Abstract
Background
We determined the frequency of cancer, neurologic degeneration and mortality in xeroderma pigmentosum (XP) patients with defective DNA repair in a four decade natural history study.
Methods
All 106 XP patients admitted to the NIH from 1971 to 2009 were evaluated from clinical records and follow-up.
Results
In the 65 percent (n=69) of patients with skin cancer, non-melanoma skin cancer (NMSC) was increased 10,000–fold and melanoma was increased 2,000-fold in patients under age 20. The 9 year median age at diagnosis of first non-melanoma skin cancer (NMSC) (n=64) was significantly younger than the 22 year median age at diagnosis of first melanoma (n= 38), a relative age reversal from the general population suggesting different mechanisms of carcinogenesis between NMSC and melanoma. XP patients with marked burning on minimal sun exposure (n=65) were less likely to develop skin cancer than those who did not. This may be related to the extreme sun protection they receive from an earlier age, decreasing their total UV exposure. Progressive neurologic degeneration was present in 24% (n=25) with 16/25 in complementation group XP-D. The most common causes of death were skin cancer (34%, n=10), neurologic degeneration (31%, n=9), and internal cancer (17%, n=5). The median age at death (29 years) in XP patients with neurodegeneration was significantly younger than those XP patients without neurodegeneration (37 years) (p=0.02).
Conclusion
This 39 year follow-up study of XP patients indicates a major role of DNA repair genes in the etiology of skin cancer and neurologic degeneration.
Keywords: genetic epidemiology, DNA repair, skin cancer, neurologic degeneration, xeroderma pigmentosum
INTRODUCTION
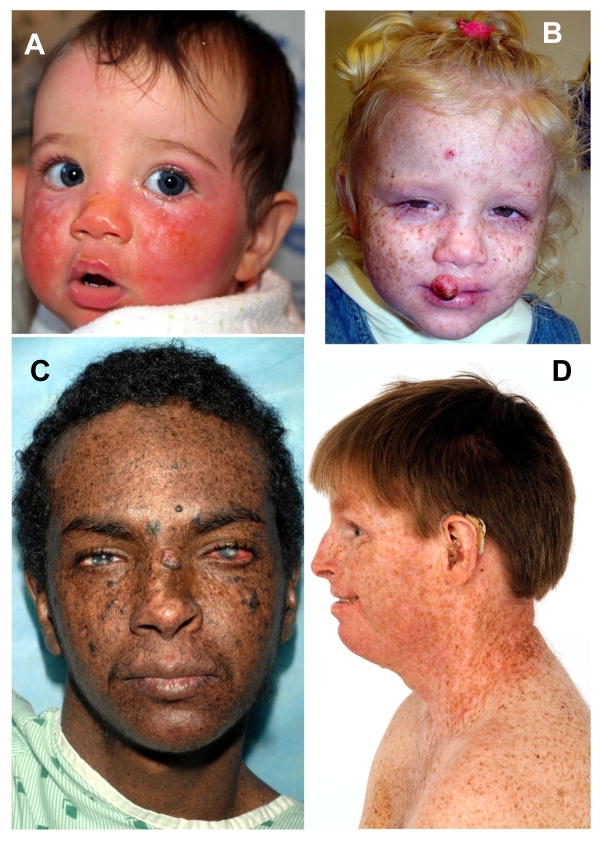
Evaluation of outcomes in patients with the neurocutaneous disorder, xeroderma pigmentosum (XP), has been limited by the number of individuals with this rare disorder and the heterogeneity of the clinical manifestations. XP is autosomal recessive with defects in repairing DNA damaged by ultraviolet radiation (UV) leading to a dramatically increased acute skin hypersensitivity to minimal sun exposure in some (Figure 1A), but not all (Figure 1B), patients and the development of non-melanoma skin cancer (NMSC) (Figure 1C) and melanoma at early ages [1–4]. Some XP patients also have progressive neurologic degeneration (Figure 1D) with some features of premature aging [2, 4–7]. XP is heterogeneous resulting from different defects in the nucleotide excision repair (NER) pathway [5, 8–11]. Seven XP complementation groups (genes), XP-A through XP-G, are associated with defective NER. The remaining group, XP variant, is deficient in DNA polymerase eta which is involved in translesional DNA synthesis.
Figure 1.
XP patients in study. A) Patient XP420BE complementation group XP-D at 9 months of age with severe blistering erythema of the malar area following minimal sun exposure. Note sparing of her forehead and eyes that were protected by a hat. B) Patient XP358BE (XP-C) at age 2 years did not sunburn easily but developed multiple hyperpigmented macules on her face. A rapidly growing SCC or keratoacanthoma grew on her upper lip and a pre-cancerous lesion appeared on her forehead. C) Northern African patient XP393BE (XP-C) [32] at age 23 years with numerous hyperpigmented macules on his face. Nodular basal cell cancer is present on his left nasal root. Pigmented basal cell cancer is present on his left cheek. His eyes show cornea scarring from unprotected sun exposure. D) Patient XP19BE (XP-A) [45] at age 35 years with neurological degeneration. He has numerous hyperpigmented macules on sun exposed areas of his face and neck. Progressive sensorineural deafness requires use of a hearing aid.
We assessed the effects of defective DNA repair genes on the frequency of skin cancer, neurologic degeneration and mortality in XP patients examined at the National Institutes of Health (NIH) beginning in 1971 and ending in 2009. In addition, we examined the influence of genetic variations in melanocortin 1-receptor (MC1R), a gene strongly associated with human pigmentation, melanoma and NMSC in the general population [12–15] and in melanoma-prone families [16], on the risk of skin cancer in XP patients.
METHODS
DATA SOURCES
Data were abstracted from medical records at the Clinical Center, NIH; from medical records of outside institutions; from research records at the National Cancer Institute (NCI); from the Social Security Death Index; from previous publications; and from personally related information.
PATIENTS
We conducted a retrospective follow-up study of clinically confirmed XP patients initially examined by a co-author (KHK) at the NIH Clinical Center from 1971 to 2009. Patients were classified as having XP, XP/Cockayne syndrome complex (XP/CS), or XP with trichothiodystrophy (XP/TTD) as previously described [2, 4, 5, 7, 17]. Patients were generally referred by health care professionals. Informed consents were obtained under NCI Institutional Review Board-approved protocols in effect at enrollment.
END POINTS
Demographic and clinical data
The primary endpoints assessed were vital status, skin and other cancer occurrence, neurologic degeneration, and cause of death. Follow-up was obtained via telephone conversation with patients, family members, physicians, and review of the Social Security death index (http://ssdi.rootsweb.ancestry.com). Clinical data were collected using a standard questionnaire [3] completed at the time of the initial visit to NIH, medical records including pathology reports of skin and other cancers, patient photographs, research records, and published reports [1–5, 7, 18–46]. Only cancers validated by pathology report review were included. Variables collected included vital status, age, sex, ethnicity, age at first skin cancer, number and types of skin and other cancers XP [2, 4, 5, 7, 42] and non-XP type neurologic abnormalities, and cause of death.
Melanocortin-1 Receptor (MC1R) variations
A family- based case-control study was conducted on a subset of subjects having appropriate DNA samples to evaluate the relationships between MC1R variants in XP patients compared to unaffected family members. Sequencing of the 951 base pair MC1R coding region was performed at the Laboratory of Molecular Technology, SAIC, Frederick, MD as described previously [16]. Variants were detected using Mutation Surveyor (SoftGenetics Inc., PA) and SAIC sequence analysis software.
XP complementation group status
XP complementation group assignment was performed using cell fusion, host cell reactivation, western blotting or DNA sequencing [4, 19–21, 23, 25–27, 29, 32–36, 38, 44, 46, 47].
