Background: The role of the Wnt/β-catenin signaling pathway in breast cancer bone metastasis is not well understood.
Results: β-Catenin signaling is activated in highly bone metastatic breast cancer cells; modification of this pathway alters the bone lesion phenotype.
Conclusion: β-Catenin acts as an important determinant in breast cancer-induced bone lesions.
Significance: Understanding the regulation of the β-catenin signaling pathway in bone metastasis is of key importance for developing new and effective therapeutic approaches.
Keywords: Bone, Breast Cancer, Catenin, Metastasis, Signal Transduction, β-Catenin Signaling, Bone Metastasis, Bone Remodeling, Cancer-induced Bone Lesions
Abstract
Breast cancer patients have an extremely high rate of bone metastases. Morphological analyses of the bones in most of the patients have revealed the mixed bone lesions, comprising both osteolytic and osteoblastic elements. β-Catenin plays a key role in both embryonic skeletogenesis and postnatal bone regeneration. Although this pathway is also involved in many bone malignancy, such as osteosarcoma and prostate cancer-induced bone metastases, its regulation of breast cancer bone metastases remains unknown. Here, we provide evidence that the β-catenin signaling pathway has a significant impact on the bone lesion phenotype. In this study, we established a novel mouse model of mixed bone lesions using intratibial injection of TM40D-MB cells, a breast cancer cell line that is highly metastatic to bone. We found that both upstream and downstream molecules of the β-catenin pathway are up-regulated in TM40D-MB cells compared with non-bone metastatic TM40D cells. TM40D-MB cells also have a higher T cell factor (TCF) reporter activity than TM40D cells. Inactivation of β-catenin in TM40D-MB cells through expression of a dominant negative TCF4 not only increases osteoclast differentiation in a tumor-bone co-culture system and enhances osteolytic bone destruction in mice, but also inhibits osteoblast differentiation. Surprisingly, although tumor cells overexpressing β-catenin did induce a slight increase of osteoblast differentiation in vitro, these cells display a minimal effect on osteoblastic bone formation in mice. These data collectively demonstrate that β-catenin acts as an important determinant in mixed bone lesions, especially in controlling osteoblastic effect within tumor-harboring bone environment.
Introduction
Breast cancer is the second leading cause of cancer deaths in women and metastasizes to bone in >80% of patients with advanced disease (1). Although the dominant lesion is lytic and destructive in cancer-induced bone metastasis in human, both resorption and formation are activated in most of the breast cancer bone metastases (2). More effective treatment strategies are urgently needed to prevent breast cancer cells from spreading to the bone and to improve patient survival rates. Understanding the molecules and their signaling mechanisms involved in the bone metastasis are of key importance for developing new and effective therapeutic approaches.
Secreted Wnt glycoproteins are important regulators of cellular differentiation and embryogenesis (3). The Wnt pathway consists of secreted ligands (Wnts) and various secreted and membrane-bound antagonists of Wnt signaling. These components activate transmembrane receptors such as low density lipoprotein receptor related protein 5/6 (Lrp-5/6)2 and Frizzled (4). There are at least three different Wnt signaling pathways, including the canonical Wnt pathway which regulates β-catenin (Wnt/β-catenin pathway); the planar cell polarity pathway; and the Wnt/Ca2+ pathway. Signaling through the canonical Wnt pathway is initiated by Wnt ligands activating Frizzleds and Lrp-5/6 (5). In the absence of appropriate Wnt ligands, β-catenin is targeted for phosphorylation, ubiquitination, and proteosomal degradation by a multiprotein complex comprising glycogen synthase kinase-3β, adenomatous polyposis coli and Axin (6–8). In the presence of an appropriate Wnt ligand, this multiprotein complex does not target β-catenin for degradation, and β-catenin can translocate to the nucleus, where in concert with members of the T cell factor (TCF)/lymphoid enhancer factor family, activates the transcription of a wide range of genes, such as c-myc and cyclin D1 (9, 10).
The Wnt pathway plays a central role in controlling embryonic bone development and bone mass (5, 11, 12). It is also essential in postnatal bone regenerative process, such as fracture healing (13, 14). Although the Wnt pathway is well recognized to be important for breast cancer tumorigenesis (15, 16), little is known about its role in breast cancer-induced bone metastasis. Bu et al. showed for the first time that breast cancer cell line MDA-MB-231, which preferentially forms osteolytic bone metastases, exhibited increased levels of Wnt/β-catenin signaling and Dickkopf-1 (Dkk-1) expression, and the tumor cell-produced Dkk-1 blocked Wnt-3A-induced osteoblast differentiation (17). Schwaninger et al. reported that Dkk-1 mRNA was expressed in the osteolytic MDA-MB-231 cell line, whereas osteoblastic breast cancer cell lines T47D and ZR75-1 did not express Dkk-1 (18). Recent studies suggested that the Wnt pathway is critically involved in some other bone malignancy, including osteosarcoma (19–21), multiple myeloma (22), and prostate cancer bone metastasis (23). We therefore hypothesize that this pathway may also be responsible for breast cancer bone metastasis.
For this purpose, we established an experimental model of tumor-bone cell interaction by intratibial injection of mouse mammary TM40D-MB tumor cells. TM40D-MB cells were developed in our laboratory previously that have the capability to metastasize to bone spontaneously in syngeneic mice (24). Interestingly, we found that this experimental model displayed a mixed type of osteoblastic and osteolytic lesions, rather than pure osteolytic lesions, which is especially useful for studying the tumor-bone cell interaction and the changes in the bone lesion between osteoblastic bone formation and osteolytic bone destruction. Utilizing this model, we aim at investigating the regulation of the β-catenin signaling pathway in breast cancer cells-induced bone lesion.
EXPERIMENTAL PROCEDURES
Cell Culture
Two mouse breast cancer cell lines, TM40D and TM40D-MB, were used in this study. TM40D mammary cells were derived from the FSK4 mammary epithelial cell line established in vitro from normal mouse mammary gland (25). The TM40D outgrowth line in vivo produces mammary tumors that were metastatic to lung but not to bone (26). TM40D-MB cells were isolated from bone lesions in mice that had been treated with intracardiac injection of TM40D cells. A previous study from our laboratory demonstrated that TM40D-MB cells induced bone metastasis very efficiently after being injected into mammary pad in BALB/c mice (24).
