Abstract
This study describes the functioning of a novel sensor to measure cortisol concentration in the interstitial fluid (ISF) of a human subject. ISF is extracted by means of vacuum pressure from micropores created on the stratum corneum layer of the skin. The pores are produced by focusing a near infrared laser on a layer of black dye material attached to the skin. The pores are viable for approximately three days after skin poration. Cortisol measurements are based on electrochemical impedance (EIS) technique. Gold microelectrode arrays functionalized with Dithiobis (succinimidyl propionate) self-assembled monolayer (SAM) have been used to fabricate an ultrasensitive, disposable, electrochemical cortisol immunosensor. The biosensor was successfully used for in-vitro measurement of cortisol in ISF. Tests in a laboratory setup show that the sensor exhibits a linear response to cortisol concentrations in the range 1 pm to 100 nM. A small pilot clinical study showed that in-vitro immunosensor readings, when compared with commercial evaluation using enzyme-linked immunoassay (ELISA) method, correlated well with cortisol levels in saliva and ISF. Further, circadian rhythm could be established between the subject's ISF and the saliva samples collected over 24 hours time-period. Cortisol levels in ISF were found reliably higher than in saliva. This Research establishes the feasibility of using impedance based biosensor architecture for a disposable, wearable cortisol detector. The projected commercial in-vivo real-time cortisol sensor device, besides being minimally invasive, will allow continuous ISF harvesting and cortisol monitoring over 24 hours even when the subject is asleep. Forthcoming, this sensor could be interfaced to a wireless health monitoring system that could transfer sensor data over existing wide-area networks such as the internet and a cellular phone network to enable real-time remote monitoring of subjects.
Keywords: Interstitial fluid (ISF), Cortisol monitoring, enzyme-linked immunoassay (ELISA), stratum corneum, biological liquids, biomedical transducers, medical services, cortisol, self assembled monolayer (SAM), electrochemical impedance, immunosensor, Dithiobis(succinimidyl propionate), disposable biosensor
Introduction
We are proposing to make a rapid device for detecting cortisol levels in interstitial fluid (ISF). A rapid cortisol test developed during our NIH funded Phase I (1R43MH085474-01, SBIR) proposal in collaboration with the University of South Florida (USF). [1,Arya et. al. 2010, 2Arya et. al, 2010] will be integrated in this device to develop a portable test for real-time and continuous measurements of personal exposure to psychosocial stress via measuring cortisol levels in ISF. Sampling of biochemical markers in the body via blood or saliva plays an integral role in the diagnosis and management of several diseases tied to cortisol levels in the body. However, due to its invasive nature blood sampling is less suitable for use in nonclinical settings especially when a continuous measurement is desirable. Saliva, on the other hand, requires constant compliance from the patient. ISF is especially attractive for cortisol monitoring, as it can be drawn continuously from the dermis through an ablated stratum corneum by simply applying a small amount of vacuum. ISF offers less invasive and compliance free method for monitoring levels of cortisol in the body. ISF is an extra cellular fluid that surrounds the cells in the human body and consists of small and moderate sized molecules, including glucose, ethanol, and cortisol. In composition, ISF is similar to blood plasma. The homeostatic feedback loop in the body ensures that these molecules have a direct correlation to the concentration of molecules in blood [3Stout et. al., 2004; 4Bentle and Thomas, 1997]. Metabolites and proteins move into ISF as they move from capillaries to cells. Consequently, the metabolite concentration in ISF is correlated to their concentration in the capillaries. The difference in concentration is based in part on molecular weight [5Fischer, 1994 and 6Sternberg, 1995]. ISF can be harvested using a minimally invasive method. The site of ISF extraction can be almost anywhere on the human body (arms, abdomen, legs) with no loss of accuracy. The application of vacuum pressure to extract ISF significantly reduces the integration time required for accurately determining the metabolite concentration. The lag between blood and ISF levels can contribute to measurement error in continuous monitoring systems. 3Stout et. al., showed that the modulated pressure application mitigated the ISF physiological error by an average of 95%. Clinical tests involving diabetic patients have shown that the correlation between ISF glucose concentration and blood glucose levels is as high as 0.90 in the 60–400 mg/dl glucose range [7Gebhart, et. al., 2003]. Using this methodology, Gebhart et al., reported correlations of 0.87 and 0.95 between blood and ISF glucose. This technique has also been successfully used for continuous monitoring of glucose [8Daniloff, 2005]. In general, small to moderate sized molecules, including glucose and ethanol, are found in ISF in the same proportion as in blood. Thus, periodic calibration using blood sampling is not required to obtain the concentration of these metabolites from ISF (larger molecules such as certain lipids also are detectable in this body fluid, but at a reduced concentration relative to blood [9Bentle and Thomas, 1997 and 10Klonoff, 1997].
