Abstract
Ca2+-dependent synaptic vesicle recycling is critical for maintenance of neurotransmission. However, uncoupling the roles of Ca2+ in synaptic vesicle fusion and retrieval has been difficult, as studies probing the role of Ca2+ in endocytosis relied on measurements of bulk synaptic vesicle retrieval. Here, to dissect the role of Ca2+ in these processes, we used a low signal-to-noise pHluorin-tagged vesicular probe to monitor single synaptic vesicle recycling in rat hippocampal neurons. We show that Ca2+ increases synaptic vesicle fusion probability in the classical sense, but surprisingly decreases the rate of synaptic vesicle retrieval. This negative regulation of synaptic vesicle retrieval is blocked by the Ca2+ chelator, EGTA, as well as FK506, a specific inhibitor of Ca2+-calmodulin-dependent phosphatase calcineurin. The slow time course of aggregate synaptic vesicle retrieval detected during repetitive activity could be explained by a progressive decrease in the rate of synaptic vesicle retrieval during the stimulation train. These results indicate that Ca2+ entry during single action potentials slows the pace of subsequent synaptic vesicle recycling.
Introduction
Maintenance of synaptic transmission requires constant retrieval and reuse of synaptic vesicles (Murthy and De Camilli, 2003; Sudhof, 2004). Several mutants with deficiencies in synaptic vesicle endocytosis manifest rapid run-down of neurotransmitter release during activity, supporting a dynamic role of vesicle endocytosis and recycling in neurotransmission (Kavalali, 2007). The executive role of intrasynaptic Ca2+ transients in synaptic vesicle exocytosis is well established (Bollmann et al., 2000; Schneggenburger and Neher, 2000; Sun et al., 2007). However, the impact of Ca2+ on synaptic vesicle endocytosis remains unclear. Ca2+ has been suggested as an essential trigger required to initiate synaptic vesicle retrieval (Marks and McMahon, 1998; Wu et al., 2009), but experiments manipulating Ca2+ concentrations directly or genetically have reported an inhibition of endocytosis (von Gersdorff and Matthews, 1994; Cousin and Robinson, 2000; Wan et al., 2010) as well as acceleration of endocytosis (Hosoi et al., 2009; Wu et al., 2009; Sun et al., 2010; Yamashita et al., 2010). A major drawback of these studies is their reliance on bulk measurements of synaptic vesicle endocytosis, which can be affected by a multitude of factors, such as the number of vesicles involved, the kinetics and duration of Ca2+ signals, and accumulation of released substances that may retrogradely alter release and retrieval processes. Indeed, there is evidence that the rate and pathway of synaptic vesicle retrieval can be altered with increasing stimulus strength and endocytic load [i.e., the number of vesicles waiting to be retrieved (Kawasaki et al., 2000; Sankaranarayanan and Ryan, 2000, 2001; Sun et al., 2002; Wu and Wu, 2007; Clayton et al., 2008)].
Here, to investigate the role of action potential-evoked Ca2+ signals in fusion and retrieval of individual synaptic vesicles, we took advantage of the improved optical signal-to-noise characteristics of pHluorin-tagged vesicular glutamate transporter (Vglut1-pHluorin) (Voglmaier et al., 2006; Balaji and Ryan, 2007; Santos et al., 2009). This setting circumvented the need for potentially toxic maneuvers, such as pre-photobleaching of surface membrane fluorescence, to improve signal-to-noise. Lentiviral expression of Vglut1-pHluorin in hippocampal neurons enabled us to visualize fusion and retrieval of single synaptic vesicles at individual release sites (boutons). We show that increasing extracellular Ca2+ increases release probability in the classical sense, but also recruits release-reluctant boutons to a more active state. We also detected a decrease in the rate of vesicle retrieval with increasing extracellular Ca2+. Furthermore, we found that this decrease was dependent on intracellular Ca2+ and activation of calcineurin. Together, these findings indicate that Ca2+ entry into the nerve terminal acts to slow synaptic vesicle endocytosis of individual vesicles and antagonize the pace of synaptic vesicle reuse. We tested this premise during 1 Hz stimulation, which triggers aggregate fusion and retrieval of multiple vesicles. The relatively slow kinetics of vesicle retrieval after 1 Hz stimulation could be accounted for by a gradual decrease in the rate of individual vesicle retrieval during the stimulation train. These results indicate a critical role for Ca2+ entry during single action potentials by setting the pace of subsequent vesicle retrieval and recycling.
Materials and Methods
Dissociated cultures.
Dissociated hippocampal cultures were prepared from postnatal day 1–3 Sprague Dawley rats of either sex, as described previously (Kavalali et al., 1999). The vGlut-pHluorin construct was a generous gift from Drs. Robert Edwards and Susan Voglmaier (University of California, San Francisco). At 5 d in vitro (DIV), cultures were infected with lentivirus expressing vGlut-pHluorin and experiments were conducted after 15 DIV when synapses reach maturity (Mozhayeva et al., 2002). In these experiments, we relied on a lentiviral expression system. This is a rather robust and sustained gene expression system that enables protein expression in large fraction of neurons (thus presynaptic terminals) in dissociated cultures. This argument is supported by our previous observation that lentiviral expression of synaptobrevin-2 restores synaptic transmission to near wild-type levels in synaptobrevin-2 knock-out cultures (Deák et al., 2006). For all experiments, the extracellular solution was a modified Tyrode's solution containing the following (in mm): 150 NaCl, 4 KCl, 10 glucose, 10 HEPES, 2 MgCl, and varying concentrations of CaCl2 (1, 2, 4, and 8 mm), pH 7.4 (310 Osm). To prevent network activity, postsynaptic ionotropic glutamate receptors antagonists 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; Sigma) and aminophosphonopentanoic acid (AP-5; Sigma) were added to the modified Tyrode's solution at concentrations of 10 and 50 μm, respectively.
Ca2+ chelation and calcineurin inhibition.
In experiments where we introduced an exogenous internal Ca2+ buffer, cultures were incubated for 20 min with 10 μm EGTA-AM (Sigma) in the modified Tyrode's solution lacking extracellular Ca2+. Similarly, to inhibit calcineurin, we incubated neurons in modified Tyrode's solution containing 1 μm FK506 (Tocris Bioscience) for 20 min and performed optical recordings in the continued presence of FK506.
Fluorescence imaging.
Experiments were performed from 17 to 22 DIV at room temperature. Experiments were performed using an Andor iXon+ back-illuminated EMCCD camera (Model no. DU-897E-CSO-#BV). Images were acquired at 5 Hz with an exposure time of 100 ms and binning of 4 to optimize the signal-to-noise ratio. Data were collected using MetaFluor and transferred to Excel for analysis.
Fluorescence analysis.