Both TM40D and TM40D-MB cell lines were cultured in DMEM (HyClone), supplemented with 5% fetal bovine serum (FBS, HyClone) and 1% penicillin/streptomycin at 37 °C in a 5% CO2 incubator. The mouse osteoblastic cell line, MC3T3, and mouse leukemic monocyte macrophage cell line, RAW 264.7, were maintained in α-MEM (Invitrogen) containing 10% FBS and 1% penicillin/streptomycin at 37 °C in a 5% CO2 incubator.
Quantitative Real-time PCR (qPCR)
Total RNAs were isolated from cell cultures using TRIzol Reagent (Invitrogen) and synthesized to cDNA using M-MLV reverse transcriptase (Invitrogen). qPCR was performed with the primers listed in supplemental Table 1. The cDNA was amplified and quantified using the Power SYBR Green PCR Master Mix (Applied Biosystems). The specificity of the PCR products was confirmed using DNA gel electrophoresis after real-time PCR.
Western Blot Analysis
Cells were lysed with Reporter Lysis buffer (Promega), and were cell lysates were loaded onto 12% SDS-PAGE. Proteins were transferred to Hybond neutral nylon membrane (Amersham Biosciences). Mouse anti-active β-catenin polyclonal (Millipore) and rabbit anti-total β-catenin monoclonal antibodies (Cell Signaling) were used at a dilution of 1:1,000, and horseradish peroxidase (HRP)-conjugated donkey anti-mouse and HRP-conjugated goat anti-rabbit antibodies (Cell Signaling) were used at a dilution of 1:10,000. Antibody complexes were detected using SuperSignal West Pico chemiluminescent substrate (Thermo Scientific). To normalize for differences in protein loading, the blots were stripped and reprobed with a rabbit anti-actin polyclonal antibody (Sigma).
Reporter Activity Assay
The TM40D-MB cells were treated with adenovirus expressing human β-catenin (Ad-β-catenin), or dominant negative human TCF4 (Ad-TCF4_DN), or a control virus (Ad-control, all from Vector Biolabs) at a multiplicity of infection (m.o.i.) of 100 for 24 h. Cells were resuspended and plated in 6-well plate at 2 × 105/well overnight. Cells were then transfected with the β-catenin-responsive firefly luciferase reporter plasmids TOPflash (wild-type promoter) or FOPflash (mutant promoter) (obtained from Benjamin Alman at the University of Toronto) using Lipofectamine 2000 reagent (Invitrogen). Twenty-four hours after transfection, the luciferase activity was determined using a Dual Luciferase Assay kit (Promega). All measurements were performed in triplicate.
Cell Proliferation Assay
The TM40D-MB cells were infected with Ad-β-catenin, Ad-TCF4_DN, and Ad-control at a m.o.i. of 100. Cells were washed 24 h after infection and plated into a 94-well plate at 1 × 104/well in 100 μl of Complete medium in triplicate. Twenty-four hours later, 10 μl of MTT (10 mg/ml) was added into each well and incubated for 1–2 h until a purple precipitate was clearly visible under the microscope. The formazan product was dissolved in dimethyl sulfoxide, and absorbancies were read at 570 nm on a microtiter plate reader.
In Vitro Osteoblast Differentiation in Co-culture System
The TM40D-MB cells were transduced with Ad-β-catenin or Ad-Control at a m.o.i. of 100. Twenty-four hours later, cells were resuspended and plated into 24-well formatted cell culture inserts (BD Biosciences). The tumor cells were then co-cultured with MC3T3 cells in osteogenic medium (α-MEM containing 10% FBS, 50 μg/ml ascorbic acid, and 10 mm β-glycerophosphate). The medium was replaced every 3 days.
Six days after confluence, MC3T3 cells were washed with PBS, and cell lysates were harvested. The ALP activity assay was performed using QuantiChromTM ALP Assay kit (BioAssay Systems). Twelve days after confluence, other MC3T3 cells were washed and fixed with 4% paraformaldehyde, followed by von Kossa staining.
In Vitro Osteoclast Differentiation in Co-culture System
The TM40D-MB cells were transduced with either Ad-β-catenin or Ad-TCF4_DN, and Ad-control at a m.o.i. of 100. Twenty-four hours later, cells were resuspended and plated into 24-well formatted cell culture inserts. The tumor cells were then co-cultured with RAW 264.7 cells in α-MEM containing 10% FBS and 1% penicillin/streptomycin. To initiate osteoclast differentiation, recombinant human RANKL protein (R&D Systems) was immediately added into the co-culture medium at a concentration of 5 ng/ml. Five days after co-culture, many multinucleated osteoclasts were observed in the cultures. Tartrate-resistant acid phosphatase (TRAP) staining was performed using a kit from Sigma. The number of multinucleated osteoclasts was counted in each well and compared between different groups.
Animal Study
Eight-week-old female BALB/c mice were inoculated with TM40D or TM40D-MB cells. Before injection, TM40D-MB cells were treated with Ad-β-catenin, Ad-TCF4_DN, or Ad-control at a m.o.i. of 100 for 24 h. Cells were washed with PBS and resuspended in a mixture with equal amount of PBS and Matrigel (BD Biosciences).
Animals were divided into four groups. Mice in group 1 were injected with tumor cells transduced with Ad-β-catenin; mice in group 2 were injected with cells expressing Ad-TCF4_DN; mice in group 3 were injected with cells treated with Ad-control. Mice in group 4 were injected with vehicles only as a negative control. There were 9 animals in each group. For intratibial injection, animals were anesthetized with a mixture of ketamine (100 mg/kg body weight) and xylazine (10 mg/kg body weight). The left hind limb was shaved and prepared with 70% alcohol. A 3-mm longitudinal incision was made with a blade over the patellar ligament. A microsyringe (Hamilton) was inserted through the tibial plateau with the knee flexed, and 10 μl of the mixture containing 1 × 105 cells was injected into the bone marrow cavity ∼2 mm below the growth plate. Animals were allowed free, unrestricted weight bearing in cages after recovery from anesthesia.
