Background: Cystatin F is a protease inhibitor normally found within the endocytic pathway, but can be secreted.
Results: Secreted cystatin F can be internalized thereby inhibiting multiple targets and causing the accumulation of cathepsin L.
Conclusion: Cystatin F inhibits the CatL convertase AEP and stabilizes CatL protein levels.
Significance: Secreted cystatin F can be activated in trans expanding its inhibitory potential beyond its site of synthesis.
Keywords: Dendritic Cells, Endocytosis, Lysosomes, Macrophages, Protease, Protease Inhibitor, Cathepsin, Cell Biology, Cystatin, Asparagine Endopeptidase (AEP)
Abstract
Cystatin F is an unusual member of the cystatin family of protease inhibitors, which is made as an inactive dimer and becomes activated by proteolysis in the endo/lysosome pathway of the immune cells that produce it. However a proportion is secreted and can be taken up and activated by other cells. We show here that cystatin F acquired in this way induces a dramatic accumulation of the single-chain form of cathepsin L (CatL). Cystatin F was observed in the same cellular compartments as CatL and was tightly complexed with CatL as determined by co-precipitation studies. The observed accumulation of single-chain CatL was partly due to cystatin F-mediated inhibition of the putative single-chain to two-chain CatL convertase AEP/legumain and partly to general suppression of cathepsin activity. Thus, cystatin F stabilizes CatL leading to the dramatic accumulation of an inactive complex composed either of the single-chain or two-chain form depending on the capacity of cystatin F to inhibit AEP. Cross-transfer of cystatin F from one cell to another may therefore attenuate potentially harmful effects of excessive CatL activity while paradoxically, inducing accumulation of CatL protein. Finally, we confirmed earlier data (Beers, C., Honey, K., Fink, S., Forbush, K., and Rudensky, A. (2003) J. Exp. Med. 197, 169–179) showing a loss of CatL activity, but not of CatL protein, in macrophages activated with IFNγ. However, we found equivalent loss of CatL activity in wild type and cystatin F-null macrophages suggesting that an inhibitory activity other than cystatin F quenches CatL activity in activated macrophages.
Introduction
Cystatins are low molecular weight cysteine protease inhibitors that target the cysteine cathepsins including L, B, S, H, C, and K. Some cystatins also inhibit the unrelated cysteine protease asparagine endopeptidase (AEP),3 also known as legumain, through a second binding site (1, 2). There are 12 cystatins in humans and the largest group known as type 2 cystatins are made with signal sequences and can therefore be secreted (1). In principle, these cystatins can regulate intracellular protease activity although the fact that they are secreted would make extracellular proteases their likely targets. Although the functions of most of the cystatins remain to be fully revealed their loss or mutation can cause serious pathology. For example, mice lacking cystatin E/M show abnormalities in the development of the epidermis, which ultimately prove fatal (3). Similarly, patients with hereditary cystatin C amyloid angiopathy (HCCAA) suffer paralysis and dementia due to brain hemorrhages (4). This condition is caused by a point mutation in cystatin C (Leu-68 to Gln), which results in misfolding and aggregate accumulation. Whereas the canonical family member cystatin C is widely expressed, the expression of other cystatins is more tissue restricted. For example, cystatin E/M is expressed in lung epithelia, skin keratinocytes and sweat glands (5, 6) while cystatin F, also known as leukocystatin, is expressed almost exclusively in immune cells, particularly CD8 T cells, NK cells, neutrophils, dendritic cells, and mast cells (7–9). The expression of some other cystatins is confined to the salivary glands (1).
We and others have recently shown that cystatin F diverges from other family members in several important respects. Cystatin F is the only family member to form a di-sulfide linked dimeric structure (10), a configuration that renders the molecule inactive because of mutual steric hindrance of one protease inhibitory site by the other subunit (9, 11). Although the dimer can be rendered active in vitro by reduction (12), high levels of reducing agent are required and the monomeric form generated can inhibit some but not all physiological targets of cystatin F (9, 12). Moreover, the endogenous monomeric form detectable in cells is N-terminally truncated by 15 residues consistent with a proteolytic activation mechanism (9). Activation of cystatin F takes place following targeting from the secretory to the endocytic pathway. This step depends on N-linked glycosylation of either of two adjacent motifs one being an unusual Asn-X-Cys motif (13). Although most cystatin F is directed to the endocytic pathway, a significant proportion is secreted in the inactive dimeric form. We recently showed that this form can be internalized and activated by other cells using the mannose-6-phosphate receptor system (13). However the consequences for the recipient cell were not investigated in detail.
The cysteine cathepsins that are targeted by cystatin F and other cystatin family members are now known to have a range of biological functions that extend beyond catabolism of proteins that enter the endocytic and phagocytic pathway (14–17). Cathepsin L (CatL) is a key lysosomal cysteine protease. In addition to its role in generalized lysosomal proteolysis it has specific roles in epidermal homeostasis (18), regulation of hair follicle morphogenesis and cycling (19), class II MHC restricted antigen processing, particularly in cortical thymic epithelial cells (20), normal cardiomyocyte function (21), and neovascularization of ischemic tissue (22).
Autoactivation of the lysosomal pro-form generates single-chain CatL (30 kDa) which is then converted to varying degrees depending on cell type, to a two-chain form consisting of a 24/25 kDa heavy chain and a 5 kDa light chain. The two-chain form of CatL is not found in cells lacking AEP indicating that AEP may be the convertase responsible for single to two-chain conversion (23). Other cell type specific factors can also regulate the levels of CatL: the p41 isoform of the class II MHC chaperone invariant chain (Ii) binds to the CatL active site and acts as a chaperone, boosting the levels of two-chain CatL in macrophages and in the macrophage conditioned extracellular milieu (24, 25). In addition, CatL protease activity can be influenced by pro-inflammatory signals, although the mechanism remains unresolved (26).
Several cysteine cathepsins, including CatL, have been implicated in a variety of pathologies such as cancer and uncontrolled inflammation and some enzymes are undergoing trials as drug targets. In principle, the cystatins might counter the adverse effects of excessive cysteine cathepsin activity but how this would be achieved and which family members might be most important is unknown.