STATISTICAL ANALYSES
Cancer risk and mortality
For comparison to general population rates of cancer or mortality, follow-up began at birth, and ended on the date of diagnosis of cancer for the cancer rates, date the patient was last known to be cancer free (for cancer outcome), date of death for the mortality analyses, date last known to be alive (for mortality), or the end of follow-up (December 31, 2009), whichever came first. The observed number of NMSC was compared to the expected number from the Kaiser Permanente skin cancer database [48] after adjustment for age, sex, race, and birth cohort. The observed number of melanoma skin cancers was compared with the expected number in the general population (O/E ratio) based on the NCI Surveillance, Epidemiology, and End Results (SEER) cancer database [49].
The relative risk for each cause of death was estimated by calculating the standardized mortality ratio (SMR) and the exact Poisson 95% confidence interval (CI). The expected number of deaths was calculated by applying the US mortality rates (by 5-year age, 5 calendar year, and sex-specific categories) to the appropriate person-time accrued by XP survivors in the cohort [50]. Kaplan-Meier estimates were used to compare survival between different groups. PROC LIFETEST of SAS (Version 9.1.3) was used to test for differences in survivor function using the Wilcoxon test. Results with p<0.05 were regarded as significant. All statistical tests were two-sided.
MC1R and skin cancer risk
We evaluated each MC1R variant individually comparing 1+ variant to the consensus MC1R sequence. Because many MC1R variants were too rare to examine their individual associations with skin cancer risk, we also used the following MC1R variables in the analyses: carriers of any MC1R variant compared with wild-type MC1R; carriers of multiple (1,2+) variants compared with the consensus sequence; carriers of 1 non-red hair color (NRHC) variant. 2+ NRHC variants, 1 red hair color variant (RHC), 2+ RHC variants, or carriers of both RHC and NRHC variants compared with wild-type MC1R[16]. We also examined whether MC1R variants influenced the number of skin cancers the patient had as well as whether the number of MC1R variants influenced the age at diagnosis of the patient’s first skin cancer. Chi-square and Fisher exact tests were used to measure the association between skin cancer risk and RHC variables. The nonparametric Jonckheere-Terpstra test [51] was used to test the hypothesis of no differences among the ages at diagnosis of skin cancer according to number or type of MC1R variants against the alternative that the ages at diagnosis decreased as number or type of MC1R variants increased.
RESULTS
Study Demographics
All 106 XP patients examined at the NIH Clinical Center from 1/1971 through 12/2009 were included in this retrospective study (Table 1). Of these, 97 were classified as XP, 2 as XP/CS complex and 7 as XP/TTD; 78 were non-Hispanic whites (NHW). The median age at last observation or death was 26 years. The age at the last NIH visit was used for the 3 patients who were lost to follow-up. Always/sometimes burning on minimal sun exposure was reported in 63% of the patients (n=65) (Table 1, Figure 1A and online Table 1).
Table 1.
Xeroderma pigmentosum patients at the NIH, 1971–2009
| Total | Gender | Race/Ethnicity | ||||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | NHW | African/AA | Asian | HW | NA | ||
| Total | 106 | 48 | 58 | 78 | 16 | 4 | 6 | 2 |
|
| ||||||||
| Age at Last Observation/Age at Death | ||||||||
| Mean | 28 | 27 | 28 | 30 | 24 | 16 | 21 | 23 |
| Median | 26 | 22 | 28 | 29 | 22 | 17 | 16 | 23 |
| Youngest | 1 | 4 | 1 | 1 | 5 | 8 | 7 | 14 |
| Oldest | 73 | 73 | 57 | 73 | 57 | 21 | 45 | 31 |
|
| ||||||||
| Burning phenotype | ||||||||
| Always/sometimes | 65 | 24 | 41 | 52 | 9 | 1 | 3 | 0 |
| Never | 38 | 23 | 15 | 25 | 5 | 3 | 3 | 2 |
| Unknown | 3 | 1 | 2 | 1 | 2 | 0 | 0 | 0 |
|
| ||||||||
| Complementation Group (Phenotype) | ||||||||
| A (XP) | 10 | 5 | 5 | 6 | 3 | 1 | 0 | 0 |
| B (XP/CS) | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| C (XP) | 46 | 24 | 22 | 28 | 8 | 3 | 5 | 2 |
| D (XP) | 23 | 8 | 15 | 18 | 3 | 0 | 1 | 0 |
| D (XP/TTD) | 7 | 3 | 4 | 7 | 0 | 0 | 0 | 0 |
| E (XP) | 3 | 1 | 2 | 3 | 0 | 0 | 0 | 0 |
| G (XP) | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 0 |
| G (XP/CS) | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Variant (XP) | 7 | 4 | 3 | 7 | 0 | 0 | 0 | 0 |
| Unknowna (XP) | 6 | 1 | 5 | 5 | 1 | 0 | 0 | 0 |
|
| ||||||||
|
Skin cancer?
| ||||||||
| Yes | 69 | 31 | 38 | 52 | 7 | 4 | 4 | 2 |
| No | 33 | 15 | 18 | 22 | 9 | 0 | 2 | 0 |
| Unknown | 4 | 2 | 2 | 4 | 0 | 0 | 0 | 0 |
|
| ||||||||
| Type of Skin Cancer | ||||||||
| NMSC | 64 | 29 | 35 | 47 | 7 | 4 | 4 | 2 |
| Melanoma | 38 | 20 | 18 | 34 | 0 | 1 | 2 | 1 |
|
| ||||||||
| Neurologic phenotype | ||||||||
| Degeneration present | 25 | 12 | 13 | 21 | 4 | 0 | 0 | 0 |
| No degeneration | 70 | 31 | 39 | 50 | 9 | 3 | 6 | 2 |
| Non-XP neuro abnormality | 10 | 5 | 5 | 6 | 3 | 1 | 0 | 0 |
| Unknown | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
Six patients were tested for complementation groups and did not have A, B, C, D, E, F, G, ERCC1 or Variant defects
Abbreviations: NHW-Non Hispanic Whites; AA-African Americans; HW-Hispanic Whites; NA-Native Americans XP-Xeroderma pigmentosum, CS-Cockayne syndrome, TTD-Trichothiodystrophy; NMSC-nonmelanoma skin cancer
Complementation group assignments were determined for 100 of the patients [4, 19–21, 23, 25, 27–29, 32–36, 38, 41, 42, 44, 46] and unpublished observations (Table 1 and online Table 1). XP-C was the most common complementation group (43%, n=46) followed by XP-D (n=30). No XP-F or ERCC-1 patients were examined at NIH although defects in these genes were identified in cell lines from patients with late onset severe neurological degeneration who were not seen at NIH [52]. As we reported previously, [25] cells from 6 XP patients could not be assigned to any of the known complementation groups and may thus have defects in presently unidentified genes.
Skin Cancer Analysis
Sixty-nine patients (65%) had one or more skin cancers and 64 of these patients had NMSC (Table 1, Figure 2, 3 and online Table 1). Some patients had hundreds of primary skin cancers; for example we reported that patient XP29BE had 284 BCC, 12 SCC, and 24 melanomas documented histologically [20, 44]. Melanoma was diagnosed in 38 patients; 33 of these also had NMSC (Table 1, Figure 2). The NMSC preceded or was diagnosed at the same time as the melanoma in 29 of the 31 patients where age at first NMSC and melanoma was known (Figure 3B).
