Abstract
Introduction:
Allosensitization is a significant obstacle to retransplantation for patients with primary renal graft failure.
Methods:
We assessed the impact of allograft nephrectomy (Group I) and weaning of immunosuppression (Group II) on percent panel reactive antibody (%PRA) at various time points after graft failure in 132 patients with a median follow-up of 47 months. Of these, 68% had allograft nephrectomy while 32% were placed on the waiting list and were either taken off immunosuppression, left on prednisone or on low-dose immunosuppressive therapy.
Results:
When groups were stratified into early (<6 months) and late (>6 months) graft failure, patients who had transplant nephrectomy for early failure demonstrated a decline in %PRA from 46% at time of graft failure to 27% at last follow-up (p = 0.02); conversely, %PRA continued to rise in Group II experiencing early allograft failure. Both Groups I and II patients with late graft failure maintained elevated %PRA at last follow-up.
Conclusion:
Allograft nephrectomy may play a role in limiting allosensitization in patients with early but not late graft failures.
Résumé
Introduction :
L’allosensibilisation est un obstacle important à la retransplantation chez les patients présentant un échec primaire de la greffe rénale.
Méthodologie :
Nous avons évalué l’impact d’une néphrectomie du greffon (groupe I) et du sevrage de l’immunosuppression (groupe II) sur le taux d’immunisation (PRA pour panel reactive antibody) à différents points dans le temps après l’échec de la greffe chez 132 patients; le suivi médian était de 47 mois. Sur les 132 patients, 68 % ont subi une néphrectomie du greffon, tandis que 32 % ont été placés sur la liste d’attente, et on a soit mis fin à leur traitement d’immunosuppression, soit poursuivi leur traitement par prednisone ou par un agent immunosuppresseur à faible dose.
Résultats :
Lorsque les groupes ont été stratifiés en fonction de l’échec précoce (< 6 mois) et tardif (> 6 mois) de la greffe, les patients qui ont subi une néphrectomie du greffon en raison d’un échec précoce ont montré une baisse du PRA, passant de 46 % au moment de l’échec de la greffe à 27 % lors du dernier suivi (p = 0,02); en revanche, le PRA a continué d’augmenter chez les patients du groupe II qui ont présenté un échec précoce de la greffe. Dans les deux groupes, les patients ayant présenté un échec tardif de la greffe présentaient toujours un PRA élevé lors du dernier suivi.
Conclusion :
La néphrectomie du greffon peut contribuer à limiter l’allosensibilisation dans les cas d’échec précoce de la greffe, mais pas dans les cas d’échec tardif.
Introduction
The number of patients returning to dialysis due to poor renal allograft function is significant and represents over 10% of the total dialysis population each year.1,2 Unfortunately, allosensitization presents a considerable barrier to re-transplantation in these patients.2,3 Percent panel reactive antibody (%PRA), a surrogate marker of allosensitization, has been reported to rise significantly after a failed renal allograft, as the graft continues to be a source of antigenic stimulation for anti-human leukocyte antigen (HLA) antibodies.4 As a consequence, these highly sensitized recipients may be disadvantaged by prolonged waiting times, as well as inferior repeat allograft survival rates; these recipients often suffer from complications secondary to increased immunosuppressive requirements.5,6
Considerable debate persists regarding the optimal management of patients with a failed renal allograft. However, it is widely accepted that not all failed allografts need removal.7,8 While early post-transplant allograft nephrectomy (AN) for vascular thromboses, infections and irreversible or accelerated rejections remain mandatory, the management of the chronically rejected kidney poses a challenge. Certain indications, such as prolonged fever, graft tenderness, hematuria, uncontrolled hypertension and recurrent infections, are accepted indications for AN in the chronically rejected graft, yet several centres continue to perform AN to also prevent allosensitization.9 Although previous studies, including our own, confirm that %PRA increases after renal transplantation and that AN does not appear to mitigate this sensitization, it is not known whether the timing of AN affects allosensitization.7,10,11 For patients who are not candidates for AN or for those with chronically rejected grafts, immunosuppression may be discontinued while they continue to wait for a second transplant.2,12 Surprisingly, the effects of this widely accepted strategy on allosensitization are not well-documented.
