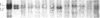Abstract
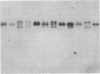
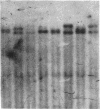
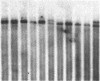
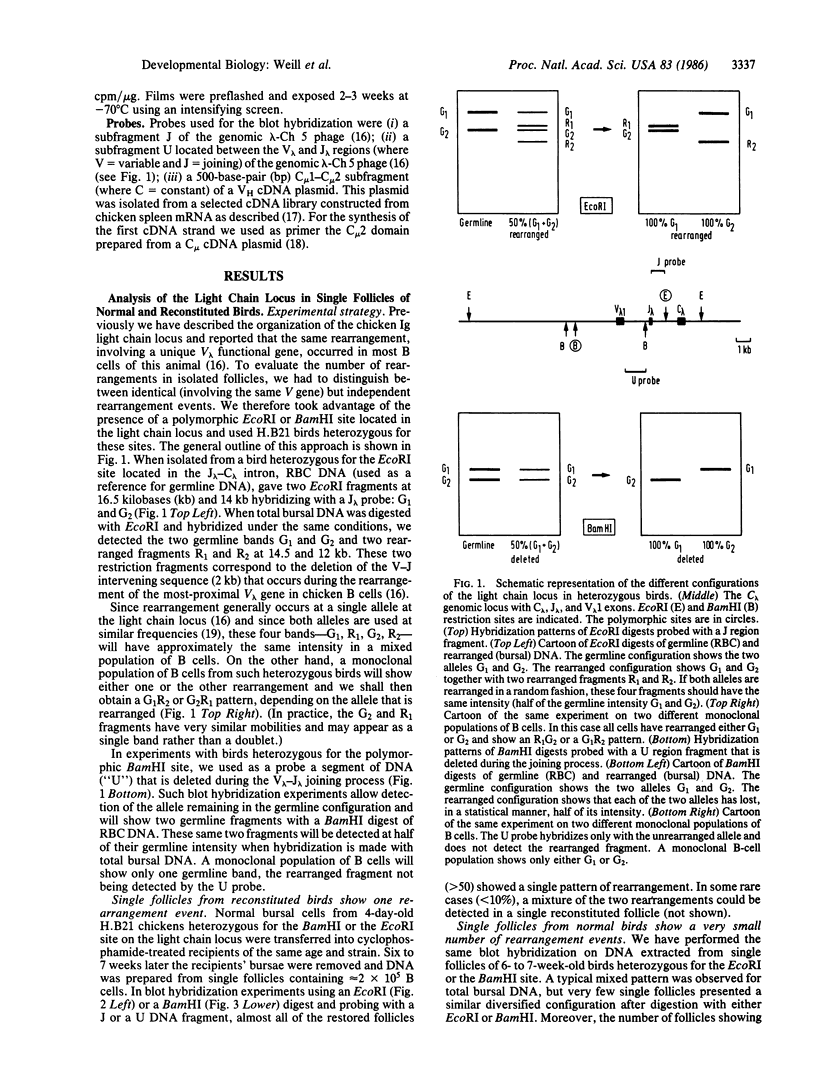
We report a molecular analysis of the chicken Ig loci in single bursal follicles from 3- to 7-week-old chickens. Each follicle contained between 10(5) and 3 X 10(5) cells. The Ig gene rearrangement patterns obtained were compared to the pattern observed with the corresponding total bursal DNA. The results obtained for the light chain locus imply that a very small number (two on average) of rearrangement events takes place in each follicle. For the heavy chain locus similar results were obtained, each follicle showing a more restricted pattern than the total bursa. These data favor a model in which each follicle is colonized by a very few prebursal stem cells that are committed to a particular Ig gene rearrangement at the very beginning of the development of the embryonic bursa. The role of the bursa as the organ in which such a committed stem cell population for the B-cell lineage arises is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson S., Miller R. G., Phillips R. A. The identification in adult bone marrow of pluripotent and restricted stem cells of the myeloid and lymphoid systems. J Exp Med. 1977 Jun 1;145(6):1567–1579. doi: 10.1084/jem.145.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt F. W., Yancopoulos G. D., Blackwell T. K., Wood C., Thomas E., Boss M., Coffman R., Rosenberg N., Tonegawa S., Baltimore D. Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J. 1984 Jun;3(6):1209–1219. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles W. E., Bumstead N., Ewert D. L., Gilmour D. G., Gogusev J., Hála K., Koch C., Longenecker B. M., Nordskog A. W., Pink J. R. Nomenclature for chicken major histocompatibility (B) complex. Immunogenetics. 1982;15(5):441–447. doi: 10.1007/BF00345903. [DOI] [PubMed] [Google Scholar]
- Coleclough C., Perry R. P., Karjalainen K., Weigert M. Aberrant rearrangements contribute significantly to the allelic exclusion of immunoglobulin gene expression. Nature. 1981 Apr 2;290(5805):372–378. doi: 10.1038/290372a0. [DOI] [PubMed] [Google Scholar]
- Dahan A., Reynaud C. A., Weill J. C. Nucleotide sequence of the constant region of a chicken mu heavy chain immunoglobulin mRNA. Nucleic Acids Res. 1983 Aug 25;11(16):5381–5389. doi: 10.1093/nar/11.16.5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick J. E., Magli M. C., Huszar D., Phillips R. A., Bernstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell. 1985 Aug;42(1):71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- Fleischman R. A., Mintz B. Development of adult bone marrow stem cells in H-2-compatible and -incompatible mouse fetuses. J Exp Med. 1984 Mar 1;159(3):731–745. doi: 10.1084/jem.159.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossi C. E., Velardi A., Cooper M. D. Postnatal liver hemopoiesis in mice: generation of pre-B cells, granulocytes, and erythrocytes in discrete colonies. J Immunol. 1985 Oct;135(4):2303–2311. [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Herzenberg L. A., Herzenberg L. A. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985 Jun 1;161(6):1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houssaint E., Belo M., Le Douarin N. M. Investigations on cell lineage and tissue interactions in the developing bursa of Fabricius through interspecific chimeras. Dev Biol. 1976 Oct 15;53(2):250–264. doi: 10.1016/0012-1606(76)90227-x. [DOI] [PubMed] [Google Scholar]
- Keller G., Paige C., Gilboa E., Wagner E. F. Expression of a foreign gene in myeloid and lymphoid cells derived from multipotent haematopoietic precursors. Nature. 1985 Nov 14;318(6042):149–154. doi: 10.1038/318149a0. [DOI] [PubMed] [Google Scholar]
