Abstract
Background
Segmental bony defects resulting from congenital facial anomalies, facial trauma, infection or oncologic surgical resection represent a common and significant clinical problem. Currently, these defects are reconstructed with autologous or allogeneic bone grafts, or prosthetic devices. These options are limited by bone supply for grafting, donor site morbidity, risk of infection and extrusion.
This study investigated the in vivo osteogenic capability of poly (ethylene glycol)-diacrylate (PEG-DA) and a protease sensitive PEG, (PEG-MMP), photoencapsulated with mesenchymal stem cells and BMP-2, in healing a critical size rat calvarial defect.
Methods
PEG-DA and PEG-MMP scaffolds photoencapsulated with rat mesenchymal stem cells (rMSCs) and/or BMP-2 were implanted into a critical size defect. Micro-CT analysis was completed 1, 4 and 8 weeks after implantation. Bone growth was histologically evaluated. Micro-CT data was analyzed using ASPIProVM software to calculate percent closure of cranial defects.
Results
PEG-MMP and PEG-MMP + BMP2 showed significantly enhanced bone growth as compared to controls. PEG-DA appeared to inhibit bone growth regardless of biofactor and rMSCs. The addition of rMSCs did not enhance bone regeneration.
Conclusion
PEG sensitive to proteolysis significantly improved bone repair in a critical size calvarial defect.
Background
Segmental bony defects resulting from congenital facial anomalies, facial trauma, infection or oncologic surgical resection represent a common and significant clinical problem. Data from the US Health Cost and Utilization project shows that 12,700 craniotomies and craniectomies were performed in 2001, and procedures to correct defects associated with facial trauma numbered 20,616. The national costs for these procedures are estimated to be approximately $549 million and $400 million, respectively.1
Currently, these tissue defects are reconstructed with autologous bone or rib grafts, allogeneic bone grafts, or biocompatible prosthetic devices. While these solutions have been shown to improve bone regeneration, each method has a unique set of drawbacks. Autologous grafting is limited by the supply of suitable bone or rib and complications arising from donor site morbidity. Allogeneic bone grafts from donors or cadavers can present a risk of disease transmission. Prosthetic devices are plagued with the potential risk of infection and extrusion.2
Tissue engineering attempts to repair or regenerate damaged tissue by using engineered tissue substitutes that can sustain functionality during regeneration and eventually integrate with the host tissue or resorb. With tissue engineered grafts, the appropriate signals (e.g. osteoconductive surface, growth factors, and osteoprogenitor cells) can be delivered in a controlled fashion. Growth factors associated with osteoinduction are bone morphogenic proteins (BMPs), transforming growth factor-beta (TGF-β), fibroblast growth factor-2 (FGF-2), vascular endothelial growth factor (VEGF) and insulin like growth factor-1 IGF-1.3
Most research has focused on the use of the BMPs and, in particular BMP-2, because they have been shown to stimulate mesenchymal cell chemotaxis and proliferation, and promote the differentiation of these cells into chondrocytes and osteoblasts.4 These osteoinductive capabilities are primarily evident by the induction of new bone formation via a process of endochondral ossification when implanted at ectopic sites.5
Bone-derived mesenchymal stem cells (MSCs) may be an ideal cell type for bone regeneration applications. MSCs can be cultured in vitro, allowing for the rapid expansion of multipotent cells 6 that are capable of differentiating into several distinct cell types including osteoblasts, chondrocytes, adipocytes, and myocytes. 7 Given this potential, experiments have been initiated to test the efficacy of MSCs to induce in vivo bone regeneration in animals.
To facilitate retention of BMP-2 and MSCs at the treatment site and reduce the effective dose, an appropriate carrier is required. The preferred carrier consists of a scaffold that is both biocompatible and resorbable in order to limit tissue rejection and allow for bone growth.