The aim of this study is to determine the relationship between the timing of AN and the changes in %PRA. Additionally, we hypothesize that the management of immunosuppression in patients with failed allografts may affect the %PRA in patients placed on the waiting list for re-transplantation.
Materials and methods
Between May 1994 and June 2001, 132 patients were diagnosed with primary renal graft failure at our centre. All appropriate approvals from our Institutional Review Board were obtained prior to starting this analysis.
Overall, the mean patient age was 48 ± 12 years (90 males, 42 females). Median primary allograft survival was 5.2 years with a median patient follow-up of 2.9 years after graft failure. Of these patients, 90 had undergone AN (Group I, 64 males, 26 females), whereas the remaining 42 patients were placed on the transplant waiting list (Group II, 26 males, 16 females) under varying degrees of immunosuppression.
We evaluated various parameters, including patient demographics, cause of original end-stage renal disease, graft survival, %PRA levels before and at various intervals after transplant, reasons for AN and any associated complications. The PRA testing was carried out using a complement-dependent cytotoxicity assay (AHG-enhanced in the case of T cells). No patients received blood transfusions while in hospital, however, we could not ascertain whether any transfusions were given at satellite dialysis centres. All patients undergoing AN had their immunosuppression terminated after the procedure. Patients who did not receive AN remained on 1 of 3 protocols of immunosuppression: (1) no wean (maintained on low-dose calcineurin inhibitor and prednisone); (2) partial wean (maintained on low-dose prednisone); and (3) total wean (withdrawn from immunosuppression at time of graft loss). Subgroup analysis was carried out to determine if the timing of AN resulted in a change in overall %PRA within subgroups.
Statistics were carried out using Students t-test (SPSS 11.0, Chicago, IL). All data are reported as mean ± standard deviation. Statistical significance was accepted at the 95% confidence interval.
Results
There was no significant age difference between patients in Group I and II (45 ± 12 years, 48 ± 11 years, respectively) and there were more males in Group I (71% vs. 26%, p = 0.03) (Table 1). The etiologies of renal dysfunction were similar between the 2 groups and were congruent with previous reports.13 Although graft survival was not statistically significant between the 2 groups (Group I: 5.4 ± 5.7 years vs. Group II: 6.8 ± 4.3 years, p = 0.15), the time to the last follow-up after the AN in Group I or graft failure in Group II was greater in the latter cohort (35 ± 32 months vs. 60 ± 50 months, p = 0.14). Of the 90 patients who received AN, 21% were due to technical failure, 20% to acute onset rejection and hemorrhage, 2% to hyperacute rejection, 3% due to primary non-function, 19% to permit weaning of immunosuppression, 12% to chronic or recurrent infections and 23% to other non-specific causes (Table 1). Significant complications related to AN included 9 minor complications (postoperative hemorrhage [n = 1]; cerebrovascular accident [n = 1]; incisional hernia [n = 2]; wound infection [n = 3]; deep vein thromboses [n = 1]; infectious colitis [n = 1]) and 4 major complications which led to perioperative death (hemorrhage [n = 1]; cerebrovascular accidents [n = 2]; pulmonary embolism [n = 1]).
Table 1.
