Background: The transcriptional corepressor G9a also activates genes by an unknown mechanism.
Results: The N-terminal region of G9a binds estrogen receptor α and is necessary and sufficient for enhancing estrogen-induced gene activation and for occupancy of G9a on target genes.
Conclusion: The domains of G9a responsible for activation and inhibition of transcription are different.
Significance: G9a has inherent coactivator as well as corepressor activity.
Keywords: Chromatin Immunoprecipitation (ChIP), Histone Methylation, Nuclear Receptors, Transcription Coactivators, Transcription Regulation, G9a, Estrogen Receptor
Abstract
Histone methyltransferase G9a has been understood primarily as a corepressor of gene expression, but we showed previously that G9a positively regulates nuclear receptor-mediated transcription in reporter gene assays. Here, we show that endogenous G9a contributes to the estradiol (E2)-dependent induction of some endogenous target genes of estrogen receptor (ER)α in MCF-7 breast cancer cells while simultaneously limiting the E2-induced expression of other ERα target genes. Thus, G9a has a dual and selective role as a coregulator for ERα target genes. The ERα binding regions associated with the pS2 gene, which requires G9a for E2-induced expression, are transiently occupied by G9a at 15 min after beginning E2 treatment, suggesting that G9a coactivator function is by direct interaction with ERα target genes. Transient reporter gene assays with deletion mutants of G9a demonstrated that domains previously associated with the corepressor functions of G9a (C-terminal methyltransferase domain, ankyrin repeat domain, and cysteine-rich domain) were unnecessary for G9a coactivator function in ERα-mediated transcription. In contrast, the N-terminal domain of G9a was necessary and sufficient for enhancement of ERα-mediated transcription and for E2-induced occupancy of G9a on ERα binding sites associated with endogenous target genes of ERα. In addition to a previously identified activation domain, this region contains a previously uncharacterized ligand-dependent ERα binding function, indicating how G9a is recruited to the target genes. Therefore, the coactivator and corepressor functions of G9a involve different G9a domains and different molecular mechanisms.
Introduction
Transcription regulation is directed by thousands of transcriptional activator and repressor proteins, which bind to a wide variety of specific regulatory DNA sequences that control transcription of associated genes. Once bound to DNA, these activator and repressor proteins recruit a large number of coregulator proteins, which carry out the processes of chromatin remodeling and transcription complex assembly/disassembly and regulation, resulting in increased or decreased transcription from the associated transcription start site (1–3). As suggested by the amazing number of different posttranslational modifications of histones and other proteins involved in transcriptional regulation, many coregulators are enzymes that add or remove these modifications (4). One of the most heavily studied histone-modifying enzymes is G9a, which methylates histone H3 at Lys-9 (H3K9),2 a modification which is generally associated with repressed genes (5–7). G9a plays critical roles in regulation of genes required for normal embryonic development (8). In addition, G9a is one of the major H3K9 methyltransferases and is localized in euchromatin, where it is recruited to specific genes by DNA-binding transcriptional repressor proteins and non-coding RNAs; it is associated with corepressor complexes and plays a major role in repression of genes that are potentially active (8–13). G9a exists in cells primarily in a complex with G9a-like protein, a highly homologous H3K9 methyltransferase (6). The corepressor function of G9a has been well characterized. Repression of some genes by G9a involves methylation of H3K9 by G9a, leading to recruitment of proteins such as HP1, which bind preferentially to histone H3 methylated at Lys-9 and establish a repressive chromatin conformation (8). However, more recently, it has been shown that G9a contributes to repression of other genes by a mechanism that does not require G9a methyltransferase activity but instead involves the ankyrin repeat domain and its ability to bind DNA methyltransferases (14).
Although the corepressor functions of G9a have been thoroughly documented, there have also been several reports indicating that G9a contributes to gene activation. We previously demonstrated that transiently overexpressed G9a cooperates synergistically with two other coactivators, GRIP1 and CARM1, as a coactivator for steroid hormone receptors in transient reporter gene assays (15). Subsequently, several other groups reported that reduction or loss of endogenous G9a expression resulted in increased expression of some endogenous genes (suggesting that G9a functions as a repressor of these genes) but reduced expression of other genes (suggesting a positive contribution by G9a to the expression of these genes) (16–18). It is possible some of the positive effects of G9a on expression of endogenous genes may be due to an indirect effect where G9a acts as a corepressor of a second gene which produces a protein that represses expression of the gene of interest. However, one group has shown that G9a associates with a broad region containing an active gene that requires G9a for maximum expression, indicating that G9a may act directly on this target gene. G9a also binds to RNA polymerase II, suggesting that it may play a role in establishing a preinitiation complex at the promoter of the gene (16).
To investigate further the mechanism by which G9a enhances gene expression, we have employed the MCF-7 breast cancer cell culture model, a very well characterized experimental system for studying the mechanism of estrogen-regulated gene expression mediated by estrogen receptor (ER)α. ERα is a steroid hormone receptor and a member of the nuclear receptor family of proteins, many of which function as ligand-activated transcription factors which bind specific regulatory DNA sequences associated with target genes and recruit coregulators to those genes to activate or repress transcription (1–3, 19). The natural estrogen estradiol (E2) binds to ERα and causes an altered conformation of ERα, which allows it to bind DNA and recruit coregulators, resulting in activation or repression of transcription of well characterized sets of target genes (20). In the current study, we identified specific genes that are regulated positively by E2 and that also require G9a for their E2-induced expression. To explore the mechanism of G9a action on these genes, we tested whether G9a is recruited to the regulatory sites of these genes; this addresses the question of whether G9a action on these genes is direct or indirect. We also defined the specific domains of G9a, which are required for its ability to enhance E2-induced gene expression. Our results indicate G9a acts directly on genes that it regulates positively, and the mechanism of positive gene regulation by G9a on E2-induced genes is completely different from the previously defined mechanisms of corepressor function by G9a.
