Background: We hypothesize that mice heterozygous for disruption of cystathionine β-synthase (Cbs+/−) are susceptible to obesity-related cardiolipotoxicity because of impaired liver glutathione synthesis.
Results: Cbs+/− mice with diet-induced obesity have augmented cardiac lipotoxicity and disturbances in glutathione homeostasis.
Conclusion: Cbs maintains heart glutathione homeostasis and protects against cardiolipotoxicity.
Significance: Cbs plays a role in the pathology of cardiolipotoxicity.
Keywords: Glutathione, Heart, Lipotoxicity, Metabolism, Obesity, Triglyceride, Cysteine
Abstract
Obesity-related cardiac lipid accumulation is associated with increased myocardial oxidative stress. The role of the antioxidant glutathione in cardiac lipotoxicity is unclear. Cystathionine β-synthase (Cbs) catalyzes the first step in the trans-sulfuration of homocysteine to cysteine, which is estimated to provide ∼50% of cysteine for hepatic glutathione biosynthesis. As cardiac glutathione is a reflection of the liver glutathione pool, we hypothesize that mice heterozygous for targeted disruption of Cbs (Cbs+/−) are more susceptible to obesity-related cardiolipotoxicity because of impaired liver glutathione synthesis. Cbs+/+ and Cbs+/− mice were fed a high fat diet (60% energy) from weaning for 13 weeks to induce obesity and had similar increases in body weight and body fat. This was accompanied by increased hepatic triglyceride but no differences in hepatic glutathione levels compared with mice fed chow. However, Cbs+/− mice with diet-induced obesity had greater glucose intolerance and lower total and reduced glutathione levels in the heart, accompanied by lower plasma cysteine levels compared with Cbs+/+ mice. Higher triglyceride concentrations, increased oxidative stress, and increased markers of apoptosis were also observed in heart from Cbs+/− mice with diet-induced obesity compared with Cbs+/+ mice. This study suggests a novel role for Cbs in maintaining the cardiac glutathione pool and protecting against cardiac lipid accumulation and oxidative stress during diet-induced obesity in mice.
Introduction
Cardiac lipid accumulation is a major consequence of obesity in human subjects and animal models (1, 2). Studies over the last 2 decades have unraveled signaling events underlying “cardiolipotoxicity” and the resulting oxidative stress and cardiac dysfunction (3, 4). However, the role of the endogenously synthesized antioxidant glutathione and factors controlling its homeostasis in cardiolipotoxicity remain unclear. Glutathione is a sulfur-containing tripeptide that functions to protect against oxidative stress, especially in the heart (5). Given the minimal level of catalase in the heart, hydrogen peroxide, a major reactive oxygen species, is mainly neutralized by glutathione peroxidase and the reduced form of glutathione, GSH (6).
Glutathione synthesis involves the sequential steps of complexing cysteine and glutamate to form γ-glutamylcysteine, catalyzed by glutamate cysteine ligase (GCL),3 followed by the addition of glycine to form glutathione, accomplished by glutathione synthase. Cysteine is considered the rate-limiting amino acid for glutathione synthesis (7). Although the importance of oxidative stress has been shown in multiple tissues affected by lipotoxicity (8–10), the role of cysteine provision and its capacity to maintain glutathione levels in the heart during lipotoxicity is not known.
Cysteine can be obtained from exogenous sources (diet and supplements) or endogenously from intracellular protein catabolism and homocysteine trans-sulfuration (11). Supplemental N-acetylcysteine increases plasma cysteine and tissue glutathione concentrations and is protective in conditions associated with oxidative stress such as diabetes (12), ischemia reperfusion (13), and in obesity-related fatty liver disease (14). It has been estimated that at least 50% of the cysteine required for endogenous glutathione synthesis in the liver is supplied by the trans-sulfuration of homocysteine (15). The first and rate-limiting step in this pathway involves conversion of homocysteine to cystathionine, catalyzed by cystathionine β-synthase (Cbs), followed by conversion to cysteine, accomplished by cystathionase. Current views suggest that the glutathione pool in the liver is responsible for maintaining glutathione concentrations in other tissues such as the heart (16). For example, in studies using chronic bile duct ligated rats, cholestatic liver disease led to diminished glutathione levels in the liver (17, 18) and subsequently in heart, brain, and kidney (18). Therefore, disturbances in liver glutathione synthesis could have profound consequences on glutathione concentrations in other tissues and impair the ability to combat oxidative stress.
The goal of this study is to test the hypothesis that mice with targeted disruption of the gene for Cbs (Cbs+/− mice) are more susceptible to cardiac lipotoxicity because of impaired synthesis of liver glutathione. We demonstrate that Cbs+/− mice with diet-induced obesity (fed a high fat diet) have greater glucose intolerance, disturbances in glutathione homeostasis with increased triglyceride accumulation, enhanced oxidative stress, and pro-apoptotic signaling in heart despite no differences in liver glutathione levels compared with Cbs+/+ mice with diet-induced obesity. We attribute these findings to our demonstration that plasma cysteine levels are lower in Cbs+/− mice with diet-induced obesity suggesting that Cbs and plasma cysteine may play roles in regulating the pool of glutathione in the heart, especially under conditions of oxidative stress, such as that observed in diet-induced obesity.
EXPERIMENTAL PROCEDURES
Animals
C57BL/6 mice heterozygous for targeted disruption of the gene for Cbs (+/−) and mice without (Cbs+/+) were studied. Genotyping for the disrupted Cbs allele, Cbstm1Unc, and the wild-type allele was conducted by PCR as described (19). Cbs+/− and Cbs+/+ mice were fed a high fat diet (HFD) from weaning for 13 weeks. The HFD contained 60% energy from fat (mix of 30:30:30:10 of butter/lard/shortening/soybean oil, respectively), 20% energy from protein (vitamin-free casein) plus 3 g/kg cysteine, and 20% energy from carbohydrate providing 5250 kcal/kg (see Table 1). The HFD also contained 10 g/kg of AIN-93 vitamin mix (Harlan Teklad), 35 g/kg of AIN-93 mineral mix (Harlan Teklad), and 2.5 g/kg choline bitartrate to provide vitamin and minerals at levels that meet National Research Council requirements for mice (20, 21). We also assessed Cbs+/+ and Cbs+/− mice fed a standard laboratory chow (Pico-Vac Lab Rodent Diet, LabDiet, PMI Nutrition International, St. Louis, MO) for 13 weeks to demonstrate diet-induced obesity in the mice fed the HFD and to compare the effects of diet-induced obesity between Cbs+/+ and Cbs+/− mice. We recognize that in addition to differences in macronutrient composition and total energy provision, additional unknown variables present in the chow diet compared with the HFD is a caveat for the interpretation of the findings of the study. An equal mix of male and female mice was studied in each diet/Cbs genotype group except where otherwise stated. At the end of the feeding period, mice were anesthetized with isofluorane, and blood was collected by cardiac puncture into EDTA (final concentration, 5 mm). Blood was immediately centrifuged at 3000 × g for 20 min at 4 °C, and plasma was collected and stored at −80 °C until later analysis. Samples of liver and heart were immediately flash-frozen in liquid nitrogen and stored at −80 °C for later studies. The protocol was approved by the University of British Columbia Animal Care Committee.