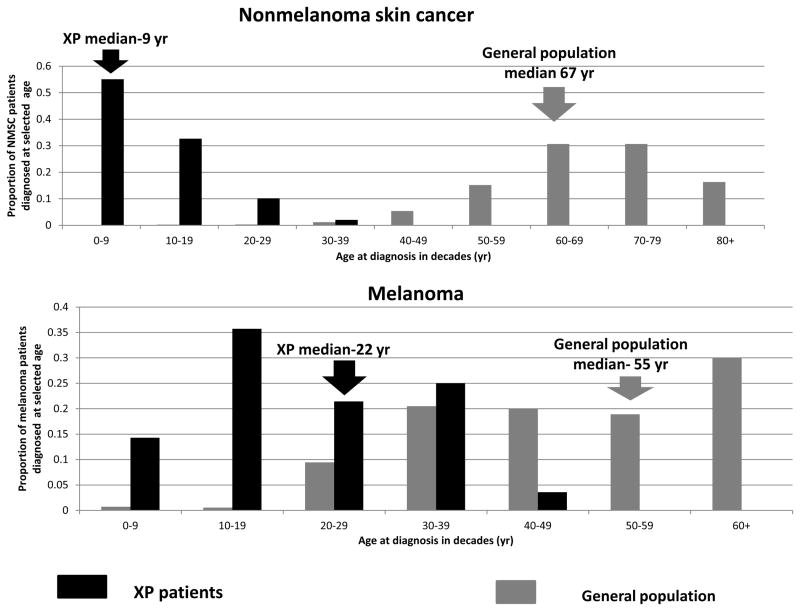
Figure 2.
XP skin cancer by age at first skin cancer diagnosis and skin cancer type compared to U.S. general population. A. Proportion of NMSC patients diagnosed at selected ages. B. Proportion of melanoma patients diagnosed at selected ages. Individuals with both NMSC and melanoma were used for both analyses. General population data taken from [48].
Figure 3.
Skin cancer and mortality in XP patients. A. Probability of the absence of skin cancers for all XP patients (n=106). At age 12 years, 50% of the patients had been diagnosed with NMSC or melanoma skin cancer (arrows) B. Scatter plot age of diagnosis of first skin cancer in patients with both NMSC and melanoma. NMSC was diagnosed earlier or at the same time as melanoma in 29/31 patients.. C. Probability of the absence of skin cancer stratified by burning phenotype. Patients that “never” burned on minimal sun exposure (n= 38) were significantly more likely to develop NMSC or melanoma skin cancer at an earlier age than those that “always or sometimes” burned (n=65) on minimal sun exposure (p=0.006). D. Probability of the absence of skin cancer stratified by XP complementation group. Patients in complementation groups XP-A, XP-B, XP-D and XP-G developed skin cancer at a significantly older age than those in complementation groups XP-C, XP-E and variant (p=0.009). E. Kaplan Meier curve of xeroderma pigmentosum patient survival compared to US general population. 30% of XP patients had died by age 32. The survival of the XP patients was significantly less than the general population (p<0.001). F. Kaplan Meier curve of xeroderma pigmentosum patient survival stratified by neurologic phenotype. Patients with neurologic degeneration had poorer survival rates than those without neurologic degeneration (p=0.04).
Among the XP patients, the median age at diagnosis of first NMSC was 9 years (range: 1–32 years). The median age at diagnosis of first melanoma (22 years, range: 2–47 years) was significantly older than NMSC (p<0.001). The age at diagnosis of XP skin cancer was substantially reduced compared to the general population: NMSC (median age 67 years) and melanoma (median age 55 years) (Figure 2A, 2B). There were 57 XP patients diagnosed with NMSC under the age of 20 years, producing a relative risk about 10,000 [95% CI 7,388 – 12,639] (Table 2) times the US population. Similarly, there were 14 XP patients diagnosed with melanoma under the age of 20 years, resulting in a relative risk more than 2,000 [95% CI 1,027–3,154] (Table 2) times the US population.
Table 2.
Observed/Expected rates of skin cancer in XP patients by age
| Attained Age | Selected Events | Observed | Expected | O/E# | 95% CI Lower | 95% CI Upper |
|---|---|---|---|---|---|---|
| <10 | All Skin Cancers | 40 | 0.01+ | 5,434 | 3,882 | 7,400 |
| <10 | NMSC | 36 | 0.00+ | 11,139 | 7,802 | 15,421 |
| <10 | Melanoma | 4 | 0.00+ | 969 | 264 | 2,480 |
| 10–19 | All Skin Cancers | 31 | 0.01+ | 5,229 | 3,553 | 7422 |
| 10–19 | NMSC | 21 | 0.00+ | 8,043 | 4,979 | 12294 |
| 10–19 | Melanoma | 10 | 0.00+ | 3,014 | 1,446 | 5544 |
| 20–39 | All Skin Cancers | 26 | 0.11+ | 239 | 156 | 351 |
| 20–39 | NMSC | 7 | 0.01+ | 525 | 211 | 1082 |
| 20–39 | Melanoma | 19 | 0.10+ | 199 | 120 | 311 |
| Total | All Skin Cancers | 98 | 0.37+ | 264 | 214 | 322 |
| Total | NMSC | 64 | 0.20+ | 327 | 252 | 418 |
| Total | Melanoma | 34 | 0.18+ | 193 | 134 | 270 |
all values were P < 0.05
The required age or year was not found in the referent rate table, therefore the closest age/year was used to obtain the rate.
NMSC was present in 60% of the NHW patients (Table 1) (median age at diagnosis-9 years, n=47). Similarly 44% of the African/African American (A/AA) patients had NMSC (median age-12 years, n=7) (p=0.8). Two A/AA patients (but no NHW patients) had SCC of the anterior tongue (p=0.04). No skin melanomas were diagnosed in the A/AA patients (p=0.1).
By age 13 years, 50% of the patients had developed skin cancer (Figure 3A). When stratified by burning phenotype, those XP patients that never burned were more likely to be diagnosed with skin cancer at an earlier age than those who had a history of always or sometimes burning on minimal sun exposure (p=0.006) (Figure 3C). This difference was also found for age at first NMSC and age at first melanoma. Many patients in complementation group XP-C, XP-E and variant never burned but were more likely to have skin cancer than patients in complementation groups XP-A, XP-B, XP-D and XP-G (p<0.001) (Figures 1B, 1C and 3D, Online table 1). Some of the XP-D and XP-A patients had severe blistering burns after a few minutes sun exposure (Figure 1A) but this extreme sun sensitivity was not reported for XP-C patients.
Neurological status
Progressive neurologic degeneration was observed in 25 (24%) XP patients. This included loss of intellectual functioning, deterioration of neurologic status, impaired hearing, abnormal speech, areflexia, ataxia, peripheral neuropathy, and loss of ability to walk and talk. Pathologically this is due to a primary neuronal loss reflected as cortical atrophy and dilated ventricles on MRI [2, 4, 5, 7, 42]. Patients with progressive neurologic degeneration were primarily in complementation group XP-D (n=16) and XP-A (n=6) (Online table 1). Two patients had XP/CS complex with skin features of XP and neurological degeneration of CS including retinal degeneration with defects in the XPB and XPG genes[4, 7, 33, 35, 36] (Table 1). Ten patients had non-XP related neurologic abnormalities; these included XP-C patients with hypoglycinemia [26, 37], with hearing loss and intellectual impairment without loss of coordination [28] or with neurological disorders and systemic lupus erythematosus [53].
Patient Mortality
Table 3 shows causes of death for the 29 XP patients who died. The median age of death was 32 years. XP survival rates were significantly lower than in the general population (p<0.001) (Figure 3E). The major causes of death were skin cancer (34%, n=10), neurologic degeneration (31%, n=9), and internal cancer (17%, n=5). Six deaths were secondary to metastatic melanoma (SMR=14, 95% CI=3–25), and 4 were secondary to invasive SCC (SMR=38, 95% CI=1–75). The SMR of death from internal cancers was 16.4 (95% CI 4.3 – 28.6). All 6 internal cancers occurred in patients who also had many skin cancers. There were 3 central nervous system cancers (SMR=11, 95% CI=1–23), and a peripheral nerve cancer (Table 3). [22]. These occurred in XP-C patients who had no neurological abnormalities. We previously reported that patient XP3BE, who died from lung cancer at age 37 years, had smoked cigarettes since age 18 years and also had many skin cancers and metastatic melanoma [3, 4, 41]. Similarly, our patient XP1BE had more than 100 skin cancers including NMSC and melanomas but died at age 49 years from metastatic endocervical adenocarcinoma of the uterus [4, 39, 42].
