Background: Flavin nucleotide metabolism in plants is incompletely understood.
Results: Gene At1g79790 encodes an FMN-specific hydrolase (AtcpFHy1) that localizes to plastids.
Conclusion: This is the first study reporting cloning and characterization of an FMN hydrolase from plastids.
Significance: This study advances the knowledge of flavin nucleotide metabolism in plants.
Keywords: Chloroplast, Flavin, FMN, Hydrolases, Plant
Abstract
FMN hydrolases catalyze dephosphorylation of FMN to riboflavin. Although these enzymes have been described in many organisms, few had their corresponding genes cloned and their recombinant proteins biochemically characterized, and none had their physiological roles determined. We found previously that FMN hydrolase activity in pea chloroplasts is Mg2+-dependent, suggesting an enzyme of the haloacid dehalogenase (HAD) superfamily. In this study, a new FMN hydrolase was purified by multistep chromatography after ammonium sulfate precipitation. The molecular weight of the native protein was estimated at ∼59,400, a dimer of about twice the predicted molecular weight of most HAD superfamily phosphatases. After SDS-PAGE of the partially purified material, two separate protein bands within 25–30 kDa were extracted from the gel and analyzed by nanoLC-MS/MS. Peptide sequence matching to the protein samples suggested the presence of three HAD-like hydrolases. cDNAs for sequence homologs from Arabidopsis thaliana of these proteins were expressed in Escherichia coli. Activity screening of the encoded proteins showed that the At1g79790 gene encodes an FMN hydrolase (AtcpFHy1). Plastid localization of AtcpFHy1 was confirmed using fluorescence microscopy of A. thaliana protoplasts transiently expressing the N-terminal fusion of AtcpFHy1 to enhanced green fluorescent protein. Phosphatase activity of AtcpFHy1 is FMN-specific, as assayed with 19 potential substrates. Kinetic parameters and pH and temperature optima for AtcpFHy1 were determined. A phylogenetic analysis of putative phosphatases of the HAD superfamily suggested distinct evolutionary origins for the plastid AtcpFHy1 and the cytosolic FMN hydrolase characterized previously.
Introduction
All organisms require FMN and FAD for vital metabolic processes. Examples include photosynthesis, mitochondrial electron transport, fatty acid oxidation, DNA repair, photoresponse, bioluminescence, biosynthesis of secondary metabolites, and metabolism of vitamins B6, B12, and folic acid. The biosynthetic pathway leading to FMN and FAD is well understood in plants, yeast, and bacteria (1–3). In these organisms, the precursor riboflavin is first synthesized from GTP and ribulose 5-phosphate, and then, sequentially, phosphorylated to FMN by riboflavin kinase and adenylylated to FAD by FAD adenylyltransferase (1–3).
Flavin nucleotide turnover is less understood. Enzymes that hydrolyze FMN (FMN hydrolase) (4–14) or FAD (FAD pyrophosphatase) (4, 7, 15–18) have been described in various organisms, but little is known about the responsible catalysts and their physiological roles. To advance the knowledge of flavin nucleotide turnover and to expand on our earlier work on flavin nucleotide metabolism in plants (9, 19), this study focuses on an FMN-hydrolyzing enzyme we detected in chloroplast extracts.
Known FMN-hydrolyzing enzymes fall into two classes: acid phosphatases and haloacid dehalogenases (HADs).4 FMN-hydrolyzing acid phosphatases (EC 3.1.3.2), which are generally nonspecific catalysts with broad substrate specificity, have been described in plants (7, 8, 20) and in other organisms (4, 10–14). These enzymes function best in an acidic environment, consistent with their predicted subcellular localization in the vacuole in eukaryotes, and they become inactive at pH > 5.5–6.0 (7, 8, 11).
An FMN-hydrolyzing enzyme from Arabidopsis thaliana, putatively localized in the cytosol as a fusion to a riboflavin kinase (9), is the only HAD superfamily FMN hydrolase from plants that has the corresponding gene cloned and the recombinant enzyme characterized. This Mg2+-dependent enzyme catalyzes FMN hydrolysis most effectively at pH 6.0–8.0, in agreement with its predicted localization in the cytosol. Several HAD superfamily phosphatases from Escherichia coli hydrolyze FMN plus a variety of other substrates, including nucleotide, deoxynucleotide, and sugar phosphates and phosphorylated cofactors (21). Substrate specificity of the cytosolic FMN hydrolase from A. thaliana (9) has yet to be investigated.
We report here on cDNA cloning, recombinant expression, and biochemical characterization of a plastid-localized FMN hydrolase from A. thaliana (AtcpFHy1) that belongs to the HAD enzyme superfamily. Unlike the HAD superfamily phosphatases from E. coli (21), AtcpFHy1 is FMN-specific. A phylogenetic analysis of a subgroup of the HAD superfamily phosphatases from A. thaliana suggests distinct evolutionary origins for the plastid AtcpFHy1 and the cytosolic FMN hydrolase characterized previously (9).
EXPERIMENTAL PROCEDURES
Materials and Plant Growth Conditions
BugBuster reagent and recombinant enterokinase were obtained from Novagen (Madison, WI). Percoll and the gel filtration calibration kits were from GE Healthcare. IMP and glucose 6-phosphate were from Fluka. Pyridoxal 5′-phosphate was from Research Organics (Cleveland, OH). Oligonucleotides and all other substrates were from Sigma.
Peas, Pisum sativum cv. Bohatyr, were grown in coarse vermiculite under 16 h of light (∼75 μE m−2 s−1) for 10 days. A. thaliana plants, ecotype Columbia, were grown in potting soil under 12 h of light (∼100 μE m−2 s−1) for 3 weeks. Temperature was set at 22 °C during the light period and at 18 °C during the dark period.
Isolation of Pea Chloroplasts
Pea chloroplasts were isolated by differential centrifugation followed by centrifugation on Percoll density gradients using published procedures (22, 23) as described before (19).
