Background: Isothiocyanates, membrane-permeable electrophiles that form adducts with thiols, have been suggested to have important medical benefits.
Results: Thiolated isothiocyanate showed electrophilic response, targeted cellular Hsp90β, and stimulated heat shock response.
Conclusion: Thiolated isothiocyanates are an active metabolite that could contribute to cellular responses through transthiocarbamoylation of cellular proteins.
Significance: These findings would extend our understanding of how isothiocyanates show their medical benefits as “functional foods.”
Keywords: Chemical Biology, Heat Shock Protein, Hsp90, Post-translational Modification, Sulfhydryl, Isothiocyanate
Abstract
Isothiocyanates, membrane-permeable electrophiles that form adducts with thiols, have been suggested to have important medical benefits. Here we shed light on isothiocyanate-thiol conjugates and studied their electrophilic potential transferring an isothiocyanate moiety to cellular proteins. When we examined the effect of sulfhydryl molecules on cellular response induced by 6-methylsulfinylhexyl isothiocyanate (6-HITC), an analog of sulforaphane isolated from broccoli, we observed significant induction of heme oxygenase-1 by 6-HITC even in the presence of N-acetyl-l-cysteine or glutathione (GSH). In addition, the authentic 6-HITC-β-mercaptoethanol (6-HITC-ME) conjugate markedly up-regulated the enzyme expression, suggesting the electrophilic potential of thiolated isothiocyanates. To gain a chemical insight into the cellular response induced by thiolated isothiocyanates, we studied the occurrence of transthiocarbamoylation of sulfhydryl molecules by 6-HITC-ME and observed that, upon incubation of 6-HITC-ME with GSH, a single product corresponding to the GSH conjugate of 6-HITC was generated. To test the functional ability of thiolated isothiocyanates to thiocarbamoylate proteins in living cells, we designed a novel probe, combining an isothiocyanate-reactive group and an alkyne functionality, and revealed that the transthiocarbamoylation of proteins occurred in the cells upon exposure to 6-HITC-ME. The target of thiocarbamoylation included heat shock protein 90 β (Hsp90β), a chaperone ATPase of the Hsp90 family implicated in protein maturation and targeting. To identify the sites of the Hsp90β modification, we utilized nano-LC/MALDI-TOF MS/MS and suggested that a thiol group on the peptide containing Cys-521 reacted with 6-HITC, resulting in a covalent adduct in a 6-HITC-treated recombinant Hsp90β in vitro. The site-selective binding to Cys-521 was supported by in silico modeling. Further study on the thiocarbamoylation of Hsp90β suggested that the formation of 6-HITC-Hsp90β conjugate might cause activation of heat shock factor-1, rapidly signaling a potential heat shock response. These data suggest that thiolated isothiocyanates are an active metabolite that could contribute to cellular responses through transthiocarbamoylation of cellular proteins.
Introduction
Epidemiological studies have demonstrated that the consumption of cruciferous vegetables is associated with a lower incidence of cancers (1–3). An important group of compounds that have this property are organosulfur compounds, such as the isothiocyanates. The isothiocyanates are compounds that occur as glucosinolates in a variety of cruciferous vegetables, such as the Brassica species. Glucosinolates are found in the cell vacuoles of various plants in the family Cruciferae, such as horseradish, mustard, broccoli, and wasabi. When plant cells are damaged, glucosinolates are hydrolyzed by myrosinase (thioglucoside glucohydrolase, EC 3.2.3.1) to produce isothiocyanates. They are among the most appreciated nutraceutical components of what have become known as “functional foods” and have been suggested to have important medical benefits. They not only inhibit microbes but can also help treat or prevent blood clotting and asthma (4). They are also a class of phytochemicals with a recognized anti-cancer activity. They can act in a chemopreventive capacity via inhibition of carcinogen-activating phase 1 enzymes (5) and induction of phase 2 detoxification enzymes (6). Isothiocyanates are also active in the postinitiation phase of tumorigenesis and are, therefore, proposed to have a chemotherapeutic potential (7, 8). The isothiocyanate-mediated disruption of cancer progression is achieved by a variety of mechanisms, including modulation of cell growth, inhibition of angiogenesis, suppression of metastasis, and induction of apoptosis. Isothiocyanates can also modulate inflammatory pathways via inhibition of the transcription factor nuclear factor κB (9). Among the varieties of isothiocyanates, the ω-methylsulfinylalkyl isothiocyanates, such as 4-methylsulfinylbutyl isothiocyanate (sulforaphane) and its analog 6-methylsulfinylhexyl isothiocyanate (6-HITC)2 present in broccoli and wasabi, respectively, have attracted much attention.
It is generally believed that isothiocyanates mediate cellular responses due to the high reactivity of the isothiocyanate moiety (10). Nucleophilic attack of isothiocyanates by thiols forms dithiocarbamates. The main pathway for biotransformation of isothiocyanates in mammals is glutathione (GSH) conjugation catalyzed by glutathione S-transferase (11). The resulting dithiocarbamate formation was described as the first step in the metabolic pathway of isothiocyanates. It has been shown that conjugation of benzyl isothiocyanate to GSH results in greatly reduced toxicity, especially at high GSH concentrations (12). Similar results were obtained for phenylalkyl isothiocyanate-cysteine conjugates (13). Thus, the conjugation with thiol leads to the reduction of isothiocyanate toxicity. Meanwhile, it was also speculated that isothiocyanate-thiol conjugates could be regarded as a transport form of isothiocyanates (14). The reaction of isothiocyanates with thiols, unlike other nucleophiles like amine and hydroxyl groups, is reversible (10). From the product dithiocarbamate, the thiol and free isothiocyanate can be regenerated. The addition of a second thiol derivative to a solution of a dithiocarbamate is suggested to result in transfer of the isothiocyanate to the second thiol group. Thus, GSH conjugates of isothiocyanates can act as “transport forms” of the parent compound (15–17).
The present study was designed to investigate the transthiocarbamoylation of sulfhydryl molecules by thiolated isothiocyanates. Transthiocarbamoylation of cellular proteins in living cells after exposure to a thiolated isothiocyanate was also studied using click chemistry. In addition, based on the identification of a heat shock protein as the major cellular target of transthiocarbamoylation, we studied its functional consequences.
EXPERIMENTAL PROCEDURES
Materials
6-HITC was a kind gift of Dr. Y. Morimitsu (Ochanomizu University). 17-AAG was purchased from Wako. The anti-Hsp70 and anti-Hsp90β goat polyclonal antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The anti-Hsp90α rabbit polyclonal antibody and recombinant human Hsp90β protein were obtained from Stressgen. The anti-lamin A rabbit polyclonal antibody was purchased from Sigma. The anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mouse monoclonal antibody was obtained from Millipore. The horseradish peroxidase (HRP)-linked anti-rabbit IgG, the HRP-linked anti-mouse IgG, and ECL (enhanced chemiluminescence) Western blotting detection reagents were obtained from GE Healthcare. The HRP-linked anti-goat IgG immunoglobulin was purchased from Dako. NeutrAvidin Plus UltraLink Resin was obtained from Thermo. Biotin-N3 was obtained from Invitrogen. TAMRA-N3 was essentially synthesized as described previously for the synthesis of related fluorescent tags (18). The detailed experimental procedures for the synthesis of the alkynyl-tagged 6-HITC (Al-ITC) and its derivatives are described in the supplementary material.
Transthiocarbamoylation of Sulfhydryl Molecules by Thiolated Isothiocyanates
The β-mercaptoethanol conjugate of 6-HITC (6-HITC-ME) was prepared by adding 6 μmol of β-mercaptoethanol in 180 μl of 0.1 m potassium phosphate buffer, pH 8, with 50% ethanol, to 1 μmol of 6-HITC. After overnight incubation, the β-mercaptoethanol conjugate of 6-HITC was purified by reverse-phase HPLC using a Develosil ODS-HG-5 column (4.6-mm inner diameter × 250 mm; Nomura Chemicals), eluted with a linear gradient of water containing 0.1% TFA (solvent A)-acetonitrile (solvent B) (time = 0 min, 10% B; 40 min, 80% B) at a flow rate of 0.8 ml/min. The elution profiles were monitored by absorbance at 190–650 nm. About 70% conversion of 6-HITC to the β-mercaptoethanol conjugate was obtained. Methanol was removed under N2, after which a stock solution of 6-HITC-ME was stored at −80 °C. A product of 95% purity was obtained as judged with reverse-phase HPLC analysis. Transthiocarbamoylation of sulfhydryl molecules by thiolated isothiocyanates was carried out upon incubation of 1 mm 6-HITC-ME with 1 mm GSH or 1 mm N-acetyl-l-cysteine (NAC) in 50 mm sodium phosphate buffer (pH 7.4) for 4 h at 37 °C. After incubation for 4 h at 37 °C, the reaction mixtures were analyzed by reverse-phase HPLC.
