Abstract
Prevention of relapse after allogeneic hematopoietic stem cell transplantation is the most likely approach to improve survival of patients treated for hematologic malignancies. Herein we review the limits of currently available transplant therapies and the innovative strategies being developed to overcome resistance to therapy or to fill therapeutic modalities not currently available. These novel strategies include nonimmunologic therapies, such as targeted preparative regimens and posttransplant drug therapy, as well as immunologic interventions, including graft engineering, donor lymphocyte infusions, T cell engineering, vaccination and dendritic cell-based approaches. Several aspects of the biology of the malignant cells as well as the host have been identified that obviate success of even these newer strategies. To maximize the potential for success, we recommend pursuing research to develop additional targeted therapies to be used in the preparative regimen or as maintenance post-transplant, better characterize the T-cell and dendritic cells subsets involved in graft-versus-host disease and the graft-versus-leukemia/tumor effect, identify strategies for timing immunologic or nonimmunologic therapies to eliminate the noncycling cancer stem cell, identify more targets for immunotherapies, develop new vaccines that will not be limited by HLA, and develop methods to identify population at very high risk for relapse in order to accelerate clinical development and avoid toxicity in patients not at risk for relapse.
Keywords: Allogeneic hematopoietic cell transplantation, Relapse prevention, Acute graft-versus-host disease, Immunotherapy, Resistant leukemia
INTRODUCTION
The goal of allogeneic hematopoietic stem cell transplantation (HSCT) is well-established as long-term disease-free survival. Recent improvements in treatment plans and supportive care have reduced treatment-related mortality, and disease relapse has now emerged as the principle reason for treatment failure after transplantation. As reviewed in a previous workshop report [1]risk factors for relapse after transplantation vary with the diagnosis of the underlying malignancy, but patients transplanted not in remission are at especially high risk for post-transplant recurrence independent of diagnosis. Factors that influence the duration of survival after relapse include age, performance status, comorbidities, remission duration, tumor burden at relapse, and presence of mixed chimerism. With rare exception, however, post-transplant relapse is ultimately fatal. Consequently, development of new strategies to prevent relapse is imperative if survival of transplanted patients is to improve.
Other workshop committees have provided detailed reviews of potential and actual therapeutic targets based on the cellular biology underlying oncogenesis [2]and the biological basis of the effector immune response to hematological malignancies[3]. Table 1 provides a partial listing of potential mechanisms by which these diseases may evade the potent actions of high-dose chemo-radiotherapy and the graft-versus-leukemia/tumor (GVT) effect. The potential causes involve the tumor microenvironment, the biology of the malignant cell, the biology of the host including the immune system, and exogenous factors. Some of these factors, such as the cell cycle status, blood supply or transcriptional dysregulation, may be temporary, while the genetic constitution is likely immutable.
Table 1.
Potential causes of resistance to therapy that may predispose to relapse
| Resistance to chemotherapy |
| Drug impermeable niche |
| Noncycling state |
| Altered function of drug transporters |
| Upregulation of DNA repair mechanisms |
| Upregulation of DNA damage sensing mechanisms |
| Upregulation of intracellular drug detoxifying molecules |
| Upregulation of intracellular drug degradation mechanisms |
| Dysregulation of apoptosis |
| Gain of function mutation of the target molecule |
| Amplification of the target molecule |
| Reduced activation of prodrug |
| Rapid metabolism of active drug |
| Resistance to radiation |
| Hypoxic niche |
| Noncycling state |
| Up regulation of DNA repair mechanisms |
| Up regulation of DNA damage sensing mechanisms |
| Dysregulation of apoptosis |
| Resistance to immunologic mechanisms |
| Microenvironment |
| Stromal barriers to chemokines |
| Local myeloid-derived suppressor cells (MDSC) |
| Local essential nutrient depletion |
| Tumor |
| Antigenic modulation or lack of a tumor specific antigen |
| Down regulation of MHC or co-stimulatory expression |
| Down regulation of death receptors |
| Resistance to perforin or granzyme |
| Expression of inhibitory ligands (FasL, PD-L, VEGF, KIR-L) |
| Production of soluble antigen |
| Production of immunosuppressive cytokines |
| Dysregulation of apoptosis |
| Immune system |
| Disruption of lymphoid architecture/trafficking |
| Defective APC function |
| Development of Treg |
| Upregulation of inhibitory receptors (PD-1) |
| Exogenous Factors |
| Concomitant use of immunosuppressive drugs |
In this manuscript we discuss the current results for conventional transplant therapies in order to identify the barriers to disease control posed by these potential resistance mechanisms. We also review the promising innovative treatments designed to eliminate or circumvent these barriers. The areas of review include both the non-immunologic therapies, such as preparative regimens and post-transplant drug therapy, as well as immunologic interventions, including graft engineering, donor leukocyte/lymphocyte infusions (DLI), T cell engineering, vaccination and dendritic cell-based approaches. Finally, based on these data, we provide our recommendations for critical strategies to prevent relapse after transplantation and the challenges that must be addressed to ensure success.
PREPARATIVE REGIMENS
Allogeneic HSCT is a widely used form of therapy for patients with hematological malignancies. In 2009 alone, between 15,000 and 20,000 patients were treated with this procedure worldwide. Although the procedure is often effective, post-transplant relapse is a common occurrence. According to reports from the Center for International Blood and Marrow Transplant Research, relapse rates following administration of ablative transplant preparative regimens and human leukocyte antigen (HLA)-matched transplantation range from approximately 25% for good-risk patients [acute leukemias in first complete remission (CR) or chronic myeloid leukemia (CML) in chronic phase] to over 60% for patients transplanted in relapse[4–6]. Efforts to decrease post-transplant relapse rates have focused largely on intensification of cytoreductive therapy, either by increasing the total body irradiation (TBI) dose or adding additional or alternative chemotherapy. Controlled randomized studies have shown that relapse rates can be reduced by increasing the TBI dose. A randomized trial in patients with acute myeloid leukemia (AML) in first CR found that the relapse rate was 12% after 15.75 Gy, compared to 35% after 12 Gy [7]. A similar study in patients with chronic phase CML found that the recurrence rate was 0% after 15.75 Gy, compared with 25% after 12 Gy[8]. However, in both these studies the non-relapse mortality was increased with the higher TBI dose, leading to no improvement in overall survival. Additional support for the importance of the TBI dose has recently been demonstrated in a retrospective study examining different conditioning regimens for patients undergoing sibling allografts for ALL[9].
Similar to the findings with TBI, higher chemotherapy doses in the preparative regimen have also been shown to decrease relapse rates. Investigators from the Fred Hutchinson Cancer Research Center studied 45 patients with chronic or accelerated phase CML who received a preparative regimen consisting of busulfan (BU) and cyclophosphamide (CY) and received transplants from HLA-identical related donors to determine the influence of variations in BU plasma concentration on the rate of relapse[10]. Of 22 patients with steady-state BU levels below the median, 7 developed persistent cytogenetic relapse, and 3 of these patients died. In contrast, there were no relapses in patients with BU steady-state levels above the median, and the difference in the hazard of relapse between the two groups was statistically significant (P = 0.0003). A similar trend with the use of a more intensive preparative regimen was seen in a non-randomized comparison in patients with advanced morphology myelodysplastic syndromes (MDS), where the addition of BU to CY and 12 Gy TBI was associated with a lower relapse rate (28% vs. 54%) compared to historical controls receiving CY and 12 Gy TBI alone[11]. Although these studies demonstrate the benefit for improved tumor control with escalated doses of therapy, they also confirm the clinical impression that conventional transplant preparative regimens are currently at the limit of normal organ tolerance. The higher radiation doses used in the studies noted above for patients with AML and CML were associated with greater regimen-related toxicities (RRT) and non-relapse mortality (NRM) [7, 8]. Similarly, adding BU to a regimen of CY and TBI led to higher NRM [10]The toxicities that occurred as a result of efforts to increase the dose of therapy can be attributed to the non-specific targeting of the therapeutic agents.
Attempts to develop improved preparative regimens with increased anti-tumor effects and less toxicity have met with only limited success, likely because virtually all of the various regimens so far are composed of relatively nonspecific agents, such as TBI or high-dose alkylating agents. Recent advances offer the potential to develop substantially improved preparative regimens. First, in order to gain the benefit of a GVT effect without the toxicities associated with a standard HSCT regimen, alternatives to conventional preparative regimens have been investigated[12–14]. In particular, by carefully manipulating both pre- and post-transplant immunosuppression, complete allogeneic engraftment can be achieved reliably with very low-dose preparative regimens, a variety of which are shown in Figure 1.
Figure 1.
Conditioning Regimens for Allogeneic HSCT (figure courtesy of H.J. Deeg).
Identification of Risk Factors Prior to Allogeneic HSCT that Predict Relapse: How Important is Disease Status Prior to Allogeneic HSCT?
Decisions of whether to transplant a patient often remain a difficult one, and a great deal of consideration has been given to the identification of factors that will predict HSCT outcome. Besides cytogenetics, many patient-, disease-, and treatment-related specific factors have been recognized that serve as predictors for outcome such as age, comorbidities, and HLA disparities, among others. The attractiveness of reduced-intensity and non-myeloablative preparative regimens emanates predominantly from thier favorable toxicity profile. The reduced intensity of the employed conditioning regimens markedly attenuates early morbidity and mortality rates. However, this same attribute enhances the risk of early relapse since disease control is almost entirely reliant on the GVT effect, which may require 30–60 days to develop. Moreover, studies assessing risk factors for post-transplant relapse in patients undergoing reduced-intensity allogeneic HSCT have suggested that failure to achieve a CR prior to HSCT exerted the greatest adverse impact on the risk of relapse after transplantation. Additional results from a second series of non-Hodgkin’s lymphoma (NHL) patients treated with reduced-intensity allogeneic HSCT identified response to chemotherapy as the only significant independent predictor of relapse, with 75% of patients with chemotherapy-resistant lymphoma progressing within one year after transplant, compared to 25% of patients with chemotherapy-sensitive disease (p= 0.001). Data evaluating 64 chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) patients undergoing reduced-intensity allogeneic HSCT confirm these findings and demonstrate a 2-year relapse-rate of 52% in patients with tumor masses greater than 5 cm in diameter at the time of transplantation, compared to 14% for patients with tumors less than or equal to 5 cm (p= 0.009)[15].
Interestingly, little attention has been given so far to the prognostic impact of minimal residual disease (MRD), as determined by multiparametric flow cytometry (MFC), at the time of presentation for transplant. MFC employing a standardized panel of monoclonal antibodies enables the detection of small numbers of occult cells that persist during therapy using technology adaptable by most clinical laboratories. Determination of MRD levels during aplasia, early after induction, and/or after consolidation chemotherapy has proven useful to predict relapse and poor outcome after autologous HSCT and may help identifying patients requiring allogeneic HSCT for treatment intensification. It is thus conceivable that these minute populations of persistent cells increase the likelihood of adverse outcome after HSCT, in particular disease relapse. However, it is currently unclear what role pre-transplant MRD, if any, plays on post-transplant outcome for many patients. To help shed light on this issue, the predictive role of MRD for adverse outcome was recently evaluated in a large cohort of adults with AML in first remission undergoing myeloablative HSCT. Five-year estimates of overall survival as determined by MFC were 26% and 79% for MRD+ and MRD− patients, respectively (Fig. 2) (R. Walter et al. in press). Two-year estimates of relapse were 58% and 14%, respectively. After adjustment for various covariates, patients who were MRD+ before HSCT remained associated with a higher risk of relapse (hazard ratio [HR] = 7.47, 2.67–20.91; p<0.0001) and overall mortality (HR = 5.16, 2.17–12.27; p<0.0001). These data suggest that pre-HSCT MRD may be an independent factor for disease relapse in many patients in first CR after myeloablative HSCT. A major goal remains to develop preparative regimens with greater anti-tumor effects to eliminate MRD and continued overall less toxicity.
Figure 2.
Estimates of the probability of overall survival and disease-free survival for AML CR1 patients with negative vs. positive MFC results pre-HSCT (R. Walter, et al., in press)
Development of Improved Therapeutic Regimens Prior to HSCT
Conventional chemotherapeutic drugs and external beam radiation therapy expose normal and neoplastic cells to identical doses of cytotoxic agents and depend upon the enhanced sensitivity of rapidly dividing cancer cells to achieve preferential killing. In theory, therapeutic efficacy could be markedly enhanced and toxicity greatly diminished if tumoricidal agents could be selectively focused on malignant cells, with minimal exposure of normal cells to cytotoxic agents. Exploration of non-targeted regimens (e.g. HDAC inhibitors, hypomethylating agents) used prior to HSCT as part of a conditioning regimen warrant further attention. Multiple targeted agents, such as inhibitors of ABL or FLT3 could conceivably be utilized prior to HSCT: however, a detailed description and rationale of multiple agents will not be described here due to space constraints. The use of antibody (Ab)-targeted approaches targeted specifically to sites of disease while relatively sparing normal organs have offered significant hope for improved tumor control with minimally increased rates of toxicity. There are several compelling reasons to utilize Abs to reduce the risk of disease relapse after allogeneic HSCT, in particular for B-cell NHL. Abs, such as the anti-CD20 Ab rituximab, may slow the growth of residual lymphoma and provide a longer window for robust graft-versus-lymphoma effects to develop. In addition to its anti-tumor effects, Ab-directed therapies may enhance phagocytosis of apoptotic malignant cells, and promote cross-priming of cytotoxic T lymphocytes. These properties may be particularly effective in enhancing the GVT effect after allogeneic HSCT by promoting the development of disease-specific donor cytotoxic T lymphocytes.
Despite the promise of unconjugated Abs, the need remains to explore alternative immunotherapy strategies and various antigenic targets for therapy. With the use of radiolabeled monoclonal Abs it is possible to deliver high doses of radiation relatively specifically to bone marrow, spleen and other sites of hematological malignancies while sparing normal organs. Two basic strategies have emerged for the use of radioimmunotherapy (RIT) as part of conditioning regimens. The first approach emphasizes the efficacy of the radiolabeled Ab by escalating the dose of the RIT and giving this either with or without high-dose chemotherapy. Potential advantages to the escalated RIT regimen include delivering potentially curative doses of radiation therapy to all disease sites that may overcome chemoresistance. Limitations include technical aspects of dealing with very high doses of radioisotopes as well as specific dosimetry issues. The second method utilizes standard non-myeloablative radioimmunotherapy combined with myeloablative chemotherapy. Advantages of this treatment design primarily include ease of administration and the potential to escalate therapy above full chemotherapy-based conditioning regimens, whereas disadvantages primarily center around the relatively lose dose of radiation that is delivered to tumor sites.
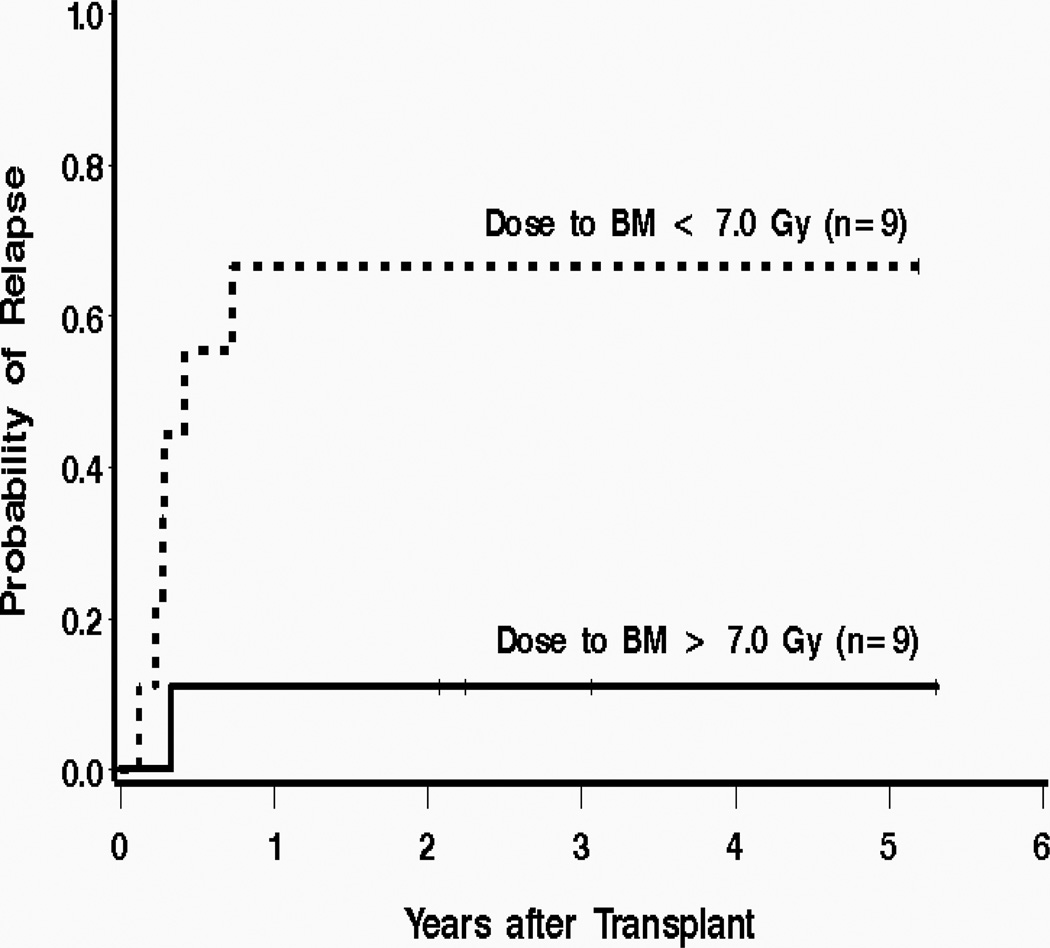
A variety of radioimmunoconjugates have been employed for the treatment of hematologic malignancies. Most investigators have utilized CD20 or CD22 as a target for RIT of NHL and either CD33 or CD45 for myeloid neoplasms. After early dose finding studies to establish maximum tolerated doses (MTD) of RIT, select groups have investigated the feasibility of adding high dose chemotherapy to the high dose radiolabeled Ab regimen. For example, it is feasible to combine 131I-anti-CD45 Ab with standard high-dose preparative regimens for treatment of AML, acute lymphocytic leukemia (ALL), and MDS[16–18]. An initial study of 131I-anti-CD45 Ab in high-risk patients with advanced AML, MDS, or ALL was performed to determine the biodistribution of the targeted radiotherapy, as well as estimate the MTD of radiation delivered by 131I-anti-CD45 Ab when combined with 120 mg/kg CY and 12 Gy TBI[16, 17]. Based on the average estimates of radiation-absorbed doses, treatment at the MTD was projected to deliver supplemental radiation doses of 24 Gy to bone marrow and 50 Gy to spleen. The same regimen was given in a small Phase II study where only 1 of 9 patients receiving a dose to bone marrow greater than 7 cGy/mCi relapsed, in contrast to 6 of 9 who relapsed after receiving doses less than 7 cGy/mCi to bone marrow (Fig. 3)[19]. These same investigators have also shown that an 131I-anti-CD45 Ab can be combined with standard high-dose preparative regimens for treatment of younger patients with leukemia, and that in the specific setting of AML in first remission, a regimen combining radiolabeled Ab with standard BU and CY has given encouraging results[18]. More recently it has been shown that high doses of targeted radiotherapy can be safely combined with a reduced-intensity preparative regimen in older relapsed or refractory patients with myeloid malignancy[20]. In this study designed to estimate the MTD, the estimates of survival and relapse were highly encouraging for this extremely high-risk patient population where the standard reduced-intensity regimens are likely not active enough in patients with relapsed disease. Treatment with this approach produced a complete remission in all patients, and all had 100% donor-derived CD3+ and CD33+ cells in the blood by day 28 after the transplant. The estimated probability of recurrent malignancy at 1 year is 40%, and the 1-year survival estimate is 41%. The 1-year survival estimate was 48% (95% CI, 26–67%) among the patients who received the MTD (Fig. 4). While the estimated probability of relapse at 1 year remains high, these results appear to be encouraging considering that 86% of the patients in the study had active AML or MDS with > 5% blasts at the beginning of the conditioning regimen, in contrast to results from studies using a fludarabine/TBI-based reduced-intensity conditioning alone where a GVT effect appeared to be most effective in patients with a low burden of malignant cells[21, 22].
Figure 3.
Probability of relapse for advanced AML patients who received 131I-BC8 Ab combined with CY/TBI. Thick solid line = dose to BM > 7 cGy/mCi; thick dashed line = dose to BM < 7 cGy/mCi.[19]
Figure 4.
Estimates of the probability of OS, DFS, TRM, and relapse among patients treated at the MTD of 24 Gy of radiation delivered to the liver by the 131I-BC8 Ab, followed by TBI/FLU.[19]
Unanswered Clinical Challenges and Program Initiative
Despite current advances in allogeneic HSCT, the central cause of failure in the vast majority of cases is relapse of disease. Thus, new strategies are needed to improve outcomes associated with allogeneic HSCT. Achieving this goal depends on the development of more effective and safer modalities for maximizing the anti-tumor potential of allogeneic HSCT. To achieve optimal elimination of tumor cells, a number of challenges remain. Outlined here are non-targeted and targeted approaches to improving the conditioning regimens that have the potential to increase the therapeutic success of allogeneic HSCT. Clinical trials focusing on these approaches should be considered.
Drug Therapy Post Allogeneic Transplant to Prevent Relapse
The role of chemotherapy following HSCT has been poorly studied. Most of these approaches have been limited to ABL kinase inhibitors in patients with Philadelphia chromosome positive (Ph+) leukemias. However, other strategies including the use of interleukins, monoclonal antibodies, immunomodulatory agents (thalidomide and lenalidomide), DNA methyltransferase inhibitors and histone deacytlase inhibitors are currently being explored. The goal of these approaches is to treat minimal residual disease while minimizing adverse side effects and avoiding the impediment of donor cell engraftment. The administration of chemotherapy after HSCT remains an open area for exploration.
Philadelphia chromosome positive leukemias (CML and ALL)
The treatment of patients with Ph+ leukemias has undergone a marked change over the past several years [23]. The current approach for patients with a Ph+ abnormality, is to administer a tyrosine kinase inhibitor (TKI), such as imatinib [24, 25]. For those patients with chronic phase CML, the current standard is imatinib at 400 milligrams per day. Given the recent data from the IRIS trial [23, 26] as well as from a German randomized study [27] imatinib remains the standard of care for patients with chronic phase CML. Given the high response rates to the second generation TKIs, dasatinib and nilotinib, most patients who fail imatinib will receive a trial of one or both of these agents prior to HSCT[27, 28]. Stem cell transplantation, therefore, has been relegated to patients whose disease fails to respond or progress while on a second generation TKI, and therefore, most patients who have received at least one second generation tyrosine kinase inhibitor. In the case of chronic phase CML, the use of TKI may not eradicate the resistant clone.
