Abstract
pMF1 is the only autonomously replicating plasmid that has been recently identified in myxobacteria. This study characterized the partitioning (par) system of this plasmid. The fragment that significantly increased the retaining stability of plasmids in Myxococcus cells in the absence of selective antibiotics contained three open reading frames (ORFs) pMF1.21-pMF1.23 (parCAB). The pMF1.22 ORF (parA) is homologous to members of the parA ATPase family, with the highest similarity (56%) to the Sphingobium japonicum ParA-like protein, while the other two ORFs had no homologs in GenBank. DNase I footprinting and electrophoretic mobility shift assays showed that the pMF1.23 (parB) product is a DNA-binding protein of iteron DNA sequences, while the product of pMF1.21 (parC) has no binding activity but is able to enhance the DNA-binding activity of ParB to iterons. The ParB protein autogenously repressed the expression of the par genes, consistent with the type Ib par pattern, while the ParC protein has less repressive activity. The ParB-binding iteron sequences are distributed not only near the partitioning gene loci but also along pMF1. These results indicate that the pMF1 par system has novel structural and functional characteristics.
Introduction
Myxobacteria are well known for their unique multicellular social behavior [1] and the production of diverse and novel bioactive secondary metabolites [2]. However, autonomously replicating plasmids have not been reported until recently. In 2008, we reported the discovery of the first and as yet only endogenous myxobacterial plasmid, pMF1, which was isolated from Myxococcus fulvus strain 124B02 [3]. Except for a few ORFs that are highly homologous to those in myxobacterial genomes, most ORFs in the Myxococcus plasmid pMF1 do not have homologs in the GenBank database, including the replication-associated sequence. We localized the replication region to the pMF1.14 ORF using a vector that cannot replicate in M. xanthus, and shuttle vectors between Escherichia coli and M. xanthus were thus successfully constructed [3]. Because they lack a stabilizing sequence, these low-copy-number shuttle vectors are not stably inherited in M. xanthus and are easily lost in the absence of selective antibiotics.
Low-copy-number plasmids are dependent on plasmid-encoded partitioning (par) systems for stable segregation into daughter cells [4]. The par loci of a plasmid typically consist of an operon containing an autogenously regulated gene pair, parA and parB [5], [6], [7], [8], [9], and one or more centromere-like iteron regions [5], [10], [11]. ParA is an ATPase (other two classes of motor proteins are GTPases ParM and TubZ) involved in the segregation of plasmids or chromosomes [12], [13], [14], [15], while ParB is a DNA-binding protein that recognizes specific iteron sequences of the centromere-like sites that are normally located upstream or downstream in the par loci [16], [17], [18]. In general, the two plasmid-encoded trans-acting partitioning proteins and the cis-acting centromeric site are all essential for stable segregation of plasmid. The plasmid partitioning systems have been identified into three types. The type I par loci encode an ATPase containing Walker motifs, while types II and III encode actin-like and tubulin-like proteins, respectively [4], [19]. Type I par system is further divided into two sub-groups, designated type Ia and type Ib, based on the regulation manner of the operon, the properties of ParB, and the location of the cis-acting sites [4]. In the type Ia par system, represented by P1 and F plasmids, the sizes of ParA and ParB proteins are 321–420aa and 312–342aa, respectively [4]. The Ia ParA contains an N-terminal DNA–binding domain, which plays an autoregulation role for the transcription of operon. In contrast to the type Ia par system, the type Ib, represented by pTAR, TP228, pB171 and pSM19035, encodes a ParA protein of 192–308aa, lacking the N-terminal DNA–binding domain [4], [20], [21], [22], [23]. The ParB protein of this sub-group is 46–131aa in size, and is able to bind to the par operon promoter and regulates the transcription of the operon [4].
In this work, the pMF1 partitioning system region was screened using pZJY41, a shuttle plasmid containing the replication region of pMF1, and was able to replicate in Myxococcus cells. The partitioning system, containing three ORFs, pMF1.21-pMF1.23, was characterized. The results showed that the pMF1 partitioning system has novel characteristics with respect to its special organization, functioning patterns and dispersed distribution of the cis-acting centromeric sequences.
Results
Isolation of the partition-associated region of plasmid pMF1
The shuttle vector pZJY41 can replicate in M. xanthus DZ1 but contains no additional stabilizing sequence, and is thus easily lost in Myxococcus cells during the subculture in the absence of selective antibiotics [3]. To screen the partitioning system region of pMF1, we established a small library of pMF1 fragments, and inserted these fragments into the pZJY41 vector, respectively (Table 1). Unpublished data suggest that the shuttle plasmid pZJY41 is sometimes structurally unstable in Myxococcus cells when a fragment that is larger than 8 kb is inserted. Thus, the pMF1 insertion fragments were all between 1.5 kb and 8 kb. The constructed plasmids were transformed into M. xanthus DZ1. Electrophoresis results demonstrated that none of the plasmids became smaller, suggesting that they were structurally stable in DZ1.
Table 1. Bacterial strains and plasmids used in this study.
| Designation | Genotype or descriptiona | Source or reference |
| Strains | ||
| M. fulvus 124B02 | wild type strain, possessing pMF1 plasmid | 3 |
| M. xanthus DZ1 | nonmotile, nonfruiting, dispersed-growing | D. R. Zusman, University of California, Berkeley |
| E. coli DH5α | supE44, ΔlacU169 (ϕ80lacZΔM15), hsdR17, recA1, endA1, gyrA96, thi-1, relA1 | Life Technologies Inc. |
| Plasmids | ||
| pMF1 | a cryptic plasmid from M. fulvus 124B02, 18.634 kb | 3 |
| pSQ37 | Ampr, pSP72 with the insertion of a 10.8-kb EcoRI fragment of pMF1(16382-8545), 13.2 kb | This study |
| pSQ38 | Ampr, pSP72 with the insertion of a 7.8-kb EcoRI fragment of pMF1(8545-16382), 10.2 kb | This study |
| pZJY41 | shuttle plasmid from a DZ1 transformant of pZJY7 | 3 |
| pXS1 | pZJY41 with the insertion of a 3.8-kb SacII fragment of pSQ37(3680-7410 of pMF1) | This study |
| pXS2 | pZJY41 with the insertion of a 4.9-kb SacII fragment of pSQ37(17270-3680 of pMF1) | This study |
| pXS3 | pZJY41 with the insertion of a 3.2-kb EcoRI-NsiI fragment of pSQ37(16382-971 of pMF1) | This study |
| pXS4 | pZJY41 with the insertion of a 7.5-kb NsiI-EcoRI fragment of pSQ37 (971-8545 of pMF1) | This study |
| pXS5 | pZJY41 with the insertion of a 3.7-kb EcoRI-PvuI fragment of pSQ38 (8525-12280 of pMF1) | This study |
| pXS6 | pZJY41 with the insertion of a 4.1-kb PvuI-EcoRI fragment of pSQ38 (12280-16382 of pMF1) | This study |
| pXS7 | pZJY41 with the insertion of a 1.5-kb EcoRI-BstEII fragment of pSQ38 (8545-10132 of pMF1) | This study |
| pXS8 | pZJY41 with the insertion of a 6.2-kb BstEII- EcoRI fragment of pSQ38 (10132-16382 of pMF1) | This study |
| pXS11 | pZJY41 with the insertion of a 2.5-kb fragment from pMF1(17242-50) | This study |
| pXS12 | pZJY41 with the insertion of a 2.5-kb fragment from pMF1(17242-1136) | This study |
| pXS13 | Ampr, Kmr, pXS11 with the deletion of a pMF1.21 | This study |
| pXS14 | Ampr, Kmr, pXS11 with the deletion of a pMF1.22 | This study |
| pXS15 | Ampr, Kmr, pXS11 with the deletion of a pMF1.23 | This study |
| pXS16 | Ampr, Kmr, pXS11 with the deletion of pMF1.22 and pMF1.23 | This study |
| pET15b | Expression vector | Novagen |
| p15b21 | Ampr, pMF1.21 cloned into pET15b | This study |
| P15b23 | Ampr, pMF1.23 cloned into pET15b | This study |
Depending on the inserted fragment, the inheritance stability of these plasmids in DZ1 varied greatly (Fig. S1). Compared to pZJY41, the plasmids pXS2 (containing nt 17270 to 3680 of pMF1) and pXS3 (containing nt 16382 to 971) were highly stable in DZ1. In the absence of the selective antibiotic kanamycin, colonies harboring the pXS3 and pXS2 plasmids comprised respectively 86% and 60% of the bacterial population after 48 h (approximately 12 generations; the double time of DZ1 is 4 h, ref 40) and 77% and 57% of the bacterial population after 120 h (approximately 30 generations), whereas the pZJY41-carrying colonies comprised <25% of the total population after 48 h and <10% after 120 h. These results suggest that the inserted fragments in pXS3 and pXS2 contained the partitioning system of pMF1.