Table 3.
Xeroderma pigmentosum patient deaths, 1971–2009
| XP Number | Comp Group | Phenotype | Age at Death (yr) | Cause of Death | Reference |
|---|---|---|---|---|---|
| XP20BE | G | XP/CSa | 6 | Neurological degeneration | [7, 33, 36] |
| XP422BE | UNK | XP | 11 | Metastatic melanoma | This study |
| XP286BE | D | XP | 16 | Neurological degeneration | This study |
| XP15BE | C | XP | 16 | Glioblastoma | [3, 4] |
| XP120BE | A | XP | 24 | Neurological degeneration | This study |
| XP13BE | VAR | XP | 25 | Struck by lightning | [4, 18] |
| XP17BE | D | XP | 26 | Neurological degeneration | [18] |
| XP4BE | VAR | XP | 27 | Metastatic melanoma | [4, 18, 76] |
| XP5BE | D | XP | 28 | Neurological degeneration | [4, 18] |
| XP423BE | UNK | XP | 28 | Invasive SCC | This study |
| XP10BE | C | XP | 28 | Invasive SCC | [4, 18] |
| XP6BE | D | XP | 29 | Invasive SCC/Neurological degenerationb | [4, 18, 20] |
| XP424BE | C | XP | 29 | Metastatic melanoma | This study |
| XP23BE | C | XP | 31 | Metastatic melanoma/astrocytoma cordc | [3, 22, 24, 27, 31] |
| XPTTD384BE | D | XP/TTDa | 32 | Metastatic melanoma | This study |
| XP27BE | C | XP | 32 | Unknown | [77] |
| XP8BE | C | XP | 32 | Metastatic melanoma | [4] |
| XP26BE | C | XP | 33 | Invasive SCC | This study |
| XP11BE | B | XP/CSa | 33 | Arteriosclerosis | [4, 35] |
| XP24BE | C | XP | 35 | Glioblastoma | [24, 31, 59] |
| XPLABE | D | XP | 36 | Drug overdose | [78] |
| XPBHBE (XP1MI) | C | XP | 37 | Unknown | [53] |
| XP3BE | C | XP | 37 | Lung cancer/Metastatic melanomad | [3, 4, 18, 41] |
| XP29BE | D | XP | 37 | Neurological degeneration | [44, 59] |
| XP7BE | D | XP | 42 | Neurological degeneration | [4, 18] |
| XP12BE | A | XP | 44 | Neurological degeneration | [4, 18, 24, 31, 45, 45, 79] |
| XP32BE | D | XP | 46 | Neurological degeneration | This study |
| XP1BE | C | XP | 49 | Uterine cancer | [4, 18, 24, 27, 39, 41, 41, 42, 59] |
| XP14BE | C | XP | 73 | Schwannoma | [4] |
XP/CS indicates Xeroderma pigmentosum/Cockayne syndrome complex. XP/TTD indicates Xeroderma pigmentosum/Trichothiodystrophy
XP6BE died from invasive SCC; however, the patient also had neurologic degeneration
XP23BE died from metastatic melanoma; however, the patient also had astrocytoma of spinal cord
XP3BE died from lung cancer; however, the patient also had metastatic melanoma
XP patients with neurologic degeneration had poorer survival rates than those patients who had no neurologic degeneration. The median age at death (29 years) in XP patients with neurological degeneration was significantly lower than those XP patients without neurological degeneration (37 years) (p=0.02; Figure 3F). The median age at death was 32 years in XP patients with defects in complementation groups that tended to be associated with neurologic degeneration (complementation groups XP-A, XP-B, XP-D and XP-G) compared to age 37 years in XP patients with defects in complementation groups less associated with neurologic degeneration (XP-C, XP-E and variant) (p=0.05). Deaths in patients with defects in the XPC gene were predominantly from cancer (metastatic or locally invasive skin cancer or nervous system cancers). In contrast, deaths in patients with defects in the XPD gene were predominantly related to progressive neurologic degeneration despite having large numbers of skin cancers.
MC1R Analysis
We included 79 XP patients (cases) and 101 unaffected XP heterozygotes/family members (controls) in analyses of the role of MC1R variants on the risk of skin cancer. As noted in previous studies of the general population [54–56], NHW had the majority of MC1R variants among these XP families (Online table 2).
There were no significant differences in the number or types of MC1R variants between cases and controls for any of these comparisons for all subjects or for NHW subjects (data not shown). MC1R variants were not associated with the occurrence of skin cancer in XP patients (all or NHW only), the number of skin cancers (single vs multiple), or the type of skin cancers (NMSC vs melanoma) (data not shown). Contrary to expectation, patients with RHC variants were significantly older at first melanoma diagnosis than those that did not have RHC variants (age=32 years, n=5, vs age=22 years, n=15; p=0.01); however, these data involved only a 20 patients with melanoma. Overall this small study of XP patients suggests that defective DNA repair has a greater influence on skin cancer risk than variants of MC1R.
DISCUSSION
In 1968 James Cleaver reported defective DNA repair in XP [57]. At NIH, Jay Robbins then initiated a study to evaluate XP patients; Kenneth Kraemer joined the study in 1971 and has led it for decades. We report an up to 39 year follow-up including 15 patients reported in 1974 [4] plus additional patients through 2009. We found a >10,000 fold increased risk of NMSC and >2,000 fold increased risk of melanoma under age 20. These rates, which vary somewhat from our earlier studies that included some of these patients [1, 3], are based on longer follow-up and increased numbers, thus contributing substantially more person years at risk The occurrence of UV type mutations in the tumor suppressor genes p53 in NMSC [58] and PTEN in melanomas [59] provide molecular evidence of a direct effect of UV exposure in skin cancer in XP patients. Compared to the general population [48, 60–63], the XP patients had a 58-year reduction in age at first NMSC, and a 33-year reduction in age at first melanoma. As in the general population, we found that the anatomic site distribution of NMSC in the XP patients was different from that of melanomas [3]. These differences suggest differences in mechanisms of carcinogenesis between NMSC and melanoma and emphasizes the importance of DNA repair in the protection against NMSC. Indeed, we found that XP-C patients with only a few percent of normal XPC mRNA resulting from a splice lariat mutation have a lower frequency of NMSC skin cancer than other XPC patients with different splice mutations leading to undetectable levels of XPC mRNA [46, 64].
Acute ultraviolet (UVB) exposure of the skin produces sunburn, an inflammatory response with erythema and blistering characterized histologically by a mixed dermal neutrophilic and lymphocytic infiltrate [65]. In the general population sunburning is a skin cancer risk factor [66]. Surprisingly, 38 of the XP patients reported never burning but these XP patients were more likely to be diagnosed with skin cancer at an earlier age than the 61% who had a history of always/sometimes burning on minimal sun exposure. This may be partly related to early initiation of rigorous sun protection because XP patients often experience severe blistering sunburn on minimal exposure (Figure 1A) (primarily in XP-A and XP-D). XP patients in complementation groups XP-A, XP-B, XP-D and XP-G (those with higher frequency of neurologic disease) were more likely to develop skin cancers at a later age than those patients in complementation groups (XP-C, variant) with no neurologic disease. These patients may also have less mobility. Many XP-C patients did not report burning on minimal sun exposure but tan normally and develop freckle-like pigmentation at an early age followed by skin cancer [28]. Similarly, mice with a defect in the XPC gene do not burn on minimal UV exposure [67]. Fibroblasts from XP patients with a history of sunburning on minimal exposure (in XP-A and XP-D) were reported to be more sensitive to killing by UV than fibroblasts from XP patients who did not burn easily [18, 39]. It is thus possible that transcription coupled DNA repair (which is defective in XP-A and XP-D but not XP-C) [11] mediated cytokine generation [24, 43, 68] may play a major role in generation of the inflammatory sunburn response.