Cell Culture
We used Caco-2 cells, derived from a human colorectal carcinoma, in this study. They are known to resemble normal small intestinal epithelial cells and serve as a useful in vitro model to further ascertain the mechanisms contributing to gut cell function. The cells were grown in Dulbecco's modified essential medium (Sigma) supplemented with 10 mm HEPES (pH 7.4), 100 units/ml penicillin, 100 mm streptomycin, and 10% (v/v) heat-inactivated fetal calf serum at 37 °C in an atmosphere of 95% air and 5% CO2. During the culture, the non-differentiated cells were passaged upon reaching 80% confluence using 0.05% trypsin in phosphate-buffered saline (PBS) with 0.5 mm EDTA.
Click Chemistry
Caco-2 cells were treated with the Al-ITC analogues dissolved in acetonitrile or the vehicle control (acetonitrile only) for 2 h at 37 °C. The cells were then washed twice with cold PBS, lysed in radioimmune precipitation assay buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, protease inhibitor mixture, and phosphatase inhibitor mixtures 1 and 2), and centrifuged at 10,000 rpm for 5 min at 4 °C. Click chemistry was performed with cellular lysates containing 2.0 mg/ml protein with 1 mm CuSO4, 1 mm ascorbic acid, 0.1 mm tris((1-benzyl-1H-1,2,3-triazol-4-yl)methyl)amine, 3 μm TAMRA-N3, or 20 μm biotin-N3. After incubation in the dark for 1 h at room temperature, the cell lysate proteins were diluted with loading buffer and separated on an SDS-polyacrylamide gel, or the avidin pull-down was performed.
Image Analysis and Spot Identification
The lysates prepared from the cells were treated with SDS sample buffer and immediately boiled for 5 min. The protein concentrations were determined using the BCA protein assay kit (Thermo). The proteins were separated by SDS-PAGE in the presence of 2-mercaptoethanol. The gel was fixed in 7% acetic acid, 10% methanol for 30 min and then scanned using a Typhoon 9200 (GE Healthcare).
MALDI-TOF MS Analysis for Protein Identification
Gel pieces were washed in water containing 0.1 and 50% methanol for 1 h, dehydrated in acetonitrile, and dried in a SpeedVac for 30 min. Samples were proteolyzed with sequence grade modified trypsin (Promega) in 50 mm NH4HCO3 buffer in the presence of 0.01% Protease MAX surfactant (Promega) for 3 h at 37 °C. The supernatant was collected and desalted by ZipTip C18 reverse-phase microcolumns (Millipore). Peptide mass fingerprints were generated with an ABI 4700 Proteomics Analyzer MALDI-TOF-TOF mass spectrometer with version 3.6 software (AB-Sciex). Proteins were identified with the MASCOT (Matrix Science, London) searching algorithms using the NCBInr data base.
Nano-LC-MALDI Analysis
Human recombinant Hsp90β was incubated with 25 μm 6-HITC for 3 h. The 6-HITC-labeled Hsp90β was digested by sequence grade modified trypsin (Promega) in the presence of 0.01% Protease MAX surfactant (Promega). The recovered peptides were then resolved by reverse-phase nano-LC (DiNa Nano LC system, KYA TECH Corp., Tokyo, Japan) and then directly fractionated onto a MALDI target plate with α-cyano-4-hydroxycinnamic acid by a spotter (DiNa Map system). MALDI-MS and MS/MS were performed on a 4700 Proteomics Analyzer. The MS/MS data were analyzed by MASCOT.
Database Analysis
MASCOT Distiller was used to obtain the monoisotopic peak list from the raw mass spectrometry files. For the MS/MS database query, the searching parameter was set up as follows. The taxonomy was selected as Homo sapiens; the mass tolerance was ±0.1 Da; the MS/MS tolerance was ±0.3 Da; the missed cleavage sites were allowed up to one; the variable modification was selected as carbamidomethyl (cysteine), oxidation (methionine), deamidation (asparagine, glutamine), and 6-HITC addition (cysteine).
Modeling Analysis
The putative three-dimensional structures of Hsp90β were built with the Phyre homology-modeling server (19) using three structures of the Hsp90 homologs as templates: yeast Hsp90 in complex with an ATP analog and the co-chaperone (closed form I, Protein Data Bank entry 2CG9) (20); apoHsp90 ortholog from Escherichia coli (open form, Protein Data Bank entry 2IOP) (21); and endoplasmic reticulum Hsp90 ortholog complexed with AMP-PNP (closed form II, Protein Data Bank entry 2O1U) (22). The 6-HITC and cysteine adduct molecule were generated and energy-minimized using the Dundee PRODRG2 Server (23). The position and conformation of the 6-HITC-cysteine Michael adduct were adjusted using Coot (24) and MolFeat (FiatLux Co.) to avoid any steric clash and to correct the direction of the isothiocyanate group of 6-HITC.
Western Blot Analysis
The homogenates prepared from the cells were treated with the SDS sample buffer and immediately boiled for 5 min. The protein concentrations were determined using the BCA protein assay kit (Thermo). The proteins separated by SDS-PAGE in the presence of 2-mercaptoethanol were electrotransferred onto a PVDF membrane. To detect the immunoreactive proteins, we used ECL reagents.
Reverse Transcription (RT)-PCR
The total RNA was isolated from the cells using TRIzol reagent (Invitrogen) according to the manufacturer's protocol and spectrophotometrically quantified. The total RNAs were reverse-transcribed into cDNA and used for the RT-PCR analysis (Qiagen Inc., Hilden, Germany). Glyceraldehyde-3-phosphate dehydrogenase was used as the internal standard. The PCR products were separated on a 1% agarose gel, and the positive signals were quantified by a densitometry analysis after staining with ethidium bromide. The following primer pairs (Sigma) were used: GAPDH, 5′-AACCCATCACCATCTTCCAGGAGC-3′ (forward) and 5′-CACAGTCTTCTGAGTGGCAGTGAT-3′ (reverse); Hsp70, 5′-ATGGCCAAAGCCGCGGCAGTCGGCATCGACCTGGG-3′ (forward) and 5′-CGGCCGTGGGCTCGTTGATGATCCGCAGCACGTTG-3′ (reverse).
RNA Interference Using siRNA
The Caco-2 cells were transfected with siRNA against HSF-1 (sc-35611) from Santa Cruz Biotechnology, Inc. as per the manufacturer's protocol. Briefly, the subconfluent (40–50%) Caco-2 cells were transfected using the Lipofectamine2000TM transfection reagent (Invitrogen); 4 μl of the siRNA stock (10 μm) and 8 μl of Lipofectamine2000TM were each diluted with 200 μl of Opti-MEM (Invitrogen). After 5 min at room temperature, they were combined and incubated for 20 min. The reaction mixtures were overlaid on the cell culture for 6 h. The medium was then added to an equal volume of culture medium without penicillin and streptomycin, and the next day, the medium was changed to a fresh medium. It was incubated at 37 °C in a CO2 incubator until ready to assay (48 h post-transfection). For the control, we used StealthTM RNAi negative control duplexes (Invitrogen).
Chemical Cross-linking of HSF-1
To prepare the whole cell extracts, cells were lysed in 50 mm sodium phosphate buffer (pH 7.4) containing 150 mm NaCl, 1% Nonidet P-40, protease inhibitor mixture (Sigma), and phosphatase inhibitor mixtures 1 and 2 (Sigma). The cross-linking of HSF-1 was performed by adding bis(sulfosuccinimidyl)suberate (Thermo) to the whole cell extract and incubated for 30 min at room temperature. The cell extracts were then subjected to Western blot analysis using the rabbit anti-HSF-1 polyclonal antibody (Cell Signaling Technology Inc., Danvers, MA), using the Can Get Signal (TOYOBO Biochemicals, Osaka, Japan).
Nuclear Translocation of HSF-1
For the immunocytochemistry, cells were washed twice in cold PBS and fixed in PBS containing 4% paraformaldehyde for 10 min at room temperature. After the membranes were permeabilized by exposing the fixed cells to PBS containing 0.5% Triton X-100, the cells were sequentially incubated in PBS solutions containing blocking serum (2% BSA) and immunostained with the anti-HSF-1 polyclonal antibody overnight at 4 °C. After being washed with PBS, the cells were incubated in Alexa Fluor 488-labeled anti-rabbit secondary antibody (Invitrogen) for 2 h at room temperature. After labeling with the secondary antibody, the cells were incubated in RNase for 10 min and propidium iodide (160 nm; Sigma) for 5 min at room temperature. The images were digitally captured using a laser-scanning confocal microscope and Pascal LSM software (LSM5 Pascal, Zeiss, Jena, Germany).