EXPERIMENTAL PROCEDURES
Plasmids
Protein expression vectors for mouse G9a (mG9a) and G9a fragments, ERα, MMTV(ERE)-luciferase, GRIP1, and CARM1 were described previously (15) except as indicated below. Briefly, proteins expressed with HA or FLAG epitope tags in human cells or in vitro were encoded by pSG5.HA or pSG5.FLAG vectors containing SV40 and T7 promoters. cDNAs encoding the mG9a ΔANK (Δ734–934), ΔCys (Δ518–591), and Δ252 (Δ1–252) deletion mutants were cloned into the EcoRI and XhoI sites of pSG5.HA. cDNA encoding mG9a fragments 1–730 and 1–600 were cloned in between the EcoRI and XhoI sites of pSG5.HA. cDNA encoding human G9a (hG9a) 93–211 with an N-terminal His-tag was cloned into pMCSG7 between the SspI and BamHI restriction sites. The cDNA encoding full length hG9a was obtained from Invitrogen and cloned between the EcoRI and XhoI sites of pSG5.HA. cDNA encoding FLAG-hG9a and FLAG-hG9a 1–280 was PCR amplified and cloned between the EcoRI and XhoI sites of pSG5.FLAG to result in a 2×FLAG epitope.
Cell Culture and Reporter Gene Assay
CV-1, Cos-7, and MCF-7 cells were maintained in Dulbecco's modified Eagle's medium with 10% fetal bovine serum at 37 °C and 5% CO2. For reporter gene assays, CV-1 cells were plated in hormone-free medium (phenol red-free Dulbecco's modified Eagle's medium with 5% charcoal-stripped serum) at 1 × 105 cells/well in 12-well plates the day before transfection. Transfection of expression plasmids was performed using BioT transfection agent (Bioland) according to the manufacturer's protocol. Molar amounts of total transfected promoters were balanced with empty pSG5 vector, and total DNA mass was balanced with the promoterless pCAT basic plasmid. After transfection, the cells were grown in hormone-free medium for 48 h in the presence or absence of 100 nm E2. Cell lysis and luciferase assays on cell extracts were performed with Promega luciferase assay kit. Internal controls were not used to normalize results because expression of so-called constitutive reporter genes is enhanced by overexpression of many coactivators, including GRIP1 and CARM1, which were used in these studies. To control for variations in transfection efficiency, multiple independent transfection experiments were performed, and multiple plasmid preps for key plasmids were tested in these assays. The results are presented as the mean ± S.D. of three transfected wells and are representative of at least three independent experiments. An aliquot of the cell lysate was reserved for immunoblot analysis.
Coimmunoprecipitation and Immunoblot
For coimmunoprecipitation, Cos-7 cells were seeded 1 × 106 cells/10-cm dish the day before transfection. Transfection of 3 μg of expression vectors was performed with Bio-T (Bioland) according to the manufacturer's protocol. Cells were incubated for 48 h in the presence of the indicated ligand (100 nm 17β-estradiol (E2), 4-hydroxytamoxifen, or ICI164,384, or the comparable volume of ethanol as vehicle) before whole cell extracts were made in 1 ml of radioimmune precipitation assay buffer (50 mm Tris·HCl (pH 8.0), 150 mm NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS). Coimmunoprecipitation and immunoblotting were performed as described previously (21). The lysate was precleared by incubation with protein A/G beads for 1 h and then immunoprecipitated overnight with 1 μg of the indicated antibodies and A/G beads. Immunoblotting was conducted with primary antibodies against HA epitope (3F10, Roche Applied Science), G9a (Sigma), ERα (HC20, Santa Cruz Biotechnology), RNA polymerase II (CTD4H8, Millipore), actin (Santa Cruz Biotechnology), and FLAG epitope (M2, Sigma). Secondary antibodies from Santa Cruz Biotechnology (anti-rat) and Promega (anti-rabbit and anti-mouse) were used for chemiluminescence detection by film, or antibodies were purchased from LI-COR Biosciences for quantitative infrared imaging.
RNA Isolation and Quantitative Reverse Transcriptase PCR (qRT-PCR)
For RNA extraction, cells were lysed in TRIzol (Invitrogen) according to the manufacturer's instructions. Reverse transcription was accomplished using iScript (Bio-Rad) according to specifications with 0.8 μg of total RNA for cDNA template. Real-time PCR amplification of this cDNA was performed on a Roche Lightcycler 480 using SYBR Green I mastermix (Roche Applied Science) and primers specific for the indicated mRNAs (see supplemental Table 1). All mRNA levels were normalized to the level of GAPDH mRNA.
G9a Depletion
For depletion of G9a with siRNA, SMARTpool siRNA targeting G9a (Dharmacon) was transfected into MCF-7 cells using Oligofectamine (Invitrogen) according to the manufacturer's protocol. Transfected MCF-7 cells were cultured for 72 h in hormone-free medium prior to E2 treatment. Cells were harvested either in TRIzol (Invitrogen) for qRT-PCR or radioimmune precipitation assay buffer for immunoblot analysis.
Chromatin Immunoprecipitation (ChIP)
ChIP was performed as described previously (15). Briefly, MCF-7 cells were cultured in hormone-free medium for 3 days before addition of E2. After exposure to 100 nm E2 for the indicated time, cells were fixed in formaldehyde for 15 min before addition of glycine solution to stop cross-linking. After washing with PBS, cells were pelleted by centrifugation and resuspended in hypotonic buffer before being passed through a 25-gauge needle three times. Cromatin was pelleted and resuspended for sonication in the Bioruptor apparatus (Diagenode) for 15 min (30 s on, then 30 s off, repeated) until chromatin fragment sizes were <0.5 kb as determined by agarose gel electrophoresis. Sonicated chromatin was diluted 1:10 for immunoprecipitation and precleared by incubating with AG-agarose beads. Immunoprecipitation was performed with the indicated antibodies by incubation overnight with AG-agarose beads. Washing and elution of the beads occurred the next day followed by overnight incubation at 65 °C to reverse cross-linking. DNA purification was performed by extraction with phenol and chloroform followed by ethanol precipitation, and DNA was resuspended for quantitative PCR (qPCR) using primers specific for the indicated DNA sites (see supplemental Table 1). G9a antibodies used were as follows: 1 μg (Sigma); 0.5 μg (R&D Systems); and Abcam whole antiserum, 10 μl. The signal from the immunoprecipitated DNA was normalized to the signal from DNA prepared from the same amount of chromatin before immunoprecipitation (input).
