Abstract
BACKGROUND
Survival after a glioblastoma multiforme (GBM) diagnosis remained static during the several decades before 1999. We hypothesized that the progressive increase in temozolomide use for GBM treatment that began in 1999 in the United States would be paralleled by a corresponding improvement in survival.
METHODS
We included 19,674 GBM cases, ages 20 years or greater, diagnosed 1993–2007 in the population-based Surveillance, Epidemiology, and End Results Program database. We used proportional hazards models to calculate calendar period hazard ratios (HR) and 95% confidence intervals (CI), adjusted for demographic covariates. We compared survival across periods using the Kaplan-Meier method.
RESULTS
Starting with cases diagnosed in 1999–2001, we observed a progressive decrease in HRs compared with cases diagnosed in 1993–1995. The multivariate-adjusted HR for 2005–2007 versus 1993–1995 was 0.69 (95% CI, 0.65–0.72). Age-stratified analyses revealed that this progressive decrease occurred in all age groups except 80+ years. Two-year survival increased from 7% among cases diagnosed in 1993–1995 and 1996–1998 to 9% among cases diagnosed in 1999–2001, 13% in 2002–2004, and 17% in 2005–2007. The disparity in survival between young and old patients increased in the temozolomide era, with two-year survival of 39% among cases diagnosed at ages 20–44 years and 1% among cases diagnosed at 80+ years in 2005–2007.
CONCLUSIONS
We observed a modest, but meaningful, population-based survival improvement for GBM patients in the United States. Widespread adoption of temozolomide represents the most likely explanation, although other treatment advances, such as increased extent of surgical resection, also may have played a role.
Keywords: glioblastoma, brain neoplasms, survival, temozolomide, SEER Program
INTRODUCTION
Glioblastoma multiforme (GBM), the most malignant form of brain cancer, has a dismal prognosis.1 In the several decades before 1999, survival after a diagnosis of GBM remained static.2–4 However, in 1999, temozolomide (TMZ), an oral alkylating agent that penetrates the blood-brain barrier, was approved by the FDA for treatment of recurrent anaplastic astrocytoma.5 Between 1999 and 2005, off-label use of TMZ for treatment of GBM (both first-line and after relapse) became increasingly widespread6–10 due to the drug's ease of administration and tolerability,7,11 promotion of its off-label use by the manufacturer,12 and the lack of an attractive chemotherapy alternative.11
Then, in 2005, a landmark phase 3 trial demonstrated that surgical resection, followed by radiotherapy plus concomitant TMZ, followed by adjuvant TMZ, resulted in a statistically significant and clinically meaningful survival benefit compared to therapy with surgery followed by radiotherapy, with minimal added toxicity.13 Median survival was 14.6 months in the group that received radiotherapy plus TMZ (with two-year survival of 26.5%) compared with median survival of 12.1 months in the group that received radiotherapy alone (with two-year survival of 10.4%). In March 2005, the U.S. Food and Drug Administration (FDA) approved this new regimen for patients with newly diagnosed GBM.14 Maximum safe surgical resection, followed by radiotherapy with concomitant TMZ, followed by adjuvant TMZ, became the new standard of care for first-line treatment.
However, patients enrolled in clinical trials differ from and may have better outcomes than patients treated outside the clinical trial setting due to selection of patients with better prognoses for clinical trials.15 Participants in the landmark TMZ trial were required to be between ages 18 and 70; to have a relatively high performance status; to have adequate hematologic, renal, and hepatic function; and to have received a stable or decreasing dose of corticosteroids (if on corticosteroids) for at least 14 days before randomization.13 Thus, the level of benefit found in the landmark trial may not translate into the same level of benefit in an unselected general population of GBM patients. The level of benefit in a general population compared to clinical trial patients may be eroded further by possible suboptimal access to or delivery of the standard treatment. Even after a phase 3 trial has established efficacy for a new treatment, it is essential to use observational databases to determine its “real world” effectiveness.
In the current study, we utilized data from the population-based cancer registries of the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) Program to determine whether survival among adult GBM patients has improved in the United States since the advent of TMZ. We hypothesized that starting in 1999 we would observe a progressive improvement in survival, corresponding with the increasing prevalence of TMZ use.