Transient Transfection of Hsp90β
The Halo-tagged human Hsp90β vector was obtained from Promega (FHC01815). Transient transfections were performed using Lipofectamine2000TM. Briefly, 8 μg of plasmid DNA and 16 μl of Lipofectamine2000TM were each diluted with 200 μl of Opti-MEM. After 48 h, the Caco-2 cells transiently expressing Halo-tagged Hsp90β were treated with 6-HITC or DMSO for 2 h. The cells were then washed twice with cold PBS, lysed in 20 mm HEPES (pH 7.4) containing 150 mm NaCl and 1% Triton X-100, and centrifuged at 10,000 rpm for 5 min at 4 °C; the supernatant was incubated with HaloLink resin (Promega) for 6 h at 4 °C. After extensive washing with 20 mm HEPES (pH 7.4) containing 150 mm NaCl and 0.05% Triton X-100, the bound materials were eluted with SDS sample buffer and subjected to immunoblot analysis using the anti-HSF-1 antibody.
Avidin Pull-down Assay
UltraLink immobilized NeutrAvidin beads (Thermo) was added to the cell lysate, and the samples were rotated at 4 °C for 1 h to preclear nonspecific binding to NeutrAvidin beads. Next, click chemistry was performed with the supernatant in the dark for 2 h at 4 °C. After incubation, the lysate containing the biotin-N3 conjugation to the Al-ITC-modified proteins was incubated with NeutrAvidin beads for 2 h at 4 °C. The beads were then washed three times in radioimmune precipitation assay buffer, and bound proteins were eluted into SDS sample buffer by boiling for 15 min. Biotinylated proteins were resolved by SDS-PAGE and subjected to immunoblot analysis with anti-Hsp90β antibody.
RESULTS
Induction of Cellular Response by Thiolated Isothiocyanates
Electrophiles, having a chemical feature to react with the sulfhydryl groups by oxido-reduction, alkylation, or disulfide interchange, are inducers of the phase 2 and antioxidant enzymes. Hence, we first examined the effect of sulfhydryl molecules, NAC and GSH, on the 6-HITC-induced HO-1 expression in human intestinal Caco-2 cells. The cells were treated with 25 μm 6-HITC in the presence and absence of the sulfhydryl molecules for 24 h, and the HO-1 expression was examined by an immunoblot analysis. As shown in Fig. 1A, HO-1 expression was significantly induced by treatment with 6-HITC alone, whereas no significant induction of HO-1 was observed when cells were treated with NAC or GSH alone. Of interest, the HO-1 expression was also observed when the cells were treated with 6-HITC in the presence of NAC or GSH. The data suggest that the dithiocarbamate derivatives generated upon incubation of 6-HITC with sulfhydryl molecules might have an HO-1-inducing electrophilic potential. To prove this hypothesis, we prepared an authentic 6-HITC-ME upon incubation of 6-HITC with β-mercaptoethanol and treated Caco-2 cells with the conjugate. As expected, 6-HITC-ME markedly up-regulated the HO-1 expression (Fig. 1B). Moreover, based on the fact that the Keap1 (Kelch-like ECH-associated protein 1)-Nrf2 system is the major regulatory pathway of these enzyme gene expressions (25), we examined the effect of 6-HITC-ME on the induction of Nrf2 expression in Caco-2 cells and observed that the conjugate significantly induced Nrf2 expression prior to the expression of HO-1 (Fig. 1C). Thus, 6-HITC-ME was speculated to be an electrophile that acts on the Keap1-Nrf2 pathway.
FIGURE 1.
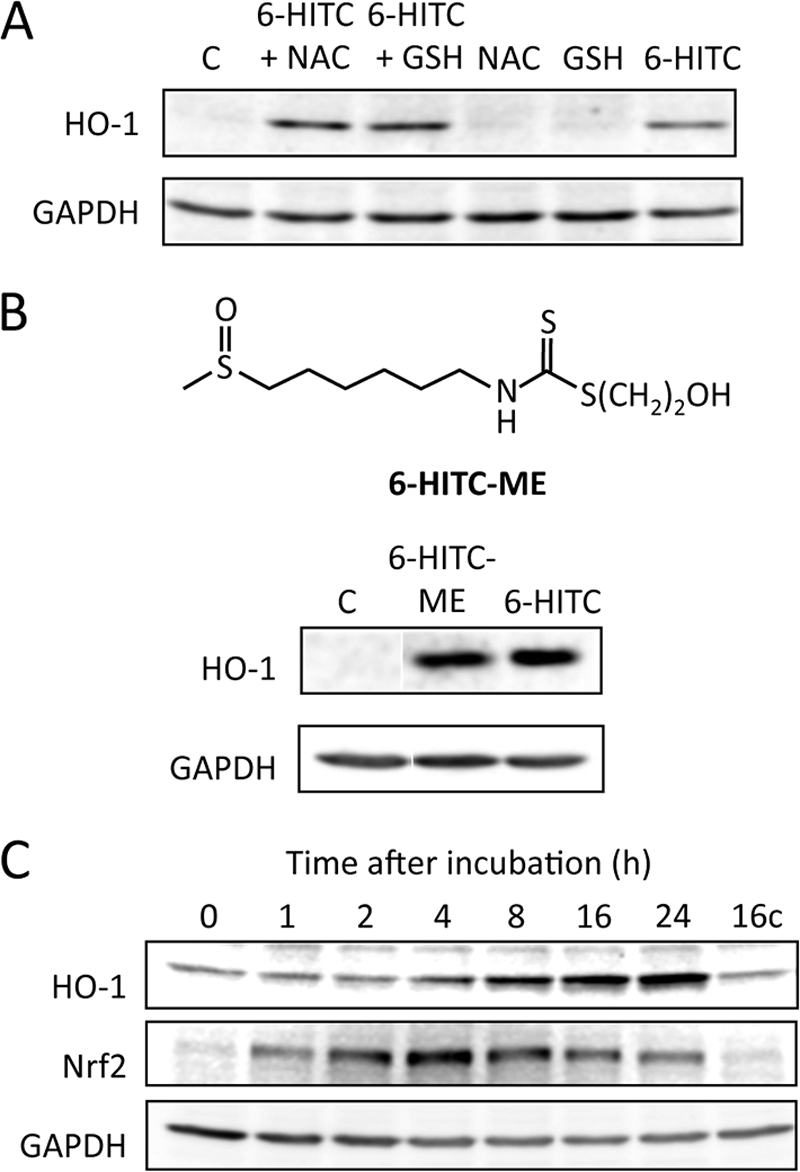
Induction of cellular response by thiolated isothiocyanates. A, immunoblot analysis of HO-1 in Caco-2 cells treated with 6-HITC in the presence and absence of sulfhydryl molecules. The cells were treated with 25 μm 6-HITC, in the presence and absence of 25 μm sulfhydryl molecules, NAC, and GSH, for 24 h. B, immunoblot analysis of HO-1 in Caco-2 cells treated with 6-HITC-ME. The cells were treated with 25 μm 6-HITC-ME for 24 h. Lane C, control. C, time-dependent up-regulation of HO-1 and Nrf2 by 6-HITC-ME. The cells were treated with 25 μm 6-HITC-ME for the indicated time intervals. Lane C, control.
Transthiocarbamoylation of Sulfhydryl Molecules by a Thiolated Isothiocyanate
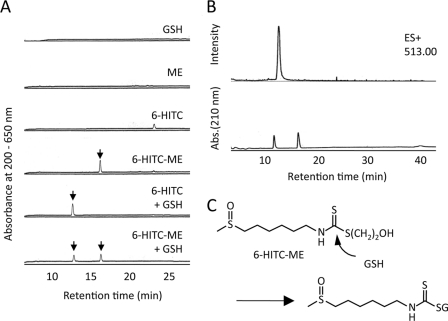
We speculated that, if the isothiocyanate-thiol conjugates function as an electrophile, they should react with sulfhydryl molecules, such as NAC and GSH, through the retro Michael cleavage followed by binding with the sulfhydryl molecules (transthiocarbamoylation). To gain a chemical insight into the cellular response induced by the thiolated isothiocyanates, we studied the occurrence of the transthiocarbamoylation of GSH by the isothiocyanate derivatives. 6-HITC-ME was incubated with an equal amount of GSH, and the transfer of the 6-HITC moiety to GSH was followed by detection of 6-HITC-GSH on reverse-phase HPLC. As shown in Fig. 2A, when the 6-HITC-ME conjugate was incubated with GSH, a single product that was also formed upon incubation of 6-HITC and GSH was detected, suggesting that the product represents the 6-HITC-GSH conjugate. Selected ion monitoring of the 6-HITC-GSH conjugate ion [M + H]+ with m/z 513 indeed detected the product (Fig. 2B), which was expected to be the GSH conjugate (Fig. 2C). Similar transthiocarbamoylation also occurred when 6-HITC-ME was incubated with NAC (supplemental Fig. S1). Thus, the 6-HITC-thiol conjugate is reactive toward thiol exchange in the presence of a suitable sulfhydryl reagent.
FIGURE 2.
