Background: β2-Glycoprotein I (β2GPI) binds to negatively charged lipids, but its physiological role remains unknown.
Results: β2GPI containing LPS, but not β2GPI depleted of LPS activity, stimulated macrophages in a TLR4-dependent manner.
Conclusion: β2GPI interacts specifically with LPS.
Significance: Apparent TLR4-mediated activation of macrophages by β2GPI is due to the presence of LPS.
Keywords: Glycoprotein, Innate Immunity, Lipid-binding Protein, Lipopolysaccharide (LPS), Macrophages, Toll-like receptors (TLRs), β2-Glycoprotein I, Anti-phospholipid Antibodies
Abstract
β2-Glycoprotein I (β2GPI) is an abundant plasma protein that binds to the surface of cells and particles expressing negatively charged lipids, but its physiological role remains unknown. Antibodies to β2GPI are found in patients with anti-phospholipid syndrome, a systemic autoimmune disease associated with vascular thrombosis and pregnancy morbidity. Although it has been suggested that anti-β2GPI antibodies activate endothelial cells and monocytes by signaling through TLR4, it is unclear how anti-β2GPI antibodies and/or β2GPI interact with TLR4. A number of mammalian proteins (termed “endogenous Toll-like receptor (TLR) ligands”) have been reported to bind to TLR4, but, in most cases, subsequent studies have shown that LPS interaction with these proteins is responsible for TLR activation. We hypothesized that, like other endogenous TLR ligands, β2GPI interacts specifically with LPS and that this interaction is responsible for apparent TLR4 activation by β2GPI. Here, we show that both LPS and TLR4 are required for β2GPI to bind to and activate macrophages. Untreated β2GPI stimulated TNF-α production in TLR4-sufficient (but not TLR4-deficient) macrophages. In contrast, neither polymyxin B-treated nor delipidated β2GPI stimulated TNF-α production. Furthermore, β2GPI bound to LPS in a specific and dose-dependent manner. Finally, untreated β2GPI bound to the surface of TLR4-sufficient (but not TLR4-deficient) macrophages. Polymyxin B treatment of β2GPI abolished macrophage binding. Our findings suggest a potential new biological activity for β2GPI as a protein that interacts specifically with LPS and point to the need to evaluate newly discovered endogenous TLR ligands for potential interactions with LPS.
Introduction
β2-Glycoprotein I (β2GPI)6 is a plasma protein found at a reasonably high concentration (∼200 μg/ml) in blood. Despite its abundance, its physiological role remains unknown. β2GPI binds to cells and particles that express negatively charged lipids, such as activated platelets, apoptotic cells, and oxidized lipoproteins (LDLs) (1). Anti-β2GPI autoantibodies are found in individuals with anti-phospholipid syndrome, a systemic autoimmune disease associated with an increased risk of arterial and venous thrombosis and pregnancy morbidity (2). Anti-β2GPI autoantibodies have been reported to activate monocytes (3–5), endothelial cells (6, 7), and platelets (8). Cell activation has been proposed to occur through TLR4 (7, 9), but it is unclear whether anti-β2GPI autoantibodies and/or β2GPI bind to this receptor.
Toll-like receptors (TLRs) mediate the innate immune response to microbial pathogens (10, 11) and recognize microbial ligands through a number of highly conserved pathogen-associated molecular patterns (12). Despite TLR specificity for microbial ligands, TLR recognition of mammalian proteins has also been reported, in particular by TLR4. These proteins, which are termed “endogenous TLR ligands” because of their mammalian (rather than microbial) origin, include the heat shock proteins HSP60 and HSP70, α-lactalbumin, and adiponectin (13–17). There is controversy, however, whether these endogenous ligands are themselves capable of binding to and activating TLR or whether TLR interaction occurs indirectly by bridging through a known TLR ligand.
We hypothesized that, like other endogenous TLR ligands, β2GPI may interact specifically with LPS and initiate TLR4-dependent signaling through complexed LPS. We show here that a commercial preparation of human β2GPI stimulated macrophages to produce TNF-α in a TLR4-dependent manner. However, β2GPI-stimulated TNF-α production by macrophages was inhibited by either pretreatment of β2GPI with polymyxin B or delipidation of β2GPI. We further show that β2GPI and LPS interacted specifically. Our findings indicate that β2GPI can activate macrophages, but only when LPS is present, and that β2GPI itself is not a ligand for TLR4. We propose that, in the presence of physiological concentrations of LPS, β2GPI may act as a LPS-interacting protein and thus enable TLR4-dependent activation of macrophages.
EXPERIMENTAL PROCEDURES
Materials
Unless stated otherwise, all chemicals were obtained commercially and used without further purification. Human β2GPI (apolipoprotein H) derived from serum was obtained from Crystal Chem (Downers Grove, IL), and human prothrombin (PT) and Glu-plasminogen (GluP) were obtained from Hematologic Technologies Inc. (Essex Junction, VT). LPS (Escherichia coli-derived, serotype O111:B4) was obtained from List Biological Laboratories (Campbell, CA). Agarose bead-bound polymyxin B (pmB-B) and soluble polymyxin B sulfate (pmB-S) were both obtained from Sigma. Thioglycollate, mouse Fc BlockTM (clone 2.4G2), and rat anti-mouse CD14 antibody (clone 4C1) were obtained from BD Biosciences. Rat anti-mouse TLR4-MD2 complex (clone MTS510) and rat IgG2a isotype control antibodies were obtained from BioLegend (San Diego, CA) and eBioscience (San Diego), respectively.
Murine monoclonal anti-human β2GPI (12A1-A17.3) and relevant isotype control (29J3-119) antibodies were produced in our laboratory (J. R.) and purified as described previously (18). Murine polyclonal anti-human β2GPI, anti-human PT, and anti-human GluP antibodies were produced in our laboratory (J. R.) by immunization with the relevant protein as described for human β2GPI (19). The murine antisera were characterized for binding to the protein used for immunization and to other proteins by ELISA, and were found to be specific for the immunogen. The serum antibodies were purified on protein A (nProtein A-Sepharose 4 Fast Flow, GE Healthcare Bio-Sciences AB, Uppsala) and eluted with 0.1 m glycine HCl (pH 3). Anti-LPS antibody (clone WN1 222-5) was obtained from HyCult Biotechnology (Uden, The Netherlands), and the murine UPC-10 IgG2a isotype control was obtained from ICN Biomedicals Inc. (Aurora, OH). FITC-conjugated goat anti-mouse IgG was obtained from Jackson ImmunoResearch Laboratories (West Grove, PA).
Mice
Specific pathogen-free C57BL/6 (wild-type) female mice (8–12 weeks of age) were purchased from Harlan Sprague-Dawley (Indianapolis, IN). The TLR4-deficient mouse strain was generated by Dr. S. Akira, Osaka University, Japan, and was backcrossed onto the C57BL/6 background for ≥8 generations before being obtained from Dr. Salman Qureshi (McGill University, Montreal, Canada). Mice were maintained and bred according to Canadian Council on Animal Care (CCAC) guidelines. Both WT and TLR4-deficient mice were maintained on food and water ad libitum. All animal experiments were approved by the McGill University Animal Care Committee.