The role of pigmentation in protection from skin cancer is complex. In agreement with other studies of A/AA patients [69–71], cancers occurred on less pigmented sites, including the anterior tongue, at a greater frequency than in the NHW patients.[1, 3, 32] This sun-exposed site is an extremely unusual location for tongue neoplasms [72]. Perhaps the dark skin offers some protection despite defective DNA repair; thus these patients may experience greater sun exposure than lighter skinned patients. The frequency of NMSC in A/AA (3/100,000) is about 100-fold lower than in NHW [73]. In contrast, in A/AA XP patients the frequency of NMSC (44%) was not significantly different than NHW patients (61%). Thus a normally functioning DNA repair system may provide greater protection from NMSC than the dark pigmentation present in A/AA skin.
The most common cause of death was skin cancer (metastatic melanoma or invasive SCC). Despite an early age of skin cancer diagnosis, ~45% live into their 40’s and the oldest patient died at age 73 years. As reported by others [58, 74] we found an increased mortality rate from CNS tumors in patients with defects in the XPC gene who did not have XP neurological degeneration. There is some evidence that these tumors may result from exposure to oxidative damage [58, 74]. Lung cancer was present in two cigarette-smoking patients which may be a consequence of the hypersensitivity of XP cells to mutagenic effects of components of cigarette smoke [11].
Progressive neurologic degeneration, which may have resulted from primary degeneration of previously normally developed neurons [5–7], was a major cause of death among the 25% of the susceptible patients (in XP-D, XP-A, XP-G and XP-B). Fibroblasts from XP patients with neurological degeneration were reported to be more sensitive to killing by UV than cells from XP patients without neurological degeneration [18, 40]. These findings implicate a role for DNA repair in maintenance of the viability of neurons.
Specific variants of the pigmentation related gene, MC1R, are associated with increased UV-induced erythema response [75] and increased risk of both melanoma and NMSC [12–15] in the general population, and in families with mutations in the melanoma susceptibility gene, CDKN2A [16]. In contrast, based on limited data, MC1R variants do not appear to dramatically affect the risks of skin cancer in individuals with XP.
Patients ascertained for this NIH study may not reflect the general population of XP patients. Many of our patients have been sun protected from very early ages, which could influence their subsequent development of skin cancer. This 39 year study characterizes the major morbidity and mortality of XP and suggests a major role of DNA repair in the etiology of skin cancer and neurologic degeneration.
Acknowledgments
Funding: This study was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics and the Center for Cancer Research.
We are indebted to Dr. Jay Robbins for initiating these XP studies. We thank Joe Zou and Nathan Appel (Information Management Services, Inc.) for assistance with statistical analyses; Caren Nadem, Engin Gozukara, and Tala Shalavi (Kraemer lab) who helped in complementation group analysis; and Scott Coccodrilli, John Elser, Viktoriya Grinberg, Robin Stewart, Hue Vong, and David Sun (Laboratory of Molecular Technology, NCI) for MC1R analysis.
Footnotes
Previous presentations: This study was presented on May 8, 2010 at the American Dermatoepidemiology Network Symposium during the Annual Meeting of the Society of Investigative Dermatology in Atlanta, GA. An abstract was published in the Journal of Investigative Dermatology:130: S61, 2010.
BMJ Journals Statement
“I Kenneth H. Kraemer, the Corresponding Author of this article (the Contribution”) has the right to grant on behalf of all authors and does grant on behalf of all authors, a licence to the BMJ Publishing Group Ltd and its licensees, to permit this Contribution (if accepted) to be published in Journal of Medical Genetics (JMG) and any other BMJ Group products and to exploit all subsidiary rights, as set out in our licence set out at: (http://jmg.bmj.com/site/about/licence.pdf). I am a National Institute of Health (“NIH”) employee, contractor or trainee, and the following cover sheet will be accepted by the BMJ Group and NIH and incorporated into the above Licence (http://sourcebook.od.nih.gov/oversight/NIHCover%20Sheet.pdf ).
Competing Interest: None declared.
Reference List
- 1.Kraemer KH, Lee MM, Scotto J. DNA repair protects against cutaneous and internal neoplasia: evidence from xeroderma pigmentosum. Carcinogenesis. 1984 Apr;5(4):511–4. doi: 10.1093/carcin/5.4.511. [DOI] [PubMed] [Google Scholar]
- 2.Kraemer KH, Lee MM, Scotto J. Xeroderma pigmentosum. Cutaneous, ocular, and neurologic abnormalities in 830 published cases. Arch Dermatol. 1987 Feb;123(2):241–50. doi: 10.1001/archderm.123.2.241. [DOI] [PubMed] [Google Scholar]
- 3.Kraemer KH, Lee MM, Andrews AD, Lambert WC. The role of sunlight and DNA repair in melanoma and nonmelanoma skin cancer. The xeroderma pigmentosum paradigm. Arch Dermatol. 1994 Aug;130(8):1018–21. [PubMed] [Google Scholar]
- 4.Robbins JH, Kraemer KH, Lutzner MA, Festoff BW, Coon HG. Xeroderma pigmentosum. An inherited disease with sun sensitivity, multiple cutaneous neoplasms, and abnormal DNA repair. Ann Intern Med. 1974;80:221–48. doi: 10.7326/0003-4819-80-2-221. [DOI] [PubMed] [Google Scholar]
- 5.Kraemer KH, Patronas NJ, Schiffmann R, Brooks BP, Tamura D, DiGiovanna JJ. Xeroderma pigmentosum, trichothiodystrophy and Cockayne syndrome: A complex genotype-phenotype relationship. Neuroscience. 2007 Jan 31;145(4):1388–96. doi: 10.1016/j.neuroscience.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindenbaum Y, Dickson D, Rosenbaum P, Kraemer K, Robbins I, Rapin I. Xeroderma pigmentosum/cockayne syndrome complex: first neuropathological study and review of eight other cases. Eur J Paediatr Neurol. 2001;5(6):225–42. doi: 10.1053/ejpn.2001.0523. [DOI] [PubMed] [Google Scholar]