Mechanisms of resistance in patients with CML include tyrosine kinase domain (TKD) mutations as well as many other host and disease markers [29]. In those patients who have an identified TKD mutation that renders the disease resistant to the available TKIs, e.g., T315I, the role of TKI therapy post stem cell transplant is unclear [30, 31]. In patients who fail TKI therapy prior to undergoing a HSCT, the additional administration of TKIs post-HSCT is unlikely to provide significant benefit.
The role of TKI therapy following HSCT in other Ph+ leukemias is actively being explored and is more sensible. The standard of care for patients with Ph+ ALL is allogeneic HSCT in first remission, assuming that the patient has an available donor and is of reasonable health. For these patients, a course of imatinib or dasatinib is typically administered prior to HSCT [25]. The use of TKI therapy post-HSCT is currently being explored in several studies including a trial being conducted by Cancer and Leukemia Group B (CALGB 10001; NCT00039377) [32].Similarly, allogeneic HSCT remains a standard approach for patients with myeloid or lymphoid blast crisis (BP-CML) as well as accelerate phase CML (AP-CML). Two groups of patients exist, those patients who present with de novo disease and those patients who transform from underlying CP-CML. For the newly diagnosed patient, exposure to TKI therapy prior to HSCT is often limited, and therefore the use of TKI therapy post-HSCT is extremely reasonable. For patients with CP-CML who transform or progress while on TKI therapy, the use of TKI therapy post-transplant is less likely to be efficacious owing largely to the presence of TKD mutations.
The current TKIs that are currently available (imatinib, dasatinib, and nilotinib) are all resistant to the T315I mutation. Therefore, patients who contain a T315I mutation will not benefit from more TKI therapy. Several agents are under development to address this problem. MK-0457, an aurora kinase inhibitor, blocked autophosphorylation of the T315I BCR-ABL mutant in preclinical assays. This agent also showed anti-proliferative effects against patient-derived CML cells harboring this mutation [33–35]. Early phase I/II data demonstrated responses in 3 of 3 patients with the T315I mutation[36]. However, as of this writing, clinical trials with MK-0457 had been suspended due to toxicity concerns.
Several other small-molecule TKIs have demonstrated activity against the T315I mutation. The aurora kinase inhibitor PHA-739358 has shown activity among patients with the T315I mutation in early stage clinical trials. The TKIs AP-24534 and XL228 have shown promising results both in cell culture and in mice bearing xenograft tumors expressing the T315I BCR-ABL mutant. A phase I randomized, open-label trial of XL228 has recently been initiated in patients with Ph+ leukemia, and clinical trials are planned for AP-24534 in patients with drug-resistant CML. Another small molecule inhibitor, WP1130, has been shown to decrease native BCR-ABL and T315I mutant protein levels in CML cells. [37]
Chronic lymphocytic leukemia/small lymphocytic lymphoma
CLL/SLL has also undergone a paradigm shift over the last decade [38, 39]. Nucleoside analog therapy, most commonly with fludarabine-based regimens, is now the standard of care. In addition, given the high expression of CD20, most patients will receive rituximab. The addition of rituximab to standard nucleoside analog therapy has improved the response rates as well as disease-free survival [40]. Although it is difficult to assess its specific effect on overall survival, the majority of patients are currently receiving rituximab therapy, and therefore rituximab therapy post-HSCT is unlikely to be of benefit. However, additional monoclonal antibodies are currently being explored. Lumiliximab is a monoclonal antibody directed against CD23. This is currently being tested in a large, randomized phase II registration trial with fludarabine, cyclophosphamide, and rituxan with or without lumiliximab. Given the absence of any complete or partial responses as a single agent, its use as a single agent in the maintenance period post-stem cell transplantation would be unlikely to derive benefit.
There are CD20 epitopes to which rituximab does not bind. For example, ofatumumab is another monoclonal anti-CD20 antibody that interacts with a distinct small loop epitope on the CD20 molecule [41]. Studies have demonstrated impressive clinical response rates in the 20 to 60 percent range. Therefore, contrary to the fact that most patients with CLL/SLL have been previously treated with rituximab, very few patients would have been treated with ofatumumab, and therefore ofatumumab may be a therapeutic option for patients with CLL/SLL following HSCT period.
Other approaches for the treatment of CLL/SLL post-HSCT include the CAL101, a small molecule inhibitor of the p110-delta PI3 kinase (PI3K)[42]. This is currently undergoing exploration in a phase I multicenter trial. In addition, two BCL2 inhibitors are currently being explored in CLL/SLL. This includes the ABT263 as well as obatoclax (GX015-070)[43–45]. The difficult problem with ABT263 is dose-limiting thrombocytopenia, which is related to BCL-XL inhibition. Another monoclonal antibody is alemtuzumab (Campath), which is directed against CD52. Lin and colleagues reported the experience from the CALGB in which case there were significant upfront deaths in patients treated with alemtuzumab after fludarabine and rituximab-based chemotherapy in hopes of treating minimal residual disease.[46] Unfortunately, alemtuzumab showed significant toxicity when used in a maintenance therapy. Lenalidomide is another agent with high single-agent activity in fludarabine-refractory patients with CLL/SLL. Its use as post-transplant therapy may be of benefit [47, 48].
Acute Myeloid Leukemia/Myelodysplastic Syndromes
Therapy following HSCT for patients with AML has been poorly studied. The use of immune-modulating agents such as interleukin 2 (IL-2) was investigated given the success of adaptive immunotherapy (i.e. DLI) in salvaging patients following allogeneic transplant. Recombinant IL-2 has activity in renal cell carcinoma and malignant melanoma can activate T cells as well as natural killer (NK) cells to generate activated cytotoxic effectors such as lymphokine activated killer (LAK) cells. These cells are able to lyse tumor targets.
The administration of rIL-2 was tested in a patients with AML following high-dose cytarabine (ara-C) as consolidation therapy [49]. This was a non-randomized study and was reasonably tolerated. rIL2 was associated with a 5-fold increase in circulating NK lymphocyte levels and a three-fold increase in circulating CD4 and CD8 T-lymphocyte levels. The CALGB evaluated the use of rIL-2 immunotherapy for minimal residual disease in a phase III trial in patients with AML in first CR after completing all planned chemotherapy[50]. Post-remission therapy was based on cytogenetic risk factors: patients with core binding factor (CBF) AML received 3 courses of high-dose ara-C (HiDAC), while all others were assigned to receive a 2-step autologous HSCT regimen [51]. Although on an attempt-to-treat basis, there was no significant improvement in disease-free (56% vs. 45%, p = 0.11) or overall survival (68% vs. 61%, p = 0.09). The problem with this study was that few patients received their full scheduled dose rIL-2 therapy, therefore making interpretation of the analysis somewhat complicated. Similar studies were also ineffective in patients over age 60 with AML [52].
The use of hypomethylating agents (e.g. 5-azacitidine) in patients with MDS has become a standard approach, specifically in those patients with high-risk disease. Recent data demonstrate that patients with high-risk MDS treated with hypomethylating agents have a survival benefit as compared to best supportive care (24 months vs. 15 months)[53]. 5-azacitidine and decitabine share similar structures and presumably similar mechanisms of action. They seem to target DNA methyltransferase activity in leukemic cells. Decitabine is a deoxyribonucleic sugar base as opposed to 5-azacitidine, which is a ribonucleic acid sugar base. Toxicity profiles of both agents have been recently reported, and both agents are approved in the United States for patients with myelodysplastic syndrome as well as early AML (20 to 30 percent myeloblasts).
In early clinical trials, the most common side effect was prolonged myelosuppression. However, in general these agents have been well tolerated. At high doses, both agents have significant cytotoxic activity. When used a low doses, however, they seem to contribute more as a differentiating agent owing predominantly through DNA methyltransferase inhibition. Initial studies from the CALGB using low-dose azacitadine at 75 mg/m2 per day subcutaneously for seven days of each month resulted in a longer median time to AML progression (p = 0.0034) and a decreased probability of leukemic transformation (p = 0.003) as compared to best supportive care [54]. The optimal dose of decitabine is unclear. In the initial studies decitabine was administered at a dose of 15 mg/m2 over 3 hours every 8 hours for 9 doses [23]. The M.D. Anderson Cancer Center employed a different dosing schedule using 20 mg/m2 per day for five days a week with repeat cycles every four to five weeks [55].
The addition of hypomethylating agents, either 5-azacitadine or decitabine, is currently being studied in patients with both MDS and AML following completion of chemotherapy[56, 57]. The CALGB is administering decitabine as a maintenance therapy in patients with AML in first CR who have recovered from an autologous HSCT (NCT00416598).
One of the most important histone post-translational modifications is acetylation of lysine residues of histone subunit H3. Acetylation at lysine residues can result in transcriptional activation of the DNA associated with the acetylated histone. In contrast, acetyl groups are removed by histone deacetylases (HDACs), of which there are four classes with at least 11 enzyme members, with both class-specific and enzyme-specific biological effects[53, 58–60]. A number of HDAC inhibitors with varying potency and chemical structural class are currently enrolling patients with hematological malignancies [59]Entinostat (SNDX-275/MS-275) and MGCD0103 selectively inhibit only class I HDACs (i.e. HDACs 1, 2, 3, and 8), while other agents, such as panobinostat (NVP-LBH589,), inhibit HDACs more broadly [60]. In clinical trials, most HDAC inhibitors have been associated with severe fatigue and gastrointestinal adverse events, such as nausea and diarrhea.
Multiple Myeloma
Thalidomide exerts its effect on myeloma cells by the enhancement of T-cell and NK-cell mediated immunological response, disruption of adhesion between multiple myeloma (MM) cells and surrounding stromal cells, and induction of caspase-8 mediated apoptosis [61].Thalidomide-based therapies have proven to be effective in patients with relapsed and refractory MM, as well as in those with newly diagnosed disease[62, 63]. Along with its conventional role, thalidomide has been evaluated as maintenance therapy following autologous HSCT [64]. There are reasons that this approach should be advantageous. First, a substantial number of MM patients who undergo autologous HSCT exhibit evidence of persistent disease following autologous HSCT. Moreover, even patients who achieve a CR ultimately relapse, demonstrating that the MM clone persists despite aggressive cytotoxic chemotherapy.
Several studies have provided evidence in support maintenance therapy. A randomized phase II study by Stewart and colleagues demonstrated the efficacy of thalidomide as maintenance therapy and established a feasible dosing schedule[65]. Attal and colleagues subsequently conducted a randomized study in newly diagnosed 597 patients who were randomization to either no maintenance, pamidronate alone, or pamidronate and thalidomide following post-autologous HSCT[66]. Thalidomide-containing maintenance therapy was associated with a significant improvement in both 3-year event-free survival and 4-year overall survival. The greatest benefit was seen in patients who achieved less than a VGPR and with favorable cytogenetics. Barlogie and colleagues, conducted a study in 668 newly diagnosed patients with MM who received the Total Therapy with or without thalidomide from the initiation of therapy until disease progression[61]. The addition of thalidomide resulted in a significant improvement in the CR rate and 5-year event-free survival, although there was no improvement in overall survival.
A shorter post-relapse median overall survival time was noted in patients who received thalidomide. Ongoing thalidomide treatment could contribute to disease resistance, thus decreasing the likelihood of successful retreatment at the time of relapse. This concept was supported by the preliminary analysis of the Medical Research Council’s Myeloma IX study, in which 820 newly diagnosed patients were randomization to either thalidomide maintenance or no maintenance. Interestingly, thalidomide was not associated with a significant benefit in PFS for the group as a whole, but a significant improvement was seen in patients who failed to achieve at least a very good partial response with intensive induction therapy. Current approaches are administering thalidomide following a reduced-intensity allogeneic HSCT platform (NCT00777998). In addition lenalidomide and bortezomib, two extremely active drugs for the treatment of both newly diagnosed and relapsed/refractory patients with MM are currently being studied as maintenance therapy following autologous HSCT as well as reduced-intensity allogeneic HSCT approaches (NCT00847639; NCT00778752; NCT00504634).
Unanswered Clinical Challenges and Program Initiative in Drug Therapy
In order to improve allogeneic HSCT outcomes, it will be important to better define populations at the greatest risk of relapse. Differences based on disease status at the time of transplant will likely impact the kinetics of relapse and therefore define the time interval available for chemotherapy intervention. Monitoring of MRD, which is currently available in only certain diseases, may serve as a powerful tool to identify appropriate patients for drug therapy post-HSCT. Future studies will need to define dose as well as timing of drug therapy post-HSCT. It may be reasonable to explore the safety of administering drug therapy while patients are still on immune suppression.
GRAFT ENGINEERING
Hematopoietic stem cell grafts contain immune cells that contribute to engraftment, immune reconstitution, a GVT effect and graft-versus-host disease (GVHD). The administration of immunosuppressive drugs or anti-lymphocyte antibodies is necessary to prevent GVHD caused by alloreactive donor T cells that are present in unmanipulated HSC grafts. Unfortunately, immunosuppressive drugs also suppress the function of T cells that provide protection from pathogens and promote a GVT effect. Thus, transplantation with unmanipulated stem cell grafts poses a conundrum for employing cellular immunotherapy to selectively target malignant cells and prevent or reduce relapse. GVHD and/or the drugs used to prevent or treat GVHD may also interfere with the absorption, pharmacokinetics, and toxicity of drug therapies that might be administered after transplant to target residual malignancy.
The notion of manipulating or engineering hematopoietic stem cell grafts to mitigate GVHD and retain or augment the GVT effect has long held conceptual appeal, but logistics and technical complexity have limited clinical evaluation, and a method to reproducibly avoid GVHD and retain GVT activity remains elusive. Here, strategies that are being investigated for the engineering of hematopoietic stem cell grafts and issues for future investigation will be discussed.
Removal of T cells from HSC grafts to prevent GVHD
Complete T cell depletion (TCD)
The complete removal of T cells from stem cell grafts can be accomplished using clinically approved devices for positive selection of CD34+ hematopoietic progenitors, and is effective for preventing GVHD after myeloablative conditioning and HLA matched or haploidentical HSCT without the need for post grafting immunosuppression [67, 68]. An alternative method that depletes T cells from the graft is to add the monoclonal antibody alemtuzumab to the stem cell product and/or administer it to the patient during conditioning [69]. In initial studies, TCD was associated with an increased risk of graft failure and leukemia relapse, and with delayed reconstitution of pathogen-specific immunity. A study in HLA identical HSCT recipients at Memorial Sloan-Kettering Cancer Center achieved engraftment with a low incidence (<10%) of both acute and chronic GVHD using fludarabine, thiotepa, and TBI for conditioning and a CD34 selected HSC graft [68]. CD4 T cell recovery was improved compared with prior studies in which antithymocyte globulin (ATG) was used in the conditioning regimen, although absolute CD4 counts remained less than 200 cells/µl for greater than 7 months in a large fraction of patients. Relapse and survival were comparable to results reported by other centers in which patients were transplanted with unmodified grafts [68]. A multicenter phase 2 trial of complete TCD in AML in first or second remission has been completed in the US, and formal publication of the data is expected soon. The absence of GVHD and pharmacologic immunosuppression, and the elevation of IL-15 and IL-7 due to lymphopenia suggest that TCD may provide a platform for targeted T cell therapies to prevent or treat relapse. However, strategies to resolve the immunodeficiency that occurs after complete TCD such as promoting the production of naïve T cells by the recipient thymus will be necessary to improve outcome [70].
Selective depletion of alloreactive T cells
It would be ideal if alloreactive T cells could be selectively removed from stem cell grafts to mitigate GVHD. Such an approach could preserve immunity to pathogens, hasten immune reconstitution, and improve the prospects for post transplant adoptive T cell therapy or vaccination to augment the GVT effect. A theoretical advantage of selective depletion of alloreactive T cells is that a GVT effect might still arise de novo from T cells in the graft that recognize nonpolymorphic leukemia antigens such as Wilms’ tumor gene (WT-1) and proteinase 3. Methods for removal of alloreactive T cells typically rely on co-incubating donor lymphocytes with allogeneic recipient stimulator cells, and then depleting the alloreactive subset based by linking an antibody specific for a cell surface activation marker to an immunotoxin or an immunomagnetic bead. Several activation markers have been evaluated for this purpose including CD25, CD69, CD137, and CD134 [71–73]. A key issue for these strategies is the choice of recipient APC to activate donor T cells in vitro, since it is uncertain whether alloantigens that are targets for GVHD are expressed in all recipient cell types.
The clinical translation of selective depletion of alloreactive T cells has been challenging, in part due to the need for specialized reagents and for in vitro manipulation of the stem cell products. Depletion of T cells that express CD25 after activation with recipient cells has been evaluated clinically in both haploidentical and HLA matched transplants, and may confer a reduced incidence of GVHD [74, 75]. Insufficient data is available to determine if the GVT effect of the graft is compromised, although it is logical to assume that it will be to some degree.
An alternative to depleting alloreactive T cells from the stem cell graft is to administer a large dose of cyclophosphamide early after infusion of a T cell replete HSC graft to eliminate alloreactive T cells that have been induced to proliferate by antigen engagement. Although this approach does not completely eliminate the need for immunosuppressive drugs, it has reduced the incidence of severe GVHD after T cell replete haploidentical and HLA identical HSCT [76].
Depletion of naïve T cells
The identification of phenotypic and functional subsets of T cells including TREG, antigen inexperienced naïve T cells (TN), and antigen experienced memory T cells (TM), has provided opportunities for manipulation of allogeneic grafts that might reduce GVHD without the severe T-cell deficiency associated with complete TCD. Studies in murine models have revealed that GVHD develops as a consequence of tissue injury, activation and proliferation of alloreactive donor T-cells in lymphoid organs, and the migration of these T-cells to tissue sites. In mice, the induction of GVHD is attenuated by CD4+ CD25+ TREG in lymph nodes early after HSCT and the transfer of CD4+ CD25+ TREG is being investigated for reducing GVHD in humans [77]. An alternative approach that is effective for GVHD prevention in murine models and preserves transfer of immunity to pathogens is based on the removal of TN cells [78]. The intent in these murine studies was to deplete TN, but the cell selection targeted CD62L and would also eliminate the TCM subset of TM, which express CD62L. Recent experiments in which purified TN, TCM, or TEM were transplanted with TCD bone marrow in a murine model of GVHD, showed that TN caused severe GVHD, TCM caused mild GVHD, and TEM did not cause GVHD [79].
Human TN and TM can also be distinguished based on phenotype: TN are CD45RA+, CD62L+ and CCR-7+, while TM are CD45RO+ and either CD62L+ CCR-7+ (TCM) or CD62L− (TEM) [80]. Sequencing of TCR genes from purified human TN and TM to estimate the diversity of αβ TCRs shows that the TN repertoire contains the greater overall TCR diversity than the TM subset [81, 82]. Functional studies show that a major component of all CD4+ and CD8+ TM are specific for persistent viruses such as CMV, EBV, HSV, and VZV. Although virus-specific T-cells can cross react with alloantigens, this typically represents recognition of allogeneic MHC rather than minor H antigens and appears to be rare [83, 84]. Direct evidence for differences in the alloreactive T cell repertoire between CD8+ TN and TM subsets has been shown using limiting dilution analysis of rigorously purified T-cells from nonparous, untransfused HLA identical sibling pairs [85]. A multicenter phase 2 trial in which naïve T cells will be depleted from HSC grafts for prevention of GVHD in HLA identical siblings has been initiated, and if effective may provide a platform for adoptive T cell therapy with leukemia-reactive T cells to augment the GVT effect without GVHD.
Addition of cells to enhance GVL effect without GVHD
A variety of immune effector cells and target molecules on leukemic cells have been identified and linked to an effective GVL response [85]. Studies to evaluate the antitumor activity of defined effector cells in patients who have relapsed after HSCT have been initiated. If safety is established in these studies, it may be reasonable in the future to consider supplementing the stem cell graft with such tumor-reactive cells to prevent relapse from occurring, or to select donors based on genotyping that would predict that alloreactive NK cells or T cells capable of promoting GVT without GVHD will be contained in the graft.
NK cells
Since the original description of the capacity of NK cells to lyse certain tumor cells in vitro without priming, a wealth of information has emerged on NK cell differentiation and how input signals from receptors expressed on these cells regulate their activation [86]. A feature of NK cells is the expression of killer inhibitory receptors (KIR) that recognize groups of class I HLA alleles, and inhibit NK cytotoxicity. A subset of haploidentical HSCT recipients may lack a class I MHC allele needed to interact with KIR on donor NK cells in the graft, and in such “KIR-ligand” mismatched haploidentical settings in which the HSC graft is rigorously T cell depleted, an accentuated GVL effect mediated by alloreactive NK cells has been observed for AML without GVHD [67, 87]. Subsequent studies attempting to document beneficial effects of NK cell alloreactivity in other HSCT settings have suggested that factors including stem cell dose, degree of T cell depletion, donor source, and GVHD prophylaxis determine the potency of the antileukemic effect mediated by donor NK cells [87]. Efforts to adoptively transfer NK cells to treat leukemia relapse both after HSCT and in the non transplant setting have been initiated to determine the safety of this approach and establish principles for NK cell therapy [88].
T cells
Several methods of increasing the number of T cells that could potentially mediate a selective GVL effect in the patient without causing GVHD are being investigated including the adoptive transfer of T cells specific for minor H antigens that are restricted in their expression to cells in the hematopoietic lineage, or T cells specific for leukemia associated antigens such as proteinase 3 or WT-1. Additionally, any donor T cell can be engineered by gene transfer to express a T cell receptor or chimeric antigen receptor that targets a molecule on leukemic cells [89, 90]. These approaches involve significant technical complexity and are discussed later in this review, but offer the potential to provide an anti-leukemic effect without GVHD. The ability of transferred T cells to function optimally in vivo is likely to depend on developing transplant regimens that do not require prolonged pharmacologic immunosuppression to prevent or treat GVHD. If the safety and efficacy of T cell therapy is established, it may be more efficient to supplement the HSC graft with anti-leukemic T cells or to administer the cells soon after transplant.
An alternative to increasing the GVT effect of the HSC graft that might be suitable for HLA identical family member transplants is to vaccinate the donor to increase the frequency of T cells in the donor (and the HSC graft) that are specific for leukemia associated determinants. In the case of minor H antigens, this would require genotyping the donor and recipient to define appropriate targets for vaccination, and may carry some risk to the donor. Vaccination to induce T cell responses to self-antigens such as WT-1 or proteinase-3 has been evaluated in patients with leukemia and yielded provocative results [91–93], but safety concerns make this impractical for donors. Vaccines might also be given to the recipient after HSCT and may be more effective if the production of naïve T cells capable of responding to vaccination by the recipient thymus were increased [94]. A potential impediment is that the best strategies for inducing T cell responses in humans by vaccination have not been established.