We show here that cells exposed to exogenous cystatin F show striking changes in both the level and configuration of CatL and we dissect the mechanisms involved. Our studies demonstrate that lysosomal protease activity is not only regulated by biosynthesis, turnover and the expression of endogenous chaperones such as p41 Ii, but additionally regulation can be achieved in trans by cystatin F. The production and secretion of cystatin F by immune cells may modulate protease activity and stability in bystander cells.
EXPERIMENTAL PROCEDURES
Chemical Reagents
Antibodies used for Western blots include; goat anti-CatB and mouse anti-cystatin C (R&D systems), sheep polyclonal antibodies to CatL and CatH (described in Ref. 27, rabbit anti-ERK2 (Santa Cruz Biotechnology), rat anti-tubulin (Abcam), rabbit anti-cystatin F (9), and sheep anti-AEP (28). Secondary antibodies include those that were HRP conjugated (Jackson Immunoresearch) and Alexa-488 or Alexa-594 conjugated (Clontech). The pan-specific protease inhibitor E-64d was purchased from Calbiochem. The AEP inhibitor MVO26630 was synthesized as described elsewhere (29).
Cell Isolation and Culture
Spleen dendritic cells and bone marrow dendritic cells (BMDC) from C57Bl6 femurs and tibia were derived exactly as described (30). Bone marrow macrophages (BMM) were derived from bone marrow precursors essentially as described (31). Briefly, precursor cells were cultured in conditioned media (RPMI (Invitrogen), l-glutamine, penicillin, and streptomycin, 10% (v/v) low endotoxin calf serum containing 10% (v/v) l-929 conditioned media for 3 days. Nonadherent cells were transferred to low attachment 6-well plates (Costar 3471) and cultured at a density of 2 × 105 cells per well. BMM were fully adherent and were F4/80 positive by day 8. BMM from AEP KO mice (32) and Ii KO mice (kind gift of Dr. Liz Bikoff) were grown as described above.
Recombinant Proteins
Recombinant cystatin c was purchased from Genway Biotech. His-tagged cystatin F and AEP were produced as previously described by gene amplification and secretion from CHO cells (9). Dimeric cystatin F was purified from culture supernatants by sequential Ni-NTA-agarose, size exclusion and cation exchange chromatography as previously described (9). Mutagenesis of AEP at residue Asn-65 to a lysine was performed using the QuikChange Site-directed Mutagenesis kit (Stratagene) targeting the following sequence 5′-GTTCAACAACTGCACGAAAGACATGTTCTTGTTCAAGGAGTC-3′ (mutated site underlined). Mutant protein was purified from culture supernatant following transient transfection of 293T cells. Recombinant proteins were found to be free of endotoxin contamination both by Limulus Amoebocyte Lysate assay (BioWhittaker) and by lack of cell surface CD40 up-regulation upon incubation with BMDC. CatL expression in MyD88 and TRIF knock-out BMM and BMDCs exposed to recombinant proteins displayed equivalent phenotypes as wild-type cells (data not shown). Recombinant proteins were added to culture media for the indicated times and concentrations and were allowed to internalize. Pilot experiments were performed to establish the concentration range over which changes in cathepsin L levels occurred. Activation of internalized cystatins and AEP was followed by Western blot and active-site labeling as described below. Densitometry of autoradiographs were performed using ImageJ. Cell permeable small molecule inhibitors of cathepsins (E64d) and AEP (MVO26630) were added to cultured cells at 5 μm and 20 μm, respectively for 24 h.
Immunoprecipitation and Nickel Chromatography
Cell lysates were prepared by the addition of ice-cold lysis buffer (20 mm Tris, 150 mm NaCl, and 1% Triton-X-100, pH 7.4). For immunoprecipitation, post-nuclear supernatants containing equivalent total protein were pre-cleared using protein G (GE Healthcare) coupled to non-immune antibody (Santa Cruz Biotechnology). Complexes of recombinant cystatin F and endogenous CatL were identified by purification of His-tagged cystatin F using Ni-NTA chromatography (Qiagen) according to the manufacturer's instructions.
Protease Activity
For active-site labeling of cathepsins in intact cells, 125I-labeled JPM-OEt (a generous gift from Matt Bogyo, Stanford University) was used as described in detail elsewhere (33). BMM were left untreated or were treated with 20 nm cystatin F or 1 ng/ml recombinant IFNγ (R&D Systems) for 48 h. Cells were then exposed to 80 nm 125I-JPM-OEt for 2 h. Labeled cathepsins were liberated from cells by glass bead homogenization (34) followed by centrifugation to remove intact nuclear and cellular debris. Equivalent total cellular protein (5–20 μg) was separated by 4–20% SDS-PAGE. Labeled CatL was further purified by immunoprecipitation (as described above) using protein G coupled to a sheep polyclonal antibody recognizing both the pro- and processed forms of the protease.
AEP activity was determined by following the hydrolysis of the AEP/legumain-specific substrate Z-Ala-Ala-Asn-7-amino-4-methylcoumarin (AMC) (Bachem) (35). Cells were lysed in sodium citrate buffer (pH 5.5) containing 100 mm citrate, 150 mm NaCl, and 0.5% Triton-X-100. The rate of hydrolysis was followed in assay buffer containing 10 μg of total protein, citrate buffer containing 20 mm citric acid, 60 mm Na2HPO4, 1 mm EDTA, 1 mm DTT, pH 5.5, and 50 μm substrate at 37 °C for 90 min. Fluorescence was quantified by measuring excitation at 360 nm and emission at 460 nm on the Fluostar Optima (BMG Labtech) plate reader and expressed as rate of hydrolysis.
Molecular Modeling/Docking
Molecular modeling of monomeric cystatin F (PDB:2CH9) with CatL was previously described elsewhere (11) using the stefin B-papain complex (PDB: 1STF). Surface display and highlighting of putative AEP interaction sites were performed in Pymol.
Immunofluorescence Microscopy
BMM were transferred from low adherence plates to Teflon-coated glass slides using Versene (Invitrogen). Cells were plated at a concentration of 2–4 × 104 per well and allowed to adhere to the glass slides. Cells were cultured with 20 nm cystatin F or solvent control for an additional 24 h. Cells were washed in PBS, fixed in 4% PFA, and permeabilized in 0.2% Triton-X-100. Cells were blocked in 2% BSA and stained with antibodies to cystatin F, and AEP or CatL, as described above. Stained cells were analyzed on the LCM510 confocal microscope (Zeiss) as described previously (13).