- 7.Rapin I, Lindenbaum Y, Dickson DW, Kraemer KH, Robbins JH. Cockayne syndrome and xeroderma pigmentosum. Neurology. 2000 Nov 28;55(10):1442–9. doi: 10.1212/wnl.55.10.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kraemer KH, Ruenger TM. Genome instability, DNA repair and cancer. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick’s Dermatology in General Medicine. 7. New York: McGraw Hill; 2008. pp. 977–86. [Google Scholar]
- 9.Ruenger TM, DiGiovanna JJ, Kraemer KH. Hereditary Diseases of genome instability and DNA repair. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick’s Dermatology in General Medicine. 7. New York: McGraw Hill; 2008. pp. 1311–25. [Google Scholar]
- 10.Sugasawa K. Xeroderma pigmentosum genes: functions inside and outside DNA repair. Carcinogenesis. 2008 Mar;29(3):455–65. doi: 10.1093/carcin/bgm282. [DOI] [PubMed] [Google Scholar]
- 11.Hanawalt PC. Controlling the efficiency of excision repair. Mutat Res. 2001 Feb 25;485(1):3–13. doi: 10.1016/s0921-8777(00)00071-9. [DOI] [PubMed] [Google Scholar]
- 12.Bastiaens MT, ter Huurne JA, Kielich C, Gruis NA, Westendorp RG, Vermeer BJ, Bavinck JN. Melanocortin-1 receptor gene variants determine the risk of nonmelanoma skin cancer independently of fair skin and red hair. Am J Hum Genet. 2001 Apr;68(4):884–94. doi: 10.1086/319500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Box NF, Duffy DL, Irving RE, Russell A, Chen W, Griffyths LR, Parsons PG, Green AC, Sturm RA. Melanocortin-1 receptor genotype is a risk factor for basal and squamous cell carcinoma. J Invest Dermatol. 2001 Feb;116(2):224–9. doi: 10.1046/j.1523-1747.2001.01224.x. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy C, ter HJ, Berkhout M, Gruis N, Bastiaens M, Bergman W, Willemze R, Bavinck JN. Melanocortin 1 receptor (MC1R) gene variants are associated with an increased risk for cutaneous melanoma which is largely independent of skin type and hair color. J Invest Dermatol. 2001 Aug;117(2):294–300. doi: 10.1046/j.0022-202x.2001.01421.x. [DOI] [PubMed] [Google Scholar]
- 15.Sturm RA. Skin colour and skin cancer - MC1R, the genetic link. Melanoma Res. 2002 Oct;12(5):405–16. doi: 10.1097/00008390-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein AM, Landi MT, Tsang S, Fraser MC, Munroe DJ, Tucker MA. Association of MC1R variants and risk of melanoma in melanoma-prone families with CDKN2A mutations. Cancer Epidemiol Biomarkers Prev. 2005 Sep;14(9):2208–12. doi: 10.1158/1055-9965.EPI-05-0321A. [DOI] [PubMed] [Google Scholar]
- 17.Broughton BC, Berneburg M, Fawcett H, Taylor EM, Arlett CF, Nardo T, Stefanini M, Menefee E, Price VH, Queille S, Sarasin A, Bohnert E, Krutmann J, Davidson R, Kraemer KH, Lehmann AR. Two individuals with features of both xeroderma pigmentosum and trichothiodystrophy highlight the complexity of the clinical outcomes of mutations in the XPD gene. Hum Mol Genet. 2001 Oct 15;10(22):2539–47. doi: 10.1093/hmg/10.22.2539. [DOI] [PubMed] [Google Scholar]
- 18.Andrews AD, Barrett SF, Robbins JH. Xeroderma pigmentosum neurological abnormalities correlate with colony-forming ability after ultraviolet radiation. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1984–8. doi: 10.1073/pnas.75.4.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyle J, Ueda T, Gonzalez V, Oh KS, Imoto K, Inui H, Busch DB, Khan SG, Tamura D, DiGiovanna JJ, Kraemer KH. Splice mutations in the XPD gene and absence of neurological symptoms. J Invest Dermatol. 2006;126:79. [Google Scholar]
- 20.Boyle J, Ueda T, Oh KS, Imoto K, Tamura D, Jagdeo J, Khan SG, Nadem C, DiGiovanna JJ, Kraemer KH. Persistence of repair proteins at unrepaired DNA damage distinguishes diseases with ERCC2 (XPD) mutations: cancer-prone xeroderma pigmentosum vs. non-cancer-prone trichothiodystrophy. Hum Mutat. 2008 Oct;29(10):1194–208. doi: 10.1002/humu.20768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christen-Zaech S, Imoto K, Khan SG, Oh KS, Tamura D, DiGiovanna JJ, Boyle J, Patronas NJ, Schiffmann R, Kraemer KH, Paller AS. Unexpected occurrence of xeroderma pigmentosum in an uncle and nephew. Arch Dermatol. 2009 Nov;145(11):1285–91. doi: 10.1001/archdermatol.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DiGiovanna JJ, Patronas N, Katz D, Abangan D, Kraemer KH. Xeroderma pigmentosum: spinal cord astrocytoma with 9-year survival after radiation and isotretinoin therapy. J Cutan Med Surg. 1998 Jan;2(3):153–8. doi: 10.1177/120347549800200308. [DOI] [PubMed] [Google Scholar]
- 23.Emmert S, Slor H, Busch DB, Batko S, Albert RB, Coleman D, Khan SG, bu-Libdeh B, DiGiovanna JJ, Cunningham BB, Lee MM, Crollick J, Inui H, Ueda T, Hedayati M, Grossman L, Shahlavi T, Cleaver JE, Kraemer KH. Relationship of neurologic degeneration to genotype in three xeroderma pigmentosum group G patients. J Invest Dermatol. 2002 Jun;118(6):972–82. doi: 10.1046/j.1523-1747.2002.01782.x. [DOI] [PubMed] [Google Scholar]
- 24.Gaspari AA, Fleisher TA, Kraemer KH. Impaired interferon production and natural killer cell activation in patients with the skin cancer-prone disorder, xeroderma pigmentosum. J Clin Invest. 1993 Sep;92(3):1135–42. doi: 10.1172/JCI116682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inui H, Oh KS, Nadem C, Ueda T, Khan SG, Metin A, Gozukara E, Emmert S, Slor H, Busch DB, Baker CC, DiGiovanna JJ, Tamura D, Seitz CS, Gratchev A, Wu WH, Chung KY, Chung HJ, Azizi E, Woodgate R, Schneider TD, Kraemer KH. Xeroderma pigmentosum-variant patients from America, Europe, and Asia. J Invest Dermatol. 2008 Aug;128(8):2055–68. doi: 10.1038/jid.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan SG, Levy HL, Legerski R, Quackenbush E, Reardon JT, Emmert S, Sancar A, Li L, Schneider TD, Cleaver JE, Kraemer KH. Xeroderma pigmentosum group C splice mutation associated with autism and hypoglycinemia. J Invest Dermatol. 1998 Nov;111(5):791–6. doi: 10.1046/j.1523-1747.1998.00391.x. [DOI] [PubMed] [Google Scholar]
- 27.Khan SG, Oh KS, Shahlavi T, Ueda T, Busch DB, Inui H, Emmert S, Imoto K, Muniz-Medina V, Baker CC, DiGiovanna JJ, Schmidt D, Khadavi A, Metin A, Gozukara E, Slor H, Sarasin A, Kraemer KH. Reduced XPC DNA repair gene mRNA levels in clinically normal parents of xeroderma pigmentosum patients. Carcinogenesis. 2006 Jan;27(1):84–94. doi: 10.1093/carcin/bgi204. [DOI] [PubMed] [Google Scholar]