Cell Culture
The B10R macrophage cell line (henceforth referred to as “B10R macrophages”), kindly provided by Dr. Danuta Radzioch (McGill University), was grown in DMEM with 4.5 g/liter glucose and 110 mg/ml sodium pyruvate (Invitrogen) supplemented with 7% heat-inactivated FBS (Invitrogen) and used at passage 4. B10R macrophages were plated in 96-well plates at a density of 100,000 cells/well. Twenty-four hours prior to the experiment, the medium was removed, and the cells were fed DMEM supplemented with reduced FBS (1%; DMEM-1) to rest the macrophages.
Peritoneal macrophages were obtained from WT or TLR4-deficient C57BL/6 mice. Briefly, mice were injected intraperitoneally with 1 ml of thioglycolate (3.85 g/dl), and 72 h later, macrophages were harvested from the peritoneal cavity by lavage with 10 ml of sterile RPMI 1640 medium. Macrophages were washed, counted, resuspended in RPMI 1640 medium supplemented with 10% FBS (RPMI-10), and plated at a density of 200,000 cells/well. In experiments done in the absence of FBS, macrophages were resuspended and maintained in RPMI 1640 medium without FBS.
B10R or peritoneal macrophages were incubated with their respective medium (DMEM-1 or RPMI-10; 100 μl/well) containing untreated versus treated LPS, human β2GPI, or PBS for 2, 4, or 6 h at 37 °C (5% CO2). The medium was then removed and analyzed for TNF-α.
For fluorescence microscopy experiments, B10R or peritoneal macrophages were cultured in medium containing β2GPI-depleted FBS to ensure that binding and detection of exogenously added human β2GPI would be specific and optimal. Depletion of β2GPI was accomplished using a heparin column (HiTrap heparin HP, GE Healthcare) and confirmed by both immunoblotting and ELISA (data not shown).
TNF-α ELISA
TNF-α was measured using a commercially available TNF-α immunoassay kit (BD OptEIA TNF ELISA Set II, BD Biosciences) according to the protocol provided by the manufacturer.
Inhibition or Removal of LPS
Three methods were used to inhibit or remove LPS from β2GPI or other LPS-containing preparations: 1) treatment with pmB-S, 2) treatment with pmB-B, and 3) delipidation. Briefly, for pmB-S treatment, 200 μg of human β2GPI or 3 μg of LPS was incubated with 80 or 30 μg of pmB-S, respectively, for 1 h at 25 °C with constant mixing on a rotator. For pmB-B treatment, 250 μg of human β2GPI or 2.5 μg of LPS (or PBS as a control) was incubated with 270 or 135 μg of pmB-A, respectively, for 1 h at 25 °C with constant mixing on a rotator. For delipidation, human β2GPI was subjected to a modified Folch lipid extraction protocol as described previously (20). Briefly, 250 μg of human β2GPI was incubated with an extraction solution composed of chloroform/methanol (2:1) for 2 h at 25 °C with constant mixing on a rotator. After centrifugation, the aqueous supernatant containing lipid-free β2GPI was retained. All treated preparations were then sterilized using a 0.2-μm filter and tested for endotoxin activity using a commercial Limulus amebocyte lysate (LAL) assay (Cape Cod Inc., Falmouth, MA) according to the protocol provided by the manufacturer. The lower limit of endotoxin detection of this LAL assay was 0.005 endotoxin units (EU)/ml.
LPS and β2GPI Binding ELISAs
Detection of LPS Binding to β2GPI
96-well high binding immunoassay plates (Greiner Bio-One, Monroe, NC) were coated with human β2GPI at 15 μg/ml in PBS for 16–20 h at 37 °C. Plates were similarly coated with PT, GluP, or gelatin as irrelevant control proteins. The following day, the plate was blocked with PBS containing 10% FBS and 0.5% gelatin for 90 min and then washed with PBS. Varying concentrations of LPS (0.05, 0.1, 0.2, or 0.4 μg/ml) were added to the coated wells and incubated for 2 h at 25 °C. The plates were then washed with PBS, and anti-LPS antibody (or isotype-matched IgG control) was added at 0.25 μg/ml and incubated for 2 h at 25 °C. Following three washes with PBS, alkaline phosphatase-conjugated goat anti-mouse IgG (Southern Biotechnology Associates, Birmingham, AL) was added to the wells and incubated for 16 h at 4 °C to detect bound LPS. The plates were washed and developed with p-nitrophenol phosphate for 20 min at 37 °C, and the absorbance at 405 nm was read using an ELISA reader (Model EL800, from BioTek Instruments, Inc., Winooski, VT).
Detection of β2GPI Binding to LPS
96-well immunoassay plates (Costar 3369, Corning Inc.) were coated with LPS at 30 μg/ml in 0.1 m Na2CO3 buffer containing 20 mm EDTA for 3 h at 37 °C. The plates were then washed with deionized water and air-dried for 16–20 h. The next day, the plates were blocked with PBS containing 1% BSA (1% BSA/PBS) for 30 min at 37 °C and washed with 0.1% BSA/PBS. Varying concentrations (5, 10, 20, or 40 μg/ml) of human β2GPI (or PT or GluP as irrelevant control proteins) were added to the coated wells and incubated for 3 h at 37 °C. The plates were washed three times with 0.1% BSA/PBS, and purified anti-β2GPI (monoclonal or polyclonal), anti-PT, or anti-GluP IgG (or isotype-matched control monoclonal or polyclonal IgG) antibody was added and incubated for 2 h at 37 °C. Following three washes with PBS, alkaline phosphatase-conjugated goat anti-mouse IgG was added and incubated for 1 h at 25 °C to detect bound β2GPI. The plates were washed, developed with p-nitrophenol phosphate, and read as described above for the LPS binding ELISA.
Competitive Inhibition of Binding to LPS or β2GPI
Competitive inhibition assays were developed for each of the LPS and β2GPI binding ELISAs. A fixed concentration of ligand (human β2GPI or LPS) that resulted in ∼50% maximum binding on the binding curve (Fig. 5) was selected. For competitive inhibition of β2GPI binding to solid-phase LPS, 10 μg/ml human β2GPI was incubated with increasing concentrations of LPS in solution (100, 250, and 500 μg/ml) for 20 h at 4 °C with constant mixing on a rotator. Alternatively, for competitive inhibition of LPS binding to solid-phase β2GPI, 0.1 μg/ml LPS was incubated with increasing concentrations of human β2GPI in solution (10, 50, and 100 μg/ml) for 20 h at 4 °C with constant mixing. Following incubation, the mixtures were collected by centrifugation and assayed using the LPS or β2GPI binding ELISA described above.
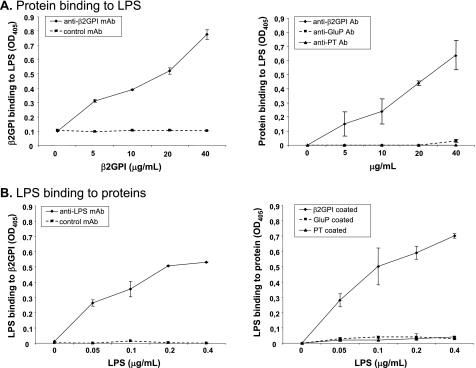
FIGURE 5.