Structural characteristics of the partitioning system of pMF1
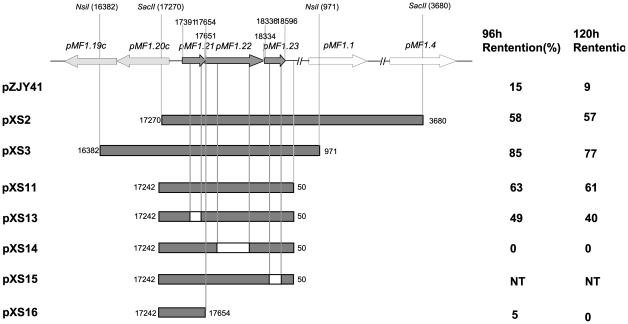
The common region of the fragments inserted in plasmids pXS2 and pXS3 was identified to be nt 17270 to 971 of pMF1, which contained three ORFs, pMF1.21, pMF1.22 and pMF1.23 (Fig. 1). It is noted that although the presence of pMF1.20 gave a further increase to the stability of plasmid (pXS2 vs pXS3, 40% improved stabilization), the gene has a reverse transcription direction to the above three gene operon, and was thus probably not included in the partitioning system. However, pMF1.20 or together with pMF1.19, may play a role on plasmid maintenance. The products of both pMF1.19 and pMF1.20 have high similarities to M. xanthus DK1622 chromosome genes MXAN6992 and MXAN 6330 [3].
Figure 1. Schematic representation of pXS2, pXS3 and the in-frame deletion regions in pMF1.21, pMF1.22 and pMF1.23 and their influence on the stability of the plasmids.
The white portion within the gray bar stands for the deletion of the corresponding genes in pXS13, pXS14 and pXS15. The corresponding stability of these plasmids in M. xanthus after growth for 96 h and 120 h without selective pressure is indicated on the right. NT, not tested.
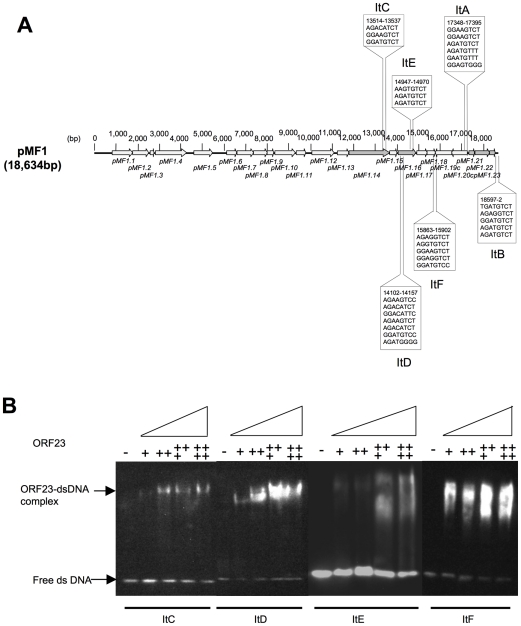
The product of pMF1.22 has extensive homology to the ParA family of ATPases, with the highest similarity of 56% to the amino acid sequence of the Sphingobium japonicum uncharacterized ParA-like protein. Amino acid sequence alignment analysis showed that the pMF1.22 protein contains the four conserved motifs of type I ParA proteins (Fig. 2A), which belong to the Walker-type ATPases [4]. It was thus suggested that the unknown pMF1.22 is a ParA-like protein, and the pMF1.22 gene was designated parA. To determine the function of this gene in vitro, we attempted to express the pMF1.22 gene in E. coli using several different hosts and vectors but did not obtain soluble product (data not shown). However, following genetic analyses indicated that the pMF1.22 gene was essential for stably partitioning of plasmids in Myxococcus cells (see below). The other two ORFs, pMF1.21 and pMF1.23, had no significant similarity to any entries in GenBank. These three ORFs were suggested to be the potential components of the partitioning system of the plasmid pMF1. The neighboring upstream and downstream sequences of these three genes contained some imperfect repeats, designated iterons ItA and ItB, respectively. In addition, there were several copies of “long repeats” (underlined) immediately downstream of the ItB sequence (Fig. 2B).
Figure 2. Genetic organization of the par loci of pMF1.
(A) Alignment of the amino acid sequences encoded by pMF1 pMF1.22 and other parA genes of other par systems. Database accession numbers: Myxocuccus fulvus pMF1 ParA, ABX46805; Agrobacterium tumefaciens pTiC58 RepA, AAF00012; A. tumefaciens pTAR ParA, P07175; A. rhizogenes pRiA4b VirC1, P05682; Bacilus subtilis MinD, Q01464; E. coli F SopA, P08866; E. coli RK2 IncC, P07673; Enterobacteria phage N15 ParA, AA19064; Enterobacteria phage P1 ParA, P07620; Mycobacterium celatum pCLP ParA-like protein, AAD42964; Pseudomonas alcaligenes pRA2 ParA, AAD40334; and Salmonella newport TP228 ParF, AAF74217. The framed boxes labeled ATP I and ATP II represent the ATPase motif regions, while those marked Motif III and Motif IV represent the additional conserved motifs in ParA family ATPases. The consensus amino acids are shaded. This figure was adapted from Motallebi-Vershareh et al, 1990 [32]. (B) The genes pMF1.19 to pMF1.1 of pMF1. The arrows indicate the coding genes and their transcriptional orientation. The three black arrows are the pMF1.21 through pMF1.23 genes in the pMF1 par operon. Nucleotide sequences of the region upstream (nt 17309 to 17566) and downstream (nt 18596 to 971) of the par operon are shown. The iterons ItA and ItB are bold and underlined and the “long repeats” are underlined.