Quantitative Real Time PCR
RNA was extracted from BMM using the RNeasy RNA kit (Qiagen) and contaminating DNA was removed using DNase I (Qiagen). RT-PCR was performed with qScript cDNA synthesis kit (Quanta) using primers containing poly-dT and random hexamers. Real time PCR was performed on an iCycler (Bio-Rad) using iQ SYBR Green detection (Bio-Rad). Primers include; CatL: Forward: 5′-ATCAAACCTTTAGTGCAGAGTGG-3′ Reverse: 5′-CTGTATTCCCCGTTGTGTAGC-3′; and normalization primers for GAPDH; Forward: 5′-TGAAGGTCGGTGTGAACGGATTTGG-3′ Reverse: 5′-ACGACATACTCAGCACCGGCCTCAC-3′ and HPRT; Forward: 5′-AGGTTGCAAGCTTGCTGGT-3′ Reverse 5′-TGAAGTACTCATTATAGTCAAGGGCA-3′.
RESULTS
Exposure to Cystatin F Induces an Accumulation of Single-chain CatL
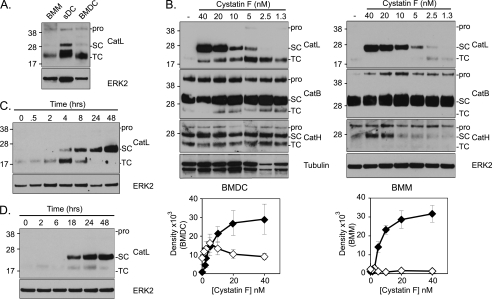
In contrast to other cystatins which are thought to engage proteases in the extracellular space, the protease binding site of secreted cystatin F is protected due to its dimeric structure (11). Although inactive dimer to active monomer conversion could occur extracellularly, a more likely scenario is that secreted cystatin F is internalized by adjacent cells and activated. To assess the consequences of exposure to cystatin F we cultured murine bone marrow-derived dendritic cells (BMDC) or macrophages (BMM) with graded doses of recombinant dimeric cystatin F. The effect on potential cysteine protease targets in the recipient cells was initially monitored by Western blotting. Exposure to cystatin F had a dramatic effect on the configuration of CatL in both BMDC and BMM. Under normal conditions in BMM, BMDC and spleen DC (sDC), CatL is almost exclusively found in its fully mature two-chain form albeit at rather modest levels (Fig. 1A). However, exposure to cystatin F levels as low as 5 nm induced a clear accumulation of both the two-chain (p24/25) and the single-chain (p30) form in BMDC (Fig. 1B, left panel) and BMM (Fig. 1B, right panel). Further increases in cystatin F concentration to as little as 10 nm eliminated the two-chain form in BMM and induced a dramatic accumulation of p30 CatL (Fig. 1B, right panel). Quantitation of multiple experiments confirmed that low nm concentrations of cystatin F induced an accumulation of two-chain CatL (more evident in BMDC than in BMM) with higher levels inducing a progressive and dramatic accumulation of single-chain CatL (Fig. 1B, lower panels). CatB and H are also found in both single-chain and two-chain forms and like CatL accumulate in their single-chain forms in the absence of AEP (23). CatB was found mostly in its single-chain form in BMM and BMDC, however exposure to cystatin F did not result in any further accumulation (Fig. 1B). CatH was present in both forms in BMDC but cystatin F had no discernable effect on either its configuration or level. In BMM CatH was mostly present in its pro and single-chain form, which was modestly increased upon exposure to cystatin F (Fig. 1B, right panel). Thus the impact of exposure to cystatin F was rather selective: CatL accumulated and underwent a major change in its configuration while CatB & H were minimally affected. Importantly, the cystatin F-induced accumulation of CatL was not replicated when cells were exposed to similar concentrations of cystatin C even though this inhibitor, like cystatin F, was taken up by cells (supplemental Fig. S1).
FIGURE 1.
Exogenous cystatin F induces the accumulation of single-chain CatL. A, CatL processing in untreated BMM, BMDC, and sDC monitored by SDS-PAGE gel under reducing conditions. CatL migrates at a zymogen (pro), single-chain (SC), and two-chain (TC) form. B, BMDC (left panel) or BMM (right panel) were co-cultured with varying doses of cystatin F (nm) for 48 h. Note accumulation of TC CatL at low cystatin F concentrations and more dramatic SC CatL accumulation at higher concentrations. Quantitative data from 3 (BMDC) or 4 (BMM) experiments are shown below the gel panels (means ± S.E.) (black diamond, SC; white diamond, TC). Processing of CatL, B and H was monitored following exposure to 20 nm cystatin F for different times (C) or when cystatin F was initially present for the times shown but then withdrawn and the cells cultured for a total of 48 h (D). Antibodies to ERK2 or tubulin were used to assess equivalent protein loaded. Data are representative of more than three independent experiments.
We next assessed the kinetics of CatL accumulation. Exposure to cystatin F for 4 h was sufficient to induce the appearance of the single-chain form and by 24 h this was the only form discernable (Fig. 1C). We also tested the effect of transient times of exposure to cystatin F over a 48 h period. As shown in Fig. 1D, cells exposed to cystatin F for 18 h and then cultured in the absence of cystatin F for a further 30 h maintained substantial amounts of CatL single-chain form, while a 24 h exposure followed by 24 h culture in the absence of cystatin F gave a stronger signal. Thus once induced, cystatin F induced CatL single-chain is stable and does not depend on continuous exposure to cystatin F. The accumulation of CatL is similar to what has been observed previously in p41 Ii knock-out macrophages (24, 25). However, the perturbation of CatL levels by cystatin F was not due to indirect effects on p41 invariant chain expression or stability since BMM lacking both p31 and p41 isoforms still accumulated 30 kDa single-chain CatL upon exposure to cystatin F (supplemental Fig. S2).