Genetic Modification of the Graft to Regulate Donor T Cell Survival
The insertion of an inducible suicide gene in donor T cells in the HSC graft offers the ability to eliminate T cells that cause GVHD if necessary, and potentially retain GVT effects that are mediated prior to activation of T cell death. The most extensively studied suicide gene is the Herpes Simplex Virus thymidine kinase (HSV-TK) gene, which encodes an enzyme that phosphorylates the drug ganciclovir into moieties that inhibit DNA elongation and kill dividing cells. Several phase 1 and 2 studies in humans have examined the immediate or delayed transfer of donor T cells modified to express HSV-TK [95, 96]. These studies have demonstrated feasibility and safety, and affirmed the principle that ganciclovir administration can eliminate HSV-TK modified T cells and control GVHD. It is less clear how effectively GVL responses are maintained, and this may depend on how long donor T cells persist in vivo before elimination. The culture conditions used for gene transfer, the potential for immune responses to HSV-TK to develop, and alterations in the TK gene that results in a non-functional protein have been identified as limitations of this approach[97, 98].
These limitations have encouraged the development of alternative conditional suicide genes. An ideal suicide gene would be nontoxic until activated, derived from or highly homologous to a human protein to reduce recognition by host immunity, and efficiently trigger cell death when activated. Chimeric FKBP-Fas or FKBP-caspase molecules that can be dimerized by binding of non-toxic chemical dimerizer to the FKBP moieties have shown promise[99]. Although both Fas and caspase are effective, the caspase based vectors confer greater sensitivity to dimerizer drug[100]. Since these chimeric molecules are human proteins, the chances of an immune response to the transgene product are less. In vivo experiments in nonhuman primates have demonstrated the safety and efficacy of inducing cell suicide in adoptively transferred T cells that expressed a Fas based suicide gene [101], and a clinical trial of a caspase construct is in progress.
Unanswered Clinical Challenges and Program Initiative in Graft Engineering
Although conceptually appealing, the use of graft engineering to achieve segregation of GVT activity from GVHD remains speculative. This reflects both the technical complexity of manipulating the cellular content of stem cell grafts and our incomplete understanding of the role of individual cells in mediating beneficial and deleterious consequences in the recipient. Several issues must be addressed to move the field forward. The most immediate issue is to determine the best platform/graft composition to enable post transplant cellular or pharmacologic therapy to prevent or treat relapse. Additional research is needed to define the role of individual innate and adaptive effector cells in mediating a GVT effect and understanding how these cells interact. Finally, the role of individual cell subsets in GVHD and GVT is likely to differ depending the HLA disparity, minor H antigen disparity, other genetic polymorphisms in the donor and recipient that affect the immune response, and the type of conditioning the patient receives and these factors are likely to influence how HSC grafts might be modified to achieve the best patient outcome.
DENDRITIC CELLS IN TRANSPLANTATION AND IMMUNE-BASED THERAPIES
Dendritic cells (DCs) comprise a complex system of bone marrow-derived leukocytes that are critical to the onset of both innate and adaptive immunity [102–104]. The anatomic distribution of DCs in blood, tissue, and lymphoid organs segregates with specific subsets and functions. In this way DCs control lymphocyte priming and determine the type of immune response. We will therefore focus here on the afferent sensitization of cellular immunity by DCs, in the context of strategies to prevent or treat post-transplant relapse. We will also explore how desired GVT effects might be distinguished from GVHD at the level of antigen-presentation.
Hematopoietic Development of Dendritic Cells
DCs differentiate along two main pathways from cycling CD34+ hematopoietic progenitor cells (HPCs). One pathway leads to plasmacytoid DCs (pDCs), which respond particularly to viral infections and secrete enormous amounts of type I interferons that can activate NK cells and NKT cells. These in turn secrete inflammatory cytokines that activate the myeloid or conventional DC progeny of the other main differentiation pathway from CD34+ HPCs. These myeloid or conventional DCs comprise Langerhans cells (LCs) that populate all stratified epithelia, including skin and mucosal surfaces, and dermal-interstitial DCs (DDC-IDCs), which populate their eponymous tissues.
In addition, non-dividing DC precursors circulate in blood, as do trace populations of already differentiated DCs. The non-dividing precursors of plasmacytoid DCs are again highly responsive to viral products for differentiation and activation. Blood monocyte precursors of conventional DCs, termed monocyte-derived DCs (moDCs), show the greatest plasticity in terms of differentiation in response to various cytokines, which determine the specific types of immune responses, e.g. inflammatory, autoimmune, or allergic responses. The exact counterparts of these moDCs have not been clearly identified in vivo, so they are still defined by their characteristics after cytokine generation in vitro. Nevertheless, circulating blood monocytes remain the most readily accessible DC precursors and are therefore most often used by translational investigators for DC-based vaccines in clinical trials. This is irrespective of recent evidence that LCs generated in vitro with recombinant cytokines from mobilized CD34+ HPCs are more potent than moDCs in stimulating antigen-specific CTLs, despite the lack of IL12p70 secreted by LCs [105–108]. Mature moDCs secrete ample IL12p70, which instead supports activation of resting NK cells [109].
Tissue Distribution and the State of DC Maturation and Activation Determine the Type of Lymphocyte Response
Under non-inflammatory steady-state conditions, immature DCs are most adept at antigen uptake and processing and are hence distributed throughout the periphery at sites most likely to encounter antigen. DCs are also a major component of lymphoid tissues in the steady state where they have migrated without the same cytokine transcriptional profile as occurs under conditions of inflammation [110]. DCs in these non-inflamed conditions express C-type lectin receptors, which bind carbohydrate moieties of glycoprotein self antigens and harmless environmental antigens for processing and presentation on MHC molecules to induce and maintain tolerance [110–112].
DCs require some form of terminal maturation and activation stimulus to become fully immunogenic, and this is a pivotal event in the control of innate and adaptive immunity. Microbial products provide a physiologic activation stimulus via Toll-like receptors (TLRs) on both plasmacytoid and conventional DCs. Such products are plentiful in the early peritransplant period and certainly underlie the cytokine storm that contributes to DC activation and the onset of GvHD [113]. Activated T cells expressing CD40L (CD154) or multimeric recombinant CD40L can also mature DCs. Ligand binding of TLRs upregulates cytokine secretion, costimulatory molecules, the maturation marker CD83, and CCR7, which supports DC migration to T cell areas of draining lymph nodes.
Early activated DCs stimulate and recruit NK and NK T cells, which then secrete IFN-gamma and other inflammatory cytokines that support the bystander activation of other DCs. The ensuing adaptive immune response generates CD4+ and CD8+ T cells, as well as other effectors like Th17 and suppressor Tregs [104, 114, 115]. Appropriate stimulation of TLRs on DCs by their respective ligands can thus initiate the entire spectrum of innate, and in turn, acquired immunity, as well as regulatory responses that probably serve to counter an otherwise unchecked immune response. Not all TLR agonists yield the same activation profile in mature DCs. A combination of inflammatory cytokines that includes IL-1-beta, TNF-alpha, IL-6, and PGE2 is often used to mature DCs for study in vitro and for use in clinical vaccine trials [116]. This mimics most of the sequelae of physiologic TLR ligand binding.
Antigen Uptake, Processing, and Presentation: DCs as cross-presenters
DCs have the same machinery as other antigen-presenting cells (APCs) to process and present antigen on class I and II MHC, though they express an enormous surplus of MHC molecules allowing simultaneous presentation of many antigenic peptides. DCs are specially endowed, however, with the ability to cross-present exogenous antigens on the DCs’ own class I, as well as the usual class II, MHC molecules to autologous T cells. This occurs irrespective of the MHC alleles expressed by the antigen source[117–119]. Cytokine-induced, CD34+ HPC-derived LCs are much less phagocytic than the more commonly studied and used moDCs, yet LCs elicit more potent T cell responses by cross-presentation [106]. Hence LCs must process much more phagocytosed antigen for MHC-restricted presentation than do moDCs, which instead sequester and degrade much of what they take in.
Investigators have also emphasized distinctions between apoptotic and necrotic cell death as a source of cross-presented antigen. The key operative, however, is whether antigen remains intact or denatured during apoptosis or necrosis, as well as whether there are any additional danger signals. These are the greater determinants of effective cross-presentation and a tolerant or immune outcome[120].
Questions
How Might We Separate GVHD from GVT at the Level of Antigen-presentation?
To begin to address this question, one must conceptually distinguish the afferent sensitization of GVH and GVT interactions by distinct DC subtypes from the effector responses mediated by responding lymphocytes. Monocyte precursors can circulate and survey tissues, rapidly differentiate into potent moDCs in response to ambient cytokines in the microenvironment, and then migrate to draining lymph nodes to sensitize circulating T cells. In some ways, monocytes provide “DCs on demand”. MoDCs are therefore prime candidates to stimulate robust immune responses under conditions of severe inflammation. Such inflammation may exist in the early post-transplant period, creating the ‘perfect (cytokine) storm’ to support moDC initiation of GVH [113]. T cells activated in this setting then have greater access into inflamed tissues where they can target resident populations of DCs like LCs and DDC-IDCs that normally have slower turnover [121, 122]. When inflammation that promotes LC or DDC/IDC migration to draining lymph nodes exceeds the capacity of local precursors to replenish these populations, marrow progenitors move into the tissues to fill that void [121, 122]. In allogeneic HSCT, then, donor-derived DC subtypes can in this way replace long-lived host LCs and DDC-IDCs in the peripheral tissues, sometimes long after blood myeloid donor chimerism has been established.
Interestingly, monocyte precursors and their moDC progeny express CD52, targeted by alemtuzumab [123]. One might hypothesize then that the use of alemtuzumab for TCD ex vivo and/or in vivo, might also target host monocytes and moDCs that could drive the afferent sensitization of donor T cells against the host. Longer-lived, slower turnover, resident populations of LCs and DDC-IDCs in the epithelia and tissues are CD52 negative and not targeted by alemtuzumab. This could then account for the clinical finding that preparative regimens using alemtuzumab ex vivo and/or in vivo often result in less GVHD for the same degree of T-cell depletion achieved by physical methods, even with HLA mismatching, because alemtuzumab additionally targets monocyte precursors and moDCs[124]. LCs and DDC-IDCs sharing minor histocompatibility Ags unique to other hematopoietic cells would remain intact long enough to stimulate the desired GVT effects mediated by the allograft[125]. Unfortunately the use of alemtuzumab to treat steroid-refractory GvHD after the fact has been plagued by excessive immunosuppression [126/ and unpublished] likely owing to the fact that it also targets effector B and T lymphocytes.
What is the Nature of Antigen Required for Sensitization of GVT
Tumor antigens for immunization segregate primarily between those that are mutated and unique to the malignancy and those that are shared with normal tissues often as self-differentiation antigens. On the one hand the goal is induction of immunity and the other is to break tolerance and induce antigen-specific autoimmunity. For induction of immunity, using more complete antigen sources, like dying tumor cells or mRNA of full length antigens, is more likely to provide both types of antigen than single peptide pulsing. So also is epitope spreading as tumor cells are killed by DC-stimulated CTL and provide additional antigenic epitopes for cross-presentation. Overcoming tolerance to induce controlled autoimmunity is more challenging, because the antigens are often weaker and less immunogenic. In such cases DCs could provide the necessary extra boost needed to stimulate an immune response. That said, Tregs specific for shared self-antigens are often present that may thwart attempts to break tolerance.
How Do Autologous Versus Allogeneic Transplant Platforms Differ for Immunotherapeutic Prevention of Relapse Post-transplant
For any immunotherapeutic applications of DCs, one must choose between active immunization versus lymphocyte sensitization ex vivo by DCs followed by adoptive or passive immunization with the resulting T cell effectors. Autologous HSCT therefore offers an excellent platform on which to test active DC immunization against the primary malignancy. In the early post-autologous HSCT period, Tregs have been eliminated or substantially reduced and have insufficiently recovered to interfere with active DC immunization. Prevention of relapse is also a clearcut response assessment, given that this is one of the main, if not the principal reason for most autologous HSCT failures.
Allogeneic HSCT is more challenging. Following the transplantation of TCD allografts, there is insufficient recovery of T cells to respond to active DC immunization. Hence one needs to sensitize T cells ex vivo for adoptive immunotherapy. While many types of APCs can be used to generate tumor antigen-specific CTLs, most of these require multiple rounds of stimulation and often substantial cytokine supplementation. Defined DCs, in particular CD34+ HPC-derived LCs, will generate potent CTL even against self-differentiation tumor antigens like WT1, against which tolerance should exist. LCs accomplish this after short periods of culture and without the confounding effect of exogenous cytokines [108]. For recipients of unmodified grafts on prolonged pharmacologic immunosuppression for prevention or treatment of GVHD, the passive transfer of activated T cells is more problematic as the pharmacologic immune suppression also compromises their function. Perhaps targeted depletion of the DC subset most responsible for fueling the fire of GVHD would reduce the need for as much or as prolonged immune suppression, thus allowing the transfer of tumor antigen-specific T cells with low risk of causing broader GVH reactivity.
What is the Role of DCs and NK cells for Prevention of Post-transplant Relapse?
NK cells would seem to provide another answer to the challenges of preventing post-transplant relapse. The prevention of GVHD, the support of early engraftment, and the promotion of GVT have all been attributed to NK cells [127, 128]. Moreover, NK cells can circumvent tolerance early after transplant by expressing inhibitory KIR for non-self HLA and executing NK cell effector functions [129]. Less is known about NKT cells. We do know, however, that pDCs responding to viral infections are potent early inducers of NK and NKT cell reactivity via production of enormous amounts of type I interferons. We also know that moDCs secrete the most IL12p70 upon activation and hence are the only conventional DC subtype capable of stimulating resting NK cells [109]. LCs, lacking IL12p70 but providing IL-15, cannot stimulate NK cell reactivity but can support their viability [109]. Returning to the theme of afferent sensitization, however, an important unknown remains with regard to how and which DCs can influence KIR expression by NK cells, potentially to maintain reactivity against tumors expressing host HLA, beyond the initial wave of autoreactive T cells.
Unanswered Clinical Challenges and Program Initiative in Dendritic Cells and Immune based Therapy
The divisions of labor among distinct human DC subtypes achieve the most effective balance between steady state tolerance and the induction of innate and adaptive immunity against pathogens, tumors, and other harmful insults. Current approaches to exploit defined DCs for immunotherapy are still mostly labor-intensive, although broader approaches for loading antigens that allow DCs to process and tailor presented peptides to their own MHC are increasingly promising. Conjugating antigen to specific receptors on DCs is also yielding progress. Rodent models are now revealing important data about distinct DC precursors, homeostasis of tissue-resident DCs, and DC turnover in response to inflammation and pathologic conditions like GVHD. The selective elimination of defined DC subsets that are responsible for GVHD, while expanding those that are critical to the onset and maintenance of GVT, represent a holy grail for the controlled afferent sensitization of lymphocyte responses in HSCT. Unfortunately to date, there are no stably tolerogenic human DCs for experimental therapeutic use to reduce GVHD while maintaining GVT. Eliminating the DC subtype most likely to fuel the fire of GVHD, together with the use of defined DC subtypes to stimulate both innate and adaptive immunity, may prove most useful clinically.
DONOR LYMPHOCYTE INFUSIONS
In the two decades since the first reports by Kolb and Slavin of using of DLI to treat patients with CML who had relapsed after allogeneic transplantation, a large number of studies have helped identify the diseases most responsive to DLI. These studies have also established doses of DLI to be used in defined clinical settings and explored methods to enhance the GVL effect of DLI and to limit toxicity [130, 131]. These studies have established that DLI is effective in treating minimal residual disease in some situations.
A small number of studies have addressed the role of prophylactic DLI. Studies in this area have been limited, and a number of factors present challenges to studying the effectiveness of DLI in this setting. First, patients who are at sufficiently high risk of relapse after transplantation to warrant additional therapeutic intervention need to be identified. These populations may be defined by disease type or state of disease at the time of transplant, or by post transplant factors such as persistent minimal residual disease or presence of mixed chimerism. Second, an appropriate platform for DLI needs to be established in the context of non-myeloablative allogeneic HSCT. Several studies have demonstrated that prophylactic DLI administration is often precluded because patients had developed complications after transplant. The most common complication is chronic graft versus host disease. Transplantation performed using T-cell depleting agents such as ATG and alemtuzumab have been most successful in creating a platform for prophylactic DLI. Third, measurable endpoints to define the success of DLI in preventing relapse are needed. Traditional endpoints such as overall and progression free survival may be used, but it is not clear if the conversion of mixed chimerism to full donor chimerism may also serve as surrogate markers of DLI activity. In this section we will attempt to identify the current role of DLI in the prevention of relapse and propose future areas to explore.
Diseases Where DLI is Effective
Registry reports from Europe and North America have identified diseases which respond to DLI. This information combined with outcome data from reduced-intensity allogeneic HSCT studies have identified diseases which appear most responsive to the GVL effect. Diseases which have a high sensitivity to GVT include CML, low-grade lymphomas, CLL and multiple myeloma. Diseases with intermediate sensitivity include Hodgkin lymphoma, AML and MDS. Diseases with a lower sensitivity include ALL and large cell lymphoma.
Chronic Myeloid Leukemia
DLI has been studied most intensively in patients with CML. Treatment in a minimal disease state is associated with improved outcome. Studies consistently demonstrate that patients with CML in more advanced stages of relapse, accelerated phase or blast crisis, have a much lower response rate following DLI [132]. An analysis of 593 DLI demonstrated responses for patients with CML in molecular relapse, cytogenetic relapse, chronic phase, and accelerated/blastic phase relapse were 100%, 90%, 75%, and 35%, respectively [133]. Responses observed in patients with CML after DLI appear to be durable. Studies have reported low relapse rates as low as 9% for patients receiving DLI [134]. The three-year overall survival for this group of patients was 95%. When relapse does occur, isolated extramedullary involvement without evidence of systemic disease can be observed. The mechanism of “immune escape” for cells in these myeloblastomas is not clear. DLI is also effective in treating patients with CML relapsing after unrelated donor transplant [135]. The degree of donor chimerism at the time of DLI is not predictive of response in patients with CML. While a high degree of donor chimerism was associated with a more rapid response, patients with less than 10% donor chimerism at the time of DLI had a similar complete remission rate as patients with high degrees of donor chimerism at the time of DLI [136].
T cell dose also appears to impact both response rate and risk of development of GHVD. Prospective trials of unmanipulated DLI have analyzed T cell number and the impact on response and GVHD. Studies have demonstrated high response rates and a low incidence of GVHD in patients receiving 1 × 107 CD3+ cells/kg [137]. A subsequent trial demonstrated that GVHD was significantly lower with the escalating dose regimen (10%) compared with the single bulk infusion (44%) (p = 0.011)[138].
The role of additional agents combined with DLI is not clear. The addition of alpha-interferon allowed much lower doses of donor cells to be infused and was associated with similar response rates to those seen with higher cell doses [139]. Imatinib and DLI have also been explored although data is limited[140].
Multiple Myeloma
The overall response rate to DLI in patient with myeloma which has relapsed approaches 45%, with complete responses noted in about 25% of patients. Both dose of cells infused and timing of DLI after transplantation for myeloma may influence response rates. When treating relapse, patients receiving greater than 1 × 108 CD3+ cells/kg had an improved response, however, responses have been noted in patients with infusion of doses as low as 1 × 107 CD3+ cells/kg [141]. Early administration of DLI after allogeneic transplantation may improve response rates and improve the graft versus myeloma effect after transplantation.
Prophylactic DLI has been explored in patients with multiple myeloma after myeloablative transplantation. In one study, prophylactic DLI was given to 14 MM patients 6 to 9 months after T-cell depleted, myeloablative allogeneic HSCT [142]. Eleven of the 14 patients receiving DLI had evidence of disease at the time of DLI with 10 demonstrating significant GVT responses, and 6 obtaining complete remissions. A limitation of this study was that only 58% of the patients were able to receive DLI after transplantation. Patients could not receive DLI if they had developed complications such as GVHD limiting the utility of this approach. Using a similar strategy after an in-vivo T cell depleted allogeneic reduced-intensity HSCT, DLI was administered to patients with residual or progressive myeloma [143]. Fourteen of 20 patients received escalating dose DLI for residual/progressive disease more than 6 months post-transplant. Fifty percent of patients had a clinical response. Significant factors associated with response included the development of acute and chronic GVHD. A common finding among studies is that not all responses are durable, suggesting the need for either repeat DLI or other agents.
DLI combined with other immune modulator agents has been explored in hopes of improving the response to DLI. Low-dose thalidomide in combination with DLI in patients with relapsed myeloma resulted in an overall response rate of 67% and a complete response rate of 22% [144]. Limited toxicity was observed with only 11% of patients developing evidence of chronic GVHD. The safety and efficacy of combining bortezimib or lenolinomide with DLI will need to be explored in clinical trials.
Myelodysplastic Syndromes and Acute Leukemias
The results of DLI in patients with relapsed acute leukemias and MDS have not been as encouraging as in patients with CML and multiple myeloma. Administration of chemotherapy followed by DLI did not improve the outcome for patients who had relapsed after transplant and was associated with significant toxicity.
Studies using prophylactic DLI are starting to emerge. Forty six patients with AML received preemptive DLI 120 days after transplant if they were off immune suppressive medications and there were no contraindications [145]. Improved survival was noted in the patients receiving DLI compared with case-matched controls. DLI may be combined with other active agents such as decitibine; however, limited data is currently available.
DLI is associated with a low response rate in ALL. In patients who respond, the duration of response is limited. There is some suggestion that T-cell diseases may respond better to DLI.
Chronic Lymphocytic Leukemia and Lymphomas
DLI experience in patients with CLL and low-grade (follicular) lymphomas is emerging. Treatment of patients with CLL in a minimal disease state appears to be associated with improved outcome [146]. Seven of 9 patients with CLL achieved durable molecular remissions following DLI. Data of the efficacy of DLI in more advanced lymphomas are lacking.
EBV-associated Lymphoproliferative Disorders after HSCT
DLI is a highly effective treatment for post-transplant EBV associated lymphoproliferative disorders (EBV-LPD). Five patients with post BMT EBV-LPD received DLI at a dose of 1.0 ×106 CD3+ T cells per kilogram and a 100% pathologic and clinical response was noted without significant GVHD [147]. As an extension of this strategy, researchers have now demonstrated that administration of in vitro cultivated EBV-specific CTLs alone is sufficient to eradicate EBV-LPD [148–150]. PCR tests are now available which can quantify EBV DNA and offer a method of diagnosing patients prior to the onset of clinically evident EBV-LPD [151]. With this tool for early detection, prophylactic administration of EBV-specific CTLs can now be used as preemptive therapy against EBV-LPD after BMT[149, 150]. DLI has also been used to treat other viral illness such as human herpesvirus-6 encephalitis [92].