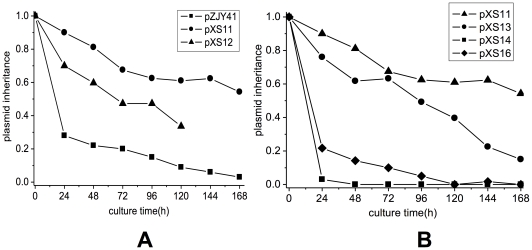
To identify the components of the hypothesized pMF1 partitioning system, we constructed plasmids pXS11 and pXS12 carrying parts of the above common region; pXS11 contained all three genes and the ItA and ItB iterons, while pXS12 contained the same components and the “long repeat” downstream of ItB (Fig. 2B). Similar to pXS2 and pXS3, the plasmids pXS11 and pXS12 were also highly inheritably stable in M. xanthus DZ1 (Fig. 3A). After 120 h of incubation without antibiotic selection, plasmids pXS11 and pXS12 were retained in 61% and 34% of the bacterial population. The “long repeat” in pXS12 seemed to have small negative effects on plasmid stabilization. We concluded that the fragment in pXS11 containing the pMF1.21, pMF1.22 and pMF1.23 ORFs and the ItA and ItB iterons constituted the partitioning cassette of pMF1 (Fig. 1).
Figure 3. The inheritance stability of the plasmids carrying different fragments of pMF1 in M. xanthus DZ1 in the absence of the selective antibiotic kanamycin (panels A and B).
The cultures grown in CTT medium were analyzed for plasmid retention at 24 h intervals until 168 h. The data presented are the average of two independent experiments.
Essentiality of pMF1.21, pMF1.22 and pMF1.23 in plasmid partitioning in Myxococcus
Bioinformatics analysis suggested that the pMF1.21-pMF1.23 genes were in the same transcriptional unit, which was further confirmed using reverse transcription PCR of the cDNA from M. xanthus DZ1 transformants of pXS11 using the primer pair of parC-up and parB-down (Table 2; detailed results not shown). This suggested that the pMF1 partitioning system contained three genes. However, the partitioning system of a plasmid typically contains two genes, parA and parB. To determine the essentiality of the three genes for plasmid segregation, we made in-frame deletion mutations in pMF1.21, pMF1.22 and pMF1.23, respectively, producing pXS13, pXS14 and pXS15 (Fig. 1). These plasmids were then transferred into the M. xanthus DZ1 strain, respectively. The pXS13 and pXS14 plasmids extracted from the M. xanthus DZ1 transformants were structurally stable, while pXS15 yielded only one positive clone, which, however, became smaller than the original size. This result suggested that the pMF1.23 gene played an essential role in the maintenance of the plasmid and the mutation in pMF1.23 was lethal to the Myxococcus cells that harbored the plasmid.
Table 2. Oligonucleotides used in this study.
| Primer | Sequence | pMF1 coordinates |
| par-up | 5′ccggatccCGCATCGGGTGAGCGTAGAG3′ | 17242-17261 |
| par-down1 | 5′ccggaattcCCCACCCCAAAACGACAAGAGG3′ | 50-29 |
| par-down2 | 5′ccggaattcTCGCCGCAGCTTCAGCCCTC3′ | 1136-1117 |
| *parCmu-up2 | 5′CGACTCGCTCATTTCCGACCC3′ | 17427-17447 |
| *parCmu-down1 | 5′GGGTCGGAAATGAGCGAGTCGATGGTCGCGCAGGCGGTGGAG3′ | 17427-17447 17610-17630 |
| *parAmu-up2 | 5′TTCGCCAGCTCCACCGCCTGC3′ | 17618-17638 |
| *parAmu-down1 | 5′GCAGGCGGTGGAGCTGGCGAAGTCCTCTCCGCCTCAACCACC3′ | 17618-17638 18146-18166 |
| *parBmu-up2 | 5′AACCTCGGCCGGCGGGGCACG3′ | 18372-18392 |
| *parBmu-down1 | 5′CGTGCCCCGCCGGCCGAGGTTCAAATTGCCGAGGAGTTGTTCGAC3′ | 18372-18392 18555-18578 |
| **parC-u: | 5′gggaattc CATATGGGCGGGCGTCACAGGG3′ | 17392-17409 |
| **parC-d: | 5′cgcggatcc TCATCGGCCCGCCTTCTTCGC3′ | 17634-17654 |
| **parB-u: | 5′gggaattc CATATGCGTGCCCCGCCGGCCGA3′ | 17370-17388 |
| **parB-d: | 5′cgcggatcc CTACTTGGGGAGATACTTGTCGA3′ | 17574-17596 |
| DupupBiotin | 5′CGCATCGGGTGAGCGTAGAG3′ | 17242-17261 |
| Dupdo | 5′CGACTCGCTCATTTCCGACCC3′ | 17428-17447 |
| ItA | 5′GGAAGTCTGGAAGTCTAGATGTCTAGATGTTTGAATGTTTGGAGTGGG3′ | 17348-17395 |
| ItB | 5′GTGATGTCTAGAGGTCTGGATGTCTAGATGTCTAGATGTCT3′ | 18596-3 |
| ItC | 5′AGACATCTGGAAGTCTGGATGTC3′ | 13514-13537 |
| ItD | 5′AGAAGTCCAGACATCTGGACATTCAGAAGTCTAGACATCTGGATGTCCAGATGGGG 3′ | 14102-14157 |
| ItE | 5′AAGTGTCTAGATGTCTAGATGTCT3′ | 14947-14970 |
| ItF | 5′AGAGGTCTAGGTGTCTGGAAGTCTGGAGGTCTGGATGTC3′ | 15863-15902 |
| ***parC-up | 5′GGAAGAGGTCAGGGCGGCAAAG3′ | 17480-17501 |
| ***parC-down | 5′GCCTTCTTCGCCAGCTCCAC3′ | 17625-17644 |
| ***parA-up | 5′ATGATTGTCGCGGTCGTGTCCC3′ | 17651-17672 |
| ***parA-down | 5′TCCGCATCCACCACCAGCACG3′ | 17743-17763 |
| ***parB-up | 5′TCCGAGCGACCCTCAACTTG3′ | 18449-18468 |
| ***parB-down | 5′CCTCGGCAATTTGGGAGTGTTC3′ | 18546-18567 |
| 16S-up | 5′CGCCGTAAACGATGAGAA3′ | |
| 16S-down | 5′TTGCGT CGAATTAAACCAC3′ |
*primers used in gene deletion.