Exposure to cystatin F might induce CatL accumulation by a number of non-mutually exclusive mechanisms. First, cystatin F might induce increased synthesis of CatL, which might explain or partly explain its accumulation. Second, uptake and activation of cystatin F could lead to blockade of normal CatL single-chain to two-chain conversion. Third, the normal turnover of CatL might be attenuated by cystatin F blockade of one or more of the proteases normally involved in CatL turnover. Fourth, cystatin F might bind to CatL stabilizing it and protecting it from degradation. Given the dramatic and selective accumulation of CatL observed, we decided to investigate these various possibilities.
Activation of Cystatin F Following Uptake by Primary Immune Cells
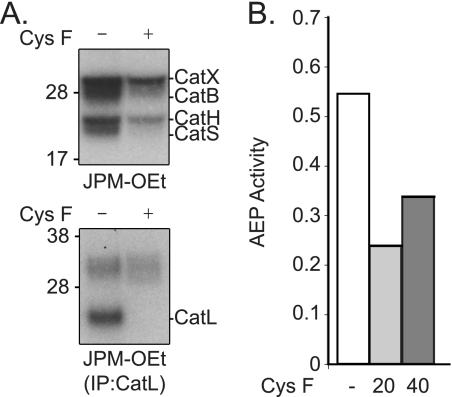
Interestingly, cystatin F exposure induced a modest increase in CatL mRNA levels (supplemental Fig. S3) but this did not reach significance and was not enough to explain the accumulation of CatL observed. To confirm that cystatin F was converted to an active protease inhibitor, we first looked to see if internalized cystatin F was converted from its inactive dimeric form to its active monomeric form. Cystatin F secreted by and purified from 293T cells is exclusively in the dimer configuration (supplemental Fig. S4). As shown previously in fibroblasts (13), cystatin F internalized by BMDC is indeed partially converted to the active monomeric form (supplemental Fig. S4). Next we performed protease active-site labeling studies with 125I-labeled JPM-OEt (34). This reagent is cell permeable and reacts with the active sites of most cysteine cathepsins (34). As shown in Fig. 2A, cathepsins X, B, H, S, and L were readily labeled with this reagent in untreated cells but following exposure to 20 nm cystatin F, labeling of most of cathepsins was substantially reduced. (Note that CatL labeling was analyzed following immunoprecipitation.) Despite the dramatic accumulation of 30kD CatL protein in the presence of cystatin F, no increase in labeling of this form was observed and labeling of the 24/25 kDa form was undetectable. We also measured the level of AEP activity since this protease does not react with 125I-JPM-OEt, but has been shown previously to be a target of type II cystatins (2). Exposure to either 20 or 40 nm cystatin F reduced the activity of AEP in cell lysates by ∼50% (Fig. 2B). Thus, consistent with our earlier studies using fibroblast cell lines, cystatin F enters primary cells, becomes activated and occupies the active site of several lysosomal cysteine proteases.
FIGURE 2.
Exogenous cystatin F inhibits lysosomal protease activity. A, active-site labeling of proteases in BMM in the absence or presence of cystatin F (±Cys F) (top panel). Labeled CatL was immunoprecipitated from BMM lysates and equal concentration of total protein analyzed (bottom panel). B, AEP activity in lysates of BMM left untreated (white bar) or exposed to increasing concentrations of cystatin F (gray bars). Data represent rate of AEP substrate cleavage. Data are representative of at least three independent experiments.
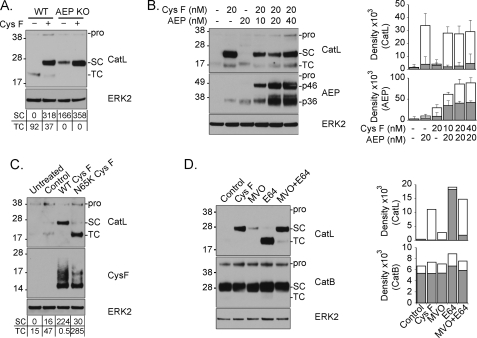
To explore further the possibility that blockade of AEP by cystatin F contributes to p30 CatL accumulation, we repeated the experiment using cells that lack AEP. As already noted, in these cells CatL is already present entirely in the single-chain p30 form (23). Nonetheless, exposure of AEP null BMM to cystatin F induced a further substantial increase in the amount of p30 CatL protein observed (Fig. 3A). Thus cystatin F blockade of AEP may contribute to p30 CatL accumulation but cystatin F must exert its effect by additional mechanisms. Consistent with this, the cystatin F-induced accumulation of p30 CatL could not be reversed by co-incubation with recombinant pro-AEP (56 kDa) which is taken up by cells and activated to its two active forms (46 kDa and 36 kDa; see Fig. 3B and Ref. 28). Quantitation of the levels of both CatL and AEP in cells treated with cystatin F and AEP confirmed accumulation of CatL and also showed that the presence of cystatin F increased the amount of active AEP (Fig. 3B, left and right panels, compare AEP (20 nm) with and without cystatin F (20 nm). This is discussed further below. We probed the contribution of AEP inhibition to CatL accumulation with a further experiment. Cystatins inhibit AEP via a motif distinct from the motif that binds to the active site of cathepsins and mutation of Asn-39 to Lys completely removed the ability of cystatin C to inhibit AEP (2). Incubating BMM with an equivalent dose of the analogous mutant in cystatin F (N65K) (supplemental Fig. S5) no longer resulted in accumulation of p30 CatL but instead resulted in enhanced accumulation of the heavy chain of the two-chain form (Fig. 3C), Thus, CatL accumulates in cystatin F-exposed cells due to at least two distinct effects of cystatin F working through its different domains: first the domain that targets the papain-like cysteine proteases retards normal turnover of the CatL two-chain form. Second, the distinct AEP inhibitory domain retards single-chain to two-chain conversion.
FIGURE 3.