Purification of an FMN Hydrolase from Pea Chloroplasts
Intact pea chloroplasts were resuspended in 50 mm Tris-HCl buffer, pH 7.8, and broken by four cycles of freezing and thawing. The chloroplast extract was cleared by centrifugation at 22,800 × g for 15 min at 4 °C followed by desalting the supernatant into buffer A (50 mm Tris-HCl, pH 7.8, 10 mm MgCl2, 1 mm DTT, and 0.2% Tween 20) using an ÄKTA FPLC system equipped with a HiPrep 26/10 desalting column (GE Healthcare). To the soluble protein extract was added powdered (NH4)2SO4 to a 1.5 m final concentration, and the precipitate was removed by centrifugation at 1,500 × g for 10 min at 4 °C. The remaining soluble protein was purified by hydrophobic interaction chromatography on a 5-ml butyl-Sepharose HP column (GE Healthcare) equilibrated with buffer A plus 1.5 m (NH4)2SO4 and eluted with buffer B (20 mm Tris-HCl, pH 7.8, 10 mm MgCl2, 1 mm DTT, and 0.2% Tween 20) over 20 column volumes. FMN hydrolase activity eluted at about 180 mm (NH4)2SO4. The pooled fraction with FMN hydrolase activity was desalted into buffer C (10 mm potassium phosphate, pH 7.2, 10 mm MgCl2, 1 mm DTT, and 0.2% Tween 20) and purified by hydroxyapatite chromatography on a 3.0-ml CHT ceramic hydroxyapatite column (Bio-Rad) equilibrated with buffer D (1 mm potassium phosphate, pH 7.2, 10 mm MgCl2, 1 mm DTT, and 0.2% Tween 20), and eluted with buffer E (100 mm potassium phosphate, pH 7.2, 10 mm MgCl2, 1 mm DTT, and 0.2% Tween 20) over 35 column volumes. FMN hydrolase activity eluted at about 40 mm potassium phosphate. The pooled FMN hydrolase fraction was diluted three times in buffer A and purified by anion exchange chromatography on a Mono Q 5/50 GL column (GE Healthcare) equilibrated with buffer A and eluted with buffer A plus 700 mm NaCl over 17 column volumes. FMN hydrolase activity eluted at about 200 mm NaCl. The pooled FMN hydrolase fraction was desalted into buffer F (50 mm Tris-HCl, pH 7.0, 10 mm MgCl2, 1 mm DTT, and 0.2% Tween 20) and reapplied to a Mono Q 5/50 GL column equilibrated with buffer F and eluted with buffer F plus 1 m NaCl over 10 column volumes. FMN hydrolase activity eluted at about 350 mm NaCl. The pooled FMN hydrolase fraction was concentrated using an ultrafiltration membrane of a 10,000-molecular weight cutoff value (Pall Life Sciences, Ann Arbor, MI) and purified by gel filtration chromatography on a Superdex 200 10/300 column (GE Healthcare) equilibrated with buffer A. Reference proteins used to estimate the molecular weight of the native protein were ribonuclease A (13,700), carbonic anhydrase (29,000), ovalbumin (43,000), conalbumin (75,000), and ferritin (440,000). To reduce the sample volume, FMN hydrolase-containing fractions were pooled, desalted into buffer F, and applied to a Mono Q 5/50 GL column equilibrated with buffer F and eluted with buffer F plus 1 m NaCl over 10 column volumes. FMN hydrolase activity eluted at about 350 mm NaCl. The purified FMN hydrolase material was desalted into water using 2-ml Zeba desalt spin columns (Pierce Biotechnology), concentrated using a centrifugal concentration system, and then separated by SDS-PAGE. The gel was stained with colloidal Coomassie Blue G-250 using the SimplyBlue SafeStain (Invitrogen). Two separate protein bands within 25–30 kDa were extracted from the gel and digested with Trypsin Gold (Promega, Madison, WI) following the manufacturer's procedures. The digested protein samples were analyzed by nanoLC-MS/MS.
NanoLC-MS/MS Analysis of the FMN-hydrolyzing Enzyme Preparation
Purified protein material was analyzed using an 1100 series HPLC–Chip-Cube system, coupled with an LC/MSD Trap XCT Ultra mass spectrometer (Agilent Technologies). For instrument control, the ChemStation B.01.03 and TrapControl 6.1 programs (Agilent Technologies) were used. Solvent A contained 0.1% formic acid, solvent B 99% acetonitrile in 0.1% formic acid. The flow rate for analytical chromatography was set at 0.3 μl/min. An 8-μl sample was first injected onto the 40-nl enrichment column of a G4240-62001 chip column (Agilent Technologies) in solvent A at 4 μl/min. After a 5-min wash with 3% solvent B, the flow path was then diverted to a ZORBAX 300SB-C18 column (75 × 43 mm, 5-μm particle size; Agilent Technologies). Solvent composition was ramped to 45% solvent B over 27 min for sample separation, then changed to 95% solvent B for 3 min to clean the column, and then changed to initial conditions for 6.5 min to re-equilibrate the column. The total time of the chromatographic separation was 42.5 min. The electron spray ionization was enabled by a capillary voltage of 1,900 V and a nitrogen gas flow of 4 liters/min at 325 °C. Parent spectra were measured in positive mode, standard-enhanced with an integrated ion current smart target setting of 200,000 and a maximum cumulative time of 100 ms. The scan range was from 200 to 1,400 m/z. Collision-induced dissociation fragmentation was carried out automatically with preference for multiply charged precursor ions. The fragmentation energy was adjusted online by smart parameter setting.
Because no genome information on pea was available, a custom database containing predicted protein sequences and translated expressed sequence tags of related plants of the tribe Trifolieae was created using search and analysis tools at the National Center for Biotechnology Information. To enable a target-decoy search strategy with estimation of false positive rates, a decoy database with reverse sequences was generated and merged with the original one. The final search database consisted of ∼24,000 entries.
The XCT Ultra data files, rendered in .yep format, were converted to .mzXML format using the CompassXport program (Bruker). Next, the .mzXML-formatted files were automatically processed by a DOS BATCH script OMSSAVALIDATION.BAT using the Open Mass Spectrometry Search Algorithm (OMSSA) (24) as a database search engine and PeptideProphet (25) and ProteinProphet (26) for hit validation. Manual calculations of fragmentation spectra were conducted using mMass 3.7 (27).
Raw data and identification results were converted to standard-compliant PRIDE XML using the PRIDE 2.3.2 converter (28) with a ProteinProphet probability score set to ≥0.9. This PRIDE XML file was submitted to the European Bioinformatic Institute (EBI) PRIDE server, where the data can be found under accession number 12272, project name “cpFHy,” and sample description “RL2.”
cDNA Cloning, Constructs, Sequence Analysis, and Expression in E. coli
Full-length cDNA clones for At1g79790, At3g48420, and At4g11570 were obtained from the Arabidopsis Biological Resource Center. Sequence comparison and ChloroP 1.1 prediction were used in combination to estimate transit peptide lengths for the three proteins. Recombinant At1g79790 was truncated by 26 amino acids, following the exact ChloroP 1.1 prediction. At3g48420 and At4g11570 were truncated by 53 and 52 amino acids, respectively. The decision to render these two recombinant proteins slightly longer than their ChloroP 1.1 predictions (65 and 70 amino acids, respectively) was based on visual inspection of At3g48420, At4g11570, and their sequence homologs from other plant species.