Evaluation of Bone Lesions
Mice tibiae were harvested and fixed in 4% paraformaldehyde. Each bone sample was radiographed with a MX-20 x-ray system (Faxitron x-ray Corporation). Bone samples were also examined with micro-computed tomography (μCT, MicroCT-40, SCANCO Medical) using 45-kV tube voltage, 88-μA tube current, and 300-ms integration time for each of 1,000 projections. Each of the 160–200 contiguous slices was reconstructed on 2,048 × 2,048 grid with 10-μm isotropic volume elements (voxels). Bone lesions were quantified in a region of interest from 480 to 680 μm below the growth plate of the proximal tibia. After radiographic examination, the bone samples were decalcified in 20% EDTA (pH 7.4) for 10 days and embedded in paraffin. Sections of 5 μm thick were prepared and stained with hematoxylin and eosin (H&E).
Statistical Analyses
Data were expressed as mean ± S.D. Statistical differences were calculated using Student's t test. A minimum of five animals were analyzed for each group for radiographic parameters. A p value < 0.05 was considered statistically significant.
RESULTS
Up-regulation of β-Catenin Signaling Activity in Bone Metastatic TM40D-MB Cells
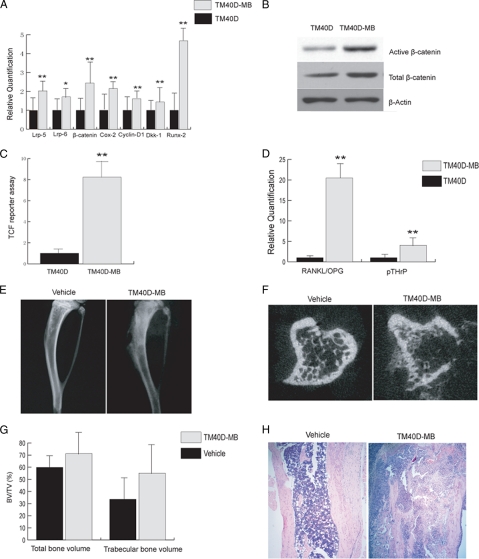
To investigate whether the β-catenin pathway is up-regulated in bone metastatic TM40D-MB as compared with non-bone metastastic TM40D cells, we performed qPCR to examine gene expression of some Wnt/β-catenin signaling molecules in both cells. As shown in Fig. 1A, the mRNA levels of both Lrp-5 and Lrp-6, which mediate the canonical Wnt pathway, were higher in TM40D-MB cells than that in TM40D cells. The β-catenin mRNA level was also up-regulated in TM40D-MB cells. We also found higher gene expression of Cox-2 and Cyclin D1 in TM40D-MB cells, both of which are target genes that are regulated by β-catenin mediated TCF/ lymphoid enhancer factor-dependent transcription (10, 27, 28). Using Western blotting, we observed a stronger induction of active β-catenin and total β-catenin in TM40D-MB cells than in TM40D cells (Fig. 1B). TCF reporter assay showed that TM40D-MB cells have a higher luciferase reporter activity than TM40D cells (Fig. 1C). These data suggest that bone metastatic TM40D-MB cells display an increased endogenous β-catenin activity. Interestingly, we noticed that the Runx-2 was up-regulated in TM40D-MB cells. It has been shown that Runx-2 is the key transcription factor for osteoblast differentiation (29). This indicates that high level β-catenin signaling in TM40D-MB cells may have the ability to promote osteoblastic bone-forming activity.
FIGURE 1.
β-Catenin signaling pathway is activated in TM40D-MB cells, which induces a mixed bone lesion. A, real-time PCR showed that Lrp-5, Lrp-6, β-catenin, Cox-2, Cyclin-D1, Dkk-1, and Runx-2 were all up-regulated in bone metastatic TM40D-MB cells compared with non-bone metastatic TM40D cells. B, Western blot analysis demonstrated a strong induction of both active and total β-catenin expression in TM40D-MB cells than in TM40D cells. C, TM40D-MB cells showed a higher TCF reporter activity than TM40D cells. D, real-time PCR showed that both RANKL/OPG ratio and pTHrP expression were significantly up-regulated in bone metastatic TM40D-MB tumor cells compared with nonmetastatic TM40D cells. TM40D-MB cells were injected into the tibia of each mouse, and equal amounts of vehicle were injected as a negative control. E–H, 5 weeks after the injection, x-radiographs (Faxitron MX-20) showed no tumor formation in vehicle-injected tibia (E). TM40-MB cell-injected tibia showed both osteoblastic and osteolytic lesions. F, μCT slices confirmed normal bone structure in vehicle treated tibia, but tumor cell injection induced a osteoblastic/osteolytic phenotype. G, μCT showed no difference in total bone volume fraction and trabecular bone volume fraction between tumor cells and vehicle injected animals. H, H&E staining on tissue sections showed normal bone structure with intact cortical matrix and bone marrow cells in marrow cavity in vehicle-treated mice. In TM40D-MB-injected tibiae, tumor cells filled the majority of marrow cavity, and cortical bone was eroded. There were also bone formation activities in some areas in the tibia, confirming a mixed bone lesion. *, p < 0.05; **, p < 0.01. Error bars, S.D.
Another interesting finding is that Dkk-1 mRNA was also expressed at a higher level in TM40D-MB cells than in TM40D cells (Fig. 1A). Dkk-1 is not only one of the direct target genes of β-catenin-mediated transcription (30, 31), but also an antagonist that binds to Lrp-5/6 and Kremen proteins to block Wnt to β-catenin signaling (32, 33). The up-regulation of Dkk-1 in TM40D-MB cells implicates that activation of the Wnt/β-catenin pathway might induce an endogenous negative feedback loop to maintain its signal activity at an appropriate level.
RANKL/OPG ratio and parathyroid hormone related peptide expression are two major mediators of osteoclastic lesions. We performed qPCR and showed that TM40D-MB cells have a significantly elevated RANKL/OPG ratio (20-fold) and pTHrP expression (4-fold) than TM40D cells, as shown in Fig. 1D.
Intratibial Injection of TM40D-MB Cells Induced a Mixed Bone Lesion in Mice
To study the interaction between breast tumor cells and bone in an experimental model, we injected 1 × 105 TM40D-MB cells directly into the tibia of BALB/c mice. We also injected an equal amount of vehicle (a mixture of PBS and Matrigel) into the animals as a negative control. After 5 weeks, x-rays revealed that all animals injected with TM40D-MB cells exhibited lesions displaying both osteoblastic bone formation and osteolytic bone destruction. No lesions were noted in the tibiae of mice treated with vehicle only (Fig. 1E). μCT confirmed the existence of both osteoblastic bone formation and lytic destruction in the area of tibiae where tumor cells were injected (Fig. 1F). Neither trabecular bone volume fraction (BV/TV%) nor total bone volume fraction (BV/TV%) differed for TM40D-MB cell injected mice compared with vehicle-treated animals (Fig. 1G). Histology confirmed the mixed bone lesions in the area of tumor inoculation. In TM40D-MB injected tibiae, tumor cells surrounding the woven bone replaced the majority of the bone marrow, and vehicle-injected mice showed a normal bone structure with intact cortical shaft (Fig. 1H).