**primers used in gene expression in E.coli.
***primers used in quantitative PCR.
The inheritance of the pXS13, pXS14 and pXS16 (with the deletion of pMF1.22 and pMF1.23) plasmids in DZ1 is shown in Fig. 3B. The plasmid pXS14 (pMF1.22 deleted) had low stability without selective pressure. The retention of the plasmid was less than 5% after 6 generations (24 h) and 0% after 12 generations (48 h). Similar result was observed in pXS16 (pMF1.22 and pMF1.23 were both deleted) plasmid stability test, 0% after 42 generations (120 h) (Fig. 3B). The mutation in pMF1.21 (pXS13) alone also decreased the inheritance stability of the plasmid. Retention of the pXS13 plasmid in DZ1 was 49% after 24 generations (96 h) and 40% after 42 generations (120 h), which was still higher than that of pZJY41 but lower than the intact par-containing plasmid pXS11. These results indicated that the three genes played different roles in stable plasmid partitioning. The parA-like gene (pMF1.22) and pMF1.23 genes are suggested to be essential, whereas pMF1.21 plays an accessory role for stably partitioning plasmids in Myxococcus cells.
The DNA-binding protein of the Myxococcus pMF1 par system
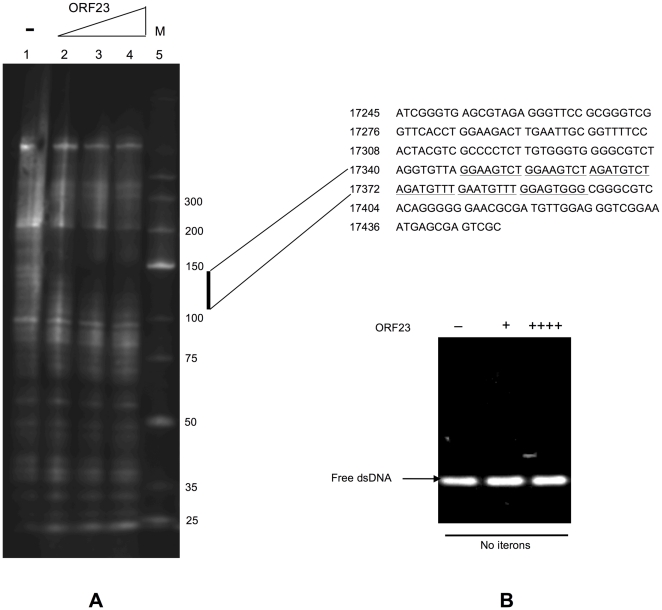
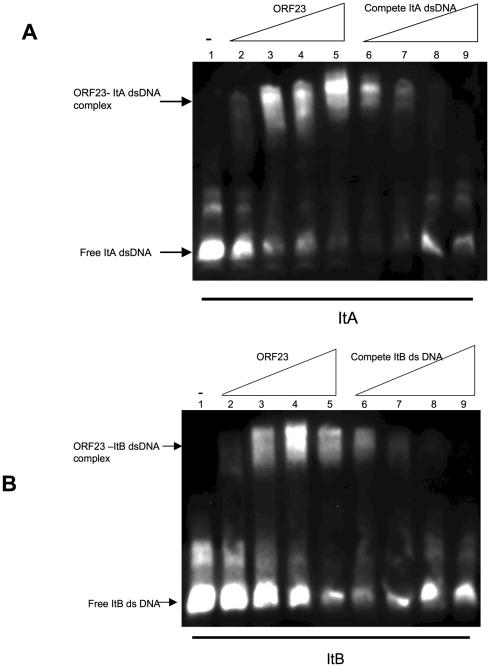
In a plasmid partitioning system, a ParB or ParB-like component is required to recognize and bind to the corresponding centromere-like sequences to form a nucleoprotein complex, which localizes at a particular position in cell and moves into daughter cells for plasmid segregation when cell division occurs [21], [23], [24], [25], [26], [27]. To determine if one or both of the pMF1.21 and pMF1.23 products were responsible for DNA binding, we expressed these two genes separately in E. coli and obtained soluble proteins. A DNase I footprint assay was used to assess the binding abilities of the purified pMF1.21 and pMF1.23 proteins to DNA sequences containing the upstream fragment with the putative repeat ItA (nt 17348–17395). The fragment (206 bp, see Fig. 4A) was biotin-labeled at the 5′ end of the coding strand. After incubation with sufficient pMF1.23 product, an approximately 100-bp sequence containing the putative iteron ItA (nt 17348 to 17447 of pMF1) was bound to the pMF1.23 protein, and protected from DNase degradation (Fig. 4A). This 100-bp sequence was then divided into two fragments, one of which contained only the ItA sequence. These two fragments were labeled separately with biotin at the 3′ end to serve as probes for an electrophoretic mobility shift assay (EMSA). The pMF1.23 protein bound to the ItA sequence (nt 17348–17395) (Fig. 5A) but not to the other fragment (nt 17396–17447) (Fig. 4B). The amount of the nucleoprotein complex increased with an increase in the concentration of the pMF1.23 product, and the addition of unlabeled ItA fragment was competitive, reducing the amount of labeled complex. To exclude possible effects of His-tag on the binding activities [28], we further constructed His-tag free pMF1.23 protein. Compared to the pMF1.23 protein with a His-tag, the protein without a His-tag had a similar binding affinity to ItA (Fig. S2), which suggested that the His-tag at the N-terminal did not affect the affinity of the protein. In contrast to pMF1.23, the pMF1.21 protein did not bind and thus did not protect either of the fragments (data not shown). The pMF1.21 and pMF1.23 genes were thus designated parC and parB, respectively. Because the product of the pMF1.23 gene is 75 amino acids long, the pMF1 par system probably belongs to type Ib (the ParB proteins of type Ia partition systems are much larger than that of type Ib, see reference 4).
Figure 4. DNA-binding activity of ORF23.
(A) DNase I footprinting analysis of the binding activity of ORF23 with the DNA fragment corresponding to nt 17242–17447 upstream of the par operon of pMF1. After incubation, the mixture was partially digested with DNase I and separated using denaturing gel electrophoresis. Lanes 1 to 4 contained 0, 250 ng, 1250 ng, and 3750 ng of ORF23, respectively. The ladder marker was in lane 5. The bar on the right indicates the tested DNA fragment containing the protected region of the iterons (underlined). (B) Electrophoretic mobility shift assays (EMSAs) showing the no DNA binding activity of ORF23 to non-iteron fragment nt 17395–17447. The concentration of labeled DNA used in each lane was 12 pmol. The amounts of purified ORF23 added in lanes 1 to 3 from left to right were 0 (−), 250 ng (+) and 1000 ng (++++), respectively.
Figure 5. EMSAs showing DNA binding activity of ORF23 to the ItA sequence nt 17348–17395 (A) and the ItB sequence nt 18580-3 (B).