A calvarial wound model has many similarities to the maxillofacial region. Morphologically and embryologically, the calvarium develops from a membrane precursor and thus resembles the membranous bones of the face. Tagaki8 and Urist determined that an 8-mm diameter defect created in the calvaria of six-month-old Sprague-Dawley rats reduced to 5 mm in four weeks, but no further healing of the defect was noted at 12 weeks. This work supported the 8-mm calvarial wound as a critical size defect in this species. Utilizing this model the osteogenic potential of an implant may be considered unequivocal.9
This preliminary project was directed at optimizing bone regrowth in a critically-sized, non-healing calvarial defect. We tested the osteoconductive and osteoinductive capability of two poly (ethylene glycol) scaffolds embedded with bioreactive growth factors and mesenchymal stem cells. We hypothesized that PEG scaffolds would be osteoconductive and that the presence of biofactors and mesenchymal stem cells would provide increased osteoinduction and thus improved bone regrowth when compared to negative control.
Methods
Preparation of PEG monomers
PEG-DA formation was prepared as previously described.10 PEG-MMP consisted of a 4-arm 20,000 mw PEG-(tetra) Norbornene hub and an enzymatically degradable di-cysteine peptide linker.
Synthesis of Norbornene anhydride
Under inert atmosphere 5-norbornene-2-carboxylic acid (1.38 g, Sigma) was dissolved in 30ml DCM, diisopropylcarbanoamide (DIC) (1.26 g, Sigma) was added slowly to reaction mixture and stirred for 30min. Reaction mixture was filtered under inert conditions; filtrate was reacted with PEG mixture as described below.
Synthesis of PEG-tetra-norbornene
4-arm poly (ethylene glycol) MW 20,000 (10 g, JenKem USA) was dissolved in 100ml DCM and cooled to 0 °C under an inert atmosphere. Pyridine (0.8 g, Sigma) and a catalytic amount of dimethylaminopyridine (DMAP 0.012 g, Sigma) were added to the reaction flask under an inert atmosphere and stirred. The symmetric Norbornene anhydride (2.5 eq, 1.38 g as prepared above) was added to the reaction mixture under inert atmosphere and stirred at 0 °C overnight. The reaction mixture was filtered and subsequently PEG-tetra-norbornene was precipitated in ethyl ether (1 L) 0 °C. The filter cake was placed in a soxlet extractor; remaining impurities were extracted with ethyl ether (48 hrs). 1H NMR (CDCl3): δ = 3.35–3.75 (broad, PEG chain protons), 4.2–4.4 (t, 8H 4x-CH2OCOR), 5.8–6.4 (multi, endo/exo 5-norborn“ene” protons) ppm.
Rat Mesenchymal Stem Cell Culture
Rat mesenchymal stem cells (rMSCs) were obtained from Tulane University Health Science Center, New Orleans, Louisiana, and cultured successfully in the Anseth Laboratory.
Photoencapsulation of rMSCs
Photoencapsulation of rMSCs has been tested on rMSC cultures, and the encapsulation process showed no abnormal effects on cell viability. For PEGDA hydrogels, a solution containing 4600 KDa Poly(ethylene) glycol DiAcrylate (PEG4600DA) at 10%wt./vol. was prepared in PBS containing the photoinitiator 4-(2-hydroxyethoxy) phenyl-(2-hydroxyl-2-propyl)ketone (I2959 Ciba).
I2959 is a water-soluble photoinitiator that initiates the polymerization of acrylate functionalities in the presence of long wave ultraviolet light and has been shown to exhibit low toxicity to cells under these photoencapsulation conditions. The CRGDS peptide serves as an extracellular matrix adhesion mimic that crosslinks into the network via the thiol contained within the cysteine residue and allows integrin-mediated cell attachment to the scaffold. Rat mesenchymal stem cells were trypsinized from cell culture and centrifuged to pellet the cells. The cells were then resuspended in the PEG monomer solution at a concentration of 5 million cells per mL. Approximately 40 μL of cell/polymer solution was then placed into sterile Teflon wells, 7mm in diameter. The wells were placed under ultraviolet light for 10 min at room temperature to polymerize the samples. Upon polymerization, the disk-shaped constructs were removed from the wells and placed into tissue culture media and incubated at 37°C in 5% CO2 until the time of implantation. After 24 hours, Live/Dead assays (Molecular Probes, Eugene, OR) were performed on the hydrogel constructs to ensure that the rMSCs remained viable throughout the encapsulation process.