Patient characteristics in Group I (allograft nephrectomy) and Group II (wean protocol without allograft nephrectomy)
| Category | Group I | Group II | pvalue |
|---|---|---|---|
| n | 90 | 42 | -- |
| Age (years) | 45 ± 12 | 48 ± 11 | NS |
| Sex (n, %) | |||
| Male | 64 (71) | 26 (62) | 0.03 |
| Female | 26 (29) | 16 (38) | 0.03 |
| Cause of ESRD (n, %) | |||
| HTN | 22 (24) | 9 (21) | NS |
| DM | 19 (21) | 8 (19) | NS |
| GN | 20 (22) | 9 (21) | NS |
| VUR | 12 (13) | 7 (17) | NS |
| PCKD | 3 (3) | 2 (4) | NS |
| SLE | 1 (1) | 1 (2) | NS |
| Other | 13 (14) | 6 (14) | NS |
| Graft survival (years) | 5.4 ± 5.7 | 6.8 ± 4.3 | NS |
| Follow-up (months) | 35 ± 32 | 60 ± 50 | 0.14 |
| Reason for AN (n, %) | |||
| Technical failure | 19 (21) | – | – |
| Acute rejection | 18 (20) | – | – |
| Hyperacute rejection | 2 (2) | – | – |
| Primary non-function | 3 (3) | – | – |
| Chronic allograft nephropathy | 17 (19) | – | – |
| Infection | 10 (12) | – | – |
| Other | 21 (23) | – | – |
| AN (n, %) | |||
| Early (<6 months) | 39 (43) | – | – |
| Late (>6 months) | 51 (57) | – | – |
| Complications | |||
| None | 77 (86) | – | – |
| Minor | 9 (10) | – | – |
| Major | 4 (4) | – | – |
| Immunosuppression wean protocol (n, %) | |||
| No wean | 7 (17) | – | |
| Partial wean | 21 (50) | – | |
| Total wean | 90 (100) | 14 (33) | – |
ESRD: End-stage renal disease; HTN: hypertension; DM: diabetes mellitus; GN: glomerulonephritis; VUR: vesicouretric reflux; PCKD: polycystic kidney disease; SLE: systemic lupus erythematosus; AN: allograft nephrectomy; NS: not significant.
All patients in Group 1 were completely weaned off their immunosuppression, whereas in Group II, 17% (n = 7), 50% (n = 21) and 33% (n = 33) of patients received no weaning, partial weaning or total weaning of their maintenance immunosuppression (Table 1). Decisions for the type of weaning therapy were based on timing of the graft failure, residual renal function at the time of graft failure and physician preference.
Patients in Group I had %PRA levels increase from a baseline of 9.1 ± 15.3% pre-transplantation to 34.2 ± 30.4% at the time of AN; levels continued to increase up to 6 months after the procedure to 45.0 ± 38.1% and then declined by the time of last follow-up to 33.8 ± 30.4% (p = 0.01 vs. the levels at 6 months). Although a similar rise in %PRA was observed following transplantation in Group II patients (pre-transplant: 9.2 ± 18.6% vs. last follow-up: 35.4 ± 35.2%, p = 0.001), these patients did not exhibit a peak %PRA at 6 months after graft failure, as seen in Group I, but demonstrated a gradual rise in %PRA.
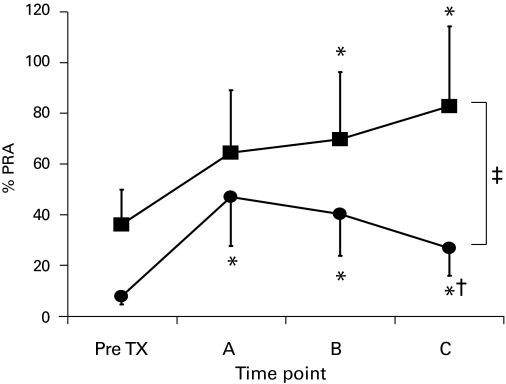
In the subgroup analysis, we found that in the 39 Group I patients who underwent early AN (<6 months following transplantation), %PRA levels declined within the first 6 months after AN and decreased until the time of last follow-up (pre-transplant: 7.7 ± 15.2%; time of AN: 46.2 ± 29.7% and last follow-up: 26.8 ± 28.9%, p = 0.02 vs. time of AN) (Fig. 1). In comparison, the subgroup of 6 Group II patients who also had early graft failure (<6 months following transplantation) and were maintained on maintenance immunosuppression showed a gradual increase in %PRA from baseline of 36.2 ± 36.8% (pre-transplant) to 82.8 ± 29.4% at the time of last follow-up (p = 0.02) (Fig. 1). At the time of last follow-up, %PRA levels were significantly lower between patients who received AN for early graft failure versus those who were maintained on immunosuppression (p < 0.03). Interestingly, 5 people in Group II were totally weaned off their immunosuppression and only 1 was left on partial immunotherapy.
Fig. 1.
Percent panel reactive antibody (%PRA) taken at various time points (A: at time of graft failure/allograft nephrectomy, B: 6 months after graft failure/allograft nephrectomy, and C: last follow-up) in patients who received early allograft nephrectomy (<6 months post-transplant, black circles) and those who were maintained on immunosuppression despite early graft failure (<6 months post-transplant, black squares). *p < 0.05 vs. pre-transplant, †p = 0.02 vs. A, ‡p < 0.05 between the 2 groups; TX: transplant.