Cortisol has been identified as a key element in the psychobiology of the stress response and therefore it can be used as a biomarker of stress. Cortisol has a range of roles in the body. It helps break proteins, glucose, and lipids, maintain blood pressure, and regulate the immune system. Inadequate amounts of cortisol can cause nonspecific symptoms such as weight loss, muscle weakness, fatigue, low blood pressure, and abdominal pain. Too much cortisol can cause increased blood pressure, high blood sugar, obesity, fragile skin, purple streaks on the abdomen, muscle weakness and osteoporosis. Abnormal levels of cortisol may also influence certain pathological conditions such as type 2 diabetes [11Abdallah et al., 2005; 12Wren and Garner, 2005], constant stress, obesity, and metabolic syndrome. In order to understand any of these factors doctors often have to order a cortisol test. Typically blood will be drawn from a vein in the arm (blood drawn at 8 am, when cortisol is at its peak and then at 4 pm, when level should have dropped), but sometimes urine (24-hour urine test, usually requires collecting all the urine produced during a day and night) or saliva (patients compliance is required to ensure they have not had anything to eat or drink 20 minutes before sample collection) may be used to perform a test. All of these methods of sample collection and testing are not only inconvenient, protracted, and painstaking but also take time to get the results back from the doctor or a laboratory.
Current methods for cortisol testing include the saliva test [13Petkus et al., 2006], the Fluorometric assay [14Appel et al., 2005], Fluorescence Polarization [15Cullum, et. al., 2006] and Reverse Phase Chromatography [16,Gatti et al., 2005]. These methods are, however, limited in sensitivity, time of analysis and cost [17,Cook 1997; 18Kaptein et al., 1997]. None of these methods are rapid and consequently results for cortisol cannot be obtained sequentially. Salivary Cortisol concentrations have become a valuable tool for both basic scientists and clinicians [19Papanicolaou et. al, 2002]. Testing of salivary cortisol has become the method of choice despite many disadvantages. However, none of these methods will allow us to get the nighttime minimum value which can identify the precise point in sleep cycle at which cortisol begins its morning cycle. To date, no rapid, reliable real-time and sequential test is available for cortisol. The major issues limiting the usefulness of prevailing tests are compliance, collection device and contamination of saliva sample. A single time assessment of cortisol level is not ideal as cortisol fluctuates throughout the day due to circadian rhythms and therefore results will differ in “early birds” and “night owls”. Researchers take samples at a specific time during the day, but patients are often non-compliant which can invalidate their results and mask potential differences between subject groups.
In this work, we demonstrate a new technique for ISF sampling for the purpose of near to continuous monitoring of cortisol concentration in the human body over 24 hours. No previous correlation exists for this molecule between ISF and blood or saliva. Therefore, our current work will help to fill up this gap. Our proposed device will be a portable diagnostic tool and will be capable of non-invasively measuring cortisol in real time in ISF. Cortisol sensor being described here will automatically provide sequential cortisol data from the patient over 24 hours with minimal compliance from the patient. Further, a pilot clinical study has been designed to validate the feasibility of making in-vitro measurement of cortisol using the sensor system being developed in collaboration with USF. We developed this electrochemical Impedance system (EIS) for sensing cortisol levels in ISF and saliva [1, 2Arya et. al., 2010]. Our ultimate goal is to combine the ISF harvesting design with the cortisol sensor system in order to develop a rapid sensor that can sequentially measure cortisol in real-time.
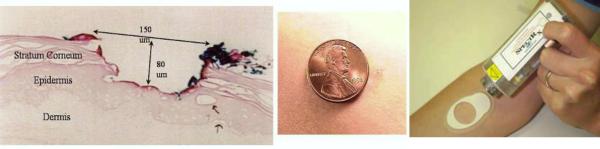
A less invasive and bloodless method for metabolite monitoring involves sampling ISF, which is an extra cellular fluid that surrounds the cells in the human body. ISF is present just below the skin, but the low permeability of the epidermal keratinized layer (the stratum corneum or SC) blocks the permeation of the fluid through the skin. In this paper, we briefly describe a minimally invasive technique to access the ISF through the skin and measure levels of cortisol in the collected fluid. We have used a minimally invasive method for continuously extracting ISF from SC [U.S. patent 6,183,434; Altea MicroPor(TM) Laser]. This method entails focusing a near infrared laser (low-energy) on a layer of black dye material affixed to the skin by an adhesive (Figure 1-Right, 20Venugopal et al., 2008). The interaction of the light at the dye layer causes a pyrotechnic event; thereby creating tiny pores (called micropores) in SC (Figure 1-Left and Middle). The diameter of the micropores is approximately equal to that of a human hair. The micropores only penetrate the SC and, hence, this procedure is essentially painless. ISF is drawn through these micropores continuously by application of a small amount of vacuum pressure.
Figure 1.
Left: Cross-section of a micropore. Note that the pore does not extend to the dermal layer. Middle: Four pores relative to the size of a penny. Right. Micropores are made using a handheld laser source that is focused on black dye attached to the skin.