A similar process was performed for MMP-degradable scaffolds (PEG-MMP). For these gels, 20K-PEG-norbornene was resuspended in PBS at a concentration of 10% wt./vol. with 0.25mM CRGDS, 0,05% I2959, and the degradable peptide crosslinker (KKCGGPQGIAGQGCKK) containing two cysteine residues flanking an MMP-cleavable sequence.
BMP-2 was purchased from R&D Systems and included in monomer solutions before polymerization at a concentration of 5ng/80μl for PEG-DA-based scaffolds. To account for volume changes resulting from free swelling of PEG-MMP hydrogels after polymerization, a concentration of 15ng/80μl BMP-2 was used.
Rat Cranial Surgery
Operations were performed on Albino male Sprague Dawley rats aged 10–11 weeks (300–350 grams). General anesthesia was administered by intraperitoneal injection of ketamine hydrochloride (40 mg/kg) mixed with xylazine (10 mg/kg). After induction of anesthesia, the surgical site was shaved of fur. The head was placed in a stereotaxic frame. An incision was made along the sagittal suture and the periosteum was elevated. An 8-mm in diameter, full thickness calvarial bone defect was created without dura perforation using a surgical microdrill fitted with a diamond bur. The wound was thoroughly irrigated with warmed saline to remove residual bone dust. After implantation of the appropriate scaffold, the periosteum was closed with 4.0 Vicryl suture and the skin was closed with staples. Animals were sacrificed by CO2 asphyxiation 9 weeks after scaffold implantation.
A total of 54 animals were randomly assigned to nine groups, testing two scaffold chemistries, PEG-DA, and PEG-MMP. The total number of animals per group was determined by power calculation analysis. Six animals for each group were utilized as follows: 1) surgical defect 2) PEG-DA scaffold 3) PEG-MMP 4) PEG-DA + BMP-2 (5ng/80μl) 5) PEG-MMP + BMP-2 (5ng/80μl) 6) PEG-DA + rMSCs (1×10^7 cells/ml) 7) PEG-MMP + rMSCs (1×10^7 cells/ml) 8) PEG-DA + rMSCs + BMP-2 9) PEG-MMP + rMSCs + BMP-2
Microcomputed tomography (micro-CT)
Micro-CT was applied to longitudinally assess bone formation in the time frame of 8 weeks. Each animal underwent micro-CT with a spatial resolution of 70 microns at 1 week, 4 weeks, and 8 weeks after implantation. Using ASPIProVM software to analyze the micro-CT data, the percentage (%) of new bone formation was presented as the ratio of new bone volume versus total defect volume.
Histology
Following sacrifice, the skulls were fixed in 10% formalin overnight, then dehydrated in 70% ethanol, decalcified in EDTA solution, and embedded in paraffin. For histochemical analysis, paraffin sections were made in the midline of the calvarial defect. Three representative animals in each group were evaluated in this way. A Masson’s trichrome stain was utilized for detection of cells and bone regrowth. Digital images from stained sections were taken by means of a transmission and polarized light Axioskop Microscope.
Statistics
Defect volume data was analyzed using the SAS statistical package.
Results
We were successfully able to prefabricate PEG-DA and PEG-MMP scaffolds. Live/Dead assays performed on the hydrogel constructs showed that the rMSCs remained viable throughout the encapsulation process. Rats serving as a negative control illustrated mean closure of defect of 32% at 4 weeks, and 50% at 8 weeks. No animals in the control group had complete closure of their defects.