GST Pulldown Experiments
Protein fragments labeled with 35S were synthesized by transcription and translation in vitro using TnT T7 Quick kit (Promega) and the appropriate plasmid as template. mG9a protein fragments 72–200, 150–265, and 200–333 were synthesized using PCR amplification products as the DNA templates. The GST fusion protein attached to beads was incubated with the 35S-labeled proteins overnight at 4 °C. After washing with NETN buffer (22) containing 0.5% and 0.01% Nonidet P40, the beads were boiled in SDS lysis buffer for analysis by SDS-PAGE and autoradiography. For pulldown of a His-tagged protein fragment, hG9a 93–211 was bacterially expressed and purified using a His Bind purification kit (Novagen). The His-tagged G9a protein was incubated with the GST fusion protein in the same manner as above.
RESULTS
G9a Depletion Differentially Affects Expression of ERα Target Genes
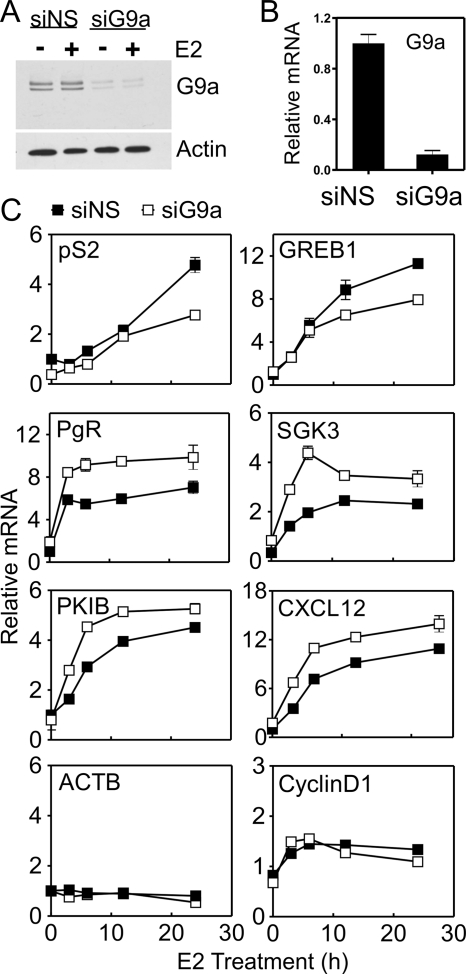
To determine the importance of G9a for ERα-mediated gene expression, we depleted G9a in MCF-7 human breast cancer cells using a pool of four siRNAs (Fig. 1, A and B) and measured mRNA levels of several well known ERα target genes at various time points after beginning E2 treatment (Fig. 1C). E2-induced levels of pS2 and GREB1 mRNA were reduced by G9a deficiency, indicating a positive regulatory effect of G9a. In contrast, induction of Protein Kinase Inhibitor β (PKIB), SKG3, PgR, and CXCL12 mRNAs by E2 in the absence of G9a was greater than in non-depleted cells. However, induction of cyclin D1 mRNA by E2 was not altered by G9a depletion. These results indicate that endogenous G9a is required for full induction of some endogenous genes by E2, limits the extent of induction of other ERα target genes, and has no effect on E2-induced expression of a third subset of ERα target genes.
FIGURE 1.
Selective positive and negative effects of G9a depletion on E2-regulated gene expression. MCF-7 cells were transfected with SMARTpool siRNA targeting G9a (siG9a) or siRNA against a nonspecific sequence (siNS). Cells transfected with siRNA were cultured for 3 days in hormone-free medium prior to addition of 100 nm E2 for the indicated time. A, whole cell extracts from cells at the zero time point of E2 treatment were analyzed by immunoblot with antibodies for G9a and actin. B, total cellular RNA was extracted from cells at the zero time point of E2 treatment and used to measure G9a mRNA level by qRT-PCR. C, total cellular RNA was extracted from cells at the indicated time point of E2 treatment and analyzed by qRT-PCR to determine levels of the indicated mRNAs, which are normalized to the level of GAPDH mRNA. Results shown are the mean and S.D. for three PCR reactions performed with cDNA samples from a single experiment, and are representative of at least three independent biological experiments performed on different days.
Measurement of Pre-mRNA Reveals Effect of G9a Depletion on Transcription
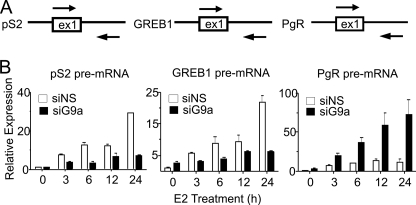
The mRNA level for a particular gene depends on the rate of synthesis and the rate of degradation. In contrast, the level of primary transcript provides a more accurate measure of the instantaneous transcriptional activity or rate for a given gene. Therefore, we measured pre-mRNA levels of pS2, GREB1, and PgR at different time points after E2 addition. Pre-mRNA levels for pS2 and GREB1 (which require G9a for full E2 induction) increased substantially after E2 treatment and were substantially inhibited by G9a depletion (Fig. 2). In contrast, in the same cells, G9a depletion increased the E2-induced levels of pre-mRNA for PgR (Fig. 2), matching the effect of G9a depletion on PgR mRNA (Fig. 1C). Similar results were obtained when the pool of four siRNAs was split into two pairs of siRNAs (supplemental Fig. 1). Thus, endogenous G9a contributes to the transcriptional activation of some ERα target genes while simultaneously limiting the E2-induced transcriptional activation of other ERα target genes.
FIGURE 2.
G9a affects ERα target gene expression at the level of transcription. A, to detect pre-mRNA for the indicated gene, primers were designed to amplify a DNA sequence that spans the boundary of exon 1 (ex1) and intron 1 for each gene. B, total RNA samples prepared as described in the legend to Fig. 1C were analyzed by qRT-PCR, using primers designed as illustrated in A. Results are presented relative to GAPDH mRNA levels as the mean and S.D. for three PCR reactions performed with the cDNA samples from a single experiment and are representative of at least three independent biological experiments performed on different days. siG9a, siRNA targeting G9a; siNS, siRNA against a nonspecific sequence.