ISF Harvesting Head (HH) Device
It is well known that the collection device itself has a major influence on accuracy of testing. Some steroid hormones tend to bind to plastic tube, so the most appropriate collection device would be glass which creates serious handling disadvantages. Although special plastic tubes are commercially available from IBL-Hamburg, especially for cortisol collection, subject's compliance and ability to follow instructions correctly affect the quality of the sample. Contamination of saliva samples with trace amounts of blood can also create false elevated levels. In addition, smoking, eating, and drinking beverages containing alcohol, caffeine, or fruit juices can produce false high levels of cortisol. Subjects are, therefore, not allowed to ingest these substances for 20 minutes prior to saliva sample collection. The test described here will be free of the majority of these complications.
During many years of transdermal (TD) ISF harvesting experience, we at Guided Therapeutics have developed several tethered and tetherless devices for harvesting ISF. The tetherless device designs are mostly based on the concept of creating a vacuum difference21. The pressure difference between the fluid volume and the vacuum in the collection tube forces fluid through the needle and into the tube. In this paper we are presenting one of the tetherless harvesting devices that were designed specifically for carefree harvesting of ISF over a period of 24 hours, in intervals of 6 hours, during a pilot clinical study with 6 subjects. Later, this device was also modified for another application for measuring alcohol (funded by NIH, NIDA, 1U01DA023812-01). Details of this work are included in another publication (in preparation).
We designed a harvesting device for sequential harvesting of ISF. First, an alignment ring containing a black dye dot is applied to SC and a porator device is used to make 4 micropores in SC. To harvest ISF, a light vacuum is applied to the poration spot. This was accomplished by using a device as shown in Figure 2. A circular disc containing a small suction cup (1 mm diameter) was attached to another disc such that a continuous path was made when the centers of the bottom and the top disc pieces were aligned, bonded together (Figure 2- Left) and placed on top of the alignment ring, thereby extending the continuous path from the disc to SC. An open ended needle was UV bonded to the top of the continuous channel. A 3 ml glass vial, evacuated to 20 inHg, is inserted into the hollow tube such that when the needle pierces the ice-blue septum in the black lid, the light vacuum from the evacuated vial pulls the porated area of SC with 4 micropores into the skin distention port and the harvesting of ISF begins (Figure 2-Right).
Figure 2.
Left: Design of the current Harvesting Head with top piece bonded with a needle and a bottom piece containing the skin distention port (Left side). A 3 ml glass vial fitted with a silicon septum in the lid is evacuated to 20 inHg with an anemometer and inserted into the hollow tube bonded to the top piece containing the open ended needle (Right side).
Previously, we have used an electrical vacuum pump for continuous harvesting of ISF for a glucose sensor application. For convenience of the subjects during clinical studies for cortisol, we replaced the electrical vacuum pump with a mechanical tetherless version described above. In order to accurately measure cortisol levels in ISF throughout the day, close to continuous supply of ISF is desired. Therefore, ISF was collected in four aliquots every 6 hours over a total of 24 hours. These sequential measurements will be able to show trends in cortisol levels, in contrast to isolated cortisol readings obtained from traditional saliva method. Under the application of small vacuum pressure (15–20 inHg), ISF was drawn continuously at a rate of about 15–20 μl per hour into an ISF collection unit (3 ml vial for this study) which is affixed to the skin with a medical adhesive. The harvested fluid was used for external or in-vitro testing of cortisol using the bench top EIS cortisol sensing device developed at USF. To make this system more robust to any vacuum leaks we evaluated different types of septa and selected the one with least rate of vacuum leak. In Figure 3, continuous path for vacuum application can be seen going from SC to the skin distention port, in the bottom disc, through the conical tunnel in the middle piece followed by the needle piercing through the ice blue septa and finishing inside the vial where the ISF accumulates. We are now using “iceblue” septa from Restek, Part No. 27156, that shows no vacuum leak over 24 hours. This harvesting device was used to collect ISF from subjects during the pilot Phase I clinical study. Even though we only require ISF harvested for 4 sequential measurements over 24 hours, usage of the “iceblue” septa enables us to accomplish un-tethered and sequential harvesting of ISF even over 72 hours, after which the micropores tend to heal due to natural healing process of the skin.
Figure 3.

Harvesting Head, showing the skin distention port in the bottom disc. The porated section of SC gets sucked into the cup inside the bottom disc upon the application of vacuum to the cup via an evacuated vial. Lateral view of black lid with ice blue septa from Restek Inc. can be seen in the vial inserted into the hollow tube.
Our technique offers several advantages. A relatively large amount of ISF is harvested (about 10μl-20μl per hour) so that the requirements for the sensor system are relatively liberal. For clinical validation, we used the ISF harvested from 6 subjects for in-vitro cortisol measurement and analysis. Due to the nature of the skin's ability to heal itself from inside out, clotting factors will appear at the sensor/skin interface and eventually block the flow of ISF from the skin to the sensor. This was especially evident when vials were changed every 6 hours during Phase I clinical study. Changing of the vial every 6 hours was done so we could keep aliquots of ISF separate to verify the feasibility of diurnal cycle over 24 hours. Increased exposure to air accelerates clotting at the poration sites. Due to this clotting, we were unable to collect ISF over the last and final period from some of the subjects even though the HH devices used during the 6 subject clinical study were pre-coated with a mix of hydrophilic Hydak B-23KX/L-110 (Reference #P 19115, Biocoat, Horsham, PA 19044) and antimicrobial additive. Based on our experience with Phase I clinical study, we will include coating with heparin for any future clinical studies especially if there is a plan to separate out ISF aliquots every 6 hours. In ultimate device design, the ISF collected will be automatically directed every 6 hours, with the ISF path completely closed to air and hence clotting process will be totally eliminated.