DLI after Non-myeloablative and Reduced-intensity Allogeneic HSCT
The role of DLI after reduced-intensity allogeneic HSCT remains to be defined. DLI has been used after reduced-intensity allogeneic HSCT in two ways: 1) treatment of persistent or relapsed disease, or 2) as a method to convert patients from a mixed chimeric state to full donor chimerism. Use of DLI after reduced-intensity allogeneic HSCT has been limited by the high incidence of chronic GVHD seen after non-TCD reduced-intensity allogeneic HSCT, which commonly develops as immune suppression is tapered. Trials exploring DLI administration while patients are on immune suppressive medications have not been performed, and their safety and efficacy are uncertain.
Prophylactic DLI has been more successfully used after TCD transplantation. When anti-T cell agents such as alemtuzumab or ATG are used as part of the reduced-intensity conditioning regimen, the incidence of recurrent disease after transplantation is increased, and many patients demonstrate mixed chimerism after transplantation. The risk of developing GVHD is also reduced thus allowing for more patients to receive DLI. A strategy of using dose escalated DLI in this setting has been shown to be associated with a low incidence of GVHD while inducing GVT effects in a variety of diseases [143, 152]. These studies have also demonstrated that mixed chimerism can be converted to full donor chimerism using DLI. In an effort to limit toxicity related to DLI, another approach focused on prophylactic CD8+ T cell-depleted DLI after non-myeloablative allogeneic HSCT[153]. In that study, 11 of 23 patients were able to receive prophylactic DLI. Patients receiving CD8+ T cell-depleted DLI demonstrated accelerated immune reconstitution and minimal GVHD.
Methods to Enhance the GVT Response Mediated by DLI
Strategies to enhance the GVT effect mediated by DLI have included infusion of activated cells as well as methods to improve potential target antigen presentation. Infusion of antigen specific cells in diseases such as CML also have the potential to increase efficacy while limiting toxicity. As previously described, selective populations of cells, such as CD8+ T cell-depleted DLI, have been explored and appear to be associated with a reduced incidence of GVHD without compromising the efficacy of DLI.
Regulatory T cells (Treg) are naturally occurring CD4+CD25+FOXP3+ T cells that constitute approximately 5–10% of the circulating CD4+ T cell population and dominantly suppress autoreactive lymphocytes and control immune responses [154, 155]. Treg suppress both the innate and the adaptive immune systems [156–158]. A trial to assess whether Treg depletion may enhance the immunologic GVT effect of DLI is currently ongoing in patients who have relapsed after transplant. Preliminary results demonstrate a greater than 2-log depletion of CD4+CD25+FOXP3+ cells has been achieved. The cell infusions are well tolerated, and minimal GVHD has been observed. Results from this trial, if successful, may inform the use of a similar strategy in patients prior to relapse.
Unanswered Clinical Challenges and Program Initiatives in DLI
Defining the risk of relapse is critical to the development of future studies of DLI. Both data for single institutions and cooperative databases will help define potential candidates for prophylactic DLI studies. This information should identify populations at high risk for relapse. Understanding the kinetics of relapse will be equally important. Differences based on both disease type and disease status at the time of transplant will impact the kinetics of relapse and define the time interval available for intervention. MRD monitoring, which is available in certain diseases, may serve as a powerful tool to identify appropriate patients for clinical trials. In diseases where a significant GVT effect has been demonstrated, DLI using current methods may be sufficient although a better understanding of dose and time of administration is needed. In diseases where GVT is less effective, novel approaches to enhance the efficacy of DLI through vaccines or target specific DLI should be explored.
Another challenge is to define the appropriate platform for prophylactic DLI in the reduced-intensity and non-myeloabaltive settings. The majority of trials of prophylactic DLI reported to date are in patients who have received T-cell depleted transplantation. Despite TCD, a number of patients are still not able to receive DLI because they have developed post-transplant complications or experience early progression of their disease. Future studies will need to define dose, timing and composition of cells to be infused in the T depleted setting. DLI in the non-T cell depleted setting has been limited to patients who have been tapered off their immune suppressive therapy. It may be reasonable to explore the safety and efficacy of administering DLI while patients are on immune suppression as a method to enhance the efficacy of DLI.
The durability of DLI is not clear, and there is a need to explored maintenance immune therapy. Risk of relapse after DLI varies by disease. Future strategies may need to explore repeated DLI in order to maintain remission. Extramedullary sites of relapse have been noted after DLI. As there is a better understanding of what factors play a role in this pattern of relapse, DLI strategies will need to change.
A significant effort should be directed toward exploring DLI combined with other promising therapeutic interventions. Future trials should combine DLI with disease modifying agents in diseases such as MDS/AML and multiple myeloma. Selected T cell infusions, activated and antigen-specific T cell infusions are also possible areas of study. DLI may also be explored in augmenting responses to vaccines. To date neither the dose, timing, nor cell type have been explored.
Finally, defined endpoints for clinical trials of DLI need to be established. Overall and progression-free survival are the most important endpoints in a randomized trial, but these types of trials will be difficult to perform outside of a cooperative group setting. For smaller trials these endpoints can only be used in well defined, homogeneous populations where there is well documented historical control data. Elimination of MRD can serve as a surrogate when available. The persistence of MRD without progression to relapse after DLI may challenge our preconceived notion that disease must be completely eliminated. It may force us to consider a disease state where the disease is suppressed but not eliminated by the donors immune system. In such cases, time to relapse would be the most important endpoint. Chimerism as a surrogate endpoint is also possible in some diseases. Data support that high levels of donor chimerism are associated with improved outcome in some diseases. In other diseases, high levels of donor chimerism do not appear to have an impact on risk of relapse suggesting that the GVT effect and the graft-versus-hematopoiesis effect in these diseases are distinct. Augmenting donor chimerism by DLI when patients are off immune suppression or perhaps converting low donor chimerism to high donor chimerism early after transplant by DLI should also be explored.
T CELL ENGINEERING
The ability of adoptively transferred T cells administered as DLI following allogeneic bone marrow transplantation to induce long-term remission in some hematological malignancies, as reviewed above, is well established [159]. The reasons why CML responds well to this therapy, while ALL and AML do not, are still poorly understood. Whether the resistance of the latter is due to poor tumor antigen recognition or the malfunction of tumor-reactive T cells in the tumor microenvironment, or both, T cell engineering offers an exciting prospect for overcoming these deficiencies. The advent of efficient methods for human T cell engineering has opened the possibility of introducing antigen receptors into T cells and thus to rapidly generate tumor-reactive T cells. Gene transfer can be easily performed in readily accessible peripheral blood T lymphocytes. Over the past decade, T cell engineering has mostly focused on redirecting T cell antigen specificity, which is normally determined by the T cell antigen receptor (TCR). While the TCR is the physiological receptor and the preferred tool for some investigators, other investigators have focused on chimeric antigen receptors (CARs) [90], which are artificial structures designed to ligate cell surface antigens and provide activating and costimulatoty signals to T cells. In addition to tumor targeting, T cell engineering additionally provides a means to augment T cell function and/or overcome tumor escape mechanisms that stifle T cell responses. The latter include HLA down-regulation, which deprives the T cell antigen receptor of a ligand on the tumor cell [165/[160]], the intra-tumoral accumulation of regulatory T cells and myeloid tumor cells [161] and the expression by tumor cells of various suppressive molecules such as prostaglandin E2, TGFβ or B7× (Table 1) [159–161].
Peripheral blood T cells can be retargeted to any chosen tumor antigen by the genetic transfer of an antigen-specific receptor. The transduced receptors may be either HLA-restricted, heterodimeric TCRs or CARs that typically recognize native cell-surface antigens. Considerable progress has been made in recent years to address the challenges posed by the transfer of either receptor type. Vector and protein modifications enable the expression of TCR chains in human T cells at functional levels and with a reduced risk of mis-pairing with endogenous TCR chains. The combinatorial inclusion of activating and costimulatory domains in CARs has dramatically enhanced the signaling properties of the chimeric receptors described over a decade ago. Based on effective T cell transduction and expansion procedures now available to support clinical investigation, improved designer TCRs and second generation CARs targeting an array of antigens, including CD19 and WT-1, are ready to be evaluated in a range of hematological malignancies.
TCR gene transfer
The clonotypic αβ or γδ heterodimer borne by every T cell associates with the CD3 complex, itself comprising α, β, γ and δ chains. T cell activation is mediated by the CD3 complex following engagement of the TCR on the MHC-peptide complex borne by the antigen-presenting cell. Since all T cells naturally express their own TCR, TCR transfer into mature lymphocytes sets the stage for competition and recombination between the two TCRs. Much research over the past few years has focused on how to appropriately express a second TCR in T lymphocytes.
Transgenic TCR Expression
TCR function is known to be dependent on its level of expression, which is limited by the amount of available CD3 complex [162]. Following gene transfer, the transduced TCR must compete with the endogenous TCR for association with CD3. While different α/β chain heterodimers seem to intrinsically differ in their competitiveness vis-à-vis the endogenous TCR [163, 164] a key point is to insure high-level expression of the transduced TCR. Much progress has been accomplished in this area, based on improvements in transcription, dual chain co-expression and codon optimization. Several retroviral enhancer/promoters have been compared in terms of their promoter strength, cell cycle dependence, and susceptibility to transcriptional inactivation or silencing. Long-term in vivo comparisons are still lacking. The need to co-express two chains is best addressed using bicistronic vector designs, which can be achieved by using either viral internal ribosomal entry sites (IRES) [165] or 2A elements [171/[166]] to link the two open reading frames. Finally, codon optimization has been shown to improve cell surface expression of several TCR heterodimers [167–169].
Controlled TCR Pairing
The re-assortment of transduced and endogenous TCR chains is a major concern in this approach, since the coexpression of 2 α and 2 β chains by the same T cell could theoretically result in the formation of 4 different TCRs, raising the prospect of inducing autoimmunity. Several molecular and cellular approaches have been proposed to resolve this issue. The first rests on the incorporation of murine constant regions into the TCR, which results in higher cell surface expression and decreased mispairing with the endogenous TCR in human T cells [170–172]. The immunogenicity of such chains is a concern, but no TCR immunogenicity was observed in the first clinical study making use of such receptors [173]. The second approach is to introduce cystein bonds into the α and β chains, which are designed to favor dimerization of the transduced TCR [174–176]. Although variable, this approach may ultimately solve this concern, and poses a lesser risk of immunogenicity than the use of extended murine sequences. An alternative, non-molecular approach is to utilize γδ T cells for adoptive therapy, in which αβ heterodimers can be introduced without the concern of heterogeneous pairing [177, 178]. It is presently unknown, whether γδ T cells will function and persist as well as αβ T cells in the adoptive T cell therapy setting.
CAR Gene Transfer
The first generation of CARs utilized the CD3ζ cytoplasmic domain to elicit T cell activation. However, it wasn’t until costimulatory properties were incorporated into the next generation of CARs that a greater strength and quality of antigen-induced signaling could be provided to T cells. An impressive array of second generation CARs has been developed in the past decade.
Antigen Recognition by CARs
Most CARs typically utilize an antibody-derived antigen-binding motif to recognize antigen. Some utilize receptor or ligand domains as their targeting moiety, such as heregulin [179] or IL-13, that bind to their cognate ligand or receptor counterpart. All of these CARs recognize native cell-surface antigens independently of antigen processing or MHC-restricted presentation. Importantly, CARs therefore do not have to be matched to the patient HLA and can recognize tumors that have down-regulated HLA expression. The molecules targeted by CARs include proteins, carbohydrates and glycolipids. Most current CARs incorporate an scFv derived from a murine monoclonal antibody, but some have been selected from phage display libraries. The rules for identifying the best-suited scFv for a particular target molecule are not yet fully elucidated.
CAR Signaling
The first CARs were reported as receptors capable of redirecting the cytotoxic activity of CTL clones and hybridomas. The emergence of CARs enabling T cells to survive repeated antigenic stimulation came with the development of CD28-CD3ζ dual-signaling receptors. These receptors increased IL-2 secretion in response to antigen and permitted absolute expansion of retargeted T cells in response to antigen in the absence of exogenous costimulation [180]. A number of CD28/CD3ζ fusion receptors have been reported, which utilize different domains and fusion points. A side-by-side comparison of different receptors expressed at similar level in the same cells type has not yet been reported. New fusion receptors are emerging. Triple-fusion receptors that encompass CD3ζ, CD28 and 4-1BB signaling motifs appear to enhance in vivo effector functions relative to the dual-fusion receptors [181, 182]. These are promising receptors but more studies are needed, including in vivo studies, to assess their therapeutic potential and safety profile. What is clear is that second generation CARs have considerably superior signaling properties compared to their CD3ζ and FcRγ forbearers, which opens up real perspectives for the therapeutic use of CARs.
The T cell Engineering Process
T cell engineering begins with the selection of adequate gene transfer tools and T cells. Whereas many tools are now available to genetically modify human T cells, the T cell type best suited for use in adoptive therapies remains to be defined. A key aspect of adoptive cell therapies is how to activate and expand T cells to generate large number of T cells without impairing their function or life span.
Methods for Gene Transfer
Several vector types are now available for efficient transduction of human primary T lymphocytes. Gamma-retroviral vectors pseudotyped with the Gibbon Ape Leukemia Virus Envelope were the first to permit efficient transduction [183–185]. Transduction conditions using mitogen- or antigen-activated T cells have since been highly optimized by many investigators and are currently in use in several clinical trials. Lentiviral vectors have also been used with success [186–188]. In contrast to gamma-retroviral vectors, these do not require cell division for stable gene transfer, but cytokine pretreatment is nonetheless a prerequisite [189–191]. Comparisons of vector silencing or vector safety are still lacking. Whereas lentiviral vector have an inherent reduced risk of transforming hematopoietic progenitors in comparison to gamma-retroviral vectors [192], it is presently unknown whether this difference extends to T cells. In contrast to hematopoietic progenitors, T cells are difficult to transform in mice [191, 193]. Stable gene transfer may also be attained using non-viral transposon/transposase systems [194], but the efficacy and safety of this approach still needs to be worked out. Transient systems have also been optimized, using electroporated DNA or RNA encoding TCRs [195, 196]. These rapid and efficient systems are useful for basic studies, but it is uncertain whether the short duration of transgene expression will be useful in clinical applications.
Transferring the Right T Cell Subset
There is much debate what T cell type, in terms of its differentiation stage [80], may be the most effective. It appears that highly lytic, activated effector T cells are not the most active in vivo [197]. Ongoing research by several investigators aims to compare the relative merits of naïve T cells, central memory cells and virus-specific T cells. The former are endowed with the greatest differentiation potential [198], but central memory T cells are imprinted at the time of their initial activation to acquire survival properties that ultimately prolong their function [199, 200]. Virus-specific memory T cells that are chronically reactivated by antigen, such as EBV-reactive T cells, may also serve as effective cellular vectors for tumor-reactive TCRs and CARs [163, 201]. More studies are needed to define the relative advantages of these different subsets. Alternative T cell subsets, such as γδ T cells and NKT cells are still little known. Another twist on this approach is to utilize lymphoid progenitors, which become tolerant of the host in which they develop and can be genetically redirected to tumor antigens [70, 202, 203].
Methods for T Cell Expansion
Beyond the T cell purification and transduction steps, the T cell expansion process is of critical importance. Indeed, T cell proliferation does not occur without affecting T cell differentiation and hence the functional and survival potentials of the cells to be adoptively transferred. The provision of adequate activating and costimulatory signals during the expansion is essential to generate effective T cells. The design of artificial antigen presenting cells (AAPCs) holds the promise to yield optimized antigen-specific stimulators [204–208], as well as non-specific T cell amplifiers [209–211]. Panels of AAPCs expressing common HLAs have recently been reported, which express single HLA molecules and should be useful to generate appropriately restricted CTLS, especially in the haploidentical transplant setting [212]. These cellular systems are poised for clinical investigation. Clinical grade antibody-coated beads are already available and currently in use for the activation, transduction and expansion of T cells in protocols in leukemia and prostate cancer [213]. This remains a vital area of investigation that will play a decisive role in the development of effective, safe and affordable T cell-based therapies.
Clinical Trials
Clinical Studies Utilizing First Generation CARs
Completed clinical studies are limited to phase I studies evaluating first generation CARs targeting the folate receptor in ovarian cancer [214], carbonic anhydrase in renal cancer [215] CD20 in lymphoma and GD2 in neuroblastoma [201]. The clinical responses have overall been very modest, with the exception of one partial response in the neuroblastoma study. Immunogenicity of CARs was observed in the first two studies, but not the latter two. These studies utilized first generation CARs and suboptimal T cell expansion procedures such as OKT3-mediated T cell expansion.The field is thus keenly awaiting studies that utilize second generation CARs and improved T cell expansion procedures that provide appropriate costimulation and cytokine stimulation prior to T cell infusion. Studies targeting CD19 are especially awaited as these hold the promise of activity in several B cell malignancies. At least seven trials targeting CD19 with second generation CARs are programmed in the U.S. and Europe, most of which utilize a CD19-CD28ζ CAR. Other investigated targets include CD20, CD23, CD33, and CD74.
Clinical Studies Utilizing TCR Gene Transfer
Two clinical studies utilizing TCR gene transfer have been reported to date, both in melanoma. Morgan and his colleagues at the NCI were the first to establish the feasibility of this approach [216]. Having transferred an HLA A2.1restricted, MART-1-specific TCR in peripheral blood T cells of patients with metastatic melanoma, the obtained partial responses in 2 out the first 15 treated patients showed partial responses. Utilizing a higher affinity MART-1-specific TCR to treat another 36 patients with metastatic lymphoma, the same groups recently reported objective cancer regressions in 30% and 19% of patients who received the human or mouse TCR, respectively [173]. However, patients exhibited destruction of normal melanocytes in the skin, eye, and ear, which subsided with steroid treatment. These encouraging results point to the possible efficacy of this approach, as well as the risk of on-target secondary effects with some if not all non-tumor-specific antigens. WT-1 targeting is currently under study by several groups. Other investigated antigens include minor histocompatibility antigens and cancer-testis antigens.
Perspectives and Challenges
T cell engineering is a rapidly evolving field of research that aims to provide tumor-targeted T cells for all patients and to generate T cells with enhanced anti-tumor function. Effective tools for genetic engineering are now available and significant advances have been made to express transduced antigen receptors at functional levels in human T lymphocytes. Both αβ heterodimeric TCRs and CARs show great promise for redirecting T cell specificity. CARs are attractive because they are not restricted by HLA, but their signaling function does not benefit from the support provided by the CD3 complex. However, CARs with vastly enhanced signaling properties have been developed in recent years.
A large number of clinical trials using either antigen receptor type are on the verge of opening in the US, Europe and Japan. The multiplicity of trials targeting CD19 in B cell malignancies will provide the ground for comparing the effectiveness of different vector designs and T cell expansion processes. The identification of the best-suited T cell subsets to engineer and the advent of novel tools such as artificial antigen presenting cells will likely contribute to further enhance the efficacy of this approach in the near future.
While the design of recombinant antigen receptors and the ability to transduce various T cell subsets has made great strides in the past few years, the clinical experience with these new tools is still very limited. The first evidence of efficacy is emerging[173, 201, 217], and so is the potential for toxicity [218–220]. The relationship between antigen receptor design, T cell dose, T cell type, conditioning, tumor burden and disease type or stage is presently unknown. Much more clinical investigation is needed to gain concrete insights into this complex immuno-pharmacology. An additional challenge will be to “export” technologies from one medical center to another, which is essential for the objective evaluation and greater accrual of patients on trials that otherwise run the risk of remaining local phenomena. This will require strong support from funding agencies, not only to support research, manufacturing and clinical investigation but also regulatory and legal to lift the obstacles to exchanging manufactured cell products between collaborating institutions.
THE ROLE OF VACCINATION IN THE PREVENTION OF RELAPSE
Despite the early identification of tumor antigens as transplantation antigens [221–225], cancer immunity remains poorly understood in the allogeneic HSCT setting. In part, this is because transplantation brings more complexity to the challenge of determining how host immune cells distinguish “self” and “non-self” [226, 227], and it expands the number of possible interactions of recipient- and donor-derived cells in priming and maintaining tumor immunity. Yet, the rich experience of allogeneic transplantation offers perhaps the strongest clinical evidence that active tumor immunity can rid patients of malignant cells such as leukemia and prevent relapse [228, 229]. If the critical cells and molecular events that drive the GVT effect were better understood, for example, rational immune therapy strategies might be devised that deliver the “Holy Grail” of allogeneic HSCT: induction of GVT without GVHD. Nevertheless, it is still early days in our understanding of the biological basis of this immunity, and for turning that “actionable intelligence,” in present jargon, into targeted immune therapy approaches to improve clinical outcome by preventing relapse after HSCT.
Vaccines are one treatment approach with the potential to selectively boost GVT. Inducing or enhancing host and donor immunity against malignant cells by vaccination is an old idea [230], but it offers many theoretical advantages over existing treatments. For example, they are relatively safe. They are antigen-specific, with the potential to reduce the risk of GVHD. They may be used to treat existing disease as well as prevent relapse by inducing long-term and possibly life-long immunity. They can be administered to both donor and recipient to increase effectiveness. Strategies that combine vaccination with cell therapies might be applied in a staged approach to first prime immunity in the donor, followed by adoptive transfer of primed donor-derived lymphocytes to transplanted recipients, and then further vaccination of the recipient to sustain the transferred donor-derived immunity. Because vaccines are less toxic than most other treatments, they could be combined with other drug therapies, such as targeted single-agent drugs in complementary or additive strategies in the post-transplant setting. Finally, eliminating GVHD may not be necessary and it may not be beneficial. Allogeneic immune responses that drive GVHD, which may not be directed specifically at tumor or tumor tissue-derived antigens, may nonetheless heighten overall immunity by increasing chemokine or cytokine expression [231], thereby indirectly driving GVT [232]. This mechanism is supported by the clinical observation that GVT often occurs in the setting of GVHD [233, 234]. Therefore, rather than excluding GVHD patients from clinical vaccine trials, the heightened immunity from low-grade GVHD might produce a more favorable milieu for eliciting GVT by vaccination in patients that don’t require steroids, for example.