The open reading frames minus the regions encoding putative organellar-targeting peptides were amplified by PCR using Pfu DNA polymerase (Stratagene, La Jolla, CA) and the primer pairs 5′-GACGACGACAAGATGGCCTCGGGTTCTTGCTGT-3′ (forward) and 5′-GAGGAGAAGCCCGGTTCATAATTTGGGTACAGA-3′ (reverse) for At1g79790, 5′-GACGACGACAAGATGGTATACAGATCATCT-3′ (forward) and 5′-GAGGAGAAGCCCGGTTTAACTAACGAACTGTTT-3′ (reverse) for At3g48420, and 5′-GACGACGACAAGATGACGCAGAGTGTGAAA-3′ (forward) and 5′-GAGGAGAAGCCCGGTTCAGAAATCATCAACTGC-3′ (reverse) for At4g11570. To generate the expression vectors, the resulting PCR fragments were purified using Wizard PCR prep mini-columns (Promega), treated with T4 DNA polymerase (Promega), and then ligated into the pET-30 Ek/LIC vector (Novagen). All procedures were carried out in accordance with the manufacturer's protocols. Each construct was verified by DNA sequencing. The expression vectors were then introduced into the BL21 Star strain of E. coli (Invitrogen) to produce the recombinant proteins. Bacteria carrying the expression vectors were cultured at 37 °C in LB medium containing 100 μg/ml kanamycin until the absorbance at 600 nm reached 0.6–1. After the addition of isopropyl β-d-thiogalactopyranoside (1 μm final concentration), the culture was further incubated at 15 °C overnight. The induced bacteria were spun down at 5,000 × g for 15 min at 4 °C. The collected bacteria were frozen in liquid N2 and stored at −80 °C until use.
Purification of Recombinant At1g79790, At3g48420, and At4g11570
Bacterial pellets were resuspended in 5 ml of lysis buffer (50 mm Tris-HCl, pH 8.0, 10 mm MgCl2, 1 mm Tris(hydroxypropyl)phosphine, and 1× BugBuster reagent). After a 15-min incubation at room temperature, cell lysates were cleared by centrifugation at 22,800 × g for 15 min at 4 °C. Cleared cell lysates were incubated with 0.5 ml of Ni-NTA His·Bind resin (Novagen) at 8 °C on a rotary shaker set at 120 rpm for 1 h followed by filtering the sample-resin mixture through a disposable polypropylene filter column (Fisher). Binding, wash, and elution buffers contained 50 mm Tris-HCl, pH 8.0, 300 mm NaCl, 10 mm MgCl2, 10 (binding), 20 (wash), or 250 mm (elution) imidazole, 0.5 mm Tris(hydroxypropyl)phosphine, 10% glycerol, and 0.2% Tween 20. Eluate fractions were immediately desalted using PD-10 desalting columns (GE Healthcare) equilibrated with buffer G (50 mm Tris-HCl, pH 8.0, 10 mm MgCl2, 1 mm Tris(hydroxypropyl)phosphine, 10% glycerol, and 0.2% Tween 20) and then digested with recombinant enterokinase to release the tag. The digested enzyme was purified on a Superdex 200 10/300 GL column equilibrated with buffer G. Purified At1g79790 was frozen in liquid N2 and stored at −80 °C until use. Freezing did not affect the enzyme activity.
Transient Expression of EGFP-fused AtcpFHy1 in A. thaliana Protoplasts
A construct carrying the in-frame fusion of the full-length AtcpFHy1 sequence to the N terminus of EGFP in the p2GWF7 vector (29) was generated using the Gateway cloning system (Invitrogen) as instructed by the manufacturer. The full-length coding sequence for AtcpFHy1 was amplified using Pfu DNA polymerase and the primer pair 5′-AAAAAGCAGGCTATGGCGGCGGCGGCGATGCA-3′ and 5′-AGAAAGCTGGGTGTAATTTGGGTACAGAGACGT-3′. The resulting PCR fragments were re-amplified using attB adapter primers (Invitrogen), purified using Wizard PCR columns, and subcloned into the pDONR 221 vector (Invitrogen) using BP recombination. The coding sequence for AtcpFHy1 was then introduced into the p2GWF7 vector from pDONR 221 using LR recombination.
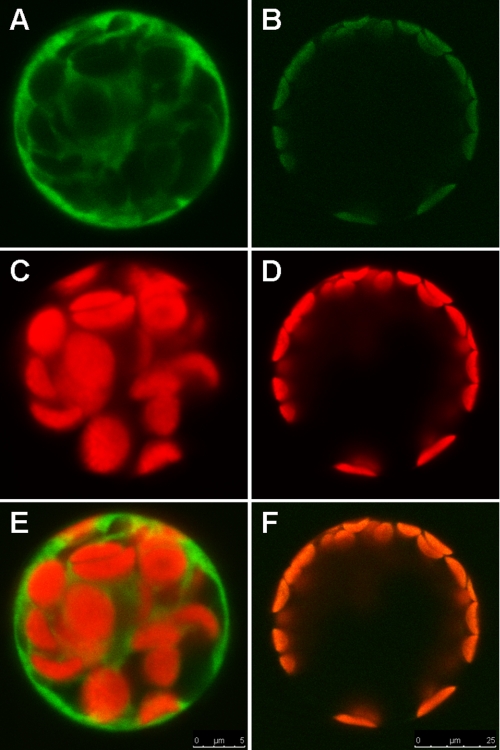
A. thaliana protoplasts were isolated from leaves of 3-week-old plants and transformed using published methods (30). Fluorescence was monitored using a TCS SP5 confocal laser-scanning microscope (Leica Microsystems, Exton, PA). EGFP fluorescence was excited at 488 nm and measured at 505–530 nm. Chlorophyll fluorescence was excited at 488 nm and measured at >650 nm.
Purification of FMN
An aqueous FMN solution (100 mm) was subjected to reversed-phase preparative chromatography on a SunFire Prep C18 OBD column (19 × 50 mm, 5-μm particle size; Waters, Milford, MA) as described before (19). FMN-containing fractions were evaporated to dryness; purified FMN was dissolved in water and stored at −20 °C until use.
Enzyme Activity Assays
FMN hydrolase activity was measured as described before (19) in buffer H (100 mm Tris-HCl, pH 8.0, 15 mm MgCl2, 1 mm Tris(hydroxypropyl)phosphine, and 0.05% Tween 20) containing 0.05 mm FMN, at 30 °C. Unless otherwise indicated, the procedures described below were used. Initial reaction rates at steady state were measured. Substrates were saturating, and product formation was proportional to enzyme concentration and time. Less than 10% of the substrates was typically consumed. Final assay volumes were 50 μl. The apparent kinetic parameters were determined at pH 8.0, a value close to that of the chloroplast stroma. Apparent kinetic parameters (Km, Vmax) were found by fitting initial reaction rates against various FMN concentrations to the Michaelis-Menten model using nonlinear regression (Enzyme Kinetics Module, SigmaPlot 9.0.1). The standard error for catalytic efficiency, kcat/Km, was calculated by error propagation (31).