TABLE 1.
Energy content of high fat diet used to induced obesity in Cbs+/+ and Cbs+/− mice
| Chow | HFD | |
|---|---|---|
| Total energy (kcal/kg) | 4020 | 5250 |
| Carbohydrates (% total energy) | 64.5 | 20.0 |
| Protein (% total energy) | 23.6 | 20.0 |
| Fat (% total energy) | 11.9 | 60.0 |
Glucose Tolerance
At 1–2 days prior to the end of the feeding period, a subset of male mice (n = 5–6 per diet/genotype group) underwent an intraperitoneal glucose tolerance test to assess glucose tolerance (22). Following a 5-h fast, mice were given an i.p. injection of glucose (2 g/kg body weight). Blood (20 μl) was collected from the saphenous vein at 0 min (base line) and at 15, 30, 60, 90, and 120 min post-glucose injection. Blood glucose levels were quantified using a blood glucose meter (Breeze2, Bayer). Fasting insulin levels were quantified in the base-line blood samples using the mouse insulin ultrasensitive ELISA (Alpco Diagnostics).
Biochemical Analyses
Total glutathione (reduced (GSH) + oxidized (GSSG)) and GSH concentrations and glutathione reductase activity were quantified using commercial kits (Trevigen). Oxidative stress-induced lipid peroxidation was estimated by direct quantification of lipid hydroperoxides in heart tissue using a commercial kit (Cayman Chemicals) as described previously (23). Plasma triglycerides were quantified by a commercial colorimetric kit (Wako Diagnostics). Protein concentrations were determined using a commercial protein assay kit (Bio-Rad) based on the method of Bradford (24).
Total lipids were extracted from liver and heart by the method of Folch et al. (25). The organic phase was evaporated under nitrogen; the lipids were solubilized in chloroform/methanol/acetone/hexane (2.0:3.0:0.5:0.5, v/v), and individual classes of lipids were separated by a 2690 Alliance HPLC (Waters). The separated lipid classes were detected and quantified by evaporative light scattering detection (model 2000, Alltech, Mandel Scientific, Guelph, Canada) as described previously (26). Plasma total homocysteine, cysteine, and methionine were quantified by HPLC-MS/MS as described previously (27). Quantification of tissue lipids and plasma thiols were conducted in the Metabolomics Core of the Nutrition and Metabolism Research Program (directed by Dr. S. Innis) at the Child and Family Research Institute.
Immunoblotting
Sections of the heart ventricles were homogenized in ice-cold homogenization buffer with 50 mm Tris-HCl, pH 7.5, 0.5 mm PMSF, 50 μg/ml leupeptin, 1 mm EDTA, 1 mm EGTA, and 3 μm pepstatin A for 30 s, followed by centrifugation at 5,000 × g for 10 min at 4 °C. The pellets were discarded, and the concentration of the supernatant was determined using a commercial protein assay kit (Bio-Rad) based on the method of Bradford (24). Samples (50 μg of protein) were denatured and separated by SDS-PAGE (10%) followed by electrotransfer to nitrocellulose membranes and immunoblotting as described (23). The following primary antibodies were used to detect the respective proteins: goat anti-human GCLc (Santa Cruz Biotechnology (sc)-2267), goat anti-human Gpx-1 (sc-22146), rabbit anti-human acyl CoA oxidase 1 (Acox-1) (sc-98499), rabbit anti-human peroxisome proliferator-activated receptor γ coactivator-1α (Pgc-1α) (sc-13067), rabbit anti-human β-actin (sc-130657), rabbit anti-nitrotyrosine (sc-55256), mouse anti-human X-linked inhibitor of apoptotic factors (XIAP) (BD Biosciences), rabbit anti-human cleaved caspase-3 (Cell Signaling Technologies), rabbit anti-human NF-κB p65 subunit (sc-372), rabbit anti-human IκB (sc-371), and phosphorylated IκB (sc-101713). The secondary antibodies used were mouse anti-goat (sc-2355), goat anti-mouse (sc-2047), and donkey anti-rabbit (sc-2315) antibodies conjugated to alkaline phosphatase. For loading controls, rabbit anti-human β-actin (sc-130656) was used. Two different blots were used for the study, and corresponding actin bands are depicted as actin-1 (blot 1) and actin-2 (blot 2) in figures. Gpx-1, caspase-3, PGC-1, IκB, and p-IκB were probed on blot 1 using Restore Plus stripping buffer (Thermo Scientific) for 10 min each time. ACOX, GCLc, NF-κB p65 subunit, nitrotyrosine, and XIAP were probed on blot 2. Detection was accomplished using the Amersham Biosciences ECL kit and visualization and quantification of band density using a Chemigenius system (Syngene, MD). The obtained images were analyzed using Image J (National Institutes of Health) and expressed as a ratio to β-actin levels.
Mitochondrial Density
Mitochondrial density was estimated by quantifying the mitochondrion-encoded cytochrome b gene (mt-Cytb) copy number relative to the nuclear encoded β-actin gene (Actb) copy number using real time PCR (28). Genomic DNA was extracted from heart using the DNeasy kit (Qiagen). The following primers were used: for mt-Cytb, CytbF, 5′-CCACTTCATCTTACCATTTATTATCGC-3′, and CytbR, 5′-TTTTATCTGCATCTGAGTTTAATCCTGT-3′; and for Actb, ActbF, 5′-CTGCCTGACGGCCAGG-3′, and ActbR, 5′-GGAAAAGAGCCTCAGGGCAT-3′. The following FAM-labeled probes were used: for mt-Cytb, cytbFAM, 5′-FAM-AGCAATCGTTCACCTCCTCTTCCTCCAC-3′,and for Actb, ActbFAM, 5′-FAM-CATCACTATTGGCAACGAGCGGTTCC-3′. Copy numbers were quantified using TaqMan PCR reagents and an ABI 7500 real time PCR system (Applied Biosystems).
Statistical Analyses
Two-way analysis of variance (ANOVA) was used to determine the effect of the diet and Cbs genotype. If significant interactions were found, the effect of diet was assessed separately for each Cbs genotype by one-way ANOVA. Analyses were accomplished by SPSS Version 16.0 (SPSS Inc., Chicago).