- 28.Khan SG, Oh KS, Emmert S, Imoto K, Tamura D, DiGiovanna JJ, Shahlavi T, Armstrong N, Baker CC, Neuburg M, Zalewski C, Brewer C, Wiggs E, Schiffmann R, Kraemer KH. XPC initiation codon mutation in xeroderma pigmentosum patients with and without neurological symptoms. DNA Repair (Amst) 2009 Jan 1;8(1):114–25. doi: 10.1016/j.dnarep.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kraemer KH, Coon HG, Petinga RA, Barrett SF, Rahe AE, Robbins JH. Genetic heterogeneity in xeroderma pigmentosum: complementation groups and their relationship to DNA repair rates. Proc Natl Acad Sci U S A. 1975 Jan;72(1):59–63. doi: 10.1073/pnas.72.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kraemer KH. Xeroderma pigmentosum. A prototype disease of environmental-genetic interaction. Arch Dermatol. 1980 May;116(5):541–2. doi: 10.1001/archderm.116.5.541. [DOI] [PubMed] [Google Scholar]
- 31.Kraemer KH, DiGiovanna JJ, Moshell AN, Tarone RE, Peck GL. Prevention of skin cancer in xeroderma pigmentosum with the use of oral isotretinoin. N Engl J Med. 1988 Jun 23;318(25):1633–7. doi: 10.1056/NEJM198806233182501. [DOI] [PubMed] [Google Scholar]
- 32.Mahindra P, DiGiovanna JJ, Tamura D, Brahim JS, Hornyak TJ, Stern JB, Lee CC, Khan SG, Brooks BP, Smith JA, Driscoll BP, Montemarano AD, Sugarman K, Kraemer KH. Skin cancers, blindness, and anterior tongue mass in African brothers. J Am Acad Dermatol. 2008 Nov;59(5):881–6. doi: 10.1016/j.jaad.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moriwaki S, Stefanini M, Lehmann AR, Hoeijmakers JH, Robbins JH, Rapin I, Botta E, Tanganelli B, Vermeulen W, Broughton BC, Kraemer KH. DNA repair and ultraviolet mutagenesis in cells from a new patient with xeroderma pigmentosum group G and cockayne syndrome resemble xeroderma pigmentosum cells. J Invest Dermatol. 1996 Oct;107(4):647–53. doi: 10.1111/1523-1747.ep12584287. [DOI] [PubMed] [Google Scholar]
- 34.Oh KS, Schmidt D, Kraemer KH. A new xeroderma pigmentosum group E kindred with a R273H mutation in the DDB2 gene has features mimicking XP variant cells. J Invest Dermatol. 2005;124:A81. [Google Scholar]
- 35.Oh KS, Khan SG, Jaspers NG, Raams A, Ueda T, Lehmann A, Friedmann PS, Emmert S, Gratchev A, Lachlan K, Lucassan A, Baker CC, Kraemer KH. Phenotypic heterogeneity in the XPB DNA helicase gene (ERCC3): xeroderma pigmentosum without and with Cockayne syndrome. Hum Mutat. 2006 Nov;27(11):1092–103. doi: 10.1002/humu.20392. [DOI] [PubMed] [Google Scholar]
- 36.Okinaka RT, Perez-Castro AV, Sena A, Laubscher K, Strniste GF, Park MS, Hernandez R, MacInnes MA, Kraemer KH. Heritable genetic alterations in a xeroderma pigmentosum group G/Cockayne syndrome pedigree. Mutat Res. 1997 Nov;385(2):107–14. doi: 10.1016/s0921-8777(97)00031-1. [DOI] [PubMed] [Google Scholar]
- 37.Quackenbush EJ, Kraemer KH, Gahl WA, Schirch V, Whiteman DA, Levine K, Levy HL. Hypoglycinaemia and psychomotor delay in a child with xeroderma pigmentosum. J Inherit Metab Dis. 1999 Dec;22(8):915–24. doi: 10.1023/a:1005691424004. [DOI] [PubMed] [Google Scholar]
- 38.Rao T, DiGiovanna JJ, Tamura D, Ueda T, Boyle J, Nadem C, Oh KS, Khan SG, Patronas NJ, Schiffmann R, Brooks BP, Kraemer KH. Features of both xeroderma pigmentosum and trichothiodystrophy presenting in patients with mutations in the XPD gene. Journal of Investigative Dermatology. 2007;(supplement 127):S105. [Google Scholar]
- 39.Robbins JH, Moshell AN. DNA repair processes protect human beings from premature solar skin damage: evidence from studies on xeroderma pigmentosum. J Invest Dermatol. 1979 Jul;73(1):102–7. doi: 10.1111/1523-1747.ep12532789. [DOI] [PubMed] [Google Scholar]
- 40.Robbins JH, Polinsky RJ, Moshell AN. Evidence that lack of deoxyribonucleic acid repair causes death of neurons in xeroderma pigmentosum. Ann Neurol. 1983;13:682–4. doi: 10.1002/ana.410130621. [DOI] [PubMed] [Google Scholar]
- 41.Robbins JH, Brumback RA, Moshell AN. Clinically asymptomatic xeroderma pigmentosum neurological disease in an adult: Evidence for a neurodegeneration in later life caused by defective DNA repair. Eur Neurol. 1993;33:188–90. doi: 10.1159/000116932. [DOI] [PubMed] [Google Scholar]
- 42.Robbins JH, Kraemer KH, Merchant SN, Brumback RA. Adult-onset xeroderma pigmentosum neurological disease--observations in an autopsy case. Clin Neuropathol. 2002 Jan;21(1):18–23. [PubMed] [Google Scholar]
- 43.Suzuki H, Kalair W, Shivji GM, Wang B, Toto P, Amerio P, Kraemer KH, Sauder DN. Impaired ultraviolet-B-induced cytokine induction in xeroderma pigmentosum fibroblasts. J Invest Dermatol. 2001 Nov;117(5):1151–5. doi: 10.1046/j.0022-202x.2001.01525.x. [DOI] [PubMed] [Google Scholar]
- 44.Ueda T, Compe E, Catez P, Kraemer KH, Egly JM. Both XPD alleles contribute to the phenotype of compound heterozygote xeroderma pigmentosum patients. J Exp Med. 2009 Dec 21;206(13):3031–46. doi: 10.1084/jem.20091892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robbins JH, Brumback RA, Mendiones M, Barrett SF, Carl JR, Cho S, Denckla MB, Ganges MB, Gerber LH, Guthrie RA. Neurological disease in xeroderma pigmentosum. Documentation of a late onset type of the juvenile onset form. Brain. 1991;114:1335–61. doi: 10.1093/brain/114.3.1335. [DOI] [PubMed] [Google Scholar]
- 46.Khan SG, Yamanegi K, Zheng ZM, Boyle J, Imoto K, Oh KS, Baker CC, Gozukara E, Metin A, Kraemer KH. XPC branch-point sequence mutations disrupt U2 snRNP binding, resulting in abnormal pre-mRNA splicing in xeroderma pigmentosum patients. Hum Mutat. 2010 Feb;31(2):167–75. doi: 10.1002/humu.21166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Imoto K, Oh KS, Boyle J, Inui H, Nadem C, Ueda T, Busch DB, Gozukara E, Khan SG, Tamura D, DiGiovanna JJ, Kraemer KH. Twelve novel mutations in 14 xeroderma pigmentosum group A families: phenotype-genotype correlation and haplotype analysis. J Invest Dermatol. 2006;126(S1):79. [Google Scholar]
- 48.Glass AG, Hoover RN. The emerging epidemic of melanoma and squamous cell skin cancer. JAMA. 1989 Oct 20;262(15):2097–100. [PubMed] [Google Scholar]
- 49.Ries LAG, Melbert D, Krapcho M. SEER cancer statistics review, 1975 – 2005. National Institures of Health. 2010 February 25; Available from: URL: seer.cancer.gov/csr/1975_2006.