Despite these potential advantages, cancer vaccines thus far have not produced significant clinical results [235]. Most vaccine trials have involved patients with solid tumors treated outside the transplant setting. However, several observations can be drawn from our failures thus far, including the identification of mechanisms of tolerance and anergy. For instance, tumors can escape immunity by decreasing MHC expression, tumor antigen expression [236], or by the emergence of cells with mutated antigens that are no longer recognized by T cells [237]. Nevertheless, appropriate antigen selection and increasing antigen expression by drug treatment might overcome these problems [238]. Furthermore, when specific immunity is induced by vaccination, it is most often in the setting of MRD, in part because host immune deficits, such as broad anergy, may not yet have developed. Alternatively, antigen-specific immune tolerance may also develop in the setting of high tumor burden, such as in patients that overexpress the leukemia-associated antigen proteinase 3 (P3) and the P3-derived epitope PR1 [239]. For example, CML cells that overexpress P3 induce selective apoptosis of high affinity PR1-specific T cells, thereby eliminating the most effective T cells against CML and creating tolerance by deletion, favoring the outgrowth of leukemia.
A critical factor when considering the lackluster results of cancer vaccines is that the trials have been designed as therapeutic rather than preventative, involving patients with large tumor burdens, including many with metastatic disease. Historically, however, vaccines have been most successful in the prevention of infectious diseases such as pneumonia or influenza, but this approach has not been well tested in the cancer setting largely due to the lack of a sufficiently immunogenic vaccine preparation and the failure to test vaccines in a healthy patient population. In part this is because of a fear of inducing side effects in patients who may have minimal disease or no disease yet at all. For example, cancer vaccines have largely been comprised of self-antigens or tumor-specific antigens that have similarity to self-antigens and the trials have therefore been focused on phase I testing in patients that already have cancer to avoid complications such as autoimmunity, despite evidence that autoimmunity may be beneficial [240, 241]. However, the allogeneic transplant setting offers a potential advantage to test whether leukemia vaccines may be useful in primary and secondary prevention since vaccines comprised of hematopoietic-restricted minor histocompatibility antigens (mHAgs) would be unlikely to induce immunity against donor hematopoietic cells in the recipient. The preventive setting is well-suited to testing cancer vaccines, however. The recently approved HPV vaccine [242], for example, is used to prevent cervical cancer, although therapeutic vaccines are also being developed for this disease [243, 244].
Vaccines for hematological malignancies have only recently been explored, mostly in the setting of myeloid leukemias and MDS. The identification of mHAgs, leukemia-specific antigens, and leukemia-associated antigens has facilitated such trials. While it is beyond the scope of this review to examine all potential vaccine targets, it is worthwhile to describe some as examples.
The allogeneic GVT effect is closely associated with donor T cells, although alloreactive NK cells[245] are also involved in anti-AML effects [246, 247]. T cells, as mediators of the adaptive immune response, recognize specific peptides within the peptide-binding groove of self-MHC molecules, and mHAgs are an obvious target of GVL in the HLA-matched SCT setting. Ubiquitously expressed mHAgs such as HY [266/147], HA-3, HA-4, HA-6, HA-7 [248], and HA-8 [249] may play a role in both GVHD and GVT effects. mHAgs exclusively expressed on recipient cells of hematopoietic origin, however, such as HA-1 and HA-2 [250] or on lineage-specific hematopoietic cells such as HB-1 [251] may result in GVT reactivity in the absence of severe GVHD. T cells recognizing hematopoiesis-restricted mHAgs may induce a relatively specific immune response against malignant hematopoietic recipient cells and induce no or only minor GVHD [252]. Despite this potential, however, the requirement for identical HLA matching and for selective mHAg mismatching of low frequency minor alleles imposes practical limits on the broad clinical implementation of mHAg-based vaccine strategies. Therefore, vaccination with leukemia-associated antigens may also be considered.
WT-1 encodes a zinc finger transcription factor involved in apoptosis, cell proliferation, and organ development [253]. WT-1 is overexpressed in many tumors including lymphoid and myeloid leukemia, and it has been strongly linked to leukemogenesis [254]. A number of putative HLA-binding motifs have been documented within the WT-1 protein, some of which bind relevant HLA allele and elicit a peptide specific CTL response [255]. Xue and colleagues modified the TCR in a leukemia mouse model to target WT-1 and demonstrated the ability of these cells to kill leukemia cells and produce cytokines in vitro [256]. Subsequent administration of TCR-transduced T cells to NOD/SCID mice harboring human leukemia cells showed elimination of leukemia in animals that received the WT-1-specific CTL, compared with controls. Tsuji et al transduced TCR genes obtained from WT-1 peptide-specific CTL into polyclonally activated Th1 and CTL. TCR gene-modified CTL and Th1 cells showed cytotoxicity, as well as IFN-γ and IL-2 production, in response to WT-1-expressing leukemia targets [257]. Thus, TCR gene-modification may provide the means to generate leukemia-specific CTL for use in AML immunotherapy.
Early clinical studies showed that WT-1-specific T cells could be elicited in AML and MDS patients following vaccination with WT-1 peptide, and clinical effects were noted during the induction of these CTL in peripheral blood [92]. A phase I/II trial reported CR in an HLA-A2 patient with relapsed AML who received four biweekly and then monthly vaccinations with WT-1 peptide plus the T helper protein keyhole limpet hemocyanin (KLH) and GM-CSF for a total of 15 vaccinations[258]. More recently, Scheibenbogen and colleagues reported the results of a phase II trial of sixteen HLA-A2-positive patients with AML and one patient with MDS who received up to 18 vaccinations (median = 8) of WT-1 peptide with KLH and GM-CSF. Twelve patients had elevated blast counts at study entry and 5 patients were in CR with a high relapse risk. In patients with elevated blast counts, six demonstrated evidence of anti-leukemia activity; one patient achieved CR for 12 months. Furthermore, tetramer and intracellular cytokine staining demonstrated WT-1-specific T cell responses in peripheral blood and bone marrow [259].
PR1, another leukemia-associated antigen, is an HLA-A201 restricted nonomer peptide (VLQELNVTV) that is derived from the differentiation stage-specific neutral serine proteases P3 and neutrophil elastase (NE), which share 54% amino acid (aa) sequence homology and are normally stored in primary azurophil granules of myeloid progenitor cells [260]. The pre-pro-forms of both proteins contain a 25 aa leader sequence which traffics them to the endoplasmic reticulum for processing and enzyme activation [261, 262]. P3 and NE are aberrantly expressed in myeloid leukemia (2–5 fold higher versus normal cells) and rheumatologic disorders such as Wegener’s granulomatosis and small vessels vasculitis [263–266]. The leukemogenic and immunogenic properties of these proteins, the latter demonstrated by their essential role in generating autoimmunity characteristic of the aforementioned rheumatologic diseases, makes them ideal targets for the development of anti-leukemia immunotherapy. PR1-specific CTL that recognize and kill PR1 expressing HLA-A2 CML cells have been correlated with clinical responses to IFN-α2b therapy in 11 of 12 patients with CML; in contrast, PR1-specific CTLs were absent in all non-responders (n=7) [260, 267]. Similarly, PR1 specific CTLs were detected in 6 of 8 patients with CML receiving allogeneic HSCT, while they were absent in patients who failed to respond to allogeneic HSCT and in those who received cytotoxic chemotherapy.
A Phase I/II vaccine study in patients with refractory or relapsed myeloid leukemia has been conducted which combined PR1 peptide and GM-CSF in 15 CML and AML patients with progressive disease. PR1-speicfic CTL, measured using PR1/HLA-A2 tetramers, were detected in 8 patients, 5 of whom obtained a clinical response [268]. In further follow-up, a total of 66 patients (AML, CML, and MDS) have been treated with the PR1 peptide vaccine and immune responses were noted in 54%, which correlated with clinical responses including complete molecular remission of t(15;17) AML, inv(16) AML, and t(9;22) CML assessed by RT-PCR [269]. The effectiveness of peptide vaccination for inducing T-cell immunity and clinical responses has also been shown in a separate trial conducted at the National Institutes of Health by Rezvani, Barrett and co-workers. WT-1 and PR1 combined vaccination resulted in immune responses in 8 of 8 patients with myeloid malignancies and a reduction of WT1 RNA in some patients as a marker of minimal residual disease [93]. Importantly, clinical responses in both trials occurred more frequently in patients with low leukemia burden or MRD.
As another example of a leukemia-associated antigen, the receptor for hyaluronic acid (HA) mediated motility (RHAMM, or CD168) has also been used as a target for vaccine therapy for AML. RHAMM is a glycophosphatidylinositol (GPI)-anchored receptor that is involved in cell motility [270]. In addition, it is oncogenic when overexpressed, is critical for ras-mediated transformation [271], and has been reported in blasts of more than 80% of patients with AML, MDS, and MM [272]. In a phase I/II vaccine study, clinical and immunological responses were noted following administration of RHAMM R3 peptide emulsified with incomplete Freund's adjuvant and GM-CSF to patients with AML, MDS, and MM overexpressing RHAMM/CD168 [273]. Furthermore, a reduction of the RHAMM/CD168 antigen by real-time RT-PCR and a decreased number of CD33+ cells were also noted following vaccine administration.
An example of a widely studied leukemic antigen as a potential target for immunotherapy is the CML bcr-abl protein. The fusion region peptide encoded by the b3a2 or b2a2 translocation is uniquely expressed in Ph+ leukemia cells, and expression of the protein is essential and sufficient for the development of CML. The fusion protein results from the reciprocal translocation t(9;22)(q34;q11), which transcribed into one of the most common chimeric bcr/abl mRNA (b3a2), and translated into BCR/ABL protein (p210 BCR-ABL). In addition, the protein fusion creates a codon split, which produces a new amino acid, lysine, in the b3a2 BCR-ABL protein; it is therefore considered as a truly leukemia-specific antigen. Peptides derived from this protein were found to bind to a number of HLA molecules, including HLA-A2-, -A3-, -A11- and –B8- molecules and were demonstrated to elicit reactive T cells in-vitro that recognize peptide-pulsed target cells. Bcr-abl-specific T cells were also detected in the peripheral blood of b3a2 CML patients and in some healthy donors. The presence of these tetramer positive cells in patients was associated with a lower tumor burden, suggesting that BCR-ABL specific T cells may participate in disease control. Nevertheless, ex-vivo peptide stimulations were necessary in some cases to visualize tetramer positive cells and were required to observe specific cytotoxic activity against CML targets. In another study, Posthuma et al could not detect nor induce high avidity BCR-ABL specific T cells from a CML patient responding to DLI. This may, at least in part, be due to the low affinity of BCR-ABL peptides. Recently, the modification of amino acid anchor residues in BCR-ABL peptide sequences showed greater immunogenicity without loss of original sequence specificity [274]. Immunity to b3a2 fusion region-derived peptides has also been elicited in 16 CML patients that received the peptides with an incomplete Freund’s adjuvant in addition to imatinib or interferon-alpha. Significantly, cytogenetic responses, including 2 patients with complete cytogenetic responses were observed in patients with demonstrable immunity [275]. The confounding effect of receiving additional treatments prevents a clear connection between the induced T cells and the responses, but future trials are ongoing that will hopefully answer this important question.
In addition to these T-cell target antigens, immunization with NKT cell epitopes may also be useful either alone or in combination. The glycolipid antigen α-galactosylceramide (α-GalCer), which binds CD1d, has demonstrated effective activation of NKT cells [276] and anti-tumor activity against a broad spectrum of malignancies in murine models and humans. Although to date most trials using α-GalCer have been conducted in patients with solid tumors, imatinib-treated CML-chronic phase patients in complete cytogenetic response were shown to have NKT cells capable of producing IFN-γ, in contrast to patients in partial remission [277]. These data, in addition to prior reports demonstrating CD1d expression by AML cells [278], provide promising evidence for use of α-GalCer/α-GalCer -pulsed DC for therapy of AML.
Despite the increasing potential for vaccine development for hematological malignancies in the allogeneic HSCT setting, significant hurdles remain for further development. While acknowledging there are more obstacles than those discussed here, I propose three broad categories that must be addressed.
First, we do not yet know what constitutes the most effective vaccine preparation. Should the vaccine be based on component antigens (DNA, peptide, protein, or glycolipid), or on whole cells (recipient-derived leukemia, dendritic cells, or genetically-modified cancer cells) with or without exogenous antigens? While antigen-specific approaches have been discussed, another approach has used lethally irradiated autologous leukemia cells genetically modified to secrete GM-CSF [279]. All of these approaches have yet to be validated in controlled trials in leukemia patients, however.
Second, we do not yet know when to give vaccines and which is the most ideal setting to test a vaccine. There are potential advantages of testing vaccines in the allogeneic setting because vaccinating recipients after HSCT offers the opportunity to immunize a healthy and potentially non-tolerized immune system. This could be the ideal preventative setting if HSCT recipients are vaccinated in CR or with MRD post-transplant. Similarly, because leukemia vaccine trials have shown clinical responses only in patients with low leukemia burden or minimal residual disease [269], this might represent an ideal clinical situation to test vaccination in a controlled clinical trial. While single agents that target molecular defects yield short-lived responses without cure in AML [280], and single agents such as imatinib require continuous treatment to maintain response in CML, they might be combined with vaccines [281, 282] to improve clinical outcome. Other agents, such as decitabine, which can potentially up-regulate antigen expression in leukemia [283], might improve vaccine effectiveness. In addition, vaccination before and after lymphodepletion chemotherapy [241], which may reset homeostatic lymphocyte proliferation, may yield a more robust immune response in the autologous or allogeneic setting [284].
Third, we need to understand how best to monitor vaccine interventions for biological activity. In the case of T cell antigens, it seems that simply measuring the number of antigen-specific T cells at specified time points may not be sufficient to characterize the tumor immune response. For example, TCR avidity, measuring the breadth of the clonal T cell response, and measuring antigen-specific cytokine production (ELISPOT, cytokine flow cytometry, or bead-based cytokine secretion assays), for example. Perhaps the most direct, albeit difficult, assay is to show that antigen-induced T cells or other vaccine-induced cells specifically lyse malignant cells, and that they show relative inactivity against non-malignant cells. Achieving this critical biological measurement in a large number of patients would help advance the concept of vaccination.
In summary, the strategy of vaccination to induce GVT offers great potential to be a nontoxic treatment that can be applied to both donors and recipients to maximize potential clinical benefit. Challenges remain, however. Before realizing the full clinical potential, we must focus on understanding the most relevant biological endpoints of effective immunity and not necessarily the ones that are most easily measured. In addition to quantitative measures, we must use qualitative assays that measure cytotoxicity or assays that directly correlate with cytotoxicity since this is likely the most relevant biological event for effective anti-tumor immunity. The setting with perhaps the most potential for clinical benefit, based on the importance of GVT and results of early vaccine trials in leukemia, is in patients with myeloid malignancies with low leukemia burden or minimal residual disease, such as patients with molecular disease only. The ideal setting to test vaccines may be in the setting of secondary prevention, such as in post-transplant patients at high risk for relapse. Finally, do not yet know what components of a vaccine are most critical for eliciting immunity, but the identification of mHAgs, leukemia-specific and leukemia-associated antigens has yielded many potential target antigens. Vaccines that direct immunity against these targets seem most likely to produce significant results.
SUMMARY AND RECOMENDED AREAS OF INVESTIGATION
Although it is clear that allogeneic HSCT is the major means for curing patients with hematologic malignancies, this degree of efficacy is not uniform across all patients. We have noted that current standard transplant therapies are limited especially by toxicity. Selective or targeting tactics, summarized in Table 2, would be expected to have less toxicity. These strategies include the use of novel targeted drugs which do not increase toxicity of the conditioning regimen or interact with other medications taken routinely post-transplant, selective depletion of the donor graft to eliminate cells most likely to cause GVHD while maintaining GVL, prophylactic treatment with T cells specifically targeting malignant cells and not causing GVHD, and new vaccines that might be applicable to a broader array of patients than with the single peptide approach.
Table 2.
Summary of strategies for prevention of relapse after allogeneic stem cell transplantation
| Limits of Current Therapy |
New Strategies | Challenges | Recommendations | |
|---|---|---|---|---|
| Preparative Regimens | Toxicity of nontargeted TBI and chemotherapy limits further dose intensification and/or selection of drugs for combinations | Add targeted therapies to reduce toxicities to nontarget tissues | Heterogeneity of the molecular etiology of leukemia yields multiple targets. |
|
| Posttransplant Drugs | Conventional chemotherapy and cytokines (IL-2) are too toxic for use in the early post BMT setting | Use targeted therapeutics as maintenance | Drug-drug interactions, potential immuno-or myelosuppression, unclear when it is best to use these drugs (cancer stem cell is not cycling) |
|
| Graft Engineering | Bulk T cell depletion enhances relapse potential | Selective depletion of alloreactive cells, depletion of the naive T cells subset | Insufficient information of which T cell subsets in humans is responsible for GVH vs GVL and whether that differs by MHC-mismatch and leukemia type |
|
| Donor Lymphocyte Infusion | Treatment efficacy limited to certain leukemias, efficacy in prophylaxis limited by GVHD | In the prophylaxis setting, use NK cells, leukemia-selective CTLs or suicidal CTLs to get GVL without a great risk of GVHD | Treg may reduce the efficacy of CTLs, there may be a paucity of targets in the HLA-identical setting |
|
| T Cell Engineering | Not available | Retarget T cell receptor genetically to ensure a high proportion with GVL activity | Leukemias may mediate anti-CTL effects or have dysregulated apoptosis, CTLs may not be long-lived |
|
| Vaccine Therapies | Not available | Peptide-based vaccines against leukemia-selective proteins | HLA-restriction limits applicable population, optimal dose schedule in humans is unknown, the leukemia may down regulate the target protein |
|
| Dendritic Cell Therapies | Not available | Eliminate the GVHD-inducing DC subset, use DC subsets that stimulate innate immunity | DCs are not expected to be effective in the absence of T cells to be stimulated |
|
Developing these innovative therapies is hampered by a lack of critical information about the biology of the human host and the biology of the malignant cell. We therefore have additional recommendations (Table 2) to close the knowledge gap regarding leukemia-specific therapeutic targets, the means to approach elimination of the noncycling cancer stem cell, control of Treg subsets, and better characterization of the T cell and dendritic cell subsets specific for GVHD or GVT.
Finally, we also recommend additional epidemiologic and biomarker studies that will be needed to accelerate clinical development. Studies of therapeutics to prevent relapse are difficult due to the extended period of time needed to observe the study endpoint (relapse and/or death). In addition, if the population is heterogeneous and contains a large proportion of patients already cured of their disease, a relatively large number of patients would be needed in a study in order to be able to detect a statistically significant improvement in outcome for the population overall. Clinical or laboratory factors that identify patients with a nearly uniform risk for relapse would enrich the study population and potentially reduce the number of patients needed to arrive at a meaningful conclusion. Additionally, identification of biomarkers followed post-transplant that are direct surrogates for the study endpoint would enable shorter follow-up times and allow for progression to the next line of investigation more rapidly.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part at the NCI 1st International Workshop on The Biology, Prevention, and Treatment of Relapse After Allogeneic Hematopoietic Stem Cell Transplantation. The opinions expressed here are those of the authors and do not represent the official position of the National Institutes of Health nor the US Government.