RESULTS
Diet-induced Obesity and Greater Glucose Intolerance in Cbs+/− Mice
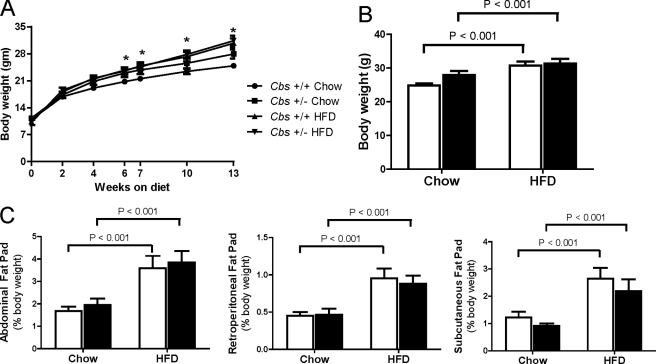
We assessed the effects of feeding a HFD (60% energy from fat) from weaning for 13 weeks to induce obesity in Cbs+/+ and Cbs+/− mice. Cbs+/+ and Cbs+/− mice fed the HFD had similar weight gain (p < 0.01) and increases (p < 0.01) in subcutaneous (inguinal), abdominal (gonadal), and retroperitoneal fat pads compared with Cbs+/+ and Cbs+/− mice fed chow (Fig. 1). These findings demonstrate that Cbs+/− mice are susceptible to diet-induced obesity to a similar extent as Cbs+/+ mice.
FIGURE 1.
Weight gain and adiposity in Cbs+/+ and Cbs+/− mice fed HFD from weaning for 13 weeks to induce obesity. A, body weight gain from weaning for 13 weeks. B, terminal body weight at 13 weeks. C, abdominal (gonadal), retroperitoneal, and subcutaneous (inguinal) fat pad weight at the end of the 13-week feeding period. Data are presented as means ± S.E., n = 9–20 mice per diet/genotype group. Open bars, Cbs+/+ mice; filled bars, Cbs+/− mice. Significance was determined by two-way ANOVA.
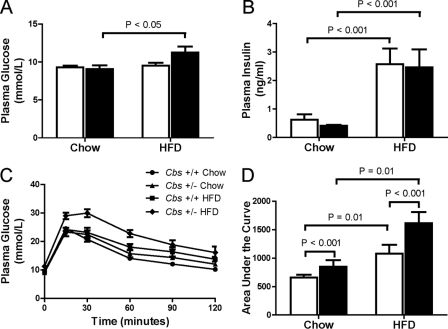
As reported for C57BL/6J mice (22), we found that Cbs+/+ and Cbs+/− mice fed the HFD had similar elevations (p < 0.001) in fasting plasma insulin levels than mice fed chow. However, only Cbs+/− mice fed the HFD had moderately elevated fasting plasma glucose levels (Fig. 2, A and B). Although both Cbs+/+ mice and Cbs+/− mice fed the HFD had mild glucose intolerance, this was more pronounced in Cbs+/− mice (Fig. 2, C and D). The area under the curve for plasma glucose levels during the 2-h intraperitoneal glucose tolerance test was greater (p < 0.001) in Cbs+/− mice than Cbs+/+ mice, and this was further amplified in Cbs+/− mice fed the HFD (Fig. 2D).
FIGURE 2.
Impaired glucose tolerance in Cbs+/− mice with diet-induced obesity. Fasting base-line plasma glucose (A) and insulin (B) are shown. C, plasma glucose concentrations following an i.p. injection of 2 g/kg glucose. D, area under the curve for plasma glucose levels during the 2-h intraperitoneal glucose tolerance test. Data are presented as means ± S.E., n = 5–6 mice per diet/genotype group. Open bars, Cbs+/+ mice; filled bars, Cbs+/− mice. Significance was determined by two-way ANOVA.
Cardiac Lipotoxicity Is Augmented in Cbs+/− Mice with Diet-induced Obesity
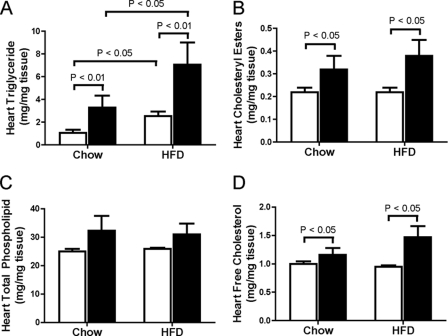
To address our hypothesis that Cbs+/− mice are more susceptible to cardiac lipotoxicity, we first quantified major lipid classes in the heart. Cbs+/− mice had higher (p < 0.05) concentrations of triglyceride, cholesteryl ester, free cholesterol, phosphatidylinositol, phosphatidylserine, lysophosphatidylcholine, and sphingosine than Cbs+/+ mice (Fig. 3 and Table 2). As expected, triglyceride concentrations in heart were higher (p < 0.05) in mice with diet-induced obesity, and this occurred to a greater extent (p < 0.01) in Cbs+/− mice compared with Cbs+/+ mice (Fig. 3A).
FIGURE 3.
Lipid accumulation in heart from Cbs+/− mice with diet-induced obesity. Triglyceride (A), cholesteryl ester (B), total phospholipids (C), and free cholesterol (D) concentrations in heart from Cbs+/− and Cbs+/− mice fed chow or the HFD. Data is presented as means ± S.E., n = 5–6 mice per diet/genotype group. Open bars, Cbs+/+ mice; filled bars, Cbs+/− mice. Significance was determined by two-way ANOVA.
TABLE 2.
Changes in phospholipids in heart from mice with diet-induced obesity
Values shown are means ± S.E.
| Lipid classes (mg/mg tissue) | Chow |
HFD |
||
|---|---|---|---|---|
| Cbs+/+ (n = 4–5) | Cbs+/− (n = 5) | Cbs+/+ (n = 6) | Cbs+/− (n = 5–6) | |
| Phosphatidylcholine | 9.88 ± 0.29 | 12.04 ± 1.63 | 9.87 ± 0.14 | 11.28 ± 1.42 |
| Phosphatidylethanolamine | 6.04 ± 0.38 | 7.10 ± 0.85 | 7.63 ± 0.12 | 7.73 ± 1.16 |
| Phosphatidylinositol | 1.14 ± 0.04 | 1.46 ± 0.22a | 1.13 ± 0.02 | 1.48 ± 0.16a |
| Phosphatidylserine | 1.04 ± 0.07 | 1.56 ± 0.31a | 0.87 ± 0.06 | 1.45 ± 0.29a |
| Lysophosphatidylcholine | 2.42 ± 0.13 | 4.28 ± 1.34a | 1.93 ± 0.11 | 3.73 ± 0.95a |
| Sphingosine | 0.98 ± 0.05 | 1.36 ± 0.27a | 0.80 ± 0.04 | 1.35 ± 0.23a |
| Cardiolipin | 3.54 ± 0.16 | 4.54 ± 0.68 | 3.63 ± 0.11 | 3.95 ± 0.63 |
a p < 0.05 versus Cbs+/+ mice, as determined by two-way analysis of variance.