METHODS
Study population
We utilized data from the Surveillance, Epidemiology, and End Results (SEER) Program population-based cancer registries (SEER Research Data [1973–2007], released April 2010, based on the November 2009 submission).16 These data are available to the public from the National Cancer Institute, Division of Cancer Control and Population Sciences, Surveillance Research Program, Cancer Statistics Branch, subject to a Research Data Agreement. The Yale School of Medicine Human Investigation Committee considered this research to be exempt from review.
We defined GBM as a malignant brain neoplasm (International Classification of Diseases for Oncology, third edition [ICD-O-3] topography codes C710–C719 and behavior code 3) with ICD-O-3 morphology codes of 9440 (glioblastoma, NOS), 9441 (giant cell glioblastoma), or 9442 (gliosarcoma).17 Our analyses included adult GBM cases (age at diagnosis 20 years or greater) diagnosed between January 1993 and December 2007. Cases diagnosed between 1993 and 1999 were from the SEER 13 Registries Database (Alaska Native, Atlanta, Connecticut, Detroit, Hawaii, Iowa, Los Angeles, New Mexico, Rural Georgia, San Francisco-Oakland, San Jose-Monterey, Seattle-Puget Sound, and Utah). Cases diagnosed between 2000 and 2007 were from the SEER 17 Registries Database (SEER 13 plus Greater California, Kentucky, Louisiana, and New Jersey). The SEER 13 registries cover about 14% of the total U.S. population and the SEER 17 registries cover about 26% of the total U.S. population.18
There were a total of 24,793 adult GBM cases diagnosed between January 1993 and December 2007 in these SEER registries. However, we restricted our cohort to microscopically-confirmed cases experiencing their first primary cancer and not diagnosed by death certificate or autopsy only (Figure 1). Epidemiologists and neuropathologists have reached a consensus that a microscopically-confirmed diagnosis of GBM is likely to be valid without further centralized pathology review.19 Our final analytic cohort consisted of 19,674 GBM cases.
Figure 1.
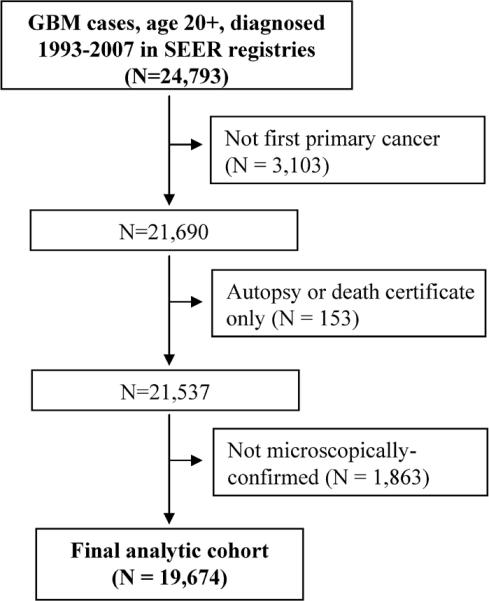
Selection of the glioblastoma multiforme (GBM) analytic cohort from the Surveillance, Epidemiology, and End Results Program (SEER) database
Data analysis and statistical methods
The study endpoint was overall survival. The cause of death for patients diagnosed with GBM is likely GBM due to their characteristic short duration of survival. The SEER dataset provides follow-up time in number of completed months. We measured overall survival time as the number of completed months from the date of GBM diagnosis to the earliest of the date of death, date last known to be alive, or the follow-up cutoff date of December 31, 2007. Those living past this date were censored.
We grouped calendar year of diagnosis, the primary predictor variable, into five three-year periods (1993–1995, 1996–1998, 1999–2001, 2002–2004, and 2005–2007). Because age is an important prognostic factor for GBM survival,1 we performed age-stratified analyses with age groups 20–44 years, 45–64 years, 65–79 years and 80+ years. We also performed analyses stratified by first course of surgical or radiotherapy treatment. Surgical treatment was recoded from SEER codes to resection, biopsy, or unknown. Radiotherapy treatment was recoded to radiotherapy (external beam), none, or other (implants or isotopes)/unknown. The SEER database does not provide data on chemotherapy treatment.
We used SAS version 9.2 for all analyses. We used Proc Phreg to estimate calendar period hazard rate ratios (HR) and 95% confidence intervals (CI) for death from univariate and multivariate Cox proportional hazards regression models. In multivariate models we adjusted for age (five-year age groups), sex, race/ethnicity (non-Hispanic White, Hispanic White, Black, Asian/Pacific Islander, American Indian/Alaskan Native, and other/unknown), and marital status (married, not married [single, widowed, separated or divorced], and unknown). We included marital status because it has been shown to influence overall survival in GBM patients.20,21 In age-stratified analyses, we continued to include age as a covariate with five-year age groups. To determine a p-value for heterogeneity, we entered the appropriate cross-product term into the model and conducted a likelihood ratio test for its addition, with the appropriate degrees of freedom.