REFERENCES
- 1.Pavletic SZ, S K, Mohty M, et al. NCI First International Workshop on the Biology, Prevention and Treatment of Relapse After Allogeneic Hematopoietic Stem Cell Transplantation: Report from the committee on the epidemiology and natural history of relapse following allogeneic cell transplantation. BBMT. doi: 10.1016/j.bbmt.2010.04.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cairo MS, C J, Maley CC, et al. NCI First International Workshop on the Biology, Prevention and Treatment of Relapse After Allogeneic Hematopoietic Stem Cell Transplantation: Report from the committee on the biologic considerations of hematologic relapse following allogeneic HSCT unrelated to graft-versus-tumor effects: state of the science. BBMT. doi: 10.1016/j.bbmt.2010.03.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller JS, E W, van den Brink MR, et al. NCI First International Workshop on the Biology, Prevention and Treatment of Relapse After Allogeneic Hematopoietic Stem Cell Transplantation: Report from the committee on the biology underlying recurrence of malignant disease following allogeneic HSCT: graft-versus-tumor/leukemia reaction. BBMT. doi: 10.1016/j.bbmt.2010.02.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrant A, Labopin M, Frassoni F, et al. Karyotype in acute myeloblastic leukemia: prognostic significance for bone marrow transplantation in first remission: a European Group for Blood and Marrow Transplantation study. Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Blood. 1997;90(8):2931–2938. [PubMed] [Google Scholar]
- 5.Copelan EA, Biggs JC, Thompson JM, et al. Treatment for acute myelocytic leukemia with allogeneic bone marrow transplantation following preparation with BuCy2. Blood. 1991;78(3):838–843. [PubMed] [Google Scholar]
- 6.Sierra J, Radich J, Hansen JA, et al. Marrow transplants from unrelated donors for treatment of Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 1997;90(4):1410–1414. [PubMed] [Google Scholar]
- 7.Clift RA, Buckner CD, Appelbaum FR, et al. Allogeneic marrow transplantation in patients with acute myeloid leukemia in first remission: a randomized trial of two irradiation regimens. Blood. 1990;76(9):1867–1871. [PubMed] [Google Scholar]
- 8.Clift RA, Buckner CD, Appelbaum FR, et al. Allogeneic marrow transplantation in patients with chronic myeloid leukemia in the chronic phase: a randomized trial of two irradiation regimens. Blood. 1991;77(8):1660–1665. [PubMed] [Google Scholar]
- 9.Marks DI, Forman SJ, Blume KG, et al. A comparison of cyclophosphamide and total body irradiation with etoposide and total body irradiation as conditioning regimens for patients undergoing sibling allografting for acute lymphoblastic leukemia in first or second complete remission. Biol Blood Marrow Transplant. 2006;12(4):438–453. doi: 10.1016/j.bbmt.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 10.Slattery JT, Clift RA, Buckner CD, et al. Marrow transplantation for chronic myeloid leukemia: the influence of plasma busulfan levels on the outcome of transplantation. Blood. 1997;89(8):3055–3060. [PubMed] [Google Scholar]
- 11.Anderson JE, Anasetti C, Appelbaum FR, et al. Unrelated donor marrow transplantation for myelodysplasia (MDS) and MDS-related acute myeloid leukaemia. Br J Haematol. 1996;93(1):59–67. doi: 10.1046/j.1365-2141.1996.4811022.x. [DOI] [PubMed] [Google Scholar]
- 12.Giralt S, Estey E, Albitar M, et al. Engraftment of allogeneic hematopoietic progenitor cells with purine analog-containing chemotherapy: harnessing graft-versus-leukemia without myeloablative therapy. Blood. 1997;89(12):4531–4536. [PubMed] [Google Scholar]
- 13.Slavin S, Nagler A, Naparstek E, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91(3):756–763. [PubMed] [Google Scholar]
- 14.Niederwieser D, Maris M, Shizuru JA, et al. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood. 2003;101(4):1620–1629. doi: 10.1182/blood-2002-05-1340. Epub 2002 Oct 3. [DOI] [PubMed] [Google Scholar]
- 15.Sorror ML, Maris MB, Sandmaier BM, et al. Hematopoietic Cell Transplantation After Nonmyeloablative Conditioning for Advanced Chronic Lymphocytic Leukemia. J Clin Oncol. 2005 doi: 10.1200/JCO.2005.04.569. [DOI] [PubMed] [Google Scholar]
- 16.Matthews DC, Appelbaum FR, Eary JF, et al. Development of a marrow transplant regimen for acute leukemia using targeted hematopoietic irradiation delivered by 131I-labelled anti-CD45 antibody, combined with cyclophosphamide and total body irradiation. Blood. 1995;85:1122–1131. [PubMed] [Google Scholar]
- 17.Matthews DC, Appelbaum FR, Eary JF, et al. Phase I study of (131)I-anti-CD45 antibody plus cyclophosphamide and total body irradiation for advanced acute leukemia and myelodysplastic syndrome. Blood. 1999;94(4):1237–1247. [PubMed] [Google Scholar]
- 18.Pagel JM, Appelbaum FR, Eary JF, et al. 131I-anti-CD45 antibody plus busulfan and cyclophosphamide before allogeneic hematopoietic cell transplantation for treatment of acute myeloid leukemia in first remission. Blood. 2006;107(5):2184–2191. doi: 10.1182/blood-2005-06-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pagel J, Gooley T, Rajendran J, et al. Targeted Radiotherapy Using 131I-anti-CD45 Antibody Followed By Allogeneic Hematopoietic Cell Transplantation (HCT): The Relationships Among Dosimetry, Bone Marrow Uptake, and Relapse. European Journal of Nuclear Medicine and Molecular Imaging. 2006;33 Supp 2:S193. [Google Scholar]
- 20.Pagel J, Appelbaum F, Sandmaier B, et al. 131I-anti-CD45 Antibody Plus Fludarabine, Low-Dose Total Body Irradiation and Peripheral Blood Stem Cell Infusion for Elderly Patients with Advanced Acute Myeloid Leukemia (AML) or High-Risk Myelodysplastic Syndrome (MDS) Blood. 2005;106(11) doi: 10.1182/blood-2009-03-213298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laport GG, Sandmaier BM, Storer BE, et al. Reduced-intensity conditioning followed by allogeneic hematopoietic cell transplantation for adult patients with myelodysplastic syndrome and myeloproliferative disorders. Biol Blood Marrow Transplant. 2008;14(2):246–255. doi: 10.1016/j.bbmt.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hegenbart U, Niederwieser D, Sandmaier BM, et al. Treatment for acute myelogenous leukemia by low-dose, total-body, irradiation-based conditioning and hematopoietic cell transplantation from related and unrelated donors. J Clin Oncol. 2006;24(3):444–453. doi: 10.1200/JCO.2005.03.1765. [DOI] [PubMed] [Google Scholar]
- 23.Druker BJ, Guilhot F, O'Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 24.Thomas DA. Philadelphia chromosome positive acute lymphocytic leukemia: a new era of challenges. Hematology Am Soc Hematol Educ Program. 2007:435–443. doi: 10.1182/asheducation-2007.1.435. [DOI] [PubMed] [Google Scholar]
- 25.Thomas DA, Faderl S, Cortes J, et al. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood. 2004;103(12):4396–4407. doi: 10.1182/blood-2003-08-2958. [DOI] [PubMed] [Google Scholar]
- 26.O'Brien SG, Guilhot F, Goldman JM, et al. International randomized study of interferon versus STI571(IRIS) 7-year follow-up: sustained survival, low rates of transformation and increased rate of molecular response (MMR) in patients with newly diagnosed chronic myeloid leukemia in chronic phase treated with imatinib. Blood. 2008;112(11):76a. [Google Scholar]
- 27.Hehlmann R, Berger U, Pfirrmann M, et al. Drug treatment is superior to allografting as first-line therapy in chronic myeloid leukemia. Blood. 2007;109(11):4686–4692. doi: 10.1182/blood-2006-11-055186. [DOI] [PubMed] [Google Scholar]
- 28.Hochhaus A, Kantarjian HM, Baccarani M, et al. Dasatinib induces notable hematologic and cytogenetic responses in chronic-phase chronic myeloid leukemia after failure of imatinib therapy. Blood. 2007;109(6):2303–2309. doi: 10.1182/blood-2006-09-047266. [DOI] [PubMed] [Google Scholar]
- 29.Druker BJ. Circumventing resistance to kinase-inhibitor therapy. N Engl J Med. 2006;354(24):2594–2596. doi: 10.1056/NEJMe068073. [DOI] [PubMed] [Google Scholar]
- 30.O'Hare T, Eide CA, Deininger MW. Bcr-Abl kinase domain mutations, drug resistance, and the road to a cure for chronic myeloid leukemia. Blood. 2007;110(7):2242–2249. doi: 10.1182/blood-2007-03-066936. [DOI] [PubMed] [Google Scholar]
- 31.O'Hare T, Walters DK, Stoffregen EP, et al. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 2005;65(11):4500–4505. doi: 10.1158/0008-5472.CAN-05-0259. [DOI] [PubMed] [Google Scholar]
- 32.Wetzler M, Stock W, Donohue KA, et al. Autologous stem cell transplantation following sequential chemotherapy and imatinib for adults with newly diagnosed Philadelphia chromosome positive acute lymphoblastic leukemia - CALGB study 10001. Blood. 2007;110(11):843a. [Google Scholar]
- 33.Carter TA, Wodicka LM, Shah NP, et al. Inhibition of drug-resistant mutants, of, ABL, KIT, and EGF receptor kinases. Proc Natl Acad Sci U S A. 2005;102(31):11011–11016. doi: 10.1073/pnas.0504952102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harrington EA, Bebbington D, Moore J, et al. VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nat Med. 2004;10(3):262–267. doi: 10.1038/nm1003. [DOI] [PubMed] [Google Scholar]
- 35.Young MA, Shah NP, Chao LH, et al. Structure of the kinase domain of an imatinib-resistant Abl mutant in complex with the Aurora kinase inhibitor VX-680. Cancer Res. 2006;66(2):1007–1014. doi: 10.1158/0008-5472.CAN-05-2788. [DOI] [PubMed] [Google Scholar]
- 36.Giles FJ, Cortes J, Jones D, et al. MK-0457, a novel kinase inhibitor, is active in patients with chronic myeloid leukemia or acute lymphocytic leukemia with the T315I BCR-ABL mutation. Blood. 2007;109(2):500–502. doi: 10.1182/blood-2006-05-025049. [DOI] [PubMed] [Google Scholar]
- 37.Bartholomeusz GA, Talpaz M, Kapuria V, et al. Activation of a novel Bcr/Abl destruction pathway by WP1130 induces apoptosis of chronic myelogenous leukemia cells. Blood. 2007;109(8):3470–3478. doi: 10.1182/blood-2006-02-005579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Byrd JC, Gribben JG, Peterson BL, et al. Select High Risk Genetic Features Predict Earlier Progression Following Chemoimmunotherapy with Fludarabine and Rituximab in Chronic Lymphocytic Leukemia (CLL): Preliminary Justification for Risk-Adapted Therapy. Blood. 2004 doi: 10.1200/JCO.2005.03.1021. [DOI] [PubMed] [Google Scholar]
- 39.Byrd JC, Lin TS, Grever MR. Treatment of relapsed chronic lymphocytic leukemia: old and new therapies. Semin Oncol. 2006;33(2):210–219. doi: 10.1053/j.seminoncol.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 40.Byrd JC, Rai K, Peterson BL, et al. Addition of rituximab to fludarabine may prolong progression-free survival and overall survival in patients with previously untreated chronic lymphocytic leukemia: an updated retrospective comparative analysis of CALGB 9712 and CALGB 9011. Blood. 2005;105(1):49–53. doi: 10.1182/blood-2004-03-0796. [DOI] [PubMed] [Google Scholar]
- 41.Hagenbeek A, Gadeberg O, Johnson P, et al. First clinical use of ofatumumab, a novel fully human anti-CD20 monoclonal antibody in relapsed or refractory follicular lymphoma: results of a phase 1/2 trial. Blood. 2008;111(12):5486–5495. doi: 10.1182/blood-2007-10-117671. [DOI] [PubMed] [Google Scholar]
- 42.Cuni S, Perez-Aciego P, Perez-Chacon G, et al. A sustained activation of PI3K/NF-kappaB pathway is critical for the survival of chronic lymphocytic leukemia B cells. Leukemia. 2004;18(8):1391–1400. doi: 10.1038/sj.leu.2403398. [DOI] [PubMed] [Google Scholar]
- 43.Shoemaker AR, Mitten MJ, Adickes J, et al. Activity of the Bcl-2 family inhibitor ABT-263 in a panel of small cell lung cancer xenograft models. Clin Cancer Res. 2008;14(11):3268–3277. doi: 10.1158/1078-0432.CCR-07-4622. [DOI] [PubMed] [Google Scholar]
- 44.Tse C, Shoemaker AR, Adickes J, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68(9):3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 45.O'Brien SM, Claxton DF, Crump M, et al. Phase I study of obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist, in patients with advanced chronic lymphocytic leukemia. Blood. 2009;113(2):299–305. doi: 10.1182/blood-2008-02-137943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Linn YC, Goh YT, Tan HC. Relapse of leukemia and lymphoma after marrow transplant: a review of cases with extramedullary relapse. Leuk Lymphoma. 2000;38(1–2):137–146. doi: 10.3109/10428190009060327. [DOI] [PubMed] [Google Scholar]
- 47.Chanan-Khan A, Miller KC, Musial L, et al. Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase II study. J Clin Oncol. 2006;24(34):5343–5349. doi: 10.1200/JCO.2005.05.0401. [DOI] [PubMed] [Google Scholar]
- 48.Ferrajoli A, Lee BN, Schlette EJ, et al. Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood. 2008;111(11):5291–5297. doi: 10.1182/blood-2007-12-130120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stone RM, DeAngelo DJ, Janosova A, et al. Low dose interleukin-2 following intensification therapy with high dose cytarabine for acute myelogenous leukemia in first complete remission. Am J Hematol. 2008;83(10):771–777. doi: 10.1002/ajh.21253. [DOI] [PubMed] [Google Scholar]
- 50.Kolitz JE, Hars V, DeAngelo DJ, et al. Phase III trial of immunotherapy with recombinant interleukin-2 (rIL-2) versus observation in patients <60 with acute myeloid leukemia in first remission: preliminary results from CALGB19808. Blood. 2007;110(11):53a. [Google Scholar]
- 51.Linker CA, Ries CA, Damon LE, et al. Autologous stem cell transplantation for acute myeloid leukemia in first remission. Biol Blood Marrow Transplant. 2000;6(1):50–57. doi: 10.1016/s1083-8791(00)70052-8. [DOI] [PubMed] [Google Scholar]
- 52.Baer MR, George SL, Caligiuri MA, et al. Low-dose interleukin-2 immunotherapy does not improve outcome of patients age 60 years and older with acute myeloid leukemia in first complete remission: Cancer and Leukemia Group B Study 9720. J Clin Oncol. 2008;26(30):4934–4939. doi: 10.1200/JCO.2008.17.0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10(3):223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20(10):2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 55.Kantarjian H, Oki Y, Garcia-Manero G, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007;109(1):52–57. doi: 10.1182/blood-2006-05-021162. [DOI] [PubMed] [Google Scholar]
- 56.Field T, Perkins J, Huang Y, et al. 5-Azacitidine for myelodysplasia before allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2009 doi: 10.1038/bmt.2009.134. [DOI] [PubMed] [Google Scholar]
- 57.Ravandi F, Kantarjian H, Cohen A, et al. Decitabine with allogeneic peripheral blood stem cell transplantation in the therapy of leukemia relapse following a prior transplant: results of a phase I study. Bone Marrow Transplant. 2001;27(12):1221–1225. doi: 10.1038/sj.bmt.1703028. [DOI] [PubMed] [Google Scholar]
- 58.Kortenhorst MS, Carducci MA, Shabbeer S. Acetylation and histone deacetylase inhibitors in cancer. Cell Oncol. 2006;28(5–6):191–222. doi: 10.1155/2006/760183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moradei O, Vaisburg A, Martell RE. Histone deacetylase inhibitors in cancer therapy: new compounds and clinical update of benzamide-type agents. Curr Top Med Chem. 2008;8(10):841–858. doi: 10.2174/156802608784911581. [DOI] [PubMed] [Google Scholar]
- 60.Stimson L, Wood V, Khan O, et al. HDAC inhibitor-based therapies and haematological malignancy. Ann Oncol. 2009;20(8):1293–1302. doi: 10.1093/annonc/mdn792. [DOI] [PubMed] [Google Scholar]
- 61.Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341(21):1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 62.Palumbo A, Bringhen S, Caravita T, et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomised controlled trial. Lancet. 2006;367(9513):825–831. doi: 10.1016/S0140-6736(06)68338-4. [DOI] [PubMed] [Google Scholar]
- 63.Rajkumar SV, Blood E, Vesole D, et al. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2006;24(3):431–436. doi: 10.1200/JCO.2005.03.0221. [DOI] [PubMed] [Google Scholar]
- 64.Spencer A, Prince HM, Roberts AW, et al. Consolidation therapy with low-dose thalidomide and prednisolone prolongs the survival of multiple myeloma patients undergoing a single autologous stem-cell transplantation procedure. J Clin Oncol. 2009;27(11):1788–1793. doi: 10.1200/JCO.2008.18.8573. [DOI] [PubMed] [Google Scholar]
- 65.Stewart AK, Chen CI, Howson-Jan K, et al. Results of a multicenter randomized phase II trial of thalidomide and prednisone maintenance therapy for multiple myeloma after autologous stem cell transplant. Clin Cancer Res. 2004;10(24):8170–8176. doi: 10.1158/1078-0432.CCR-04-1106. [DOI] [PubMed] [Google Scholar]
- 66.Attal M, Harousseau JL, Leyvraz S, et al. Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood. 2006;108(10):3289–3294. doi: 10.1182/blood-2006-05-022962. [DOI] [PubMed] [Google Scholar]
- 67.Aversa F, Tabilio A, Velardi A, et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med. 1998;339(17):1186–1193. doi: 10.1056/NEJM199810223391702. [DOI] [PubMed] [Google Scholar]
- 68.Jakubowski AA, Small TN, Young JW, et al. T cell depleted stem-cell transplantation for adults with hematologic malignancies: sustained engraftment of HLA-matched related donor grafts without the use of antithymocyte globulin. Blood. 2007;110(13):4552–4559. doi: 10.1182/blood-2007-06-093880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barge RM, Starrenburg CW, Falkenburg JH, et al. Long-term follow-up of myeloablative allogeneic stem cell transplantation using Campath "in the bag" as T-cell depletion: the Leiden experience. Bone Marrow Transplant. 2006;37(12):1129–1134. doi: 10.1038/sj.bmt.1705385. [DOI] [PubMed] [Google Scholar]
- 70.Zakrzewski JL, Goldberg GL, Smith OM, et al. Enhancing T cell reconstitution after hematopoietic stem cell transplantation: a brief update of the latest trends. Blood Cells Mol Dis. 2008;40(1):44–47. doi: 10.1016/j.bcmd.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ge X, Brown J, Sykes M, et al. CD134-allodepletion allows selective elimination of alloreactive human T cells without loss of virus-specific and leukemia-specific effectors. Biol Blood Marrow Transplant. 2008;14(5):518–530. doi: 10.1016/j.bbmt.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hartwig UF, Nonn M, Khan S, et al. Depletion of alloreactive donor T lymphocytes by CD95-mediated activation-induced cell death retains antileukemic, antiviral, and immunoregulatory T cell immunity. Biol Blood Marrow Transplant. 2008;14(1):99–109. doi: 10.1016/j.bbmt.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 73.Wehler TC, Nonn M, Brandt B, et al. Targeting the activation-induced antigen CD137 can selectively deplete alloreactive T cells from antileukemic and antitumor donor T-cell lines. Blood. 2007;109(1):365–373. doi: 10.1182/blood-2006-04-014100. [DOI] [PubMed] [Google Scholar]
- 74.Andre-Schmutz I, Le Deist F, Hacein-Bey-Abina S, et al. Immune reconstitution without graft-versus-host disease after haemopoietic stem-cell transplantation: a phase 1/2 study. Lancet. 2002;360(9327):130–137. doi: 10.1016/S0140-6736(02)09413-8. [DOI] [PubMed] [Google Scholar]
- 75.Solomon SR, Mielke S, Savani BN, et al. Selective depletion of alloreactive donor lymphocytes: a novel method to reduce the severity of graft-versus-host disease in older patients undergoing matched sibling donor stem cell transplantation. Blood. 2005;106(3):1123–1129. doi: 10.1182/blood-2005-01-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Luznik L, O'Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Socie G, Blazar BR. Acute graft-versus-host disease, from the bench to the bedside. Blood. 2009 doi: 10.1182/blood-2009-06-204669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7(5):340–352. doi: 10.1038/nri2000. [DOI] [PubMed] [Google Scholar]
- 79.Zheng H, Matte-Martone C, Jain D, et al. Central memory CD8+ T cells induce graft-versus-host disease and mediate graft-versus-leukemia. J Immunol. 2009;182(10):5938–5948. doi: 10.4049/jimmunol.0802212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 81.Arstila TP, Casrouge A, Baron V, et al. A direct estimate of the human alphabeta T cell receptor diversity. Science. 1999;286(5441):958–961. doi: 10.1126/science.286.5441.958. [DOI] [PubMed] [Google Scholar]
- 82.Robins HS, Campregher PV, Srivastava SK, et al. Comprehensive assessment of T cell receptor {beta} chain diversity in {alpha}{beta} T cells. Blood. 2009 doi: 10.1182/blood-2009-04-217604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rist M, Smith C, Bell MJ, et al. Cross-recognition of HLA DR4 alloantigen by virus-specific CD8+ T cells: a new paradigm for self-/nonself-recognition. Blood. 2009;114(11):2244–2253. doi: 10.1182/blood-2009-05-222596. [DOI] [PubMed] [Google Scholar]
- 84.Archbold JK, Macdonald WA, Burrows SR, et al. T-cell allorecognition: a case of mistaken identity or deja vu? Trends Immunol. 2008;29(5):220–226. doi: 10.1016/j.it.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 85.Bleakley M, Riddell SR. Molecules and mechanisms of the graft-versus-leukaemia effect. Nat Rev Cancer. 2004;4(5):371–380. doi: 10.1038/nrc1365. [DOI] [PubMed] [Google Scholar]
- 86.Long EO. Negative signaling by inhibitory receptors: the NK cell paradigm. Immunol Rev. 2008;224:70–84. doi: 10.1111/j.1600-065X.2008.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gill S, Olson JA, Negrin RS. Natural killer cells in allogeneic transplantation: effect on engraftment, graft- versus-tumor, and graft-versus-host responses. Biol Blood Marrow Transplant. 2009;15(7):765–776. doi: 10.1016/j.bbmt.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105(8):3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 89.Uckert W, Schumacher TN. TCR transgenes and transgene cassettes for TCR gene therapy: status in 2008. Cancer Immunol Immunother. 2009;58(5):809–822. doi: 10.1007/s00262-008-0649-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sadelain M, Brentjens R, Riviere I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol. 2009;21(2):215–223. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Keilholz U, Letsch A, Busse A, et al. A clinical and immunologic phase 2 trial of Wilms tumor gene product 1 (WT1) peptide vaccination in patients with AML and MDS. Blood. 2009;113(26):6541–6548. doi: 10.1182/blood-2009-02-202598. [DOI] [PubMed] [Google Scholar]