Design and Fabrication of the Photolithographic Cortisol Electrodes and its Evaluation
Standard photolithographic technique was used to fabricate the immunoelectrochemical device on oxidized silicon wafer in a clean room environment [22,23Sun, et al., 2007]. Gold microelectrode arrays functionalized with Dithiobis (succinimidyl propionate (DTSP) self-assembled monolayer (SAM) have been used to bind anti-cortisol (Mab) and to fabricate an ultrasensitive, disposable, electrochemical cortisol immunosensor. Ethanol amine (EA) was used to block non specific binding sites. EIS has been utilized to characterize the EA/Mab/DTSP/Au and to estimate cortisol as a function of its concentration. EIS measurements were carried out using phosphate buffer Potentiostat/Galvanostat (Eco Chemie, Netherlands). These studies have been described in details in two publications [1, 2Arya et al., 2010]. To account for the variation in initial impedance values for individual EA/Mab/DTSP/Au bioelectrode, all experiments were carried out with increasing cortisol concentration and the resulting change in charge transfer resistance (Rct) data set was normalized to (charge transfer resistance for desired concentration)/ (charge transfer resistance of blank EA/Mab/DTSP/Au cortisol sensor chip) and plotted against cortisol concentration. The use of step-by-step approach and normalization ensures that the observed change in impedance was due to surface modification occurring by cortisol binding and not due to superimposed effects of multi electrode measurement. It was observed that, using normalization, all electrodes with different impedance for EA/Mab/DTSP/Au show similar response within error of 5% for desired concentration and reveal the linear range of 1 pM to 100 nM with sensitivity of 0.325 M1. Selectivity test was conducted using EA/Mab/DTSP/Au electrode against corticosterone which is very similar to cortisol in steroid family. The results indicated that cortisol sensor was selective and can be used for selective estimation of cortisol1.
Clinical Validation and Performance
A small human subject pilot study has been conducted for clinical validation of the rapid cortisol sensor developed to date. This human subject study meets the definition of `Clinical Research' and was conducted under IRB approval (CA-10-08-0016) and Good Clinical Practices. Six participants were recruited for saliva and ISF samples for clinical validation and sensor evaluation. This study was conducted at Guided Therapeutics in Norcross, GA. A Case Report Form (CRF) was used per subject as an additional screening measure to evaluate the subjects' inclusion/exclusion criteria and to document the poration procedure. The ISF and saliva samples, collected over 24 hours for the cortisol sensor validation, were aliquoted into duplicate eppendorf tubes. Eppendorf tubes were individually placed in a biohazard bag, each labeled with date, time, and subject ID number. Labeled tubes were immediately frozen and sent to the Diagnostics Systems Laboratory (Dr. Clemens Kirschbaum laboratory in Dresden, Germany) for between the sites analysis of cortisol and to USF for validation of our current cortisol sensor test performance using EIS. Dr. Clemens's lab uses the commercially available Cortisol Luminescence Immunoassay manufactured by IBL, Hamburg.
For validating the circadian rhythm for cortisol, we used the non-invasive laser method, described previously, to collect ISF from six subjects at four different time intervals during a 24-hour period. Levels of cortisol in the body change throughout the day [24Liu et al., 2005]. These levels are highest in the morning, dropping rapidly until mid-day, and gradually declining throughout the rest of the day and lowest at night [25Kurina et al., 2004]. Cortisol is secreted with a circadian periodicity and peaks just prior to waking in the morning [26Krieger, 1975]. The amount of cortisol production in the body undergoes diurnal variation, with the highest levels present in the early morning, and the lowest levels present around midnight. As a result, four ISF samples were collected at four suggested intervals of 8 am to 2 pm, 2 pm to 8 pm, 8 pm to 2 am, and 2 am to 8 am. To get these samples evaluated using the commercially available kit, we needed 40–50 μl of sample volume for duplicate measurements. Using the non-invasive method described previously, we can accomplish ISF flow rate of ~10–15 μl/hour. Therefore, ISF was collected continuously over 6 hour time-range so we could harvest 60–90 ul during each of the four time slots. These samples were collected in duplicate per subject using a vacuum level setting of 20 inHg per location. ISF from both the harvesting heads were pooled together and then divided into two separate aliquots for duplicate measurements. In the end, we had four ISF vials per subject, ~190–200 μl ISF per vial per time slot. Saliva samples were collected every two hours and three of these chronological samples in each time-slot were pooled in equal volumes to prepare a single saliva sample representing each time-slot. Each subject was given a special tube called a “Salivette®” (From SARSTEDT, a German company, Item Number 51.1534.500) for saliva collection. Subjects were instructed to abstain from eating or drinking 20 minutes before each saliva sample collection. Three saliva tubes were collected per time slot. In the end equal volumes of saliva from each of the 3 tubes, collected per time slot, were mixed together and divided into two parts for duplicate measurements. Saliva collected in this way best mimics the sequential ISF collection over each of the six hour time-slot.