Amplitudes were determined as the difference between the average of 3 points after stimulus and the average of 8 points before stimulus. Successful events were those that had amplitudes greater than twice the SD of 8 frames (∼1.5 s) before the event. For the analysis of decay constants, single-exponential Levenberg–Marquardt least sum of squares minimizations were used to fit data in Clampfit (Molecular Devices). Events were selected for decay analysis only if their decay stabilized, but did not assume events returned to prestimulus baseline. Dwell times were calculated as the time between the initial fluorescence step and the start of fluorescence decay predicted by the best fit decay in Clampfit.
Stimulation protocol.
Cultures were stimulated using parallel bipolar electrodes (FHC) delivering 15–20 mA pulses. Cultures stimulated at 0.025–0.2 Hz for 5–7 min, followed by a rest period before 10 action potentials (APs) delivered at 1 Hz. After the fluorescence returned to pre-1 Hz fluorescence, a 20 Hz stimulus for 20 s was delivered to help select active puncta.
Reconstructions of endocytic retrieval during 1 Hz stimulation.
Reconstructions of 1 Hz stimulus responses were performed using custom macros written in Microsoft Excel by summation of “ideal” single-vesicle events derived from averages of amplitude, decay time, and dwell time. Therefore, each Ca2+ concentration had its own ideal event (i.e., the 8 mm Ca2+ 1 Hz reconstruction uses properties of 8 mm Ca2+ events). Least sum of squares error minimization was used to determine the best fitting sequential increase in decay time. Goodness of fit was determined by fitting fluorescence data with a single-exponential decay, then fitting hypothetical decays with the same curve.
Results
Detection of synaptic vesicle fusion and retrieval during low-frequency stimulation
To investigate the properties of single synaptic vesicle exocytosis and endocytosis, we expressed the pHluorin-tagged version of the vesicular glutamate transporter (vGlut-pHluorin) in dissociated hippocampal cultures (Voglmaier et al., 2006) using a lentiviral expression system (Ertunc et al., 2007). Earlier work has shown that vGlut-pHluorin expression results in low levels of surface fluorescence, enabling reliable detection of single-vesicle fusion events (Balaji and Ryan, 2007). Moreover, unlike pHluorin-tagged synaptobrevin and synaptotagmin-based probes, vGlut-pHluorin shows limited lateral diffusion upon vesicle fusion, thus providing a higher-fidelity marker for synaptic vesicle exocytosis and endocytosis (Wienisch and Klingauf, 2006; Balaji and Ryan, 2007). These earlier findings also agree well with the detection of limited copy numbers of vGlut in purified synaptic vesicles (Takamori et al., 2006).
In mature neurons expressing vGlut-pHluorin, we assessed synaptic vesicle fusion and retrieval by applying low-frequency stimulation (0.025–0.1 Hz) followed by 20 Hz high-frequency burst stimulation to identify synaptic boutons (Fig. 1A). The low-frequency stimulation protocol triggered positive fluorescence fluctuations with amplitudes 2 SDs above the mean baseline noise (Fig. 1B, left) typically corresponding to 2.1 ± 0.2 fluorescence units (Fig. 1C, top). Each positive fluorescence fluctuation was followed by swift decay back to baseline fluorescence consistent with rapid vesicle reacidification and retrieval (Fig. 1D). Most importantly, this experimental strategy did not require measures, such as pre-photobleaching, to reduce background surface fluorescence levels, which may have adverse effects (Gandhi and Stevens, 2003; Atluri and Ryan, 2006; Balaji and Ryan, 2007).
Figure 1.

Single-vesicle fusion events are distinguishable from noise and decay rapidly. A, Example frame showing numerous punctate release sites, left, and corresponding vGlut1-pHluorin signal, right, evoked by low-frequency single-AP stimulation (0.025–0.2 Hz) in 2 mm CaCl2 followed by 20 Hz stimulation. B, Raw fluorescence data (gray trace) can be fit with Levenberg–Marquardt single-exponential decay (black trace). Red trace shows the moving average of five points (∼1 s). Scale bar shows 1 arbitrary fluorescence unit (a.u.) amplitude over 2 s. C, Comparing the amplitude distribution of evoked events with and without folimycin shows no difference in distribution and mean, suggesting that the events observed in 2 mm extracellular Ca2+ are composed of single quanta. White bars show a normal distribution of noise (with Gaussian fit, blue trace, mean = 0.14 ± 0.52 a.u.). Gray bars depict successful events [with Gaussian fit, red trace, mean = 2.12 ± 0.23 a.u. without folimycin and 2.05 ± 0.48 a.u. with folimycin (KStest = 1)]. D, The distribution decay constants of 570 events from 355 boutons across six coverslips is exponential with mean = 3.4 s and median = 2.2 s. E, In some cases, events did not decay immediately but spent some dwell time on the surface (example inset). The frequency distribution of dwell times (from same population of events in D) shows a bimodal distribution with the vast majority of events decaying immediately (<200 ms).
To test whether the positive fluorescence fluctuations we detected were indeed due to synaptic vesicle fusion and retrieval, we performed similar experiments in the presence of the vacuolar ATPase inhibitor folimycin. The decay phase of the vGlut-pHluorin signal reports the rate of synaptic vesicle reacidification that follows vesicle retrieval. Therefore, in the presence of folimycin, fusion events take on a staircase-like fluorescence pattern (Fig. 1B, bottom) as inhibition of reacidification traps vesicles in an alkaline state. The amplitudes of fusion events we detected in these experiments were comparable to the distribution of events we analyzed above in the absence of folimycin (Fig. 1C, bottom), indicating that our detection and analysis criteria can reliably uncover vesicle fusion and retrieval during low-frequency, sparse stimulation.
In 2 mm extracellular Ca2+, the fluorescence decay time (τ) constants had a mean around 3 s (τ = 3.4 ± 3.3 s, Fig. 1D). The decay times followed an exponential distribution with ∼72% of events decaying with time constants <4 s after fusion. At this near-physiological Ca2+ concentration, fusion events were rarely followed by a plateau phase, suggesting that vGlut-pHluorin probes did not manifest a detectable period of residency at the surface membrane (Fig. 1E). This result implies that in these experiments, single synaptic vesicle endocytosis is initiated within our acquisition rate (200 ms) after vesicle exocytosis.
