). These results show that the β-oxidation cycle is not essential for biotin biosynthesis in E. coli.
FIGURE 5.
β-Oxidation cycle is not required for biotin synthesis in E. coli. Cells were grown on medium supplemented with biotin (right) or on biotin-depleted medium containing avidin (left). The strains used were biotin-auxotrophic ΔbioD (sector 1); control BW25113 (sector 2); and deletion mutants ΔfadA (sector 3), ΔfadB (sector 4), ΔfadE (sector 5), ΔfadK (sector 6), ΔfadJ (sector 7), and ΔfadI (sector 8). Plates were incubated under aerobic (upper) or anaerobic (lower) conditions.
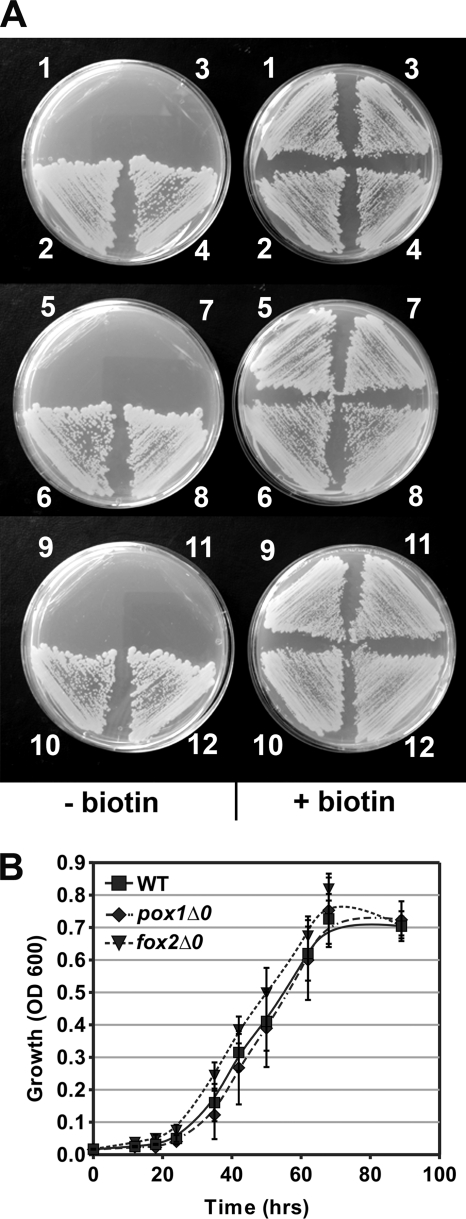
Although no structural ortholog of bioF is found in S. cerevisiae, AON synthase is encoded in this organism and in closely related species by BIO6, a structural paralog of the BIO3 gene, which encodes the bacterium-type DAN synthase (12, 30). Because no peroxisomal targeting sequence was identified in Bio6p, we investigated the roles of the peroxisome and β-oxidation cycle in S. cerevisiae biotin synthesis. Many S. cerevisiae laboratory strains, including reference strain BY4742 used in the Saccharomyces Genome Deletion Project, require biotin due the absence of the syntenic BIO6 and BIO1 genes (12, 30). The BIO6-BIO1 gene cluster of prototrophic strain A364a was cloned and introduced into BY4742 and the deletions mutants derived from it, and all strains were tested for biotin prototrophy. The BIO6/BIO1-transformed mutants pox1Δ0, fox2Δ0, and pot1Δ0, deficient in peroxisomal acyl-CoA oxidase, multifunctional protein, and 3-ketothiolase, respectively, were all biotin prototrophs despite the complete block of the β-oxidation cycle (Fig. 6A). Furthermore, BIO6/BIO1-transformed mutants pex5Δ0 and pex7Δ0, defective in peroxisomal protein import of PTS1- and PTS2-tagged proteins, respectively, were also biotin prototrophs (Fig. 6A) (31). The growth kinetics of the β-oxidation-deficient strains pox1Δ0/BIO and fox2Δ0/BIO on biotin-deficient medium were similar to those of the BY4742/BIO control strain (Fig. 6B). Thus, in contrast to A. nidulans, the β-oxidation cycle does not contribute to biotin synthesis in S. cerevisiae.
FIGURE 6.