Free-phosphate formation was measured spectrophotometrically using the BIOMOL Green assay (Enzo Life Sciences, Plymouth Meeting, PA) in buffer H, but with 0.01% Tween 20. FMN served as a positive assay control. Substrates tested were ADP, AMP, ATP, CTP, GTP, IMP, pyridoxal 5′-phosphate, UTP, xanthosine 5′-monophosphate, dATP, NADP+, phosphoglyceric acid, sodium pyrophosphate, 4-nitrophenyl phosphate, glucose 1-phosphate, glucose 6-phosphate, glucose 1,6-bisphosphate, and fructose 1,6-bisphosphate. Protein concentration was determined by the RC DC assay (Bio-Rad) or the Bradford assay (32) with bovine serum albumin as the standard.
Phylogenetic Analysis
We conducted similarity searches of the A. thaliana protein, genome, and expressed sequence tag sequences in GenBankTM using the BLASTP and TBLASTN prediction programs with AtFHy (9) as the query sequence. These searches identified 21 protein sequences: At1g14310, At1g56500, At1g79790, At2g32150, At2g33255, At2g38740, At2g41250, At3g10970, At3g48420, At3g62040, At4g11570, At4g21470 (AtFMN/FHy, (9)), At4g25840, At4g39970, At5g02230, At5g10460, At5g44730, At5g45170, At5g57440, At5g59480, and At5g59490. These protein sequences were first processed to eliminate the N- and C-terminal protrusions, and then aligned using the MUSCLE algorithm (33). The phylogenetic tree was assembled using the PhyML program, and drawn using the TreeDyn program (from the Laboratoire Information Génomique et Structurale (IGS)).
RESULTS
Pea Chloroplasts Contain Mg2+-dependent FMN Hydrolase Activity
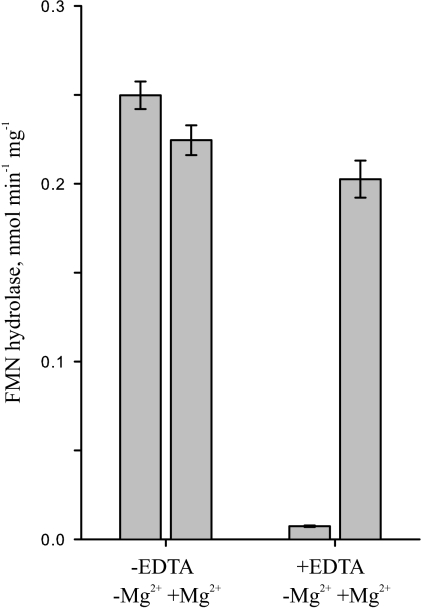
Previous work showed the presence of FMN hydrolase activity in crude extracts of pea chloroplasts (19). We checked the dependence of this activity on Mg2+ to find out whether the responsible enzyme may belong to the HAD superfamily, as does the cytosolic FMN hydrolase from A. thaliana characterized previously (9). Diminished FMN hydrolase activity in the extracts of pea chloroplasts when EDTA replaced MgCl2 in the assays (Fig. 1) suggested that the responsible enzyme is a member of the Mg2+-dependent HAD superfamily.
FIGURE 1.
FMN hydrolase activity in pea chloroplasts is Mg2+-dependent. FMN hydrolase activity was measured at pH 8.0 in extracts of Percoll-isolated pea chloroplasts. Assay mixtures contained 20 mm MgCl2 or 20 mm EDTA. Data are mean ± S.E. of three independent organelle extractions, each assayed in triplicate.
Bioinformatic Sequence Analysis
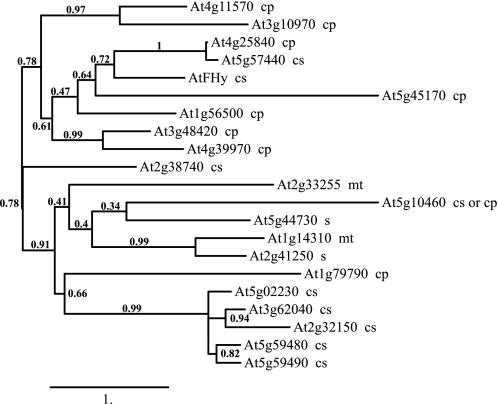
We first attempted to identify a candidate enzyme for FMN hydrolase activity in plastids using bioinformatic sequence analysis of predicted HAD superfamily proteins. By using the Superfamily 1.75 HMM library in the genome assignments server through the University of Bristol, a sequence search for HAD domain-containing proteins returned 210 potential candidates in A. thaliana. Similarity searches of the A. thaliana protein and DNA sequences in GenBank with AtFHy (9) as the query sequence identified 21 protein sequences. A bioinformatic analysis of these sequences predicted that at least eight enzymes localize in plastids (Fig. 2). The predicted plastid enzyme most similar to the cytosolic FMN hydrolase was previously reported to be a glycerol-3-phosphate phosphatase (34), suggesting that the FMN hydrolases from plastids and the cytosol have divergent protein sequences. Consistently, analysis of HAD superfamily phosphatases from E. coli has shown that multiple enzymes with divergent protein sequences hydrolyze FMN, as well as a variety of other substrates (21).
FIGURE 2.
Molecular phylogenetic tree and predicted subcellular localization of the HAD superfamily phosphatases from A. thaliana. Multiple alignment of the predicted sequences was made using MUSCLE (30). The phylogenetic tree was estimated by maximum likelihood, with bootstrap probability values given at the partition branches (using the PhyML and TreeDyn programs). Subcellular localization, indicated next to the enzyme, was predicted using the TargetP 1.1 server. cp, plastids; mt, mitochondria; cs, cytosol; s, secreted.
The reported enzyme activity assays, the bioinformatic analysis, and the reference literature collectively suggested that the plastid FMN hydrolase belongs to the HAD superfamily, but may be too divergent from AtFHy to be readily identified by bioinformatic sequence analysis and screening candidate enzymes for activity, considering the large number of predicted HAD superfamily enzymes in plants. We therefore pursued protein purification from chloroplasts to identify the target enzyme.
Purification of an FMN Hydrolase from Pea Chloroplasts
Purification of chloroplasts from A. thaliana was inefficient in our hands. As a result, we sought to purify the FMN hydrolase from pea chloroplasts, to determine its protein sequence, and to identify homologous protein sequences in the A. thaliana genome. Next, we sought to clone and recombinantly express the corresponding gene from A. thaliana and to biochemically characterize the encoded recombinant enzyme.
