Background: Alternative splicing generates calcium channel splice variants with altered electrophysiological properties.
Results: Exclusion of exons encoding the IQb domain or proximal/distal domains attenuates Ca2+-dependent inactivation of the CaV1.3 channels.
Conclusion: Alternative splicing at the C terminus alters the critical Ca2+ inhibitory feedback property of CaV1.3 channels.
Significance: Alternative splicing is an exquisite mechanism for customizing channel function within diverse biological niches.
Keywords: Calcium Channels, Immunohistochemistry, Ion channels, Patch Clamp Electrophysiology, RNA Splicing, Alternative Splicing, Calcium-dependent Inactivation, Cav1.3 Calcium Channel, L-type Calcium Channels
Abstract
CaV1.3 channels are unique among the high voltage-activated Ca2+ channel family because they activate at the most negative potentials and display very rapid calcium-dependent inactivation. Both properties are of crucial importance in neurons of the suprachiasmatic nucleus and substantia nigra, where the influx of Ca2+ ions at subthreshold membrane voltages supports pacemaking function. Previously, alternative splicing in the CaV1.3 C terminus gives rise to a long (CaV1.342) and a short form (CaV1.342A), resulting in a pronounced activation at more negative voltages and faster inactivation in the latter. It was further shown that the C-terminal modulator in the CaV1.342 isoforms modulates calmodulin binding to the IQ domain. Using splice variant-specific antibodies, we determined that protein localization of both splice variants in different brain regions were similar. Using the transcript-scanning method, we further identified alternative splicing at four loci in the C terminus of CaV1.3 channels. Alternative splicing of exon 41 removes the IQ motif, resulting in a truncated CaV1.3 protein with diminished inactivation. Splicing of exon 43 causes a frameshift and exhibits a robust inactivation of similar intensity to CaV1.342A. Alternative splicing of exons 44 and 48 are in-frame, altering interaction of the distal modulator with the IQ domain and tapering inactivation slightly. Thus, alternative splicing in the C terminus of CaV1.3 channels modulates its electrophysiological properties, which could in turn alter neuronal firing properties and functions.
Introduction
Ca2+ influx through voltage-gated calcium channels regulates numerous physiological functions in the nervous system, including neurotransmitter release, generation of dendritic Ca2+ spikes, and induction of activity-dependent gene regulation. To support this diverse range of functions, neurons express a diverse complement of voltage-gated calcium channels, N-type (CaV2.2) and P/Q-type (CaV2.1) channels to mediate rapid influx of Ca2+ into the presynaptic terminal, triggering synaptic vesicle fusion and neurotransmitter release (excitation-secretion coupling) (1), and L-type (CaV1.2 and CaV1.3) channels to couple neuronal activity to gene transcription (excitation-transcription coupling) (2, 3). Furthermore, as CaV1.3 channels activate at comparatively more negative potentials (4, 5), they also serve pacemaker function and shape neuronal firing (6, 7). Alternative splicing in the CaV1.3 α1 subunit C terminus gives rise to a long form (usage of exon 42, CaV1.342) and a short form (usage of exon 42A, CaV1.342A) (8), which could functionally alter voltage- and Ca2+-dependent gating. Activation of calcium current through CaV1.342A channels was more pronounced at negative voltages, and inactivation was faster due to the enhanced calcium dependent inactivation (CDI)2 (9). CDI develops in response to local elevations of intracellular Ca2+ sensed by channel-bound calmodulin (CaM) (10, 11). However, modulation of CDI is an important prerequisite in some cells, such as in outer hair cells of the cochlea, where a splice variant that is deleted of the CaM-binding IQ domain of CaV1.3 (CaV1.3IQΔ) was reported to diminish CDI (12). Although mRNA expression of both long and short CaV1.3 isoforms was found in several mouse and human tissues, the protein expression profile of these splice isoforms has not been studied.
In this study, we generated two splice variant-specific antibodies, pAb_CaV1.342 and pAb_CaV1.342A, to analyze the expression profiles of both CaV1.3 channel isoforms. Interestingly, CaV1.3 channel truncation experiments by Singh et al. (9) restricted modulator activity to the last 116 amino acids of the C terminus, with CaV1.3ΔC116 channels showing similar gating properties as CaV1.342A. However, biochemical evidence for CaV1.3 C-terminal cleavage is lacking and does not appear to function as a transcriptional regulator (13). Therefore, although CaV1.342 and CaV1.342A channels prevailed as the dominant isoforms, we employed the transcript-scanning method (14, 15) to systematically identify novel and functional C terminus splice variants of CaV1.3 that could be important in modulating gating properties of the channel. In addition to the CaV1.3IQΔ (12), we have identified and characterized the biophysical properties and subcellular localization of 4 novel splice isoforms: exon 41 (CaV1.3Δ41), exon 43 (CaV1.343S-2), exon 44 (CaV1.3Δ44), and exon 48 (CaV1.348S). Another splice isoform in exon 43 (CaV1.343S) was described in our accompanying article (16). Alternative splicing in the C terminus causes hyperpolarized shifts in the activation and inactivation properties and modulates the degree of CDI, via changes in the IQ domain, or conserved proximal and distal domains (termed PRCD and DCRD), which could alter its C-terminal gating modulator (CTM) activity. All alternatively spliced CaV1.3 channels examined in this study were functional and may contribute differentially to the overall firing property of neurons in specific nuclei, particularly in physiological and disease states.
EXPERIMENTAL PROCEDURES
Generation of Polyclonal Antibodies against CaV1.342 and CaV1.342A
The rat CaV1.342 splice variant peptide (CCEDDSSPTWSRQNYSYYNRYPGSSMD) was subcloned in-frame at EcoRI and XhoI sites of expression plasmid pGEX-4T-1 (Ambersham Biosciences). The resulting fusion protein was expressed in the host Escherichia coli BL21 (DES) cells. This GST-fused CaV1.342 protein was purified and eluted with glutathione-agarose (Sigma, G4501). Purified CaV1.342-GST protein was used to immunize female New Zealand White rabbits once a month. Complete Freund's adjuvant (Sigma, F5881) was first mixed with GST fusion protein for immunization, and incomplete Freund's adjuvant (Sigma, F5506) was used in subsequent injections once a month. Serum was pre-absorbed overnight at 4 °C with excess GST protein to remove contaminating GST IgG in the serum and the antibody of interest was affinity purified from immobilized GST fusion protein with an IgG elution buffer (Pierce). The concentration of the resulting antibody was ∼1.5 μg/μl, and was designated as pAb_CaV1.342.
The polyclonal peptide antibody pAb_CaV1.342A was raised (Alpha Diagnostic International, San Antonio, TX) against the peptide containing exon 42A (6 amino acids MLERML; AF370009.1) and two amino acids from exon 41 (LQ). The peptide CLQMLERML was synthesized and used for generation of peptide antibody against CaV1.342A channels in rabbits. The inclusion of an additional residue C (cysteine) for pAb_CaV1.342A is to stabilize and increase the ease of affinity purification of the peptide (12). The concentration of antibody was ∼0.8 μg/μl.
Protein Western Blotting
Tissues from mouse brains, wild type and CaV1.3−/− knock-out, were homogenized in cold lysis buffer containing the following (in mm): 50 Tris, pH 8.0, 1 EDTA, and 150 NaCl. All processes were done at 4 °C. The homogenate was centrifuged at 8,000 × g for 15 min, followed by 40,000 × g for 1 h. Membrane proteins were extracted from the pellet with cold lysis buffer supplemented with 1% Triton X-100 for 1 h. The pellet was then centrifuged at 40,000 × g for 1 h. Membrane proteins were extracted from the supernatant, and 20 μg of protein was separated in 6% SDS-polyacrylamide gel under reducing conditions, and transferred onto a PVDF membrane using a semi-dry transfer system (Bio-Rad).