Transthiocarbamoylation of sulfhydryl molecules by 6-HITC-β-mercaptoethanol conjugate. A, reaction of 6-HITC-ME with GSH. The reaction mixture, containing 1 mm GSH, was incubated with 1 mm 6-HITC-ME in 50 mm sodium phosphate buffer (pH 7.4). After incubation for 4 h at 37 °C, the reaction mixtures were analyzed by reverse-phase HPLC on a Develosil ODS-HG-5 column (4.6-mm inner diameter × 250 mm; Nomura Chemicals) eluted with a linear gradient of water containing 0.1% TFA (solvent A)-acetonitrile (solvent B) (time = 0 min, 10% B; 40 min, 80% B) at a flow rate of 0.8 ml/min. The elution profiles were monitored by absorbance at 190–650 nm. B, LC-MS analysis of 6-HITC-ME incubated with GSH. Top, selected ion current chromatograms obtained from the LC-MS analysis monitored with m/z 513. Bottom, HPLC profile. C, a proposed mechanism for the rearrangement reaction between the 6-HITC-bound β-mercaptoethanol and GSH.
Transthiocarbamoylation of Cellular Proteins by Thiolated Isothiocyanates
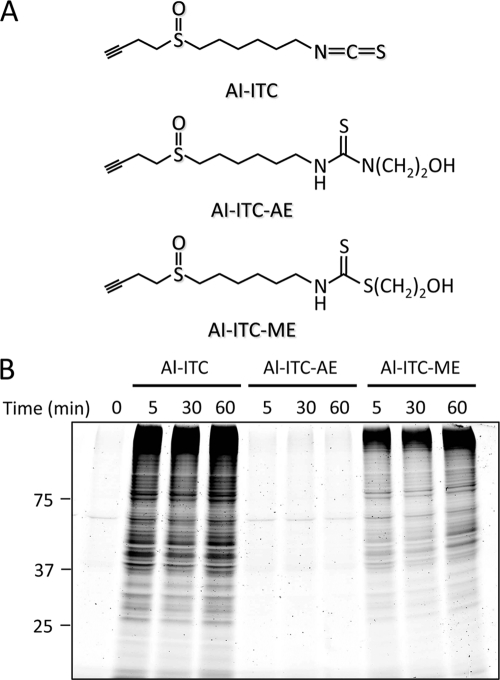
To test the functional ability of thiolated isothiocyanates to transthiocarbamoylate proteins in living cells, a novel probe was designed, combining an isothiocyanate-reactive group and an alkyne functionality. The presence of the alkyne functionality could then be detected in a later step upon the Cu(I)-catalyzed Huisgen [3 + 2] cycloaddition (“click chemistry”) reaction (26) with an azide-functionalized fluorescent group (supplemental Fig. S2). The alkynylated 6-HITC analogues, such as Al-ITC and its 2-aminoethanol derivative (Al-ITC-AE) and β-mercaptoethanol derivative (Al-ITC-ME) (Fig. 3A), were synthesized (supplemental Fig. S3 and supplemental material) and examined for transthiocarbamoylation of cellular proteins in human intestinal Caco-2 cells. After exposure of the cells to these alkynylated 6-HITC analogues, click chemistry was performed with cellular lysates followed by the separation of the lysate proteins on SDS-PAGE. In accordance with the reversible nature of the reactions, a dramatic increase of protein-bound label was observed when the cells were incubated with Al-ITC and Al-ITC-ME, whereas no labeling was observed in the Al-ITC-AE-treated cells (Fig. 3B), suggesting that thiolated isothiocyanates, not aminated isothiocyanates, are reversible conjugates that react with sulfhydryl molecules through transthiocarbamoylation.
FIGURE 3.
Transthiocarbamoylation of cellular proteins by the alkynylated 6-HITC analogues in Caco-2 cells. A, chemical structures of the alkynylated 6-HITC analogues, Al-ITC, Al-ITC-AE, and Al-ITC-ME, used in this study. B, fluorescent detection of protein-bound isothiocyanate. Caco-2 cells were pretreated with the alkynylated 6-HITC analogues or vehicle for 2 h. The cell lysates were subjected to the click reaction with TAMRA-N3 followed by separation on SDS-polyacrylamide gel.
Identification of Thiocarbamoylated Proteins
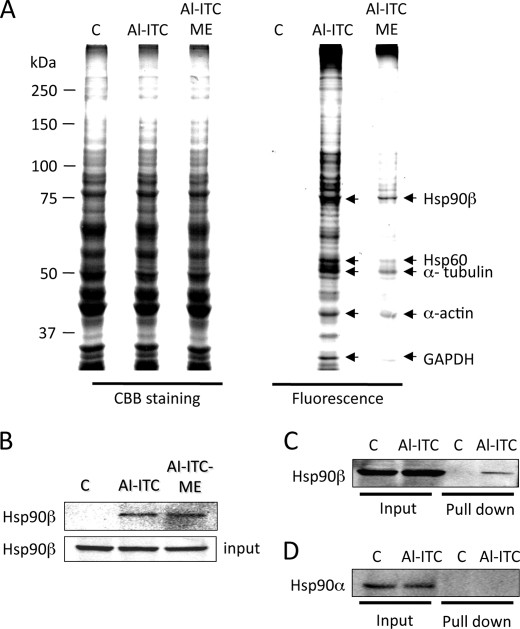
To further analyze the target proteins of transthiocarbamoylation in living cells, fluorescent profiling of the total cell lysates revealed many proteins labeled by Al-ITC or Al-ITC-ME (Fig. 4A). To identify the proteins tagged with the “click” reagents, the bands resolved by Coomassie Brilliant Blue staining were excised and analyzed by MALDI-TOF MS. A survey of the protein hits from the cell lysates labeled with the alkynylated 6-HITC analogues revealed candidate isothiocyanate target proteins. From three independent proteomic experiments, we identified five proteins, including Hsp90β, Hsp60, α-tubulin, β-actin, and GAPDH as the targets (supplemental Table S1). Although Al-ITC-ME was a less efficient alkylation reagent, the majority of the selectively labeled proteins were consistent with those targeted by Al-ITC.
FIGURE 4.
Identification of Hsp90β as a target of thiocarbamoylation. A, SDS-PAGE of proteins labeled with the alkynylated 6-HITC analogues. Left three lanes, Coomassie Brilliant Blue (CBB) staining; right three lanes, TAMRA fluorescence gel images. The proteins indicated by the arrows represent the targets identified by MALDI-TOF MS. The Caco-2 cells were pretreated with the Al-ITC analogues or vehicle for 2 h. The cell lysates were subjected to the click reaction with TAMRA-N3 followed by separation on SDS-polyacrylamide gel. B–D, validation of Hsp90β as the selected target of the 6-HITC analogues in cells. The Caco-2 cells were treated with Al-ITC, Al-ITC-ME, or vehicle for 2 h. The cell lysates were subjected to the click reaction with biotin-N3, and the labeled proteins were precipitated with NeutrAvidin beads followed by Western blot with an anti-Hsp90β antibody (B and C) or an anti-Hsp90α antibody (D).
Hsp90β, among the identified target proteins, was the most clearly visible for the Al-ITC- and Al-ITC-ME-treated cell preparations. However, the protein was simply identified from gel bands that co-migrated with fluorescence signal. The band may therefore contain both adducted and unadducted proteins. Hence, the binding of the Al-ITC analogues into endogenous Hsp90β was confirmed by a pull-down assay. To this end, the Caco-2 cells were treated with 25 μm Al-ITC or Al-ITC-ME, and then the biotin-N3 conjugation to the Al-ITC-modified proteins from the cellular lysates was done using click chemistry. Pull-down with NeutrAvidin beads followed by Western blot with an anti-Hsp90β antibody consistently demonstrated the binding of the alkynyl-6-HITC analogues into endogenous Hsp90β (Fig. 4B). Of interest, the pull-down assay also revealed that Al-ITC did not bind to Hsp90α, suggesting the specificity of the interaction between Hsp90β and the isothiocyanates (Fig. 4, C and D). Transthiocarbamoylation of other cellular proteins, including Hsp70, Keap1, and GST P1, were also confirmed by this assay (supplemental Fig. S4).