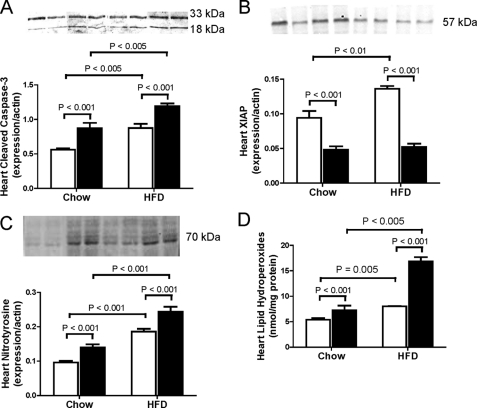
We further determined whether the greater triglyceride deposition in heart from Cbs+/− mice with diet-induced obesity was accompanied by indicators of lipotoxicity by assessing markers of apoptosis, oxidative stress, and mitochondrial dysfunction. To assess apoptosis, we quantified expression of cleaved caspase-3 and XIAP. Levels of cleaved caspase-3 were higher (p < 0.001) and levels of XIAP were lower (p < 0.001) in Cbs+/− mice than Cbs+/+ mice (Fig. 4, A and B). Levels of cleaved caspase-3 were higher (p < 0.005) in both groups of mice fed the HFD (Fig. 4A), whereas levels of XIAP were only increased in Cbs+/+ mice fed the HFD (Fig. 4B). We quantified levels of nitrotyrosine and lipid hydroperoxides as indicators of oxidative stress and found that Cbs+/− mice had higher (p < 0.001) levels of nitrotyrosine and hydroperoxides in heart than Cbs+/+ mice and that diet-induced obesity was associated with higher (p < 0.005) levels of nitrotyrosine and hydroperoxides in both groups of mice (Fig. 4, C and D).
FIGURE 4.
Enhanced apoptosis and oxidative stress in heart from Cbs+/− mice with diet-induced obesity. Markers of apoptosis were assessed by quantifying expression levels of cleaved caspase-3 (A) and XIAP (B). Indicators of oxidative stress were assessed by quantifying expression of nitrotyrosine (C) and lipid hydroperoxides (D). Data are presented as means ± S.E., n = 5–6 mice per diet/genotype group. Open bars, Cbs+/+ mice; filled bars, Cbs+/− mice. Inset depicts representative immunoblots for analyzed proteins in comparison with β-actin bands from the same blot. Significance was determined by two-way ANOVA.
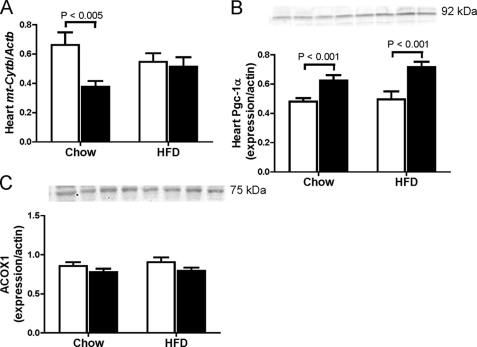
Effects of the increased lipid deposition in Cbs+/− mice with diet-induced obesity on mitochondrial damage were assessed by quantifying the mitochondrion-encoded cytochrome b gene (mt-Cytb) copy number relative to the nucleus-encoded β-actin gene (Actb) and by assessing protein expression of Pgc-1α, a prime regulator of mitochondrial biogenesis in heart (29). We found that in chow-fed mice, Cbs+/− mice had less (p = 0.005) copies of mt-Cytb/Actb than Cbs+/+ mice, but no effect of diet-induced obesity was observed (Fig. 5A). We also found higher (p < 0.001) levels of Pgc-1α in heart from Cbs+/− mice compared with Cbs+/+ mice, and this was also unaffected by diet (Fig. 5B). This suggests that Pgc-1α expression is up-regulated as a compensatory mechanism to induce mitochondrial biogenesis (30). We further assessed if the higher levels of Pgc-1α in Cbs+/− mice were accompanied by altered expression of Acox1, required for peroxisomal fatty acid β-oxidation (31), we found no effect of Cbs genotype or diet-induced obesity on Acox1 protein levels in heart (Fig. 5C). Together, these findings suggest that Cbs+/− mice demonstrate mitochondrion-specific damage and are more susceptible to obesity-related cardiac lipotoxicity and oxidative stress.
FIGURE 5.
Mitochondrial dysfunction in heart from Cbs+/− mice with diet-induced obesity. Mitochondrial dysfunction was assessed by quantifying the mitochondrion-encoded cytochrome b gene (mt-Cytb) copy number relative to the nucleus-encoded β-actin gene (Actb) copy number (A) and by assessing expression of Pgc-1α (B). C, expression of Acox-1. Data are presented as means ± S.E., n = 5–6 mice per diet/genotype group. Open bars, Cbs+/+ mice; filled bars, Cbs+/− mice. Inset depicts representative immunoblots for analyzed proteins in comparison with β-actin bands from the same blot. Significance was determined by two-way ANOVA.
Cardiac Lipotoxicity in Cbs+/− Mice with Diet-induced Obesity Is Accompanied by Decreased Glutathione Status and Decreased NF-κB Signaling in Heart
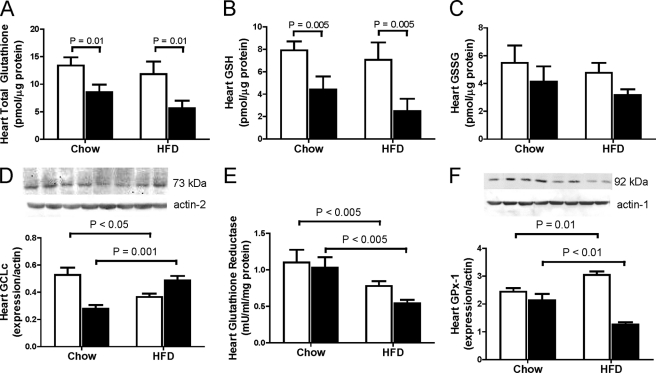
As a first step toward assessing the mechanisms underlying the augmented cardiac lipotoxicity and oxidative stress in Cbs+/− mice with diet-induced obesity, we assessed changes in cardiac glutathione homeostasis. We found that Cbs+/− mice had lower levels of total glutathione (GSH + GSSG) (p < 0.01) and GSH (p = 0.005) in heart compared with Cbs+/+ mice, an effect observed in both chow-fed and HFD-fed mice (Fig. 6, A--C). To investigate this further, we assessed expression of proteins involved in glutathione synthesis and metabolism. Synthesis of glutathione is catalyzed by the rate-limiting enzyme GCL, a heterodimer that consists of a catalytic subunit (GCLc) and a modifier subunit (GCLm), with GCLc activity up-regulated by oxidative stress (32). We found that the effect of the HFD on the expression of GCLc in heart was dependent on Cbs genotype. Cbs+/− mice fed the HFD had higher (p = 0.001) levels of GCLc expression in heart compared with those fed chow, whereas GCLc expression in heart was lower (p < 0.05) in Cbs+/+ mice fed the HFD compared with those fed chow (Fig. 6C). An additional pathway important in maintaining tissue glutathione homeostasis is recycling of GSSG to GSH, catalyzed by glutathione reductase (33). Cbs+/+ and Cbs+/− mice fed the HFD had lower (p < 0.005) levels of glutathione reductase activity compared with those fed chow (Fig. 6E). We also assessed expression of GPx-1, an antioxidant enzyme that utilizes GSH (5). Similar to what we observed for GCLc expression, the effect of the HFD on GPx-1 expression in heart was also dependent on Cbs genotype, with lower (p < 0.01) GPx-1 expression in Cbs+/− mice and higher (p = 0.01) expression in Cbs+/+ mice fed the HFD compared with their respective genotypes fed chow (Fig. 6F). Taken together, these findings suggest that a reduced pool of glutathione and reduced ability to utilize glutathione as an antioxidant in the heart may contribute to the enhanced cardiac lipotoxicity in Cbs+/− mice with diet-induced obesity.