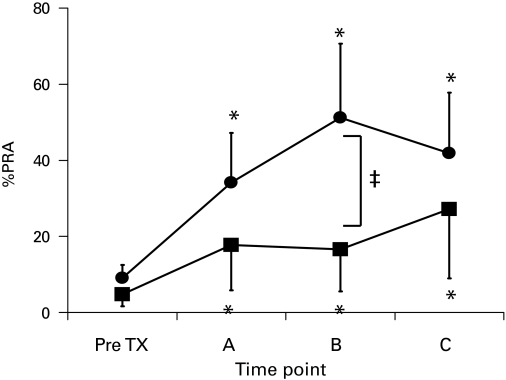
In comparison, 51 of the Group I patients who underwent late AN (>6 months after transplantation) revealed that %PRA continued to rise from pre-transplant values of 9.0 ± 11.1% to 34.2 ± 30.4% at the time of nephrectomy and to 41.9 ± 30.1% at the time of last follow-up (p = 0.002) (Fig. 2). Group II patients who developed late graft failure (>6 months after transplantation) followed a similar rising trend in %PRA levels from 4.8 ± 9.0% at baseline to 17.7 ± 26.2% at time of graft failure and to 27.7 ± 29.4% at the time of last follow-up (p = 0.02) (Fig. 2). Within this cohort of Group II patients (n = 36), 9 underwent total weaning, 18 had partial weaning and 8 had no weaning of immunosuppression.
Fig. 2.
Percent panel reactive antibody taken at various time points (A: at time of graft failure, B: 6 months after graft failure/allograft nephrectomy, and C: last follow-up) in patients who received late allograft nephrectomy (>6 months post transplant, black circles) and those who were maintained on immunosuppression despite late graft failure (>6 months post transplant, black squares). *p < 0.05 vs. pre-transplant, ‡p < 0.05 between the 2 groups; TX: transplant.
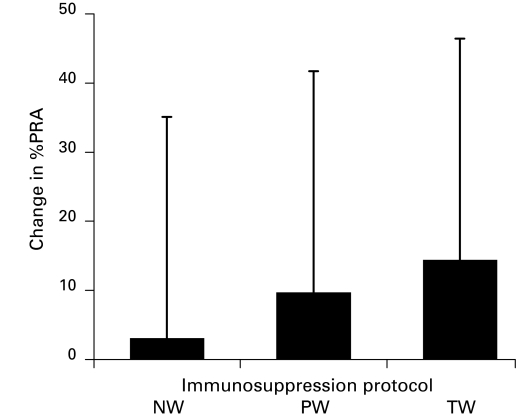
Fig. 3 demonstrates the change in %PRA in Group II patients who were placed on various immunosuppressive regimens following graft failure. Although not statistically significant, patients who were completely weaned off immunosuppression had greater elevations in the %PRA (increase of 15%) at the time of last follow-up compared with patients who had their immunotherapy partially weaned (increase of 9%) or not weaned (increase of 3%).
Fig. 3.
Change in percent panel reactive antibody from time of graft failure to time of last follow-up in various immunosuppression withdrawal protocols. NW: No wean; PW: partial wean; TW: total wean, p = NS.
Discussion
Re-transplantation occurs in up to 25% of patients requiring renal replacement therapy.14 This paper highlights the importance of minimizing the extent of allosensitization in renal transplant patients to not only maximize the lifespan of the allograft, but also to abrogate the potentially detrimental immunological effects of a failed graft on re-transplantability.
It has been argued that a failed renal transplant is a continuous source of antigenic stimulation for anti-HLA antibodies and thus may decrease the possibility of finding a crossmatch-negative second kidney.11 Apart from this immunological effect, a failed allograft left in situ may also induce a chronic inflammatory response leading to erythropoietin resistance, hypoalbuminemia and infection.15,16 On the contrary, others have argued that the removal of a nonfunctional allograft is followed by a rise in HLA-antibodies, suggesting that the graft may be acting as an “immunological sponge” to absorb low levels of allo-antibodies or may regulate the capacity of the recipient’s immune system to mount a response to the donor’s major histocompatibility complex (MHC) antigens through the activation of regulatory T cells.17–19 In addition, the failing graft may provide some residual diuresis and solute clearance which may assist in fluid balance during dialysis.