Peroxisomal β-oxidation cycle or import of peroxisomal proteins is dispensable for biotin synthesis in S. cerevisiae. A, strains transformed with either empty vector p416 or a plasmid carrying the BIO6/BIO1 gene cluster (BIO) were grown on medium containing biotin (right) or on biotin-depleted medium supplemented with avidin (left). The strain/plasmid combinations were BY4742/p416 (sector 1), BY4742/BIO (sector 2), pox1Δ0/p416 (sector 3), pox1Δ0/BIO (sector 4), fox2Δ0/p416 (sector 5), fox2Δ0/BIO (sector 6), pot1Δ0/p416 (sector 7), pot1Δ0/BIO (sector 8), pex5Δ0/p416 (sector 9), pex5Δ0/BIO (sector 10), pex7Δ0/p416 (sector 11), and pex7Δ0/BIO (sector 12). B, growth kinetics of strains BY4742/BIO (WT), pox1Δ0/BIO, and fox2Δ0/BIO in liquid biotin-depleted medium supplemented with avidin. Absorbance at 600 nm (OD 600; 0.6-cm light path) was recorded. Error bars represent S.E. (n = 6).
DISCUSSION
We have shown that A. nidulans AON synthase encoded by the bioF gene is a peroxisomal enzyme and that its proper localization is essential for biotin synthesis. While this work was under review, Tanabe et al. (32) reported that the AON synthase of both Aspergillus oryzae and the plant Arabidopsis thaliana are found in peroxisomes. Our current work further extends this observation by showing that fungal orthologs of A. nidulans BioF also contain PTS1 sequences, which strongly suggests that functional localization of AON synthase in the peroxisome is conserved throughout the fungal kingdom. However, notable exceptions are the alternative AON synthase (Bio6p) found in S. cerevisiae and other members of the Saccharomyces genus (see further discussion below). In contrast, biotin synthase is an iron-sulfur protein localized in the mitochondria of S. cerevisiae, and all fungal orthologs are predicted to be mitochondrial, including A. nidulans BioB (33). A mitochondrial targeting sequence is predicted for the A. nidulans BioDA bifunctional protein with a low probability and C-terminal fusion between A. oryzae BioDA and GFP was shown recently to be located in mitochondria (32). However, no mitochondrial targeting sequences are detectable in S. cerevisiae Bio3p and Bio4p, which suggests that DAN and DTB synthase steps occur in the cytosol in ascomycete yeasts. Thus, the biotin biosynthetic pathway of most fungi would be physically divided over at least two distinct compartments.
Mutations in the aoxA or foxA genes strongly reduced the colonial growth rate of A. nidulans on biotin-deficient medium, suggesting that the peroxisomal β-oxidation cycle contributes to biotin synthesis. The addition of pimelic acid to the medium substantially improved growth of the ΔaoxA and ΔfoxA mutants, but their growth was still not as robust as that of the control biotin-prototrophic strain, possibly due to a limitation in the uptake of pimelate or its activation to pimeloyl-CoA. These results indicate that pimeloyl-CoA must be generated primarily in the peroxisomes by the β-oxidation of an unknown acyl-CoA with a chain length longer than that of pimelic acid (C7). Although it remains possible that the residual growth of the ΔaoxA and ΔfoxA mutants on biotin-deficient medium was due to a minor contribution of a pathway other than peroxisomal β-oxidation for pimeloyl-CoA synthesis, it is more likely that this residual growth was due to the presence of other peroxisomal enzymes contributing to a residual β-oxidation capacity. Indeed, previous studies describing the ΔaoxA (28) and ΔfoxA (27) mutants reported substantial residual growth of both mutants on medium with oleic acid as the main carbon source, indicating residual β-oxidation activity in these strains. Although bioF appears to be unique in the A. nidulans genome, explaining the lack of growth of the ΔbioF mutant on biotin-deficient medium, considerable redundancy exists among the β-oxidation genes. For example, A. nidulans contains at least eight proteins with similarity to acyl-CoA dehydrogenases containing PTS1 sequences, in addition to two peroxisomal acyl-CoA oxidases, AoxA and AoxB (28). Genes other than foxA are also known in A. nidulans that might encode enzymes with enoyl-CoA hydratase and/or 3-hydroxyacyl-CoA dehydrogenase activity (27, 34).
Although AON synthase has been demonstrated to be active with pimeloyl-CoA, it is unknown whether it also has activity toward pimeloyl-ACP (35, 36). BioC-BioH in E. coli and BioI in B. subtilis are postulated to result in the formation of pimeloyl-ACP, whereas BioW is a pimeloyl-CoA synthetase (9). However, the activities of BioW toward ACP and BioI toward acyl-CoAs are undetermined. Thus, the identity of the pimeloyl thioester used in vivo in both E. coli and B. subtilis remains to be determined. In this context, the localization of AON synthase in peroxisomes in fungi and the participation of the β-oxidation cycle in biotin synthesis in A. nidulans imply that pimeloyl-CoA is the substrate for the AON synthase in fungi. Furthermore, the absence of involvement of the β-oxidation cycle in E. coli implies that the BioC-BioH systems must directly generate the pimelate thioester rather than longer odd-chain dicarboxylate thioesters that would require chain shortening via the β-oxidation cycle.