- Kurland J. I., Ziegler S. F., Witte O. N. Long-term cultured B lymphocytes and their precursors reconstitute the B-lymphocyte lineage in vivo. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7554–7558. doi: 10.1073/pnas.81.23.7554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassila O., Eskola J., Toivanen P., Dieterlen-Lièvre F. Lymphoid stem cells in the intraembryonic mesenchyme of the chicken. Scand J Immunol. 1980;11(4):445–448. doi: 10.1111/j.1365-3083.1980.tb00011.x. [DOI] [PubMed] [Google Scholar]
- Le Douarin N. M., Houssaint E., Jotereau F. V., Belo M. Origin of hemopoietic stem cells in embryonic bursa of Fabricius and bone marrow studied through interspecific chimeras. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2701–2705. doi: 10.1073/pnas.72.7.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottenburg C., Weissman I. L. Cmu gene rearrangement of mouse immunoglobulin genes in normal B cells occurs on both the expressed and nonexpressed chromosomes. Proc Natl Acad Sci U S A. 1981 Jan;78(1):484–488. doi: 10.1073/pnas.78.1.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olah I., Glick B. The number and size of the follicular epithelium (FE) and follicles in the bursa of Fabricius. Poult Sci. 1978 Sep;57(5):1445–1450. doi: 10.3382/ps.0571445. [DOI] [PubMed] [Google Scholar]
- Paige C. J., Kincade P. W., Moore M. A., Lee G. The fate of fetal and adult B-cell progenitors grafted into immunodeficient CBA/N mice. J Exp Med. 1979 Sep 19;150(3):548–563. doi: 10.1084/jem.150.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige C. J., Kincade P. W., Shinefeld L. A., Sato V. L. Precursors of murine B lymphocytes. Physical and functional characterization, and distinctions from myeloid stem cells. J Exp Med. 1981 Jan 1;153(1):154–165. doi: 10.1084/jem.153.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios R., Steinmetz M. Il-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell. 1985 Jul;41(3):727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- Pink J. R., Ratcliffe M. J., Vainio O. Immunoglobulin-bearing stem cells for clones of B (bursa-derived) lymphocytes. Eur J Immunol. 1985 Jun;15(6):617–620. doi: 10.1002/eji.1830150616. [DOI] [PubMed] [Google Scholar]
- Pink J. R., Vainio O., Rijnbeek A. M. Clones of B lymphocytes in individual follicles of the bursa of Fabricius. Eur J Immunol. 1985 Jan;15(1):83–87. doi: 10.1002/eji.1830150116. [DOI] [PubMed] [Google Scholar]
- Ratcliffe M. J., Ivanyi J. Allotype suppression in the chicken. IV. Deletion of B cells and lack of suppressor cells during chronic suppression. Eur J Immunol. 1981 Apr;11(4):306–310. doi: 10.1002/eji.1830110408. [DOI] [PubMed] [Google Scholar]
- Ratcliffe M. J., Lassila O., Pink J. R., Vainio O. Avian B cell precursors: surface immunoglobulin expression is an early, possibly bursa-independent event. Eur J Immunol. 1986 Feb;16(2):129–133. doi: 10.1002/eji.1830160204. [DOI] [PubMed] [Google Scholar]
- Reth M. G., Ammirati P., Jackson S., Alt F. W. Regulated progression of a cultured pre-B-cell line to the B-cell stage. 1985 Sep 26-Oct 2Nature. 317(6035):353–355. doi: 10.1038/317353a0. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Anquez V., Dahan A., Weill J. C. A single rearrangement event generates most of the chicken immunoglobulin light chain diversity. Cell. 1985 Feb;40(2):283–291. doi: 10.1016/0092-8674(85)90142-4. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Dahan A., Weill J. C. Complete sequence of a chicken lambda light chain immunoglobulin derived from the nucleotide sequence of its mRNA. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4099–4103. doi: 10.1073/pnas.80.13.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Toivanen P., Toivanen A. Bursal and postbursal stem cells in chicken. Functional characteristics. Eur J Immunol. 1973 Sep;3(9):585–595. doi: 10.1002/eji.1830030912. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. M., Till J. E., Siminovitch L., McCulloch E. A. Cytological evidence for a relationship between normal hemotopoietic colony-forming cells and cells of the lymphoid system. J Exp Med. 1968 Mar 1;127(3):455–464. doi: 10.1084/jem.127.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]