ERα Binding Regions of pS2 Gene Are Occupied by G9a in Hormone-dependent Manner
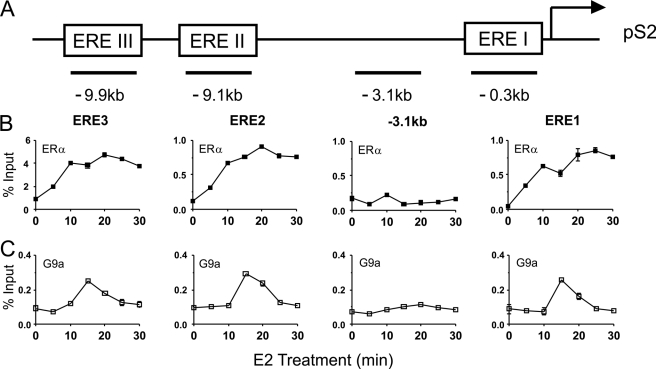
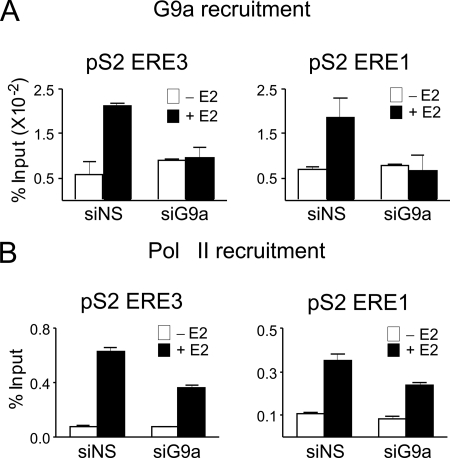
The actions of G9a as a corepressor involve direct G9a occupancy of regulatory sites on the regulated gene, resulting in methylation of Lys-9 of histone H3 by G9a or recruitment of other corepressor proteins (8, 14). In contrast, the mechanism by which G9a contributes to gene activation is unknown. G9a could occupy regulatory regions of the target gene and thereby contribute directly to enhanced transcription. Alternatively, positive effects on transcription of a specific gene by G9a could be indirect: G9a could occupy and repress a second gene that encodes a repressor protein for the first gene. Direct action of G9a as a coactivator would require that G9a occupy regulatory sites of the gene. We therefore tested for E2-regulated occupancy of G9a on the ERα binding sites of the pS2 gene in MCF-7 cells. ERα binding sites are located 9.9, 9.1, and 0.3 kb upstream of the transcription start site of the pS2 gene (Fig. 3A) (23). Using ChIP assays, we observed E2-induced binding of ERα to these three sites, but not to a control site at −3.1 kb, beginning at 5 min and continuing through 30 min of E2 treatment (Fig. 3B). At ∼15 min after beginning E2 exposure, G9a occupancy of the pS2 gene also increased sharply and transiently at the ERα binding regions but not at the −3.1 kb control region (Fig. 3C). By 25 min of E2 exposure, G9a occupancy returned to pre-E2 treatment levels. These results were obtained with G9a antibodies from R&D Systems, and similar results were obtained with G9a antibodies from Sigma-Aldrich and Abcam (data not shown). The specificity of the G9a antibodies was further confirmed by repeating the ChIP analysis after depletion of G9a. Upon G9a depletion, the E2-induced occupancy of G9a at the ERα binding sites of the pS2 gene after 15 min of E2 exposure was abolished (Fig. 4A). G9a depletion also caused diminished E2-induced occupancy of RNA polymerase II at the pS2 gene measured after 45 min of E2 exposure (Fig. 4B), which is near the peak time for RNA polymerase II occupancy. This result is consistent with the reduced expression of pS2 mRNA and pre-mRNA after depletion of G9a. The E2-induced occupancy of G9a at ERα binding sites indicates that G9a acts directly on the target gene in a hormone-dependent manner.
FIGURE 3.
E2-induced recruitment of G9a to ERα binding sites associated with the pS2 gene. A, the upstream regulatory region of the pS2 gene, showing three ERα binding sites (ERE), the transcription start site (arrow), and the locations of amplicons used for qPCR analysis of DNA from ChIP (horizontal bars with distance from the transcription start site indicated in kb). B and C, MCF-7 cells were treated with E2 for the indicated time before cross-linking of chromatin with formaldehyde. ChIP was performed with antibodies against ERα (B) and G9a (C), and the indicated amplicons were quantified by qPCR in the immunoprecipitated DNA and the input DNA before immunoprecipitation. Results shown for G9a were performed with antibodies from R&D Systems; similar results were obtained with anti-G9a antibodies from Abcam and Sigma. Results are shown as percentage of input, are the mean and S.D. of three PCR reactions performed with the DNA samples from a single experiment, and are representative of at least three independent biological experiments performed on different days.
FIGURE 4.
G9a depletion reduces occupancy of G9a and RNA polymerase II at ERα binding sites associated with the pS2 gene. MCF-7 cells were transfected with siNS or siG9a, grown for 3 days in hormone-free medium, and treated with 100 nm E2 for 0, 15, or 45 min as described in the legend to Fig. 1. A, ChIP was performed on chromatin from the 0- and 15-min E2-treated cells using antibodies against G9a. B, ChIP was performed on chromatin from the 0- and 45-min E2-treated cells using antibodies against RNA polymerase II (Pol II). DNA regions including the indicated pS2 EREs were amplified by qPCR from the DNA isolated by ChIP. Results are shown as percentage of input before ChIP, are the mean and S.D. for three PCR reactions from the DNA samples from a single experiment, and are representative of at least three independent biological experiments performed on different days. siG9a, siRNA targeting G9a; siNS, siRNA against a nonspecific sequence.