FIGURE 6.
Disturbances in glutathione metabolism in heart from Cbs+/− mice with diet-induced obesity. Glutathione metabolism in heart was assessed by quantifying levels of total glutathione (A), GSH (B), GSSG (C), Gclc expression (D), glutathione reductase activity (E), and Gpx-1 expression (F). Data are presented as means ± S.E., n = 5 mice per diet/genotype group. Open bars, Cbs+/+ mice; filled bars, Cbs+/− mice. Inset depicts representative immunoblots for analyzed proteins in comparison with β-actin bands from the same blot. Significance was determined by two-way ANOVA.
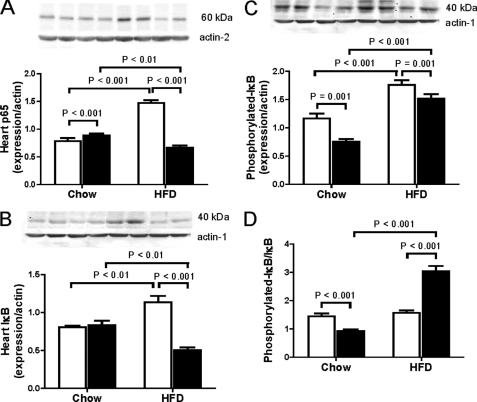
As glutathione homeostasis is intimately connected to NF-κB signaling, one of the key redox-controlled nuclear transcription factors in the heart (34), we assessed expression of proteins involved with NF-κB signaling. The effect of diet-induced obesity on expression of the NF-κB p65 subunit and IκBα in heart was affected by the Cbs genotype. In this regard, Cbs+/− mice fed the HFD had lower (p < 0.01) levels of the NF-κB p65 subunit and IκBα than the same mice fed chow (Fig. 7, A and B), whereas Cbs+/+ mice fed the HFD had higher (p < 0.001) levels of the NF-κB p65 subunit and IκBα than the same mice fed chow (Fig. 7, A and B). Although similar increases in expression of phosphorylated IκBα in Cbs+/− and Cbs+/+ mice with diet-induced obesity were observed (Fig. 7C), only Cbs+/− mice fed the HFD had a higher ratio of phosphorylated IκBα/IκBα compared with the same mice fed chow and Cbs+/+ mice fed the HFD (Fig. 7D). These findings suggest that the decreased total glutathione concentrations in heart from Cbs+/− mice with diet-induced obesity is accompanied by a decrease in p65 expression and activation of IκBα, which would suggest an inhibition of NF-κB signaling.
FIGURE 7.
NF-κB signaling in heart from Cbs+/− mice with diet-induced obesity. NF-κB signaling was assessed by quantifying levels of the NF-κB p65 subunit (A), IκB (B), phosphorylated-IκB (C), and the ratio of phosphorylated-IκB/IκB (D) in heart. Data are presented as means ± S.E., n = 5 mice per diet/genotype group. Open bars, Cbs+/+ mice; filled bars, Cbs+/− mice. Inset depicts representative immunoblots for analyzed proteins in comparison with β-actin bands from the same blot. Significance was determined by two-way ANOVA.
Diet-induced Obesity in Cbs+/− Mice Is Not Associated with Changes in Liver Glutathione but Is Accompanied by Alterations in Liver and Plasma Methyl Metabolites
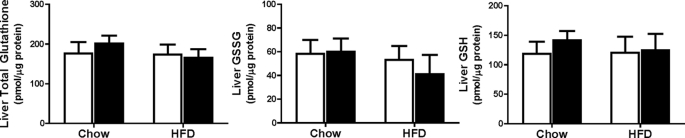
Given that the glutathione pool in the liver is thought to be responsible for maintaining glutathione concentrations in other tissues such as the heart (16–18), we next assessed whether there was an effect of diet-induced obesity in Cbs+/− mice on liver glutathione concentrations. Interestingly, we found similar levels of total glutathione, GSH, and GSSG in liver from Cbs+/+ and Cbs+/− mice fed the HFD compared with those fed chow (Fig. 8). This occurred despite higher (p < 0.01) triglyceride levels in liver from Cbs+/+ and Cbs+/− mice fed the HFD compared with those fed chow (Table 3). Interestingly, we found no effect of Cbs+/− genotype on liver triglyceride levels as we observed in heart.
FIGURE 8.
No disturbances in glutathione metabolites in liver from Cbs+/− mice with diet-induced obesity. Total glutathione, GSSG, and GSH levels were quantified in liver. Data are presented as means ± S.E., n = 7–11 mice per diet/genotype group. Open bars, Cbs+/+ mice; filled bars, Cbs+/− mice. Significance was determined by two-way ANOVA.
TABLE 3.
Changes in major lipid classes in liver from mice with diet-induced obesity
Values shown are means ± S.E.
| Lipid classes (μg/mg protein) | Chow |
HFD |
||
|---|---|---|---|---|
| Cbs+/+ (n = 5) | Cbs+/− (n = 5) | Cbs+/+ (n = 5) | Cbs+/− (n = 6) | |
| Triglycerides | 23.44 ± 2.14 | 24.26 ± 3.63 | 49.92 ± 12.55a | 49.90 ± 7.90a |
| Cholesteryl esters | 6.20 ± 0.38 | 5.88 ± 0.38 | 3.18 ± 0.19a | 2.70 ± 0.04a |
| Free cholesterol | 7.68 ± 0.18 | 8.05 ± 0.45 | 7.141 ± 0.16 | 7.67 ± 0.52 |
| Total phospholipids | 130.0 ± 3.20 | 135.9 ± 3.43b | 123.1 ± 2.42 | 132.3 ± 4.40b |
| Phosphatidylcholine | 57.48 ± 1.11 | 59.20 ± 1.10 | 57.35 ± 0.74 | 60.47 ± 1.56 |
| Phosphatidylethanolamine | 27.98 ± 1.40 | 31.60 ± 1.02b | 25.92 ± 1.35 | 28.68 ± 1.15b |
| Phosphatidylinositol | 12.54 ± 0.28 | 13.18 ± 0.28b | 11.79 ± 0.15a | 12.16 ± 0.25a,b |
| Phosphatidylserine | 5.60 ± 0.09 | 5.74 ± 0.20 | 4.85 ± 0.14a | 5.03 ± 0.33a |
| Lysophosphatidylcholine | 12.18 ± 1.60 | 11.18 ± 0.60 | 9.31 ± 0.25 | 11.54 ± 1.61 |
| Sphingosine | 7.36 ± 0.14 | 7.68 ± 0.30 | 7.32 ± 0.36 | 7.71 ± 0.25 |
| Cardiolipin | 6.89 ± 0.12 | 7.26 ± 0.19 | 6.56 ± 0.05a | 6.67 ± 0.16a |
a p < 0.01 versus chow-fed mice.
b p < 0.05 versus Cbs+/+ mice, as determined by two-way analysis of variance.