PEG-DA
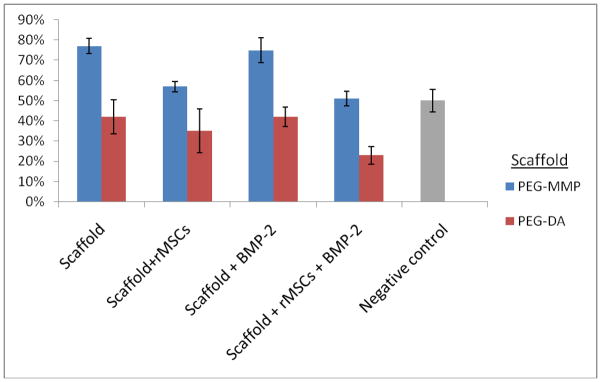
Micro-CT analysis at 4 weeks after implantation with PEG-DA, PEG-DA +rMSCs, PEG-DA + BMP-2, and PEG-DA + rMSCs + BMP-2 illustrated mean reductions in defect volume of 27%, 20%, 8%, 5%, respectively. At 8 weeks the mean reduction in defect volume had increased to 42%, 35%, 42%, 23% (Table 1 and Figure 1).
Table 1.
Micro-CT: Percent Reduction in Wound Volume
| Mean (SE) | |||||
|---|---|---|---|---|---|
| Time | Control (n=6) | PEG-DA (n=6) | PEG-DA+rMSCs (n=6) | PEG-DA+BMP-2 (n=6) | PEG-DA+rMSCs+BMP-2 (n=6) |
| Week 4 | 32% (10.57) | 27% (7.56) | 20% (5.45) | 8% (5.2) | 5% (4.19) |
| Week 8 | 50% (5.63) | 42% (10.88) | 35% (4.84) | 42% (4.38) | 23% (8.44) |
Figure 1.
Percent (%) reduction in bony wound volume by scaffold type. Standard error bars are shown.
Histology
On gross examination, there was no resorbtion of the PEG-DA scaffold. Microscopic evaluation showed sharp demarcation of the bony wound and PEG-DA interface. There were no islands of ossification. Bone regrowth was limited deep to the scaffold and immediately adjacent to dura matter. A representative image is shown in Figure 2.
Figure 2.
Representative animal implanted with a PEG-DA scaffold, 9 weeks post-implantation. Specimen stained with Masson’s trichrome. 10 x magnification. A fibrous capsule is seen surrounding the non-degraded PEG-DA scaffold. There is sharp demarcation at the wound-PEG interface. No islands of ossification within the scaffold. Limited bone regrowth is present immediately adjacent to dura.
PEG-MMP
Micro-CT analysis at 4 weeks after implantation with PEG-MMP, PEG-MMP +rMSCs, PEG-MMP + BMP-2, and PEG-MMP + rMSCs + BMP-2 illustrated mean reductions in defect volume of 59%, 32%, 55%, 26%, respectively. At 8 weeks, 77%, 57%, 75%, 51%, mean reductions were present (Table 2 and Figure 1).
Table 2.
Micro-CT: Percent Reduction in Wound Volume
| Mean (SE) | |||||
|---|---|---|---|---|---|
| Time | Control (n=6) | PEG-MMP (n=6) | PEG-MMP+rMSCs (n=6) | PEG-MMP+BMP-2 (n=6) | PEG-MMP+rMSCs+BMP-2 (n=6) |
| Week 4 | 32% (10.57) | 59% (4.90) | 32% (6.78) | 55% (2.01) | 26% (8.72) |
| Week 8 | 50% (5.63) | 77% (7.73) | 57% (6.04) | 75% (2.44) | 51% (3.69) |
Histology
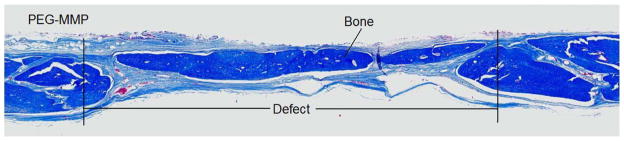
On gross examination, there was complete resorption of the PEG-MMP scaffold. Microscopic evaluation showed good infiltration of bone with near complete coverage of the defect. Bone regrowth was present along the periphery and centrally. A histological section of a representative sample is shown in Figure 3.