Ca2+-dependent regulation of single synaptic vesicle fusion probability
During low-frequency stimulation (<1 Hz), the behavior of most synapses is dictated by their baseline probability of synaptic vesicle fusion (Pr), as there is limited propensity for short-term synaptic plasticities seen at higher frequencies (Zucker and Regehr, 2002). Accordingly, in the presence of 2 mm extracellular Ca2+, we detected only a small number of fusion events per synaptic bouton during sustained low-frequency stimulation, suggesting a low Pr and preponderance of fusion failures. In the next set of experiments, we systematically investigated Ca2+ dependence of Pr by tallying events at each stimulation as failures (events within 2 SDs of baseline noise) or successes (2 SDs above the mean baseline noise as in Fig. 1) (Fig. 2A) and calculated the Pr as the ratio of successful events to the total number of stimuli (i.e., trials) applied to a given bouton. At 1 mm and 2 mm extracellular Ca2+ concentration, this analysis yielded a distribution where 88% and 84% of synapses had a Pr of <0.2, respectively, with a median of 0.1 (Fig. 2B,C). This distribution is remarkably similar to earlier single synapse measurements conducted by measuring probability of FM1-43 uptake as a marker for Pr (Murthy et al., 1997).
Figure 2.

Increasing Ca2+ causes an increase in Pr. A, Example trace showing a successful event, the shaded area shows 2 SDs from the mean of the noise. Vertical scale bar represents 2 a.u. while horizontal scale bar is 5 s. B–E, Release probability, by bouton, shows classical Ca2+ sensitivity as extracellular Ca2+ increases. Overall increase in average Pr could be described with a Hill equation with a coefficient of 4.8. However, at high Ca2+ concentrations, a bimodal distribution of release probabilities reflects two populations of boutons: one reluctant-to-release and one release competent.
Next, we examined the distributions of Pr values measured per synaptic bouton at increasing extracellular Ca2+ concentrations. Interestingly, at 1 mm Ca2+, ∼35% of synaptic boutons were largely unresponsive to low-frequency stimulation and therefore had an evoked release probability of near 0 (Pr < 0.1). At 2 mm extracellular Ca2+, this fraction decreased to ∼20% whereas at 4 and 8 mm Ca2+, ∼10% of synaptic boutons were unresponsive to low-frequency stimulation. In addition to this decrease in the fraction of extremely low Pr synapses, we also detected an increase in overall release probability in the classical sense (Fig. 2B–E). With increasing Ca2+ concentrations, Pr values showed a widespread distribution with an increasing number of synaptic boutons reaching a Pr of 1. Cumulative data from all Ca2+ concentrations could be described with a Hill function (Pr = 1/(1 + (c/[Ca2+]e)n)) yielding a corresponding Hill coefficient (n) of 4.8, which suggests a degree of cooperativity consistent with previous electrophysiological estimates of Ca2+ dependence of neurotransmitter release probability (Dodge and Rahamimoff, 1967; Mintz et al., 1995; Fernández-Chacón et al., 2001). Furthermore, these results reinforce the premise that our optical measurements report single-vesicle release from single release sites. Interestingly, increasing extracellular Ca2+ concentrations also caused a marked increase in the amplitudes of fusion events, suggesting multivesicular release (see below). However, Pr calculations we present here did not differentiate between single and multivesicular fusion events.
The effect of Ca2+ on multivesicular release
During low-frequency single action potential stimulation, fluorescent responses showed an abrupt increase in fluorescence, consistent with fast exocytosis followed by a slower decay (Fig. 3A). As extracellular Ca2+ increased, the amplitudes of fluorescence changes also increased in a quantal fashion (Fig. 3B) consistent with an increase in multivesicular fusion events. At low extracellular Ca2+ (1 mm), fluorescence amplitude distributions could be represented with a single Gaussian curve (mean = 2.2 ± 0.5 a.u., reduced χ2 = 0.97). The amplitudes of events detected at near-physiological 2 mm extracellular Ca2+ were also fit well by a single Gaussian (mean = 2.1 ± 0.2 a.u., reduced χ2 = 0.98), suggesting that single-vesicle fusion events predominate low-frequency evoked transmission at 1 and 2 mm extracellular Ca2+. At 4 mm Ca2+, the fluorescence amplitude distribution broadened and could be fit by the sum of two Gaussians (black traces) with means spaced at integer multiples of one another, indicating quantal fluorescence steps (mean1 = 2.2 ± 0.5 a.u.; mean2 = 4.4 ± 1.1 a.u.; reduced χ2 = 0.96, red traces). At high 8 mm extracellular Ca2+, the fluorescence amplitude distribution fit to the sum of three Gaussians (mean1 = 2.2 ± 0.5 a.u.; mean2 = 4.4 ± 1.1 a.u.; mean3 = 6.6 ± 1.6 a.u., reduced χ2 = 0.85). Here, it is important to note that our acquisition rate (200 ms) was not fast enough to distinguish between synchronous multivesicular fusion events and fast asynchronous fusion events. Thus our reference to fluorescence changes greater than those observed in 1 and 2 mm Ca2+ as multivesicular fusion events includes asynchronous fusion events that occur in a single release site in tandem (Rudolph et al., 2011). These findings indicate that the increase in extracellular Ca2+ concentration not only increases single-vesicle fusion probability but also increases the likelihood of multivesicular synchronous and/or asynchronous fusion events.
Figure 3.
Increasing extracellular Ca2+ promotes multivesicular release, slows decay time, increases the propensity for a vesicle to dwell on the surface, and increases that dwell time. A, Sample traces of single-vesicle events observed in extracellular 1 mm (left), 2 mm (center left), 4 mm (center right), and 8 mm (right) Ca2+. The gray trace shows raw fluorescence data, the red trace is a moving average of five points (∼1 s), and the black trace is a single-exponential decay fit using Levenberg–Marquardt least-squares minimization to the gray trace. B, The amplitude distribution of fluorescence changes in response to single-AP stimuli delivered at low (0.025–0.2 Hz) frequency. Fluorescence intensity amplitudes increase with increasing Ca2+. White bars show amplitude distribution during no stimulus and have a normal Gaussian distribution (blue dashed line). Gray bars are the change in fluorescence of successful release events (for calculation see methods) and can be fit with one (red dashed line) or multiple—quantally distributed—Gaussians (black line). C, Single-vesicle decay time increases with increasing Ca2+. Only events from B with amplitudes within 1 SD of the mean in 2 mm Ca2+ (i.e., 2.1 ± 0.2 a.u.) were selected. At 1 mm extracellular Ca2+, events decayed with an average τ of 1.9 ± 2.3 s (from 87 events from 55 puncta and 3 coverslips from 2 cultures). At 2 mm, extracellular Ca2+ events decayed slower with an average τ to 3.4 ± 3.3 s (from 572 events from 233 puncta from 5 coverslips over 3 cultures). The decay increased to an average τ of 8.1 ± 7.3 s in 4 mm Ca2+ (from 511 events from 168 puncta over 6 coverslips from 3 sets of cultures). Finally, events in 8 mm extracellular Ca2+ had an average τ of 13.1 ± 10.5 s (from 542 events from 186 puncta from 6 coverslips from 4 cultures). The inset shows the cumulative probability histogram of decay times, all conditions are significantly different (p < 0.001) according to KStest. D, The distribution of dwell times shows that increasing Ca2+ causes more vesicles to experience a delay before endocytosis. Additionally, increasing Ca2+ increases the length of that delay but appears to have some maximal effect after 4 mm Ca2+.