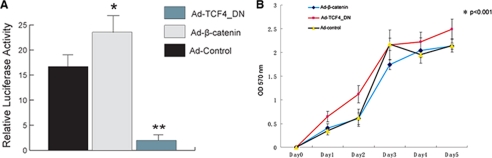
Treatment with Adenovirus Expressing β-Catenin or Dominant Negative TCF4 Modified β-Catenin Signaling in TM40D-MB Cells
To analyze the effect of β-catenin signaling on the regulation of breast cancer-induced bone lesions, we genetically modified the β-catenin signaling pathway by treating the TM40D-MB cells with Ad-β-catenin or Ad-TCF4_DN at a m.o.i. of 100. Transfected cells were harvested to determine the β-catenin activity by a luciferase reporter assay. Increased β-catenin level by Ad-β-catenin in TM40D-MB cells only slightly increased the reporter activity, whereas cells treated with Ad-TCF4_DN had a great down-regulation in luciferase activity (Fig. 2A). We also performed MTT assay to determine whether modification of the β-catenin pathway has an effect on tumor cell proliferation. As shown in Fig. 2B, TM40D-MB cells expressing Ad-TCF4_DN showed a modest increase in cell proliferation compared with the Ad-control. However, there was no difference in the proliferation rate between TM40D-MB cells treated with Ad-β-catenin and Ad-control.
FIGURE 2.
Modification of β-catenin signaling in TM40D-MB cells. A, 24 h after transfection of β-catenin-responsive plasmid TOPflash and FOPflash, reporter activity assay showed that treatment of Ad-β-catenin had a modest increase in the reporter activity, but treatment of Ad-TCF4_DN significantly decreased luciferase reporter activity. *, p < 0.05; **, p < 0.01. B, MTT assay revealed that proliferation was increased in TM40D-MB cells treated with Ad-TCF4_DN, whereas proliferation was not changed in cells treated with Ad-β-catenin. *, p < 0.001. Error bars, S.D.
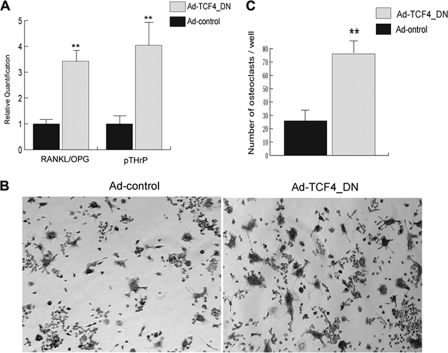
Inhibition of β-Catenin Using Dominant Negative TCF4 Increased Osteoclast Differentiation in Vitro and Enlarged Osteolytic Bone Destruction in Mice and Decreased Osteoblast Differentiation in Vitro
To study whether inactivation of the β-catenin pathway has an impact on osteoclast differentiation, we first performed qPCR for RANKL, OPG, and pTHrP in TM40D-MB cells. We found that TM40D-MB cells harboring a dominant-negative TCF4 vector increased the RANKL/OPG ratio. Furthermore, there was a drastic elevation in the level of pTHrP expression (Fig. 3A). Although the signaling mechanism for these increased gene expressions is not clear (see “Discussion” below), there are previous reports showing that increased RANKL/OPG ratio and the expression of pTHrP mRNA are two key factors in promoting osteoclast differentiation during osteolytic bone destruction accompanying breast cancer (2). Our result implied that inactivation of β-catenin in TM40D-MB cells might also increase osteoclast differentiation in bone microenvironment. To test this hypothesis, we co-cultured TM40D-MB cells expressing TCF4_DN with osteoclast precursors, RAW 264.7 cells, and performed TRAP staining after 5 days. As shown in Fig. 3, B and C, RAW 264.7 cells co-cultured with TCF4_DN-expressing TM40D-MB cells developed significantly more multinucleated osteoclasts compared with other RAW 264.7 cells co-cultured with control tumor cells.
FIGURE 3.
Inactivation of β-catenin increases osteoclast differentiation and inhibits osteoblast differentiation in vitro. A, real-time PCR assay showed that both RANKL/OPG ratio and pTHrP were up-regulated in tumor cells treated with Ad-TCF4_DN compared with cells treated with Ad-control. TM40D-MB cells were infected with Ad-TCF4_DN at a m.o.i. of 100 for 24 h; cells were then co-cultured with osteoclast precursors, RAW 264.7, for 5 days. TRAP staining was performed to detect multinucleate osteoclast formation. B and C, TRAP staining showed an increased number of multinucleated osteoclasts in cells treated with Ad-TCF4_DN. **, p < 0.01. Error bars, S.D.
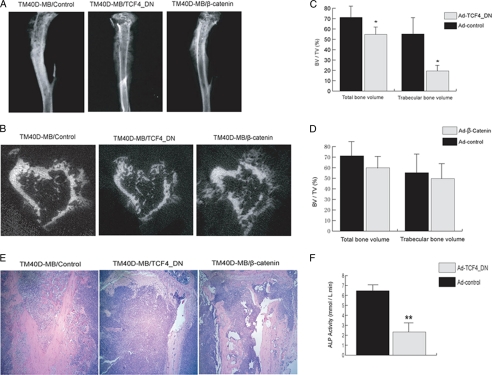
To investigate whether inactivating β-catenin in tumor cells could affect osteoclastic bone destruction in mice, we injected TM40D-MB cells expressing TCF4_DN into the mouse tibiae. Radiography was carried out 5 weeks after tumor inoculation. Mice injected with TCF4_DN-expressing TM40D-MB cells clearly showed much increased regions of lytic lesions compared with animals that were injected with control virus-treated tumor cells (Fig. 4A). μCT revealed a decrease in both total bone volume and trabecular bone volume in animals injected with tumor cells expressing TCF4_DN (Fig. 4, B–D). H&E staining also showed increased lytic bone destruction in TCF4_DN-expressing TM40D-MB compared with control TM40D-MB cells (Fig. 4E).