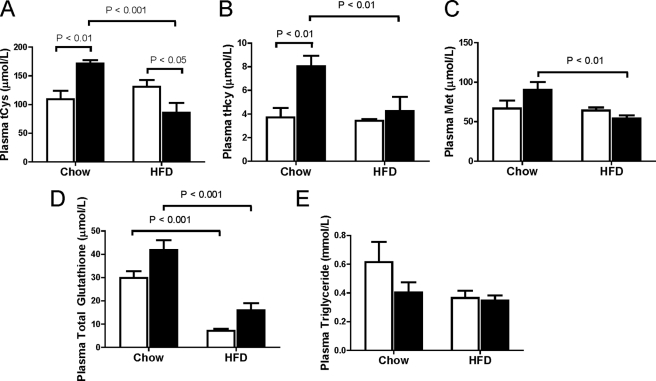
It has been estimated that at least 50% of the cysteine required for endogenous glutathione synthesis in the liver is supplied by the trans-sulfuration of homocysteine (15). However, little trans-sulfuration enzyme (Cbs and cystathionase) expression is thought to be present in heart (11), and therefore, endogenous glutathione synthesis by heart is dependent on cysteine availability. As such, we questioned whether the reduced glutathione levels and cardiac lipotoxicity in heart from Cbs+/− mice with diet-induced obesity was a result of decreased cysteine availability for endogenous glutathione synthesis by heart. As a first step, we quantified plasma concentrations of cysteine and glutathione. Plasma total cysteine and homocysteine levels were higher (p < 0.01) in chow-fed Cbs+/− mice compared with Cbs+/+ mice (Fig. 9, A and B). Interestingly, Cbs+/− mice fed the HFD had lower (p < 0.01) plasma total cysteine, homocysteine, and methionine levels than those fed chow, an effect we did not observe in Cbs+/+ mice (Fig. 9, A–C). This was accompanied by reduced plasma levels of glutathione in both Cbs+/− and Cbs+/+ mice fed the HFD (Fig. 9D). We found no effect of Cbs+/− genotype or diet-induced obesity on plasma triglyceride levels (Fig. 9E). These findings suggest there is decreased availability of circulating cysteine for glutathione synthesis by the heart in Cbs+/− mice with diet-induced obesity.
FIGURE 9.
Decreased plasma cysteine in Cbs+/− mice with diet-induced obesity. Plasma total cysteine (A) and its related metabolites total homocysteine (B), methionine (C), total glutathione (D), and triglyceride (E) levels were quantified in plasma. Data are presented as means ± S.E., n = 5–9 mice per diet/genotype group. Open bars, Cbs+/+ mice; filled bars, Cbs+/− mice. Significance was determined by two-way ANOVA.
DISCUSSION
Disturbances in glutathione homeostasis have been shown in Cbs+/− mice (35), and overexpression of the glutathione-utilizing enzyme, Gpx-1, in these mice attenuates the endothelial dysfunction found in this model (36). However, the direct role of Cbs in maintaining cardiac glutathione status has not been reported. The goal of this study was to test the hypothesis that Cbs+/− mice fed a HFD to induce obesity will have decreased glutathione concentrations and lipotoxicity in the heart because of disturbances in liver glutathione synthesis (37). There are three main findings in this study. First, Cbs+/− mice are susceptible to diet-induced obesity to a similar degree as Cbs+/− mice but have greater glucose intolerance. Cbs+/− mice had lower total glutathione and GSH concentrations in heart, and this occurred to the greatest extent in Cbs+/− mice fed the HFD. The disturbances in glutathione homeostasis in heart were accompanied by triglyceride accumulation, enhanced oxidative stress, and markers of pro-apoptotic signaling. Finally, total glutathione concentrations in liver were unaffected by Cbs+/− genotype or the HFD. However, Cbs+/− mice with diet-induced obesity had lower plasma total cysteine levels suggesting lower provision of cysteine for heart glutathione synthesis. Taken together, these findings suggest an important role for Cbs in the maintenance of cardiac glutathione and in the prevention of cardiac lipotoxicity associated with diet-induced obesity in young adult mice.
Cysteine and glutathione are linked to lipid metabolism through the methionine cycle. Previous studies have demonstrated disturbances in lipid metabolism in liver from Cbs+/− mice with hyperhomocysteinemia (19, 38, 39), but little is known regarding lipid metabolism in the heart of Cbs+/− mice or the effect of feeding a HFD. Interestingly, impaired glutathione homeostasis in heart from Cbs+/− mice was accompanied by triglyceride accumulation in heart, and this occurred to the greatest extent in Cbs+/− mice fed the HFD. The underlying mechanism to account for this finding is unknown but could be linked to reduced mitochondrial density. A decrease in glutathione status, as we observed in the heart from Cbs+/− mice fed the HFD, can lead to an accumulation of hydrogen peroxide, which decomposes to form the highly reactive hydroxyl radical causing membrane lipid peroxidation (40) and damages the mitochondria (41). We did find higher levels of lipid hydroperoxides in heart from Cbs+/− mice, suggesting oxidative stress, and this was also accompanied by increased nitrotyrosine levels in heart. These findings suggest that there may be increased peroxynitrite, a reactive nitrogen species, which nitrates tyrosine residues of proteins to form nitrotyrosine and is in accordance with previous reports that showed increased levels of nitrotyrosine in aorta from C57BL/6J mice fed a HFD (42).
Given that the pool of glutathione in liver is thought to regulate glutathione concentrations in extra-hepatic tissues (18), we hypothesized that Cbs+/− mice fed the HFD would be more susceptible to cardiac lipotoxicity because of diminished liver glutathione. However, we found no effect of the Cbs+/− genotype or HFD feeding on liver total glutathione concentrations, but we did find an effect in heart. Prior studies have either failed to detect Cbs expression (38) or found marginal Cbs activity (43) in heart. We postulated that if Cbs is expressed in vascular endothelial cells (44) and smooth muscle cells (43), which are present in the heart (from coronary arteries), it would be present in heart homogenates. We did find Cbs mRNA in heart, but at very low levels relative to other tissues, and Cbs protein was detected in heart using large amounts of protein, but we observed no effect of the Cbs+/− genotype or the HFD (results not shown).