The cortisol biosensor was tested with ISF and the measurements were compared to ELISA. This biosensor enabled cortisol detection up to 1 pM within the 40 minutes analysis time. The biosensor was successfully used for in-vitro measurement of cortisol in ISF. This research establishes the feasibility of using impedance based biosensor architecture for disposable, wearable cortisol detector.
For clinical validation, we used the ISF harvested from 6 subjects for in-vitro cortisol measurement and analysis. The HH devices used during the 6 subject clinical study were pre-coated with a mix of hydrophilic Hydak B-23KX/L-110 (Reference #P 19115, Biocoat, Horsham, PA 19044) and antimicrobial additive.
Results of Clinical Data from 6 Subjects
The impedance based cortisol biosensor developed during Phase I, showed sensitivity of 2.296 Kohms M−1 and correlation coefficient of 0.997 (To obtain correlation coefficient, data points were plotted in origin software and linear fit analysis was carried out. Linear fit analysis reveals the value of slope, intercept and correlation coefficient).
For validating the performance of cortisol sensor chip developed during Phase I, ISF and saliva samples collected from 6 subjects were tested. A total of 31 samples were evaluated from 6 subjects. Of the total number of samples tested, there were 18 saliva samples and 13 ISF samples.
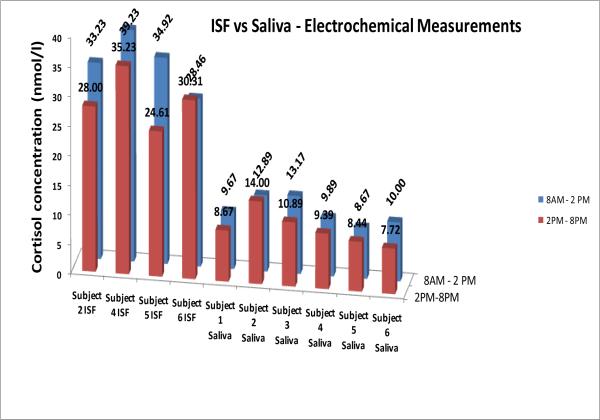
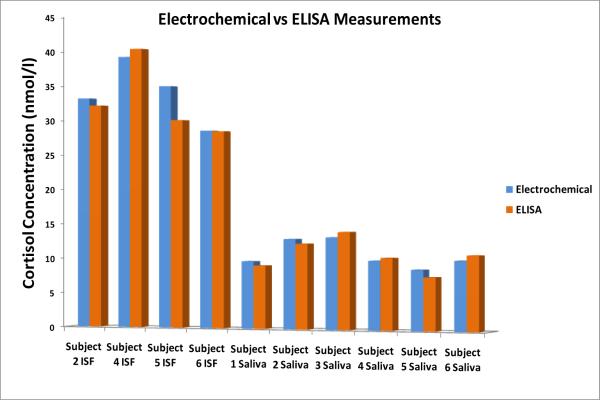
It was found that for all subjects there is an expected trend in the data (Figure 4). For each subject we see that in general all the samples collected early morning or mid-morning had higher levels of measured cortisol versus their corresponding afternoon samples. In addition, it can be seen that the cortisol levels in ISF were 3 to 4 times higher than in saliva for all six subjects. The hypothesis is that when ISF is collected continuously over a few hours range, some of the serum/plasma from capillaries might get mixed with ISF and hence the concentration of bound-cortisol increases in the collected ISF sample. A number of studies have revealed correlations between unbound cortisol in serum and plasma to be r > 0.90. However, the correlation between the total cortisol levels in blood and salivary cortisol is usually weaker due to different amounts of Cortisol Bounding Globulin (CBG) and albumin found in blood. To date, no correlation studies exist between that of saliva and ISF or blood and ISF. The device being described here will help us learn more about this relationship. It is evident that the electrochemical measurements are in excellent agreement with the ELISA results, and thus corroborates the EIS measurement technique for cortisol testing. Such agreement was seen for all the saliva and ISF samples taken at different time intervals from all subjects. Figures 4 and 5 show the correlation between the cortisol concentrations obtained using professional ELISA measurements and the impedance measurements (impedance measurement data for 40 frequencies was recorded and plotted on logarithmic distribution). Again, to obtain correlation coefficient, data points were plotted in origin software and linear fit analysis was carried out. It is evident that the impedance measurements are in excellent agreement with the ELISA results, and thus corroborates our bio-electrode measurement technique for cortisol testing. Such agreement was seen to exist for all the saliva and ISF samples collected at different time intervals from all subjects.
Figure 4.
Comparison of cortisol level in ISF and Saliva using the Electrochemical Measurements.
Figure 5.
Comparison of electrochemical vs. ELISA measurement on saliva and ISF samples collected over two separate time-slots.