N-terminal Region of G9a Is Necessary and Sufficient for G9a Coactivator Function in Transient Reporter Gene Assays
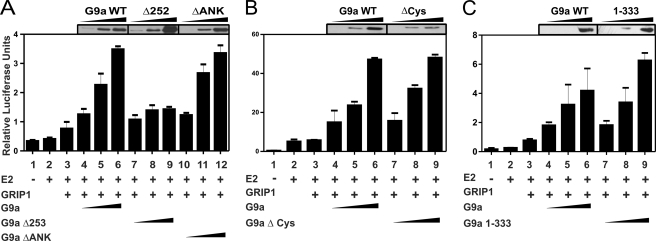
To explore further the mechanism of G9a recruitment and action as a coactivator, we tested which domains of G9a are required for its coactivator function with ERα. We previously observed enhancement of steroid hormone-induced reporter gene expression by G9a and found that the SET (Su(var), enhancer of Zeste, trithorax) domain of G9a, which contains the methyltransferase activity, was not required (15). Robust G9a coactivator function required the co-expression of p160 coactivator GRIP1/SRC-2, and G9a also exhibited dramatic three-coactivator synergy in cooperation with GRIP1 and protein arginine methyltransferase CARM1. GRIP1, a member of the p160 or steroid receptor coactivator family, binds ERα in a hormone-dependent manner and serves as a scaffold protein for recruitment of other coactivators, including CARM1 (24). Because GRIP1 can bind to the ankyrin repeat domain of G9a (25), we tested whether the ankyrin repeat domain is required for G9a coactivator function with ERα. When ERα and GRIP1 were overexpressed by transient transfection of CV-1 cells, E2-induced expression of a transiently transfected ERα reporter gene was enhanced greatly by coexpression of full-length mG9a (Fig. 5A, bars 1–6). The enhancement by G9a was only observed in the presence of E2 (supplemental Fig. 2D), indicating that G9a coactivator activity depends upon the presence of hormone-activated ERα. A mG9a mutant lacking the ankyrin repeat domain (G9aΔANK, lacking amino acids 730–965) and expressed at levels similar to full-length mG9a, exhibited coactivator activity similar to that of full-length mG9a (Fig. 5A, bars 10–12). Thus, neither the C-terminal SET domain nor the ankyrin repeat domain is required for the coactivator function of G9a in this assay, suggesting that neither the methyltransferase activity nor the ability to bind GRIP1 is required for G9a coactivator function. We also tested a G9a mutant lacking the cysteine-rich region (amino acids 518–591), which is homologous to cysteine ring domains known to bind other transcription factors. Again, the coactivator function of this deletion mutant, in cooperation with GRIP1, was similar to that of full-length G9a (Fig. 5B).
FIGURE 5.
Enhancement of E2-dependent reporter gene expression by G9a and G9a protein fragments in cooperation with GRIP1. CV-1 cells in 12-well dishes were transfected with MMTV(ERE)-luciferase reporter plasmid (200 ng) and expression vectors encoding ERα (0.2 ng); GRIP1 (100 ng); HA-tagged mG9a full-length (FL), mG9aΔ252, mG9aΔANK, or mG9aΔCys (100, 200 or 400 ng); and mG9a 1–333 (50, 100, or 200 ng). Cells were grown in the presence or absence of E2 for 48 h before measurement of luciferase activity and immunoblot analysis with antibodies against HA. Results shown are mean and S.D. from triplicate transfected cultures for each condition, and each experiment shown is representative of three or more independent experiments performed on different days.
We then focused on the only remaining untested region, the N terminus, which contains a previously characterized activation domain (15). A mG9a mutant lacking the N-terminal 252 amino acids, which was also expressed at levels similar to full-length mG9a, exhibited little or no activity as a coactivator in cooperation with GRIP1 (Fig. 5A, bars 7–9), indicating that the N-terminal region is required for coactivator function. Because the N-terminal region of G9a, but none of the other regions tested, was required for coactivator function, we tested whether the N-terminal region alone is sufficient for coactivator function. Indeed, full-length mG9a and an N-terminal mG9a fragment consisting of amino acids 1–333, expressed at similar levels, enhanced E2-induced reporter gene activity to a similar extent in cooperation with GRIP1 (Fig. 5C). Note that approximately half as much of the mutant mG9a plasmid, compared with the wild type plasmid, was required to achieve equivalent expression levels. For all of the above mG9a mutants, similar results were obtained when both GRIP1 and CARM1 were coexpressed with mG9a or the mG9a mutants (supplemental Fig. 2). However, under these conditions where GRIP1, CARM1, and G9a cooperated synergistically as coactivators, the mG9a 1–333 fragment was quite active but exhibited somewhat less activity than full-length mG9a at the higher levels of expression.
Amino acids 72–333 of mG9a are highly conserved with amino acids 15–281 of hG9a (supplemental Fig. 3). Therefore, we tested the coactivator function of the hG9a N-terminal fragment (amino acids 1–280), which is analogous to mG9a 1–333. As with mG9a, comparable levels of full-length hG9a and hG9a 1–280 exhibited similar levels of coactivator activity in cooperation with GRIP1 (supplemental Fig. 4). Thus, the N-terminal region of G9a (mouse or human) was both necessary and sufficient for G9a coactivator function in this transient reporter gene assay.
G9a Exhibits Ligand-dependent ERα Binding Activity
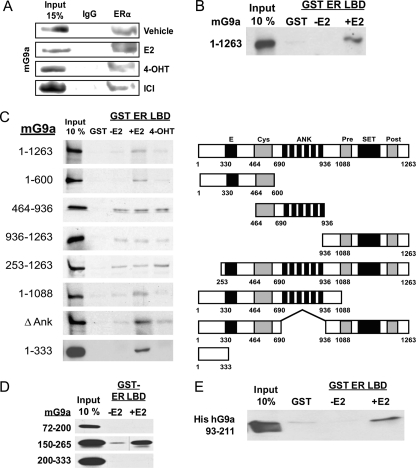
To understand further the mechanism of coactivator function by G9a and its N-terminal region in ERα-mediated transcription, we investigated how G9a is recruited to the target genes of ERα. Because our results suggested that G9a association with GRIP1 is not required for coactivator function (Fig. 5A), we considered the possibility of G9a recruitment directly by interaction with ERα. When transiently expressed ERα was immunoprecipitated from Cos-7 cells, transiently expressed HA-tagged mG9a was co-precipitated in the absence of E2, but the co-precipitation was further enhanced when cells were treated with E2 prior to harvest (Fig. 6A). ERα antagonists 4-hydroxytamoxifen and ICI164,384 failed to enhance the ERα-G9a interaction. To test whether this apparent hormone-dependent interaction of mG9a with ERα was direct, 35S-labeled mG9a was translated in vitro and incubated with GST-tagged ERα LBD bound to agarose beads. Binding of mG9a to ERα LBD in this assay was highly dependent on E2 (Fig. 6B), suggesting that the interaction was probably direct. The small amount of mG9a binding to GST alone may be due to the overloading of GST on the beads compared with GST-ERα LBD. In similar GST pulldown assays, mG9a 1–600 also bound to the ERα N-terminal AF-1 fragment and to the ERα DNA binding domain fragment (supplemental Fig. 5). However, in our experiment, the ERα DNA binding domain binds to many proteins, casting doubt on whether the interactions observed are specific or are instead in vitro artifacts of this fragment.
FIGURE 6.