β2GPI and LPS interact physically in a dose-dependent manner. A, left panel, binding of soluble β2GPI to solid-phase LPS was detected by ELISA. Increasing concentrations of β2GPI were added to LPS-coated plates. Dose-dependent binding of β2GPI to LPS was detected by murine anti-β2GPI mAb but was not observed with an isotype-matched IgG control mAb. Right panel, binding of soluble β2GPI, PT, or GluP to solid-phase LPS was detected by ELISA. Binding of increasing concentrations of β2GPI was detected by anti-β2GPI antibody, whereas binding of the other proteins (PT and GluP) was not detected by the relevant antibodies. Binding is expressed as the mean A405 (OD405) ± S.D. of duplicate samples and is shown for one example representative of four independent experiments. B, left panel, binding of soluble LPS to solid-phase β2GPI was detected by ELISA. Increasing concentrations of LPS were added to β2GPI-coated plates. Dose-dependent binding of LPS to β2GPI was detected by murine anti-LPS mAb but was not observed with an isotype-matched IgG control mAb. Right panel, binding of soluble LPS to solid-phase β2GPI, PT, or GluP was detected by ELISA. Binding of LPS to β2GPI-coated plates was detected by anti-LPS mAb. In contrast, LPS did not bind to PT or GluP. Binding is expressed as the mean A405 ± S.D. of duplicate samples and is shown for one example representative of four independent experiments.
Fluorescence Microscopy
B10R or peritoneal macrophages were fixed for 30 min at 25 °C with 0.1% glutaraldehyde. After washing with PBS, macrophages were incubated for 1 h at 25 °C with 10 μg/ml rat anti-mouse CD16/CD32 antibody (mouse Fc BlockTM), followed by another PBS wash. Untreated or pmB-B-treated human β2GPI at 20 μg/ml was added to the macrophages for 1 h at 25 °C, followed by either murine monoclonal anti-human β2GPI antibody (12A1-A17.3) or the relevant isotype control (29J3-119) at 20 μg/ml for an additional 2 h at 25 °C. After two washes with PBS, FITC-conjugated goat anti-mouse IgG (5 μg/ml) was added and incubated for 1 h to detect macrophage-bound human β2GPI. Hoechst 33342 (1 μg/ml) was added for the final 10 min of FITC-labeled antibody incubation. Macrophages were washed again and visualized using an Olympus 1X81 microscope and the Image-Pro Plus 7.0 acquisition system.
CD14 and TLR4 Blocking Experiments
B10R or peritoneal macrophages were plated as described above and then preincubated for 30 min at 37 °C (5% CO2) with blocking antibody for TLR4/MD2 (20 μg/ml) or CD14 (10 μg/ml) or with the IgG2a isotype control antibody. Then, β2GPI (10 μg/ml for B10R cells and 50 μg/ml for peritoneal macrophages) or LPS (0.1 μg/ml) was added to the macrophages and incubated for 6 h at 37 °C (5% CO2). The cell medium was removed, centrifuged, and analyzed for TNF-α by ELISA as described above.
RESULTS
β2GPI and LPS Induce TNF-α Production in Macrophages in a TLR4/CD14-dependent Manner
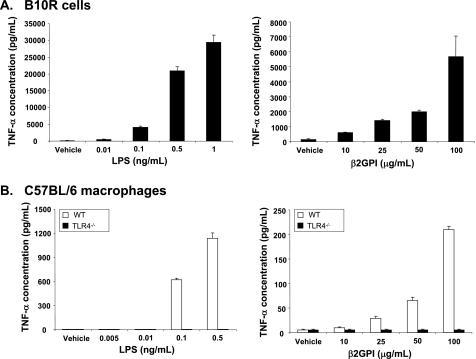
To determine whether β2GPI can itself act as an endogenous TLR ligand, we selected macrophages as our target cell, as these cells are exquisitely sensitive to TLR4 activation, especially when induced by LPS. We used two different forms of macrophages for our experiments: a macrophage cell line (B10R) and primary cultures of elicited peritoneal (WT or TLR4-deficient) macrophages. Macrophages were incubated with either LPS or commercially obtained β2GPI and evaluated for TNF-α production. As expected, B10R macrophages incubated with LPS for 6 h showed a dose-dependent production of TNF-α (Fig. 1A, left panel). B10R macrophages incubated with β2GPI also showed a dose-dependent production of TNF-α, albeit to a lower extent than that seen with LPS (Fig. 1A, right panel). Similarly, peritoneal macrophages from WT C57BL/6 mice showed a dose-dependent increase in TNF-α production in response to both LPS and β2GPI (Fig. 1B). In marked contrast, peritoneal macrophages from TLR4-deficient C57BL/6 mice showed no TNF-α production whatsoever following exposure to either LPS or β2GPI, implying a critical role for TLR4 in the response (Fig. 1B). Thus, like LPS, β2GPI appears to induce TNF-α production by macrophages through TLR4.
FIGURE 1.
β2GPI and LPS induce TNF-α production in macrophages. A, B10R macrophages were incubated with increasing concentrations of LPS (left panel) or commercially obtained human β2GPI (right panel) for 6 h, and TNF-α production was measured by ELISA. Data are mean TNF-α concentrations ± S.E. of triplicate samples for four (LPS) and three (β2GPI) independent experiments. B, peritoneal macrophages from WT C57BL/6 mice were incubated with increasing concentrations of LPS (left panel) or β2GPI (right panel) for 6 h, and TNF-α production was measured by ELISA. TLR4-deficient (TLR4−/−) macrophages did not produce TNF-α in response to LPS or β2GPI. Data are mean TNF-α concentrations ± S.E. of triplicate samples for four independent experiments each for both LPS and β2GPI.
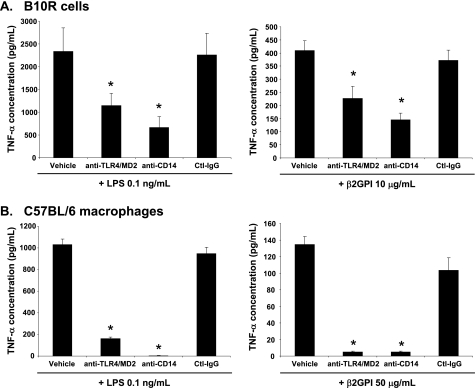
To specifically address the involvement of TLR4 in the production of TNF-α by macrophages exposed to β2GPI, we used blocking antibodies to the TLR4/MD2 and CD14 receptors and determined their effect on TNF-α production by macrophages. B10R or WT peritoneal macrophages were preincubated with anti-TLR4/MD2, anti-CD14, or isotype control antibody before stimulation with β2GPI or LPS. Both anti-TLR4/MD2 and anti-CD14 antibodies significantly inhibited TNF-α production by B10R and WT macrophages (Fig. 2). These data indicate that, similar to LPS, TLR4 and its associated co-receptors CD14 and MD2 are involved in β2GPI-induced macrophage TNF-α production.
FIGURE 2.