- 50.Monson RR. Analysis of relative survival and proportional mortality. Comput Biomed Res. 1974 Aug;7(4):325–32. doi: 10.1016/0010-4809(74)90010-x. [DOI] [PubMed] [Google Scholar]
- 51.Mielke PW, Jr, Berry KJ. The Terpstra-Jonckheere test for ordered alternatives: randomized probability values. Percept Mot Skills. 2000 Oct;91(2):447–50. doi: 10.2466/pms.2000.91.2.447. [DOI] [PubMed] [Google Scholar]
- 52.Imoto K, Slor H, Orgal S, Khan SH, Oh KS, Busch DB, Nadem C, Ueda T, Gadoth N, Jaspers NG, Kraemer KH. Xeroderma pigmentosum group F patients with late onset neurological disease. Journal of Investigative Dermatology. 2005;124:A78. [Google Scholar]
- 53.Hananian J, Cleaver JE. Xeroderma pigmentosum exhibiting neurological disorders and systemic lupus erythematosus. Clin Genet. 1980;17:39–45. doi: 10.1111/j.1399-0004.1980.tb00112.x. [DOI] [PubMed] [Google Scholar]
- 54.Gerstenblith MR, Goldstein AM, Fargnoli MC, Peris K, Landi MT. Comprehensive evaluation of allele frequency differences of MC1R variants across populations. Hum Mutat. 2007 May;28(5):495–505. doi: 10.1002/humu.20476. [DOI] [PubMed] [Google Scholar]
- 55.Kanetsky PA, Ge F, Najarian D, Swoyer J, Panossian S, Schuchter L, Holmes R, Guerry D, Rebbeck TR. Assessment of polymorphic variants in the melanocortin-1 receptor gene with cutaneous pigmentation using an evolutionary approach. Cancer Epidemiol Biomarkers Prev. 2004 May;13(5):808–19. [PubMed] [Google Scholar]
- 56.Savage SA, Gerstenblith MR, Goldstein AM, Mirabello L, Fargnoli MC, Peris K, Landi MT. Nucleotide diversity and population differentiation of the melanocortin 1 receptor gene, MC1R. BMC Genet. 2008;9:31. doi: 10.1186/1471-2156-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cleaver JE. Defective repair replication of DNA in xeroderma pigmentosum. Nature. 1968;218:652–6. doi: 10.1038/218652a0. [DOI] [PubMed] [Google Scholar]
- 58.Giglia G, Dumaz N, Drougard C, Avril MF, ya-Grosjean L, Sarasin A. p53 mutations in skin and internal tumors of xeroderma pigmentosum patients belonging to the complementation group C. Cancer Res. 1998 Oct 1;58(19):4402–9. [PubMed] [Google Scholar]
- 59.Wang Y, DiGiovanna JJ, Stern JB, Hornyak TJ, Raffeld M, Khan SG, Oh KS, Hollander MC, Dennis PA, Kraemer KH. Evidence of ultraviolet type mutations in xeroderma pigmentosum melanomas. Proc Natl Acad Sci U S A. 2009 Apr 14;106(15):6279–84. doi: 10.1073/pnas.0812401106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bivens MM, Bhosle M, Balkrishnan R, Camacho FT, Feldman SR, Fleischer AB., Jr Nonmelanoma skin cancer: is the incidence really increasing among patients younger than 40? A reexamination using 25 years of U.S. outpatient data. Dermatol Surg. 2006 Dec;32(12):1473–9. doi: 10.1111/j.1524-4725.2006.32358.x. [DOI] [PubMed] [Google Scholar]
- 61.Gray DT, Suman VJ, Su WP, Clay RP, Harmsen WS, Roenigk RK. Trends in the population-based incidence of squamous cell carcinoma of the skin first diagnosed between 1984 and 1992. Arch Dermatol. 1997 Jun;133(6):735–40. [PubMed] [Google Scholar]
- 62.Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994 May;30(5 Pt 1):774–8. doi: 10.1016/s0190-9622(08)81509-5. [DOI] [PubMed] [Google Scholar]
- 63.Rogers HW, Weinstock MA, Harris AR, Hinckley MR, Feldman SR, Fleischer AB, Coldiron BM. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010 Mar;146(3):283–7. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 64.Khan SG, Metin A, Gozukara E, Inui H, Shahlavi T, Muniz-Medina V, Baker CC, Ueda T, Aiken JR, Schneider TD, Kraemer KH. Two essential splice lariat branchpoint sequences in one intron in a xeroderma pigmentosum DNA repair gene: mutations result in reduced XPC mRNA levels that correlate with cancer risk. Hum Mol Genet. 2004 Feb 1;13(3):343–52. doi: 10.1093/hmg/ddh026. [DOI] [PubMed] [Google Scholar]
- 65.Rhodes LE, Gledhill K, Masoodi M, Haylett AK, Brownrigg M, Thody AJ, Tobin DJ, Nicolaou A. The sunburn response in human skin is characterized by sequential eicosanoid profiles that may mediate its early and late phases. FASEB J. 2009 Nov;23(11):3947–56. doi: 10.1096/fj.09-136077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kennedy C, Bajdik CD, Willemze R, De Gruijl FR, Bouwes Bavinck JN. The influence of painful sunburns and lifetime sun exposure on the risk of actinic keratoses, seborrheic warts, melanocytic nevi, atypical nevi, and skin cancer. J Invest Dermatol. 2003 Jun;120(6):1087–93. doi: 10.1046/j.1523-1747.2003.12246.x. [DOI] [PubMed] [Google Scholar]
- 67.Berg RJ, Ruven HJ, Sands AT, De Gruijl FR, Mullenders LH. Defective global genome repair in XPC mice is associated with skin cancer susceptibility but not with sensitivity to UVB induced erythema and edema. J Invest Dermatol. 1998 Apr;110(4):405–9. doi: 10.1111/j.1523-1747.1998.00173.x. [DOI] [PubMed] [Google Scholar]
- 68.Faustin B, Reed JC. Sunburned skin activates inflammasomes. Trends Cell Biol. 2008 Jan;18(1):4–8. doi: 10.1016/j.tcb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 69.Chidzonga MM, Mahomva L, Makunike-Mutasa R, Masanganise R. Xeroderma pigmentosum: a retrospective case series in Zimbabwe. J Oral Maxillofac Surg. 2009 Jan;67(1):22–31. doi: 10.1016/j.joms.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 70.Jacyk WK. Xeroderma pigmentosum in black South Africans. Int J Dermatol. 1999 Jul;38(7):511–4. doi: 10.1046/j.1365-4362.1999.00724.x. [DOI] [PubMed] [Google Scholar]
- 71.Khatri ML, Bemghazil M, Shafi M, Machina A. Xeroderma pigmentosum in Libya. Int J Dermatol. 1999 Jul;38(7):520–4. doi: 10.1046/j.1365-4362.1999.00751.x. [DOI] [PubMed] [Google Scholar]
- 72.Frazell EL, Lucas JC., Jr Cancer of the tongue. Report of the management of 1,554 patients. Cancer. 1962 Nov;15:1085–99. doi: 10.1002/1097-0142(196211/12)15:6<1085::aid-cncr2820150602>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 73.Scotto J, Fears TR, Fraumeni JF. Incidence of non-melanoma skin cancer in the United States. Bethesda, MD: U.S. Department of Health and Human Services; 1982. [Google Scholar]
- 74.Giglia G, Bouffet E, Jouvet A, Ohgaki H, Kleihues P, Sarasin A. Molecular analysis of glioma and skin-tumour alterations in a xeroderma-pigmentosum child. Int J Cancer. 1999 May 5;81(3):345–50. doi: 10.1002/(sici)1097-0215(19990505)81:3<345::aid-ijc6>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 75.Han J, Kraft P, Colditz GA, Wong J, Hunter DJ. Melanocortin 1 receptor variants and skin cancer risk. Int J Cancer. 2006 Oct 15;119(8):1976–84. doi: 10.1002/ijc.22074. [DOI] [PubMed] [Google Scholar]
- 76.Wang Y, Tan XH, DiGiovanna JJ, Richard Lee CC, Stern JB, Raffeld M, Jaffe ES, Kraemer KH. Genetic Diversity in Melanoma Metastases from a Patient with Xeroderma Pigmentosum. J Invest Dermatol. 2009 Dec 3; doi: 10.1038/jid.2009.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Plotnick H. Xeroderma pigmentosum and mucocutaneous malignancies in three black siblings. Cutis. 1980 Mar;25(3):311–3. [PubMed] [Google Scholar]
- 78.Kraemer KH, Herlyn M, Yuspa SH, Clark WH, Jr, Townsend GK, Neises GR, Hearing VJ. Reduced DNA repair in cultured melanocytes and nevus cells from a patient with xeroderma pigmentosum. Arch Dermatol. 1989 Feb;125(2):263–8. [PubMed] [Google Scholar]
- 79.Robbins JH. Xeroderma pigmentosum. Defective DNA repair causes skin cancer and neurodegeneration. JAMA. 1988 Jul 15;260(3):384–8. doi: 10.1001/jama.260.3.384. [DOI] [PubMed] [Google Scholar]