Inhibition of AEP is partially responsible for CatL single-chain accumulation. A, WT and AEP-null (AEP KO) BMM were culture in the absence or presence of 20 nm cystatin F (Cys F) for 48 h, and CatL expression and processing were assessed. B, BMM were cultured in the presence of cystatin F with or without an increasing concentration of recombinant AEP (nm) for 48 h. CatL processing and accumulation of internalized AEP were assessed by immunoblot (left panel) and quantitated (right panel, means + S.E.). Note that 56 kDa pro-AEP is activated following uptake to yield a 46 kDa and a further processed 36 kDa form. C, BMM were left untreated or treated with solvent control (Control), wild type cystatin F (WT Cys F) or an equal concentration of cystatin F mutated in the AEP interaction domain (N65K Cys F) for 48 h. Processing of CatL and internalization of cystatin F was assessed by immunoblot (D) BMM were exposed to either 20 nm cystatin F (Cys F) or membrane permeant small molecule inhibitors of AEP (MVO), cathepsins (E64), or both (MVO+E64) for 24 h. Quantitation of this experiment is shown in the right panel. Equivalent total protein concentrations are shown (ERK2). Data are representative of more than three independent experiments. Numbers beneath gel panels in A and C show raw densities (× 102) for CatL and AEP isoforms.
We attempted to mimic the action of cystatin F by incubation of BMM with small molecule protease inhibitors of AEP and cathepsins. The pan-cathepsin inhibitor E64d did not induce any change in p30 CatL levels although strongly elevated levels of p24/25 form were observed (Fig. 3D). Incubation with the AEP inhibitor MVO26630 induced some increase in the level of p30kD consistent with a role for AEP in p30 to p24/25 conversion however the effect was modest compared with that seen with cystatin F (Fig. 3D). In contrast, exposure to both E64d and MVO26630 induced a substantial accumulation of p30 CatL approximately equivalent to that induced by cystatin F (Fig. 3D). These results confirm that both cystatin F inhibitory activities are necessary for the accumulation of the p30 single-chain form of CatL.
Cystatin F Is Complexed with CatL Following Uptake
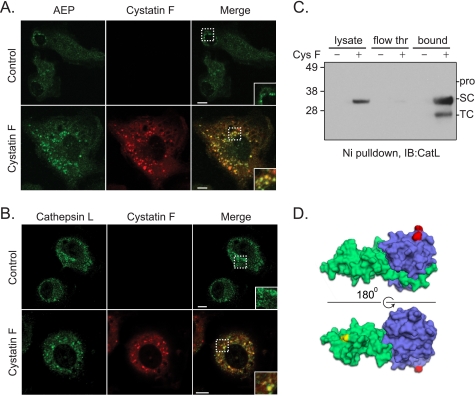
The suppression of active-site cathepsin labeling and reduced AEP activity following cystatin F exposure implied that cystatin F complexed with these cysteine proteases following uptake. To test this directly we examined the intracellular compartments accessed by cystatin F and tested the direct association of internalized cystatin F with these targets. BMM exposed to 20 nm cystatin F accumulated the inhibitor in discrete structures scattered throughout the cytoplasm. Consistent with AEP inhibition, many of these structures also harbored AEP (Fig. 4A, inset). Interestingly, cystatin F exposure increased AEP staining, as already discussed above (Fig. 3B). However, recovery of His-tagged cystatin F on Ni-agarose beads failed to reveal stably associated AEP most likely because the interaction did not persist throughout the precipitation protocol (data not shown). In contrast, internalized cystatin F not only co-localized with CatL (Fig. 4B) but was also recovered bound to the inhibitor (Fig. 4C). These results raised the possibility that cystatin F bound to p30 CatL might interfere with AEP access to the cleavage site that generates the two-chain form of CatL. To assess this possibility we generated a structural model of cystatin F monomer bound at the active site of CatL using the stefin B/CatH structure as a guide. The structure of AEP is not yet available preventing a definitive statement about whether or not its access to CatL would be affected by bound cystatin F. Nonetheless the model demonstrates that the putative cleavage point on CatL (highlighted in red) is distant from bound cystatin F (Fig. 4D) making it unlikely that cystatin F sterically hinders AEP access. Surprisingly, our attempts to test this important point by direct experimentation were unsuccessful. Addition of active AEP to isolated CatL p30 did not result in generation of p24/25 CatL, even in the absence of bound cystatin F (data not shown). Either additional factors are needed for AEP to convert CatL single-chain to two-chain or the conditions for this to occur were not optimal in our in vitro incubations.
FIGURE 4.
AEP and CatL associate with cystatin F following its internalization in macrophages. A, AEP (green) and cystatin F (red) colocalization was monitored by immunofluorescence microscopy in BMM (colocalization in yellow, see inset) following addition of solvent (Control) or 20 nm cystatin F for 24 h. B, CatL (green) and cystatin F (red) colocalization was assessed as described in A. White scale bars, 10 μm. C, Ni/NTA pull-down assay to detect interaction between His-tagged CysF and endogenous CatL using the same conditions described in A. Immunoblot of CatL in the cell lysate (lysate), portion of total protein that did not associate with His-tagged CysF (flow thr), or that which pulled down with His-CysF (bound). D, molecular model of monomeric cystatin F (green) in complex with CatL (blue) based on the stefinB/papain complex (PBD:1STF). The location of the single-chain to two-chain cleavage site is highlighted in red on CatL and the putative site involved in binding to AEP (N65) in yellow on cystatin F.