Identification of a Cysteine Residue of Recombinant Hsp90β Labeled by 6-HITC
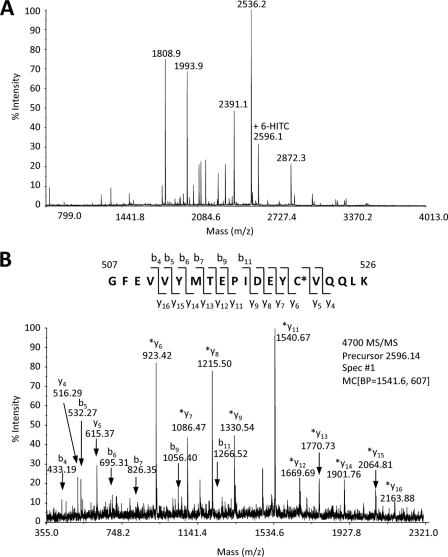
We characterized the modification of Hsp90β by 6-HITC using recombinant human Hsp90β in vitro. Hsp90β was incubated with 25 μm 6-HITC for 3 h and then trypsinized, and the recovered peptides were then resolved by reverse-phase nano-LC and were directly fractionated onto a MALDI target plate. The resulting MALDI target plate was then subjected to an MS/MS batch analysis using the MALDI-TOF/TOF. The overall sequence coverage obtained for Hsp90β was ∼50%. The cysteine-containing peptides identified by MALDI-TOF MS/MS are detailed in supplemental Table S2. The sequence coverage included three (Cys-412, Cys-521, and Cys-564) of the six total cysteine residues (Cys-366, Cys-412, Cys-521, Cys-564, Cys-589, and Cys-590) in Hsp90β. The MS/MS fragmentation data showed that one peptide had incorporated the 6-HITC. Sequencing of the tryptic peptide by tandem mass spectrometry revealed that 6-HITC is incorporated into Cys-521 (Fig. 5). However, modification of other cysteine residues (Cys-412 and Cys-564) was not observed. Thus, it was speculated that Hsp90β might sense isothiocyanates by direct modification at Cys-521. However, it remains unclear whether the same cysteine could be labeled with 6-HITC in cells.
FIGURE 5.
MALDI-TOF MS spectrum of recombinant Hsp90β labeled by 6-HITC. Hsp90β was incubated with 25 μM 6-HITC for 3 h. The 6-HITC-labeled Hsp90β was alkylated, trypsinized, and analyzed by nano-LC-MALDI (A). The resulting MALDI target plate was then subjected to an MS/MS batch analysis using the AB-Sciex 4700 MALDI-TOF/TOF (B).
Modeling Analysis
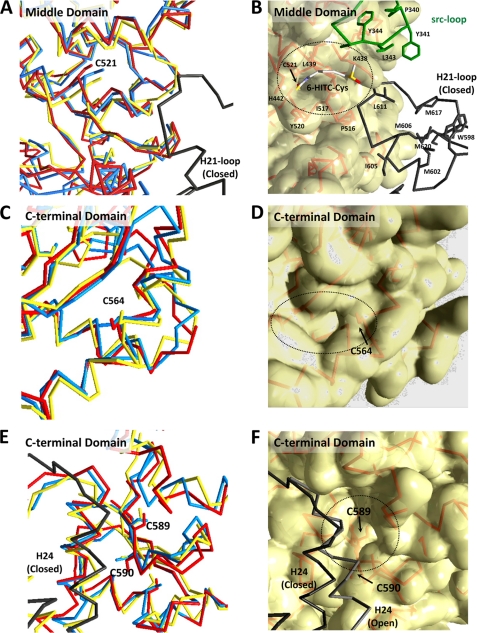
In humans, Hsp90 exists as a homodimer, which contains three highly conserved domains. These regions consist of a 25-kDa N-terminal ATP-binding domain, a 35-kDa middle domain, and a 12-kDa C-terminal dimerization domain. To gain an insight into the mechanism of the formation of the 6-HITC adduct at Cys-521 in Hsp90β, we characterized the different conformational states of the homology-based Hsp90β structures (namely the open state and two closed states) by a computational analysis. Superposition of these three structures revealed that the C-terminal halves of the middle and C-terminal domains have similar backbone traces regardless of the opening and closing motions of Hsp90β (Fig. 6). Structural changes were mainly observed in the N-terminal domain, boundary regions of the domains, and the loop regions. Interestingly, three cysteine residues, Cys-521, Cys-564, and Cys-589, are located in the C-terminal half of the middle and C-terminal domains. These cysteine residues are partially exposed to the solvent and are located at the edge or bottom of the putative ligand-binding pockets. The size of the putative ligand-binding pockets in the vicinity of Cys-564 and Cys-589 is too small for the 6-HITC binding. However, Cys-521, adjacent to a surface-exposed hydrophobic pocket and consisting of His-442, Lys-438, Leu-439, Pro-516, Ile-517, and Tyr-520, may provide a suitable environment for the 6-HITC binding. This putative 6-HITC binding pocket is sandwiched by the Src-loop and H21-loop from the partner protomer. Moreover, the side chain of Cys-521 lies at the base of the putative 6-HITC-binding cleft spaced a mere ∼10 Å from these two loops. The thiol group of Cys-366 is also exposed to the solvent but locates at the flexible loop. The side chains of Cys-411 and Cys-590 are completely buried within the protein cores.
FIGURE 6.
Predicted three-dimensional models in the vicinity of Cys-521 identified as the target of 6-HITC. A, superposition of the Cα-traces of the C-terminal halves of the middle domains. The open (yellow) and two closed form (red and blue) models of Hsp90β were constructed using the structures of yeast Hsp82, E. coli HtpG, and Canis lupus GRP94. The deduced sequence of human Hsp90β is very similar to that of yeast Hsp82 (63% identity) among the Hsp90 homologs whose three-dimensional structures are known. The Src-loop (residues 336–351) is shown in green. The H21-loop containing the exposed C-terminal helix H21 is disordered both in the open form and closed form I structure. The H21-loop, shown in black, is illustrated based on alignment with the closed form II structure. B, Van der Walls molecular surface in the vicinity of Cys-521. The residues involved in the formation of the putative 6-HITC-binding pocket and exposed hydrophobic surface by the C-terminal domain helix H21 are shown in the wire frame model. C and E, superpositions of the backbone traces of the C-terminal domains. D and F, Van der Waals molecular surface of the C-terminal domains. The putative ligand-binding pockets were depicted by the dashed circles. The H24 helices of the partner protomer in two conformational states are shown in black and gray, respectively.
Induction of Heat Shock Response via Protein Thiocarbamoylation
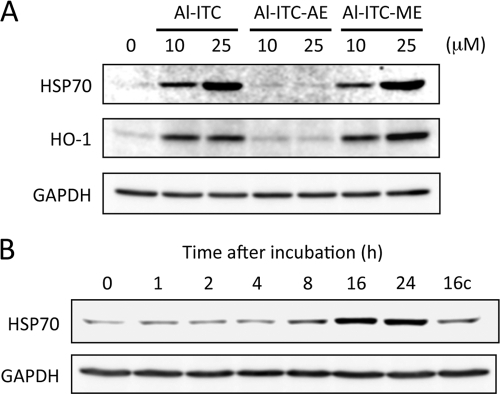
Hsp90β is a chaperone ATPase of the Hsp90 family implicated in protein maturation and targeting. Expression of Hsp90β is not inducible by stress, unlike Hsp90α, whose level increases in cells under stress. It has also been suggested that the modification of Hsp90 and other chaperones by electrophiles leads to heat shock gene expression (27). The microarray data indeed revealed a dramatic increase in the heat shock-regulated transcripts in 6-HITC-treated rat liver RL34 cells.3 We observed that repression of the Hsp90β expression with Hsp90β siRNA markedly up-regulated Hsp70 expression, and treatment with 17-(allylamino)-17-demethoxygeldanamycin, an inhibitor of Hsp90, enhanced expression of Hsp70 (supplemental Fig. S5). In addition, in accordance with the finding that treatment of the cells with the Al-ITC and Al-ITC-ME resulted in a dramatic increase of protein thiocarbamoylation (Fig. 3B), Hsp70 expression was significantly up-regulated by Al-ITC and Al-ITC-ME (Fig. 7). These data suggest that the thiocarbamoylation of Hsp90β with 6-HITC may be associated with the heat shock response.
FIGURE 7.
Induction of cellular response by the alkynylated 6-HITC analogues. A, immunoblot analysis of Hsp70 and HO-1 in Caco-2 cells treated with the alkynylated 6-HITC analogues. The cells were treated with Al-ITC, Al-ITC-AE, or Al-ITC-ME (10 or 25 μm) for 24 h. B, time-dependent up-regulation of Hsp70 by Al-ITC-ME. The cells were treated with 25 μm Al-ITC-ME for the indicated time intervals. C, control.