E2-dependent binding of ERα to the N-terminal region of G9a. A, Cos-7 cells were transfected with 3 μg each of expression vectors encoding ERα and HA-tagged full-length mG9a. Cells were treated with the indicated ERα ligand (4-OHT, 4-hydroxytamoxifen; ICI, ICI164,384) for 48 h, cell extracts were immunoprecipitated with anti-ERα antibody, and immunoblot was performed with anti-HA antibody. B, 35S-labeled full-length mG9a was synthesized in vitro and incubated in the presence and absence of E2 with GST or GST-ERα LBD bound to glutathione-agarose beads. The bound protein fraction was eluted and analyzed by SDS-PAGE and autoradiography. A 10% input sample was loaded for comparison. C and D, 35S-labeled mG9a fragments were synthesized in vitro and incubated with GST or GST-ERα LBD bound to glutathione-agarose beads in the presence and absence of E2 or 4-hydroxytamoxifen. Bound proteins and 10% input samples were analyzed as described in B. The vertical line in the G9a 150–265 gel image is a scratch on the film. E, bacterially expressed His-tagged hG9a 93–211 was incubated in the presence and absence of E2 with GST or GST- ERα LBD bound to glutathione-agarose beads. Input and bound samples were analyzed by immunoblot with an anti-His tag antibody.
Although we cannot rule out contributions from the G9a interactions with the ERα DNA binding domain and AF-1 regions, we focused on the hormone-dependent interaction of G9a with the ERα LBD, as the coactivator function of G9a also depends upon hormonal activation of ERα. To determine the sequences of G9a necessary for ligand-dependent binding to ERα LBD, multiple deletion mutants of G9a were translated in vitro and tested for their ability to bind GST-ERα LBD in the presence and absence of E2. C-terminal truncations of various sizes or deletion of the ankyrin repeat domain failed to reduce the E2-dependent binding of mG9a to ERα LBD (Fig. 6C). In contrast, deletion of the N-terminal 252 amino acids eliminated E2-dependent binding to the ERα LBD, although some fragments lacking the N-terminal end exhibited weak E2-independent binding not observed with full-length mG9a. Finally, the N-terminal mG9a fragment consisting of amino acids 1–333, which was active as a coactivator (Fig. 5), retained the ability to bind ERα LBD in an E2-dependent manner (Fig. 6C). Similar E2-dependent binding to ERα LBD was obtained with hG9a 1–280, which is the homologous fragment for mG9a 1–333 (data not shown).
Because the N-terminal sequence of G9a contained no known ERα binding motif (e.g. LXXLL where L is leucine and X is any amino acid), we sought to further define the region necessary for this novel ERα LBD binding activity. Three overlapping fragments of the N terminus were translated in vitro and incubated with GST ERα LBD in the presence and absence of E2. Of the three fragments, only the central region, consisting of mG9a amino acids 150–265, bound ERα LBD and bound in a ligand-dependent manner (Fig. 6D). As a stringent test for direct G9a binding to ERα LBD, hG9a 93–211 (which corresponds to mG9a 150–265) containing a His tag was bacterially expressed and incubated with GST-ERα LBD. The hG9a (93–211) fragment produced in bacteria was bound to ERα LBD in an E2-dependent manner (Fig. 6E). Thus, the N-terminal region of G9a, which is sufficient for coactivator function, can bind directly to ERα LBD in response to hormone, providing a potential mechanism for recruitment of G9a to the target genes of ERα.
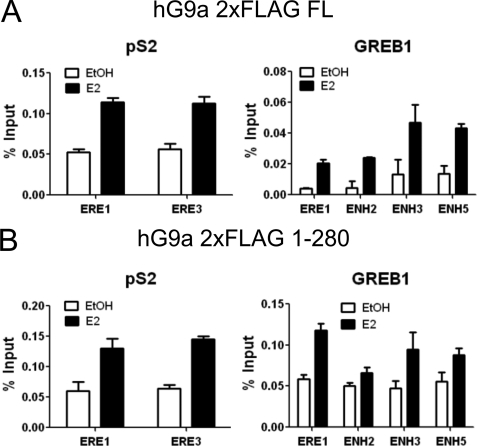
G9a N Terminus Is Sufficient for Recruitment to ERα Target Genes
The presence of a ligand-dependent ERα binding domain and coactivator function within the N-terminal region of G9a led us to investigate whether the same N-terminal region of G9a is responsible for the E2-induced occupancy of G9a on ERα binding sites associated with ERα target genes. MCF-7 cells were transfected with a plasmid encoding hG9a full-length or hG9a 1–280 containing two N-terminal FLAG tags. We observed E2-induced association of full length G9a and G9a 1–280 with the ERα binding sites associated with the pS2 and GREB1 genes (Fig. 7). Thus, the N-terminal region of G9a is sufficient for coactivator function, ligand-dependent binding to ERα, and recruitment of G9a to ERα binding sites associated with E2-regulated genes.
FIGURE 7.
The N-terminal region of G9a is sufficient for E2-induced recruitment to endogenous ERα target genes. MCF-7 cells were transfected with pSG5.2×FLAG-hG9a (A) or pSG5.2×FLAG-hG9a 1–280 (B), grown for 3 days in hormone-free medium, and treated with 100 nm E2 for 0 or 15 min. ChIP was performed using antibodies against FLAG and qPCR primers for the ERα binding sites associated with the pS2 and GREB1 genes. Results are shown as percentage of input, are the mean and S.D. for three PCR reactions from the DNA samples from a single experiment, and are representative of two independent biological experiments performed on different days.
DISCUSSION
Selective Positive and Negative Regulation of Target Genes of ERα by Coregulator G9a
By examining mRNA levels of multiple genes, which are up-regulated in response to E2 in MCF-7 cells, we found that the effects of G9a depletion fell into three different categories (Fig. 1C). 1) E2 caused a stronger induction of some genes after G9a depletion, indicating that G9a acts negatively to limit the extent of E2-induced expression for these genes. 2) G9a depletion restricted the extent of induction of a second set of ERα target genes, indicating a positive role for G9a in E2-induced expression of these genes. 3) Depletion of G9a had no effect on the E2-induced expression of a third subset of genes. For both subsets of ERα target genes regulated by G9a, the effect of G9a on the mRNA level was transcriptional, as depletion of G9a caused similar effects on the levels of E2-induced pre-mRNA and mature mRNA for the target genes (Fig. 2).