The effect of Ca2+ on single synaptic vesicle retrieval
Next, we analyzed the fluorescence decay of single-vesicle events by only selecting fusion events with fluorescence amplitudes of univesicular fusion events within the first Gaussian distribution (i.e., mean = 2.2 ± 0.5 a.u.). We then determined the decay time and dwell time of those events. Surprisingly, increases in extracellular Ca2+ increased the τ of single-vesicle events (Fig. 3C), skewing the distributions toward slower decay times. At 1 mm extracellular Ca2+, events decayed with an average τ of 1.9 s and median τ of 1.3 s, which increased to 3.4 s and median τ of 2.2 s in 2 mm extracellular Ca2+. In 4 mm extracellular Ca2+, the average τ increased to 8.1 s, although half of those events decayed within 5 s (median τ = 5.6 s). Finally, events in 8 mm extracellular Ca2+ had an average τ of 13.1 s with median τ of 10.7 s.
In addition to the apparent slowing in the time course of fluorescence decay, increasing extracellular Ca2+ caused some vesicles to show a brief delay at the high fluorescence value before the onset of fluorescence decay (Fig. 3D). The majority of events in all extracellular Ca2+ conditions did not manifest a dwell time longer than 200 ms. However, in some cases, there were significant pauses before fluorescence decay and increasing extracellular Ca2+ caused more fusion events to dwell longer at elevated fluorescence values (Fig. 3D). In 1 and 2 mm extracellular Ca2+, fusion events experienced dwell times of 3.8 ± 2.5 s and 4.2 ± 2.8 s, respectively. The dwell time distributions were not significantly different at low and near-physiological extracellular Ca2+ [Kolmogorov–Smirnov test (KStest) p = 1]. This dwell time increased to 8.3 ± 5.7 s and 7.3 ± 4.3 s in 4 and 8 mm extracellular Ca2+, respectively. The distributions of dwell times were not significantly different between 4 and 8 mm extracellular Ca2+ (KStest p = 1), but there was a significant difference between high Ca2+ and low Ca2+ dwell times (KStest p < 0.005), suggesting that a threshold level of intracellular Ca2+ delays vesicle retrieval for seconds.
The relation between probability of synaptic vesicle fusion and synaptic vesicle trafficking
We next explored the relationship between the synaptic Pr (estimated as in Fig. 2) and the kinetic properties of individual fusion events (Fig. 4A–C). We found that synapses with a high Pr were indeed more likely to release multiple vesicles in response to a single stimulation (Fig. 4A). This correlation was particularly striking in high Pr values detected in 8 mm Ca2+ (Fig. 4A). In this analysis, we observed a Ca2+-dependent increase in single-vesicle retrieval time, as documented in Figure 3C, which manifests as a vertical shift in decay times between 2 and 8 mm Ca2+ (Fig. 4B). However, we also found that synapses with a higher Pr were slower to retrieve vesicles (Fig. 4B), consistent with earlier observations (Gandhi and Stevens, 2003). Together these data suggest that intrinsic properties of synapses control the rate of endocytosis, but extrinsic factors, such as Ca2+ influx, also act to slow vesicle retrieval. Dwell times, however, were relatively independent of release probability in high Ca2+, but showed a slight increase with increasing Pr in 2 mm Ca2+ (Fig. 4C), suggesting the independence of processes underlying the initiation of the retrieval versus the actual retrieval. Finally, given that higher Pr synapses are more prone to multivesicular release, we probed how the decay times of multivesicular events compared to those of single-vesicle events (Fig. 4D). In 8 mm Ca2+, where we detect multivesicular events in high abundance, we found that the individual decay times comprising the retrieval of a multivesicular event (τm1, τm2) were slower than that of single-vesicle retrieval events (τs) (Fig. 4D) (multivesicular decay: mean τm1 of 15.1 s with median τm1 of 14.5 s vs single-vesicle decay: mean τs of 13.1 s with median τs of 10.7 s; KStest p < 0.001). Interestingly, within multivesicular events, the second decay time constant τm2 was still slower than both the single-vesicle events τs and the first τm1, with average τm2 of 17.8 s and median τm2 of 17.0 s (KStest p < 0.001). These findings suggest that multivesicular events are slower to recycle than single-vesicle events, which may point to a small but significant impact of exocytic load on the kinetics of vesicle retrieval.
Figure 4.
The probability of synaptic vesicle fusion correlates with the propensity of multivesicular fusion and a longer single-vesicle retrieval time. A, Synapses with a high release probability exhibit more multivesicular fusion events and B, slower single-vesicle retrieval times. C, Dwell times, however, are relatively unaffected by the Pr of the synapse. Error bars show SEM. D, The fluorescence decay time of multivesicular fusion events can be separated into two decay steps (τm1, τm2) D, Inset, In 8 mm Ca2+, the individual decay times comprising the retrieval of multivesicular events were slower than those of single-vesicle retrieval events (τs) (multivesicular decay: average τm1 of 15.1 s with median τm1 of 14.5 s vs single-vesicle decay: average τs of 13.1 s with median τs of 10.7 s; KStest p < 0.001). Within multivesicular events, the second decay time constant (τm2) was still slower than both the single-vesicle events τs and the first τm1, with average τm2 of 17.8 s and median τm2 of 17.0 s (KStest p < 0.001).
Intrasynaptic Ca2+ acts to modulate vesicle endocytosis
We sought to test the hypothesis that Ca2+ influx, rather than extracellular Ca2+ per se, acts to modulate the kinetics of endocytosis. To this end, we analyzed low-frequency evoked events in the presence of the internal Ca2+ chelator, EGTA-AM at near-physiological 2 mm extracellular Ca2+ (Fig. 5A) and high 8 mm extracellular Ca2+ (Fig. 5B). Low-frequency stimulation after incubation with 10 μm EGTA-AM (20 min. pretreatment) decreased decay times in both 2 mm Ca2+ and 8 mm Ca2+ to an average τ of 1.3 s and 7.7 s, respectively (KStest p < 0.001 for both 2 mm Ca2+ and 8 mm Ca2+), and median τ of 0.9 s and 5.7 s, respectively. At 2 mm Ca2+ as well as 8 mm Ca2+, EGTA-AM treatment resulted in a threefold decrease in Pr (2 mm Ca2+: without EGTA-AM average Pr ≈ 0.13, with EGTA-AM average Pr ≈ 0.04; at 8 mm Ca2+: without EGTA-AM average Pr ≈ 0.6, with EGTA-AM average Pr ≈ 0.18) and complete abrogation of multivesicular events. These results suggest that Ca2+ entry into the presynaptic terminal is required to mediate the negative action of increased extracellular Ca2+ on the rate of vesicle retrieval.