Figure 3.
Representative animal implanted with a PEG-MMP scaffold, 9 weeks post-implantation. Specimen stained with Masson’s trichrome. 10 x magnification. The scaffold is completely resorbed. There is peripheral and central bone regrowth is present within the area previously occupied by the scaffold.
Statistical Analysis
Mean values of the percent reduction in initial defect volume were calculated using a cell means model (a form of linear model useful for analyzing categorical data). Mean values for each treatment category were calculated, as were differences between treatment and the control group for the eight week time-point. Statistical tests were performed to determine if the difference between each treatment group and the control group were statistically significant. The model indicates that taken as a whole, there is a significant difference in means for the groups (p-value <.0001).
For each paired difference, the difference, test statistic and p-value are listed in Table 3. The p-value (i.e. Pr > |t|) represents the calculated probability of observing a difference in means between the control and the comparison group as or more extreme than the one observed, based on the experimental design. PEG-MMP and PEG-MMP + BMP-2 appear to be statistically greater in reducing the initial defect volume than control, with an estimated mean difference of 27.3% and 25.0% (p-values of .0041 and .0081 respectively).
Table 3.
Differences in Mean Wound Volume and Control
| Comparison Group | Difference in Mean Wound Volume and Control | Standard Error | t value | Pr > |t| |
|---|---|---|---|---|
| PEG-DA | −0.08 | 0.09 | −0.86 | 0.39 |
| PEG-DA+rMSC | −0.14 | 0.09 | −1.72 | 0.09 |
| PEGDA + BMP-2 | −0.08 | 0.09 | −0.88 | 0.38 |
| PEG-DA+rMSC+BMP-2 | −0.27 | 0.09 | −2.99 | 0.005 |
| PEG-MMP | 0.27 | 0.09 | 3.02 | 0.004 |
| PEG-MMP+rMSC | 0.07 | 0.09 | 0.79 | 0.43 |
| PEG-MMP+BMP2 | 0.25 | 0.09 | 2.77 | 0.008 |
| PEG-MMP+rMSC+BMP2 | 0.01 | 0.09 | 0.10 | 0.92 |
Comment
We attempted to utilize PEG scaffolds as osteoconductive moieties and as an effective delivery vehicle for the cytokine BMP-2. By encapsulating mesenchymal stem cells, which have the potential to commit to an osteoblastic lineage, we aimed to establish new centers of bone formation within a defect. In theory, combining scaffold, cytokines, and cells to generate ex vivo tissue-engineered constructs should provide more effective bone regeneration in vivo in comparison to biomaterial matrices alone11 or negative control.
We were successfully able to prefabricate PEG-DA and PEG-MMP, and maintain cell viability following photopolymerization in PEG. Negative control animals illustrated a mean reduction in defect volume of approximately 50% at 8 weeks, and none had complete closure of their defects. This reconfirms the validity of this calvarial critical size defect model.
Our poly (ethylene glycol)-diacrylate (PEG-DA) scaffolds were non-degradable by cell initiated proteolysis and did not enhance osteoconduction in this model. In fact, we showed a trend toward decreased bone growth regardless of encapsulated biofactor or stem cell. These findings were clear by micro-CT, gross post-mortem evaluation, and on histologic analysis. Sharp demarcation of the bony wound edge and a lack of islands of ossification were seen radiographically and histologically (Figure 1). When bone regrowth occurred it was deep to the scaffold and immediately adjacent to the dura mater. This is not surprising given the large body of evidence supporting the osteogenic potential of dura mater. 12
By comparison, PEG scaffolding susceptible to cell triggered proteolysis (PEG-MMP) showed substantially more bone formation than negative control and thus appeared osteoconductive. This increased bone formation was seen in both peripheral new bone (in continuity with the native calvaria) and central new bone (not in continuity with the calvarial edges) (Figure 2).