β2GPI and LPS induce TNF-α production in macrophages through TLR4. B10R macrophages (A) or peritoneal macrophages from WT C57BL/6 mice (B) were preincubated with medium or with anti-TLR4/MD2, anti-CD14, or isotype control IgG (Ctl-IgG) antibody for 30 min, followed by 6 h of incubation with either β2GPI (10 μg/ml for B10R macrophages and 50 μg/ml for peritoneal macrophages) or LPS (0.1 ng/ml). Antibody blocking of access to TLR4/MD2 or CD14 significantly decreased macrophage TNF-α production induced by β2GPI (right panels) or LPS (left panels). TNF-α production was measured by ELISA. Data are mean TNF-α concentrations ± S.E. of triplicate samples for four (B10R macrophages) and two (peritoneal macrophages) independent experiments. *, p < 0.05 by Student's two-tailed t test for cells treated with anti-TLR4/MD2 or anti-CD14 antibody compared with cells treated with isotype control antibody.
LPS Contamination of β2GPI Is Responsible for Induction of TNF-α Secretion by Macrophages
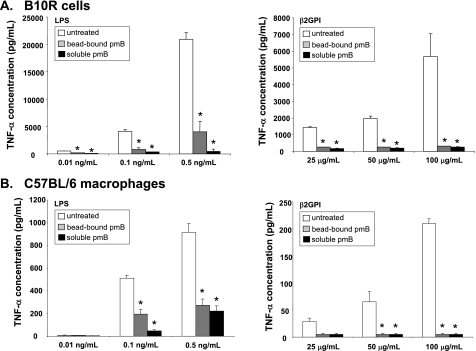
To ensure that β2GPI did not contain contaminating endotoxin activity that might be responsible for the macrophage activation we observed, we tested the β2GPI used in our macrophage experiments for endotoxin activity using a sensitive LAL assay. There was indeed endotoxin activity (9.774 ± 1.320 EU/ml) in the β2GPI preparation at 10 μg/ml (Table 1). We neutralized or removed endotoxin activity in the β2GPI preparation by three distinct methods and then tested the treated β2GPI for its ability to induce TNF-α production by macrophages. Table 1 shows the levels of endotoxin activity before and after treatment. As expected, treatment of LPS with pmB-S or pmB-B abrogated TNF-α production in B10R macrophages (Fig. 3A). Similarly, treatment of β2GPI with either form of polymyxin B inhibited TNF-α production by B10R macrophages (Fig. 3A). These findings were replicated in peritoneal macrophages from WT mice, which showed little or no TNF-α production when incubated with polymyxin B-treated LPS or β2GPI (Fig. 3B). Finally, delipidated β2GPI, having little or no remaining endotoxin activity (0.047 ± 0.040 EU/ml), failed to stimulate TNF-α production by B10R macrophages (data not shown).
TABLE 1.
Endotoxin activity detected in LPS and β2GPI preparations
Endotoxin activity was measured in LPS (0.1 ng/ml) and β2GPI (10 μg/ml) preparations by LAL assay with a lower limit of sensitivity of 0.005 EU/ml. The data shown are the mean EU/ml ± S.E. (n = two independent experiments, each consisting of two independent samples and measurements). The quantity of endotoxin was significantly reduced in LPS and β2GPI preparations that had been treated with pmB-B or pmB-S or delipidated.
| Preparation | Endotoxin activity |
|---|---|
| EU/ml | |
| LPS | 1.227 ± 0.092 |
| pmB-B-treated LPS | 0.011 ± 0.006 |
| pmB-S-treated LPS | 0.236 ± 0.053 |
| β2GPI | 9.774 ± 1.320 |
| pmB-B-treated β2GPI | 0.156 ± 0.090 |
| pmB-S-treated β2GPI | 0.800 ± 0.060 |
| Delipidated β2GPI | 0.470 ± 0.040 |
FIGURE 3.
LPS contamination of β2GPI is responsible for induction of TNF-α secretion by macrophages. B10R macrophages (A) or peritoneal macrophages from WT C57BL/6 mice (B) were incubated for 6 h with varying concentrations of untreated or treated LPS (left panels) or untreated or treated β2GPI (right panels). TNF-α production was measured by ELISA. LPS (0.01, 0.1, and 0.5 ng/ml) was left untreated or was treated with pmB-B (bead-bound pmB) or with pmB-S (soluble pmB). Similarly, β2GPI (25, 50, and 100 μg/ml) was left untreated or was treated with pmB-B or pmB-S. For both B10R (A) and C57BL/6 (B) macrophages, removal or inhibition of endotoxin activity in LPS (left panels) or β2GPI (right panels) by polymyxin B resulted in significantly decreased production of TNF-α by macrophages. *, p < 0.03 by Student's two-tailed t test for comparison of each treated condition with the untreated control. Data are mean TNF-α concentrations ± S.E. of triplicate samples for two (B10R macrophages) and three (C57BL/6 peritoneal macrophages) independent experiments.
It is important to note that pmB-S or pmB-B treatment of β2GPI or delipidation abrogated its ability to induce TNF-α production without affecting other biological activities of the protein. In all cases, treated β2GPI behaved similarly to untreated β2GPI in ELISAs measuring binding to cardiolipin or anti-β2GPI antibodies (data not shown). Clearly, however, these treatments were effective in removing endotoxin activity from β2GPI (Table 1), as indicated by a dramatic decrease in the capacity of treated β2GPI to stimulate TNF-α production by macrophages (Fig. 3). These results suggest that endotoxin contamination of β2GPI is responsible for inducing TNF-α production by macrophages (Fig. 1) and that β2GPI alone cannot stimulate macrophage TNF-α production.
β2GPI Does Not Affect the Stimulatory Effect of LPS
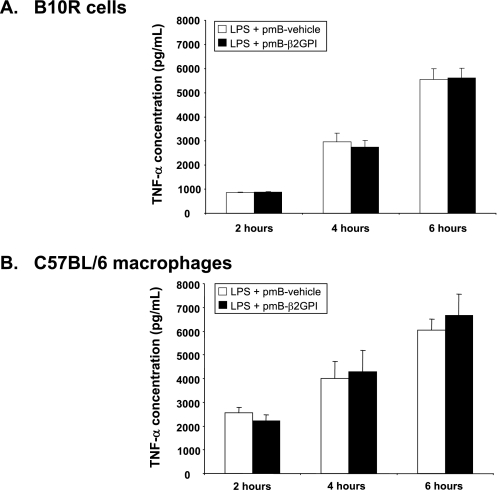
We next investigated whether the addition of LPS to polymyxin B-treated β2GPI, which had been rendered free of (or extremely low in) endotoxin activity, would restore its ability to induce TNF-α production by macrophages. Fig. 4 shows that the addition of very small amounts (0.5 ng/ml) of LPS to polymyxin B-treated β2GPI indeed restored the capacity of β2GPI to induce TNF-α production in both B10R and WT peritoneal macrophages. Notably, the level of TNF-α production was similar to that produced by macrophages exposed to the same concentration of LPS alone. Thus, no synergistic (or inhibitory) effects were observed when polymyxin B-treated β2GPI was combined with LPS. Similar findings were observed under FBS-free conditions (data not shown). These findings suggest that contaminating LPS (i.e. endotoxin) alone is responsible for the macrophage activation induced by untreated β2GPI and that β2GPI does not modulate the response to LPS by macrophages.