Figure 5.
The rate of endocytosis is controlled by intracellular Ca2+ influx. A, B, The decay time of events evoked by single action potential stimulation in 2 mm (A) or 8 mm (B) extracellular Ca2+ decreases with application of the slow intracellular Ca2+ chelator EGTA-AM. Cumulative probability histograms show that incubation in EGTA-AM decreases the decay time of events in both 2 mm Ca2+ (KStest p < 0.001) and 8 mm Ca2+ (KStest p < 0.001).
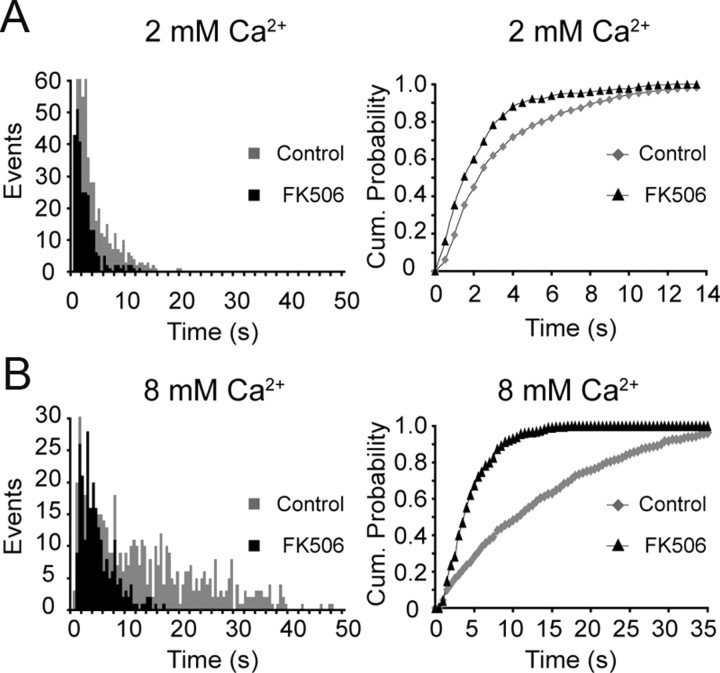
Intrasynaptic Ca2+ acts via calcineurin to modulate vesicle endocytosis
Previous work has suggested that the Ca2+/calmodulin-dependent phosphatase, calcineurin, acts as a key regulator of synaptic vesicle endocytosis through dephosphorylation of endocytic proteins (Marks and McMahon, 1998; Wu et al., 2009; Sun et al., 2010). Next, we sought to test the hypothesis that Ca2+ influx acts via calcineurin to modulate the kinetics of endocytosis. We analyzed low-frequency evoked events in the presence of a specific calcineurin inhibitor, FK506, at near-physiological 2 mm extracellular Ca2+ (Fig. 6A) and high 8 mm extracellular Ca2+ (Fig. 6B). Under both conditions, as with internal Ca2+ chelation, inhibition of calcineurin using 1 μm FK506 resulted in faster decay times. In 2 mm Ca2+, the mean τ decreased to 2.2 s, and in 8 mm Ca2+, the mean τ decay time decreased to 4.5 s (KStest p < 0.001 for both 2 and 8 mm Ca2+) with median τ of 1.5 s and 3.8 s for 2 and 8 mm Ca2+, respectively. Here, it is important to note that FK506 treatment induced only a small decrease in release probability at 8 mm Ca2+ from average Pr of 0.6 without FK506 to 0.5 with FK506, supporting the premise that calcineurin inhibition directly facilitates vesicle retrieval rather than acting indirectly by regulation of release probability. Together, these results suggest that Ca2+ entry into the presynaptic terminal activates calcineurin to mediate the negative action of increased extracellular Ca2+ on the rate of vesicle retrieval.
Figure 6.
Intracellular Ca2+ activates calcineurin to control the rate of endocytosis. A, B, The decay time of events evoked by single action potential stimulation in 2 mm (A) and 8 mm (B) extracellular Ca2+ decreases when calcineurin is inhibited with FK506. Cumulative probability histograms show that incubation in FK506 decreases the decay time of events in both 2 mm Ca2+ (KStest p < 0.001) and 8 mm Ca2+ (KStest p < 0.001).
Estimating single-vesicle retrieval during higher-frequency stimulation
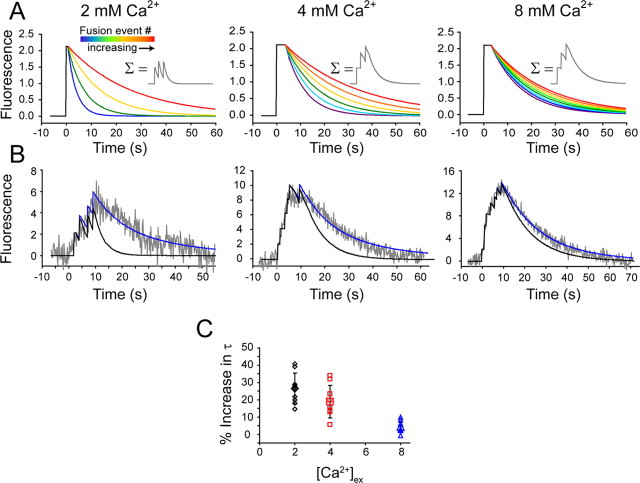
Our results so far suggest that upon fusion, synaptic vesicles are reacidified and retrieved with a relatively rapid time course. However, this decay time is tightly controlled by intrasynaptic Ca2+ and calcineurin activation. Next, we tested this premise at 1 Hz stimulation, where intrasynaptic Ca2+ accumulates (Zucker and Regehr, 2002) without inducing significant synaptic depression in this system (Ertunc et al., 2007). For this purpose, we compiled theoretical ideal single-vesicle events using mean decay times and dwell times for 2, 4, and 8 mm extracellular Ca2+ conditions (Fig. 7A). Neurons were stimulated with 10 APs delivered at 1 Hz and the resulting fluorescence traces were fit by the summation of ideal events using the probability of release estimate we obtained by fitting the rise time of each 1 Hz trace (Fig. 7B). Interestingly, predicted ideal 1 Hz traces obtained by cumulative summation of individual ideal events decayed faster than the observed experimental fluorescence decay and therefore were poor fits to the data (Fig. 7B, black traces). We hypothesized that this apparent slower decay could be the result of slowing individual retrieval events as intrasynaptic Ca2+ increases, because 1 Hz stimulation is faster than nerve terminal Ca2+ removal (Regehr et al., 1994). To seek a better fit to the observed 1 Hz decay phase, we serially increased vesicle decay time for each stimulus delivered. Increasing decay time as a function of stimuli mimics actual fluorescence data (Fig. 7B, blue traces). If endocytosis slows with each round of Ca2+ entry (i.e., stimulus) (Fig. 7C), then each stimulus slows vesicle retrieval by ∼30%, in 2 mm extracellular Ca2+. This scenario maximized the goodness of fit of idealized traces to the actual data (Rper stim2 = 0.92, compared to Rno change2 = −1.5). As extracellular Ca2+ increases, the serial deceleration observed is occluded such that at high extracellular Ca2+, succeeding vesicles show limited decrease in their rate of retrieval per stimulus (for 4 mm Ca2+ Rper stim2 = 0.94, compared to Rno change2 = −0.2) (Fig. 7B, middle). In fact, at 8 mm Ca2+, decay times must increase only slightly, if at all, with each stimulus or fusion event to account for the actual data (Rper stim2 = 0.99, compared to Rno change2 = 0.90) (Fig. 7B, left). This finding suggests that single-vesicle events at high Ca2+ concentrations are approaching a lower limit of vesicle retrieval rate.