Lair and colleagues showed, in an experimental murine cardiac transplant model and in a large cohort of human kidney transplant patients, that the presence of the first rejected graft did not influence the survival of the second transplant.4 In addition, these authors found a higher incidence of anti-HLA antibodies and a higher %PRA in re-transplant patients who underwent primary AN. More recently, Ahmad and colleagues similarly showed that nephrectomy of a failed graft did not influence the survival of a second transplant when compared to patients with retained failed allografts who also received second transplants.7 Using multivariate analysis, however, it was found that the only predictor of patient and graft survival was %PRA prior to the second transplant. Both these contemporary studies confirm older reports by Sumrani and colleagues who showed that patients undergoing AN prior to re-transplantation had a higher %PRA and thus a higher incidence of delayed graft function in subsequent transplants. Importantly, rates of acute rejection and long-term graft outcomes were similar between patients who had undergone AN and those who retained their grafts.20 These studies spanning several decades concur with our data that %PRA does increase following AN for late graft losses (>6 months following transplantation). Not surprisingly, we also demonstrate that %PRA continued to rise even if the failed graft was left in situ, albeit to a lesser degree.
Our study is the first to confirm that the time of allograft failure and subsequent AN may influence long-term allosensitization. This phenomenon is clinically important in managing patients with early graft failure. Similar to previous studies mentioned above, we reaffirm that the common practice to perform AN in patients experiencing graft failure within 6 months of transplantation should be continued to minimize further sensitization and to maximize the possibility of future retransplantation. Conversely, we were unable to demonstrate that AN has a beneficial effect against allosensitization in late graft failure. Donor-specific PRA following an AN was not evaluated in any of our patients, but should be carried out in future trials.
Although AN is technically straightforward, the potential for significant morbidity should be considered given the inherent comorbidities observed in most renal failure patients. Compared with previous authors reporting morbidity rates of up to 20% and 39%, respectively,1,21 our study demonstrated 10% morbidity and 4% mortality rates.
Subgroup analysis showed that in the small group of patients who did not receive AN for early graft failure, %PRA rose significantly throughout the period of follow-up. An explanation for these findings likely resides in their higher %PRA prior to transplantation compounded with either primary non-function (n = 2) and steroid-resistant rejection (n = 4). Although antibody-mediated rejection may have also contributed to the rapid graft loss, neither donor-specific antibody testing nor histopathologic stains were available during the time of this study to confirm this speculation. In addition, almost all of these patients had complete immunosuppression withdrawal at the time of graft failure, which may have played a significant contribution to further allosensitization in these already sensitized individuals. Further studies using a larger cohort of patients will be better apt at discerning the immunological outcomes in patients undergoing early graft failure.
The debate continues over how much and how long immunosuppression should be maintained following allograft loss. Strategies include early discontinuation of immunosuppression to prolonged or gradual tapering of medication or continuation of low-dose maintenance therapy to minimize rejection and maintain dieresis.22–26 More recently, Morales and colleagues showed that in patients who developed late allograft failure (mean 44 months after transplant), the immediate withdrawal of mycophenolate mofetil followed by progressive withdrawal of calcineurin inhibitors and prednisone over 3 months was successful in 62% of patients, whereas the remaining 38% of patients still developed episodes of acute rejection requiring pulse steroids (50%), coil embolization of the graft (48%) and AN (2%).8 Interestingly, 46% of those patients who were successfully weaned off immunosuppression developed significant increases in their %PRA which complicated their chances for re-transplantation. We show that patients with grafts left in situ, %PRA continued to rise gradually until the time of last follow-up. Interestingly, when the data were analyzed according to the type of immunosuppressive regimen, patients who were continued on low-dose immunotherapy had the least overall increase in %PRA at the time of last follow-up compared to patients that had total and immediate withdrawal that showed the greatest increase in %PRA within the same time frame. Taking into consideration that the patients in all 3 protocols had similarly low-starting %PRAs, the greater increase in the latter group suggests that the in situ graft may be a source of antigenic stimulation for anti-donor HLA antibodies to form. Due to small overall numbers, these changes in %PRA were not shown to be statistically significant. Nevertheless, although the current study was not powered enough to make a formal conclusion, we highlight a potential trend which requires further investigation.