FIGURE 4.
LPS restores the stimulatory effect of polymyxin B-treated β2GPI. B10R macrophages (A) or peritoneal macrophages from C57BL/6 mice (B) were incubated for 2, 4, or 6 h with LPS (0.5 ng/ml) mixed with vehicle or β2GPI (10 μg/ml) that had been treated with pmB-B (pmB-vehicle or pmB-β2GPI, respectively). TNF-α production was measured by ELISA. For both B10R (A) and peritoneal (B) macrophages, LPS, whether combined with pmB-B-treated vehicle or β2GPI, induced similar and substantial levels of TNF-α. Data are mean TNF-α concentrations ± S.E. of triplicate samples for two independent experiments each for both B10R and C57BL/6 macrophages.
β2GPI Interacts Specifically with LPS
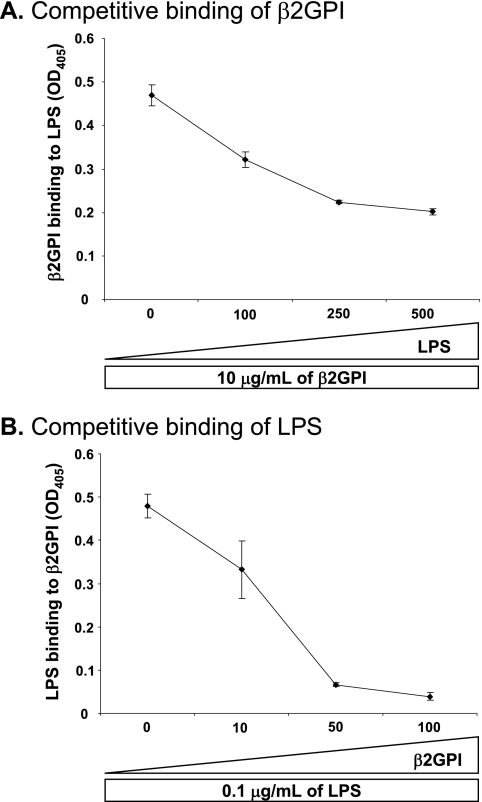
In our next set of studies, we explored a potential LPS-interacting role for β2GPI. To determine whether β2GPI can bind to LPS and, reciprocally, whether LPS can bind to β2GPI, we developed two binding assays. The first assay was an ELISA for the detection of β2GPI binding to LPS, in which the plate was coated with LPS. The second assay was an ELISA for the detection of LPS binding to β2GPI, in which the plate was coated with purified human β2GPI. We found that β2GPI bound to LPS in a dose-dependent manner when detected with anti-β2GPI antibody compared with control antibody (Fig. 5A). Similarly, we observed that LPS bound to β2GPI in a dose-dependent manner when detected with anti-LPS antibody compared with control antibody (Fig. 5B). Other plasma-derived glycoproteins, such as PT and GluP, did not interact with LPS, as shown by reciprocal binding ELISAs in which either LPS (Fig. 5A) or the protein itself (Fig. 5B) was used in the solid phase.
To ensure that these assays detected specific and biochemically relevant binding interactions for β2GPI and LPS, we performed competitive binding assays in which we added excess soluble ligand. Specifically, increasing amounts of soluble LPS were mixed with soluble β2GPI prior to assessing the binding of β2GPI to LPS-coated plates. As shown in Fig. 6A, the addition of excess soluble LPS inhibited the binding of soluble β2GPI to LPS-coated plates. Similarly, the addition of excess soluble β2GPI inhibited the binding of soluble LPS to β2GPI-coated plates (Fig. 6B). These results demonstrate a specific physical interaction between β2GPI and LPS.
FIGURE 6.
Interaction between β2GPI and LPS is specific. A, specificity of binding of soluble β2GPI to solid-phase LPS was assessed by competitive ELISA. Increasing concentrations (0, 100, 250, and 500 μg/ml) of soluble LPS were incubated with a constant concentration (10 μg/ml) of soluble β2GPI, and the mixture was then added to LPS-coated plates. Murine anti-β2GPI mAb was used to detect binding of β2GPI to plate-bound LPS. Soluble LPS inhibited the binding of β2GPI to plate-bound LPS in a dose-dependent manner. Binding is expressed as the mean A405 (OD405) ± S.D. of duplicate samples and is shown for one example representative of three independent experiments. B, specificity of binding of soluble LPS to solid-phase β2GPI was assessed by competitive ELISA. Increasing concentrations (10, 50, and 100 μg/ml) of soluble β2GPI were incubated with a constant concentration (0.1 μg/ml) of soluble LPS, and the mixture was then added to β2GPI-coated plates. Murine anti-LPS mAb was used to detect binding of LPS to β2GPI. Soluble β2GPI inhibited the binding of LPS to plate-bound β2GPI in a dose-dependent manner. Binding is expressed as the mean A405 ± S.D. of duplicate samples and is shown for one example representative of three independent experiments.
β2GPI Binds to Macrophages in the Presence (but Not Absence) of LPS
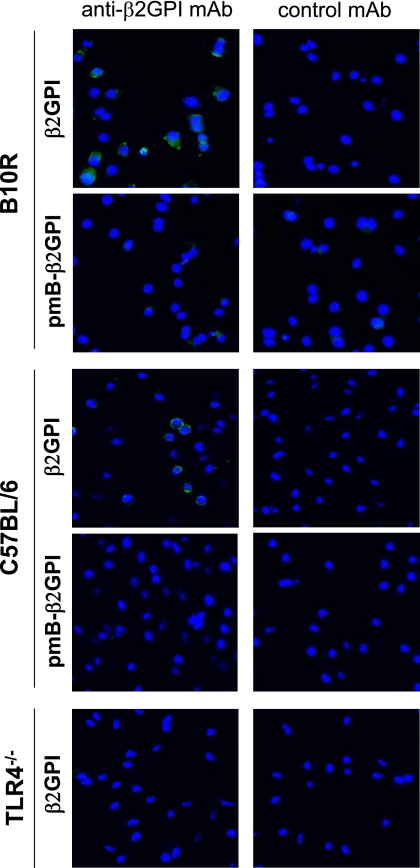
To address the biological relevance of the interaction between β2GPI and LPS, we examined the binding of β2GPI to the surface of viable macrophages in the presence or absence of LPS. Using a monoclonal anti-β2GPI antibody to detect macrophage-bound β2GPI by fluorescence microscopy, we observed that commercially obtained β2GPI bound to both B10R and WT peritoneal macrophages. Both B10R and WT peritoneal macrophages demonstrated surface staining of β2GPI at the cell membrane when incubated with the anti-β2GPI antibody (Fig. 7, left β2GPI panels), whereas no staining was observed with the negative control antibody (right β2GPI panels). Importantly, this interaction was dependent on the presence of LPS, as no binding was observed with polymyxin B-treated β2GPI (Fig. 7, pmB-β2GPI panels). Similarly, this interaction was dependent on TLR4, as no binding of β2GPI to TLR4-deficient peritoneal macrophages was observed (Fig. 7, TLR4−/− panels). These results are consistent with our hypothesis that the binding and activation of macrophages by β2GPI require both LPS and TLR4.