Figure 7.
Modeling of 1 Hz stimulation requires a serial slowing in endocytic kinetics. A, Ideal events generated by averaging all decay times and dwell times from single-vesicle events during low-frequency stimulation. Heat map colors show the effect of serial slowing on single-vesicle events. Insets show examples of the summation of three events at stimulus numbers 1 (t = 0 s), 5 (t = 4 s), and 10 (t = 9 s). B, Ideal events can be used to fit higher-frequency (1 Hz) stimulation data (gray trace). Two fits were generated where decay time is held constant (black trace) and where decay is increasing for each stimulus regardless of fusion event (blue trace). C, Increasing decay time per stimulus requires small adjustments to each vesicle fusion event as extracellular Ca2+ increases.
Discussion
Single-vesicle endocytosis is rapid
Optical measurements of single synaptic vesicle endocytosis have resulted in a widespread disagreement of vesicle retrieval kinetics and time course (Gandhi and Stevens, 2003; Granseth et al., 2006; Balaji and Ryan, 2007; Zhu et al., 2009). In this study, we use vGlut-pHluorin to optically monitor vesicle reacidification without prior photobleaching (Gandhi and Stevens, 2003) or event averaging (Granseth et al., 2006). Our results are consistent with earlier reports of fast single-vesicle endocytosis (Aravanis et al., 2003; Gandhi and Stevens, 2003; Bowser and Khakh, 2007; Zhang et al., 2009a, 2009b) that proceeds though a single process (Balaji and Ryan, 2007) during low-frequency 0.025–0.1 Hz stimulation albeit with remarkably fast kinetics (Fig. 1). During low-frequency stimulation, in physiological extracellular Ca2+, small fluorescence steps occurred faster than our rate of acquisition (200 ms), consistent with fast vesicle fusion. To confirm that the observed events were the products of single-vesicle fusion, we compared the fluorescence amplitude of events in the presence and absence of the vacuolar H+-ATPase inhibitor folimycin (Fig. 1B,C). The similarity between the two fluorescence event amplitude distributions suggests that observed fluorescence steps were the result of single-vesicle fusion events. Additionally, our observed Pr distribution at 2 mm extracellular Ca2+ (Fig. 2B) was strikingly similar to previous estimates of probability of neurotransmitter release using FM dye uptake (Murthy et al., 1997) and electrophysiological methods (Fernández-Chacón et al., 2001) in hippocampal neurons. This similarity in Pr estimates strongly supports the premise that, in our experimental system, all recycling vesicles possess a comparable number of Vglut1-pHluorin molecules and a negligible population of untagged vesicles. Furthermore, these observations strongly support our assumption that the fluorescence changes we observe are due to single-vesicle fusion events originating from individual synaptic release sites.
The rise in fluorescence amplitude was followed by rapid fluorescence decay that could be fit with a single exponential (Fig. 1D). These decay times had a median τ of 2.2 s and average of 3.4 s, at near-physiological extracellular Ca2+, indicating rapid vesicle reacidification, and rapid vesicle retrieval (Gandhi and Stevens, 2003). The majority of events (∼87%) began to decay in <0.5 s after vesicle fusion. But in some cases the fluorescence amplitude would pause before decay with an average dwell time of ∼4.2 s (Fig. 1E). Although we cannot differentiate between a pause in vesicle reacidification and a period of vesicle residency on the synaptic surface, these measurements of single-vesicle endocytosis are significantly faster than the previous estimates of vesicle reacidification (Atluri and Ryan, 2006; Granseth et al., 2006) or endocytic time courses (Balaji and Ryan, 2007) at physiological Ca2+ levels.
Ca2+ increases vesicle fusion probability and slows retrieval
Given the sensitivity of our experimental system, we were able to examine single-vesicle release probability (Fig. 2) as well as single-vesicle retrieval as a function of extracellular Ca2+ concentration (Fig. 3). We found that increasing extracellular Ca2+ elevated vesicle fusion Pr, with a corresponding Hill coefficient of 4.8, similar to what is observed using electrophysiological measures (Dodge and Rahamimoff, 1967; Mintz et al., 1995; Fernández-Chacón et al., 2001). Interestingly, in addition to an increase in overall release probability as Ca2+ increased, the number of boutons with very low release probability (Pr < 0.1) decreased, suggesting that more boutons were recruited to an active state.
Higher extracellular Ca2+ concentrations produced larger steps in fluorescence amplitude increase, which could be described with quantal Gaussian distributions with equally spaced means. These results indicate that increasing extracellular Ca2+ raises the propensity for multivesicular and/or fast asynchronous release events (Fig. 3B). This observation also raises the possibility that dual discontinuous decay times detected in earlier work (Zhu et al., 2009) could in part reflect vesicle retrieval after multivesicular fusion events (e.g., Fig. 3A). When we selected events that had fluorescence amplitudes within 1 SD of the first Gaussian mean (i.e., univesicular events), we found that increasing extracellular Ca2+ slowed vesicle retrieval (Fig. 3C), and encouraged vesicle residency at the synaptic surface membrane (Fig. 3C). Accordingly, buffering intracellular Ca2+, by incubating neurons in EGTA-AM accelerated the rate of vesicle retrieval (Fig. 5), confirming previous results that show the rate of endocytosis is closely tied to Ca2+ influx into the synaptic terminal (Yao et al., 2009). However, our findings point to a negative regulation of synaptic vesicle retrieval by Ca2+, which is consistent with earlier reports (von Gersdorff and Matthews, 1994; Wan et al., 2010). Previous work has suggested that Ca2+/calmodulin-dependent phosphatase, calcineurin, acts as a key regulator of synaptic vesicle endocytosis through dephosphorylation of endocytic proteins (Marks and McMahon, 1998; Wu et al., 2009; Sun et al., 2010). Our results indicate that calcineurin may indeed play a key role in transducing the Ca2+ signal to the endocytic machinery, thus slowing down single-vesicle retrieval, although its actual molecular target in this process remains to be determined (Fig. 6).