FIGURE 4.
Modification of β-catenin pathway affects bone lesion phenotype in vivo. After infection of TM40D-MB cells with various adenoviruses, tumor cells were injected into the mouse tibia. After 5 weeks, animals were examined by radiography, and tissue samples were harvested for histological analysis. A, x-ray examination displayed enlarged lytic lesions in tibiae of animals injected with Ad-TCF4_DN-treated tumor cells. There was no increased osteoblastic activity in animals injected with tumor cells overexpressing β-catenin. B, μCT confirmed phenotype description above. C and D, analysis using μCT revealed that both total bone volume fraction and trabecular bone volume fraction decreased in animals injected with tumor cells expressing TCF4_DN. However, no significant difference was observed in total bone volume fraction and trabecular bone volume fraction between mice injected with control virus and Ad-β-catenin; E, H&E staining demonstrated a mixed bone lesion in tibia of control virus-treated mice, as both tumor bone formation and bone resorption were observed in tumor area. In contrast, a predominantly lytic phenotype was seen in mouse tibia that had been injected with Ad-TCF4_DN-treated tumor cells. The marrow cavity was completely filled with tumor cells; bone formation was hardly detected. In mice injected with tumor cells overexpressing β-catenin, both osteoblastic and osteolytic activity were seen, which is comparable with control virus-treated mice. F, real-time PCR assay showed a decrease in ALP activity in MC3T3 cells co-cultured with TM40D-MB cells treated with Ad-TCF4_DN rather than those co-cultured with control virus-treated tumor cells. *, p < 0.05; **, p < 0.01. Error bars, S.D.
In addition, to study whether inactivating β-catenin also inhibits osteoblast differentiation, we co-cultured TM40D-MB cells expressing TCF4_DN with osteoblasts MC3T3. Six days after co-culture, we observed a decrease in ALP activity, an early osteoblast differentiation marker, in MC3T3 cells co-cultured with Ad-TCF4_DN-treated tumor cells (Fig. 4F).
Taken together, these data demonstrated that inactivation of the β-catenin pathway in breast cancer cells not only increases osteoclast differentiation and promotes osteolytic lesions, but also inhibits osteoblast differentiation.
β-Catenin Overexpression in TM40D-MB Cells Failed to Induce More Osteoblastic Bone Formation in Mice
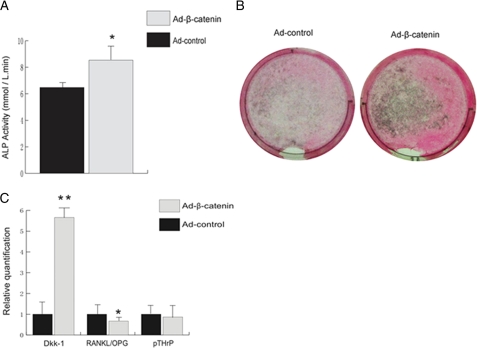
To understand whether cancer cells overexpressing β-catenin can increase osteoblast differentiation, we first co-cultured mouse osteoblasts MC3T3 with TM40D-MB cells treated with either Ad-β-catenin or control virus. Six days after co-culture, we found a slight increase in ALP activity in MC3T3 cells co-cultured with Ad-β-catenin-treated tumor cells (Fig. 5A). We also performed von Kossa staining at day 12 after co-culture. Likewise, we observed increased calcium deposition in MC3T3 cells co-cultured with TM40D-MB overexpressing β-catenin than in MC3T3 cells co-cultured with control virus-treated tumor cells (Fig. 5B). These results suggested that tumor cells overexpressing β-catenin could induce osteoblast differentiation in vitro. Interestingly, using qPCR, we noticed that treatment with Ad-β-catenin also increased Wnt antagonist Dkk-1 (Fig. 5C), suggesting that a negative feedback loop exists in response to overactivation of the Wnt/β-catenin pathway.
FIGURE 5.
TM40D-MB cells overexpressing β-catenin increases osteoblast differentiation in vitro. TM40D-MB cells were infected with Ad-β-catenin at a m.o.i. of 100 for 24 h; cells were then co-cultured with osteoblasts MC3T3 cells. A, 6 days after co-culture, ALP activity had a modest increase in MC3T3 cells treated with Ad-β-catenin. B, 12 days after co-culture, von Kossa staining showed that mineralization was also slightly increased in cells overexpressing β-catenin. C, real-time PCR demonstrated a strong induction of Dkk-1 in cells treated with Ad-β-catenin. RANKL/OPG was slightly decreased in cells overexpressing β-catenin, but there was no change in pTHrP level. *, p < 0.05; **, p < 0.01. Error bars, S.D.
To investigate whether tumor cells overexpressing β-catenin may affect osteoclasts activity, we also examined RANKL/OPG and pTHrP at their mRNA levels using qPCR. We found that TM40D-MB cells overexpressing β-catenin displayed only a slight decrease in the ratio of RANKL/OPG, and the level of pTHrP showed no difference compared with control tumor cells (Fig. 5C). These data suggest that TM40D-MB cells overexpressing β-catenin do not contribute significantly to reduce osteoclast differentiation.
Next, we explored whether overexpressing β-catenin in tumor cells also increases osteoblastic bone formation in vivo; to do so, we performed intratibial injection of TM40D-MB cells that had been treated with either Ad-β-catenin or Ad-control. To our surprise, 5 weeks after injection, mice that had been injected Ad-β-catenin-treated tumor cells did not show a significant increase in osteoblastic bone formation within the tibiae compared with those injected with control vector-treated tumor cells. μCT analysis also showed no difference in both total bone volume and trabecular bone volume between two groups. Histological examination further indicated that tibia injected with tumor cells overexpressing β-catenin remained a mixed lesion rather than a conversion from a mixed to an osteoblastic phenotype (Fig. 4, A–E).