The amount of purified ORF23 added in lanes 1 to 5 in (A) and (B) was 0 (−), 250 ng, 500 ng, 1000 ng, and 1500 ng, respectively. Increasing amounts of unlabeled DNA fragments (0, 0.8 pmol, 8 pmol, and 80 pmol) were incubated with 500 ng purified ORF23 protein in lanes 6 to 9 in (A) and (B), respectively.
Iteron sequences for plasmid partitioning and their distribution in pMF1
Because the sequences upstream and downstream in the par loci are both imperfect repeats (Fig. 3B), we investigated whether the repeat sequence (nt 18597-3) downstream of the par loci (ItB) was also a binding site for ParB. After labeling with biotin at the 3′ end, the probes were incubated with the pMF1.23 protein (ParB). The pMF1.23 product also bound to the ItB probe and formed DNA-protein complexes (Fig. 5B). Similarly, the amounts of the nucleoprotein complex increased with increasing pMF1.23 product concentration, and the addition of unlabeled ItB fragment was competitive, reducing the amount of the labeled complex of the ItB probe.
Compared to other plasmid partitioning systems, the iteron copy number in the pMF1 par operon was much lower (upstream ItA and downstream ItB). Further bioinformatics analysis of the pMF1 sequence revealed several additional centromere-like sites dispersed mainly between the replication-associated region (pMF1.14) and the partition loci, from nt 12511 to 3 of pMF1, including in the pMF1.14 and pMF1.16 ORFs (Fig. 6A). These sites, designated ItC, ItD, ItE, and ItF, as well as ItA and ItB contain repeat units similar to the motif of AGATGTCT, which is an imperfect complement repeat itself. The EMSAs indicated that ItC, ItD, ItE, and ItF were also specifically bound by the pMF1.23 product (Fig. 6B). Therefore, the iterons of pMF1 are distributed not only near the partitioning loci but also throughout the plasmid.
Figure 6. Iterons in the plasmid pMF1.
(A) Distribution of predicted iteron sequences in the plasmid pMF1. (B) The binding activity of ORF23 to ItC, ItD, ItE and ItF. The substrate DNA used is indicated at the bottom of the figure. The amounts of ORF23 added to each lane are indicated at the top of the figure as follows: 0 (−), 250 ng (+), 500 ng (++), 1000 ng (+++), 1500 ng (++++).
Autogenous regulation of expression of the par loci in pMF1
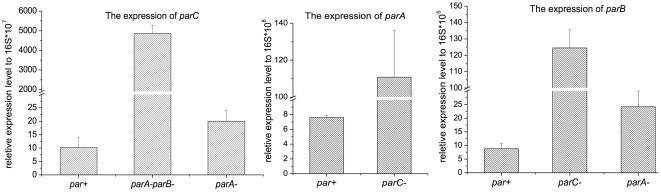
The par system is typically autogenously regulated by its products. In type Ia par loci, ParA is the regulator, while in type Ib and type II, the ParB protein regulates the expression of the partition-related proteins [4]. Because pXS15 (pMF1.23 mutated) is lethal for DZ1 cells harboring the plasmid, we constructed a par mutant containing only the promoter and the parC gene (pMF1.21) of the pMF1 par loci in the pZJY41 plasmid (designated pXS16) to determine the regulation pattern of the pMF1 par loci. Before quantify the expression profile, we assessed the copy numbers of the plasmids pZJY41, pXS11, pXS13 (parC −), pXS14 (parA −), and pXS16 (parA−parB −) by comparing the fluorescence intensities of the bands under UV light, which showed that the copy numbers of pXS11, pXS13 and pXS14 were similar to that of pZJY41, 10 to ∼20 in Myxococcus DZ1 [3]; whereas the plasmid pXS16 was some lower (about half the number of other plasmids from the band density on electrophoretic gel) (Fig. S3). Then the gene expression profile of pXS13 (parC −), pXS14 (parA −), and pXS16 (parA−parB −) was compared with that of pXS11 (par +) using quantitative RT-PCR (Fig. 7). The results revealed that the expression of parB and parC in pXS14 (parA −) was almost equivalent to that of pXS11 (par +) in the DZ1 strain, which indicates that the ParA protein did not regulate gene expression in the par loci. However, the expression of the parC gene in the parA−parB− mutant (pXS16) was greatly increased, at least 200 times higher than that in pXS11. Therefore, the pMF1.23 product (ParB) played an essential role in regulating the promoter activity of the par loci. This autogenous regulation pattern of pMF1 par loci is consistent with that of a type Ib partitioning system [4].
Figure 7. Quantitative PCR analysis.
The gene expression of parC, parA and parB of the deletion mutants of pXS13 (parC−), pXS14 (parA−) and pXS16 (parA−parB−) relative to that of the 16S gene and compared to that of the transformant of pXS11 (par+).
Function of the ParC protein
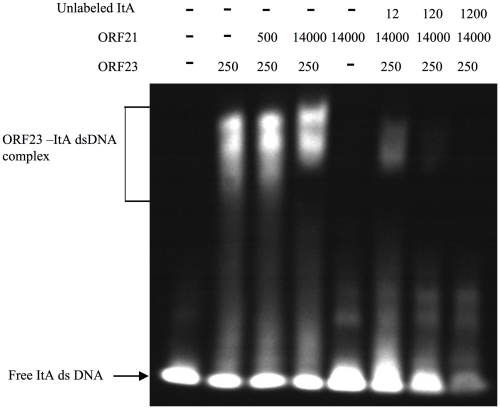
Compared to typical par systems, the pMF1 par loci contained an additional gene parC. Although the pMF1.21 product alone did not bind to the iteron fragments, the protein was able to enhance the DNA-binding activity of the pMF1.23 product (Fig. 8). If the amount of the pMF1.21 product was much higher than the pMF1.23 product (Fig. 8, lane 4), the enhancement was accordingly much greater than that when the two proteins were almost equally expressed (lane 3). The expression of the parA and parB genes in the parC mutant (pXS13) was also significantly increased, approximately 15 times higher than that in pXS11 (Fig. 7). The ParC protein thus had a repressive effect on the promoter of the par loci but much lower than that of the ParB protein. The ParC protein appears to play an accessory role in the pMF1 partitioning system by not only enhancing the DNA-binding activity of ParB but also regulating gene expression of the par loci.
Figure 8. Binding of purified ORF21 and ORF23 to the ItA fragment.
ItA was incubated with purified ORF23 and/or ORF21, the amounts of which are described at the top of the figure (ng). Indicated amounts of unlabeled ItA (pmol) was added to show the competition. The concentration of labeled DNA used in each lane was 12 pmol.