Although BioC-BioH and BioI-BioW constitute the best described pathways for the synthesis of the pimelate moiety, it is clear that several alternative pathways must exist. In bacteria in which bioH is missing, the bioC gene is often associated with another structurally unrelated gene, bioG, or bioK in cyanobacteria (37). Whether BioG or BioK shares the same activity as BioH is unknown. In bacteria lacking bioC, bioH, bioI, or bioW, the classical bioA/bioB/bioD/bioF operon is sometimes associated with genes sharing similarity with fatty acid biosynthetic genes, such as in Desulfovibrio vulgaris or Desulfovibrio desulfuricans (38). Proteins with significant similarity to BioC, BioI, BioH, or BioW are not found in fungal genomes, including A. nidulans or S. cerevisiae, which suggests the involvement of a distinct pathway in fungi for the production of the pimelate moiety.
The absence of a peroxisome-localized AON synthase and the lack of involvement of PEX5, PEX7, or peroxisomal β-oxidation enzymes in biotin synthesis by S. cerevisiae suggest that the pimelate thioester biosynthetic pathway found in S. cerevisiae and related species is distinct from that in A. nidulans and most other fungi. Hall and Dietrich (30) demonstrated a distinct evolutionary history for the genes encoding the last four steps of the biotin biosynthetic pathway in S. cerevisiae. Their work suggests that the biotin pathway from pimelate onwards was lost in the ancestor of S. cerevisiae and subsequently reacquired by a combination of horizontal gene transfer from bacteria and neofunctionalization of a duplicated gene. The distinct evolutionary history of the S. cerevisiae BIO genes is highlighted by the fact that, although the DAN and DTB synthases reside together on a single protein encoded by the bifunctional gene bioDA in most biotin-prototrophic Eukaryota (fungi, öomycetes, and plants), these two activities are encoded by distinct genes in bacteria and S. cerevisiae (10). Our present work strongly suggests that there are also differences between ascomycete yeasts and filamentous fungi concerning the synthesis of the key pimelic acid intermediate. Future studies of the early part of the biotin biosynthetic pathway (i.e. before the first committed step catalyzed by the AON synthase) in S. cerevisiae and A. nidulans are therefore complementary and likely to reveal alternative routes for the generation of pimelate thioester, which are distinct and different from those described in bacteria.
Supplementary Material
Acknowledgment
We thank Stefanie Pöggeler (University of Göttingen) for providing the pDsRed-SKL plasmid.
This work was supported in part by Fonds National Suisse de la Recherche Scientifique Grant 3100A0-122493 (to Y. P.). Work at the Instituto de Agroquímica y Tecnología de Alimentos was supported by Ministerio de Ciencia e Innovación (MCINN) Grant BIO2008-00228.

The on-line version of this article (available at http://www.jbc.org) contains supplemental data, Fig. S1, and Table S1.
- ACP
- acyl carrier protein
- AON
- 8-amino-7-oxononanoate
- DAN
- 7,8-diaminononanoate
- DTB
- dethiobiotin
- PTS1
- type 1 peroxisomal targeting sequence.
REFERENCES
- 1. Zempleni J., Wijeratne S. S., Hassan Y. I. (2009) Biofactors 35, 36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lin S., Cronan J. E. (2011) Mol. BioSyst. 7, 1811–1821 [DOI] [PubMed] [Google Scholar]
- 3. Streit W. R., Entcheva P. (2003) Appl. Microbiol. Biotechnol. 61, 21–31 [DOI] [PubMed] [Google Scholar]
- 4. Cleary P. P., Campbell A. (1972) J. Bacteriol. 112, 830–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rolfe B., Eisenberg M. A. (1968) J. Bacteriol. 96, 515–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lin S., Hanson R. E., Cronan J. E. (2010) Nat. Chem. Biol. 6, 682–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cryle M. J., Schlichting I. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 15696–15701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stok J. E., De Voss J. (2000) Arch. Biochem. Biophys. 384, 351–360 [DOI] [PubMed] [Google Scholar]