- 92.Oka Y, Tsuboi A, Taguchi T, et al. Induction of WT1 (Wilms' tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression. Proc Natl Acad Sci U S A. 2004;101(38):13885–13890. doi: 10.1073/pnas.0405884101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rezvani K, Yong AS, Mielke S, et al. Leukemia-associated antigen-specific T-cell responses following combined PR1 and WT1 peptide vaccination in patients with myeloid malignancies. Blood. 2008;111(1):236–242. doi: 10.1182/blood-2007-08-108241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jenq RR, King CG, Volk C, et al. Keratinocyte growth factor enhances DNA plasmid tumor vaccine responses after murine allogeneic bone marrow transplantation. Blood. 2009;113(7):1574–1580. doi: 10.1182/blood-2008-05-155697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bonini C, Ferrari G, Verzeletti S, et al. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science. 1997;276(5319):1719–1724. doi: 10.1126/science.276.5319.1719. [see comments] [DOI] [PubMed] [Google Scholar]
- 96.Ciceri F, Bonini C, Stanghellini MT, et al. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I–II study. Lancet Oncol. 2009;10(5):489–500. doi: 10.1016/S1470-2045(09)70074-9. [DOI] [PubMed] [Google Scholar]
- 97.Berger C, Flowers ME, Warren EH, et al. Analysis of transgene-specific immune responses that limit the in vivo persistence of adoptively transferred HSV-TK-modified donor T cells after allogeneic hematopoietic cell transplantation. Blood. 2006;107(6):2294–2302. doi: 10.1182/blood-2005-08-3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Deschamps M, Mercier-Lethondal P, Certoux JM, et al. Deletions within the HSV-tk transgene in long-lasting circulating gene-modified T cells infused with a hematopoietic graft. Blood. 2007;110(12):3842–3852. doi: 10.1182/blood-2007-04-087346. [DOI] [PubMed] [Google Scholar]
- 99.Thomis DC, Marktel S, Bonini C, et al. A Fas-based suicide switch in human T cells for the treatment of graft-versus-host disease. Blood. 2001;97(5):1249–1257. doi: 10.1182/blood.v97.5.1249. [DOI] [PubMed] [Google Scholar]
- 100.Straathof KC, Pule MA, Yotnda P, et al. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005;105(11):4247–4254. doi: 10.1182/blood-2004-11-4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Berger C, Blau CA, Huang ML, et al. Pharmacologically regulated Fas-mediated death of adoptively transferred T cells in a nonhuman primate model. Blood. 2004;103(4):1261–1269. doi: 10.1182/blood-2003-08-2908. [DOI] [PubMed] [Google Scholar]
- 102.Rossi M, Young JW. Human dendritic cells: potent antigen-presenting cells at the crossroads of innate and adaptive immunity. Journal of Immunology. 2005;175(3):1373–1381. doi: 10.4049/jimmunol.175.3.1373. [DOI] [PubMed] [Google Scholar]
- 103.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449(7161):419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 104.Palucka AK, Ueno H, Fay J, et al. Dendritic cells: a critical player in cancer therapy? Journal of Immunotherapy. 2008;31(9):793–805. doi: 10.1097/CJI.0b013e31818403bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Banchereau J, Palucka AK, Dhodapkar M, et al. Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer Research. 2001;61(17):6451–6458. [PubMed] [Google Scholar]
- 106.Ratzinger G, Baggers J, de Cos MA, et al. Mature human Langerhans cells derived from CD34+ hematopoietic progenitors stimulate greater cytolytic T lymphocyte activity in the absence of bioactive IL-12p70, by either single peptide presentation or cross-priming, than do dermal-interstitial or monocyte-derived dendritic cells. Journal of Immunology. 2004;173:2780–2791. doi: 10.4049/jimmunol.173.4.2780. (Erratum in J Immunol. 2005: 174 3818) [DOI] [PubMed] [Google Scholar]
- 107.Klechevsky E, Morita R, Liu M, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29(3):497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Romano E, Rossi M, Barreira da Silva R, et al. Human Langerhans cells stimulate robust cytolytic T-cells against tumor antigens, including WT1, by an IL15-dependent mechanism. Submitted. [Google Scholar]
- 109.Munz C, Dao D, Ferlazzo G, et al. Mature myeloid dendritic cell subsets have distinct roles for activation and viability of circulating human natural killer cells. Blood. 2005;105(1):266–273. doi: 10.1182/blood-2004-06-2492. [DOI] [PubMed] [Google Scholar]
- 110.Jiang A, Bloom O, Ono S, et al. Disruption of E-Cadherin-Mediated Adhesion Induces a Functionally Distinct Pathway of Dendritic Cell Maturation. Immunity. 2007;27(4):610–624. doi: 10.1016/j.immuni.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hawiger D, Inaba K, Dorsett Y, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. Journal of Experimental Medicine. 2001;194(6):769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bonifaz L, Bonnyay D, Mahnke K, et al. Efficient Targeting of Protein Antigen to the Dendritic Cell Receptor DEC-205 in the Steady State Leads to Antigen Presentation on Major Histocompatibility Complex Class I Products and Peripheral CD8+ T Cell Tolerance. Journal of Experimental Medicine. 2002;196(12):1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ferrara JL, Levine JE, Reddy P, et al. Graft-versus-host disease. Lancet. 2009;373(9674):1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Banerjee DK, Dhodapkar MV, Matayeva E, et al. Expansion of FOXP3high regulatory T cells by human dendritic cells (DCs) in vitro and after injection of cytokine-matured DCs in myeloma patients. Blood. 2006;108(8):2655–2661. doi: 10.1182/blood-2006-03-011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chung DJ, Rossi M, Romano E, et al. Indoleamine 2,3-dioxygenase-expressing mature human monocyte-derived dendritic cells expand potent autologous regulatory T cells. Blood. 2009;114(3):555–563. doi: 10.1182/blood-2008-11-191197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jonuleit H, Kuhn U, Muller G, et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur.J.Immunol. 1997;27:3135–3142. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 117.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 118.Guermonprez P, Saveanu L, Kleijmeer M, et al. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature. 2003;425(6956):397–402. doi: 10.1038/nature01911. [DOI] [PubMed] [Google Scholar]
- 119.Ackerman AL, Kyritsis C, Tampe R, et al. Access of soluble antigens to the endoplasmic reticulum can explain cross-presentation by dendritic cells. Nat Immunol. 2005;6(1):107–113. doi: 10.1038/ni1147. [DOI] [PubMed] [Google Scholar]
- 120.Goldszmid RS, Idoyaga J, Bravo AI, et al. Dendritic Cells Charged with Apoptotic Tumor Cells Induce Long-Lived Protective CD4+ and CD8+ T Cell Immunity against B16 Melanoma. Journal of Immunology. 2003;171(11):5940–5947. doi: 10.4049/jimmunol.171.11.5940. [DOI] [PubMed] [Google Scholar]
- 121.Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8(12):935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 122.Merad M, Manz MG. Dendritic cell homeostasis. Blood. 2009;113(15):3418–3427. doi: 10.1182/blood-2008-12-180646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ratzinger G, Reagan JL, Heller G, et al. Differential CD52 expression by distinct myeloid dendritic cell subsets: implications for alemtuzumab activity at the level of antigen presentation in allogeneic graft-host interactions in transplantation. Blood. 2003;101(4):1422–1429. doi: 10.1182/blood-2002-04-1093. [DOI] [PubMed] [Google Scholar]
- 124.Chakraverty R, Peggs K, Chopra R, et al. Limiting transplantation-related mortality following unrelated donor stem cell transplantation by using a nonmyeloablative conditioning regimen. Blood. 2002;99(3):1071–1078. doi: 10.1182/blood.v99.3.1071. [DOI] [PubMed] [Google Scholar]
- 125.Durakovic N, Bezak KB, Skarica M, et al. Host-Derived Langerhans Cells Persist after MHC-Matched Allografting Independent of Donor T Cells and Critically Influence the Alloresponses Mediated by Donor Lymphocyte Infusions. Journal of Immunology. 2006;177(7):4414–4425. doi: 10.4049/jimmunol.177.7.4414. [DOI] [PubMed] [Google Scholar]
- 126.Gomez-Almaguer D, Ruiz-Arguelles GJ, del Carmen Tarin-Arzaga L, et al. Alemtuzumab for the treatment of steroid-refractory acute graft-versus-host disease. Biology of Blood and Marrow Transplantation. 2008;14(1):10–15. doi: 10.1016/j.bbmt.2007.08.052. [DOI] [PubMed] [Google Scholar]
- 127.Davies SM, Dickinson A, Miller JS. Human polymorphism and variable outcomes of cancer chemotherapy and transplantation. Biology of Blood and Marrow Transplantation. 2008;14(1 Suppl 1):120–128. doi: 10.1016/j.bbmt.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 128.Velardi A. Role of KIRs and KIR ligands in hematopoietic transplantation. Current Opinion in Immunology. 2008;20(5):581–587. doi: 10.1016/j.coi.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 129.Yu J, Venstrom JM, Liu XR, et al. Breaking tolerance to self, circulating natural killer cells expressing inhibitory KIR for non-self HLA exhibit effector function after T cell-depleted allogeneic hematopoietic cell transplantation. Blood. 2009;113(16):3875–3884. doi: 10.1182/blood-2008-09-177055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Slavin S, Naparstek E, Nagler A, et al. Allogeneic cell therapy with donor peripheral blood cells and recombinant human interleukin-2 to treat leukemia relapse after allogeneic bone marrow transplantation. Blood. 1996;87(6):2195–2204. [PubMed] [Google Scholar]
- 131.Kolb HJ, Schattenberg A, Goldman JM, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86:2041–2050. [PubMed] [Google Scholar]
- 132.Collins RH, Jr, Shpilberg O, Drobyski WR, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation [see comments] J Clin Oncol. 1997;15(2):433–444. doi: 10.1200/JCO.1997.15.2.433. [DOI] [PubMed] [Google Scholar]
- 133.Raiola AM, Van Lint MT, Valbonesi M, et al. Factors predicting response and graft-versus-host disease after donor lymphocyte infusions: a study on 593 infusions. Bone Marrow Transplant. 2003;31(8):687–693. doi: 10.1038/sj.bmt.1703883. [DOI] [PubMed] [Google Scholar]
- 134.Dazzi F, Szydlo RM, Cross NC, et al. Durability of responses following donor lymphocyte infusions for patients who relapse after allogeneic stem cell transplantation for chronic myeloid leukemia. Blood. 2000;96(8):2712–2716. [PubMed] [Google Scholar]
- 135.Porter DL, Collins RH, Jr, Hardy C, et al. Treatment of relapsed leukemia after unrelated donor marrow transplantation with unrelated donor leukocyte infusions. Blood. 2000;95(4):1214–1221. [PubMed] [Google Scholar]
- 136.Chiorean EG, DeFor TE, Weisdorf DJ, et al. Donor chimerism does not predict response to donor lymphocyte infusion for relapsed chronic myelogenous leukemia after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2004;10(3):171–177. doi: 10.1016/j.bbmt.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 137.Mackinnon S, Papadapoulos EB, Carabasi MH, et al. Adoptive immunotherapy evaluating escalating doses of donor leukocytes for relapse of chronic myeloid leukemia after bone marrow transplantation: Separation of graft-versus-leukemia responses from graft-versus-host disease. Blood. 1995;86:1261–1268. [PubMed] [Google Scholar]
- 138.Dazzi F, Szydlo RM, Craddock C, et al. Comparison of single-dose and escalating-dose regimens of donor lymphocyte infusion for relapse after allografting for chronic myeloid leukemia. Blood. 2000;95(1):67–71. [PubMed] [Google Scholar]
- 139.Posthuma EF, Marijt EW, Barge RM, et al. Alpha-interferon with very-low-dose donor lymphocyte infusion for hematologic or cytogenetic relapse of chronic myeloid leukemia induces rapid and durable complete remissions and is associated with acceptable graft-versus-host disease. Biol Blood Marrow Transplant. 2004;10(3):204–212. doi: 10.1016/j.bbmt.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 140.Savani BN, Montero A, Kurlander R, et al. Imatinib synergizes with donor lymphocyte infusions to achieve rapid molecular remission of CML relapsing after allogeneic stem cell transplantation. Bone Marrow Transplant. 2005;36(11):1009–1015. doi: 10.1038/sj.bmt.1705167. [DOI] [PubMed] [Google Scholar]
- 141.Lokhorst HM, Schattenberg A, Cornelissen JJ, et al. Donor lymphocyte infusions for relapsed multiple myeloma after allogeneic stem-cell transplantation: predictive factors for response and long-term outcome. J Clin Oncol. 2000;18(16):3031–3037. doi: 10.1200/JCO.2000.18.16.3031. [DOI] [PubMed] [Google Scholar]
- 142.Alyea E, Schossman R, Canning C, et al. CD6 T cell Depleted Allogeneic Bone Marrow Transplant followed by CD4+ Donor Lymphocyte Infusion for Patients With Multiple Myeloma. Blood. 1999;94(No. 10):609a. doi: 10.1182/blood.v98.4.934. [DOI] [PubMed] [Google Scholar]
- 143.Peggs KS, Mackinnon S, Williams CD, et al. Reduced-intensity transplantation with in vivo T-cell depletion and adjuvant dose-escalating donor lymphocyte infusions for chemotherapy-sensitive myeloma: Limited efficacy of graft-versus-tumor activity. Biol Blood Marrow Transplant. 2003;9(4):257–265. doi: 10.1053/bbmt.2003.50009. [DOI] [PubMed] [Google Scholar]
- 144.Kroger N, Shimoni A, Zagrivnaja M, et al. Low-dose thalidomide and donor lymphocyte infusion as adoptive immunotherapy after allogeneic stem cell transplantation in patients with multiple myeloma. Blood. 2004;104(10):3361–3363. doi: 10.1182/blood-2004-05-2031. [DOI] [PubMed] [Google Scholar]
- 145.Schmid C, Schleuning M, Ledderose G, et al. Sequential regimen of chemotherapy, reduced-intensity conditioning for allogeneic stem-cell transplantation, and prophylactic donor lymphocyte transfusion in high-risk acute myeloid leukemia and myelodysplastic syndrome. J Clin Oncol. 2005;23(24):5675–5687. doi: 10.1200/JCO.2005.07.061. [DOI] [PubMed] [Google Scholar]
- 146.Ritgen M, Stilgenbauer S, von Neuhoff N, et al. Graft-versus-leukemia activity may overcome therapeutic resistance of chronic lymphocytic leukemia with unmutated immunoglobulin variable heavy-chain gene status: implications of minimal residual disease measurement with quantitative PCR. Blood. 2004;104(8):2600–2602. doi: 10.1182/blood-2003-12-4321. [DOI] [PubMed] [Google Scholar]
- 147.Papadapoulos EB, Ladanyi M, Emmanuel D, et al. Infusions of donor leukocytes to treat Epstein-Barr-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. New England Journal of Medicine. 1994;330:1185–1191. doi: 10.1056/NEJM199404283301703. [DOI] [PubMed] [Google Scholar]
- 148.Heslop HE, Brenner MK, Rooney C, et al. Administration of neomycin-resistance-gene-marked EBV-specific cytotoxic T lymphocytes to recipients of mismatched-related or phenotypically similar unrelated donor marrow grafts. Hum Gene Ther. 1994;5(3):381–397. doi: 10.1089/hum.1994.5.3-381. [DOI] [PubMed] [Google Scholar]
- 149.Rooney CM, Smith CA, Ng CY, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92(5):1549–1555. [PubMed] [Google Scholar]
- 150.Gustafsson A, Levitsky V, Zou JZ, et al. Epstein-Barr virus (EBV) load in bone marrow transplant recipients at risk to develop posttransplant lymphoproliferative disease: prophylactic infusion of EBV-specific cytotoxic T cells. Blood. 2000;95(3):807–814. [PubMed] [Google Scholar]
- 151.Rooney CM, Smith CA, Ng CY, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet. 1995;345(8941):9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- 152.Peggs KS, Thomson K, Hart DP, et al. Dose-escalated donor lymphocyte infusions following reduced intensity transplantation: toxicity, chimerism, and disease responses. Blood. 2004;103(4):1548–1556. doi: 10.1182/blood-2003-05-1513. [DOI] [PubMed] [Google Scholar]
- 153.Meyer RG, Britten CM, Wehler D, et al. Prophylactic transfer of CD8-depleted donor lymphocytes after T-cell-depleted reduced-intensity transplantation. Blood. 2007;109(1):374–382. doi: 10.1182/blood-2006-03-005769. [DOI] [PubMed] [Google Scholar]
- 154.Piccirillo CA, Shevach EM. Naturally-occurring CD4+CD25+ immunoregulatory T cells: central players in the arena of peripheral tolerance. Semin Immunol. 2004;16(2):81–88. doi: 10.1016/j.smim.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 155.Fehervari Z, Sakaguchi S. Development and function of CD25+CD4+ regulatory T cells. Curr Opin Immunol. 2004;16(2):203–208. doi: 10.1016/j.coi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 156.Azuma T, Takahashi T, Kunisato A, et al. Human CD4+ CD25+ regulatory T cells suppress NKT cell functions. Cancer Res. 2003;63(15):4516–4520. [PubMed] [Google Scholar]
- 157.Cederbom L, Hall H, Ivars F. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur J Immunol. 2000;30(6):1538–1543. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 158.Maloy KJ, Salaun L, Cahill R, et al. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med. 2003;197(1):111–119. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112(12):4371–4383. doi: 10.1182/blood-2008-03-077974. [DOI] [PubMed] [Google Scholar]
- 160.Seliger B. Molecular mechanisms of MHC class I abnormalities and APM components in human tumors. Cancer Immunol Immunother. 2008;57(11):1719–1726. doi: 10.1007/s00262-008-0515-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5(4):263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 162.Krogsgaard M, Davis MM. How T cells 'see' antigen. Nat Immunol. 2005;6(3):239–245. doi: 10.1038/ni1173. [DOI] [PubMed] [Google Scholar]
- 163.Heemskerk MH, Hoogeboom M, Hagedoorn R, et al. Reprogramming of virus-specific T cells into leukemia-reactive T cells using T cell receptor gene transfer. J Exp Med. 2004;199(7):885–894. doi: 10.1084/jem.20031110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sommermeyer D, Neudorfer J, Weinhold M, et al. Designer T cells by T cell receptor replacement. Eur J Immunol. 2006;36(11):3052–3059. doi: 10.1002/eji.200636539. [DOI] [PubMed] [Google Scholar]
- 165.Ghattas IR, Sanes JR, Majors JE. The encephalomyocarditis virus internal ribosome entry site allows efficient coexpression of two genes from a recombinant provirus in cultured cells and in embryos. Mol Cell Biol. 1991;11(12):5848–5859. doi: 10.1128/mcb.11.12.5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Szymczak AL, Vignali DA. Development of 2A peptide-based strategies in the design of multicistronic vectors. Expert Opin Biol Ther. 2005;5(5):627–638. doi: 10.1517/14712598.5.5.627. [DOI] [PubMed] [Google Scholar]
- 167.Scholten KB, Kramer D, Kueter EW, et al. Codon modification of T cell receptors allows enhanced functional expression in transgenic human T cells. Clin Immunol. 2006;119(2):135–145. doi: 10.1016/j.clim.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 168.de Witte MA, Jorritsma A, Kaiser A, et al. Requirements for effective antitumor responses of TCR transduced T cells. J Immunol. 2008;181(7):5128–5136. doi: 10.4049/jimmunol.181.7.5128. [DOI] [PubMed] [Google Scholar]
- 169.Jorritsma A, Gomez-Eerland R, Dokter M, et al. Selecting highly affine and well-expressed TCRs for gene therapy of melanoma. Blood. 2007;110(10):3564–3572. doi: 10.1182/blood-2007-02-075010. [DOI] [PubMed] [Google Scholar]
- 170.Voss RH, Kuball J, Engel R, et al. Redirection of T cells by delivering a transgenic mouse-derived MDM2 tumor antigen-specific TCR and its humanized derivative is governed by the CD8 coreceptor and affects natural human TCR expression. Immunol Res. 2006;34(1):67–87. doi: 10.1385/IR:34:1:67. [DOI] [PubMed] [Google Scholar]
- 171.Cohen CJ, Zhao Y, Zheng Z, et al. Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res. 2006;66(17):8878–8886. doi: 10.1158/0008-5472.CAN-06-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hart DP, Xue SA, Thomas S, et al. Retroviral transfer of a dominant TCR prevents surface expression of a large proportion of the endogenous TCR repertoire in human T cells. Gene Ther. 2008;15(8):625–631. doi: 10.1038/sj.gt.3303078. [DOI] [PubMed] [Google Scholar]
- 173.Johnson LA, Morgan RA, Dudley ME, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114(3):535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kuball J, Dossett ML, Wolfl M, et al. Facilitating matched pairing and expression of TCR chains introduced into human T cells. Blood. 2007;109(6):2331–2338. doi: 10.1182/blood-2006-05-023069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cohen CJ, Li YF, El-Gamil M, et al. Enhanced antitumor activity of T cells engineered to express T-cell receptors with a second disulfide bond. Cancer Res. 2007;67(8):3898–3903. doi: 10.1158/0008-5472.CAN-06-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Voss RH, Willemsen RA, Kuball J, et al. Molecular design of the Calphabeta interface favors specific pairing of introduced TCRalphabeta in human T cells. J Immunol. 2008;180(1):391–401. doi: 10.4049/jimmunol.180.1.391. [DOI] [PubMed] [Google Scholar]
- 177.Rischer M, Pscherer S, Duwe S, et al. Human gammadelta T cells as mediators of chimaeric-receptor redirected anti-tumour immunity. Br J Haematol. 2004;126(4):583–592. doi: 10.1111/j.1365-2141.2004.05077.x. [DOI] [PubMed] [Google Scholar]
- 178.van der Veken LT, Hagedoorn RS, van Loenen MM, et al. Alphabeta T-cell receptor engineered gammadelta T cells mediate effective antileukemic reactivity. Cancer Res. 2006;66(6):3331–3337. doi: 10.1158/0008-5472.CAN-05-4190. [DOI] [PubMed] [Google Scholar]
- 179.Altenschmidt U, Kahl R, Moritz D, et al. Cytolysis of tumor cells expressing the Neu/erbB-2, erbB-3, and erbB-4 receptors by genetically targeted naive T lymphocytes. Clin Cancer Res. 1996;2(6):1001–1008. [PubMed] [Google Scholar]
- 180.Maher J, Brentjens RJ, Gunset G, et al. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol. 2002;20(1):70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 181.Carpenito C, Milone MC, Hassan R, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A. 2009;106(9):3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhong X, Matsushita M, Plotkin J, et al. Chimeric antigen receptors comining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol Ther. 2009 doi: 10.1038/mt.2009.210. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bunnell BA, Muul LM, Donahue RE, et al. High-efficiency retroviral-mediated gene transfer into human and nonhuman primate peripheral blood lymphocytes. Proc Natl Acad Sci U S A. 1995;92(17):7739–7743. doi: 10.1073/pnas.92.17.7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lam JS, Reeves ME, Cowherd R, et al. Improved gene transfer into human lymphocytes using retroviruses with the gibbon ape leukemia virus envelope. Hum Gene Ther. 1996;7(12):1415–1422. doi: 10.1089/hum.1996.7.12-1415. [DOI] [PubMed] [Google Scholar]
- 185.Gallardo HF, Tan C, Ory D, et al. Recombinant retroviruses pseudotyped with the vesicular stomatitis virus G glycoprotein mediate both stable gene transfer and pseudotransduction in human peripheral blood lymphocytes. Blood. 1997;90(3):952–957. [PubMed] [Google Scholar]