Discussion
Plasmid partitioning systems typically contain two genes encoding a trans-acting protein pair ParA/ParB and cis-acting iteron sites upstream and/or downstream of these two genes [4], [27], [29]. Represented by P1 ParA, the type Ia ParA contains an N-terminal DNA–binding domain, which plays an autoregulation role for the transcription of operon; whereas the type Ib, represented by pTAR, TP228, pB171 and pSM19035, encodes a ParA protein of 192–308aa, lacking the N-terminal DNA–binding domain [4], [6], [20], [21], [22], [23]. In the type Ib par system, the ParB-like protein serves as the autogenous regulator of the par operon by repressing the transcription of the par genes. The partitioning system of Myxococcus pMF1, described in this paper, contains three coding genes, pMF1.21 (parC), pMF1.22 (parA) and pMF1.23 (parB), thus designated parCAB. Although the potential segregation-related region of Pseudomonas alcaligenes pRA2 also contains three ORFs, the parC is not required for plasmid stability and is likely not a coding region [26]. The three genes described in this paper all play roles in plasmid segregation. In the pMF1 parCAB system, the ParB protein is able to specifically bind to the iteron sites, which are not only distributed upstream and downstream of the operon but also dispersed between the replication-associated region and the par loci. Disperse centromere-like sites have also been found in the linear prophage N15 [30], the plasmid RK2 [31], [32], and streptococcal plasmid pSM19035 [20]. In the pMF1 par system, six centromere-like sites were identified. Although more stable than pZJY41, the pXS11 plasmid in M. xanthus DZ1 did not reach a similar high heritable stability as other par loci-constructed plasmids, probably because the par loci fragment in the plasmid contained only two centromere-like sites (upstream ItA and downstream ItB of the operon). In addition to its binding activity, type Ib ParB proteins also serve as autogenous regulators of the par loci by repressing the transcription of the par genes [4], [5], [26]. In plasmid pMF1, the ParB protein is essential for stability of the plasmid containing the par loci by binding to the iteron sequences for the partitioning process and regulating the expression of the par genes to avoid their over-expression. In the absence of the parB gene, the expression of the parC gene in M. xanthus DZ1 harboring the pXS16 plasmid (parA−parB −) was more than 200-fold higher than that when the parB gene was present. Mutation of the pMF1 parB with the presence of the parA wild type allele is unsuccessful, which is probably due to that disruption of parB gene may lead to over-expression of ParA, which seems to be lethal to cells harboring pXS15 (parB −). This is similar to the pRA2 partitioning system [26]. Binding of the ParB protein to the cis-acting sites functions not only in segregation of the plasmids containing the pMF1 par loci but also in regulating the expression of the operon, whereas ParA-like protein lacks the regulatory N-terminal. These results suggested that the parCAB system of pMF1 belonged to type Ib. In the type Ib plasmid pSM19035, Omega (ParB homologue) has multiple binding sites on the plasmid, serving as a global regulator [20]. The pMF1.23 also has multiple cognate sites on plasmid pMF1: ItA and ItB are close to the parCAB genes while the other centromere-like sites mainly localize between the replication–associated region (pMF1.14) [3] and the partition locus, from nt 12511 to 3 of pMF1. Furthermore, deleting parA and parB gene at the same time led a decrease in plasmid copy number (Fig. S3), whereas deleting parA alone did not have the same effect. These results probably hint potential regulating roles of pMF1.23 on plasmid replication and partition as a global regulator. The most unusual characteristic of the par loci of pMF1 is the presence of an additional gene, parC (pMF1.21). Although unable to bind to any of the DNA regions tested, ParC greatly enhanced the DNA-binding activity of ParB. ParB negatively regulated the par promoter, and qPCR results showed that ParC also repressed the expression of the par genes, although to a lesser extent than ParB. We hypothesize that the ParC protein probably binds to ParB-DNA complex to enhance the function of ParB and thus plays as a collaborator of ParB for its functions.
Materials and Methods
Plasmids and oligonucleotides
All the plasmids used in this study are described in Table 1, and the oligonucleotide sequences used in this study are given in Table 2.
Bacterial strains and growth conditions
The myxobacterial strains were cultivated in CTT [33] medium at 30°C. The E. coli strains were incubated in Luria-Bertani (LB) medium at 37°C. When required, a final concentration of 100 µg/ml ampicillin and/or 40 µg/ml kanamycin was added to the solid or liquid media for selection.
Cloning of the partition system of pMF1
pSQ37 and pSQ38 were subclones of pMF1 and contained the total sequence of pMF1. The boundaries of pMF1 fragments in pSQ37 and pSQ38 were the EcoRI sites. EcoRI/EcoRI fragments of pSQ37 and pSQ38 containing the pMF1 sequences were each digested with additional restriction enzyme. To preserve stability-associated region, we chose two different restriction enzyme pairs for each of the EcoRI/EcoRI fragments: NsiI and SacII were used with pSQ37, whereas BstEII and PvuI were used with pSQ38. A library of 1.5 kb to 8 kb fragments was generated and inserted into the EcoRV site of pZJY41. E. coli DH5α was used as the plasmid host for the majority of cloning procedures in this study. Plasmid DNA was prepared, manipulated and transformed using standard procedures [34]. The recombinant plasmids containing various pMF1 fragments were electroporated into M. xanthus DZ1 according to the protocol described by Kashefi and Hartzell [35] and characterized using the plasmid stability assay described bellow.
Plasmid stability assay
M. xanthus DZ1 cells carrying the plasmids to be tested for stability were grown to late exponential phase in CTT medium supplemented with 40 µg/ml kanamycin at 30°C. The culture was considered generation 0 and diluted 1∶25 in fresh CTT liquid medium without antibiotics and grown at 30°C for 24 h. Then, serial dilutions of the culture were plated on solid CTT medium without antibiotics. The dilutions and plating were repeated every 24 h, which represented an interval of approximately six generations. After each round, 120 single colonies from the plates were patched onto fresh CTT medium with and without kanamycin, and plasmid stability was measured as the percentage of antibiotic-resistant clones.
Protein expression and purification
The coding regions of pMF1.21 and pMF1.23 were cloned under the control of the T7 promoter of pET-15b (Novagen) and recombinant proteins with a His6-tag were expressed in E. coli BL21 (DE3) after induction with 1 mM isopropyl-β-D-thiogalactopyranoside (IPTG). Soluble proteins were purified on a Ni-agarose column according to the manufacturer's protocols (Qiagen). Proteins used in EMSAs and DNase I footprint assays were at least 90% pure based on sodium dodecyl sulfate-polyacrylamide gel estimates, according to the method of Laemmli [36].
Electrophoretic mobility shift assay (EMSA)
The DNA oligonucleotides were synthesized by Sangon Biotech Company (Shanghai) and labeled with biotin at the 3′ ends using the Biotin 3′ End DNA Labeling Kit (Pierce). Complementary biotin-labeled oligonucleotides were annealed as probes for DNA binding. The DNA probe (12 pmol) containing the iteron repeat fragment was incubated with the indicated amounts of ORF21, ORF23, or both purified protein preparations in reaction buffer (12 mM HEPES, 4 mM Tris-HCl, pH 8.0, 60 mM KCl, 5 mM MgCl2, 0.1 mM EDTA, pH 8.0, 1 µg poly(dI-dC) and 2% glycerol) for 30 min at 30°C. Unlabeled competitor DNA fragments were also used as indicated. Samples were loaded on a 5% native polyacrylamide gel and electrophoresed at 10 mA for 3 h. DNA was electrophoretically transferred to a nylon membrane, and the biotin-labeled nucleic acids were detected using the Chemiluminescent Nucleic Acid Detection Module (Pierce), according to the manufacturer's instructions.