Expected circadian rhythm and high degree of correlation can be seen in the diurnal change for both saliva and the ISF samples (Figure 6). We can clearly see the circadian rhythm in 4 subject's saliva data with both ELISA and EIS methods. The same can be seen for three ISF samples and the ELISA method. As explained earlier, a few of the ISF samples couldn't be collected but we have shown 0.997 correlations between ELISA and EIS and hence we are confident that all the ISF samples will show circadian rhythm for the remaining subjects as well.
Figure 6.
Circadian rhythm can be seen for Saliva and ISF Elisa data (1st and 3rd graphs). We have shown equivalence between ELISA and Impedance data. This means circadian rhythm can be seen in our impedance data as well. We can indeed show circadian rhythm in ISF for 3 subjects, graph in the middle.
These results are very encouraging, especially because ~70% higher cortisol numbers are seen in ISF. This could mean that ISF might be a better choice of a bodily fluid to be tested for the levels of cortisol than saliva, serum or blood. Our ability to verify the circadian variation in cortisol in these results confirms that cortisol is indeed secreted with a circadian periodicity in ISF. We have shown that cortisol can be measured discretely in ISF and should be able to correlate that to the physiological stress levels. Consequently, we should be able to show the expected variation of cortisol under various stress behavioral conditions.
Conclusions
The existing methods for detecting cortisol are limited with respect to their sensitivity, time of analysis and cost. Examples of commercially available assays are fluorometric, ELISA assay and reverse phase chromatography assay. None of these methods are rapid and hence results obtained from these are neither instantaneous nor continuous. Most of the saliva based methods are at a disadvantage because salivary cortisol is found in lower concentrations and biosensors traditionally have lower sensitivity compared to ELISA and RIA. Our system has an added advantage here as concentration of cortisol in ISF is ~4 times higher than found in saliva. In addition, contaminants in the saliva sample could interfere with the signal transduction and increase signal to noise ratio thereby projecting false reading. Our methodology is free from these sample collection compliance requirements or sample contamination issues.
A device capable of measuring cortisol in ISF would greatly expand studies which are being conducted both inside and outside the laboratory as this device is capable of sequential or continuous monitoring of cortisol in ISF without the need for patient intervention. Such a device has several advantages over existing technology. This will greatly increase the amount of information about underlying cortisol diurnal secretion. This device allows us to obtain a full 24-hour diurnal data, including sleeping hours. Current ambulatory techniques cannot be made during sleep. Therefore, these current methods limit our observations to waking hours only. Although there have been many studies of cortisol output in clinical research centers, these have typically relied on frequent blood draws that only approximate a continuous readout. Importantly, these studies are carried out in the artificial setting that at best only approximates the normal life of the subject. It is well known that overnight stays in clinical research centers disrupt sleep patterns. The ability of our proposed technique to measure cortisol continuously in the home would therefore allow us for the first time to see the full 24-hour cycle in the person's natural home setting. Therefore, this portable, real-time and easy method of cortisol assessment would provide a greatly superior tool for researchers and clinicians.
Our cortisol test will allow a sequential/continuous readout of cortisol levels in an ambulatory setting. This will provide 24-hour diurnal data for the first time by allowing us to obtain the nighttime cortisol minimum value and will identify the precise point in sleep cycle at which cortisol begins its morning cycle. It will provide easy access to the post-awakening daily peak of cortisol production. This test will provide an innovative technique to measure cortisol in the person's home environment instead of artificial setting in clinical research centers. This is a revolutionary and superior tool that will be of immense value to endocrinologists, as well as to the researchers and clinicians in the behavioral sciences community. There is great potential and commercial need for cortisol monitoring in several areas of the market, 1. Military: interest for effective monitoring of stress and fatigue of a soldier in the field. 2. Endocrinologist: for monitoring and maintaining of cortisol levels amongst patients with Cushing's Syndrome (CS), Addison's Disease (AD) and autoimmune diseases besides patients with acute social, psychosocial stress and depression. 3. Sports/Exercise Physiologist: individuals or trainers are interested in building muscle through certain exercising routines could monitor their cortisol levels during various resistance training programs. 4. Medicine field for non-invasive monitoring of cortisol levels for patients with Type 2 Diabetes. Our device will be ideal for non-invasive monitoring of cortisol levels during weight-loss programs.
Acknowledgement
This work was partially supported through NIH award 1R43MH085474-01; “Instacortisol:A realtime and continuous asessment of cortisol in ISF” and USF BITT Award.