DISCUSSION
Given the well demonstrated crucial role of β-catenin in bone development and regeneration, we predict that mammary tumor cells with different levels of β-catenin may affect the patterns of bone lesions. Due to the lack of mouse model for spontaneous breast cancer bone metastasis, we have established the current experimental model of breast cancer bone metastasis through inoculating the bone metastatic TM40D-MB cells directly into the tibiae of mice. This method has been used widely to study tumor-bone interaction during cancer metastases induced by many other cancers (23, 34, 35). This model facilitates investigation of the interaction between tumor cells and bone and allows us to quantify the bone lesions between different treatment groups very easily. Most importantly, our mouse model displayed a mixed, rather than the pure osteolytic bone lesion, faithfully mimicking the bone lesions occurring in human breast cancer patients. Therefore, this model is very helpful for studying the signaling mechanisms underlying the changes between osteoblastic bone formation and osteoclastic bone resorption.
In this report, we show that, compared with the non-bone metastatic breast cancer cells TM40D, highly bone metastatic TM40D-MB cells exhibit significantly higher endogenous β-catenin signaling activity than the non-bone metastatic TM40D cells. This suggests that the β-catenin pathway is involved in regulating the bone metastatic property. A variety of studies have shown that activation of the Wnt/β-catenin pathway is associated with osteoblast differentiation in vitro and new bone formation in vivo (14, 36, 37) and is responsible for some osteoblast bone malignancy, such as prostate cancer bone metastasis and osteosarcoma (21, 23). It is therefore not surprising that this pathway may play an important role in osteoblastic bone formation in the mixed lesion. We further noticed that TM40D-MB cells express higher level of Runx-2, and previous studies demonstrated that up-regulation of Runx-2 by canonical Wnt signaling plays a key role during osteogenesis (38). In prostate cancer bone metastasis, tumor cells can produce high level Runx-2, which promote tissue invasion, homing to bone, as well as osteoblastic differentiation (39). Furthermore, Barnes et al. reported that human breast cancer cells can produce Runx-2, which activates bone sialoprotein and contributes to an osteoblastic phenotype in bone metastasis (40). Hence, it seems likely that activated β-catenin signaling in TM40D-MB cells may contribute directly to osteoblastic bone formation through up-regulation of Runx-2.
Interestingly, two downstream target genes (i.e. Cox-2 and Dkk-1) are also up-regulated in TM40D-MB cells. Because these two gene products can also regulate osteoclast activity in bone once they are secreted (22), this may partly explain why TM40D-MB cells with high level endogenous β-catenin activity induce a mixed bone lesion, rather than a more osteoblastic phenotype. Furthermore, we observed a strong induction of both RANKL/OPG and pTHrP expression in TM40D-MB cells. Although during normal bone remodeling, Wnt signaling acts as a repressor for RANKL transcription in osteoblasts (41), this does not conflict with our finding, as Jones et al. discovered that RANKL can prominently regulate migration of epithelial cancer cells and bone-specific metastases including melanoma and breast cancer (42). pTHrP is also responsible for bone metastasis induced by breast and lung cancer (43, 44). Therefore, high level RANKL/IOPG ratio and pTHrP in TM40D-MB cells may represent a metastatic property of these tumor cells. Previous studies have proved that RANKL/OPG and pTHrP are two major mediators for osteoclast differentiation and osteolysis (2, 45). Hence, although the mechanism whereby TM40D-MB cells have high level of RANKL and pTHrP is not known, it seems likely that these two molecules play an important role in breast tumor cells to induce osteoclastic lesion through a Wnt-independent mechanism. Because TM40D-MB cells can express both activated β-catenin signaling activity as well as molecules responsible for osteoclast activity (e.g. RANKL, pTHrP, Cox-2), the balanced effect is to produce a mixed lesion consisting both osteoblastic bone formation and osteoclastic bone destruction.
However, it should be noted that our animal model omits the early events of cancer bone metastases, such as tumor migration, intravasation, and extravasations. This means that our data suggested that β-catenin in TM40D-MB cells only contributes to the bone lesion in the tumor cell-bone microenvironment after tumor injection. It is unknown whether this pathway is also critically involved in the early steps of breast cancer bone metastasis.
Next, we showed that inactivation of the β-catenin pathway in TM40D-MB cells expressing a dominant negative TCF4 (TCF4_DN) significantly increased osteoclast differentiation in vitro and enlarged osteolytic destruction in vivo. Noticeably, although TM40D-MB cells have already had a higher ratio of RANKL/OPG and an elevated level of pTHrP than nonmetastatic TM40D cells, treatment with Ad-TCF4_DN still further increased RANKL/OPG ratio and pTHrP mRNA (Fig. 3A), which led to increased osteoclast activity both in vitro and in vivo. Given that the Wnt/β-catenin pathway regulates osteoclast differentiation and bone resorption through its transcription repression of RANKL (41), it is not a surprise that inactivation of β-catenin by Ad-TCF4_DN increased RANKL expression and thus a higher RANKL/OPG ratio. However, the mechanism whereby TCF4_DN enhances pTHrP expression in TM40D-MB cells is unclear. One possibility is that β-catenin/TCF signaling may activate inhibitor(s) of pTHrP through other unknown downstream targets. Thus, treatment of TM40D-MB cells with TCF4_DN may release the suppression, resulting in highly induced pTHrP expression.
In addition, we observed that inactivation of the β-catenin pathway by Ad-TCF4_DN also inhibited osteoblast differentiation in a tumor-bone co-culture system, as indicated by decreased ALP activity. This finding is in agreement with previous reports that Wnt antagonist Dkk-1 can block osteoblast differentiation in both physiological and pathological conditions, including breast cancer bone metastasis (12, 13, 22, 36). As a result, the drastic effect on osteolytic lesion by the TCF4_DN in our model is mediated through combined mechanisms: up-regulation of osteoclast differentiation as well as down-regulation of osteoblast differentiation.
Based on our findings that inactivation of the β-catenin pathway by Ad-TCF4_DN in breast tumor cells promotes osteolytic bone lesions, we initially predicted that overexpression of β-catenin in the tumor cells might induce a more osteoblastic phenotype in mice. Surprisingly, injection of TM40D-MB cells overexpressing β-catenin did not induce a shift of bone lesion toward osteoblastic bone, but such treatment did induce a very modest increase of osteoblast differentiation in the tumor-bone co-culture system. As described above, we note that bone metastatic TM40D-MB cells already possess a higher endogenous β-catenin signaling activity, as was shown by reporter activity assay and gene expression techniques, than in control TM40D cells. Hence, the preexisiting high level of β-catenin activity might be the reason why treating TM40D-MB cells with Ad-β-catenin fails to shift the bone lesion pattern. This possibility is supported by our reporter activity assay, in which only a slight increase in the luciferase activity was observed in Ad-β-catenin-treated TM40D-MB cells compared with Ad-control-treated cells (Fig. 2).