Clearly osteoconduction was dependent on cell triggered proteolysis. Within a non-degradable scaffold, cell migration can only occur by amoeboid cell migration and thus pore size must be larger than the migrating cell’s diameter. However, the porosity of our PEG-DA scaffold is well below the critical porosity by which migration is possible. Conversely, PEG-MMP enables cell initiated proteolytic migration, and thus porosity is less critical.
Bone morphogenic protein-2 at a concentration of (5ng/80μl) was not osteoinductive in vivo, and showed no increased osteogensis beyond that seen with PEG-MMP alone. Previous in vitro work has shown improved bone regeneration utilizing significantly higher concentrations (5μg/100μl) of BMP-2 (Lutolf). 13 Our decision to utilize a low concentration was based on our previous in vitro cell culture studies. Additionally, high concentrations of BMP-2 have been shown to give rise to heterotrophic ossification. 14
The addition of rat mesenchymal stem cells did not increase osteoinduction as was hypothesized, and in fact appeared to be inhibitory (Table. 3). There is literature suggesting that rMSCs in vitro are largely unresponsive to BMP-2 without dexamethasone in the culture medium.15 Also, BMP-2 used in isolation with MSCs, has shown very low osteogenic potential in some reports. 15 Some in vitro and in vivo studies suggest that early treatment of rMSCs with bFGF and dexamethasone induced BMP-2 responsive osteoprogenitor cells, that when treated with BMP-2 differentiated into fully mature osteoblasts. 15 Our protocol did not utilize dexamethasone or bFGF.
In conclusion, this calvarial critical size defect model appeared valid and enabled evaluation of the osteogenic potential of our scaffold. Micro-CT imaging techniques allowed longitudinal analysis of bone regrowth. Poly (ethylene glycol) scaffold sensitive to cell triggered proteolysis was necessary for improving bone regrowth. BMP-2 and rMSCs in the concentration and conditions of our project did not further increase bone regrowth. This was likely due to low BMP-2 concentration and mesenchymal stem cells that despite good viability were not fully responsive to the osteogenic differentiating influence of BMP-2.
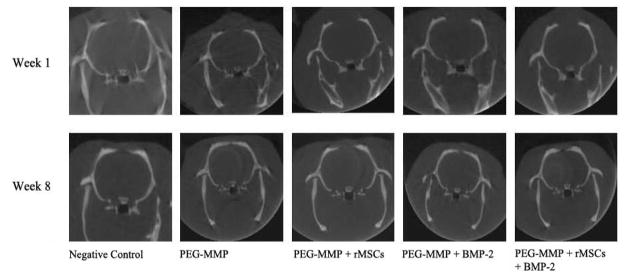
Figure 4.
Representative micro-CT images of animals implanted with PEG-DA scaffold. One-week and 8-week scans are shown.
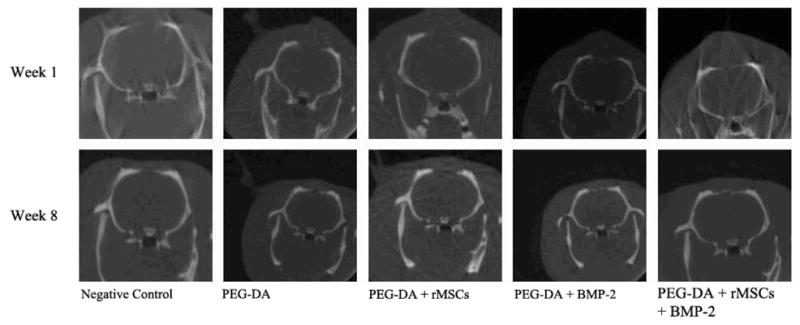
Figure 5.