We also assessed whether disturbances in glutathione homeostasis could account for the lower concentrations of reduced GSH and total glutathione in heart. In this regard, the higher GCLc expression in heart from Cbs+/− mice fed the HFD was expected as this enzyme is known to be up-regulated under conditions associated with reduced GSH depletion (45). Glutathione reductase activity, the primary enzyme responsible for recycling reduced of GSH from its oxidized form, GSSG, was the lowest in the Cbs+/− mice fed HFD, which could be an important factor underlying the lower reduced GSH levels in heart from these mice. In addition, we also observed decreased GPx-1 expression in heart from Cbs+/− mice fed the HFD, which may simply be the result of a decreased requirement for the enzyme because of decreased GSH levels, the enzyme substrate, and may result in ineffective removal of reactive oxygen species. However, although such mechanisms may explain lower reduced GSH levels per se, it does not explain lower total glutathione (sum total of GSH and GSSG) levels in the heart from Cbs+/− mice fed either diet, which is reflective of tissue glutathione biosynthesis. This suggests that other factors, such as diminished cysteine availability, may also contribute to the vastly reduced glutathione status in the heart. This idea is further supported with the observation that NF-κB signaling is altered in heart from Cbs+/− mice fed the HFD. It has been speculated that a moderate level of reduced glutathione status activates NF-κB signaling, whereas more severe depletions of glutathione status actually inhibit NF-κB activation (46).
Interestingly, we found lower plasma total cysteine levels in Cbs+/− mice with diet-induced obesity compared with Cbs+/+ mice. These findings are in contrast to reports in humans that have shown a positive relationship between plasma total cysteine levels and body mass index and fat mass (47). The mechanism underlying the decreased cysteine levels in Cbs+/− mice fed the HFD is unknown but may involve up-regulation of hepatic homocysteine trans-sulfuration and increased utilization of cysteine for glutathione synthesis in liver to combat the oxidative stress associated with HFD feeding. A prior study demonstrated increased trans-sulfuration of homocysteine in HepG2 cells treated with H2O2 to induce oxidative stress (37). We theorize that Cbs+/− mice may be more sensitive to HFD-induced oxidative stress relative to Cbs+/+ mice and, as such, up-regulate hepatic homocysteine trans-sulfuration in an attempt to provide cysteine for maintenance of liver glutathione, but other tissues, such as heart, are affected because of their reduced capacity for glutathione synthesis compared with liver under conditions of oxidative stress (48).
In summary, this study demonstrates that Cbs+/− mice are more sensitive to cardiac lipotoxicity associated with diet-induced obesity. We speculate this may be a consequence of disturbances in glutathione homeostasis in the heart resulting from diminished availability of cysteine for endogenous glutathione synthesis, rather than disturbances in the pool of glutathione in the liver. These findings imply that Cbs and the trans-sulfuration pathway may not be only limited in its protective role in the liver but may extend to the heart, especially in the pathology of cardiolipotoxicity associated with diet-induced obesity in mice.
Acknowledgments
We thank Roger Dyer (Child and Family Research Institute, Department of Pediatrics, University of British Columbia) for quantifying tissue lipids and plasma homocysteine, methionine, and cysteine concentrations. We also thank Kathy Ho and Mihai Cirstea for assistance with animal feeding and tissue collection.
This work was supported in part by a grant-in-aid from the Heart and Stroke Foundation of British Columbia and Yukon (to A. M. D.) and a Natural Sciences and Engineering Research Council of Canada Discovery Grant (to A. M. D.).
- GCL
- glutamate cysteine ligase
- GCLc
- catalytic subunit of GCL
- GLCm
- modifier subunit of GCL
- Cbs
- cystathionine β-synthase
- ANOVA
- analysis of variance
- HFD
- high fat diet
- XIAP
- X-linked inhibitor of apoptotic factor
- FAM
- 6-carboxyfluorescein.
REFERENCES
- 1. Szczepaniak L. S., Victor R. G., Orci L., Unger R. H. (2007) Circ. Res. 101, 759–767 [DOI] [PubMed] [Google Scholar]
- 2. Zhou Y. T., Grayburn P., Karim A., Shimabukuro M., Higa M., Baetens D., Orci L., Unger R. H. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 1784–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Unger R. H. (2002) Annu. Rev. Med. 53, 319–336 [DOI] [PubMed] [Google Scholar]
- 4. Keaney J. F., Jr., Larson M. G., Vasan R. S., Wilson P. W., Lipinska I., Corey D., Massaro J. M., Sutherland P., Vita J. A., Benjamin E. J. (2003) Arterioscler. Thromb. Vasc. Biol. 23, 434–439 [DOI] [PubMed] [Google Scholar]
- 5. Sies H. (1999) Free Radic. Biol. Med. 27, 916–921 [DOI] [PubMed] [Google Scholar]
- 6. Antunes F., Han D., Cadenas E. (2002) Free Radic. Biol. Med. 33, 1260–1267 [DOI] [PubMed] [Google Scholar]
- 7. Vincent B. R., Mousset S., Jacquemin-Sablon A. (1999) Eur. J. Biochem. 262, 873–878 [DOI] [PubMed] [Google Scholar]
- 8. Ghosh S., Kewalramani G., Yuen G., Pulinilkunnil T., An D., Innis S. M., Allard M. F., Wambolt R. B., Qi D., Abrahani A., Rodrigues B. (2006) Free Radic. Biol. Med. 41, 1413–1424 [DOI] [PubMed] [Google Scholar]
- 9. Aronis A., Madar Z., Tirosh O. (2005) Free Radic. Biol. Med. 38, 1221–1230 [DOI] [PubMed] [Google Scholar]