In addition, we further noticed that Ad-β-catenin-treated TM40D-MB cells had a much higher expression level of Dkk-1 compared with control TM40D-MB cells. Previous studies have widely demonstrated that Dkk-1 can inhibit osteoblast differentiation and play a crucial role in both bone regeneration and bone malignancies, including breast cancer bone metastasis and multiple myeloma (14, 17, 22, 46). Given the osteoinhibitory effects of Dkk-1, it seems plausible that the negative feedback by Dkk-1 in response to overactivation of the β-catenin signaling pathway may also participate in the prevention from inducing a more osteoblastic phenotype. Nevertheless, our study does not rule out possibilities that other Wnt/β-catenin inhibitors (e.g. Sfrp-1, Sfrp-2, and Dkk-2) might also be induced in TM40D-MB cells after being treated with Ad-β-catenin.
On the other hand, we noticed that TM40D-MB cells treated with Ad-β-catenin only showed a slight decrease in the RANKL/OPG ratio and the pTHrP was not changed. These tumor cells might only have slightly reduced osteolytic bone destruction, which provides another mechanism for the failure to induce more osteoblastic bone lesion after the β-catenin-overexpressing TM40D-MB tumor cells are inoculated into the bone.
In general, bone metastatic TM40D-MB cells possess higher endogenous β-catenin signaling activity, which mediates osteoblastic differentiation and induces bone-forming activity in the mixed bone lesion. Inactivation of this pathway can enlarge osteolytic bone destruction through both up-regulation of osteoclast differentiation and down-regulation of osteoblast differentiation. However, the role of this pathway in osteolytic destruction is still unclear. Our data suggest that osteolytic lesion may be mediated through both Wnt-dependent (e.g. Cox-2 and Dkk-1) and Wnt-independent mechanisms (e.g. pTHrP and RANKL/OPG).
Breast cancer-induced bone metastasis displayed a mixed lesion, instead of the pure osteolytic destruction as in the myeloma-induced bone lesions. The regulation of the β-catenin pathway in breast cancer bone metastasis is very complicated. Our data argue that it is still too early to develop any therapeutic approach by simply targeting β-catenin either to block bone metastasis or to reduce bone lesion. More studies are needed to understand the molecular mechanisms of β-catenin action in the bone-tumor microenvironment before any therapy can be developed to treat bone metastasis.
Supplementary Material
Acknowledgments
We thank Northwestern University for the use of the MicroCT facility.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 CA79736 (to M. Z.). This work was also supported by Susan G. Komen Breast Cancer Foundation Grant KG080231 (to M. Z.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1.
- Lrp
- low density lipoprotein receptor-related protein
- Ad
- adenovirus
- ALP
- alkaline phosphatase
- Dkk
- Dickkopf
- DN
- dominant negative
- m.o.i.
- multiplicity of infection
- μCT
- micro-computed tomography
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- OPG
- osteoprotegerin
- qPCR
- quantitative real-time PCR
- RANKL
- receptor activator of nuclear factor κB ligand
- TCF
- T cell factor
- TRAP
- tartrate-resistant acid phosphatase.
REFERENCES
- 1. Kozlow W., Guise T. A. (2005) J. Mammary Gland Biol. Neoplasia 10, 169–180 [DOI] [PubMed] [Google Scholar]
- 2. Mundy G. R. (2002) Nat. Rev. Cancer 2, 584–593 [DOI] [PubMed] [Google Scholar]
- 3. Moon R. T., Brown J. D., Torres M. (1997) Trends Genet. 13, 157–162 [DOI] [PubMed] [Google Scholar]
- 4. Johnson M. L., Kamel M. A. (2007) Curr. Opin. Rheumatol 19, 376–382 [DOI] [PubMed] [Google Scholar]
- 5. Akiyama T. (2000) Cytokine Growth Factor Rev. 11, 273–282 [DOI] [PubMed] [Google Scholar]
- 6. Behrens J. (2000) J. Cell Sci. 113, 911–919 [DOI] [PubMed] [Google Scholar]
- 7. Eastman Q., Grosschedl R. (1999) Curr. Opin. Cell Biol. 11, 233–240 [DOI] [PubMed] [Google Scholar]
- 8. Ikeda S., Kishida S., Yamamoto H., Murai H., Koyama S., Kikuchi A. (1998) EMBO J. 17, 1371–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. He T. C., Sparks A. B., Rago C., Hermeking H., Zawel L., da Costa L. T., Morin P. J., Vogelstein B., Kinzler K. W. (1998) Science 281, 1509–1512 [DOI] [PubMed] [Google Scholar]
- 10. Tetsu O., McCormick F. (1999) Nature 398, 422–426 [DOI] [PubMed] [Google Scholar]
- 11. Westendorf J. J., Kahler R. A., Schroeder T. M. (2004) Gene 341, 19–39 [DOI] [PubMed] [Google Scholar]
- 12. Yang Y. (2003) Birth Defects Res. C Embryo Today 69, 305–317 [DOI] [PubMed] [Google Scholar]
- 13. Chen Y., Alman B. A. (2009) J. Cell. Biochem. 106, 353–362 [DOI] [PubMed] [Google Scholar]
- 14. Chen Y., Whetstone H. C., Lin A. C., Nadesan P., Wei Q., Poon R., Alman B. A. (2007) PLoS Med. 4, e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boras-Granic K., Wysolmerski J. J. (2008) Organogenesis 4, 116–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mohinta S., Wu H., Chaurasia P., Watabe K. (2007) Front. Biosci. 12, 4020–4033 [DOI] [PubMed] [Google Scholar]