Conclusion
Overall, although this study is retrospective in nature, the data are the first to suggest that the time of graft failure and subsequent AN may play a significant role in allosensitization. Specifically, AN in patients experiencing graft failure within 6 months of transplantation may assist in minimizing further sensitization and maximizing the possibility of future re-transplantation. Conversely, we were unable to demonstrate that AN has a beneficial effect against allosensitization in late graft failure. In addition, the potential role of immunosuppressive therapy in modulating allosensitization in the post-graft failure period cannot be discounted and requires further investigation.
Table 2.
Overall %PRA profiles following renal transplantation in Group I (allograft nephrectomy) and Group II (wean protocol without allograft nephrectomy) patients
| Pre-transplantation | Time of graft failure/allograft nephrectomy | 6 months after graft failure | Last follow-up | |
|---|---|---|---|---|
| Group I (%) | 9.1 ± 15.3 | 34.2 ± 30.4* | 45.5 ± 38.1* | 33.8 ± 30.4* |
| Group II (%) | 9.2 ± 18.6 | 24.4 ± 32.3* | 24.4 ± 31.6*† | 35.4 ± 35.2* |
Values are mean ± standard deviation,
p < 0.05 vs. pre-transplant and
p < 0.05 vs. Group I. PRA: panel reactive antibody.
Footnotes
Competing interests: None declared.
This paper has been peer-reviewed.
References
- 1.Vanrenterghem Y, Khamis S. The management of the failed renal allograft. Nephrol Dial Transplant. 1996;11:955–7. [PubMed] [Google Scholar]
- 2.Bennett WM. The failed renal transplant: in or out? Semin Dial. 2005;18:188–9. doi: 10.1111/j.1525-139X.2005.18306.x. [DOI] [PubMed] [Google Scholar]
- 3.Schweitzer EJ, Wilson JS, Fernandez-Vina M, et al. A high panel-reactive antibody rescue protocol for cross-match-positive live donor kidney transplants. Transplantation. 2000;70:1531–6. doi: 10.1097/00007890-200011270-00023. [DOI] [PubMed] [Google Scholar]
- 4.Lair D, Coupel S, Giral M, et al. The effect of a first kidney transplant on a subsequent transplant outcome: an experimental and clinical study. Kidney Int. 2005;67:2368–75. doi: 10.1111/j.1523-1755.2005.00343.x. [DOI] [PubMed] [Google Scholar]
- 5.Hardy S, Lee SH, Terasaki PI. Sensitization 2001. Clin Transpl. 2001:271–8. [PubMed] [Google Scholar]
- 6.Speiser DE, Jeannet M. Renal transplantation to sensitized patients: decreased graft survival probability associated with a positive historical crossmatch. Transpl Immunol. 1995;3:330–4. doi: 10.1016/0966-3274(95)80019-0. [DOI] [PubMed] [Google Scholar]
- 7.Ahmad N, Ahmed K, Mamode N. Does nephrectomy of failed allograft influence graft survival after re-transplantation. Nephrol Dial Transplant. 2009;24:639–42. doi: 10.1093/ndt/gfn567. Epub 2008 Oct 13. [DOI] [PubMed] [Google Scholar]
- 8.Morales A, Gavela E, Kanter J, et al. Treatment of renal transplant failure. Transplant Proc. 2008;40:2909–11. doi: 10.1016/j.transproceed.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 9.Noel C, Hazzan M, Boukelmoune M, et al. Indication for allograft nephrectomy after irreversible rejection: is there an ideal delay? Transplant Proc. 1997;29:145–6. doi: 10.1016/s0041-1345(96)00041-3. [DOI] [PubMed] [Google Scholar]
- 10.Khakhar AK, Shahinian VB, House AA, et al. The impact of allograft nephrectomy on percent panel reactive antibody and clinical outcome. Transplant Proc. 2003;35:862–3. doi: 10.1016/s0041-1345(02)04031-9. [DOI] [PubMed] [Google Scholar]