DNase I footprinting assay
The DNA fragment from nt17242 to 17447 of the pMF1 plasmid labeled with biotin at only the 5′ end of the coding strand was PCR-amplified using the primers DupupBio and Dupdo. The DNA probe was incubated with the indicated amounts of purified ORF23 in footprinting buffer containing HEMG buffer (50 mM EDTA, pH 8.0, 50 mM KCl, 12.5 mM HEPES pH 7.6, 6 mM MgCl2, 5% glycerol), 20 mM HEPES pH7.6 and 5 µg poly(dI-dC) in a final volume of 50 µl for 30 min at 30°C followed by adding 50 µl Ca/Mg Solution (5 mM CaCl2, 10 mM MgCl2) to the mixture. Then DNase I (Promega) and its buffer were added. After 3-min digestion, the stop solution was added. The resulting products were separated on an 8% polyacrylamide sequencing gel. DNA was electrophoretically transferred to a nylon membrane, and the biotin-labeled nucleic acids were detected following the same procedure as the EMSA described above.
Quantitative real-time PCR analysis
M. xanthus DZ1 transformed using various plasmids was cultured on CTT solid medium containing 40 µg/ml kanamycin for 3–5 days and then inoculated in CTT liquid medium and incubated with shaking for 18 to 24 h at 30°C. The cells were collected, and the RNA was extracted immediately using the SV total RNA extraction kit (Promega) according to the manufacturer's instructions. Genomic DNA was eliminated using a DNA-free kit (ABI). The purified RNA was reverse transcribed to cDNA, and quantitative real-time PCR was performed in a BioRad sequence detection system with the iQ SYBR Green Supermix. 16S rRNA was used as the normalization signal [37]. The primers used for each gene were listed in Table 2.
Supporting Information
The inheritance stability of the plasmids constructed for isolation of the partition-associated region of plasmid pMF1 in M. xanthus DZ1 in the absence of the selective antibiotic kanamycin. The data presented are the averages of two independent experiments.
(TIFF)
EMSAs showing DNA binding activity of ORF23 with or without a His-tag to the ItA sequence nt 17348–17395. (A) Assays showing ORF23 with or without a His-tag specific binding to ItA. Increasing amunts of ORF23 were incubated with ItA. The amount of the purified ORF23 with a His-tag added in lanes 1 to 5 or that without a His-tag added in lanes 6 to 10 was 0 (−), 250 ng, 500 ng, 1000 ng, and 1500 ng, respectively. (B) Competition experiments showing ORF23 with or without a His-tag specific binding to the ItA. The amounts of unlabeled DNA fragments (0, 0.8 pmol, 8 pmol, and 80 pmol) were incubated with 500 ng purified ORF23 protein with a His-tag in lanes 1 to 4 or that without a His-tag in lanes 5 to 8, respectively.
(TIFF)
Agarose gel electrophoresis analysis of the pZJY41 derivates. 6×108 cells were used to extract the plasmid for each lane. Lane1, pZJY41; lane 2, pXS11; lane 3, pXS13, lane 4, pXS14; lane 5, pXS16; lane M, supercoiled DNA ladder markers. The size of each band is labeled on the right of the panel.
(TIFF)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was financially supported by the National Science Foundation for Distinguished Young Scholars (grant 30825001) and the National Natural Science Foundation (grant 30971572) of China. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Shimkets LJ. Social and developmental biology of the myxobacteria. Microbiol Rev. 1990;54:473–501. doi: 10.1128/mr.54.4.473-501.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reichenbach H, Hofle G. Biologically active secondary metabolites from myxobacteria. Biotechnol Adv. 1993;11:219–277. doi: 10.1016/0734-9750(93)90042-l. [DOI] [PubMed] [Google Scholar]
- 3.Zhao JY, Zhong L, Shen MJ, Xia ZJ, Cheng QX, et al. Discovery of the autonomously replicating plasmid pMF1 from Myxococcus fulvus and development of a gene cloning system in Myxococcus xanthus. Appl Environ Microbiol. 2008;74:1980–1987. doi: 10.1128/AEM.02143-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebersbach G, Gerdes K. Plasmid segregation mechanisms. Annu Rev Genet. 2005;39:453–479. doi: 10.1146/annurev.genet.38.072902.091252. [DOI] [PubMed] [Google Scholar]
- 5.de la Hoz AB, Ayora S, Sitkiewicz I, Fernandez S, Pankiewicz R, et al. Plasmid copy-number control and better-than-random segregation genes of pSM19035 share a common regulator. Proc Natl Acad Sci U S A. 2000;97:728–733. doi: 10.1073/pnas.97.2.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman SA, Austin SJ. The P1 plasmid-partition system synthesizes two essential proteins from an autoregulated operon. Plasmid. 1988;19:103–112. doi: 10.1016/0147-619x(88)90049-2. [DOI] [PubMed] [Google Scholar]
- 7.Hayes F, Radnedge L, Davis MA, Austin SJ. The homologous operons for P1 and P7 plasmid partition are autoregulated from dissimilar operator sites. Mol Microbiol. 1994;11:249–260. doi: 10.1111/j.1365-2958.1994.tb00305.x. [DOI] [PubMed] [Google Scholar]
- 8.Jensen RB, Dam M, Gerdes K. Partitioning of plasmid R1. The parA operon is autoregulated by ParR and its transcription is highly stimulated by a downstream activating element. J Mol Biol. 1994;236:1299–1309. doi: 10.1016/0022-2836(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 9.Mori H, Mori Y, Ichinose C, Niki H, Ogura T, et al. Purification and characterization of SopA and SopB proteins essential for F plasmid partitioning. J Biol Chem. 1989;264:15535–15541. [PubMed] [Google Scholar]
- 10.Dolowy P, Mondzelewski J, Zawadzka R, Baj J, Bartosik D. Cloning and characterization of a region responsible for the maintenance of megaplasmid pTAV3 of Paracoccus versutus UW1. Plasmid. 2005;53:239–250. doi: 10.1016/j.plasmid.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Bouet JY, Funnell BE. P1 ParA interacts with the P1 partition complex at parS and an ATP-ADP switch controls ParA activities. EMBO J. 1999;18:1415–1424. doi: 10.1093/emboj/18.5.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen RB, Gerdes K. Partitioning of plasmid R1. The ParM protein exhibits ATPase activity and interacts with the centromere-like ParR-parC complex. J Mol Biol. 1997;269:505–513. doi: 10.1006/jmbi.1997.1061. [DOI] [PubMed] [Google Scholar]
- 14.Koonin EV. A superfamily of ATPases with diverse functions containing either classical or deviant ATP-binding motif. J Mol Biol. 1993;229:1165–1174. doi: 10.1006/jmbi.1993.1115. [DOI] [PubMed] [Google Scholar]