- 186.Levine BL, Humeau LM, Boyer J, et al. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc Natl Acad Sci U S A. 2006;103(46):17372–17377. doi: 10.1073/pnas.0608138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Joseph A, Zheng JH, Follenzi A, et al. Lentiviral vectors encoding human immunodeficiency virus type 1 (HIV-1)-specific T-cell receptor genes efficiently convert peripheral blood CD8 T lymphocytes into cytotoxic T lymphocytes with potent in vitro and in vivo HIV-1-specific inhibitory activity. J Virol. 2008;82(6):3078–3089. doi: 10.1128/JVI.01812-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yang S, Cohen CJ, Peng PD, et al. Development of optimal bicistronic lentiviral vectors facilitates high-level TCR gene expression and robust tumor cell recognition. Gene Ther. 2008;15(21):1411–1423. doi: 10.1038/gt.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Unutmaz D, KewalRamani VN, Marmon S, et al. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J Exp Med. 1999;189(11):1735–1746. doi: 10.1084/jem.189.11.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cavalieri S, Cazzaniga S, Geuna M, et al. Human T lymphocytes transduced by lentiviral vectors in the absence of TCR activation maintain an intact immune competence. Blood. 2003;102(2):497–505. doi: 10.1182/blood-2003-01-0297. [DOI] [PubMed] [Google Scholar]
- 191.Newrzela S, Cornils K, Li Z, et al. Resistance of mature T cells to oncogene transformation. Blood. 2008;112(6):2278–2286. doi: 10.1182/blood-2007-12-128751. [DOI] [PubMed] [Google Scholar]
- 192.Montini E, Cesana D, Schmidt M, et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol. 2006;24(6):687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- 193.Peng PD, Cohen CJ, Yang S, et al. Efficient nonviral Sleeping Beauty transposon-based TCR gene transfer to peripheral blood lymphocytes confers antigen-specific antitumor reactivity. Gene Ther. 2009;16(8):1042–1049. doi: 10.1038/gt.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Huang X, Wilber AC, Bao L, et al. Stable gene transfer and expression in human primary T cells by the Sleeping Beauty transposon system. Blood. 2006;107(2):483–491. doi: 10.1182/blood-2005-05-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhao Y, Zheng Z, Cohen CJ, et al. High-efficiency transfection of primary human and mouse T lymphocytes using RNA electroporation. Mol Ther. 2006;13(1):151–159. doi: 10.1016/j.ymthe.2005.07.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Schaft N, Dorrie J, Muller I, et al. A new way to generate cytolytic tumor-specific T cells: electroporation of RNA coding for a T cell receptor into T lymphocytes. Cancer Immunol Immunother. 2006;55(9):1132–1141. doi: 10.1007/s00262-005-0098-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Klebanoff CA, Gattinoni L, Torabi-Parizi P, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A. 2005;102(27):9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hinrichs CS, Borman ZA, Cassard L, et al. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc Natl Acad Sci U S A. 2009;106(41):17469–17474. doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Berger C, Jensen MC, Lansdorp PM, et al. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118(1):294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Klebanoff CA, Yu Z, Hwang LN, et al. Programming tumor-reactive effector memory CD8+ T cells in vitro obviates the requirement for in vivo vaccination. Blood. 2009;114(9):1776–1783. doi: 10.1182/blood-2008-12-192419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pule MA, Savoldo B, Myers GD, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14(11):1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Papapetrou EP, Kovalovsky D, Beloeil L, et al. Harnessing endogenous miR-181a to segregate transgenic antigen receptor expression in developing versus post-thymic T cells in murine hematopoietic chimeras. J Clin Invest. 2009;119(1):157–168. doi: 10.1172/JCI37216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhao Y, Parkhurst MR, Zheng Z, et al. Extrathymic generation of tumor-specific T cells from genetically engineered human hematopoietic stem cells via Notch signaling. Cancer Res. 2007;67(6):2425–2429. doi: 10.1158/0008-5472.CAN-06-3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Latouche JB, Sadelain M. Induction of human cytotoxic T lymphocytes by artificial antigen-presenting cells. Nat Biotechnol. 2000;18(4):405–409. doi: 10.1038/74455. [DOI] [PubMed] [Google Scholar]
- 205.Papanicolaou GA, Latouche JB, Tan C, et al. Rapid expansion of cytomegalovirus-specific cytotoxic T lymphocytes by artificial antigen-presenting cells expressing a single HLA allele. Blood. 2003;102(7):2498–2505. doi: 10.1182/blood-2003-02-0345. [DOI] [PubMed] [Google Scholar]
- 206.Dupont J, Latouche JB, Ma C, et al. Artificial antigen-presenting cells transduced with telomerase efficiently expand epitope-specific, human leukocyte antigen-restricted cytotoxic T cells. Cancer Res. 2005;65(12):5417–5427. doi: 10.1158/0008-5472.CAN-04-2991. [DOI] [PubMed] [Google Scholar]
- 207.Numbenjapon T, Serrano LM, Singh H, et al. Characterization of an artificial antigen-presenting cell to propagate cytolytic CD19-specific T cells. Leukemia. 2006;20(10):1889–1892. doi: 10.1038/sj.leu.2404329. [DOI] [PubMed] [Google Scholar]
- 208.Suhoski MM, Golovina TN, Aqui NA, et al. Engineering artificial antigen-presenting cells to express a diverse array of co-stimulatory molecules. Mol Ther. 2007;15(5):981–988. doi: 10.1038/mt.sj.6300134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Maus MV, Thomas AK, Leonard DG, et al. Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4-1BB. Nat Biotechnol. 2002;20(2):143–148. doi: 10.1038/nbt0202-143. [DOI] [PubMed] [Google Scholar]
- 210.Zhang H, Snyder KM, Suhoski MM, et al. 4-1BB is superior to CD28 costimulation for generating CD8+ cytotoxic lymphocytes for adoptive immunotherapy. J Immunol. 2007;179(7):4910–4918. doi: 10.4049/jimmunol.179.7.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kim JV, Latouche JB, Riviere I, et al. The ABCs of artificial antigen presentation. Nat Biotechnol. 2004;22(4):403–410. doi: 10.1038/nbt955. [DOI] [PubMed] [Google Scholar]
- 212.Hasan AN, Kollen WJ, Trivedi D, et al. A panel of artificial APCs expressing prevalent HLA alleles permits generation of cytotoxic T cells specific for both dominant and subdominant viral epitopes for adoptive therapy. J Immunol. 2009;183(4):2837–2850. doi: 10.4049/jimmunol.0804178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hollyman D, Stefanski J, Przybylowski M, et al. Manufacturing validation of biologically functional T cells targeted to CD19 antigen for autologous adoptive cell therapy. J Immunother. 2009;32(2):169–180. doi: 10.1097/CJI.0b013e318194a6e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kershaw MH, Westwood JA, Parker LL, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12(20 Pt 1):6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lamers CH, Sleijfer S, Vulto AG, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24(13):e20–e22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 216.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314(5796):126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Brentjens R, Riviere I, Frattini M, et al. ASGCT Annual Meeting. Washington, DC: 2010. Marked regression of adenopathy follwoing infusion of autologous T cells genetically targeted to the CD19 antigen in a patient with bulky CLL. [Google Scholar]
- 218.Morgan RA, Yang JC, Kitano M, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 18(4):843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Yeh S, Karne NK, Kerkar SP, et al. Ocular and systemic autoimmunity after successful tumor-infiltrating lymphocyte immunotherapy for recurrent, metastatic melanoma. Ophthalmology. 2009;116(5):981–989. doi: 10.1016/j.ophtha.2008.12.004. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Brentjens R, Yeh R, Bernal Y, et al. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol Ther. 18(4):666–668. doi: 10.1038/mt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Burnet FM. Immunological surveillance in neoplasia. Transplant Rev. 1971;7:3–25. doi: 10.1111/j.1600-065x.1971.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 222.Apffel CA, Peters JH. Rejection of lethal tumors after subcutaneous inoculation: a phenomenon of antigenic expression? J Natl Cancer Inst. 1967;39(6):1129–1139. [PubMed] [Google Scholar]
- 223.Pollack W, Tripodi D. The role of the immune response in the establishment and rejection of neoplastic tissue. Int Arch Allergy Appl Immunol. 1967;32(5):429–435. doi: 10.1159/000229954. [DOI] [PubMed] [Google Scholar]
- 224.Breyere EJ, Williams LB. Antigens Associated with a Tumor Virus: Rejection of Isogenic Skin Grafts from Leukemic Mice. Science. 1964;146:1055–1056. doi: 10.1126/science.146.3647.1055. [DOI] [PubMed] [Google Scholar]
- 225.Eichwald EJ, Silmser CR, Weissman I. Sex-linked rejection of normal and neoplastic tissue. I. Distribution and specificity. J Natl Cancer Inst. 1958;20(3):563–575. [PubMed] [Google Scholar]
- 226.Janeway CA., Jr The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992;13(1):11–16. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- 227.Zinkernagel RM. Assessing the mechanisms that give rise to autoimmunity. Science. 2000;290(5489):11. doi: 10.1126/science.290.5489.11a. [DOI] [PubMed] [Google Scholar]
- 228.van Rhee F, Lin F, Cullis JO, et al. Relapse of chronic myeloid leukemia after allogeneic bone marrow transplant: the case for giving donor lymphocyte transfusions before the onset of hematological relapse. Blood. 1994;83:3377–3383. [PubMed] [Google Scholar]
- 229.Giralt S, Hester J, Huh Y, et al. CD8-depleted donor lymphocyte infusion as treatment for relapsed chronic myelogenous leukemia after allogeneic bone marrow transplantation. Blood. 1995;86:4337–4343. [PubMed] [Google Scholar]
- 230.Didot A. Essai sur la prophylaxie du cancer. Bull Acad Roy Belge. 1851;11:100–172. [Google Scholar]
- 231.Deeg HJ. Cytokines in graft-versus-host disease and the graft-versus-leukemia reaction. Int J Hematol. 2001;74(1):26–32. doi: 10.1007/BF02982546. [DOI] [PubMed] [Google Scholar]
- 232.Porter DL, Orloff GJ, Antin JH. Donor mononuclear cell infusions as therapy for B-cell lymphoproliferative disorder following allogeneic bone marrow transplant. Transplantation Science 4.1. 1994;4:12–14. [PubMed] [Google Scholar]
- 233.Rowlings PA, Gale RP, Horowitz MM, et al. Bone marrow transplantation in leukemia. J Hematother. 1994;3(3):235–238. doi: 10.1089/scd.1.1994.3.235. [DOI] [PubMed] [Google Scholar]
- 234.Hessner M, Endean D, Capser J, et al. Use of unrelated marrow grafts compensates for reduced graft-versus-leukemia reactivity after T-cell-depleted allogeneic bone marrow transplantation for chronic myelogenous leukemia. Blood. 1995;86:3987–3996. [PubMed] [Google Scholar]
- 235.Rosenberg SA. Shedding light on immunotherapy for cancer. N Engl J Med. 2004;350(14):1461–1463. doi: 10.1056/NEJMcibr045001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Dermime S, Mavroudis D, Jiang YZ, et al. Immune escape from a graft-versus-leukemia effect may play a role in the relapse of myeloid leukemias following allogeneic bone marrow transplantation. Bone Marrow Transplant. 1997;19(10):989–999. doi: 10.1038/sj.bmt.1700778. [DOI] [PubMed] [Google Scholar]
- 237.Restifo NP, Esquivel F, Kawakami Y, et al. Identification of human cancers deficient in antigen processing. J Exp Med. 1993;177(2):265–272. doi: 10.1084/jem.177.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.van Hall T, Wolpert EZ, van Veelen P, et al. Selective cytotoxic T-lymphocyte targeting of tumor immune escape variants. Nat Med. 2006;12(4):417–424. doi: 10.1038/nm1381. [DOI] [PubMed] [Google Scholar]
- 239.Molldrem JJ, Lee PP, Kant S, et al. Chronic myelogenous leukemia shapes host immunity by selective deletion of high-avidity leukemia-specific T cells. J Clin Invest. 2003;111(5):639–647. doi: 10.1172/JCI16398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Rosenberg SA, White DE. Vitiligo in patients with melanoma: normal tissue antigens can be targets for cancer immunotherapy. J Immunother Emphasis Tumor Immunol. 1996;19(1):81–84. [PubMed] [Google Scholar]
- 241.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298(5594):850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Koutsky LA, Ault KA, Wheeler CM, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347(21):1645–1651. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- 243.Welters MJ, Kenter GG, Piersma SJ, et al. Induction of tumor-specific CD4+ and CD8+ T-cell immunity in cervical cancer patients by a human papillomavirus type 16 E6 and E7 long peptides vaccine. Clin Cancer Res. 2008;14(1):178–187. doi: 10.1158/1078-0432.CCR-07-1880. [DOI] [PubMed] [Google Scholar]
- 244.Vambutas A, DeVoti J, Nouri M, et al. Therapeutic vaccination with papillomavirus E6 and E7 long peptides results in the control of both established virus-induced lesions and latently infected sites in a pre-clinical cottontail rabbit papillomavirus model. Vaccine. 2005;23(45):5271–5280. doi: 10.1016/j.vaccine.2005.04.049. [DOI] [PubMed] [Google Scholar]
- 245.Cudkowicz G, Bennett M. Peculiar immunobiology of bone marrow allografts. II. Rejection of parental grafts by resistant F 1 hybrid mice. J Exp Med. 1971;134(6):1513–1528. doi: 10.1084/jem.134.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 247.Davies SM, Ruggieri L, DeFor T, et al. Evaluation of KIR ligand incompatibility in mismatched unrelated donor hematopoietic transplants. Killer immunoglobulin-like receptor. Blood. 2002;100(10):3825–3827. doi: 10.1182/blood-2002-04-1197. [DOI] [PubMed] [Google Scholar]
- 248.den Haan JMM, Sherman NE, Blokland E, et al. Identification of a graft versus host disease-associated human minor histocompatibility antigen. Science. 1995;268:1476–1480. doi: 10.1126/science.7539551. [DOI] [PubMed] [Google Scholar]
- 249.Brickner AG, Warren EH, Caldwell JA, et al. The immunogenicity of a new human minor histocompatibility antigen results from differential antigen processing. J Exp Med. 2001;193(2):195–206. doi: 10.1084/jem.193.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.de Bueger M, Bakker A, Van Rood JJ, et al. Tissue distribution of human minor histocompatibility antigens. Ubiquitous versus restricted tissue distribution indicates heterogeneity among human cytotoxic T lymphocyte-defined non-MHC antigens. J Immunol. 1992;149:1788–1798. [PubMed] [Google Scholar]
- 251.Dolstra H, Fredrix H, Maas F, et al. A human minor histocompatibility antigen specific for B cell acute lymphoblastic leukemia. J Exp Med. 1999;189(2):301–308. doi: 10.1084/jem.189.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Marijt WA, Heemskerk MH, Kloosterboer FM, et al. Hematopoiesis-restricted minor histocompatibility antigens HA-1- or HA-2-specific T cells can induce complete remissions of relapsed leukemia. Proc Natl Acad Sci U S A. 2003;100(5):2742–2747. doi: 10.1073/pnas.0530192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Hewitt SM, Hamada S, McDonnell TJ, et al. Regulation of the proto-oncogenes bcl-2 and c-myc by the Wilms' tumor suppressor gene WT1. Cancer Research. 1995;55(22):5386–5389. [PubMed] [Google Scholar]
- 254.Tsuboi A, Oka Y, Ogawa H, et al. Constitutive expression of the Wilms' tumor gene WT1 inhibits the differentiation of myeloid progenitor cells but promotes their proliferation in response to granulocyte-colony stimulating factor (G-CSF) Leuk Res. 1999;23(5):499–505. doi: 10.1016/s0145-2126(99)00037-5. [DOI] [PubMed] [Google Scholar]
- 255.Bellantuono I, Gao L, Parry S, et al. Two distinct HLA-A0201-presented epitopes of the Wilms tumor antigen 1 can function as targets for leukemia-reactive CTL. Blood. 2002;100(10):3835–3837. doi: 10.1182/blood.V100.10.3835. [DOI] [PubMed] [Google Scholar]
- 256.Xue SA, Gao L, Hart D, et al. Elimination of human leukemia cells in NOD/SCID mice by WT1-TCR gene-transduced human T cells. Blood. 2005;106(9):3062–3067. doi: 10.1182/blood-2005-01-0146. [DOI] [PubMed] [Google Scholar]
- 257.Tsuji T, Yasukawa M, Matsuzaki J, et al. Generation of tumor-specific, HLA class I-restricted human Th1 and Tc1 cells by cell engineering with tumor peptide-specific T-cell receptor genes. Blood. 2005;106(2):470–476. doi: 10.1182/blood-2004-09-3663. [DOI] [PubMed] [Google Scholar]
- 258.Mailander V, Scheibenbogen C, Thiel E, et al. Complete remission in a patient with recurrent acute myeloid leukemia induced by vaccination with WT1 peptide in the absence of hematological or renal toxicity. Leukemia. 2004;18(1):165–166. doi: 10.1038/sj.leu.2403186. [DOI] [PubMed] [Google Scholar]
- 259.Keilholz U, Letsch A, Asemissen A, et al. Clinical and immune responses of WT1-peptide vaccination in patients with acute myeloid leukemia. ASCO Annual Meeting Proceedings. 2006
- 260.Molldrem JJ, Lee PP, Wang C, et al. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nat Med. 2000;6(9):1018–1023. doi: 10.1038/79526. [DOI] [PubMed] [Google Scholar]
- 261.Rao NV, Rao GV, Marshall BC, et al. Biosynthesis and processing of proteinase 3 in U937 cells. Processing pathways are distinct from those of cathepsin G. J Biol Chem. 1996;271(6):2972–2978. doi: 10.1074/jbc.271.6.2972. [DOI] [PubMed] [Google Scholar]
- 262.Lindmark A, Gullberg U, Olsson I. Processing and intracellular transport of cathepsin G and neutrophil elastase in the leukemic myeloid cell line U-937-modulation by brefeldin A, ammonium chloride, and monensin. J Leukoc Biol. 1994;55(1):50–57. doi: 10.1002/jlb.55.1.50. [DOI] [PubMed] [Google Scholar]
- 263.Franssen CF, Stegeman CA, Kallenberg CG, et al. Antiproteinase 3- and antimyeloperoxidase-associated vasculitis. Kidney Int. 2000;57(6):2195–2206. doi: 10.1046/j.1523-1755.2000.00080.x. [DOI] [PubMed] [Google Scholar]
- 264.Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89(10):3503–3521. [PubMed] [Google Scholar]
- 265.Brouwer E, Stegeman CA, Huitema MG, et al. T cell reactivity to proteinase 3 and myeloperoxidase in patients with Wegener's granulomatosis (WG) Clin Exp Immunol. 1994;98(3):448–453. doi: 10.1111/j.1365-2249.1994.tb05511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Dengler R, Munstermann U, al-Batran S, et al. Immunocytochemical and flow cytometric detection of proteinase 3 (myeloblastin) in normal and leukaemic myeloid cells. Br J Haematol. 1995;89(2):250–257. doi: 10.1111/j.1365-2141.1995.tb03297.x. [DOI] [PubMed] [Google Scholar]
- 267.Molldrem JJ, Lee PP, Wang C, et al. A PR1-human leukocyte antigen-A2 tetramer can be used to isolate low-frequency cytotoxic T lymphocytes from healthy donors that selectively lyse chronic myelogenous leukemia. Cancer Res. 1999;59(11):2675–2681. [PubMed] [Google Scholar]
- 268.Heslop HE, Stevenson FK, Molldrem JJ. Immunotherapy of hematologic malignancy. Hematology Am Soc Hematol Educ Program. 2003:331–349. doi: 10.1182/asheducation-2003.1.331. [DOI] [PubMed] [Google Scholar]
- 269.Qazilbash M, Wieder E, Rios R, Lu S, Kant S, Giralt S, Estey E, Thall P, de Lima M, Couriel D, Champlin R, Komanduri K, Molldrem J. Vaccination with the PR1 leukemia-associated antigen can induce complete remission in patients with myeloid leukemia. Blood. 2004;104:259. [Google Scholar]
- 270.Entwistle J, Zhang S, Yang B, et al. Characterization of the murine gene encoding the hyaluronan receptor RHAMM. Gene. 1995;163(2):233–238. doi: 10.1016/0378-1119(95)00398-p. [DOI] [PubMed] [Google Scholar]
- 271.Hall CL, Yang B, Yang X, et al. Overexpression of the hyaluronan receptor RHAMM is transforming and is also required for H-ras transformation. Cell. 1995;82(1):19–26. doi: 10.1016/0092-8674(95)90048-9. [DOI] [PubMed] [Google Scholar]
- 272.Greiner J, Giannopoulos K, Li L, et al. RHAMM/CD168-R3 Peptide vaccination of HLA-A2+ patients with acute myeloid leukemia(AML), myelodysplastic syndrome (MDS) and multiple myeloma (MM) American Society of Hematology Annual Meeting. 2005
- 273.Greiner J, Li L, Ringhoffer M, et al. Identification and characterization of epitopes of the receptor for hyaluronic acid-mediated motility (RHAMM/CD168) recognized by CD8+ T cells of HLA-A2-positive patients with acute myeloid leukemia. Blood. 2005;106(3):938–945. doi: 10.1182/blood-2004-12-4787. [DOI] [PubMed] [Google Scholar]
- 274.Pinilla-Ibarz J, Korontsvit T, Zakhaleva V, et al. Synthetic peptide analogs derived from bcr/abl fusion proteins and the induction of heteroclitic human T-cell responses. Haematologica. 2005;90(10):1324–1332. [PubMed] [Google Scholar]
- 275.Bocchia M, Gentili S, Abruzzese E, et al. Effect of a p210 multipeptide vaccine associated with imatinib or interferon in patients with chronic myeloid leukaemia and persistent residual disease: a multicentre observational trial. Lancet. 2005;365(9460):657–662. doi: 10.1016/S0140-6736(05)17945-8. [DOI] [PubMed] [Google Scholar]
- 276.Hayakawa Y, Godfrey DI, Smyth MJ. Alpha-galactosylceramide: potential immunomodulatory activity and future application. Current Medicinal Chemistry. 2004;11(2):241–252. doi: 10.2174/0929867043456115. [DOI] [PubMed] [Google Scholar]
- 277.Shimizu K, Hidaka M, Kadowaki N, et al. Evaluation of the function of human invariant NKT cells from cancer patients using alpha-galactosylceramide-loaded murine dendritic cells. J Immunol. 2006;177(5):3484–3492. doi: 10.4049/jimmunol.177.5.3484. [DOI] [PubMed] [Google Scholar]
- 278.Metelitsa LS, Weinberg KI, Emanuel PD, et al. Expression of CD1d by myelomonocytic leukemias provides a target for cytotoxic NKT cells. Leukemia. 2003;17(6):1068–1077. doi: 10.1038/sj.leu.2402943. [DOI] [PubMed] [Google Scholar]
- 279.Ho VT, Vanneman M, Kim H, et al. Biologic activity of irradiated, autologous, GM-CSF-secreting leukemia cell vaccines early after allogeneic stem cell transplantation. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0908358106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Lane SW, Scadden DT, Gilliland DG. The leukemic stem cell niche: current concepts and therapeutic opportunities. Blood. 2009;114(6):1150–1157. doi: 10.1182/blood-2009-01-202606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Molldrem J. Immune therapy of AML. Cytotherapy. 2002;4(5):437–438. doi: 10.1080/146532402320776099. [DOI] [PubMed] [Google Scholar]
- 282.Guilhot F, Roy L, Saulnier PJ, et al. Immunotherapeutic approaches in chronic myelogenous leukemia. Leuk Lymphoma. 2008;49(4):629–634. doi: 10.1080/10428190801927510. [DOI] [PubMed] [Google Scholar]
- 283.Lubbert M, Tobler A, Daskalakis M. Cytosine demethylation of the proteinase-3/myeloblastin primary granule protease gene during phagocyte development. Leukemia. 1999;13(9):1420–1427. doi: 10.1038/sj.leu.2401486. [DOI] [PubMed] [Google Scholar]
- 284.Rapoport AP, Stadtmauer EA, Aqui N, et al. Restoration of immunity in lymphopenic individuals with cancer by vaccination and adoptive T-cell transfer. Nat Med. 2005;11(11):1230–1237. doi: 10.1038/nm1310. [DOI] [PubMed] [Google Scholar]