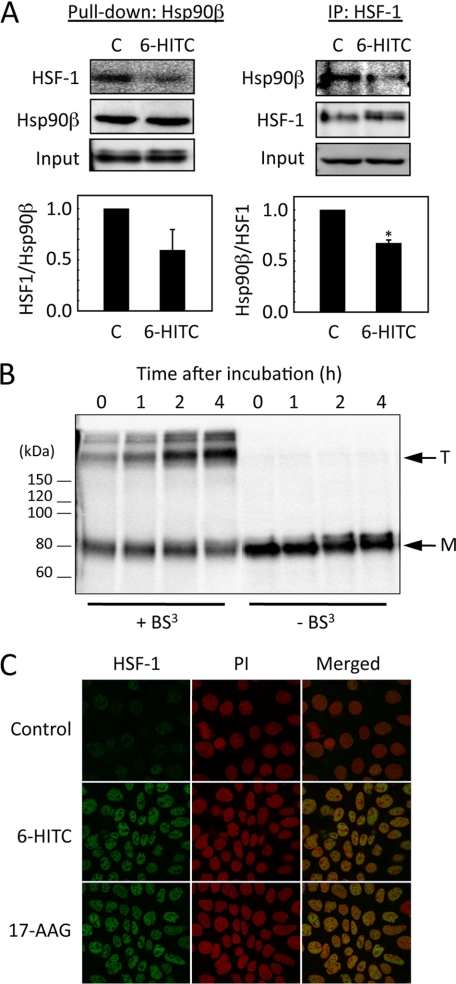
To further investigate the involvement of Hsp90β in the heat shock response, we examined the activation of a latent transcription factor, HSF-1, which principally controls the inducible expression of the heat shock genes caused by heat or chemical stress, in the 6-HITC-treated Caco-2 cells. In the absence of stress, HSF-1 is retained in the cytoplasm by inhibitory associations with Hsp90 and various co-chaperones (28). A fundamental step in the activation of HSF-1 is homotrimerization, leading to acquisition of the heat shock element DNA binding activity, followed by induction of the heat shock response. To show the correlation between the thiocarbamoylation of Hsp90β and HSF-1 activation, we performed a pull-down assay using Halo-tagged Hsp90β, demonstrating that its association with HSF-1 is disrupted by the 6-HITC treatment (Fig. 8A). In addition, 6-HITC promoted the polymerization (Fig. 8B) and nuclear translocation of HSF-1 (Fig. 8C and supplemental Fig. S6).
FIGURE 8.
Activation of HSF-1 by 6-HITC. A, left panels, Halo Tag pull-down assay. Caco-2 cells transiently expressing the Halo-tagged Hsp90β were treated with 6-HITC or DMSO for 2 h. After incubation, the cell lysates were incubated with HaloLink resin, and the bound materials were subjected to an immunoblot analysis using the anti-HSF-1 antibody. The bar graph indicates the relative HSF-1 levels after normalization for Hsp90β. The results are shown as the mean ± S.D. (error bars) for three separate experiments. Right panels, immunoprecipitation assay. Caco-2 cells were treated with 6-HITC or DMSO for 2 h. After incubation, cell lysates were incubated with anti-HSF-1 antibody, and bound materials were subjected to immunoblot analysis using anti-Hsp90β antibody. The bar graph indicates the relative Hsp90β levels after normalization for HSF-1. The results are shown as the mean ± S.D. for three separate experiments. *, p < 0.05. B, determination of oligomeric structures of HSF-1 in Caco-2 cells treated with 6-HITC. The whole cell extracts prepared from the cells treated with 6-HITC for the indicated time intervals were incubated with bis(sulfosuccinimidyl)suberate (BS3) for 30 min at room temperature. The cell extracts were then subjected to a Western blot analysis using rabbit anti-HSF-1 polyclonal antibody. Arrows, T, trimer; M, monomer. C, immunocytochemical detection of HSF-1 in Caco-2 cells treated with 6-HITC. The cells were treated with 25 μm 6-HITC, 2 μm 17-(allylamino)-17-demethoxygeldanamycin (17-AAG), or vehicle for 2 h. Localization of HSF-1 was determined by immunocytochemistry using the rabbit anti-HSF-1 polyclonal antibody.
Accompanied by the activation of HSF-1, 6-HITC up-regulated the expression of the HSF-1-responsive genes, including the Hsp70 gene (Fig. 9A). Consistent with the enhanced gene expression, both the intracellular and extracellular levels of Hsp70 were up-regulated by 6-HITC in a time-dependent manner (Fig. 9B). Finally, to assess the importance of the heat shock response in mediating the cellular response to the isothiocyanate, an siRNA-based approach was used to selectively inhibit the HSF-1 expression. Caco-2 cells were transfected with either an HSF1-specific siRNA or a negative control siRNA. The level of HSF-1 mRNA in the cells transfected with HSF-1 siRNA was reduced to ∼20% of the level in the control cells. Western blotting indicated silencing of the HSF-1 protein expression (Fig. 9C). The control and HSF-1 knockdown cells were then exposed to 6-HITC, and changes in the Hsp70 gene expression were then measured. The induction of Hsp70 by 6-HITC was robust in the control siRNA-transfected cells; however, silencing HSF1 significantly down-regulated the 6-HITC-induced Hsp70 expression, further verifying the successful knockdown of the heat shock response.
FIGURE 9.
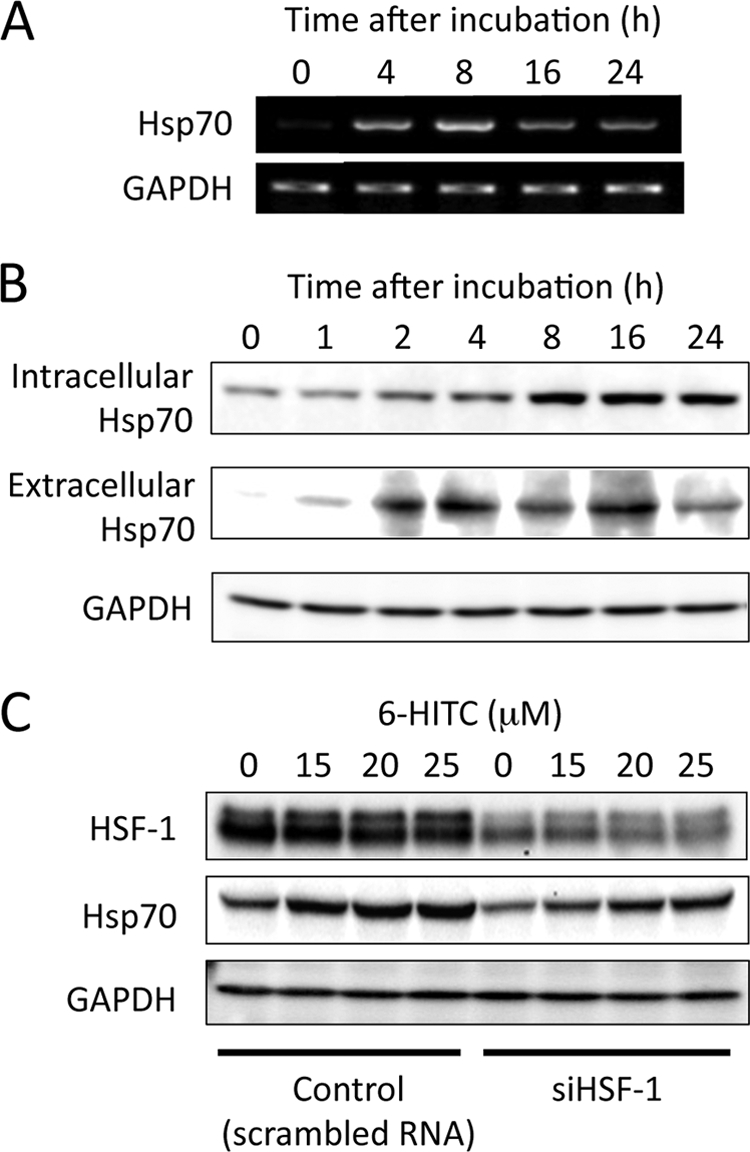
Induction of heat shock response by 6-HITC. A, RT-PCR analysis of Hsp70 in Caco-2 cells treated with 6-HITC. B, immunoblot analysis of intracellular and extracellular Hsp70 in Caco-2 cells treated with 6-HITC. Hsp70 was analyzed by immunoblot analysis using the goat anti-Hsp70 polyclonal antibody. C, effects of specific siRNA of HSF-1 upon induction of Hsp70 by 6-HITC. The cells were treated for 48 h with the indicated siRNA as described above prior to incubation with 6-HITC for 8 h. In A and B, the cells were treated with 25 μm 6-HITC for the indicated time intervals.