- 9. Bower S., Perkins J. B., Yocum R. R., Howitt C. L., Rahaim P., Pero J. (1996) J. Bacteriol. 178, 4122–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Magliano P., Flipphi M., Sanglard D., Poirier Y. (2011) Fungal Genet. Biol. 48, 208–215 [DOI] [PubMed] [Google Scholar]
- 11. Phalip V., Kuhn I., Lemoine Y., Jeltsch J. M. (1999) Gene 232, 43–51 [DOI] [PubMed] [Google Scholar]
- 12. Wu H., Ito K., Shimoi H. (2005) Appl. Environ. Microbiol. 71, 6845–6855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baba T., Ara T., Hasegawa M., Takai Y., Okumura Y., Baba M., Datsenko K. A., Tomita M., Wanner B. L., Mori H. (2006) Mol. Systems Biol. 2, 2006.0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cove D. J. (1966) Biochim. Biophys. Acta 113, 51–56 [DOI] [PubMed] [Google Scholar]
- 15. Tilburn J., Scazzocchio C., Taylor G. G., Zabicky-Zissman J. H., Lockington R. A., Davies R. W. (1983) Gene 26, 205–221 [DOI] [PubMed] [Google Scholar]
- 16. Del Campillo-Campbell A., Kayajanian G., Campbell A., Adhya S. (1967) J. Bacteriol. 94, 2065–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pokorska A., Drevet C., Scazzocchio C. (2000) J. Mol. Biol. 298, 585–596 [DOI] [PubMed] [Google Scholar]
- 18. Amann E., Ochs B., Abel K. J. (1988) Gene 69, 301–315 [DOI] [PubMed] [Google Scholar]
- 19. Nayak T., Szewczyk E., Oakley C. E., Osmani A., Ukil L., Murray S. L., Hynes M. J., Osmani S. A., Oakley B. R. (2006) Genetics 172, 1557–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Han K. H., Lee D. B., Kim J. H., Kim M. S., Han K. Y., Kim W. S., Park Y. S., Kim H. B., Han D. M. (2003) J. Microbiol. 41, 34–40 [Google Scholar]
- 21. McDonald T., Brown D., Keller N. P., Hammond T. M. (2005) Mol. Plant-Microbe Interact. 18, 539–545 [DOI] [PubMed] [Google Scholar]
- 22. Elleuche S., Poeggeler S. (2008) Fungal Genet. Rep. 55, 9–12 [DOI] [PubMed] [Google Scholar]
- 23. Lametschwandtner G., Brocard C., Fransen M., Van Veldhoven P., Berger J., Hartig A. (1998) J. Biol. Chem. 273, 33635–33643 [DOI] [PubMed] [Google Scholar]
- 24. Engh I., Würtz C., Witzel-Schlömp K., Zhang H. Y., Hoff B., Nowrousian M., Rottensteiner H., Kück U. (2007) Eukaryot. Cell 6, 831–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ruprich-Robert G., Berteaux-Lecellier V., Zickler D., Panvier-Adoutte A., Picard M. (2002) Genetics 161, 1089–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hynes M. J., Murray S. L., Khew G. S., Davis M. A. (2008) Genetics 178, 1355–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maggio-Hall L. A., Keller N. P. (2004) Mol. Microbiol. 54, 1173–1185 [DOI] [PubMed] [Google Scholar]
- 28. Reiser K., Davis M. A., Hynes M. J. (2010) Curr. Genet. 56, 139–150 [DOI] [PubMed] [Google Scholar]
- 29. Neuberger G., Maurer-Stroh S., Eisenhaber B., Hartig A., Eisenhaber F. (2003) J. Mol. Biol. 328, 581–592 [DOI] [PubMed] [Google Scholar]
- 30. Hall C., Dietrich F. S. (2007) Genetics 177, 2293–2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lazarow P. B. (2006) Biochim. Biophys. Acta 1763, 1599–1604 [DOI] [PubMed] [Google Scholar]
- 32. Tanabe Y., Maruyama J., Yamaoka S., Yahagi D., Matsuo I., Tsutsumi N., Kitamoto K. (2011) J. Biol. Chem. 286, 30455–30461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mühlenhoff U., Richhardt N., Gerber J., Lill R. (2002) J. Biol. Chem. 277, 29810–29816 [DOI] [PubMed] [Google Scholar]
- 34. Magliano P., Sanglard D., Poirier Y. (2010) Biochim. Biophys. Acta 1801, 1386–1392 [DOI] [PubMed] [Google Scholar]
- 35. Alexeev D., Alexeeva M., Baxter R. L., Campopiano D. J., Webster S. P., Sawyer L. (1998) J. Mol. Biol. 284, 401–419 [DOI] [PubMed] [Google Scholar]
- 36. Webster S. P., Alexeev D., Campopiano D. J., Watt R. M., Alexeeva M., Sawyer L., Baxter R. L. (2000) Biochemistry 39, 516–528 [DOI] [PubMed] [Google Scholar]
- 37. Rodionov D. A., Mironov A. A., Gelfand M. S. (2002) Genome Res. 12, 1507–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rodionov D. A., Dubchak I., Arkin A., Alm E., Gelfand M. S. (2004) Genome Biol. 5, R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.