FIGURE 7.
β2GPI binds to fixed viable macrophages in the presence (but not absence) of LPS. B10R or peritoneal (C57BL/6 or TLR4-deficient (TLR4−/−)) macrophages were fixed and incubated with 20 μg/ml untreated β2GPI or pmB-B-treated β2GPI (pmB-β2GPI). Binding of β2GPI to the macrophage surface was detected by murine anti-β2GPI mAb (left panels) but was not observed with an isotype-matched control mAb (right panels). Bound murine mAb was detected with FITC-conjugated anti-murine IgG antibody by fluorescence microscopy. Binding of β2GPI to the macrophage membrane occurred with untreated β2GPI (upper panel) but not pmB-B-treated β2GPI (lower panel) in TLR4-sufficient B10R and C57BL/6 macrophages but not in TLR4−/− macrophages. Micrographs are representative of two independent experiments (magnification ×40) and show an overlay of two acquisitions: FITC staining and Hoechst 33342 staining.
DISCUSSION
We have shown that β2GPI interacts specifically with LPS and that contaminating LPS enables β2GPI to activate macrophages in a TLR4-dependent manner. Serum-derived human β2GPI, not treated to remove trace amounts of LPS, had TNF-α-inducing activity equivalent to that of a low level of LPS. In marked contrast, polymyxin B-treated β2GPI (or delipidated β2GPI), with very low to negligible endotoxin content, was unable to induce TNF-α production in either B10R or primary murine peritoneal macrophages. TLR4 was also required, as untreated β2GPI failed to induce TNF-α production in TLR4-deficient macrophages, and antibodies to either TLR4/MD2 or CD14 blocked β2GPI-induced TNF-α production by TLR4-sufficient macrophages. We have shown that β2GPI interacts physically with LPS in a dose-dependent manner and that binding of β2GPI to LPS and vice versa is specifically competed by the appropriate ligand. This interaction also occurs on the cell surface, as untreated (but not polymyxin B-treated) β2GPI bound to TLR4-sufficient macrophages. Neither form of β2GPI bound to TLR4-deficient macrophages. Taken together, these findings suggest that LPS and TLR4 are required for β2GPI binding to macrophages and that β2GPI may be an important physiological carrier of LPS.
β2GPI is an abundant plasma glycoprotein implicated in various biological processes, such as platelet aggregation (8, 21, 22), lipid-dependent prothrombinase activity (23–25), and macrophage uptake of apoptotic cells (1). It binds to multiple cell types, including activated platelets (22), endothelial cells (6), and macrophages (1), but it is unclear whether its interaction with cells can induce signal transduction. Apolipoprotein E receptor 2 on platelets and endothelial cells (26, 27), annexin A2 on endothelial cells and monocytes (28), and Ro60 on apoptotic blebs (29) have all been described as potential cell membrane receptors for β2GPI, but these receptors do not explain the TLR4-like (4, 9) or TLR2-like (30) signaling events observed in the presence of antibodies to β2GPI. Recognizing that the immunostimulatory properties of anti-β2GPI antibody-β2GPI complexes are similar to those of LPS, a known TLR4 ligand, we hypothesized that LPS within the β2GPI preparation might be responsible for the observed stimulatory effects. We took advantage of the finding that a commercial preparation of human serum-derived β2GPI did indeed contain low but significant levels of LPS. Macrophage TNF-α production induced by this β2GPI preparation followed kinetics similar to that shown by LPS, and the magnitude of the effect correlated with endotoxin content in the preparation. Moreover, both polymyxin B-treated and delipidated β2GPI, which no longer contained significant endotoxin activity, were unable to stimulate TNF-α production.
To ensure the efficacy of endotoxin removal from β2GPI, we evaluated two different forms of polymyxin B (bead-bound and soluble). Both forms removed endotoxin activity, and neither form affected the biological activity of β2GPI, as demonstrated by the equivalent reactivity of treated and untreated β2GPI in anti-β2GPI and anti-cardiolipin ELISAs before and after treatment (data not shown). We also performed control experiments to confirm that the lack of macrophage-stimulating and macrophage-binding activity observed with polymyxin B-treated β2GPI was not due to residual polymyxin B. For example, the vehicle control was treated with polymyxin B in the same way that β2GPI was treated. Polymyxin B-treated vehicle had no effect on LPS-stimulated production of TNF-α by macrophages. Thus, the lack of macrophage-stimulating activity in polymyxin B-treated β2GPI can be attributed to the removal of LPS rather than an effect of residual polymyxin B.
The finding that commercially available β2GPI contains a significant amount of endotoxin suggested to us that β2GPI may act as a LPS-interacting protein. Indeed, many proteins have been reported to interact with LPS. Adiponectin (17), α-lactalbumin (16), galectins (31), and chaperone proteins, such as HSP60 (13, 15), HSP70 (14), and HSP90 (32, 33), have all been found to contain significant amounts of LPS. For each of these proteins, contaminating LPS was shown to be the component responsible for its observed biological effect on cells. Thus, the literature associated with these proteins merits careful attention, as contaminating LPS can result in misleading conclusions. In this study, we have clearly shown that β2GPI depleted of LPS is unable to reproduce the immunostimulatory effect of untreated β2GPI on macrophages. Although this finding does not absolutely exclude the possibility that β2GPI has immunomodulatory effects on macrophages, it does indicate that caution is needed when drawing conclusions about possible receptor(s) with which β2GPI may interact.
As indicated by our data, an extremely small amount of contaminating LPS is sufficient to produce considerable immunostimulatory activity in both B10R and elicited peritoneal macrophages. In previous studies, 100 pg/ml LPS (∼2 EU/ml) was found to be the minimum concentration that could effectively activate RAW 264.7 macrophages (34, 35). Our results are consistent with these previous findings. We found that 100 pg/ml LPS (1.227 EU/ml by our assay) was the minimum concentration capable of activating both B10R and C57BL/6 peritoneal macrophages. For untreated β2GPI, 10 μg/ml was the minimum concentration needed to trigger macrophage TNF-α production in our experiments. The endotoxin content of untreated β2GPI was 9.774 EU/ml (0.977 EU/μg of β2GPI) and was reduced to 0.0156 EU/μg (∼63-fold) by polymyxin B treatment. On the basis of the endotoxin content of pure LPS (1.227 × 104 EU/μg), we estimated that untreated and polymyxin B-treated β2GPI contained ∼80 and 1.3 pg of LPS/μg of β2GPI, respectively.
Publications showing an activating effect of anti-β2GPI antibodies on cells often highlight the fact that the antibodies were treated with polymyxin B. Two issues may explain the apparent discrepancy between these studies and our findings. First and more importantly, these studies focused on anti-β2GPI antibodies rather than on β2GPI, whereas our study has focused on β2GPI alone. In fact, many published findings for anti-β2GPI antibodies were obtained without the addition of exogenous β2GPI, suggesting that sufficient β2GPI was present within the cell culture system (or already bound to the cells). In such cases, endogenous LPS may also have been present, if β2GPI acts as a LPS-interacting protein in vivo. Second, as compared among different studies, the levels of endotoxin activity can differ significantly depending on the lower limit of sensitivity for the particular endotoxin assay (usually LAL assay) used. For example, the assay that we used is extremely sensitive (lower limit of sensitivity = 0.005 EU/ml), but some publications have used assays with 10-fold lower sensitivity (e.g. lower limit of sensitivity = 0.05 EU/ml) (3, 36). Despite the greater sensitivity of the LAL assay we used, the levels of contaminating endotoxin we measured are in agreement with previous studies in terms of LPS concentration required to induce macrophage stimulation.