DISCUSSION
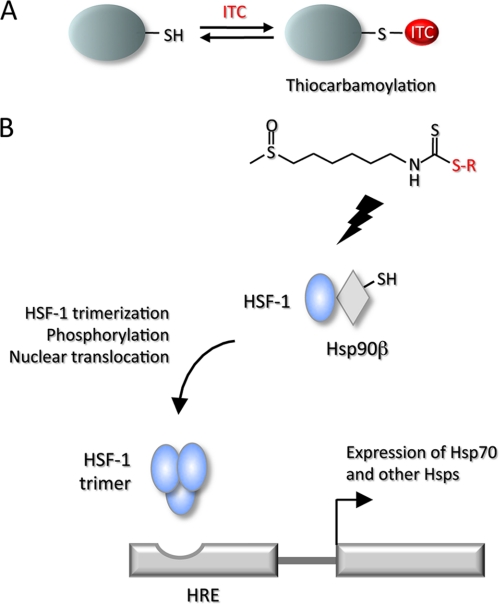
It has been suggested that isothiocyanates are transferred from one low molecular weight compound, such as GSH, to another or to reactive and accessible cysteines in proteins. Such transthiocarbamoylation is a common feature of isothiocyanates (15–17). In the present study, the transthiocarbamoylation experiments conducted with the synthetic conjugate showed that 6-HITC-ME was stable in aqueous media at physiological pH and temperature. At the end of a 4-h incubation period, less than 2% of the conjugate had dissociated to 6-HITC, whereas 6-HITC-ME underwent facile exchange in the presence of sulfhydryl molecules, such as GSH and NAC. After a 4-h incubation with GSH, 6-HITC-ME decreased to less than 30% of the original concentration. The high degree of conjugation to the sulfhydryl molecules is not consistent with an exchange reaction that takes place following liberation of free 6-HITC, because little free 6-HITC was detected after 4 h of incubation in buffer alone. Based on these results, it appears that the mechanism of the exchange reaction does not involve liberation of free isothiocyanate but rather entails a nucleophilic displacement by the sulfhydryl agent present in excess. Regardless of the exact mechanism, it is evident that the thiocarbamate conjugates of 6-HITC and related isothiocyanates, such as sulforaphane, are capable of acting as effective thiocarbamoylating agents toward thiol groups. These reversible features may be associated with the biological activity of these isothiocyanates (Fig. 10A).
FIGURE 10.
Reversible regulation of protein functions via sulfhydryl modifications (A) and schematic representation of HSF-1 regulation by Hsp90 and its activation through transthiocarbamoylation (B). HRE, heat shock response element.
Modification of proteins is recognized as a key mechanism underlying the biological activity of isothiocyanates. The electrophilic carbon residue in the isothiocyanate moiety is capable of reacting with biological nucleophiles, such as cysteine in proteins and the tripeptide GSH. Binding of isothiocyanates to cellular proteins, such as transient receptor potential channels (29), has been demonstrated to occur via the covalent modification of cysteine. More recently, using a click chemistry-based proteomic approach, Ahn et al. (30) have shown that sulforaphane was highly reactive with cysteine residues in proteins, such as Keap1, macrophage migration inhibitory factor, and thioredoxin, to form covalent bonds. They have also shown that the reactive isothiocyanates of sulforaphane can be replaced by the more gentle electrophilic sulfoxythiocarbamate group that also selectively targets cysteine residues in proteins but forms stable thiocarbamate adducts. In the present study, to achieve the specific and efficient transthiocarbamoylation of target proteins by thiolated isothiocyanates in intact cells, the novel probes with an alkyne group, such as Al-ITC and its 2-aminoethanol and β-mercaptoethanol derivatives, were designed based on 6-HITC (Fig. 3A). We introduced the alkyne rather than the azide functionality on the probe based on several chemical considerations indicated by Gillet et al. (31). Although azide-functionalized molecules seem compatible under in vivo labeling conditions (32), the chemical propensity of azides to undergo reduction into amines might be of concern. In addition, a significantly lower level of background labeling was obtained when the alkyne probe was used in combination with an azide fluorescent reporter. The presence of the alkyne functionality could then be detected in a later step upon the Cu(I)-catalyzed Huisgen [3 + 2] cycloaddition (“click chemistry”) reaction with an azide-functionalized fluorescent group (azide-rhodamine) (26). This approach enabled us to detect thiocarbamoylated proteins in the cells upon exposure to the thiolated isothiocyanate probe (Fig. 3B). They included α-tubulin, β-actin, GAPDH, Hsp60, and Hsp90β (Fig. 4A). Keap1, an electrophile sensor protein, was not identified as the target of the isothiocyanate probes in the mass spectrometry-based proteomic study. This may be due to the relative abundance of Hsp90β versus Keap1 in Caco-2 cells. Instead of the proteomics identification, we confirmed the thiocarbamoylation of endogenous Keap1 by a pull-down assay (supplemental Fig. S4).
Among the number of proteins that covalently interacted with the thiolated isothiocyanates in Caco-2 cells, we focused on Hsp90 because of the observations that Hsp90β was the most clearly visible one in the SDS-PAGE analysis of the Al-ITC-ME-treated cell preparations (Fig. 4A). Hsp90 accounts for nearly 2% of the total protein in most unstressed cells and is involved in essential physiological processes, including protein trafficking and signal transduction, protein degradation, and regulation and stabilization of a wide range of client proteins. In humans, Hsp90 exists in either the β isoform or the more prominent α isoform, which share an approximate 85% homology (33). Although Hsp90 typically exists as a homodimer, experiments in defined systems have documented a chaperoning activity by the self-oligomerization of Hsp90 and client protein binding in a thermally denaturing environment, thus maintaining the substrate protein in a folding-competent form (34). Refolding of the substrate is accomplished with the addition of Hsp70 along with other essential chaperones and co-chaperones, such as Hsp60, chaperonin 10, and the Hsp-organizing protein (35). Hsp90 is highly expressed in various cancerous tissues compared with non-cancerous tissues, which provides a cancer cell selectivity by the Hsp90 inhibitor. Therefore, inhibiting the Hsp90 chaperone activity emerged as a new molecular target for developing anticancer agents because of its high selectivity and simultaneous knockdown of various oncogenic proteins. Several Hsp90 inhibitors have been developed and tested in preclinical and clinical models for their anticancer activity (36).
Covalent modification of the cysteine residues in Hsp90 has been reported to be involved in regulating the Hsp90 chaperone cycle. Zhang et al. (37) have reported that Hsp90 undergoes nitrosylation at Cys-589. Carbone et al. (38) have shown that a lipid peroxidation product, 4-hydroxy-2-nonenal, specifically targets Cys-564 of Hsp90 in a rat model of chronic alcoholic liver disease. In the present study, although the 6-HITC-cysteine adduct was mapped only in the in vitro experiment and not in the studies with cells, we identified Cys-521 as a primary target of thiocarbamoylation by 6-HITC (Fig. 5). The reasons why 6-HITC selectively targeted Cys-521 of the six conserved cysteine residues, Cys-366, Cys-411, Cys-521, Cys-564, Cys-589, and Cys-590, remains unclear. However, the location of Cys-521 in the hydrophobic pocket within Hsp90β may explain it. In the absence of a nucleotide, Hsp90 adopts an open conformation, in which each of the three domains of both monomers present the hydrophobic region into a large dimeric cleft. Shiau et al. (21) suggested that this structure captures Hsp90 poised to bind client proteins, and the hydrophobic elements of the middle and C-terminal domains interface accommodate the diverse shapes and sizes of the Hsp90 client proteins. Because of the positioning of its exposed hydrophobic face, the H21-loop and Src-loop (residues 336–351) appear to be ideal locations for client protein binding (20–22). Interestingly, the side chain of Cys-521 lies at the base of the putative 6-HITC-binding cleft spaced a mere ∼10 Å from these two loops. The in silico modeling analysis indeed showed that the hydrophobic 6-HITC molecule bound to Cys-521 was located in the hydrophobic pocket consisting of His-442, Lys-438, Leu-439, Pro-516, Ile-517, and Tyr-520 sandwiched by the Src-loop and H21-loop from the partner protomer (Fig. 6).
Exposure of cells to heat shock and other stressors results in virtual shutdown of normal cellular protein synthesis, paralleled by a shift to high levels of Hsp synthesis. It is considered that the induction of Hsps in mammalian cells is transcriptionally regulated. Transcription is initiated by a transcription factor, HSF-1 (39, 40). This protein is thought to be constitutively present and kept in an inactive state by its association with Hsp70 in particular (41). During exposure to proteotoxic agents, Hsp70 is required to chaperone damaged proteins, and consequently, its association with HSF-1 is broken. Subsequently, the heat shock factor trimerizes, migrates to the nucleus, and activates the promoters of heat shock genes (42). Jacobs and Marnett (43) also demonstrated that 4-hydroxy-2-nonenal could induce heat shock response via expression and nuclear translocation of HSF-1. In addition to transcriptional regulation, translational regulation has been described in insect cells (44), and there is some evidence for this type of regulation in mammalian cells (45). Although the mechanisms by which 6-HITC and its thiol derivative induce the heat shock response are not fully understood, the present study hypothesized that the process by which isothiocyanates enhance the heat shock gene expression may involve the thiocarbamoylation of cysteine residue(s) in Hsp90β, causing the release and nuclear translocation of HSF1 (Fig. 10B). It is also likely that the accumulation of abnormal proteins, such as the thiocarbamoylated Hsp90, may at least in part explain the heat shock response. Hsp70 that is normally bound to HSF-1 is diverted to the abnormal proteins as chaperones. This reduces the inhibition of HSF-1 normally exerted by Hsp70, allowing for the activation of HSF-1 with transactivation of the Hsp70 gene and increased production of the heat shock protein (41). An additional mechanism that has been proposed for the heat shock response induced by isothiocyanates is the reduced degradation of a short lived protein that is a positive regulator of the heat shock protein transcription (38).