What are the mechanisms that determine whether G9a acts to enhance or inhibit gene expression, after it is recruited to the gene in response to E2? Promoter-specific effects on gene regulation by various transcription factors and coregulators is a well known but poorly understood phenomenon. We speculate that the specific protein-protein interactions or post-translational modification that G9a experiences on a particular target gene of ERα determine whether G9a acts as a coactivator or corepressor.
The selective actions of G9a on different subsets of ERα target genes suggest the interesting possibility that regulation of G9a levels, localization, or activity could alter the overall response to E2 and thus alter the physiological response elicited by E2. This may be a mechanism for fine tuning of the response to E2. Future work to determine the effects of G9a depletion or inhibition on global gene regulation in response to E2 will examine this hypothesis.
Mechanism by Which G9a Is Recruited to ERα Target Genes and Enhances E2-induced Transcription
We found that the specific domains of G9a required for the ability of G9a to enhance E2-induced transcription are completely different from the domains implicated previously in G9a-mediated repression. Repression can be achieved through methylation of H3K9 by the C-terminal SET domain of G9a, leading to recruitment of HP1 and establishment of a repressive chromatin environment (8). Methyltransferase-independent repression has been assigned to the ankyrin repeat domain and correlated with the ability of that domain to interact with DNA methyltransferases (6, 14). The cysteine-rich domain is also apparently required for repression in some cases because it is the site of interaction for some DNA-binding repressor proteins that recruit G9a to specific genes (11). We found that none of these three domains is required for the coactivator function of G9a in transient transfection assays (Fig. 5). Instead, only the N-terminal region of G9a, which contains no recognizable amino acid motifs, was necessary and sufficient for enhancement of transcription of a transient reporter gene by E2-activated ERα.
Because G9a acted synergistically with GRIP1 as a coactivator for steroid receptors (Fig. 5) (15), we proposed that G9a is recruited to the target gene by interaction with GRIP1. However, GRIP1 interacts with G9a through the ankyrin repeat domain (25), which is not required for the coactivator function of G9a (Fig. 5). What then is the mechanism by which the N-terminal region of G9a enhances transcription by E2-activated ERα? The N-terminal region of G9a binds to ERα LBD in a hormone-dependent manner (Fig. 6), and it contains a transcriptional activation domain (15). These two activities satisfy the minimum requirements for a transcriptional coactivator: a mechanism for recruitment to regulatory sequences of target genes (in this case through interaction with ERα) and a mechanism for contributing to transcriptional activation after recruitment to the gene (through the action of an activation domain, which can recruit other coactivators, make contact with other components of the chromatin or transcription machinery, or carry out some enzyme reaction, if the activation domain contains such an activity). Furthermore, we confirmed that the N-terminal fragment of hG9a, which binds ERα and is sufficient for coactivator function in transient reporter gene assays, is also sufficient for E2-induced recruitment of G9a to ERα binding sites associated with E2-regulated genes (Fig. 7). Thus, we have identified a new set of functions for G9a that allow it to serve as a coactivator. The domains required for positive regulation of transcription are located in a small region at the extreme N terminus of G9a, whereas the domains required for corepressor function occupy the C-terminal three-quarters of the G9a polypeptide.
Does G9a Enhance E2-induced Transcription by Direct or Indirect Action on Target Genes of ERα?
Enhancement of E2-induced transcription by G9a could be accomplished by direct action of G9a on the target gene or by indirect actions of G9a. The indirect mechanism could involve repressive action of G9a on another gene encoding a protein that represses expression of the ERα target gene. If the indirect mechanism is correct, we would expect it to involve the previously defined repression mechanisms of G9a carried out by some combination of the SET, ankyrin repeat, and cysteine-rich domains. Because none of these domains was required for G9a coactivator function with ERα on a transient reporter gene (Fig. 5), our results do not support the indirect repressive mode of action by G9a to enhance E2-induced expression of some ERα target genes. Furthermore, the N-terminal domain of G9a, which is necessary and sufficient for the coactivator function of G9a, has never been implicated in repression by G9a.
Instead, our results support a direct mechanism of action. The N-terminal domain is capable of binding directly to ERα LBD in a hormone-dependent manner (Fig. 6), occupying genes that are induced by E2 at the same sites occupied by ERα in response to E2 treatment (Fig. 7) and enhancing E2-induced expression of a transient reporter gene (Fig. 5). The ERα binding activity (Fig. 6) and transcriptional activation function (15) contained in the N-terminal region provide a plausible mechanism for the coactivator function of G9a. E2 causes increased occupancy by G9a on the regulatory sites of endogenous target genes of ERα, which require G9a for full E2-induced expression. The occupancy of G9a on the target gene thus satisfies the minimum condition for a direct mechanism of positive regulation by G9a. In addition, the sites occupied by G9a are the exact sites occupied by ERα in an E2-dependent manner. The E2-dependent occupancy of G9a on DNA sites occupied by ERα (Fig. 3) strongly suggests that G9a is recruited by ERα. Although we have not tested whether G9a also interacts with ERβ, ERβ is not expressed in MCF-7 cells (26). Our demonstration that G9a directly binds to ERα LBD in a hormone-dependent manner (Fig. 6) provides an explanation of why G9a occupies the same genomic sites occupied by ERα (Figs. 3 and 7). We cannot rule out some additional indirect contribution by G9a in the E2-induced expression of ERα target genes, but our results clearly show that G9a is recruited to the endogenous genes in an E2-dependent manner and thus can act directly on the target genes.
Because G9a binds in vitro to the ERα DNA binding domain and AF-1 regions as well as the LBD, further work will be required to determine whether these additional in vitro interactions are functionally relevant in vivo. However, the relatively strong hormone-dependent interaction with the ERα LBD would appear to be most relevant and sufficient for recruitment of G9a to target genes by ERα. The most commonly documented mechanism for binding of coactivators to the LBD of a nuclear receptor, including ERα, is through an LXXLL motif in the coactivator, which forms an amphipathic α helix that binds to a hydrophobic groove in the surface of the ligand-activated receptor (27, 28). However, the region of G9a responsible for binding to ERα LBD does not contain a motif resembling the LXXLL motif, suggesting that G9a binds to the ERα LBD via a novel mechanism. The small ERα-binding fragment of G9a (hG9a amino acids 93–211) defined here may be amenable to structural studies that can define the structural basis of this novel type of coactivator interaction with the ERα LBD.