For antibody detection, the membrane was first blocked with 5% nonfat milk in TBST (20 mm Tris, pH 7.6, 137 mm NaCl, and 0.05% Tween 20) for 1 h at room temperature. The membrane was incubated with diluted primary antibody pAb_CaV1.342 (used at 1:2000), diluted primary antibody pAb_CaV1.342A (used at 1:200), or pAb_CaV1.3 antibody (Alomone Labs, Jerusalem, Israel) (used at 1:200) overnight at 4 °C. After washing with TBST, the membrane was incubated with diluted goat anti-rabbit secondary antibody (used at 1:2000; Sigma) for 1 h. After washing, the antibody binding was detected with SuperSignal Ultra Chemiluminescent substrate (Pierce).
Chromogen Staining
Adult wild type C57 mice were deeply anesthetized, and perfused transcardially with ice-cold 4% paraformaldehyde buffered with PBS, pH 7.4, for 30 min. Brain and spinal cord were dissected and stored in a freezer at −80 °C. Frozen coronal sections at 40 μm thickness were cut in a cryostat. Freely floating sections were washed and blocked in 4% normal goat serum in TBSTx (TBS with 0.1% Triton X-100) for 2 h. Sections were incubated with primary antibody pAb_CaV1.342 (used at 1:200) and primary antibody pAb_CaV1.342A (used at 1:100) overnight at 4 °C on the orbital shaker. After washing, sections were incubated with biotinylated goat anti-rabbit IgG (used at 1:500, Vector). After washing, sections were incubated with avidin-biotin complex (ABC) reagent (Vector) for 2 h, followed by DAB (0.12% H2O2 and 0.05% 3,3′-diaminobenzidine) (Sigma) for 20 min before mounting onto glass slides.
Fluorescence Staining
Brains of deeply anesthetized wild type C57 mice were dissected out without perfusion. Frozen brain coronal sections at 20 μm thickness from both wild type C57 and CaV1.3−/− knock-out mice were prepared in a cryostat and mounted on glass slides. The brain sections were fixed with 4% paraformaldehyde in PBS. After washing and blocking with 4% normal goat serum in TBSTx, the sections were incubated with primary antibody pAb_CaV1.342 (used at 1:200) and primary antibody pAb_CaV1.342A (used at 1:100) alone or together with mouse anti-bassoon antibody (used at 1:500; Novus Biologicals) overnight at 4 °C. After washing, slides were treated for 2 h in FITC-conjugated goat anti-rabbit IgG (Invitrogen) against CaV1.3 protein and Cy3-conjugated goat anti-mouse IgG (Invitrogen) to detect the presence of the bassoon protein. After washing, the sections were mounted in FluorSaveTM Reagent (Calbiochem-Novabiochem) to retard fading. Preparations were analyzed using Carl Zeiss Laser Scanning System LSM 510. In control sections, one or both primary antibodies were omitted. The experiment was repeated in at least three animals.
C terminus Transcript Scanning by Nested PCR
The transcript scanning method has been described in detail by Mittman et al. (14) and Soong et al. (15) for the systematic identification of loci for alternative splicing of voltage-gated calcium channel genes. Here we briefly describe the method. Pairs of primers were used to amplify 5 amplicons covering overlapping regions of the C terminus of the CaV1.3 α1-subunit. The size of the PCR products ranged from 515 to 638 bp, spanning between 2 and 4 exons. We used 1 lot of rat brain cDNA libraries (Marathon-ReadyTM cDNA, catalog numbers 639412) for the transcript scanning reactions. Initial PCRs were conducted using rat CaV1.3-specific primers situated on exons 36 and 3′-UTR of the CaV1.342 isoform. Nested PCRs were conducted using primers flanking exons 37 to 49 (supplemental Fig. S2C). The amplicons were subcloned into pGEMp®-T Easy vector and transformed into DH10B E. coli cells. A total of 237 colonies were picked as templates for nested PCR using the exon-specific primers listed in supplemental Table S1. The different splice combinations were differentiated based on their distinct migration patterns in 3% agarose gels (supplemental Fig. S2D). To verify the accuracy of gel analysis, plasmids extracted from representative colonies were sent for DNA sequencing.
To characterize their functional properties, the splice variations in the C terminus were substituted into the full-length wild type CaV1.342 and constructs CaV1.3Δ41, CaV1.343S-2, CaV1.3Δ44, and CaV1.348S were generated. The CaV1.3Δ41 (deleted exon 41) has exon 41 alternatively spliced out, thus generating a truncated protein that is different from CaV1.342 (contains exon 41). The CaV1.343S-2 (truncated exon 43) is subjected to alternative splicing at the region just after PCRD, thus generating a truncated protein with an intact PCRD. The CaV1.3Δ44 (deleted exon 44) has exon 44 alternatively spliced out, generating an in-frame protein that lacks exon 44. The CaV1.348S construct (alternative 3′ splice acceptor site in exon 48) has a portion of exon 48 spliced out, generating an in-frame protein that lacks the first 45 amino acids of exon 48.
Electrophysiological Recordings and Data Analysis
Whole cell patch clamp recordings were used to characterize the wild type long form CaV1.342 and short form CaV1.342A, as well as the splice variants: CaV1.3Δ41, CaV1.343S-2, CaV1.3Δ44, and CaV1.348S. The Ca2+ currents were recorded at room temperature using the whole cell patch clamp electrophysiology technique from transiently transfected mammalian human embryonic kidney 293 (HEK 293) cells according to methods described previously (17, 18). Outward K+ currents were blocked by Cs+ in the internal and external solutions. Cells were transiently transfected with wild type long form CaV1.342, short form CaV1.342A, or the splice variants, CaV1.3Δ41, CaV1.343S-2, CaV1.3Δ44 or CaV1.348S, rat β2a and α2δ subunits using the standard calcium phosphate transfection method. IBa or ICa were recorded at room temperature using the whole cell patch clamp technique 48–72 h after transfection. For whole cell patch clamp recording, the internal solution (patch-pipette solution) contained the following (in mm): 138 Cs-MeSO3, 5 CsCl, 0.5 EGTA, 10 HEPES, 1 MgCl2, 2 mg/ml of Mg-ATP, pH 7.3 (adjusted with CsOH), 290 mosmol with glucose. The external solution contained the following (in mm): 10 HEPES, 140 tetraethylammonium methanesulfonate, 5 BaCl2, or 5 CaCl2 (pH adjusted to 7.4 with CsOH and osmolality to 290–310 with glucose). Pipettes, of 1.5–2 megohm resistance, were used. Whole cell currents, obtained under voltage clamp with an Axopatch 200B amplifier (Molecular Devices, Union City, CA), were filtered at 1–5 kHz and sampled at 5–50 kHz, and the series resistance was typically <5 megohm after >70% compensation. A P/4 protocol was used to subtract on-line the leak and capacitive transients.