Cystatin F Is Not the Endogenous Inhibitor of CatL Activity in IFNγ-activated BMM
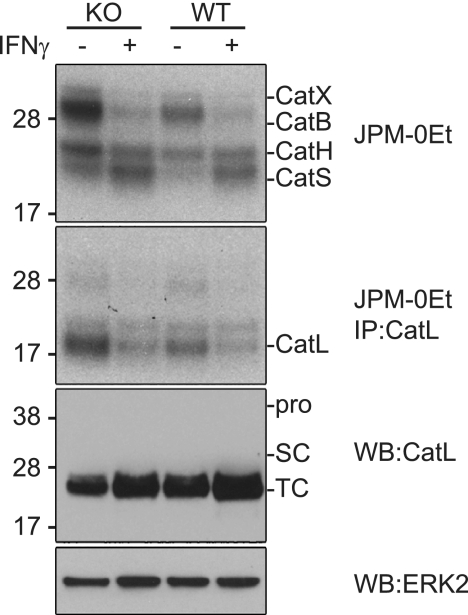
In an earlier study Beers et al. showed that when peritoneal or BMM were activated with IFNγ there was a dramatic loss of CatL activity as measured by an active-site directed probe (26). In contrast CatS activity increased in the same cells. Interestingly, levels of CatL protein were maintained in IFNγ-treated macrophages leading the authors to propose that the loss of CatL enzyme activity may be due to the generation of a specific inhibitor. In the same study it was shown that DC appear to also express a CatL inhibitor since transgenic expression of CatL in DCs resulted in high levels of CatL protein but again, no enzyme activity was detectable with an active-site directed probe. The putative CatL inhibitor (or inhibitors) was not identified in this study, but the authors suggested that it might be cystatin F because of its known expression in hematopoietic cells. This proposal would be consistent with the known ability of cystatin F to inhibit CatL in vitro and with our demonstration here that internalization of cystatin F results in increased CatL levels but loss of activity (Fig. 2A). We recently generated cystatin F null mice (to be fully analyzed elsewhere) giving us the opportunity to test this proposal. We generated BMM from wild type and cystatin F null mice and activated cells with IFNγ for 48 h. We then labeled the intact cells with the active-site directed probe 125I-JPM-OEt and analyzed the labeled proteases by SDS-PAGE. The relative labeling of cathepsins was different to that seen by Beers et al., probably due to the use of a different active-site directed probe and to the fact that we immunoprecipitated CatL following labeling to isolate its signal from the other cathepsins. Consistent with the results of Beers et al., in wild type (WT) cells labeling of CatS was increased in IFNγ-treated cells while labeling of CatL was reduced (Fig. 5, top panel). However, in BMM lacking cystatin F (KO) the results were essentially the same. In other words the loss of CatL labeling seen following IFNγ activation persisted in the absence of cystatin F (Fig. 5). Thus despite the ability of exogenous cystatin F to sequester and stabilize CatL and prevent access to its active site, it does not appear to be the putative inhibitor required to explain the results of Beers et al.
FIGURE 5.
Cystatin F is not responsible for changes in CatL and S activity following IFNγ treatment of BMM. Cystatin F knock-out (KO) and wild-type (WT) BMM were labeled with 125I-JPM-OEt in the absence or presence of 1 ng/ml IFNγ (top panel). Labeled CatL was immunoprecipitated from an equal concentration of total protein in cell extracts (bottom panel). Changes in expression or processing of CatL was assessed by immunoblot. Total protein was assessed by ERK2 immunoblot. Data are representative of more than three independent experiments.
DISCUSSION
The cysteine cathepsins are now known to fulfill many important physiological functions but like all proteases, their activity needs to be carefully regulated. For example, their premature activation in the secretory pathway is prevented by the biosynthesis of inactive pro-forms that generally require the low pH environment of the endo/lysosomal pathway for activation. Active cathepsins that escape the endo/lysosomal pathway and appear in the extracellular environment are thought to be subject to the inhibitory constraints of the cystatins but it has been less clear whether cystatins can attenuate cathepsin activity within the endo/lysosomal pathway and which family members might be involved. Cystatin F has a unique structure and mechanism of activation that suggests it regulates intracellular rather than extracellular protease activity. Unlike all other family members it is made as an inactive disulfide linked dimer and becomes activated following targeting to the endo/lysosome pathway (9, 11, 12). Although in principle activation could be achieved by reduction, high levels of reducing agent are required to convert dimeric cystatin F to its monomeric form in vitro. Our earlier data demonstrated that an N-terminal cleavage event consistent with proteolytic rather than reductive activation was required (9). The novel concept of a cystatin that is itself activated by proteolysis implies an autoregulatory feedback loop: excessive protease levels could convert inactive cystatin F to active cystatin F reducing active protease levels and in turn further cystatin F conversion. Although substantial amounts of cystatin F are retained within cells, a significant proportion is secreted (9, 10, 36). Since this material is inactive and cannot inhibit extracellular proteases, a function proposed for other cystatin family members, the question of the function and fate of secreted cystatin F arises.
Cystatin F is one of three mammalian cystatins to be glycosylated the others being cystatin E/M (5) and cystatin C, produced by rodent neural stem cell cultures (37), and we showed recently that extracellular dimeric cystatin F could be taken up by fibroblasts using the mannose-6-phosphate receptor system (13). Importantly, cystatin F was then activated by the recipient cells. Here we probed the consequences of acquisition of cystatin F in trans in more detail in primary macrophages and DCs. Following uptake and activation, cystatin F induced a remarkable change in the configuration of CatL within both DC and macrophages. Other cathepsin activities were supressed, as determined by active-site labeling, but protein levels did not accumulate to the same extent as CatL. Thus in contrast to the situation with p41 invariant chain, where the two-chain form of CatL was stabilized (24), cystatin F stabilizes the single-chain form of CatL and does so by at least two mechanisms. Cystatin F suppressed cysteine cathepsin activity which slowed the turnover of CatL, while suppression of AEP shifted the configuration of CatL to the single-chain form. In addition it is likely that by complexing with cystatin F, CatL was rendered more stable even though the site of putative AEP cleavage is not likely to be occluded. Cystatin F also boosted the level of AEP in cells when the latter was co-administered. Again, this is likely to be due to suppression of the enzymes that drive AEP turnover. We also tested an earlier prediction that cystatin F was the putative CatL inhibitor induced by IFNγ treatment of macrophages (26) but found no difference between wild type and cystatin F-null cells.
Our data demonstrate that a protease inhibitor can be secreted by one cell and then internalized, activated and used to modulate protease activity in another cell. This cross-feeding phenomenon was previously demonstrated for lysosomal proteases themselves. For example, in the thymus CatL-deficient thymocytes acquired the enzyme from CatL-sufficient thymic epithelial cells. This acquisition was functionally important because it enabled the thymocytes to present CD1-restricted antigen to NKT cells (38). Moreover, it is well documented that lysosomal cysteine proteases are up-regulated in a wide variety of cancers and following relocation to the tumor cell surface and extracellular environment, promote tumor progression (39). For example, in pancreatic islet cancers tumor associated macrophages produced CatS and CatB in an IL-4-dependent manner which promoted tumor progression (40). The source of extracellular cathepsins will be either membrane proximal lysosomes that have fused with the cell surface or cathepsin pro-forms delivered via the secretory pathway. The latter will be stable and likely inactive (at neutral pH) in the extracellular milieu but like cystatin F, can be activated following uptake by a neighboring cell.