FMN hydrolase was purified from extracts of pea chloroplasts by a series of chromatography steps. Purification steps comprised hydrophobic interaction on butyl-Sepharose, hydroxyapatite on CHT ceramic hydroxyapatite, anion exchange on Mono Q, and gel filtration on Superdex 200 (Table 1). Preliminary experiments used washed or Percoll-isolated chloroplasts to test purification strategies with a variety of chromatography columns. These tests showed that using Percoll-isolated chloroplasts provided no additional benefit to enriching the extracts in FMN hydrolase activity. Therefore, washed chloroplasts obtained from differential centrifugation were used instead during the final scaled-up purification.
TABLE 1.
Partial purification of FMN hydrolase from pea chloroplasts
| Steps | Protein | Activity | Specific activity | Yield | -Fold |
|---|---|---|---|---|---|
| mg | nmol min−1 | nmol min−1mg−1 | % | ||
| Chloroplast extract | 1,037 | 249.4 | 0.24 | 100 | 1 |
| (NH4)2SO4 precipitation | 609 | 102.3 | 0.17 | 41 | 0.7 |
| Butyl-Sepharose | 14.6 | 25.2 | 1.72 | 10 | 7 |
| Hydroxyapatite | 1.8 | 13.7 | 7.52 | 5.5 | 31 |
| Mono Q (pH 7.8) | —a | 8.9 | — | — | — |
| Mono Q (pH 7.0) | — | 6.1 | — | — | — |
| Superdex 200 | — | 1.3 | — | — | — |
| Mono Q (pH 7.0) | — | 1.0 | — | — | — |
a —, not determined because the protein concentration of the samples was too low to measure.
Adding 1.5 m (NH4)2SO4 to the chloroplast extract at the beginning of the purification work resulted in a net loss in specific activity. However, this step enabled binding of the target activity to the butyl-Sepharose column and produced a 10-fold enrichment in FMN hydrolase activity. The next purification step using hydroxyapatite resulted in an ∼4.4-fold enrichment. We were unable to calculate specific activity in subsequent purification steps, anion exchange and gel filtration, because the protein samples became too diluted to measure concentration. All active protein fractions obtained from the purification steps were analyzed by Coomassie Blue-stained gel electrophoresis (not shown).
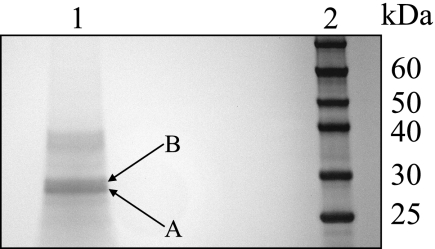
The molecular weight of the native FMN hydrolase was estimated at ∼59,400 by gel filtration chromatography on Superdex 200. The estimate suggests a dimer of about twice as much as the predicted molecular weight (20,000–35,000) of most putative HAD superfamily phosphatases from A. thaliana. Two separate protein bands within 25–30 kDa became visible after SDS-PAGE of the partially purified protein with FMN hydrolase activity (Fig. 3). Both protein bands were extracted from the gel, digested with trypsin, and analyzed by nanoLC-MS/MS.
FIGURE 3.
Partial purification of the protein fraction containing FMN hydrolase activity in extracts of pea chloroplasts. The protein fraction was purified as described under “Experimental Procedures.” Samples were separated by SDS-PAGE on a NuPAGE 10% Bis-Tris gel and stained with Coomassie Blue. Lane 1, partially purified protein fraction; lane 2, protein markers. Bands A and B, the two protein bands that were excised from the gel and analyzed by nanoLC-MS/MS.
NanoLC-MS/MS Analysis
Because no genome information on pea was available, the nanoLC-MS/MS data were compared with a custom database made of sequences from the related plants of the tribe Trifolieae. A comparative analysis of the lower protein band (Fig. 3, band A) yielded three significant hits, with probability scores over 0.75, against the putative HAD superfamily phosphatases from the tribe Trifolieae (Table 2).
TABLE 2.
Significant peptides identified by nanoLC-MS/MS analysis, the corresponding proteins from Trifolieae, and the sequence homologs from A. thaliana
Only peptides with a probability score >0.75 are shown.
| A. thaliana gene ID | Trifolieae gene ID (species) | Peptide sequence hit | Probability |
|---|---|---|---|
| At3g48420 | 149647748 (Arachis hypogaea) | AVSAIVSFLLGPER | 0.999 |
| 162291478 (Vigna unguiculata) | KPDPAIYLLAASTLGVEPSR | 0.998 | |
| 166959920 (Phaseolus angustissimus) | AVSAIVSFLLGPER | 0.999 | |
| At1g79790 | 86108494 (Trifolium pratense) | HLEVDPSYCIFIDDR | 0.912 |
| At4g11570 | 255639949 (Glycine max) | EEIYQSLQ129GGIYR | 0.781 |
Recombinant Expression, Purification, and Biochemical Characterization of AtcpFHy1
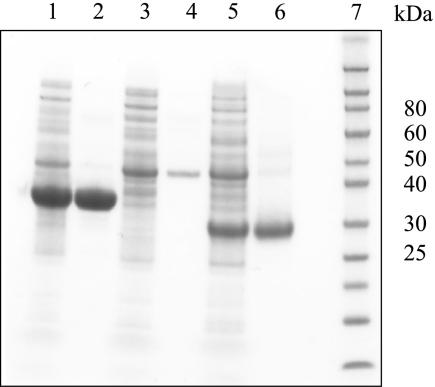
After showing high sequence similarity to the candidate FMN hydrolases from the tribe Trifolieae, full-length cDNA clones for the three proteins from A. thaliana (At1g79790, At3g48420, and At4g11570) were overexpressed in E. coli, and the encoded recombinant proteins were affinity-purified (Fig. 4). The purified His-tagged enzymes were cleaved with recombinant enterokinase and further purified by gel filtration (At1g79790) or affinity (At3g48420 and At4g11570) chromatography to produce tag-free recombinant enzymes (Fig. 5).
FIGURE 4.
Affinity purification of recombinant At3g48420, At4g11570, and At1g79790 under native conditions. The enzymes were purified as described under “Experimental Procedures.” Samples were separated by SDS-PAGE on a NuPAGE 10% Bis-Tris gel and stained with Coomassie Blue. Lane 1, soluble E. coli extract expressing At3g48420; lane 2, Ni-NTA affinity-purified At3g48420; lane 3, soluble E. coli extract expressing At4g11570; lane 4, Ni-NTA affinity-purified At4g11570; lane 5, soluble E. coli extract expressing At1g79790; lane 6, Ni-NTA affinity-purified At1g79790; lane 7, protein markers.