Data were acquired using pClamp9 software (Molecular Devices), and analyzed and fitted using GraphPad Prism IV software and Microsoft (Seattle, WA) Excel. Data are expressed as mean ± S.E. Statistical analysis was performed using paired or unpaired Student's t test. Current-voltage (I-V) curve relationships were obtained by step depolarization from a holding potential of −100 mV to various test potentials. I-V curves were fitted according to Equation 1: I = Gmax(V-Erev)/(1 + exp[(V − V1/2act)/kI-V]), where Gmax is the maximum conductance of the cell, Erev is the reversal potential, V1/2act is the voltage for half-maximal activation, kI-V is the slope of Boltzmann function, and n is the number of tested cells. Steady-state inactivation data and CDI were fitted to Equation 2: amp1 + (1-amp1)/(1 + exp[(V − V1/2inact)/SF1) + amp2/(1 + exp[(V − V1/2inact′)SF2], where amp1 is the initial current amplitude, amp2 is the final current amplitude, V is the membrane potential of the conditioning pulse, V1/2inact is the potential for half-inactivation, and SF is the slope factor. Activation data were fitted according to Equation 3: G/Gmax = Flow/{1 + exp[(V1/2,low − V)/klow]} + (1 − Flow)/{1 + exp[(V1/2,high − V)/khigh]}, where G is the tail current, Gmax is the tail current evoked by a depolarization to +120 mV, Flow is the fraction of the low threshold component, V is the membrane potential of the test pulse, V1/2,low, V1/2, high, klow, and khigh are the half-activation potentials and slope factors for the low and high threshold components and V1/2act was calculated when G = 0.5 Gmax.
RESULTS
Generation and Characterization of CaV1.3 Splice Variant-specific Antibodies
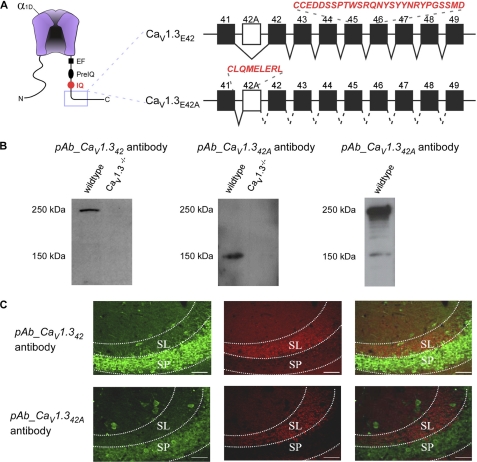
In the C terminus of CaV1.3, expression of longer isoform CaV1.342 is mediated by exon skipping of exon 42A in the pre-mRNA (8). The presence of exon 42A predicts a α1-subunit containing C termini 500 amino acids shorter than exon 42-containing subunits (GenBank accession number AF370010) (Fig. 1A). Although qualitative RT-PCR showed brain tissue expression of both CaV1.3 splice isoforms, with predominant exon 42 mRNA levels in both mouse and human (9), not much is known about differential protein expression due to lack of antibodies that differentiate between exons 42 and 42A. We therefore raised specific polyclonal antibodies against the CaV1.342 and CaV1.342A channels. For CaV1.342A channels, exons downstream of exon 42A are not encoded as the use of cassette exon 42A produced a stop codon 7 amino acids after exon 41 (Fig. 1A). A polyclonal antibody was thereby generated against a GST fusion to a 27-amino acid sequence encoded by exons 45 and 46 (Fig. 1A), yielding antibody pAb_CaV1.342. For CaV1.342A channels, a polyclonal peptide antibody was raised against exon 42A, which encodes eight amino acids downstream of exon 41 (Fig. 1A). The resulting antibody was denoted pAb_CaV1.342A. In both cases, antibodies were affinity purified before use.
FIGURE 1.
Characterization of pAb_CaV1.342 and pAb_CaV1.342A specific antibodies Western blot and immunostaining. A, left, schematic of CaV1.3 channel pore-forming α1 subunit (α1D), with hot spots for CaM/channel regulation in the carboxyl terminus (EF, EF-hand (32); pre-IQ and IQ domains (33, 34). Right, diagrammatic representation of two C-terminal splice variants of the CaV1.3 α1 subunits. Incorporation of exon 42A instead of exon 42 results in a truncated C terminus 6 amino acids after exon 41. Rabbit polyclonal antibodies were raised specifically against these two splice variants. The peptide sequences utilized are in red and italics, namely for pAb_CaV1.342, CCEDDSSPTWSRQNYSYYNRYPGSSMD, and pAb_CaV1.342A, CLQMLERL. B, Western blot of total membrane protein extracted from wild type and CaV1.3−/− knock-out mice. Left blot was probed with pAb_CaV1.342 antibody. A single band of ∼250 kDa was detected in a wild type mouse but absent in the knock-out mouse. Middle blot was probed with pAb_CaV1.342A antibody. A single band of ∼150 kDa was detected in a wild type mouse but absent in the knock-out mouse. Right blot was probed with commercial pAb_CaV1.3 antibody. C, double labeling of the CA3 region of wild type mouse dorsal hippocampus with bassoon (red) and pAb_CaV1.342 or pAb_CaV1.342A (green). Positive staining for presynaptic vesicle proteins (bassoon) is restricted to the stratum lucidum (SL), a mossy fiber recipient layer of the CA3 subfield of the hippocampus. Strong staining of pAb_CaV1.342 (top) and pAb_CaV1.342A (bottom) was observed in the stratum pyramidale (SP) of the region. No co-localization was observed between the bassoon and pAb_CaV1.342 or pAb_CaV1.342A. Scale bar, 50 μm.
To establish the specificity of pAb_CaV1.342 and pAb_CaV1.342A, we analyzed membrane proteins extracted from wild type and CaV1.3−/− knock-out mouse brain tissue via Western blotting. A single protein band with the predicted size of ∼250 kDa was observed for CaV1.342 in the wild type (Fig. 1B, left) and ∼180 kDa was observed for CaV1.342A (Fig. 1B, middle), but was absent in CaV1.3−/− knock-out mouse. In addition, multiple bands were detected with commercial pAb_CaV1.3 antibody (Fig. 1B, right) in the 250 to 150 kDa range, indicating the expression of different CaV1.3 channel splice isoforms of different sizes. Visual inspection revealed that CaV1.3 channels with a longer C terminus appear to be the dominant species.
In the CA3 region of the hippocampus, a similar immunostaining pattern was observed for both pAb_CaV1.342 and pAb_CaV1.342A antibodies (Fig. 1C). Strong labeling of both CaV1.342 and CaV1.342A proteins was restricted mostly to the pyramidal cell soma in the stratum pyramidale (SP) (Fig. 1C, left panels). Some interneurons in the CA3 region are also immunostained for CaV1.342 and CaV1.342A proteins. The most dense labeling was observed in the cell soma and proximal dendrites for both cell types, with diminishing intensity in the distal dendritic regions. Bassoon, a presynaptic vesicle marker, stained the stratum lucidum, a mossy fiber recipient layer of the CA3 subfield.
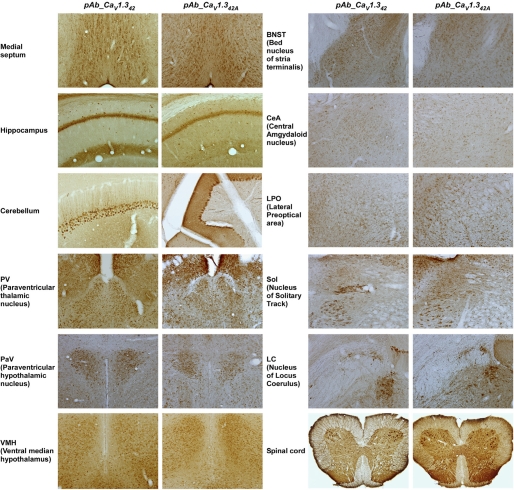
Similar Localization of Both CaV1.342 and CaV1.342A Proteins in Brain and Spinal Cord Slices
Using polyclonal antibodies pAb_CaV1.342 and pAb_CaV1.342A, we mapped the expression patterns of CaV1.3 splice variants in the mouse brain and spinal cord tissues (Fig. 2). Stronger staining was observed for both splice variants in the septum region, the cerebellar Purkinje cells, the paraventricular thalamic nucleus, the paraventricular hypothalamic nucleus, the ventral medial hypothalamus, the dorsal lateral geniculate nucleus, and some nuclei from the brain stem including hypoglossal nucleus, dorsal motor nucleus of vagus, and locus coeruleus. A weaker staining was observed in the bed nucleus of stria terminalis, the central amygdaloid nucleus, and the lateral preoptical area. In the spinal cord, immunostaining with both pAb_CaV1.342 and pAb_CaV1.342A antibodies were confined to neurons in the gray matter, and similar staining patterns were observed in all animals examined. The staining was present at all spinal levels of the gray matter, as shown in a representative coronal section from the lumbar segment (Fig. 2). The staining was most intense at the ventral horn motor neurons, with some small neurons stained in the intermediate zone and in the dorsal horn as well. In the ventral horn neuron, both soma and the proximal dendrites were strongly stained.