- 10. Chinen I., Shimabukuro M., Yamakawa K., Higa N., Matsuzaki T., Noguchi K., Ueda S., Sakanashi M., Takasu N. (2007) Endocrinology 148, 160–165 [DOI] [PubMed] [Google Scholar]
- 11. Brosnan J. T., Brosnan M. E. (2006) J. Nutr. 136, 1636S–1640S [DOI] [PubMed] [Google Scholar]
- 12. De Mattia G., Bravi M. C., Laurenti O., Cassone-Faldetta M., Proietti A., De Luca O., Armiento A., Ferri C. (1998) Diabetologia 41, 1392–1396 [DOI] [PubMed] [Google Scholar]
- 13. Ceconi C., Curello S., Cargnoni A., Ferrari R., Albertini A., Visioli O. (1988) J. Mol. Cell. Cardiol. 20, 5–13 [DOI] [PubMed] [Google Scholar]
- 14. Lin C. C., Yin M. C., Hsu C. C., Lin M. P. (2004) Lipids 39, 843–848 [DOI] [PubMed] [Google Scholar]
- 15. Mosharov E., Cranford M. R., Banerjee R. (2000) Biochemistry 39, 13005–13011 [DOI] [PubMed] [Google Scholar]
- 16. Lauterburg B. H., Adams J. D., Mitchell J. R. (1984) Hepatology 4, 586–590 [DOI] [PubMed] [Google Scholar]
- 17. Barón V., Muriel P. (1999) Biochim. Biophys. Acta 1472, 173–180 [DOI] [PubMed] [Google Scholar]
- 18. Ljubuncic P., Tanne Z., Bomzon A. (2000) Gut 47, 710–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Devlin A. M., Singh R., Wade R. E., Innis S. M., Bottiglieri T., Lentz S. R. (2007) J. Biol. Chem. 282, 37082–37090 [DOI] [PubMed] [Google Scholar]
- 20. Reeves P. G., Nielsen F. H., Fahey G. C., Jr. (1993) J. Nutr. 123, 1939–1951 [DOI] [PubMed] [Google Scholar]
- 21. Subcommittee on Laboratory Animal Nutrition (1995) Nutrient Requirements of Laboratory Animals, pp. 80–102, National Academy of Sciences Press, Washington, D.C [Google Scholar]
- 22. Andrikopoulos S., Blair A. R., Deluca N., Fam B. C., Proietto J. (2008) Am. J. Physiol. Endocrinol. Metab. 295, E1323–E1332 [DOI] [PubMed] [Google Scholar]
- 23. Ghosh S., Khazaei M., Moien-Afshari F., Ang L. S., Granville D. J., Verchere C. B., Dunn S. R., McCue P., Mizisin A., Sharma K., Laher I. (2009) Am. J. Physiol. Renal. Physiol. 296, F700–F708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bradford M. M. (1976) Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 25. Folch J., Lees M., Sloane Stanley G. H. (1957) J. Biol. Chem. 226, 497–509 [PubMed] [Google Scholar]
- 26. Innis S. M., Dyer R. A. (2002) J. Lipid Res. 43, 1529–1536 [DOI] [PubMed] [Google Scholar]
- 27. Friesen R. W., Novak E. M., Hasman D., Innis S. M. (2007) J. Nutr. 137, 2641–2646 [DOI] [PubMed] [Google Scholar]
- 28. Shen X., Zheng S., Thongboonkerd V., Xu M., Pierce W. M., Jr., Klein J. B., Epstein P. N. (2004) Am. J. Physiol. Endocrinol. Metab. 287, E896–E905 [DOI] [PubMed] [Google Scholar]
- 29. Arany Z., He H., Lin J., Hoyer K., Handschin C., Toka O., Ahmad F., Matsui T., Chin S., Wu P. H., Rybkin I. I., Shelton J. M., Manieri M., Cinti S., Schoen F. J., Bassel-Duby R., Rosenzweig A., Ingwall J. S., Spiegelman B. M. (2005) Cell Metab. 1, 259–271 [DOI] [PubMed] [Google Scholar]
- 30. Suliman H. B., Welty-Wolf K. E., Carraway M., Tatro L., Piantadosi C. A. (2004) Cardiovasc. Res. 64, 279–288 [DOI] [PubMed] [Google Scholar]
- 31. Cabrero A., Merlos M., Laguna J. C., Carrera M. V. (2003) J. Lipid Res. 44, 388–398 [DOI] [PubMed] [Google Scholar]
- 32. Huang C. S., Chang L. S., Anderson M. E., Meister A. (1993) J. Biol. Chem. 268, 19675–19680 [PubMed] [Google Scholar]
- 33. Griffith O. W. (1999) Free Radic. Biol. Med. 27, 922–935 [DOI] [PubMed] [Google Scholar]
- 34. Pechanova O., Simko F. (2010) J. Hypertens. 28, S39–S44 [DOI] [PubMed] [Google Scholar]
- 35. Vitvitsky V., Dayal S., Stabler S., Zhou Y., Wang H., Lentz S. R., Banerjee R. (2004) Am. J. Physiol. Regul. Integr. Comp. Physiol. 287, R39–R46 [DOI] [PubMed] [Google Scholar]
- 36. Weiss N., Zhang Y. Y., Heydrick S., Bierl C., Loscalzo J. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 12503–12508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vitvitsky V., Mosharov E., Tritt M., Ataullakhanov F., Banerjee R. (2003) Redox. Rep. 8, 57–63 [DOI] [PubMed] [Google Scholar]
- 38. Namekata K., Enokido Y., Ishii I., Nagai Y., Harada T., Kimura H. (2004) J. Biol. Chem. 279, 52961–52969 [DOI] [PubMed] [Google Scholar]
- 39. Werstuck G. H., Lentz S. R., Dayal S., Hossain G. S., Sood S. K., Shi Y. Y., Zhou J., Maeda N., Krisans S. K., Malinow M. R., Austin R. C. (2001) J. Clin. Invest. 107, 1263–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Srivastava S., Chan C., Srivastava S., Chan C. (2007) Free Radic. Res. 41, 38–49 [DOI] [PubMed] [Google Scholar]
- 41. Ghosh S., Pulinilkunnil T., Yuen G., Kewalramani G., An D., Qi D., Abrahani A., Rodrigues B. (2005) Am. J. Physiol. Heart Circ. Physiol. 289, H768–H776 [DOI] [PubMed] [Google Scholar]
- 42. Molnar J., Yu S., Mzhavia N., Pau C., Chereshnev I., Dansky H. M. (2005) Circ. Res. 96, 1178–1184 [DOI] [PubMed] [Google Scholar]
- 43. Chen P., Poddar R., Tipa E. V., Dibello P. M., Moravec C. D., Robinson K., Green R., Kruger W. D., Garrow T. A., Jacobsen D. W. (1999) Adv. Enzyme Regul. 39, 93–109 [DOI] [PubMed] [Google Scholar]
- 44. Wang J., Dudman N. P., Wilcken D. E., Lynch J. F. (1992) Atherosclerosis 97, 97–106 [DOI] [PubMed] [Google Scholar]
- 45. Lu S. C., Huang Z. Z., Yang J. M., Tsukamoto H. (1999) Hepatology 30, 209–214 [DOI] [PubMed] [Google Scholar]
- 46. Lou H., Kaplowitz N. (2007) J. Biol. Chem. 282, 29470–29481 [DOI] [PubMed] [Google Scholar]
- 47. Elshorbagy A. K., Nurk E., Gjesdal C. G., Tell G. S., Ueland P. M., Nygård O., Tverdal A., Vollset S. E., Refsum H. (2008) Am. J. Clin. Nutr. 88, 738–746 [DOI] [PubMed] [Google Scholar]
- 48. Malmezat T., Breuillé D., Capitan P., Mirand P. P., Obled C. (2000) J. Nutr. 130, 1239–1246 [DOI] [PubMed] [Google Scholar]