We used Proc Lifetest to generate Kaplan-Meier survival curves and to calculate the percent of subjects alive at two years and five years (and 95% confidence intervals), median survival (and 95% confidence interval) and a stratified log-rank test of equality across periods (stratified by age, sex, race/ethnicity, and marital status). The SEER dataset provides follow-up time in number of completed months (not days). Cases who survived at least n months, but fewer than n+1 months, would have n completed months of survival. On the average, these cases survived “n.5” months. For the purpose of calculating median survival we therefore added 0.5 months to the result derived from completed months.
Results (not shown) were essentially unchanged when further adjusting for SEER registry, when restricting analyses to the SEER 13 registries, or when including cases that were not first primary cancers or were not microscopically-confirmed. All statistical tests were two-sided with α = 0.05.
RESULTS
Table 1 shows the demographic and clinical characteristics of the GBM cases. Eighty-seven percent (17,110) of the 19,674 cases died and 13% (2,564) were censored. Cases diagnosed in 2005–2007 had a substantially lower proportion of deaths than cases diagnosed in earlier time periods at least partially because follow-up time was the shortest for these cases (maximum of 35 completed month). There were considerably more cases diagnosed in 1999–2001, 2002–2004, and 2005–2007 compared to 1993–1995 and 1996–1998 because after 1999 cases were included from four additional cancer registries (SEER 17 registries versus SEER 13 registries). Most cases were non-Hispanic White and were diagnosed between ages 45 and 79 years. The majority of cases were male. The first course of treatment included surgical resection for 77% of patients and radiotherapy for 73% of patients.
Table 1.
Demographic and clinical characteristics of adult glioblastoma multiforme (GBM) cases, SEER, 1993–2007
| Characteristic | GBM cases No. (% of total) | Deaths No. (% of cases) |
|---|---|---|
| Total | 19,674 (100) | 17,110 (87) |
| Calendar period diagnosed | ||
| 1993–1995 | 2,257 (11) | 2,219 (98) |
| 1996–1998 | 2,376 (12) | 2,339 (98) |
| 1999–2001 | 4,165 (21) | 4,039 (97) |
| 2002–2004 | 5,338 (27) | 5,024 (94) |
| 2005–2007 | 5,538 (28) | 3,489 (63) |
| Age at diagnosis | ||
| 20–44 years | 2,219 (11) | 1,682 (76) |
| 45–64 years | 8,976 (46) | 7,561 (84) |
| 65–79 years | 7,049 (36) | 6,497 (92) |
| 80+ years | 1,430 (7) | 1,370 (96) |
| Sex | ||
| Male | 11,382 (58) | 9,897 (87) |
| Female | 8,292 (42) | 7,213 (87) |
| Race/Ethnicity | ||
| Non-Hispanic White | 16,041 (82) | 14,119 (88) |
| Hispanic White | 1,780 (9) | 1,454 (82) |
| Black | 960 (5) | 817 (85) |
| Asian/Pacific Islander | 799 (4) | 656 (82) |
| American Indian/Alaskan Native | 60 (0.3) | 45 (75) |
| Other/Unknown | 34 (0.2) | 19 (56) |
| Marital status at diagnosis | ||
| Married | 13,017 (66) | 11,261 (87) |
| Not marrieda | 6,134 (31) | 5,389 (88) |
| Unknown | 523 (3) | 460 (88) |
| First course of treatment | ||
| Surgery | ||
| Resection | 15,130 (77) | 12,854 (85) |
| Biopsy | 4,394 (22) | 4,110 (94) |
| Unknown | 150 (1) | 146 (97) |
| Radiotherapy | ||
| Radiotherapy (external beam) | 14,371 (73) | 12,167 (85) |
| None | 4,688 (24) | 4,378 (93) |
| Otherb/unknown | 615 (3) | 565 (92) |
Single, widowed, separated, or divorced
Implant or isotope
In a Cox proportional hazards model including all cases, the calendar period effect was homogeneous according to sex (p-value for heterogeneity = 0.76), race/ethnicity (p-value for heterogeneity = 0.57), and marital status (p-value for heterogeneity = 0.66), but was heterogeneous according to age (p-value for heterogeneity = 0.016), indicating a difference in the period effect across age groups.