FIGURE 2.
Similar localization of CaV1.342 and CaV1.342A proteins in many regions of brain and spinal cord. Wild type mouse brain and spinal cord sections are immunostained with pAb_CaV1.342 and pAb_CaV1.342A antibodies, using 3,3′-diaminobenzidine as the chromogen. Left panel, brain and spinal cord sections were stained for CaV1.342 channels. Right panel, brain and spinal cord sections were stained for CaV1.342A channels. Similar staining patterns were observed for both CaV1.342 and CaV1.342A proteins in many regions of the brain and spinal cord.
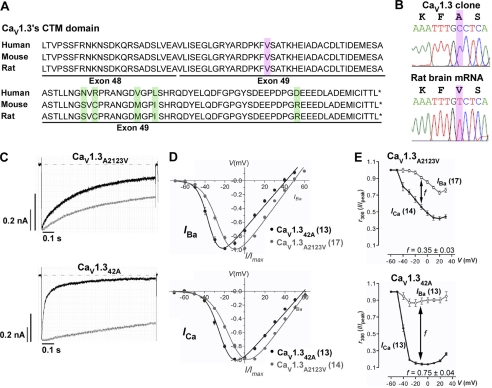
Identification and Correction of Cloning Error in Rat CaV1.342 Clone
Alternative splicing in human CaV1.3 results in CaV1.342A channels with more pronounced activation of calcium current at negative voltages and faster inactivation due to enhanced CDI (9). However, this diminished CDI of the CaV1.342 isoform was not replicated in rat clones, and was attributed to a single valine to alanine switch in its distal carboxyl tail (Fig. 3A, highlighted purple) (19). Sequencing analysis of the PCR fragment amplified with primers flanking exons 48–49 revealed GTC (coding for valine) in both rat brain (Fig. 3B) and rat heart cDNA (data not shown), instead of GCC (coding for alanine) at position 2123 in rat CaV1.342 clone (Fig. 3B). The amplified fragment was cloned into cloning vector pGEM®-T Easy, and sequencing analysis for the correct clone was performed against rat CaV1.3 mRNA (GenBank accession number NM017298) before subcloning into rat CaV1.342 clone.
FIGURE 3.
Correction of cloning error in the original CaV1.342 clone to CaV1.3A2123V. A, amino acid alignments of the CTM region for long variants of human, mouse, and rat CaV1.3 (GenBank accession numbers: human, NM000720; mouse, NM028981; rat, NM017298). Positions of exons 48 and 49 are given. Highlighted green residues mark the differences between these variants. Highlighted purple residues mark the position whereby alanine is observed in position 2123 in rat CaV1.342 clone (GenBank accession number D38101) instead of valine. B, top, direct sequencing results of rat clone CaV1.342 clone. The peptide combination is listed in black; bottom, direct sequencing results of RT-PCR amplified from rat brain. Highlighted purple residues mark the position of cloning error. C, representative IBa (gray) and ICa (black) traces during depolarization to 10 mV. The IBa and ICa traces were scaled to enable comparison between the two profiles. Current scales were drawn for both IBa (gray) and ICa (black). The time scales for each IBa and ICa pair are the same. D, normalized I-V plots for IBa (top) and ICa (bottom) of CaV1.3A2123V and CaV1.342A. The curves were fitted with the equation described under “Experimental Procedures.” E, calcium-dependent inactivation of current through CaV1.3A2123V and CaV1.342A. The fraction of the peak current, Ipeak, remained at time intervals of 300 ms upon depolarization to the indicated voltages for CaV1.3A2123V channels. The difference between the remaining current for IBa and ICa, f value, indicates the strength of CDI. The presence of CDI is also marked by “U”-shaped dependence of ICa on voltage (i.e. CaV1.342A, black curves). The curves are visual fits of the values plotted to facilitate comparison. The number of cells recorded are given in parentheses.
We systematically investigated the biophysical properties of both IBa and ICa for the corrected clone CaV1.3A2123V and CaV1.342A as illustrated in Fig. 3, C–E, and Tables 1–3. The current trace profile for CaV1.342A displayed a slower inactivating IBa compared with the corrected clone CaV1.3A2123V (Fig. 3C, gray traces), but showed an early and much pronounced inactivation of ICa (Fig. 3C, black traces). In the I-V curves, CaV1.342A showed a pronounced shift in the hyperpolarized direction, indicating a more negatively activating channel in both Ba2+ and Ca2+ (Fig. 3D). This was also reflected in a pronounced hyperpolarized shift in voltage for half-maximal activation, V½act, by 11.22 mV in Ba2+ (see Table 1) and 10.87 mV in Ca2+ (see Table 2) with respect to CaV1.3A2123V (both p < 0.001; unpaired t test). This negative shift was because of the significant decrease in slope of activation, kact, by 2.43 mV in Ba2+ (see Table 1) and 2.57 mV in Ca2+ (see Table 2). From the profiles of the exemplary traces in Fig. 3C, it is clear that the corrected CaV1.3A2123V clone is not deprived of CDI, it is 2-fold smaller than the short variant CaV1.342A (f−10, 300 ms, CaV1.3A2123V, 0.35 ± 0.03, n = 14; CaV1.342A, 0.75 ± 0.04, n = 13). Hence, correction of alanine to valine in position 2123 in the rat CaV1.342 clone was sufficient to replicate the diminished CDI observed in human CaV1.342. This confirms previous experiments by Liu et al. (19) that the valine residue is crucial for interaction of the CTM with the calcium-sensing apparatus in the proximal C terminus and could greatly repress the CDI of CaV1.3 channels.
TABLE 1.