Supplementary Material
Acknowledgments
We thank Jeong Hoon Kim (Samsung Medical Center, Sungkyunkwan University) for experimental protocols and technical advice. We thank Geoffrey Greene (University of Chicago) for the plasmid encoding GST fused to the LBD of ERα. We also thank Omar Khalid and Kelly Chang (University of Southern California) for technical assistance. LI-COR chemiluminescence detection was provided by the University of Southern California Research Center of Liver Diseases, and other material assistance was provided by the Molecular and Cell Biology Support Core and Bioreagent and Cell Culture Core of the University of Southern California Norris Comprehensive Cancer Center.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 DK055274 (to M. R. S.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1, Figs. 1–5, and additional references.
- H3K9
- histone H3 Lys-9
- ERα
- estrogen receptor α
- mG9a
- mouse G9a
- MMTV
- mouse mammary tumor virus
- ERE
- estrogen responsive enhancer element
- GRIP1
- glucocorticoid receptor interacting protein 1
- CARM1
- coactivator-associated arginine methyltransferase 1
- ANK
- ankyrin repeats
- hG9a
- human G9a
- qRT-PCR
- quantitative reverse transcription-PCR
- PgR
- progesterone receptor
- LBD
- ligand binding domain.
REFERENCES
- 1. Glass C. K., Rosenfeld M. G. (2000) Genes Dev. 14, 121–141 [PubMed] [Google Scholar]
- 2. Métivier R., Penot G., Hübner M. R., Reid G., Brand H., Kos M., Gannon F. (2003) Cell 115, 751–763 [DOI] [PubMed] [Google Scholar]
- 3. Métivier R., Gallais R., Tiffoche C., Le Péron C., Jurkowska R. Z., Carmouche R. P., Ibberson D., Barath P., Demay F., Reid G., Benes V., Jeltsch A., Gannon F., Salbert G. (2008) Nature 452, 45–50 [DOI] [PubMed] [Google Scholar]
- 4. Lee D. Y., Teyssier C., Strahl B. D., Stallcup M. R. (2005) Endocr. Rev. 26, 147–170 [DOI] [PubMed] [Google Scholar]
- 5. Tachibana M., Sugimoto K., Fukushima T., Shinkai Y. (2001) J. Biol. Chem. 276, 25309–25317 [DOI] [PubMed] [Google Scholar]
- 6. Shinkai Y., Tachibana M. (2011) Genes Dev. 25, 781–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Milner C. M., Campbell R. D. (1993) Biochem. J. 290, 811–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tachibana M., Sugimoto K., Nozaki M., Ueda J., Ohta T., Ohki M., Fukuda M., Takeda N., Niida H., Kato H., Shinkai Y. (2002) Genes Dev. 16, 1779–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nishio H., Walsh M. J. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 11257–11262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gyory I., Wu J., Fejér G., Seto E., Wright K. L. (2004) Nat. Immunol. 5, 299–308 [DOI] [PubMed] [Google Scholar]
- 11. Roopra A., Qazi R., Schoenike B., Daley T. J., Morrison J. F. (2004) Mol. Cell 14, 727–738 [DOI] [PubMed] [Google Scholar]
- 12. Duan Z., Zarebski A., Montoya-Durango D., Grimes H. L., Horwitz M. (2005) Mol. Cell Biol. 25, 10338–10351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nagano T., Mitchell J. A., Sanz L. A., Pauler F. M., Ferguson-Smith A. C., Feil R., Fraser P. (2008) Science 322, 1717–1720 [DOI] [PubMed] [Google Scholar]
- 14. Epsztejn-Litman S., Feldman N., Abu-Remaileh M., Shufaro Y., Gerson A., Ueda J., Deplus R., Fuks F., Shinkai Y., Cedar H., Bergman Y. (2008) Nat. Struct. Mol. Biol. 15, 1176–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee D. Y., Northrop J. P., Kuo M. H., Stallcup M. R. (2006) J. Biol. Chem. 281, 8476–8485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chaturvedi C. P., Hosey A. M., Palii C., Perez-Iratxeta C., Nakatani Y., Ranish J. A., Dilworth F. J., Brand M. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 18303–18308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kondo Y., Shen L., Ahmed S., Boumber Y., Sekido Y., Haddad B. R., Issa J. P. (2008) PLoS One 3, e2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lehnertz B., Northrop J. P., Antignano F., Burrows K., Hadidi S., Mullaly S. C., Rossi F. M., Zaph C. (2010) J. Exp. Med. 207, 915–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tsai M. J., O'Malley B. W. (1994) Annu. Rev. Biochem. 63, 451–486 [DOI] [PubMed] [Google Scholar]
- 20. Tee M. K., Rogatsky I., Tzagarakis-Foster C., Cvoro A., An J., Christy R. J., Yamamoto K. R., Leitman D. C. (2004) Mol. Biol. Cell 15, 1262–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee Y. H., Koh S. S., Zhang X., Cheng X., Stallcup M. R. (2002) Mol. Cell Biol. 22, 3621–3632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koh S. S., Chen D., Lee Y. H., Stallcup M. R. (2001) J. Biol. Chem. 276, 1089–1098 [DOI] [PubMed] [Google Scholar]
- 23. Pan Y. F., Wansa K. D., Liu M. H., Zhao B., Hong S. Z., Tan P. Y., Lim K. S., Bourque G., Liu E. T., Cheung E. (2008) J. Biol. Chem. 283, 32977–32988 [DOI] [PubMed] [Google Scholar]
- 24. Ma H., Hong H., Huang S. M., Irvine R. A., Webb P., Kushner P. J., Coetzee G. A., Stallcup M. R. (1999) Mol. Cell Biol. 19, 6164–6173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Collins R. E., Northrop J. P., Horton J. R., Lee D. Y., Zhang X., Stallcup M. R., Cheng X. (2008) Nat. Struct. Mol. Biol. 15, 245–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Paruthiyil S., Parmar H., Kerekatte V., Cunha G. R., Firestone G. L., Leitman D. C. (2004) Cancer Res. 64, 423–428 [DOI] [PubMed] [Google Scholar]
- 27. Darimont B. D., Wagner R. L., Apriletti J. W., Stallcup M. R., Kushner P. J., Baxter J. D., Fletterick R. J., Yamamoto K. R. (1998) Genes Dev. 12, 3343–3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shiau A. K., Barstad D., Loria P. M., Cheng L., Kushner P. J., Agard D. A., Greene G. L. (1998) Cell 95, 927–937 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.