Although our studies were performed exclusively ex vivo, they raise the question of the physiological significance of an interaction between β2GPI and LPS. Several possibilities exist. β2GPI could mimic the effect of LPS-binding protein, an acute-phase reactant produced by the liver, and catalyze the interaction of LPS with CD14. As the effect of β2GPI on TNF-α production was the same irrespective of the presence or absence of serum (data not shown), it would appear that β2GPI cannot replace LPS-binding protein. In fact, β2GPI may have little or no effect on the biological activity of LPS, as the addition of polymyxin B-treated β2GPI lacked a synergistic (or inhibitory) effect on LPS-induced TNF-α secretion by macrophages. It remains possible, however, that the effect of native β2GPI, such as that found in vivo, may differ from that of polymyxin B-treated β2GPI. Similar observations have been made for recombinant human HSP60, the initial preparations of which contained LPS. HSP60-associated LPS had a 2-fold greater effect on macrophages than pure LPS. However, when the same HSP60 was treated with polymyxin B and then mixed with pure LPS, HSP60 had little or no effect on LPS activity (13). Based on the known ability of β2GPI to bind to negatively charged particles and protect against their potential procoagulant/proinflammatory effects (37), it seems reasonable to speculate that β2GPI might have an inhibitory or sequestering effect on LPS in vivo. This effect could conceivably be modulated by the presence of antibodies to β2GPI.
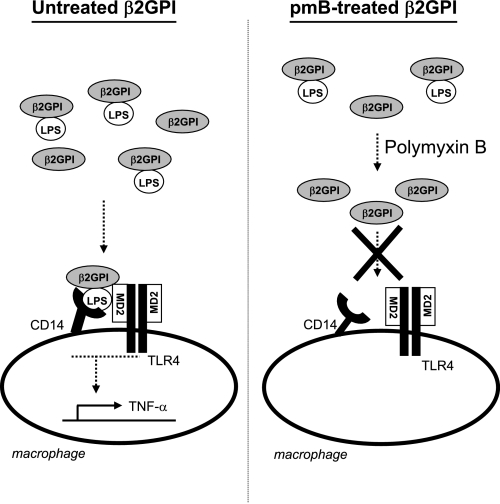
In summary, we have shown that the apparent ability of a commercial preparation of β2GPI to bind to and stimulate macrophages is due to contamination of this protein with LPS. The binding and immunostimulatory activities of the protein preparation paralleled those of LPS and were consistent with the concentration of contaminating LPS found in the β2GPI. Similar to LPS, the binding and immunostimulatory activities were dependent on TLR4. Although many proteins can be contaminated by endotoxin, we have shown that, for β2GPI, contamination likely arises because of a specific interaction between β2GPI and LPS. Thus, β2GPI binds to LPS and vice versa. Removal of LPS from β2GPI or removal of TLR4 from the cell ablated the ability of β2GPI to bind to and stimulate macrophages. We propose that β2GPI interacts with LPS in a specific manner (Fig. 8). When β2GPI binds to a cell surface, any LPS complexed to the β2GPI will also be brought to the cell surface. β2GPI-bound LPS could then conceivably interact with TLR4, resulting in cell activation and TNF-α release. In marked contrast, following purification to remove contaminating LPS, β2GPI is no longer able to trigger cell activation and TNF-α production. It remains unclear whether β2GPI plays a physiological role in vivo in modulating the effects of LPS. Circulating autoantibodies to β2GPI have been reported to trigger cell activation via the TLR4 pathway (5, 7, 9). As anti-β2GPI antibodies can stabilize binding of β2GPI to phospholipid membranes by decreasing its rate of dissociation (38), the presence of these antibodies may enhance the capacity of β2GPI to deliver LPS to TLR4 on the cell surface and thereby trigger cell activation.
FIGURE 8.
Proposed model showing that β2GPI requires LPS for macrophage binding and activation. In our proposed model, β2GPI is capable of binding to and activating the macrophage only when it is bound to LPS. For untreated β2GPI (in the presence of LPS), β2GPI is carried to the macrophage surface with LPS and is bound to the cell through the interaction of LPS with CD14. LPS is transferred to MD2 to form a monomeric LPS-MD2 complex that binds to and activates TLR4. Subsequent TLR4 oligomerization and signaling lead to the release of TNF-α production by macrophages. In contrast, for polymyxin B (pmB)-treated β2GPI (in the absence of LPS), β2GPI is unable to bind to or activate the macrophage.
Acknowledgments
We are grateful to Evan Roter for contribution to experiments related to this study and to Dr. Simone Chevalier for providing access to the fluorescence microscope (funded by the Canada Foundation for Innovation and the Research Institute of the McGill University Health Centre).
Note Added in Proof
After acceptance of this paper for publication, Agar and coworkers (39) reported that β2GPI binds to and inactivates LPS both in vitro and in vivo. These authors further demonstrated that binding of β2GPI to LPS resulted in a conformational change in β2GPI that led to binding of the β2GPI/LPS complex to monocytes and clearance of this complex. The findings of a specific interaction between β2GPI and LPS and binding of this complex to monocytes are consistent with our data, although we did not perform in vivo analyses or evaluate the conformation of β2GPI in our studies.
This was supported in part by operating grants from the Canadian Institutes of Health Research (CIHR) and the Institute of Musculoskeletal Health and Arthritis (IMHA) and by Arthritis Society Grant MOP-42391 (to J. R.), CIHR Grants MOP-67101 and MOP-97916, CIHR/IMHA Fall 2008 Priority Announcement Grant MUS-67101, and a post-doctoral fellowship from the Research Institute of the McGill University Health Centre (to P. L.).
- β2GPI
- β2-glycoprotein I
- TLR
- Toll-like receptor
- PT
- prothrombin
- GluP
- Glu-plasminogen
- pmB-B
- agarose bead-bound polymyxin B
- pmB-S
- soluble polymyxin B sulfate
- LAL
- Limulus amebocyte lysate
- EU
- endotoxin units
- mAb
- monoclonal antibody.