Comparison of IBa electrophysiological properties of CaV1.3 channels containing long form (CaV1.3A2123V), short form (CaV1.342A), and splice variants Δ41, 43S-2, Δ44, and 48S
| Construct |
I-V |
G-V |
SSI |
||||
|---|---|---|---|---|---|---|---|
| Vrev | V1/2 act | kact | V1/2 act | kact | V1/2 inact | kinact | |
| mV | mV | mV | |||||
| Cav1.3A2123V | 49.29 ± 0.61 | −24.33 ± 0.49 | −8.03 ± 0.49 | −10.50 ± 0.95 | 15.13 ± 0.91 | −44.24 ± 0.47 | 5.90 ± 0.40 |
| Cav1.342A | 41.59 ± 0.79a | −35.55 ± 0.53a | −5.60 ± 0.40a | −30.44 ± 0.84a | 8.57 ± 0.74a | −40.7 ± 0.79a | 4.79 ± 0.71a |
| Cav1.3Δ41 | 52.85 ± 0.61a | −20.37 ± 0.48a | −7.37 ± 0.33a | −12.68 ± 0.57a | 10.72 ± 0.51a | −41.05 ± 0.63 | −7.03 ± 0.55a |
| Cav1.343S-2 | 48.20 ± 1.22b | −32.34 ± 0.61a | −4.82 ± 0.47a | −32.24 ± 0.57a | 7.59 ± 0.50a | −43.02 ± 0.31 | 3.89 ± 0.23a |
| Cav1.3Δ44 | 41.59 ± 0.81a | −33.78 ± 0.60a | −5.64 ± 0.45a | −28.75 ± 0.88a | 10.23 ± 0.78a | −46.57 ± 1.10 | −4.83 ± 0.88a |
| Cav1.348S | 42.04 ± 0.74a | −35.39 ± 0.49a | −5.34 ± 0.37a | −28.72 ± 0.65a | 9.19 ± 0.65a | −45.80 ± 0.69a | −4.82 ± 0.57a |
a p < 0.001, compared to CaV1.3A2123V (unpaired t test). Values shown are mean ± S.E.
b p < 0.01, compared to CaV1.3A2123V (unpaired t test). Values shown are mean ± S.E.
TABLE 2.
Comparison of ICa electrophysiological properties of CaV1.3 channels
| Construct |
I-V |
||
|---|---|---|---|
| Vrev | V1/2 act | kact | |
| mV | |||
| CaV1.3A2123V | 62.12 ± 0.72 | −12.28 ± 0.53 | −9.39 ± 0.30 |
| CaV1.342A | 58.61 ± 0.73a | −23.15 ± 0.39a | −6.82 ± 0.27a |
| CaV1.3Δ41 | 62.21 ± 0.90 | −11.37 ± 0.71a | −9.61 ± 0.40 |
| CaV1.343S-2 | 57.89 ± 1.18 | −22.60 ± 0.74a | −8.1 ± 0.48a |
| CaV1.3Δ44 | 62.12 ± 0.72a | −12.28 ± 0.53a | −9.39 ± 0.30a |
| CaV1.348S | 58.61 ± 0.73a | −23.15 ± 0.39a | −8.10 ± 0.48a |
a p < 0.001, compared to CaV1.3A2123V (unpaired t test). Values shown are mean ± S.E.
TABLE 3.
Comparison of the kinetics of recovery from inactivation in Ba2+
| Construct | ιf | ιS |
|---|---|---|
| s | ms | |
| CaV1.3A2123V | 0.61 ± 0.12 | 7.74 ± 2.97 |
| CaV1.342A | 0.76 ± 0.22a | 20.84 ± 7.45a |
| CaV1.3Δ41 | 0.63 ± 0.11a | 7.65 ± 2.42a |
| CaV1.343S-1 | 0.44 ± 0.09a | 7.08 ± 2.86a |
| CaV1.3Δ44 | 0.40 ± 0.09a | 24.07 ± 10.05a |
| CaV1.348S | 0.53 ± 0.12a | 8.51 ± 3.69a |
a p < 0.001, compared to Cav1.3A2123V (unpaired t test). Values shown are mean ± S.E.
Identification and Expression of Novel C-terminal Spliced CaV1.3 Channels
Transcript scanning of CaV1.3 from rat brain cDNA revealed alternative splicing in five loci on the C terminus, resulting in at least eight splice variants, namely CaV1.342, CaV1.342A, CaV1.3IQΔ, CaV1.3Δ41, CaV1.343S, CaV1.343S-2, CaV1.3Δ44, and CaV1.348S. Altogether there are three alternative splice acceptor sites, two exon skippings, one cassette exon, and one intron retention. In all cases, use of the canonical “gt … ag” splice junctions in the alternative forms was preserved (supplemental Fig. S3). To quantify the frequency of various C-terminal splice isoforms, a first round RT-PCR using primers situated on flanking e37 and 3′ UTR was performed. This was followed by colony PCR of bacterial colonies containing plasmids subcloned with PCR products of various splice variants. Agarose gel electrophoresis of PCR products isolated from each colony will help identify and enumerate the frequency of occurrence of each splice variant. Of 237 bacterial colonies screened, CaV1.343S appeared to be the most abundant (44%), followed by CaV1.342 (16.1%), CaV1.3IQΔ (13.5%), CaV1.3Δ44 (9.7%), CaV1.348S (6.3%), CaV1.343S-2 (5.1%), and CaV1.3Δ41 (4.6%) (supplemental Table S1). We next characterized the four novel splice variants, which alters the C terminus of CaV1.3 (Fig. 4A). CaV1.3Δ41 deletes exon 41 and truncates the C terminus beyond the EF-hand, whereas CaV1.343S-2 deletes the constitutive “intron” in exon 43 and removes the DCRD of CaV1.342. Length altering CaV1.3Δ44 and CaV1.348S splice variants shortened the length between PCRD and DCRD, and may hence remove the secondary structures critical for CTM interaction with the proximal C terminus. All four splice variants may modulate CTM function and affect inactivation properties of the channel. To characterize these splice variants, the sequence alterations in these exons were genetically engineered into the C terminus sequence of CaV1.342 constructs, and compared against this predominant reference isoform. One other novel splice variant that alters the C terminus of CaV1.3, CaV1.343S, was further characterized in the accompanying article (16).
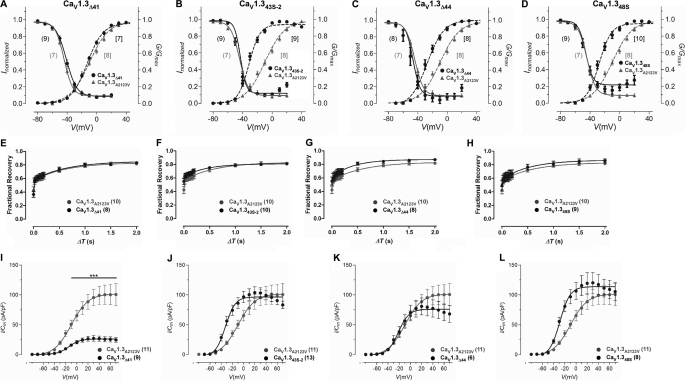
FIGURE 4.
Current-voltage relationships of CaV1.3 alternatively spliced variants. A, schematic representation of alternatively spliced CaV1.3 channel constructs. The channel backbone consists of CaV1.3 (GenBank accession number: D38101, white box), whereas the cytosolic tail consists of CaV1.3 long form (CaV1.342), or alternatively spliced variants IQΔ, Δ41, 43S, 43S-2, Δ44, and 48S (black). The stop codons for IQΔ, Δ41, 43S, and 43S-2 are indicated by black and white filled circles. Numbering follows the CaV1.3 amino acid sequence. B–E, representative IBa (gray) and ICa (black) traces during depolarization to 10 mV for alternatively spliced constructs Δ41, 43S-2, Δ44, and 48S. The IBa and ICa traces were scaled to enable comparison between the two profiles. Current scales were drawn for both IBa (gray) and ICa (black). The time scales for each IBa and ICa pair are the same. F–I, normalized I-V plots for IBa of alternatively spliced constructs Δ41, 43S-2, Δ44, and 48S. The curves were fitted with the equation described under “Experimental Procedures.” In parentheses are the number of cells recorded. J-M, same as F-I, but for ICa. N-Q, calcium-dependent inactivation of current through alternatively spliced variants Δ41, 43S-2, Δ44, and 48S. The fraction of peak current, Ipeak, remained at time intervals of 300 ms upon depolarization for IBa and ICa. f value indicates the strength of the CDI. The curves are visual fits of the values plotted to facilitate comparison. The number of cells recorded are given in parentheses.