The precise physiological consequences of cystatin F acquisition by bystander cells in vivo remain to be clarified. The lysosomal cysteine proteases perform many key physiological functions but their role in various types of pathology has also been well documented. For example, several cysteine cathepsins, including CatL, are collagenolytic and elastinolytic and are believed to contribute to lung damage observed in asthma and COPD (41, 42). CatL has also been implicated in vascular wall remodeling and both AEP and CatL are expressed at higher levels in unstable atherosclerotic plaque (43, 44). As noted above, cathepsins have also been shown to promote tumor progression and CatL has been shown to promote tissue vascularization, beneficially in ischemic tissue but less desirably in the tumor environment (22).
Given all the above examples where elevated cathepsin levels appear to promote pathology it is tempting to speculate that extracellular cystatin F may act in trans to attenuate the activity of enzymes like CatL, S, B, H, and AEP that have all been linked to pathological inflammation and tumor progression. Cystatin F is expressed by many of the immune cells frequently found in the tumor microenvironment and at sites of inflammation such as T cells, NK cells, neutrophils, mast cells, and tumor-associated macrophages. We suggest that cystatin F secretion by these cells might attenuate the undesirable effects of locally produced cysteine proteases. In addition, evidence was recently presented that the single-chain form of CatL lacked the gelatinase activity possessed by the two-chain form (44). Therefore by shifting the configuration of CatL to the single-chain form, cystatin F may diminish the destructive potential of CatL even if it is not directly complexed with cystatin F. Glycosylation-dependent internalization and activation of cystatin F could complement the action of secretory cystatins that engage extracellular proteases directly in the extracellular milieu.
Supplementary Material
Acknowledgments
We thank Drs. Liz Bikoff, Matt Bogyo, and Alex Schuettelkopf for kindly providing invariant chain null bone marrow, JPM-OEt, and the model of cystatin F with CatL, respectively.
This work was supported by a Wellcome Trust Programme grant (to C. W.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S5.
- AEP
- asparagine endopeptidase
- CatL
- cathepsin L
- BMM
- bone marrow-derived macrophage
- BMDC
- bone marrow-derived dendritic cell
- sDC
- spleen-derived dendritic cell.
REFERENCES
- 1. Abrahamson M., Alvarez-Fernandez M., Nathanson C. M. (2003) Biochem. Soc. Symp. 70, 179–199 [DOI] [PubMed] [Google Scholar]
- 2. Alvarez-Fernandez M., Barrett A. J., Gerhartz B., Dando P. M., Ni J., Abrahamson M. (1999) J. Biol. Chem. 272, 19195–19203 [DOI] [PubMed] [Google Scholar]
- 3. Zeeuwen P. L., van Vlijmen-Willems I. M., Hendriks W., Merkx G. F., Schalkwijk J. (2002) Hum. Mol. Genet. 11, 2867–2875 [DOI] [PubMed] [Google Scholar]
- 4. Olafsson I., Grubb A. (2000) Amyloid 7, 70–79 [DOI] [PubMed] [Google Scholar]
- 5. Ni J., Abrahamson M., Zhang M., Fernandez M. A., Grubb A., Su J., Yu G. L., Li Y., Parmelee D., Xing L., Coleman T. A., Gentz S., Thotakura R., Nguyen N., Hesselberg M., Gentz R. (1997) J. Biol. Chem. 272, 10853–10858 [DOI] [PubMed] [Google Scholar]
- 6. Zeeuwen P. L., Van Vlijmen-Willems I. M., Jansen B. J., Sotiropoulou G., Curfs J. H., Meis J. F., Janssen J. J., Van Ruissen F., Schalkwijk J. (2001) J. Invest. Dermatol. 116, 693–701 [DOI] [PubMed] [Google Scholar]
- 7. Ni J., Fernandez M. A., Danielsson L., Chillakuru R. A., Zhang J., Grub A., Su J., Gentz R., Abrahamson M. (1998) J. Biol. Chem. 273, 24797–24804 [DOI] [PubMed] [Google Scholar]
- 8. Halfon S., Ford J., Foster J., Dowling L., Lucian L., Sterling M., Xu Y., Weiss M., Ikeda M., Liggett D., Helms A., Caux C., Lebecque S., Hannum C., Menon S., McClanahan T., Gorman D., Zurawski G. (1998) J. Biol. Chem. 273, 16400–16408 [DOI] [PubMed] [Google Scholar]
- 9. Hamilton G., Colbert J. D., Schuettelkopf A. W., Watts C. (2008) EMBO J. 27, 499–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cappello F., Gatti E., Camossetto V., David A., Lelouard H., Pierre P. (2004) Exp. Cell Res. 297, 607–618 [DOI] [PubMed] [Google Scholar]
- 11. Schüttelkopf A. W., Hamilton G., Watts C., van Aalten D. M. (2006) J. Biol. Chem. 281, 16570–16575 [DOI] [PubMed] [Google Scholar]
- 12. Langerholc T., Zavasnik-Bergant V., Turk B., Turk V., Abrahamson M., Kos J. (2005) Febs. J. 272, 1535–1545 [DOI] [PubMed] [Google Scholar]
- 13. Colbert J. D., Plechanovová A., Watts C. (2009) Traffic 10, 425–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vasiljeva O., Reinheckel T., Peters C., Turk D., Turk V., Turk B. (2007) Curr. Pharm. Des. .13, 387–403 [DOI] [PubMed] [Google Scholar]
- 15. Colbert J. D., Matthews S. P., Miller G., Watts C. (2009) Eur. J. Immunol. 39, 2955–2965 [DOI] [PubMed] [Google Scholar]