Representative micro-CT images of animals implanted with PEG-MMP scaffold. One-week and 8-week scans area shown.
Contributor Information
Adam Terella, Department of Otolaryngology, University of Colorado School of Medicine
Peter Mariner, Howard Hughes Medical Institute, Department of Chemical and Biological Engineering, University of Colorado at Boulder
Nate Brown, Department of Chemical and Biological Engineering, University of Colorado at Boulder
Kristi Anseth, Howard Hughes Medical Institute, Department of Chemical and Biological Engineering, University of Colorado at Boulder
Sven-Olrik Streubel, Department of Otolaryngology, University of Colorado School of Medicine, The Children’s Hospital, Aurora, Colorado.
References
- 1.Steiner C, Elixhauser A, Schnaier J. The healthcare cost and utilization project: an overview. Effective Clinical Practice. 2002;5(3):143–51. [PubMed] [Google Scholar]
- 2.Rubin JP, Yaremchuk MJ. Complications and toxicities of implantable biomaterials used in facial reconstructive and aesthetic surgery: a comprehensive review of the literature. Plastic & Reconstructive Surgery. 1997;100(5):1336–1353. doi: 10.1097/00006534-199710000-00043. [DOI] [PubMed] [Google Scholar]
- 3.Linkhart TA, Mohan S, Baylink DJ. Growth factors for bone growth and repair: IGF, TGF beta and BMP. Bone. 1996;19(1 Suppl):1S–12S. doi: 10.1016/s8756-3282(96)00138-x. [DOI] [PubMed] [Google Scholar]
- 4.Urist MR, DeLange RJ, Finerman GA. Bone cell differentiation and growth factors. Science. 1983;220(4598):680–6. doi: 10.1126/science.6403986. [DOI] [PubMed] [Google Scholar]
- 5.Lindsey WH, Franz DA, Toung JS, London SD, Ogle RO. A nasal critical-size defect: an experimental model for the evaluation of facial osseous repair techniques. Archives of Otolaryngology -- Head & Neck Surgery. 1998;124(8):912–5. doi: 10.1001/archotol.124.8.912. [DOI] [PubMed] [Google Scholar]
- 7.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 9.Schmitz JP, Hollinger JO. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clinical Orthopaedics & Related Research. 1986;(205):299–308. [PubMed] [Google Scholar]
- 10.Nuttelman CR, Tripodi MC, Anseth KS. In vitro osteogenic differentiation of human mesenchymal stem cells photoencapsulated in PEG hydrogels. Journal of Biomedical Materials Research Part A. 2004;68(4):773–82. doi: 10.1002/jbm.a.20112. [DOI] [PubMed] [Google Scholar]
- 11.Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26(27):5474–91. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Spector JA, Greenwald JA, Warren SM, et al. Dura mater biology: autocrine and paracrine effects of fibroblast growth factor 2. Plastic & Reconstructive Surgery. 2002;109(2):645–54. doi: 10.1097/00006534-200202000-00035. [DOI] [PubMed] [Google Scholar]
- 13.Lutolf MP, Weber FE, Schmekel HG, et al. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nature Biotechnology. 2003;21(5):513–8. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- 14.McKay B, Sandhu HS. Use of recombinant human bone morphogenetic protein-2 in spinal fusion applications. Spine. 2002;27 (16 Suppl 1):66–85. doi: 10.1097/00007632-200208151-00014. [DOI] [PubMed] [Google Scholar]
- 15.Hanada K, Dennis J, Caplan A. Stimulatory Effect of Basic Fibroblast Growth Factor and Bone Morphogenic Protein-2 on Osteogenic Differentiation of Rat Bone Marrow-Derived Mesenchymal Stem Cells. Journal of Bone and Mineral Research. 1997;12(10):1606–1614. doi: 10.1359/jbmr.1997.12.10.1606. [DOI] [PubMed] [Google Scholar]