Alternative Splicing in CaV1.3 C-terminal Modulates CDI
The current trace profile for CaV1.343S-2 exhibited a slower inactivating IBa compared with CaV1.3A2123V or the other three splice variants (Fig. 4, B–E, gray traces), but showed an early and much pronounced inactivation of ICa (Fig. 4, B–E, black traces). All splice variants except for CaV1.3Δ41 activated at more negative potentials as observed by the hyperpolarized shift in V½ act in both Ba2+ (CaV1.343S-2 by 8.01 mV; CaV1.3Δ44 by 9.45 mV; CaV1.348S by 11.22 mV; see Fig. 4, F–I, and Table 1) and Ca2+ (CaV1.343S-2 by 7.07 mV; CaV1.3Δ44 by 12.29 mV; CaV1.348S by 10.32 mV; see Fig. 4, J–M, and Table 2) with respect to CaV1.3A2123V (p < 0.001; unpaired t test). This shift is predominantly caused by a decrease in the slope of activation kact (see Tables 1 and 2). The hyperpolarized shift observed in CaV1.3Δ44 was not observed in the equivalent human clone (9), possibly due to unidentified amino acid differences between the rat and human clones at the regions governing gating. CaV1.3Δ41 showed a slight, although statistically significant (p < 0.001; unpaired t test), depolarized shift in V½ act by 3.96 mV in Ba2+ and 0.91 mV in Ca2+, with a slight increase in the slope of activation (kact, increased by 0.66 in Ba2+). As expected, deletion of exon 41 in CaV1.3Δ41, which removed the entire IQ motif, resulted in a very small degree of inactivation by Ca2+ even at 300 ms (f−10, 0.15 ± 0.02; see Fig. 4N). Alternative splicing of exon 43 in CaV1.343S-2, which caused a frameshift and deletion of CTM, exhibited a very pronounced CDI, comparable with that of CaV1.342A (f−10, 0.74 ± 0.03; see Fig. 4O). Skipping of exon 44 and the use of the alternative acceptor site on exon 48 results in two splice variants, CaV1.3Δ44 and CaV1.348S, which retained CTM and exhibit smaller f−10 than CaV1.342A (f−10, CaV1.3Δ44, 0.64 ± 0.02; CaV1.348S, 0.68 ± 0.02; see Fig. 4, P and Q). It is plausible that the secondary structure changes brought about by these splice variants may affect the efficacy of interaction between the DCRD and the calcium-sensing apparatus.
Hyperpolarized Shifts in Activation and Inactivation Properties of CaV1.3 Splice Variants
To enable a more accurate assessment of the voltage activation of these channels, we analyzed the tail currents (G) obtained at the end of a short depolarizing pulse to various potentials. In addition, to determine the inactivation properties of the channels under steady-state conditions, transfected cells were held at various potentials for 15-s and currents evoked before and after the inactivating pulse were compared. The data obtained are displayed in Fig. 5, A–D, and Table 1.
FIGURE 5.
Voltage-dependent electrophysiological properties of splice variants CaV1.3Δ41, CaV1.343S-2, CaV1.3Δ44, and CaV1.348S. A–D, activation and steady-state inactivation properties. Normalized plots for activation (dashed lines) and steady-state inactivation (solid lines) for long form (CaV1.3A2123V) and alternatively spliced constructs Δ41, 43S-2, Δ44, and 48S. For steady-state inactivation, peak currents obtained after the 15-s inactivating pulse were normalized to that obtained before inactivation and plotted against voltage. The curves are fitted with the Boltzmann relationship. For the activation plots, the peak of the tail currents (G) were normalized against the largest peak and plotted against voltage. The curves were fitted using the equation given under “Experimental Procedures.” The number of cells recorded are given in parentheses, “(/)” for steady-state inactivation and “[/]” for activation. Plots of the long form (CaV1.3A2123V) were redrawn in each graph for comparison. E–G, recovery from inactivation. Fractional recovery was plotted as a function of ΔT for CaV1.3A2123V and splice variants Δ41, 43S-2, Δ44, and 48S, respectively. The curve for CaV1.3A2123V was redrawn for all plots for comparison. Curves were fitted as described under “Experimental Procedures.” I–L, density of Ba2+ currents through CaV1.3A2123V and splice variants Δ41, 43S-2, Δ44, and 48S. The peak of tail currents measured at the end of short depolarizing pulses evoked at different potentials were normalized against the membrane capacitance (Cm) of the recorded cell to obtain current density. The number of cells are indicated in the parentheses. ***, p < 0.001 (compared with CaV1.3A2123V) (Kruskal-Wallis test followed by Dunn's multiple comparison post test).
Just as in the I-V analyses, the novel splice variants produced a hyperpolarized shift in V½ act of the tail current analyses compared with CaV1.3A2123V. The changes observed here were also significant (V½ act, CaV1.3Δ41, −12.68 ± 0.57, n = 7; CaV1.343S-2, −32.24 ± 0.57, n = 9; CaV1.3Δ44, −28.75 ± 0.88, n = 8; CaV1.348S, −28.72 ± 0.65, n = 10; p < 0.001 unpaired t test). The activation slope factors for the splice variants were significantly smaller than CaV1.3A2123V. In addition, all four splice variants had small shifts in V½ inact.
The recovery of the channels, here, may be described as occurring in two-phases: a fast recovery phase (ΔT ≈ 0–40 ms) and a slow recovery phase (ΔT ≈ 60 ms onwards). In CaV1.3A2123V, recovery began rapidly and about 56% of the channels were recovered by 20 ms, near the beginning of the slow phase. However, the subsequent rate of recovery was decreased and only ∼82% of channels were recovered at the end of 2 s (Fig. 5E, gray traces). CaV1.3Δ41 channels displayed a similar recovery profile as CaV1.3A2123V (Fig. 5E, black traces), reflected in similar slope values of the fast and slow phases (Table 3). CaV1.343S-2, like CaV1.3A2123V, began with rapid recovery at the early part of the slow phase, where ∼66% of the channels had recovered by 100 ms (Fig. 5F). Despite the slightly more gentle slope than CaV1.3A2123V at the late slow phase, ∼81% of channels were recovered by 2 s. CaV1.3Δ44 started with rapid recovery near the early part of the slow phase, reaching ∼60% recovery of channels by 20 ms, respectively (Fig. 5G). It has a steeper slope than CaV1.3A2123V in the fast phase (τf, CaV1.3A2123V, 7.74 ± 2.91 ms; CaV1.3Δ44, 24.07 ± 10.05 ms; p = 0.06, unpaired t test) and a gentler slope in the slow phase (τs, CaV1.3A2123V, 0.61 ± 0.12 s; CaV1.3Δ44, 0.40 ± 0.09 s; p = 0.10, unpaired t test). Recovery of CaV1.3Δ44 channels reached ∼87% by 2 s in the slow phase, slightly greater than the ∼82% recovery of CaV1.3A2123V channels. CaV1.348S, like CaV1.3A2123V, began with rapid recovery at the early part of the slow phase, where ∼63% of the channels had recovered by 100 ms (Fig. 5H). Despite the slightly more gentle slope than CaV1.3A2123V at the late slow phase, ∼86% of channels were recovered by 2 s, which is slightly greater than that of CaV1.3A2123V channels.