Biographies
Biographical Sketches
Manju Venugopal specializes in the fields of biochemistry and chemistry with 20 years experience in both corporate and academic settings. She has developed, validated, performed, and transferred assay methods for new products with primary expertise in product improvement, product support, FDA/510(k), and clinical studies. She received her Ph.D. degree (1992) in chemistry from Rensselaer Polytechnic Institute, Troy, NY and completed a Postdoctoral Research Fellowship at Robert Wood Johnson Medical School, Piscataway, NJ (1994). She received her M.Sc. in Chemistry (1986) from Indian Institute of Technology (IIT), Kanpur, India and her B.Sc. in Chemistry (1984) from St. Stephen's College in New Delhi, India. At present, she is a senior scientist at Guided Therapeutics Inc. (GT) in Norcross, GA. At GT, she is managing research and development of interstitial fluid (ISF) based minimally invasive novel bio-sensors for measuring cortisol, alcohol and glucose. She has been quite active towards the goal to investigate and solve a targeted problem in monitoring stress and supporting prototype device development and testing. She is serving as a Principal Investigator, providing oversight and direction for the development of this device for measuring the stress hormone. She has provided technical leadership for mechanical design, formulation, fabrication and development of prototype biosensors systems for point-of-care instrument besides conducting internal verification testing for FDA submission resulting in approval and launch of a product. She has a strong background in immunochemistry and biomedical sensors including human pilot studies. She has participated in all aspects of clinical studies of prototype devices, data analysis, reporting, and maintaining a documentation system following GMP/ISO guidelines. She is energetically involved in creating innovative sensor opportunities to obtain additional funding for these activities.
In addition, Manju Venugopal is actively involved in the clinical application and development of a rapid and painless testing platform for the early detection of cervical cancer disease based on company's patented biophotonics technology that utilizes light to detect disease at the cellular level. At present she is actively assisting in managing the development of a non-invasive test for Barrett's Esophageal (BE) using the cervical cancer technology platform in partnership with Konica Minolta Opto. Her cancer device and diagnosis experience includes theory of device operation, device assembly, repair, working and training with clinicians, clinical data management, measurement procedures, verification of data integrity, device testing and troubleshooting using specialized test equipment and several software tools. Prior to joining GT, she developed immunoassays and prototype devices at CIBA Vision to conduct clinical studies and quantify female hormones in tears and on hydrogel based contact lenses. She designed and optimized integrated optical fluorescent biomedical metabolite sensors for point of care clinical devices at Roche Diagnostics, Osmetech Inc. and Photonic Sensor Systems.
Sunil K. Arya received B.Sc. in Chemistry in 2002, M.Sc. in Organic Chemistry in 2004 and Ph.D. in Chemistry-Biosensor in 2008 from the University of Delhi, Delhi, India. He is currently a Post Doctorate Fellow in the Department of Electrical Engineering at the University of South Florida. His primary interests are in the development of application of Nanomaterials, monolayers and MEMS for gas sensor and electrochemical biosensor. His research focus is in the areas of micro/nanofabrication, materials science and thin film sensors/biosensor.
Shekhar Bhansali received BE in metallurgical engineering (Honors) from the Malaviya Regional Engineering College (MREC), Jaipur, India (1987), MTech Aircraft Production Engineering from Indian Institute of Technology (IIT), Madras, India, (1991), and the PhD in electrical engineering from the Royal Melbourne Institute of Technology (RMIT), Melbourne, Vic., Australia (1997). He is currently a professor in the Department of Electrical Engineering at the University of South Florida. His interests are in the areas of Bio-MEMS, nano-structures, energy storage, sensors, and microsystems. He is the recipient of the NSF CAREER award and is also the director of NSF-IGERT program, coordinator for Sloan Fellowship Programs and co-director of NSF Bridges to the Doctorate minority fellowship program. He has over 70 international conference and journal publications and seven pending US patents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
reference