Table 2 shows calendar period HRs for death for all ages combined and stratified by age group. We also examined ages 20–69 years because the original landmark trial had an upper age limit of 70 years.13 The reference calendar period was 1993–1995. The risk of death among cases diagnosed in 1996–1998 did not differ significantly from the risk of death among cases diagnosed in 1993–1995. However, starting in 1999–2001, when TMZ came into use, we observed a progressive decrease in multivariate-adjusted HRs through 2005–2007, for all ages combined (20+) and for the age groups 20–44 years, 45–64 years, and 65–79 years. The HR for calendar period 2005–2007 was 0.69 (95% CI, 0.65–0.72) for all ages combined, and was similar for each of the age groups 20–44 years, 45–64 years, and 65–79 years. However, the HR for the age 80+ years age group did not differ from 1.00 across periods (p = 0.48). For the age range 20–69 years, the HR for calendar period 2005–2007 was 0.64 (95% CI, 0.60–0.68).
Table 2.
Calendar period hazard rate ratios and 95% confidence intervals for death derived from Cox proportional hazards models, adult glioblastoma multiforme cases, SEER, 1993–2007
| Univariate HR (95% CI) |
Multivariate-adjusted HRa (95% CI) |
||||||
|---|---|---|---|---|---|---|---|
| Age (years): | 20+ | 20+ | 20–44 | 45–64 | 65–79 | 80+ | 20–69 |
| Deaths/cases: | 17,110/19,674 | 17,110/19,674 | 1,682/2,219 | 7,561/8,976 | 6,497/7,049 | 1,370/1,430 | 11,487/13,695 |
| Period | |||||||
| 1993–1995 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 1996–1998 | 0.99 (0.94–1.05) | 0.97 (0.92–1.03) | 0.95 (0.80–1.12) | 0.96 (0.88–1.05) | 0.99 (0.90–1.08) | 1.00 (0.79–1.26) | 0.97 (0.90–1.04) |
| 1999–2001 | 0.91 (0.87–0.96) | 0.89 (0.85–0.94) | 0.82 (0.70–0.95) | 0.87 (0.81–0.94) | 0.91 (0.83–0.98) | 1.10 (0.90–1.35) | 0.87 (0.82–0.92) |
| 2002–2004 | 0.82 (0.78–0.86) | 0.78 (0.74–0.82) | 0.77 (0.66–0.90) | 0.75 (0.69–0.81) | 0.80 (0.74–0.87) | 0.96 (0.79–1.18) | 0.75 (0.71–0.80) |
| 2005–2007 | 0.72 (0.68–0.76) | 0.69 (0.65–0.72) | 0.66 (0.55–0.79) | 0.63 (0.58–0.69) | 0.70 (0.64–0.76) | 1.05 (0.86–1.29) | 0.64 (0.60–0.68) |
| p-value for period effect | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.48 | <0.0001 |
HR, hazard ratio; CI, confidence interval
Adjusted for sex, age, race/ethnicity, and marital status
When we added the first course of treatment variables to the model, we found that the period effect was heterogeneous according to both surgery (p = 0.0001) and radiotherapy (p < 0.0001). We therefore examined multivariate-adjusted calendar period HRs stratified by first course of treatment (Table 3) and found the period effect to be most pronounced among those treated with both surgical resection and radiotherapy. In this subgroup of 11,546 cases (59%), the HR for those diagnosed in 2005–2007 was 0.62 (95% CI, 0.57–0.66). Among the 1,482 patients (8%) who received biopsy only, the HR did not differ from 1.00 across periods (p = 0.63).
Table 3.