We found that synapses with higher release probability show an elevated propensity for multivesicular fusion and slower single-vesicle retrieval kinetics (Fig. 4A–C). In addition, we found that multivesicular fusion events were slower to be retrieved than single-vesicle events (Fig. 4D). This observation suggests that exocytic load (i.e., the number of vesicles fused with the plasma membrane) may exert a direct influence on the rate of retrieval. However, as Ca2+ appears to slow retrieval time regardless of Pr in low-release-probability boutons, this possibility does not preclude a strong direct impact of Ca2+ on vesicle retrieval kinetics (compare Fig. 4A,B).
Endocytosis progressively slows during higher-frequency stimulation
Is the observed slowing of vesicle retrieval at elevated Ca2+ concentrations significant in the context of physiological higher-frequency stimulation? We recorded fluorescence waveforms during a 10 AP train delivered at 1 Hz and reproduced the observed waveform using the summation of theoretical ideal single-vesicle events (Fig. 7). We reasoned that because a 1 Hz stimulus is faster than presumed terminal Ca2+ clearance (∼1.1 s; Regehr et al., 1994), endocytosis would slow with concomitant internal Ca2+ buildup. Indeed, reconstructing experimental fluorescent traces obtained from 1 Hz stimulations required that each subsequent fusion event be retrieved slower than the previous one (Fig. 7B,C). At near-physiological extracellular Ca2+, each successive retrieval time course had to be 15–40% slower than the previous, at physiological Ca2+. At 4 mm extracellular Ca2+, single-vesicle retrieval was already slower than at physiological Ca2+, and therefore, the gradual decrease in endocytosis rate was less significant, 13–33%. At 8 mm extracellular Ca2+ this progressive decrease in the rate of endocytosis (−0.5 to 9%) was near saturation, suggesting a lower limit to the rate of single synaptic vesicle retrieval.
Together, our findings show that the rate of single-vesicle endocytosis is tightly controlled by presynaptic Ca2+ influx and subsequent calcineurin activation. Surprisingly, during low levels of activity, this Ca2+-dependent regulation acts in a negative manner, slowing the rate of vesicle retrieval. Accordingly, we find that during higher-frequency repetitive stimulation, gradual presynaptic Ca2+ accumulation slows the rate of vesicle retrieval. These findings underscore the role of neuronal Ca2+ buffering as a key regulator of endocytic synaptic vesicle retrieval rate, which may constitute a global, neuron-wide means to set the pace of synaptic vesicle recycling and neurotransmitter release (Armbruster and Ryan, 2011). Therefore, these results go beyond the proposal that synaptic vesicle retrieval is indeed fast, and they provide a basis where earlier seemingly contradictory results can be reconciled in a single coherent scheme.
Footnotes
This work is supported by a grant from the National Institute of Mental Health (R01MH066198) to E.T.K. as well as the Cellular Biophysics of the Neuron Training Program at University of Texas Southwestern Medical Center T32 NS069562 (J.L.). E.T.K. is an Established Investigator of the American Heart Association. We thank Drs. Robert Edwards and Susan Voglmaier (University of California, San Francisco) for the original vGlut-pHluorin construct. We gratefully acknowledge Drs. Denise Ramirez and Mikhail Khvotchev for discussions and their critical insight during this project.
References
- Aravanis AM, Pyle JL, Tsien RW. Single synaptic vesicles fusing transiently and successively without loss of identity. Nature. 2003;423:643–647. doi: 10.1038/nature01686. [DOI] [PubMed] [Google Scholar]
- Armbruster M, Ryan TA. Synaptic vesicle retrieval time is a cell-wide rather than individual-synapse property. Nat Neurosci. 2011;14:824–826. doi: 10.1038/nn.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atluri PP, Ryan TA. The kinetics of synaptic vesicle reacidification at hippocampal nerve terminals. J Neurosci. 2006;26:2313–2320. doi: 10.1523/JNEUROSCI.4425-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaji J, Ryan TA. Single-vesicle imaging reveals that synaptic vesicle exocytosis and endocytosis are coupled by a single stochastic mode. Proc Natl Acad Sci U S A. 2007;104:20576–20581. doi: 10.1073/pnas.0707574105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollmann JH, Sakmann B, Borst JG. Calcium sensitivity of glutamate release in a calyx-type terminal. Science. 2000;289:953–957. doi: 10.1126/science.289.5481.953. [DOI] [PubMed] [Google Scholar]
- Bowser DN, Khakh BS. Two forms of single-vesicle astrocyte exocytosis imaged with total internal reflection fluorescence microscopy. Proc Natl Acad Sci U S A. 2007;104:4212–4217. doi: 10.1073/pnas.0607625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton EL, Evans GJ, Cousin MA. Bulk synaptic vesicle endocytosis is rapidly triggered during strong stimulation. J Neurosci. 2008;28:6627–6632. doi: 10.1523/JNEUROSCI.1445-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin MA, Robinson PJ. Ca2+ influx inhibits dynamin and arrests synaptic vesicle endocytosis at the active zone. J Neurosci. 2000;20:949–957. doi: 10.1523/JNEUROSCI.20-03-00949.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deák F, Shin OH, Kavalali ET, Südhof TC. Structural determinants of synaptobrevin 2 function in synaptic vesicle fusion. J Neurosci. 2006;26:6668–6676. doi: 10.1523/JNEUROSCI.5272-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge FA, Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967;193:419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertunc M, Sara Y, Chung C, Atasoy D, Virmani T, Kavalali ET. Fast synaptic vesicle reuse slows the rate of synaptic depression in the CA1 region of hippocampus. J Neurosci. 2007;27:341–354. doi: 10.1523/JNEUROSCI.4051-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Chacón R, Königstorfer A, Gerber SH, García J, Matos MF, Stevens CF, Brose N, Rizo J, Rosenmund C, Südhof TC. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;410:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- Gandhi SP, Stevens CF. Three modes of synaptic vesicular recycling revealed by single-vesicle imaging. Nature. 2003;423:607–613. doi: 10.1038/nature01677. [DOI] [PubMed] [Google Scholar]
- Granseth B, Odermatt B, Royle SJ, Lagnado L. Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses. Neuron. 2006;51:773–786. doi: 10.1016/j.neuron.2006.08.029. [DOI] [PubMed] [Google Scholar]