- 11.Langone AJ, Chuang P. The management of the failed renal allograft: an enigma with potential consequences. Semin Dial. 2005;18:185–7. doi: 10.1111/j.1525-139X.2005.18305.x. [DOI] [PubMed] [Google Scholar]
- 12.Naini AE, Harandi AA, Daemi P, et al. Outcome of patients without any immunosuppressive therapy after renal allograft failure. Saudi J Kidney Dis Transpl. 2008;19:59–61. [PubMed] [Google Scholar]
- 13.Suthanthiran M, Strom TB. Renal transplantation. N Engl J Med. 1994;331:365–76. doi: 10.1056/NEJM199408113310606. [DOI] [PubMed] [Google Scholar]
- 14.Coupel S, Giral-Classe M, Karam G, et al. Ten-year survival of second kidney transplants: impact of immunologic factors and renal function at 12 months. Kidney Int. 2003;64:674–80. doi: 10.1046/j.1523-1755.2003.00104.x. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Gomez JM, Perez-Gomez I, Jofre R, et al. Presence of a failed kidney transplant in patients who are on hemodialysis is associated with chronic inflammatory state and erythropoietin resistance. J Am Soc Nephrol. 2004;15:2494–501. doi: 10.1097/01.ASN.0000137879.97445.6E. [DOI] [PubMed] [Google Scholar]
- 16.Ayus JC, Achinger SG. At the peril of dialysis patients: ignoring the failed transplant. Semin Dial. 2005;18:180–4. doi: 10.1111/j.1525-139X.2005.18304.x. [DOI] [PubMed] [Google Scholar]
- 17.Carmalho I, Lopez-Carvalho T, Ostler D, et al. Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. J Exp Med. 2003;197:403–11. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lucchiari N, Panajotopoulos N, Xu C, et al. Antibodies eluted from acutely rejected renal allografts bind to and activate human endothelial cells. Hum Immunol. 2000;61:518–27. doi: 10.1016/s0198-8859(00)00109-9. [DOI] [PubMed] [Google Scholar]
- 19.Martin L, Guignier F, Mousson C, et al. Detection of donor-specific anti-HLA antibodies with flow cytometry in eluates and sera from renal transplant recipients with chronic allograft nephropathy. Transplantation. 2003;76:395–400. doi: 10.1097/01.TP.0000078895.24606.45. [DOI] [PubMed] [Google Scholar]
- 20.Sumrani N, Delaney V, Hong JH, et al. The influence of nephrectomy of the primary allograft on retransplant graft outcome in the cyclosporine era. Transplantation. 1992;53:52–5. doi: 10.1097/00007890-199201000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Sharma DK, Pandey AP, Nath V, et al. Allograft nephrectomy--a 16-year experience. BJU Int. 1989;64:122–4. doi: 10.1111/j.1464-410x.1989.tb05969.x. [DOI] [PubMed] [Google Scholar]
- 22.Jassal SV, Lok CE, Walele A, et al. Continued transplant immunosuppression may prolong survival after return to peritoneal dialysis: results of a decision analysis. Am J Kidney Dis. 2002;40:178–83. doi: 10.1053/ajkd.2002.33927. [DOI] [PubMed] [Google Scholar]
- 23.Silberman H, Fitzgibbons TJ, Butler J, et al. Renal allografts retained in situ after failure. Arch Surg. 1980;115:42–3. doi: 10.1001/archsurg.1980.01380010034006. [DOI] [PubMed] [Google Scholar]
- 24.Smak Gregoor PJ, Zieste R, van Saase JL, et al. Immunosuppression should be stopped in patients with renal allograft failure. Clin Transpl. 2001;15:397–401. doi: 10.1034/j.1399-0012.2001.150606.x. [DOI] [PubMed] [Google Scholar]
- 25.Madore F, Hebert MJ, Leblanc M, et al. Determinants of late allograft nephrectomy. Clin Nephrol. 1985;44:284–9. [PubMed] [Google Scholar]
- 26.Hansen BL, Rohr N, Svedsen V, et al. Graft failure and graft nephrectomy without severe complications. Nephrol Dial Transplant. 1987;2:189–90. [PubMed] [Google Scholar]