Calendar period multivariate-adjusted hazard rate ratios and 95% confidence intervals for death derived from Cox proportional hazards models, by first course of treatment, adult glioblastoma multiforme cases, SEER, 1993–2007
| Multivariate-adjusted HRa (95% CI) | |||||
|---|---|---|---|---|---|
| First course of treatmentb: | Allc | Resection and radiotherapy | Resection only | Biopsy and radiotherapy | Biopsy only |
| Deaths/cases: | 17,110/19,674 | 9,573/11,546 | 2,885/3,145 | 2,526/2,756 | 1,433/1,482 |
| Period | |||||
| 1993–1995 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 1996–1998 | 0.97 (0.92–1.03) | 0.99 (0.92–1.07) | 0.89 (0.76–1.04) | 1.12 (0.97–1.29) | 0.89 (0.72–1.11) |
| 1999–2001 | 0.89 (0.85–0.94) | 0.89 (0.83–0.96) | 0.85 (0.74–0.97) | 1.02 (0.90–1.16) | 0.88 (0.73–1.07) |
| 2002–2004 | 0.78 (0.74–0.82) | 0.74 (0.69–0.79) | 0.81 (0.71–0.93) | 0.88 (0.77–1.00) | 0.95 (0.79–1.15) |
| 2005–2007 | 0.69 (0.65–0.72) | 0.62 (0.57–0.66) | 0.78 (0.69–0.89) | 0.79 (0.69–0.91) | 0.89 (0.74–1.08) |
| p-value for period effect | <0.0001 | <0.0001 | 0.0048 | <0.0001 | 0.63 |
HR, hazard ratio; CI, confidence interval
Adjusted for sex, age, race/ethnicity, and marital status
The SEER database does not provide data on chemotherapy treatment.
Includes 745 cases with unknown surgical resection or radiotherapy status
Table 4 shows two-year and median survival by calendar period, for all cases and for cases treated with both surgical resection and radiotherapy. Consistent with the proportional hazards analyses, among all cases, survival for all ages combined did not improve appreciably during the pre-TMZ era (between 1993–1995 and 1996–1998), but did increase steadily starting in 1999–2001, when TMZ came into use. Thus, between 1993–1995 and 2005–2007, two-year survival increased from 7% to 17% and median survival increased from 7.5 months to 9.5 months. The stratified log-rank test indicated that the difference in survival among periods was highly statistically significant (p < 0.0001).
Table 4.
Two-year and median survival by calendar period, adult glioblastoma multiforme cases, SEER, 1993–2007
| Age (years): | 20+ | 20–44 | 45–64 | 65–79 | 80+ | 20–69 |
|---|---|---|---|---|---|---|
| Survival statistics by period | ||||||
| All cases | ||||||
| 2-year survival, percenta (95% CI) | ||||||
| 1993–1995 | 7 (6, 8) | 24 (20, 29) | 7 (5, 9) | 1 (1, 2) | 1 (0, 4) | 9 (7, 10) |
| 1996–1998 | 7 (6, 9) | 25 (21, 30) | 8 (7, 10) | 2 (1, 3) | 1 (0, 4) | 10 (9, 12) |
| 1999–2001 | 9 (9, 10) | 33 (28, 37) | 10 (9, 12) | 2 (2, 3) | 1 (0, 2) | 13 (12, 14) |
| 2002–2004 | 13 (12, 14) | 35 (31, 39) | 16 (14, 17) | 5 (4, 6) | 2 (1, 3) | 18 (16, 19) |
| 2005–2007 | 17 (16, 19) | 39 (33, 44) | 21 (19, 24) | 9 (7, 11) | 1 (0, 4) | 22 (21, 24) |
| Median survival, monthsb (95% CI) | ||||||
| 1993–1995 | 7.5 (7.5, 7.5) | 13.5 (12.5, 15.5) | 9.5 (8.5, 9.5) | 5.5 (4.5, 5.5) | 3.5 (2.5, 4.5) | 9.5 (8.5, 9.5) |
| 1996–1998 | 7.5 (7.5, 7.5) | 15.5 (14.5, 16.5) | 9.5 (9.5, 10.5) | 5.5 (4.5, 5.5) | 3.5 (3.5, 3.5) | 9.5 (9.5, 10.5) |