FIGURE 5.
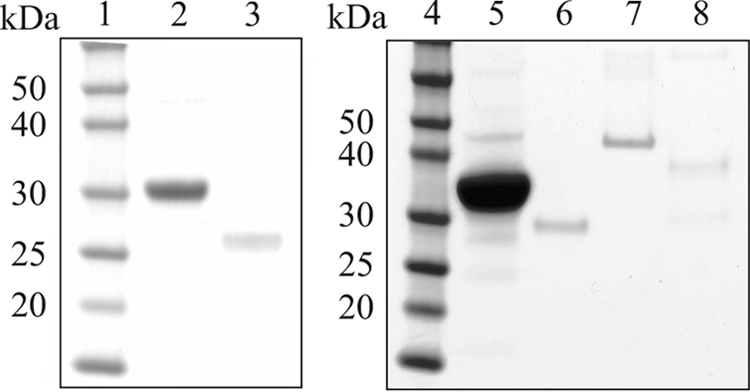
Purification of recombinant At1g79790, At3g48420, and At4g11570 under native conditions. The enzymes were purified as described under “Experimental Procedures.” Samples were separated by SDS-PAGE on a NuPAGE 10% Bis-Tris gel and stained with Coomassie Blue. Lane 1, protein markers; lane 2, Ni-NTA affinity-purified At1g79790; lane 3, final At1g79790 preparation; lane 4, protein markers; lane 5, Ni-NTA affinity-purified At3g48420; lane 6, final At3g48420 preparation; lane 7, Ni-NTA affinity-purified At4g11570; lane 8, final At4g11570 preparation.
Activity assays of the three tag-free recombinant proteins showed that only At1g79790 has significant FMN hydrolase activity (Table 3). This enzyme was found to localize in the plastid stroma by a proteomic approach (32). Its plastid localization was confirmed by fluorescence microscopy of EGFP-fused proteins transiently expressed in A. thaliana protoplasts (Fig. 6). This enzyme was accordingly named AtcpFHy1. Alignment of the deduced protein sequences of AtcpFHy1 from A. thaliana with the nearest sequence homologs from other plant species is shown in supplemental Fig. 1. Predicted subcellular localization of AtcpFHy1 and its sequence homologs as determined by ChloroP 1.1 is presented in supplemental Table 1. Protein sequences of At1g79790, At3g48430, and At4g11570 are shown in supplemental Fig. 2.
TABLE 3.
Specific activity of recombinant At3g48420, At1g79790, and At4g11570
FMN hydrolase activity was measured using tag-free recombinant enzymes purified under native conditions. Assay mixture contained 0.05 mm FMN. Data are means of triplicate determinations.
| Gene ID | Specific activity |
|---|---|
| nmol min−1mg−1 | |
| At3g48420 | <0.01 |
| At1g79790 | 1,106 |
| At4g11570 | 0.01 |
FIGURE 6.
AtcpFHy1 localizes to chloroplasts. C-terminal EGFP fusion to the full-length AtcpFHy1 was transiently expressed in A. thaliana protoplasts. Non-fused EGFP in the pUC18-GFP5T-sp plasmid was used as a control for targeting to the cytosol. Subcellular localization was analyzed by fluorescence microscopy. A and B, EGFP fluorescence; C and D, chlorophyll autofluorescence; E and F, merged images.
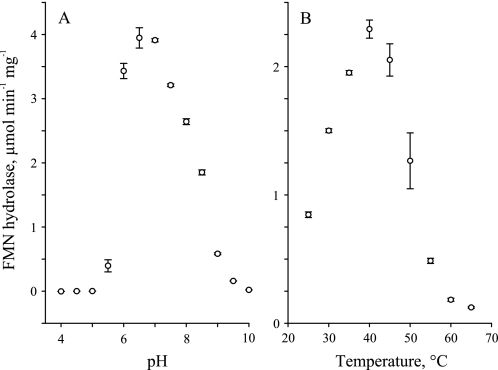
Recombinant AtcpFHy1 hydrolyzed FMN most effectively at pH 6.5–7.0 (Fig. 7A) and at 40 °C (Fig. 7B). A sharper decline in activity was observed for the acid (pH < 6) range and for the high end (>45 °C) temperatures. In comparison, the cytosolic FMN hydrolase we characterized previously (9) has a relatively close pH optimum at pH 5.5–6.0 and a 15 °C higher temperature optimum at ∼55 °C.
FIGURE 7.
Effect of pH and temperature on the FMN hydrolase activity of recombinant AtcpFHy1. A, FMN hydrolase activity was measured across pH 4–10 at 0.5-pH intervals in CHES-Hepes-citric acid buffer at 30 °C. B, FMN hydrolase activity was measured across temperatures 25°–65 °C at 0.5 °C intervals in Tris-HCl buffer at pH 8.0. Assay mixtures contained 0.05 mm FMN and 0.05% Tween 20. Data are mean ± S.E. of 1–2 triplicate determinations.
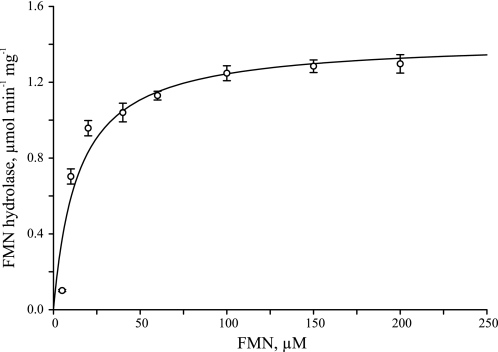
The apparent kinetic parameters for recombinant AtcpFHy1, as found by a nonlinear best fit to the Michaelis-Menten model, were Km = 14.2 ± 1.6 μm and Vmax = 1.42 ± 0.04 μmol min−1 mg−1, with derived parameters kcat = 0.59 ± 0.02 s−1 and kcat/Km = 4.15 ± 0.48 × 104 s−1 m−1. The initial reaction rates used to determine the apparent kinetic parameters are depicted in Fig. 8. The Km value of AtcpFHy1 for FMN is ∼21-fold higher, the kcat value is ∼30-fold lower, and the kcat/Km value is ∼160-fold lower than that of the cytosolic FMN hydrolase from A. thaliana characterized previously, which also belongs to the HAD superfamily (9).
FIGURE 8.
Catalytic properties of recombinant AtcpFHy1. Measurements were made at pH 8.0 with various FMN concentrations using a recombinant enzyme purified under native conditions. Data are mean ± S.E. of 3–4 triplicate determinations. The curve is a nonlinear best fit to the Michaelis-Menten model (SigmaPlot 9.0.1).