REFERENCES
- 1. Maiti S. N., Balasubramanian K., Ramoth J. A., Schroit A. J. (2008) J. Biol. Chem. 283, 3761–3766 [DOI] [PubMed] [Google Scholar]
- 2. Levine J. S., Branch D. W., Rauch J. (2002) N. Engl. J. Med. 346, 752–763 [DOI] [PubMed] [Google Scholar]
- 3. Lambrianides A., Carroll C. J., Pierangeli S. S., Pericleous C., Branch W., Rice J., Latchman D. S., Townsend P., Isenberg D. A., Rahman A., Giles I. P. (2010) J. Immunol. 184, 6622–6628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sorice M., Longo A., Capozzi A., Garofalo T., Misasi R., Alessandri C., Conti F., Buttari B., Riganò R., Ortona E., Valesini G. (2007) Arthritis Rheum. 56, 2687–2697 [DOI] [PubMed] [Google Scholar]
- 5. Zhou H., Yan Y., Xu G., Zhou B., Wen H., Guo D., Zhou F., Wang H. (2011) Clin. Exp. Immunol. 163, 189–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Del Papa N., Guidali L., Spatola L., Bonara P., Borghi M. O., Tincani A., Balestrieri G., Meroni P. L. (1995) Clin. Exp. Rheumatol. 13, 179–185 [PubMed] [Google Scholar]
- 7. Raschi E., Testoni C., Bosisio D., Borghi M. O., Koike T., Mantovani A., Meroni P. L. (2003) Blood 101, 3495–3500 [DOI] [PubMed] [Google Scholar]
- 8. Shi W., Chong B. H., Chesterman C. N. (1993) Blood 81, 1255–1262 [PubMed] [Google Scholar]
- 9. Pierangeli S. S., Vega-Ostertag M. E., Raschi E., Liu X., Romay-Penabad Z., De Micheli V., Galli M., Moia M., Tincani A., Borghi M. O., Nguyen-Oghalai T., Meroni P. L. (2007) Ann. Rheum. Dis. 66, 1327–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Antal-Szalmás P. (2000) Eur. J. Clin. Invest. 30, 167–179 [DOI] [PubMed] [Google Scholar]
- 11. Medzhitov R. (2001) Nat. Rev. Immunol. 1, 135–145 [DOI] [PubMed] [Google Scholar]
- 12. Rauch J., Dieudé M., Subang R., Levine J. S. (2010) Lupus 19, 347–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gao B., Tsan M. F. (2003) J. Biol. Chem. 278, 22523–22529 [DOI] [PubMed] [Google Scholar]
- 14. Gao B., Tsan M. F. (2003) J. Biol. Chem. 278, 174–179 [DOI] [PubMed] [Google Scholar]
- 15. Habich C., Kempe K., van der Zee R., Rümenapf R., Akiyama H., Kolb H., Burkart V. (2005) J. Immunol. 174, 1298–1305 [DOI] [PubMed] [Google Scholar]
- 16. Lin I. C., Kuo C. D. (2010) Food Chem. Toxicol. 48, 2642–2649 [DOI] [PubMed] [Google Scholar]
- 17. Turner J. J., Smolinska M. J., Sacre S. M., Foxwell B. M. (2009) Scand. J. Immunol. 69, 329–336 [DOI] [PubMed] [Google Scholar]
- 18. Price B. E., Rauch J., Shia M. A., Walsh M. T., Lieberthal W., Gilligan H. M., O'Laughlin T., Koh J. S., Levine J. S. (1996) J. Immunol. 157, 2201–2208 [PubMed] [Google Scholar]
- 19. Levine J. S., Subang R., Nasr S. H., Fournier S., Lajoie G., Wither J., Rauch J. (2006) J. Immunol. 177, 6504–6516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goldstein J. L., Basu S. K., Brown M. S. (1983) Methods Enzymol. 98, 241–260 [DOI] [PubMed] [Google Scholar]
- 21. Nimpf J., Wurm H., Kostner G. M. (1985) Thromb. Haemost. 54, 397–401 [PubMed] [Google Scholar]
- 22. Nimpf J., Wurm H., Kostner G. M. (1987) Atherosclerosis 63, 109–114 [DOI] [PubMed] [Google Scholar]
- 23. Goldsmith G. H., Pierangeli S. S., Branch D. W., Gharavi A. E., Harris E. N. (1994) Br. J. Haematol. 87, 548–554 [DOI] [PubMed] [Google Scholar]
- 24. Nimpf J., Bevers E. M., Bomans P. H., Till U., Wurm H., Kostner G. M., Zwaal R. F. (1986) Biochim. Biophys. Acta 884, 142–149 [DOI] [PubMed] [Google Scholar]
- 25. Shi T., Iverson G. M., Qi J. C., Cockerill K. A., Linnik M. D., Konecny P., Krilis S. A. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 3939–3944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lutters B. C., Derksen R. H., Tekelenburg W. L., Lenting P. J., Arnout J., de Groot P. G. (2003) J. Biol. Chem. 278, 33831–33838 [DOI] [PubMed] [Google Scholar]
- 27. Romay-Penabad Z., Aguilar-Valenzuela R., Urbanus R. T., Derksen R. H., Pennings M. T., Papalardo E., Shilagard T., Vargas G., Hwang Y., de Groot P. G., Pierangeli S. S. (2011) Blood 117, 1408–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Romay-Penabad Z., Montiel-Manzano M. G., Shilagard T., Papalardo E., Vargas G., Deora A. B., Wang M., Jacovina A. T., Garcia-Latorre E., Reyes-Maldonado E., Hajjar K. A., Pierangeli S. S. (2009) Blood 114, 3074–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reed J. H., Giannakopoulos B., Jackson M. W., Krilis S. A., Gordon T. P. (2009) Arthritis Rheum. 60, 860–869 [DOI] [PubMed] [Google Scholar]
- 30. Alard J. E., Gaillard F., Daridon C., Shoenfeld Y., Jamin C., Youinou P. (2010) J. Immunol. 185, 1550–1557 [DOI] [PubMed] [Google Scholar]
- 31. Sarter K., André S., Kaltner H., Lensch M., Schulze C., Urbonaviciute V., Schett G., Herrmann M., Gabius H. J. (2009) Biochem. Biophys. Res. Commun. 379, 155–159 [DOI] [PubMed] [Google Scholar]
- 32. Byrd C. A., Bornmann W., Erdjument-Bromage H., Tempst P., Pavletich N., Rosen N., Nathan C. F., Ding A. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 5645–5650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Triantafilou K., Triantafilou M., Dedrick R. L. (2001) Nat. Immunol. 2, 338–345 [DOI] [PubMed] [Google Scholar]
- 34. Dziarski R., Gupta D. (2005) Infect. Immun. 73, 5212–5216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang J. S., Kim H. J., Ryu Y. H., Yun C. H., Chung D. K., Han S. H. (2006) Clin. Vaccine Immunol. 13, 309–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mulla M. J., Brosens J. J., Chamley L. W., Giles I., Pericleous C., Rahman A., Joyce S. K., Panda B., Paidas M. J., Abrahams V. M. (2009) Am. J. Reprod. Immunol. 62, 96–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Di Simone N., Luigi M. P., Marco D., Fiorella D. N., Silvia D., Clara D. M., Alessandro C. (2007) Ann. N.Y. Acad. Sci. 1108, 505–514 [DOI] [PubMed] [Google Scholar]
- 38. Willems G. M., Janssen M. P., Pelsers M. M., Comfurius P., Galli M., Zwaal R. F., Bevers E. M. (1996) Biochemistry 35, 13833–13842 [DOI] [PubMed] [Google Scholar]
- 39. Agar C., de Groot P. G., Mörgelin M., Monk S. D., van Os G., Levels J. H., de Laat B., Urbanus R. T., Herwald H., van der Poll T., Meijer J. C. (2011) Blood 117, 6939–6947 [DOI] [PubMed] [Google Scholar]