| 1999–2001 | 7.5 (7.5, ,8.5) | 16.5 (15.5, 18.5) | 10.5 (9.5, 10.5) | 5.5 (5.5, 5.5) | 3.5 (2.5, 3.5) | 10.5 (9.5, 10.5) |
| 2002–2004 | 8.5 (8.5, 9.5) | 17.5 (16.5, 19.5) | 11.5 (10.5, 11.5) | 5.5 (5.5, 6.5) | 3.5 (3.5, 4.5) | 11.5 (10.5, 11.5) |
| 2005–2007 | 9.5 (9.5, 10.5) | 18.5 (17.5, 21.5) | 12.5 (12.5, 13.5) | 6.5 (6.5, 6.5) | 3.5 (2.5, 3.5) | 12.5 (12.5, 13.5) |
| Stratified log-rank testc | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.39 | <0.0001 |
| Cases treated with surgical resection and radiotherapy | ||||||
| 2-year survival, percenta (95% CI) | ||||||
| 1993–1995 | 9 (8, 11) | 29 (23, 36) | 8 (6, 11) | 3 (1, 5) | 0 | 11 (9, 13) |
| 1996–1998 | 11 (9, 13) | 30 (24, 36) | 11 (8, 13) | 3 (2, 5) | 0 | 14 (12, 16) |
| 1999–2001 | 13 (12, 14) | 38 (33, 44) | 13 (11, 15) | 3 (2, 5) | 1 (0, 5) | 17 (15, 18) |
| 2002–2004 | 18 (17, 19) | 39 (35, 44) | 20 (18, 22) | 7 (6, 9) | 5 (2, 10) | 22 (20, 24) |
| 2005–2007 | 24 (22, 26) | 45 (38, 52) | 27 (24, 30) | 14 (11, 17) | 3 (1, 9) | 28 (26, 31) |
| Median survival, monthsb (95% CI) | ||||||
| 1993–1995 | 10.5 (9.5, 10.5) | 15.5 (13.5, 17.5) | 11.5 (10.5, 12.5) | 8.5 (7.5, 8.5) | 7.5 (4.5, 8.5) | 11.5 (11.5, 12.5) |
| 1996–1998 | 10.5 (10.5, 10.5) | 16.5 (14.5, 18.5) | 11.5 (10.5, 12.5) | 7.5 (7.5, 8.5) | 5.5 (4.5, 7.5) | 11.5 (11.5, 12.5) |
| 1999–2001 | 11.5 (10.5, ,11.5) | 19.5 (18.5, 21.5) | 12.5 (12.5, 13.5) | 7.5 (7.5, 8.5) | 5.5 (4.5, 6.5) | 13.5 (12.5, 13.5) |
| 2002–2004 | 12.5 (12.5, 12.5) | 20.5 (18.5, 22.5) | 13.5 (13.5, 14.5) | 8.5 (8.5, 9.5) | 6.5 (5.5, 7.5) | 13.5 (13.5, 14.5) |
| 2005–2007 | 13.5 (13.5, 14.5) | 21.5 (18.5, 26.5) | 15.5 (15.5, 16.5) | 10.5 (9.5, 10.5) | 5.5 (4.5, 6.5) | 16.5 (15.5, 16.5) |
| Stratified log-rank testc | <0.0001 | 0.0003 | <0.0001 | <0.0001 | 0.48 | <0.0001 |
CI, confidence interval
Probability of surviving at least 24 completed months (at least 24.5 months, on average)
SEER provides follow-up time in number of completed months. Cases who survived at least n months, but fewer than n+1 months, would have n completed months of survival, which corresponds with n.5 months, on average. We therefore added 0.5 months to the result derived from completed months.
Stratified by age, sex, race/ethnicity, and marital status
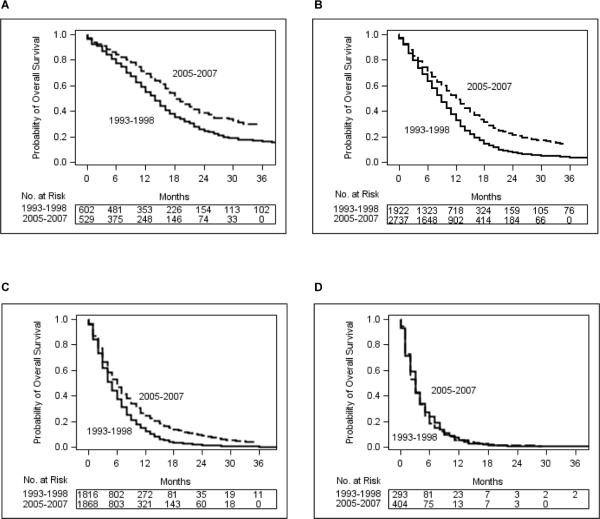
Among all cases, in each age group except for the oldest, survival increased progressively after 1998. Between 1993–1995 and 2005–2007, median survival increased from 13.5 months to 18.5 months in the 20–44 age group, from 9.5 months to 12.5 months in the 45–64 age group, and from 5.5 months to 6.5 months in the 65–79 age group. For each of these age groups, the stratified log-rank test was highly statistically significant (p < 0.0001). Age-group-specific Kaplan-Meier curves comparing cases diagnosed in 2005–2007 with cases diagnosed in the pre-TMZ era (1993–1998) clearly demonstrate the improvement in survival (Figure 2) for each of these age groups. However, survival did not improve in the age 80+ years age group, with median survival remaining constant at 3.5 months (p = 0.39).