We found that AtcpFHy1 does not hydrolyze any of the 18 other potential substrates tested. Substrates comprised phosphates of representative nucleotides (ADP, AMP, ATP, CTP, GTP, IMP, UTP, xanthosine 5′-monophosphate, and dATP), sugars (glucose 1-phosphate, glucose 6-phosphate, glucose 1,6-bisphosphate, and fructose 1,6-bisphosphate), and cofactors (pyridoxal 5′-phosphate and NADP+) plus phosphoglyceric acid, sodium pyrophosphate, and 4-nitrophenyl phosphate. This result suggests that AtcpFHy1 has evolved high specificity for FMN.
DISCUSSION
Despite the vital importance of FMN and FAD in metabolism, the factors that affect the homeostasis of these cofactors in plants and other organisms are incompletely understood. Previous work suggested that riboflavin, the precursor of FMN and FAD, is probably synthesized only in chloroplasts in plants (3). We found that plant cells contain a bifunctional riboflavin kinase-FMN hydrolase in the cytosol (9), as well as monofunctional FAD synthetases in chloroplasts (19). Also, we provided biochemical evidence for riboflavin kinase, FMN hydrolase, and FAD pyrophosphatase activities in chloroplasts and mitochondria (19). Other groups described biochemical evidence of FAD synthetase activity in mitochondria (35), as well as cloning of the corresponding genes and characterization of FAD pyrophosphatases from plastids (36).
The emerging view of flavin nucleotide metabolism in plants is that FMN and FAD can be synthesized from and degraded to riboflavin by individual subcellular compartments (plastids, mitochondria, and the cytosol). The presence of enzymes that synthesize flavin nucleotides in organelles and the cytosol is not surprising because it ensures that the individual subcellular compartments have the ability to independently regulate flavin nucleotide content in response to specific metabolic needs. It is surprising, however, to find the abundance of hydrolytic enzymes (FMN hydrolases and FAD pyrophosphatases) in organelles and the cytosol, which raises the question of the roles these enzymes play in metabolism.
Biosynthesis and hydrolysis of flavin nucleotides in the same subcellular compartment form a futile cycle that results in expenditure of ATP. One possible benefit to the plant from this expenditure is that flavin nucleotide hydrolysis provides a means to broadly and quickly down-regulate plant metabolism in response to environmental or developmental cues by removing the needed enzyme cofactors. Another possibility is that hydrolytic enzymes serve to reduce the contents of flavin nucleotides inside organelles and the cytosol in times of reduced need. The released riboflavin could then be exported to a site where is needed, or it could be degraded. Such a mechanism could reduce photodegradation of the labile flavins and the associated release of free radicals, thus preventing oxidative damage to the cell.
Toward improving the overall understanding of flavin nucleotide metabolism in plants, this study aimed to clone the corresponding gene and biochemically characterize the encoded enzyme that hydrolyzes FMN in plant chloroplasts. We favored purification from pea chloroplasts along with LC-MS/MS peptide mass identification and cloning of the sequence homolog from A. thaliana over screening for FMN hydrolase activity in all predicted HAD superfamily phosphatases from A. thaliana. Screening of the HAD superfamily phosphatases from E. coli has shown that several of the 22 enzymes hydrolyze FMN as one of the substrates (21). Thus, applying such a screening to the A. thaliana enzymes could have left doubt whether the enzyme responsible for most of the activity in plastids was identified.
Biochemical characterization of AtcpFHy1 showed that of the 19 substrates tested, this enzyme hydrolyzes only FMN. This substrate specificity is in contrast to those of FMN-hydrolyzing HAD superfamily phosphatases from E. coli, which effectively also hydrolyze other substrates. The phylogenetic analysis in Fig. 2 showed that AtcpFHy1 is less similar to the cytosolic FMN hydrolase we described previously (9) than to several other HAD superfamily phosphatases from A. thaliana. This finding implies convergent evolution of FMN hydrolase activity; it also implies that sequence similarity is a poor predictor of catalytic activity for the HAD superfamily of enzymes.
The presence of multiple predicted HAD superfamily phosphatases in organelles and the cytosol in plants (Fig. 2) raises questions about their substrates and also about their physiological roles. The HAD superfamily phosphatases from E. coli hydrolyze nucleotide, sugar, and organic acid phosphates plus phosphorylated cofactors and small phosphodonors (21); one can therefore anticipate that the HAD superfamily phosphatases from plants would hydrolyze a similar suite of substrates.
As for the physiological roles such enzymes play in plants, one can only speculate. The phosphate status of plant cells changes in response to developmental cues and also in response to environmental cues such as temperature variations and phosphate availability (37–39). The HAD superfamily phosphatases could play important roles in mediating rebalancing of the pools of organic phosphoesters and inorganic phosphate in response to those cues. In addition, sensing of the cytosolic phosphate concentration was recently proposed as the mechanism used by plant cells to monitor their phosphate status (40). Hence, one can also envision that the HAD superfamily phosphatases play a role in sensing phosphate availability. Future work is thus needed to characterize these enzymes and determine their physiological roles.
Supplementary Material
Acknowledgments
We thank Craig Whitney and Julianna Gothard-Szamosfalvi for growing pea and A. thaliana plants. We are also grateful for the support provided by the EBI and by the OMSSA team of the National Center for Biotechnology Information (NCBI).
This work was supported by the United States National Institute of Food and Agriculture under National Research Initiative Competitive Grant 2007-35318-18438 (to S. R.), the National Science Foundation Grant MCB-1052492 (to S. R.), the Murdoch Foundation, and the Instituto Tecnológico y de Estudios Superiores de Monterrey (Mexico) under Bioengineering and Nano-particles Research Chair Grant CAT 161 (to R. W.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2 and a table.
- HAD
- haloacid dehalogenase
- Ni-NTA
- nickel-nitrilotriacetic acid
- EGFP
- enhanced green fluorescent protein
- Bis-Tris
- 2-(bis(2-hydroxyethyl)amino)-2-(hydroxymethyl)propane-1,3-diol
- CHES
- 2-(cyclohexylamino)ethanesulfonic acid.