- 17. Bu G., Lu W., Liu C. C., Selander K., Yoneda T., Hall C., Keller E. T., Li Y. (2008) Int. J. Cancer 123, 1034–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schwaninger R., Rentsch C. A., Wetterwald A., van der Horst G., van Bezooijen R. L., van der Pluijm G., Löwik C. W., Ackermann K., Pyerin W., Hamdy F. C., Thalmann G. N., Cecchini M. G. (2007) Am. J. Pathol 170, 160–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haydon R. C., Deyrup A., Ishikawa A., Heck R., Jiang W., Zhou L., Feng T., King D., Cheng H., Breyer B., Peabody T., Simon M. A., Montag A. G., He T. C. (2002) Int. J. Cancer 102, 338–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hoang B. H., Kubo T., Healey J. H., Sowers R., Mazza B., Yang R., Huvos A. G., Meyers P. A., Gorlick R. (2004) Int. J. Cancer 109, 106–111 [DOI] [PubMed] [Google Scholar]
- 21. Kansara M., Tsang M., Kodjabachian L., Sims N. A., Trivett M. K., Ehrich M., Dobrovic A., Slavin J., Choong P. F., Simmons P. J., Dawid I. B., Thomas D. M. (2009) J. Clin. Invest. 119, 837–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tian E., Zhan F., Walker R., Rasmussen E., Ma Y., Barlogie B., Shaughnessy J. D., Jr. (2003) N. Engl. J. Med. 349, 2483–2494 [DOI] [PubMed] [Google Scholar]
- 23. Hall C. L., Bafico A., Dai J., Aaronson S. A., Keller E. T. (2005) Cancer Res. 65, 7554–7560 [DOI] [PubMed] [Google Scholar]
- 24. Li Z., Schem C., Shi Y. H., Medina D., Zhang M. (2008) Clin. Exp. Metastasis 25, 389–400 [DOI] [PubMed] [Google Scholar]
- 25. Kittrell F. S., Oborn C. J., Medina D. (1992) Cancer Res. 52, 1924–1932 [PubMed] [Google Scholar]
- 26. Stickeler E., Kittrell F., Medina D., Berget S. M. (1999) Oncogene 18, 3574–3582 [DOI] [PubMed] [Google Scholar]
- 27. Howe L. R., Subbaramaiah K., Chung W. J., Dannenberg A. J., Brown A. M. (1999) Cancer Res. 59, 1572–1577 [PubMed] [Google Scholar]
- 28. Shtutman M., Zhurinsky J., Simcha I., Albanese C., D'Amico M., Pestell R., Ben-Ze'ev A. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 5522–5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ducy P. (2000) Dev. Dyn. 219, 461–471 [DOI] [PubMed] [Google Scholar]
- 30. Niida A., Hiroko T., Kasai M., Furukawa Y., Nakamura Y., Suzuki Y., Sugano S., Akiyama T. (2004) Oncogene 23, 8520–8526 [DOI] [PubMed] [Google Scholar]
- 31. González-Sancho J. M., Aguilera O., García J. M., Pendás-Franco N., Peña C., Cal S., García de Herreros A., Bonilla F., Muñoz A. (2005) Oncogene 24, 1098–1103 [DOI] [PubMed] [Google Scholar]
- 32. Fedi P., Bafico A., Nieto Soria A., Burgess W. H., Miki T., Bottaro D. P., Kraus M. H., Aaronson S. A. (1999) J. Biol. Chem. 274, 19465–19472 [DOI] [PubMed] [Google Scholar]
- 33. Glinka A., Wu W., Delius H., Monaghan A. P., Blumenstock C., Niehrs C. (1998) Nature 391, 357–362 [DOI] [PubMed] [Google Scholar]
- 34. Corey E., Quinn J. E., Bladou F., Brown L. G., Roudier M. P., Brown J. M., Buhler K. R., Vessella R. L. (2002) Prostate 52, 20–33 [DOI] [PubMed] [Google Scholar]
- 35. Mourskaia A. A., Dong Z., Ng S., Banville M., Zwaagstra J. C., O'Connor-McCourt M. D., Siegel P. M. (2009) Oncogene 28, 1005–1015 [DOI] [PubMed] [Google Scholar]
- 36. Bain G., Müller T., Wang X., Papkoff J. (2003) Biochem. Biophys. Res. Commun. 301, 84–91 [DOI] [PubMed] [Google Scholar]
- 37. Hill T. P., Später D., Taketo M. M., Birchmeier W., Hartmann C. (2005) Dev. Cell 8, 727–738 [DOI] [PubMed] [Google Scholar]
- 38. Gaur T., Lengner C. J., Hovhannisyan H., Bhat R. A., Bodine P. V., Komm B. S., Javed A., van Wijnen A. J., Stein J. L., Stein G. S., Lian J. B. (2005) J. Biol. Chem. 280, 33132–33140 [DOI] [PubMed] [Google Scholar]
- 39. Baniwal S. K., Khalid O., Gabet Y., Shah R. R., Purcell D. J., Mav D., Kohn-Gabet A. E., Shi Y., Coetzee G. A., Frenkel B. (2010) Mol. Cancer 9, 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barnes G. L., Javed A., Waller S. M., Kamal M. H., Hebert K. E., Hassan M. Q., Bellahcene A., Van Wijnen A. J., Young M. F., Lian J. B., Stein G. S., Gerstenfeld L. C. (2003) Cancer Res. 63, 2631–2637 [PubMed] [Google Scholar]
- 41. Spencer G. J., Utting J. C., Etheridge S. L., Arnett T. R., Genever P. G. (2006) J. Cell Sci. 119, 1283–1296 [DOI] [PubMed] [Google Scholar]
- 42. Jones D. H., Nakashima T., Sanchez O. H., Kozieradzki I., Komarova S. V., Sarosi I., Morony S., Rubin E., Sarao R., Hojilla C. V., Komnenovic V., Kong Y. Y., Schreiber M., Dixon S. J., Sims S. M., Khokha R., Wada T., Penninger J. M. (2006) Nature 440, 692–696 [DOI] [PubMed] [Google Scholar]
- 43. Yin J. J., Selander K., Chirgwin J. M., Dallas M., Grubbs B. G., Wieser R., Massagué J., Mundy G. R., Guise T. A. (1999) J. Clin. Invest. 103, 197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Miki T., Yano S., Hanibuchi M., Kanematsu T., Muguruma H., Sone S. (2004) Int. J. Cancer 108, 511–515 [DOI] [PubMed] [Google Scholar]
- 45. Guise T. A., Yin J. J., Taylor S. D., Kumagai Y., Dallas M., Boyce B. F., Yoneda T., Mundy G. R. (1996) J. Clin. Invest. 98, 1544–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen Y., Whetstone H. C., Youn A., Nadesan P., Chow E. C., Lin A. C., Alman B. A. (2007) J. Biol. Chem. 282, 526–533 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.