- 16. Bird P. I., Trapani J. A., Villadangos J. A. (2009) Nat. Rev. Immunol. 9, 871–882 [DOI] [PubMed] [Google Scholar]
- 17. Reiser J., Adair B., Reinheckel T. (2010) J. Clin. Invest. 120, 3421–3431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reinheckel T., Hagemann S., Dollwet-Mack S., Martinez E., Lohmüller T., Zlatkovic G., Tobin D. J., Maas-Szabowski N., Peters C. (2005) J. Cell Sci. 118, 3387–3395 [DOI] [PubMed] [Google Scholar]
- 19. Roth W., Deussing J., Botchkarev V. A., Pauly-Evers M., Saftig P., Hafner A., Schmidt P., Schmahl W., Scherer J., Anton-Lamprecht I., Von Figura K., Paus R., Peters C. (2000) FASEB J. 14, 2075–2086 [DOI] [PubMed] [Google Scholar]
- 20. Nakagawa T., Roth W., Wong P., Nelson A., Farr A., Deussing J., Villadangos J. A., Ploegh H., Peters C., Rudensky A. Y. (1998) Science 280, 450–453 [DOI] [PubMed] [Google Scholar]
- 21. Petermann I., Mayer C., Stypmann J., Biniossek M. L., Tobin D. J., Engelen M. A., Dandekar T., Grune T., Schild L., Peters C., Reinheckel T. (2006) FASEB J. 20, 1266–1268 [DOI] [PubMed] [Google Scholar]
- 22. Urbich C., Heeschen C., Aicher A., Sasaki K., Bruhl T., Farhadi M. R., Vajkoczy P., Hofmann W. K., Peters C., Pennacchio L. A., Abolmaali N. D., Chavakis E., Reinheckel T., Zeiher A. M., Dimmeler S. (2005) Nat. Med. 11, 206–213 [DOI] [PubMed] [Google Scholar]
- 23. Shirahama-Noda K., Yamamoto A., Sugihara K., Hashimoto N., Asano M., Nishimura M., Hara-Nishimura I. (2003) J. Biol. Chem. 278, 33194–33199 [DOI] [PubMed] [Google Scholar]
- 24. Lennon-Duménil A. M., Roberts R. A., Valentijn K., Driessen C., Overkleeft H. S., Erickson A., Peters P. J., Bikoff E., Ploegh H. L., Wolf Bryant P. (2001) EMBO J. 20, 4055–4064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fiebiger E., Maehr R., Villadangos J., Weber E., Erickson A., Bikoff E., Ploegh H. L., Lennon-Duménil A. M. (2002) J. Exp. Med. 196, 1263–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beers C., Honey K., Fink S., Forbush K., Rudensky A. (2003) J. Exp. Med. 197, 169–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kos J., Smid A., Krasovec M., Svetic B., Lenarcic B., Vrhovec I., Skrk J., Turk V. (1995) Biol. Chem. Hoppe Seyler 376, 401–405 [DOI] [PubMed] [Google Scholar]
- 28. Li D. N., Matthews S. P., Antoniou A. N., Mazzeo D., Watts C. (2003) J. Biol. Chem. 278, 38980–38990 [DOI] [PubMed] [Google Scholar]
- 29. Loak K., Li D. N., Manoury B., Billson J., Morton F., Hewitt E., Watts C. (2003) Biol. Chem. 384, 1239–1246 [DOI] [PubMed] [Google Scholar]
- 30. West M. A., Wallin R. P., Matthews S. P., Svensson H. G., Zaru R., Ljunggren H. G., Prescott A. R., Watts C. (2004) Science 305, 1153–1157 [DOI] [PubMed] [Google Scholar]
- 31. Racoosin E. L., Swanson J. A. (1989) J. Exp. Med. 170, 1635–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Matthews S. P., Werber I., Deussing J., Peters C., Reinheckel T., Watts C. (2010) J. Immunol. 184, 2423–2431 [DOI] [PubMed] [Google Scholar]
- 33. Bogyo M., Baruch A., Jeffery D., Greenbaum D., Borodovsky A., Ovaa H., Kessler B. (2004) in Current Protocols in Protein Science, Chapter 21, Unit 21.17, John Wiley, New York: [DOI] [PubMed] [Google Scholar]
- 34. Bogyo M., Verhelst S., Bellingard-Dubouchaud V., Toba S., Greenbaum D. (2000) Chem. Biol. 7, 27–38 [DOI] [PubMed] [Google Scholar]
- 35. Chen J. M., Dando P. M., Rawlings N. D., Brown M. A., Young N. E., Stevens R. A., Hewitt E., Watts C., Barrett A. J. (1997) J-Biol-Chem. 272, 8090–8098 [DOI] [PubMed] [Google Scholar]
- 36. Nathanson C. M., Wassélius J., Wallin H., Abrahamson M. (2002) Eur. J. Biochem. 269, 5502–5511 [DOI] [PubMed] [Google Scholar]
- 37. Taupin P., Ray J., Fischer W. H., Suhr S. T., Hakansson K., Grubb A., Gage F. H. (2000) Neuron 28, 385–397 [DOI] [PubMed] [Google Scholar]
- 38. Honey K., Benlagha K., Beers C., Forbush K., Teyton L., Kleijmeer M. J., Rudensky A. Y., Bendelac A. (2002) Nat. Immunol. 3, 1069–1074 [DOI] [PubMed] [Google Scholar]
- 39. Mohamed M. M., Sloane B. F. (2006) Nat. Rev. Cancer 6, 764–775 [DOI] [PubMed] [Google Scholar]
- 40. Gocheva V., Wang H. W., Gadea B. B., Shree T., Hunter K. E., Garfall A. L., Berman T., Joyce J. A. (2010) Genes Dev. 24, 241–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bühling F., Röcken C., Brasch F., Hartig R., Yasuda Y., Saftig P., Brömme D., Welte T. (2004) Am. J. Pathol. 164, 2203–2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shapiro S. D. (2002) Biochem. Soc. Trans. 30, 98–102 [DOI] [PubMed] [Google Scholar]
- 43. Li W., Kornmark L., Jonasson L., Forssell C., Yuan X. M. (2009) Atherosclerosis 202, 92–102 [DOI] [PubMed] [Google Scholar]
- 44. Mattock K. L., Gough P. J., Humphries J., Burnand K., Patel L., Suckling K. E., Cuello F., Watts C., Gautel M., Avkiran M., Smith A. (2010) Atherosclerosis 208, 83–89 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.