- 16.Davis MA, Austin SJ. Recognition of the P1 plasmid centromere analog involves binding of the ParB protein and is modified by a specific host factor. EMBO J. 1988;7:1881–1888. doi: 10.1002/j.1460-2075.1988.tb03021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Funnell BE. The P1 plasmid partition complex at parS. The influence of Escherichia coli integration host factor and of substrate topology. J Biol Chem. 1991;266:14328–14337. [PubMed] [Google Scholar]
- 18.Funnell BE, Gagnier L. The P1 plasmid partition complex at parS. II. Analysis of ParB protein binding activity and specificity. J Biol Chem. 1993;268:3616–3624. [PubMed] [Google Scholar]
- 19.Larsen RA, Cusumano C, Fujioka A, Lim-Fong G, Patterson P, et al. Treadmilling of a prokaryotic tubulin-like protein, TubZ, required for plasmid stability in Bacillus thuringiensis. Genes Dev. 2007;21:1340–1352. doi: 10.1101/gad.1546107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dmowski M, Sitkiewicz I, Ceglowski P. Characterization of a novel partition system encoded by the delta and omega genes from the streptococcal plasmid pSM19035. J Bacteriol. 2006;188:4362–4372. doi: 10.1128/JB.01922-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ebersbach G, Gerdes K. The double par locus of virulence factor pB171: DNA segregation is correlated with oscillation of ParA. Proc Natl Acad Sci U S A. 2001;98:15078–15083. doi: 10.1073/pnas.261569598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayes F. The partition system of multidrug resistance plasmid TP228 includes a novel protein that epitomizes an evolutionarily distinct subgroup of the ParA superfamily. Mol Microbiol. 2000;37:528–541. doi: 10.1046/j.1365-2958.2000.02030.x. [DOI] [PubMed] [Google Scholar]
- 23.Kalnin K, Stegalkina S, Yarmolinsky M. pTAR-encoded proteins in plasmid partitioning. J Bacteriol. 2000;182:1889–1894. doi: 10.1128/jb.182.7.1889-1894.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dam M, Gerdes K. Partitioning of plasmid R1. Ten direct repeats flanking the parA promoter constitute a centromere-like partition site parC, that expresses incompatibility. J Mol Biol. 1994;236:1289–1298. doi: 10.1016/0022-2836(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 25.Edgar R, Chattoraj DK, Yarmolinsky M. Pairing of P1 plasmid partition sites by ParB. Mol Microbiol. 2001;42:1363–1370. doi: 10.1046/j.1365-2958.2001.02717.x. [DOI] [PubMed] [Google Scholar]
- 26.Kwong SM, Yeo CC, Poh CL. Molecular analysis of the pRA2 partitioning region: ParB autoregulates parAB transcription and forms a nucleoprotein complex with the plasmid partition site, parS. Mol Microbiol. 2001;40:621–633. doi: 10.1046/j.1365-2958.2001.02405.x. [DOI] [PubMed] [Google Scholar]
- 27.Moller-Jensen J, Jensen RB, Gerdes K. Plasmid and chromosome segregation in prokaryotes. Trends Microbiol. 2000;8:313–320. doi: 10.1016/s0966-842x(00)01787-x. [DOI] [PubMed] [Google Scholar]
- 28.Misselwitz R, de la Hoz AB, Ayora S, Welfle K, Behlke J, et al. Stability and DNA-binding properties of the omega regulator protein from the broad-host range Streptococcus pyogenes plasmid pSM19035. FEBS Lett. 2001;505:436–440. doi: 10.1016/s0014-5793(01)02865-4. [DOI] [PubMed] [Google Scholar]
- 29.Ogura T, Hiraga S. Partition mechanism of F plasmid: two plasmid gene-encoded products and a cis-acting region are involved in partition. Cell. 1983;32:351–360. doi: 10.1016/0092-8674(83)90454-3. [DOI] [PubMed] [Google Scholar]
- 30.Ravin N, Lane D. Partition of the linear plasmid N15: interactions of N15 partition functions with the sop locus of the F plasmid. J Bacteriol. 1999;181:6898–6906. doi: 10.1128/jb.181.22.6898-6906.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lukaszewicz M, Kostelidou K, Bartosik AA, Cooke GD, Thomas CM, et al. Functional dissection of the ParB homologue (KorB) from IncP-1 plasmid RK2. Nucleic Acids Res. 2002;30:1046–1055. doi: 10.1093/nar/30.4.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pansegrau W, Lanka E, Barth PT, Figurski DH, Guiney DG, et al. Complete nucleotide sequence of Birmingham IncP alpha plasmids. Compilation and comparative analysis. J Mol Biol. 1994;239:623–663. doi: 10.1006/jmbi.1994.1404. [DOI] [PubMed] [Google Scholar]
- 33.Kaiser D. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc Natl Acad Sci U S A. 1979;76:5952–5956. doi: 10.1073/pnas.76.11.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sambrook J, Russell DW. Molecular cloning: a laboratory manual, 3rd ed. 2001. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 35.Kashefi K, Hartzell PL. Genetic suppression and phenotypic masking of a Myxococcus xanthus frzF- defect. Mol Microbiol. 1995;15:483–494. doi: 10.1111/j.1365-2958.1995.tb02262.x. [DOI] [PubMed] [Google Scholar]
- 36.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 37.Bode HB, Ring MW, Schwar G, Kroppenstedt RM, Kaiser D, et al. 3-Hydroxy-3-methylglutaryl-coenzyme A (CoA) synthase is involved in biosynthesis of isovaleryl-CoA in the myxobacterium Myxococcus xanthus during fruiting body formation. J Bacteriol. 2006;188:6524–6528. doi: 10.1128/JB.00825-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The inheritance stability of the plasmids constructed for isolation of the partition-associated region of plasmid pMF1 in M. xanthus DZ1 in the absence of the selective antibiotic kanamycin. The data presented are the averages of two independent experiments.
(TIFF)
EMSAs showing DNA binding activity of ORF23 with or without a His-tag to the ItA sequence nt 17348–17395. (A) Assays showing ORF23 with or without a His-tag specific binding to ItA. Increasing amunts of ORF23 were incubated with ItA. The amount of the purified ORF23 with a His-tag added in lanes 1 to 5 or that without a His-tag added in lanes 6 to 10 was 0 (−), 250 ng, 500 ng, 1000 ng, and 1500 ng, respectively. (B) Competition experiments showing ORF23 with or without a His-tag specific binding to the ItA. The amounts of unlabeled DNA fragments (0, 0.8 pmol, 8 pmol, and 80 pmol) were incubated with 500 ng purified ORF23 protein with a His-tag in lanes 1 to 4 or that without a His-tag in lanes 5 to 8, respectively.
(TIFF)
Agarose gel electrophoresis analysis of the pZJY41 derivates. 6×108 cells were used to extract the plasmid for each lane. Lane1, pZJY41; lane 2, pXS11; lane 3, pXS13, lane 4, pXS14; lane 5, pXS16; lane M, supercoiled DNA ladder markers. The size of each band is labeled on the right of the panel.
(TIFF)