Figure 2.
Kaplan-Meier survival plots for glioblastoma multiforme cases according to year of diagnosis and age. The x-axes indicate completed months of follow-up. SEER provides follow-up time in number of completed months. Cases who survived at least n months, but fewer than n+1 months, would have n completed months of survival, which corresponds with “n.5” months, on average. Thus, the probability of surviving at least two years (24 completed months), for example, represents the probability of surviving at least 24.5 months, on average. Age group: (A) 20–44 years. (B) 45–64 years. (C) 65–79 years. (D) 80+ years.
As expected, survival among cases treated with both surgical resection and radiotherapy was superior to survival among all cases. For cases treated with both resection and radiotherapy, between 1993–1995 and 2005–2007, median survival increased from 10.5 months to 13.5 months for all ages combined, from 15.5 months to 21.5 months in the 20–44 years age group, from 11.5 to 15.5 months in the 45–64 years age group, and from 8.5 months to 10.5 months in the 65–79 years age group. Survival did not improve in the 80+ years age group (p = 0.48).
For the age range 20–69 years, comparable to the age range in the landmark trial, among all cases diagnosed in 2005–2007, two-year survival was 22% and median survival was 12.5 months. For cases treated with both surgical resection and radiotherapy, two-year survival in 2005–2007 was 28% and median survival was 16.5 months.
Age group 20–44 years was the only age group with appreciable five-year survival, which increased between 1993–1998 and 1999–2004 (data not shown in table). For all cases in this age group, five-year survival was 12% (95% CI, 9–16%) for cases diagnosed in 1993–1995, 11% (95% CI, 8–15%) in 1996–1998, 17% (95% CI, 13–20%) in 1999–2001, and 16% (95% CI, 13–20%) in 2002–2004. For cases in age group 20–44 years treated with both surgical resection and radiotherapy, five-year survival was 14% (95% CI, 10–20%) for cases diagnosed in 1993–1995, 13% (95% CI, 9–18%) in 1996–1998, 20% (95% CI, 16–25%) in 1999–2001, and 19% (95% CI, 15–23%) in 2002–2004. Because the maximum follow-up among cases diagnosed in 2005–2007 was only 35 completed months, we were unable to estimate five-year survival for cases diagnosed in this calendar period.
DISCUSSION
We observed a modest, but meaningful, progressive improvement in survival of population-based GBM patients in the United States, starting with cases diagnosed in the 1999–2001 calendar period, when off-label use of TMZ for GBM treatment commenced (whether concurrent with first course of radiotherapy, adjuvant, and/or after relapse), and continuing through the 2005–2007 calendar period, when maximal safe surgical resection followed by radiotherapy plus concomitant TMZ followed by adjuvant TMZ became the undisputed new standard of care for first-line treatment of GBM. In the age range comparable to the landmark trial (20–69 years), the two-year survival of 22% and the median survival of 12.5 months among cases diagnosed in 2005–2007 were less favorable than the two-year survival of 26.5% and the median survival of 14.6 months observed in the landmark trial.13 It is not surprising that survival in the unselected U.S. general population of GBM patients, which included patients with poor prognoses and patients who did not receive the TMZ standard of care, lagged behind survival in the landmark trial. However, among our population-based cases treated with both surgical resection and radiotherapy, survival compared favorably with survival in the landmark trial, with two-year survival of 28% and median survival of 16.5 months among cases diagnosed in 2005–2007.