- 1.Arya Sunil K., Chornokur Ganna, Venugopal Manju, Bhansali Shekhar. Dithiobis(succinimidyl propionate) modified gold microarray electrode based electrochemical immunosensor for ultrasensitive detection of cortisol. Biosensors and Bioelectronics. 2010;25:2296–230. doi: 10.1016/j.bios.2010.03.016. or on-line version Doi:10.1016/j.bios.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arya Sunil K., Chornokur Ganna, Venugopal Manju, Bhansali Shekhar. Antibody Functionalized Interdigitated μ-Electrodes (IDμE's) Based Impedimetric Cortisol Biosensor. Analyst. 2010;135:1941–46. doi: 10.1039/c0an00242a. [DOI] [PubMed] [Google Scholar]
- 3.Stout PJ, Racchini JR, Hilgers ME. Anovel approach to mitigating the physiological lag between blood and interstitial fluid glucose measurements. Diabetes Technol. Ther. 2004;6:635–644. doi: 10.1089/dia.2004.6.635. [DOI] [PubMed] [Google Scholar]
- 4.Bantle JP, Thomas W. Glucose measurement in patients with diabetes mellitus with dermal interstitial fluid. J. Lab. Clin. Med. 1997;130:436–441. doi: 10.1016/s0022-2143(97)90044-5. [DOI] [PubMed] [Google Scholar]
- 5.Fischer U, Rebrin K, von Woedtke T, Abel P. Clinical usefulness of the glucose concentration in the subcutaneous tissue—Properties and pitfalls of electrochemical biosensors. Horm Metab. Res. 1994;26:515–522. doi: 10.1055/s-2007-1001747. [DOI] [PubMed] [Google Scholar]
- 6.Sternberg F, Meyerhoff C, Mennel FJ, Bischof F, Pfeiffer EF. Subcutaneous glucose concentration in humans. Real estimation and continuous monitoring. Diabetes Care. 1995;18:1266–1269. doi: 10.2337/diacare.18.9.1266. [DOI] [PubMed] [Google Scholar]
- 7.Gebhart S, Faupel M, Fowler R, Kapsner C, Lincoln D, McGee V, Pasqua J, Steed L, Wangsness M, Xu F, Vanstory M. Glucose sensing in transdermal body fluid collected under continuous vacuum pressure via micropores in the stratum corneum. Diabetes Technol. Ther. 2003;5:159–166. doi: 10.1089/152091503321827812. [DOI] [PubMed] [Google Scholar]
- 8.Daniloff GY. Continuous glucose monitoring: Long-term implantable sensor approach. Diabetes Technol. Ther. 1999;1:261–266. doi: 10.1089/152091599317170. [DOI] [PubMed] [Google Scholar]
- 9.Bantle JP, Thomas W. Glucose measurement in patients with diabetes mellitus with dermal interstitial fluid. J. Lab. Clin. Med. 1997;130:436–441. doi: 10.1016/s0022-2143(97)90044-5. [DOI] [PubMed] [Google Scholar]
- 10.Klonoff DC. Noninvasive blood glucose monitoring. Diabetes Care. 1997;20:433–437. doi: 10.2337/diacare.20.3.433. [DOI] [PubMed] [Google Scholar]
- 11.Abdallah BM, Beck-Nielsen H, Gaster M. Eur. J. Clin. Invest. 2005;35:627–634. doi: 10.1111/j.1365-2362.2005.01552.x. [DOI] [PubMed] [Google Scholar]
- 12.Wren JD, Garner HR. J. Biomed. Biotechnol. 2005;2:104–112. doi: 10.1155/JBB.2005.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petkus MM, McLauchlin M, Vuppu AK, Rios L, Garcia AA, Hayes MA. Analyt. Chem. 2006;78:1405–1411. doi: 10.1021/ac0512204. [DOI] [PubMed] [Google Scholar]
- 14.Appel D, Schmid RD, Dragan CA, Bureik M, Urlacher VB. Anal. Bioanal. Chem. 2005;383(2):182–186. doi: 10.1007/s00216-005-0022-9. [DOI] [PubMed] [Google Scholar]
- 15.Cullum E, Duplessis M, Crepeau LJ. Method for detection of stress biomarkers including cortisol by fluorescence polarization. US: 2006. [Google Scholar]
- 16.Gatti R, Cappellin E, Zecchin B, Antonelli G, Spinella P, Mantero F, De Palo EF. J. Chromatography B. 2005;824:51–56. doi: 10.1016/j.jchromb.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 17.Cook CJ. Nat. Biotechnol. 1997;15(5):421. doi: 10.1038/nbt0597-467. [DOI] [PubMed] [Google Scholar]
- 18.Kaptein WA, Zwaagstra J, Venema K, Ruiters MHJ, Korf J. Sens. Actuators B: Chem. 1997;45(1):63–69. [Google Scholar]
- 19.Papanicolaou DA, et al. Nighttime Salivary Cortisol: A Useful Test for the Diagnosis of Cushing's Syndrome. Journal of Clinical and Endocrinology Metabolism. 2002;87(10):4515–4521. doi: 10.1210/jc.2002-020534. [DOI] [PubMed] [Google Scholar]
- 20.Venugopal Manju, Feuvrel Kathryn, Mongin David, Bambot Shabbir, Faupel Mark, Panangadan Anand, Talukder Ashit, Pidva Rishi. Clinical Evaluation of a Novel Interstitial Fluid Sensor System for Continuous Alcohol Monitoring IEEE. Sensors Journal. 2008;8(N1):71–80. [Google Scholar]
- 21.Rosenfeld L. Full text “A golden age of clinical chemistry: 1948-1960”. Clin. Chem. 2000;46(10):1705–14. PMID 11017957iyh. [PubMed] [Google Scholar]
- 22.Sun K, Ramgir N, Bhansali S. An Immunoelectrochemical Sensor for Salivary Cortisol Measurement. Sensors and Actuators B: Chemical. 2007 [Google Scholar]
- 23.Sun K, Ramgir N, Venugopal M, Bhansali S. Estimation of Cortisol level in interstitial fluid and saliva using electrochemical array. 22nd international conference, EUROSENSORS; Dresden, Germany. 2008. [Google Scholar]
- 24.Liu D, Perdue RK, Sun L, Crooks M. Langmuir. 2004;20:5905–5910. doi: 10.1021/la049605p. [DOI] [PubMed] [Google Scholar]
- 25.Kurina LM, Schneider B, Waite LJ. Stress Health. 2004;20:53–63. [Google Scholar]
- 26.Krieger DT. Rhythms of ACTH and corticosteroid secretion in health and disease and their experimental modification. J. Steroid Biochem. 1975;6:785–791. doi: 10.1016/0022-4731(75)90068-0. [DOI] [PubMed] [Google Scholar]