- Hosoi N, Holt M, Sakaba T. Calcium dependence of exo- and endocytotic coupling at a glutamatergic synapse. Neuron. 2009;63:216–229. doi: 10.1016/j.neuron.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Kavalali ET. Multiple vesicle recycling pathways in central synapses and their impact on neurotransmission. J Physiol. 2007;585:669–679. doi: 10.1113/jphysiol.2007.137745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavalali ET, Klingauf J, Tsien RW. Activity-dependent regulation of synaptic clustering in a hippocampal culture system. Proc Natl Acad Sci U S A. 1999;96:12893–12900. doi: 10.1073/pnas.96.22.12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki F, Hazen M, Ordway RW. Fast synaptic fatigue in shibire mutants reveals a rapid requirement for dynamin in synaptic vesicle membrane trafficking. Nat Neurosci. 2000;3:859–860. doi: 10.1038/78753. [DOI] [PubMed] [Google Scholar]
- Marks B, McMahon HT. Calcium triggers calcineurin-dependent synaptic vesicle recycling in mammalian nerve terminals. Curr Biol. 1998;8:740–749. doi: 10.1016/s0960-9822(98)70297-0. [DOI] [PubMed] [Google Scholar]
- Mintz IM, Sabatini BL, Regehr WG. Calcium control of transmitter release at a cerebellar synapse. Neuron. 1995;15:675–688. doi: 10.1016/0896-6273(95)90155-8. [DOI] [PubMed] [Google Scholar]
- Mozhayeva MG, Sara Y, Liu X, Kavalali ET. Development of vesicle pools during maturation of hippocampal synapses. J Neurosci. 2002;22:654–665. doi: 10.1523/JNEUROSCI.22-03-00654.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VN, De Camilli P. Cell biology of the presynaptic terminal. Annu Rev Neurosci. 2003;26:701–728. doi: 10.1146/annurev.neuro.26.041002.131445. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Sejnowski TJ, Stevens CF. Heterogeneous release properties of visualized individual hippocampal synapses. Neuron. 1997;18:599–612. doi: 10.1016/s0896-6273(00)80301-3. [DOI] [PubMed] [Google Scholar]
- Regehr WG, Delaney KR, Tank DW. The role of presynaptic calcium in short-term enhancement at the hippocampal mossy fiber synapse. J Neurosci. 1994;14:523–537. doi: 10.1523/JNEUROSCI.14-02-00523.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph S, Overstreet-Wadiche L, Wadiche JI. Desynchronization of multivesicular release enhances Purkinje cell output. Neuron. 2011;70:991–1004. doi: 10.1016/j.neuron.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan S, Ryan TA. Real-time measurements of vesicle-SNARE recycling in synapses of the central nervous system. Nat Cell Biol. 2000;2:197–204. doi: 10.1038/35008615. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan S, Ryan TA. Calcium accelerates endocytosis of vSNAREs at hippocampal synapses. Nat Neurosci. 2001;4:129–136. doi: 10.1038/83949. [DOI] [PubMed] [Google Scholar]
- Santos MS, Li H, Voglmaier SM. Synaptic vesicle protein trafficking at the glutamate synapse. Neuroscience. 2009;158:189–203. doi: 10.1016/j.neuroscience.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R, Neher E. Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature. 2000;406:889–893. doi: 10.1038/35022702. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Sun JY, Wu XS, Wu LG. Single and multiple vesicle fusion induce different rates of endocytosis at a central synapse. Nature. 2002;417:555–559. doi: 10.1038/417555a. [DOI] [PubMed] [Google Scholar]
- Sun J, Pang ZP, Qin D, Fahim AT, Adachi R, Südhof TC. A dual-Ca2+-sensor model for neurotransmitter release in a central synapse. Nature. 2007;450:676–682. doi: 10.1038/nature06308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Wu XS, Xu J, McNeil BD, Pang ZP, Yang W, Bai L, Qadri S, Molkentin JD, Yue DT, Wu LG. The role of calcium/calmodulin-activated calcineurin in rapid and slow endocytosis at central synapses. J Neurosci. 2010;30:11838–11847. doi: 10.1523/JNEUROSCI.1481-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori S, Holt M, Stenius K, Lemke EA, Grønborg M, Riedel D, Urlaub H, Schenck S, Brügger B, Ringler P, Müller SA, Rammner B, Gräter F, Hub JS, De Groot BL, Mieskes G, Moriyama Y, Klingauf J, Grubmüller H, Heuser J, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Voglmaier SM, Kam K, Yang H, Fortin DL, Hua Z, Nicoll RA, Edwards RH. Distinct endocytic pathways control the rate and extent of synaptic vesicle protein recycling. Neuron. 2006;51:71–84. doi: 10.1016/j.neuron.2006.05.027. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Matthews G. Inhibition of endocytosis by elevated internal calcium in a synaptic terminal. Nature. 1994;370:652–655. doi: 10.1038/370652a0. [DOI] [PubMed] [Google Scholar]
- Wan QF, Zhou ZY, Thakur P, Vila A, Sherry DM, Janz R, Heidelberger R. SV2 acts via presynaptic calcium to regulate neurotransmitter release. Neuron. 2010;66:884–895. doi: 10.1016/j.neuron.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienisch M, Klingauf J. Vesicular proteins exocytosed and subsequently retrieved by compensatory endocytosis are nonidentical. Nat Neurosci. 2006;9:1019–1027. doi: 10.1038/nn1739. [DOI] [PubMed] [Google Scholar]
- Wu W, Wu LG. Rapid bulk endocytosis and its kinetics of fission pore closure at a central synapse. Proc Natl Acad Sci U S A. 2007;104:10234–10239. doi: 10.1073/pnas.0611512104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XS, McNeil BD, Xu J, Fan J, Xue L, Melicoff E, Adachi R, Bai L, Wu LG. Ca(2+) and calmodulin initiate all forms of endocytosis during depolarization at a nerve terminal. Nat Neurosci. 2009;12:1003–1010. doi: 10.1038/nn.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Eguchi K, Saitoh N, von Gersdorff H, Takahashi T. Developmental shift to a mechanism of synaptic vesicle endocytosis requiring nanodomain Ca2+ Nat Neurosci. 2010;13:838–844. doi: 10.1038/nn.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao CK, Lin YQ, Ly CV, Ohyama T, Haueter CM, Moiseenkova-Bell VY, Wensel TG, Bellen HJ. A synaptic vesicle-associated Ca2+ channel promotes endocytosis and couples exocytosis to endocytosis. Cell. 2009;138:947–960. doi: 10.1016/j.cell.2009.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Li Y, Tsien RW. The dynamic control of kiss-and-run and vesicular reuse probed with single nanoparticles. Science. 2009a;323:1448–1453. doi: 10.1126/science.1167373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Li Y, Tsien RW. Response to comment on “the dynamic control of kiss-and-run and vesicular reuse probed with single nanoparticles.”. Science. 2009b;325:1499. doi: 10.1126/science.1167373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Xu J, Heinemann SF. Two pathways of synaptic vesicle retrieval revealed by single-vesicle imaging. Neuron. 2009;61:397–411. doi: 10.1016/j.neuron.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]