Deletion of Exon 41 in CaV1.3 Decreases Current Density
Alternative splicing of CaV1.3 in the C terminus results in short variant CaV1.342A with a gross increase in current density (9). We hence measured the current density from the novel CaV1.3 C terminus splice variants and compared the density against CaV1.3A2123V. The current density of CaV1.3Δ41 was 3.3 times smaller than CaV1.3A2123V (see Fig. 5I, CaV1.3A2123V: 88.39 ± 12.48 pA/pF, CaV1.3Δ41: 26.53 ± 4.22 pA/pF; p < 0.001, Kruskal-Wallis test, followed by Dunn's multiple comparison post test). Even when depolarized to −40 mV, the current density was also significantly smaller (CaV1.3A2123V: 8.15 ± 1.29 pA/pF, CaV1.3Δ41: 2.06 ± 0.30 pA/pF; p < 0.001, Kruskal-Wallis test, followed by Dunn's multiple comparison post test). CaV1.3Δ44 also sustained a small, but not statistically significant decrease in current density compared with CaV1.3A2123V (see Fig. 5K). In contrast, however, CaV1.343S-2 and CaV1.348S channels displayed small increases in current density to CaV1.3A2123V (see Fig. 5, J and L), but these were not statistically significant.
DISCUSSION
Here we present systematic identification and functional characterization of the biophysical properties and subcellular localization of alternative splice variants in the C terminus of CaV1.3. Such study has not been described for CaV1.3 channels before. Alternative splicing of the C terminus affects the modulatory effects of CTM in CaV1.342, providing functional diversity to CaV1.3 channels in rat brain tissues. Activation at negative voltages is one hallmark of CaV1.3 channels crucial for adequate neurotransmitter release in inner hair cells and pacemaker function in the sinoatrial node (20) and in neuronal excitability (6, 21). Deletion of CTM and shortening the intervals between PCRD and DCRD causes a hyperpolarized shift of V½ act by about 10 mV by decreasing the slope factor of the activation curves.
The functional properties of CaV1.342 and CaV1.342A channels have been studied in previous reports. Activation of calcium current through CaV1.342A channels was more pronounced at negative voltages, and inactivation was faster because of enhanced CDI. It was determined by CaV1.3 channel truncations that the modulatory activity was restricted to the last 116 amino acids of the C terminus and involves interaction of a DCRD with a PCRD as shown by mutation analysis (9). Gating properties of these CaV1.3Δ116 channels, which lack the DCRD, were reversed by co-expression of corresponding C-terminal peptide C116. Intramolecular protein interaction in the C terminus of CaV1.3 channels modulates calmodulin binding (9, 19, 22). Our study used two splice variant-specific antibodies, one recognizing the long terminus of CaV1.342, whereas the other recognizes the short terminus of CaV1.342A, to show similar expression patterns for both splice isoforms in mouse brain. Stronger expression of both splice isoforms was selectively detected in specific brain regions, namely the paraventricular hypothalamic nucleus and locus coeruleus of the brain stem (Fig. 2), where CaV1.3 were shown to be selectively stimulated by Bay K8644 in CaV1.2DHP−/− mice (23). Surprisingly, weaker staining of both splice isoforms were observed in the central amygdala, the bed nucleus of the stria terminalis, and the lateral preoptical area, where significant Bay K8644-induced Fos expression was observed (23). It was speculated that selective stimulation of CaV1.3 channels restricts neuronal activation to a specific set of mainly limbic, hypothalamic and brainstem areas, which are associated with brain functions concerning integration of emotion/depression-related behavior (24). It would be interesting to characterize the expression profile of CaV1.3 splice variants in these specific regions, especially because quantitative PCR using the TaqMan gene expression assay in our accompanying article (16) has shown ∼60% CaV1.342 and ∼5% CaV1.342A mRNA expression in amygdala, leaving 35% unaccounted C-terminal splice variants. Although quantitative differences at the mRNA levels may not necessarily correlate with protein expression differences, it allows us to speculate on the channel isoform primarily responsible for the observed activity-dependent responses.
Our work has shown that besides exons 42 and 42A, alternative splicing in other exons of the C-terminal generates three classes of functionally distinct CaV1.3 channel variants. Alternative splicing of exon 41 causes either deletion of only the IQ domain or the entire exon 41, causing a premature stop codon and CaV1.3 channel without the IQ domain pertinent to CaM interaction and hence CDI. Downstream of exon 42, alternative splicing occurring in exons 43, 44, and 48 could either delete the CTM or shorten the distance between PCRD and DCRD. The implications of retaining just the PCRD when alternative splicing occurs at exon 43 (CaV1.343S) were examined in our accompanying article (16). A similar degree of hyperpolarized shift in activation and inactivation properties was observed in splice variant CaV1.343S-2, with robust CDI that closely resembles that of CaV1.342A. Alternative splicing in exons 44 and 48 are in-frame. The resultant secondary protein structure (i.e. α-helices) between PCRD and DCRD and hence, efficacy of interaction between these two domains may be altered, causing a less robust CDI. Alternative splicing in the C terminus generates CaV1.3 channel isoforms with a spectrum of electrophysiological properties. Differences in the inactivation pattern of CaV1.3 C-terminal splice isoforms could underlie different shapes or firing rates of action potentials, as observed in different types of neurons (25). Interaction of CaV1.3 with the dihydropyridine (DHP) antagonist is state-dependent, and alternative splicing of the CaV1.3 C-terminal should also have a strong impact on the efficiency of DHP inhibition as this correlates with the amount of CaV1.3 channels inactivated (4, 5).
Two studies using CaV1.342 and CaV1.342A have shown that CaM-like Ca2+-binding proteins (CaBPs) are integral subunits and modulators of CaV channels, suppressing its inactivation (26, 27). In both studies, CaBP1 strongly inhibits inactivation of ICa and IBa through interactions with the IQ domain and N-terminal of CaV1.3, whereas CaBP4 appears to modulate CDI differentially depending on the CaV1.3 isoform used. CaBP family members (CaBP1 through CaBP5) have varying expression patterns, and only CaBP1 and CaBP2 can be expressed as multiple, alternatively spliced variants in the brain and retina, whereas CaBP3 through CaBP5 are restricted to retinal rod and cone cells (28). Hence, it might be interesting to examine the modulation of these CaM-like proteins in the brain on electrophysiological properties of CaV1.3 C-terminal splice variants, especially given the differential modulation on CDI and current densities observed in CaV1.3Δ41, CaV1.3Δ44, and CaV1.348S, which could potentially expand the baseline CaM regulatory profile of the various Ca2+ signaling proteins in the nervous system. Besides CaBPs, CaVβ subunits were found to increase membrane expression of CaV1.2 channels via ubiquitination and stability of the calcium channel complex (29). Two endoplasmic reticulum retention motifs were identified in the proximal C-terminal region of these CaV1.2 channels, which are highly conserved in CaV1.3 channels. Although co-expression of the CaVβ subunit did not alter retention of the CaV1.2 C terminus in the endoplasmic reticulum, it mediated a marked increase in cell surface and total protein expression of the full-length channel (29). In CaV1.3Δ41, both endoplasmic reticulum retention motifs are removed via alternative splicing and could alter the cell surface expression and hence decrease the current density. For exploring the mechanism by which splicing alters calcium currents, we further characterized ON-gating currents (QON) via depolarizing cells to positive potentials at which no ionic inward and outward currents were observed (see supplemental Fig. S4). QON reflects the capacitative voltage-sensor movements upon depolarization during channel gating. Alternative splicing of exon 42A removes the CTM and results in a ∼2-fold greater QON compared with CaV1.342, whereas removal of exon 41 causes a ∼4-fold decline in QON compared with CaV1.342. This suggests that alternative splicing of exon 41 may decrease current density via down-regulation of functional surface expression of the channels. However, although the current densities of CaV1.343S-2 and CaV1.3Δ44 are not significantly altered as compared with CaV1.342, their QON values were significantly decreased as well.