Supplementary Material
Acknowledgment
We thank Dr. Y. Morimitsu (Ochanomizu University) for the kind gift of 6-HITC.
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas (Research in a Proposed Research Area), Japan (Grant 20117007, to K. U.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1 and S2 and Figs. S1–S6.
T. Kumagai and K. Uchida, unpublished data.
- 6-HITC
- 6-methylsulfinylhexyl isothiocyanate
- Al-ITC
- alkynyl-tagged 6-HITC
- Al-ITC-AE
- 2-aminoethanol derivative of Al-ITC
- Al-ITC-ME
- β-mercaptoethanol derivative of Al-ITC
- 6-HITC-ME
- 6-HITC-β-mercaptoethanol
- HO-1
- heme oxygenase-1
- HSF-1
- heat shock factor-1
- Hsp
- heat shock protein
- NAC
- N-acetyl-l-cysteine
- AMP-PNP
- 5′-adenylyl-β,γ-imidodiphosphate
- TAMRA
- tetramethylrhodamine.
REFERENCES
- 1. Lee H. P., Gourley L., Duffy S. W., Esteve J., Lee J., Day N. E. (1989) Int. J. Cancer 43, 1007–1016 [DOI] [PubMed] [Google Scholar]
- 2. Olsen G. W., Mandel J. S., Gibson R. W., Wattenberg L. W., Schuman L. M. (1991) Cancer Causes Control 2, 291–297 [DOI] [PubMed] [Google Scholar]
- 3. Mehta R. G., Liu J., Constantinou A., Thomas C. F., Hawthorne M., You M., Gerhüser C., Pezzuto J. M., Moon R. C., Moriarty R. M. (1995) Carcinogenesis 16, 399–404 [DOI] [PubMed] [Google Scholar]
- 4. Depree J. A., Howard T. M., Savage G. P. (1999) Food Res. Int. 31, 329–337 [Google Scholar]
- 5. Nakajima M., Yoshida R., Shimada N., Yamazaki H., Yokoi T. (2001) Drug Metab. Dispos. 29, 1110–1113 [PubMed] [Google Scholar]
- 6. Zhang Y., Talalay P., Cho C. G., Posner G. H. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 2399–2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chiao J. W., Wu H., Ramaswamy G., Conaway C. C., Chung F. L., Wang L., Liu D. L. (2004) Carcinogenesis 25, 1403–1408 [DOI] [PubMed] [Google Scholar]
- 8. Pham N. A., Jacobberger J. W., Schimmer A. D., Cao P., Gronda M., Hedley D. W. (2004) Mol. Cancer Ther. 3, 1239–1248 [PubMed] [Google Scholar]
- 9. Heiss E., Herhaus C., Klimo K., Bartsch H., Gerhäuser C. (2001) J. Biol. Chem. 276, 32008–32015 [DOI] [PubMed] [Google Scholar]
- 10. Wu X., Zhou Q. H., Xu K. (2009) Acta Pharmacol. Sin. 30, 501–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang Y., Kolm R. H., Mannervik B., Talalay P. (1995) Biochem. Biophys. Res. Commun. 206, 748–755 [DOI] [PubMed] [Google Scholar]
- 12. Bruggeman I. M., Temmink J. H., van Bladeren P. J. (1986) Toxicol. Appl. Pharmacol. 83, 349–359 [DOI] [PubMed] [Google Scholar]
- 13. Zheng G. Q., Kenney P. M., Lam L. K. (1992) J. Med. Chem. 35, 185–188 [DOI] [PubMed] [Google Scholar]
- 14. Talalay P., Fahey J. W., Holtzclaw W. D., Prestera T., Zhang Y. (1995) Toxicol. Lett. 82, 173–179 [DOI] [PubMed] [Google Scholar]
- 15. Baillie T. A., Slatter J. G. (1991) Acc. Chem. Res. 24, 264–270 [Google Scholar]
- 16. Baillie T. A., Kassahun K. (1994) Adv. Pharmacol. 27, 163–181 [DOI] [PubMed] [Google Scholar]
- 17. Kassahun K., Davis M., Hu P., Martin B., Baillie T. (1997) Chem. Res. Toxicol. 10, 1228–1233 [DOI] [PubMed] [Google Scholar]
- 18. Speers A. E., Cravatt B. F. (2004) Chem. Biol. 11, 535–546 [DOI] [PubMed] [Google Scholar]
- 19. Kelley L. A., Sternberg M. J. (2009) Nat. Protoc. 4, 363–371 [DOI] [PubMed] [Google Scholar]
- 20. Ali M. M., Roe S. M., Vaughan C. K., Meyer P., Panaretou B., Piper P. W., Prodromou C., Pearl L. H. (2006) Nature 440, 1013–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shiau A. K., Harris S. F., Southworth D. R., Agard D. A. (2006) Cell 127, 329–340 [DOI] [PubMed] [Google Scholar]
- 22. Dollins D. E., Warren J. J., Immormino R. M., Gewirth D. T. (2007) Mol. Cell. 28, 41–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schüttelkopf A. W., van Aalten D. M. (2004) Acta Crystallogr. D Biol. Crystallogr. 60, 1355–1363 [DOI] [PubMed] [Google Scholar]
- 24. Emsley P., Cowtan K. (2004) Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 25. Itoh K., Wakabayashi N., Katoh Y., Ishii T., O'Connor T., Yamamoto M. (2003) Genes Cells 8, 379–391 [DOI] [PubMed] [Google Scholar]
- 26. Rostovtsev V. V., Green L. G., Fokin V. V., Sharpless K. B. (2002) Angew. Chem. Int. Ed. Engl. 41, 2596–2599 [DOI] [PubMed] [Google Scholar]
- 27. Jacobs A. T., Marnett L. J. (2010) Acc. Chem. Res. 43, 673–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Voellmy R. (2004) Cell Stress Chaperones 9, 122–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Macpherson L. J., Dubin A. E., Evans M. J., Marr F., Schultz P. G., Cravatt B. F., Patapoutian A. (2007) Nature 445, 541–545 [DOI] [PubMed] [Google Scholar]
- 30. Ahn Y. H., Hwang Y., Liu H., Wang X. J., Zhang Y., Stephenson K. K., Boronina T. N., Cole R. N., Dinkova-Kostova A. T., Talalay P., Cole P. A. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 9590–9595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gillet L. C., Namoto K., Ruchti A., Hoving S., Boesch D., Inverardi B., Mueller D., Coulot M., Schindler P., Schweigler P., Bernardi A., Gil-Parrado S. (2008) Mol. Cell. Proteomics 7, 1241–1253 [DOI] [PubMed] [Google Scholar]
- 32. Speers A. E., Adam G. C., Cravatt B. F. (2003) J. Am. Chem. Soc. 125, 4686–4687 [DOI] [PubMed] [Google Scholar]
- 33. Hickey E., Brandon S. E., Smale G., Lloyd D., Weber L. A. (1989) Mol. Cell. Biol. 9, 2615–2626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yonehara M., Minami Y., Kawata Y., Nagai J., Yahara I. (1996) J. Biol. Chem. 271, 2641–2645 [DOI] [PubMed] [Google Scholar]
- 35. Johnson B. D., Schumacher R. J., Ross E. D., Toft D. O. (1998) J. Biol. Chem. 273, 3679–3686 [DOI] [PubMed] [Google Scholar]
- 36. Adams J., Elliott P. J. (2000) Oncogene 19, 6687–6692 [DOI] [PubMed] [Google Scholar]
- 37. Zhang Y., Keszler A., Broniowska K. A., Hogg N. (2005) Free Radic. Biol. Med. 38, 874–881 [DOI] [PubMed] [Google Scholar]
- 38. Carbone D. L., Doorn J. A., Kiebler Z., Ickes B. R., Petersen D. R. (2005) J. Pharmacol. Exp. Ther. 315, 8–15 [DOI] [PubMed] [Google Scholar]
- 39. Lis J., Wu C. (1993) Cell 74, 1–4 [DOI] [PubMed] [Google Scholar]
- 40. Morimoto R. I. (1993) Science 259, 1409–1410 [DOI] [PubMed] [Google Scholar]
- 41. Abravaya K., Myers M. P., Murphy S. P., Morimoto R. I. (1992) Genes Dev. 6, 1153–1164 [DOI] [PubMed] [Google Scholar]
- 42. Sarge K. D., Murphy S. P., Morimoto R. I. (1993) Mol. Cell. Biol. 13, 1392–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jacobs A. T., Marnett L. J. (2007) J. Biol. Chem. 282, 33412–33420 [DOI] [PubMed] [Google Scholar]
- 44. DiDomenico B. J., Bugaisky G. E., Lindquist S. (1982) Proc. Natl. Acad. Sci. U.S.A. 79, 6181–6185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Joshi-Barve S., De Benedetti A., Rhoads R. E. (1992) J. Biol. Chem. 267, 21038–21043 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.