REFERENCES
- 1. Bacher A., Eberhardt S., Eisenreich W., Fischer M., Herz S., Illarionov B., Kis K., Richter G. (2001) Vitam. Horm. 61, 1–49 [DOI] [PubMed] [Google Scholar]
- 2. Bacher A., Eberhardt S., Fischer M., Kis K., Richter G. (2000) Annu. Rev. Nutr. 20, 153–167 [DOI] [PubMed] [Google Scholar]
- 3. Roje S. (2007) Phytochemistry 68, 1904–1921 [DOI] [PubMed] [Google Scholar]
- 4. Barile M., Brizio C., De Virgilio C., Delfine S., Quagliariello E., Passarella S. (1997) Eur. J. Biochem. 249, 777–785 [DOI] [PubMed] [Google Scholar]
- 5. McCormick D. B., Russell M. (1962) Comp. Biochem. Physiol. 5, 113–121 [Google Scholar]
- 6. Kumar S. A., Vaidyanathan C. S. (1963) Biochim. Biophys. Acta 73, 98–104 [DOI] [PubMed] [Google Scholar]
- 7. Mitsuda H., Tomozawa Y., Tsuboi T., Kawai F. (1965) J. Vitaminol. (Kyoto) 11, 20–29 [DOI] [PubMed] [Google Scholar]
- 8. Mitsuda H., Tsuge H., Tomozawa Y., Kawai F. (1970) J. Vitaminol. (Kyoto) 16, 52–57 [DOI] [PubMed] [Google Scholar]
- 9. Sandoval F. J., Roje S. (2005) J. Biol. Chem. 280, 38337–38345 [DOI] [PubMed] [Google Scholar]
- 10. Fuchs K. R., Shekels L. L., Bernlohr D. A. (1992) Biochem. Biophys. Res. Commun. 189, 1598–1605 [DOI] [PubMed] [Google Scholar]
- 11. Granjeiro J. M., Ferreira C. V., Jucá M. B., Taga E. M., Aoyama H. (1997) Biochem. Mol. Biol. Int. 41, 1201–1208 [DOI] [PubMed] [Google Scholar]
- 12. Taga E. M., Van Etten R. L. (1982) Arch. Biochem. Biophys. 214, 505–515 [DOI] [PubMed] [Google Scholar]
- 13. Sensabaugh G. F., Golden V. L. (1978) Am. J. Hum. Genet. 30, 553–560 [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang Z. Y., Van Etten R. L. (1990) Arch. Biochem. Biophys. 282, 39–49 [DOI] [PubMed] [Google Scholar]
- 15. Kumar S. A., Rao N. A., Vaidyanathan C. S. (1965) Arch. Biochem. Biophys. 111, 646–652 [DOI] [PubMed] [Google Scholar]
- 16. Byrd J. C., Fearney F. J., Kim Y. S. (1985) J. Biol. Chem. 260, 7474–7480 [PubMed] [Google Scholar]
- 17. Lee R. S., Ford H. C. (1988) J. Biol. Chem. 263, 14878–14883 [PubMed] [Google Scholar]
- 18. Shin H. J., Mego J. L. (1988) Arch. Biochem. Biophys. 267, 95–103 [DOI] [PubMed] [Google Scholar]
- 19. Sandoval F. J., Zhang Y., Roje S. (2008) J. Biol. Chem. 283, 30890–30900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tejera García N. A., Olivera M., Iribarne C., Lluch C. (2004) Plant Physiol. Biochem. 42, 585–591 [DOI] [PubMed] [Google Scholar]
- 21. Kuznetsova E., Proudfoot M., Gonzalez C. F., Brown G., Omelchenko M. V., Borozan I., Carmel L., Wolf Y. I., Mori H., Savchenko A. V., Arrowsmith C. H., Koonin E. V., Edwards A. M., Yakunin A. F. (2006) J. Biol. Chem. 281, 36149–36161 [DOI] [PubMed] [Google Scholar]
- 22. Cline K. (1986) J. Biol. Chem. 261, 14804–14810 [PubMed] [Google Scholar]
- 23. Douce R., Bourguignon J., Brouquisse R., Neuburger M. (1987) Methods Enzymol. 148, 403–415 [Google Scholar]
- 24. Geer L. Y., Markey S. P., Kowalak J. A., Wagner L., Xu M., Maynard D. M., Yang X., Shi W., Bryant S. H. (2004) J. Proteome Res. 3, 958–964 [DOI] [PubMed] [Google Scholar]
- 25. Keller A., Nesvizhskii A. I., Kolker E., Aebersold R. (2002) Anal. Chem. 74, 5383–5392 [DOI] [PubMed] [Google Scholar]
- 26. Nesvizhskii A. I., Keller A., Kolker E., Aebersold R. (2003) Anal. Chem. 75, 4646–4658 [DOI] [PubMed] [Google Scholar]
- 27. Strohalm M., Kavan D., Novák P., Volný M., Havlícek V. (2010) Anal. Chem. 82, 4648–4651 [DOI] [PubMed] [Google Scholar]
- 28. Barsnes H., Vizcaíno J. A., Eidhammer I., Martens L. (2009) Nat. Biotechnol. 27, 598–599 [DOI] [PubMed] [Google Scholar]
- 29. Karimi M., Inzé D., Depicker A. (2002) Trends Plant Sci. 7, 193–195 [DOI] [PubMed] [Google Scholar]
- 30. Sheen J. (2001) Plant Physiol. 127, 1466–1475 [PMC free article] [PubMed] [Google Scholar]
- 31. Meyer S. L. (1975) Data Analysis for Scientists and Engineers, pp. 39–48, John Wiley & Sons, Inc., New York [Google Scholar]
- 32. Bradford M. M. (1976) Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 33. Dereeper A., Guignon V., Blanc G., Audic S., Buffet S., Chevenet F., Dufayard J. F., Guindon S., Lefort V., Lescot M., Claverie J. M., Gascuel O. (2008) Nucleic Acids Res. 36, W465–W469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Caparrós-Martín J. A., Reiland S., Köchert K., Cutanda M. C., Culiáñez-Macià F. A. (2007) Plant Mol. Biol. 63, 505–517 [DOI] [PubMed] [Google Scholar]
- 35. Giancaspero T. A., Locato V., de Pinto M. C., De Gara L., Barile M. (2009) FEBS J. 276, 219–231 [DOI] [PubMed] [Google Scholar]
- 36. Ogawa T., Yoshimura K., Miyake H., Ishikawa K., Ito D., Tanabe N., Shigeoka S. (2008) Plant Physiol. 148, 1412–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lin W. Y., Lin S. I., Chiou T. J. (2009) J. Exp. Bot. 60, 1427–1438 [DOI] [PubMed] [Google Scholar]
- 38. Doerner P. (2008) Curr. Opin. Plant Biol. 11, 536–540 [DOI] [PubMed] [Google Scholar]
- 39. Rouached H., Arpat A. B., Poirier Y. (2010) Mol. Plant 3, 288–299 [DOI] [PubMed] [Google Scholar]
- 40. Pratt J., Boisson A. M., Gout E., Bligny R., Douce R., Aubert S. (2009) Plant Physiol. 151, 1646–1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.