In the brain, CaV1.3 channels couple neuronal activity to transcriptional events, mediating long term potentiation in the amygdala and participating in the consolidation of fear memory (30). The CTM in the long CaV1.342 variant may be suitable for longer lasting Ca2+ signals triggered by stronger depolarization inducing CREB phosphorylation and synaptic plasticity, and the ITTL motif in CTM is crucial for interaction with the macromolecular signaling complex formed by Shank (31). It would be interesting to determine whether alternative splicing in the C terminus of CaV1.3 alters its predominantly soma-dendritic localization or its synaptic clustering, and whether substitution of the ITTL motif may alter its coupling with Shank and other adaptor proteins at the postsynaptic density to influence pCREB signaling.
Supplementary Material
Acknowledgments
We greatly appreciate the gift of rat CaV1.342 and CaV1.342A cDNA from Dr. Diane Lipscombe (Brown University, RI) and rat β and rat α2δ cDNA from Dr. Terry P. Snutch (University of British Columbia, Vancouver, British Columbia, Canada). We also thank Dr. Jörg Striessnig and Dr. Alexandra Koschak for invaluable advice on the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant RO1DC00276 and the Singapore Biomedical Research Council.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S5 and Table S1.
- CDI
- calcium-dependent inactivation
- CaM
- calmodulin
- CTM
- C-terminal modulator
- PCRD
- primal C-terminal regulatory domain
- DCRD
- distal C-terminal regulatory domain
- CaBP
- CaM-like calcium-binding protein
- DHP
- dihydropyridine
- SSI
- steady-state inactivation.
REFERENCES
- 1. Dunlap K., Luebke J. I., Turner T. J. (1995) Trends Neurosci. 18, 89–98 [PubMed] [Google Scholar]
- 2. Hell J. W., Westenbroek R. E., Warner C., Ahlijanian M. K., Prystay W., Gilbert M. M., Snutch T. P., Catterall W. A. (1993) J. Cell Biol. 123, 949–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lipscombe D., Helton T. D., Xu W. (2004) J. Neurophysiol. 92, 2633–2641 [DOI] [PubMed] [Google Scholar]
- 4. Xu W., Lipscombe D. (2001) J. Neurosci. 21, 5944–5951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koschak A., Reimer D., Huber I., Grabner M., Glossmann H., Engel J., Striessnig J. (2001) J. Biol. Chem. 276, 22100–22106 [DOI] [PubMed] [Google Scholar]
- 6. Olson P. A., Tkatch T., Hernandez-Lopez S., Ulrich S., Ilijic E., Mugnaini E., Zhang H., Bezprozvanny I., Surmeier D. J. (2005) J. Neurosci. 25, 1050–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Helton T. D., Xu W., Lipscombe D. (2005) J. Neurosci. 25, 10247–10251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hui A., Ellinor P. T., Krizanova O., Wang J. J., Diebold R. J., Schwartz A. (1991) Neuron 7, 35–44 [DOI] [PubMed] [Google Scholar]
- 9. Singh A., Gebhart M., Fritsch R., Sinnegger-Brauns M. J., Poggiani C., Hoda J. C., Engel J., Romanin C., Striessnig J., Koschak A. (2008) J. Biol. Chem. 283, 20733–20744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liang H., DeMaria C. D., Erickson M. G., Mori M. X., Alseikhan B. A., Yue D. T. (2003) Neuron 39, 951–960 [DOI] [PubMed] [Google Scholar]
- 11. Soldatov N. M., Zühlke R. D., Bouron A., Reuter H. (1997) J. Biol. Chem. 272, 3560–3566 [DOI] [PubMed] [Google Scholar]
- 12. Shen Y., Yu D., Hiel H., Liao P., Yue D. T., Fuchs P. A., Soong T. W. (2006) J. Neurosci. 26, 10690–10699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gomez-Ospina N., Tsuruta F., Barreto-Chang O., Hu L., Dolmetsch R. (2006) Cell 127, 591–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mittman S., Guo J., Agnew W. S. (1999) Neurosci. Lett. 274, 143–146 [DOI] [PubMed] [Google Scholar]
- 15. Soong T. W., DeMaria C. D., Alvania R. S., Zweifel L. S., Liang M. C., Mittman S., Agnew W. S., Yue D. T. (2002) J. Neurosci. 22, 10142–10152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bock G., Gebhart M., Scharinger A., Jangsangthong W., Busquet P., Poggiani C., Sartori S., Mangoni M., Sinnegger-Brauns M. J., Herzig S., Striessnig J., Koschak A. (2011) J. Biol. Chem. 286, 42736–42748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Patil P. G., Brody D. L., Yue D. T. (1998) Neuron 20, 1027–1038 [DOI] [PubMed] [Google Scholar]
- 18. Peterson B. Z., DeMaria C. D., Adelman J. P., Yue D. T. (1999) Neuron 22, 549–558 [DOI] [PubMed] [Google Scholar]
- 19. Liu X., Yang P. S., Yang W., Yue D. T. (2010) Nature 463, 968–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mangoni M. E., Couette B., Bourinet E., Platzer J., Reimer D., Striessnig J., Nargeot J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 5543–5548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bean B. P. (2007) Nature 447, 1059–1060 [DOI] [PubMed] [Google Scholar]
- 22. Singh A., Hamedinger D., Hoda J. C., Gebhart M., Koschak A., Romanin C., Striessnig J. (2006) Nat. Neurosci. 9, 1108–1116 [DOI] [PubMed] [Google Scholar]
- 23. Hetzenauer A., Sinnegger-Brauns M. J., Striessnig J., Singewald N. (2006) Neuroscience 139, 1005–1015 [DOI] [PubMed] [Google Scholar]
- 24. Manji H. K., Drevets W. C., Charney D. S. (2001) Nat. Med. 7, 541–547 [DOI] [PubMed] [Google Scholar]
- 25. Bean B. P. (2007) Nat. Rev. Neurosci. 8, 451–465 [DOI] [PubMed] [Google Scholar]
- 26. Cui G., Meyer A. C., Calin-Jageman I., Neef J., Haeseleer F., Moser T., Lee A. (2007) J. Physiol. 585, 791–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang P. S., Alseikhan B. A., Hiel H., Grant L., Mori M. X., Yang W., Fuchs P. A., Yue D. T. (2006) J. Neurosci. 26, 10677–10689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haeseleer F., Sokal I., Verlinde C. L., Erdjument-Bromage H., Tempst P., Pronin A. N., Benovic J. L., Fariss R. N., Palczewski K. (2000) J. Biol. Chem. 275, 1247–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Altier C., Garcia-Caballero A., Simms B., You H., Chen L., Walcher J., Tedford H. W., Hermosilla T., Zamponi G. W. (2011) Nat. Neurosci. 14, 173–180 [DOI] [PubMed] [Google Scholar]
- 30. Gamelli A. E., McKinney B. C., White J. A., Murphy G. G. (2011) Hippocampus 21, 133–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang H., Fu Y., Altier C., Platzer J., Surmeier D. J., Bezprozvanny I. (2006) Eur. J. Neurosci. 23, 2297–2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peterson B. Z., Lee J. S., Mulle J. G., Wang Y., de Leon M., Yue D. T. (2000) Biophys. J. 78, 1906–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Erickson M. G., Liang H., Mori M. X., Yue D. T. (2003) Neuron 39, 97–107 [DOI] [PubMed] [Google Scholar]
- 34. Pitt G. S., Zühlke R. D., Hudmon A., Schulman H., Reuter H., Tsien R. W. (2001) J. Biol. Chem. 276, 30794–30802 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.