Although we demonstrated a close ecologic association between improved survival and increasing TMZ usage, because the SEER database did not include chemotherapy treatment information, we were unable to evaluate whether the patients treated with TMZ were, in fact, the ones with improved survival. Nevertheless, the increasing usage of TMZ represents the most obvious explanation for at least the majority of the improvement in survival. First, the landmark 2005 trial clearly established the efficacy of TMZ.13 Second, introduction of TMZ was the major change that occurred in GBM treatment during the overall period of observation, and there is no doubt that by 2005–2007 most non-elderly patients were being treated with the TMZ standard of care.22,23 Third, survival improved the most among cases with a first course of treatment that included both surgical resection and radiotherapy. These were the cases that had the potential to receive the complete TMZ standard of care regimen (maximum safe surgical resection, followed by radiotherapy with concomitant TMZ, followed by adjuvant TMZ). Finally, other, smaller studies of patients treated in the non-clinical trial setting in Europe observed a meaningful survival improvement with TMZ treatment.24–27
We were able to perform gross adjustment for surgery (resection vs. biopsy) and for radiotherapy (radiotherapy versus none) during the first course of treatment, but the data available in the SEER database do not permit finer adjustment. It is possible that advances in neurosurgical techniques resulting in greater extent of resection contributed to the improvement in GBM survival.28–32 Other secular trends in care, such as more aggressive treatment of tumor recurrence or enhancements in palliative care, may have contributed as well.33,34 However, we know of no data on temporal trends in extent of surgical resection or other changes in care that would allow us to test these conjectures. It is unlikely that advances in radiotherapy contributed to the survival improvement, as treatment with the more advanced technique of intensity-modulated radiotherapy does not appear to convey a survival benefit over treatment with conventional three-dimensional conformal radiotherapy.35
Other than the introduction of TMZ or other improvements in GBM treatment, there is no obvious explanation for the observed improvement in GBM survival. To control for potential confounding, we adjusted for age, sex, race/ethnicity, and marital status in the proportional hazards models. The observed survival improvement would be explained if there had been a secular diagnostic trend, starting in 1999, toward classification of less aggressive gliomas, previously considered grade III, as GBMs (grade IV gliomas). However, that the last major change in diagnostic criteria for GBM occurred with the 1993 revision of the World Health Organization Classification of Tumours of the Central Nervous System36 argues against such a “grade migration” starting in 1999. Thus, although we cannot rule out that our results are an artifact of unknown confounding or bias, the survival improvement appears to be real.
As expected, among cases diagnosed within each period, survival decreased with increasing age at diagnosis.1 Among cases diagnosed in 2005–2007, median survival decreased progressively from 18.5 months in the 20–44 years age group to a dismal 3.5 months in the 80+ years age group, the only age group that did not benefit from a survival improvement in the TMZ era. Improved survival in the youngest age group combined with static survival in the oldest age group means that the survival disparity between young and old GBM cases is now greater than ever.
Among cases diagnosed in 2005–2007 in the 20–44 years age group, the median survival of 18.5 months and the two-year survival of 39% among all cases and the median survival of 21.5 months and the two-year survival of 45% among those treated with both surgical resection and radiotherapy were substantially more encouraging than survival statistics typically quoted for GBM patients as a whole. Because the maximum follow-up among these cases was only 35 months, we were unable to obtain estimates of longer term survival during the 2005–2007 calendar period. However, even in the pre-TMZ era, we observed five-year survival among cases diagnosed in this age group to be 11–12% among all cases and 13–14% among cases treated with both surgical resection and radiotherapy, comparable to that observed in other pre-TMZ era patient series in this age range.37 Five-year survival among cases diagnosed in the 20–44 years age group improved to 16–17% for all cases and to 19–20% for cases treated with both resection and radiotherapy in the TMZ era calendar periods in which we could measure five-year survival (1999–2001 and 2002–2004). Further advances in five-year survival since 2005 in this age group, when the TMZ standard of care was definitively adopted, would not be surprising.
Reasons for the grim population-based survival among the elderly, which has been documented previously,1,3,15,38 are not totally clear. Because patients over age 70 were not included in the landmark clinical trial,13 the TMZ standard of care has not been established for this age group. The extent to which the lack of improvement in survival in the 80+ years age group was due to resistance to TMZ therapy versus patients not receiving or not tolerating TMZ therapy is a question for future research.
In conclusion, the progressive, widespread adoption of TMZ for adult GBM treatment that began in 1999 in the United States was paralleled by a modest, but meaningful, population-based survival improvement among all GBM patients except those aged 80+ years. While the increased usage of TMZ represents the most likely explanation for the survival improvement, it is possible that other treatment advances, such as increased extent of surgical resection, also played a role. Nevertheless, there remains an urgent need to develop novel efficacious treatments to build on the platform provided by the current standard of care.
Condensed abstract.
In the United States, there has been a modest, but meaningful, progressive survival improvement among adult glioblastoma multiforme patients diagnosed at all ages except 80+ years, starting with cases diagnosed in 1999–2001 and continuing through cases diagnosed in 2005–2007. This improvement coincided with the progressive, widespread adoption of temozolomide for glioblastoma treatment that also started in 1999.
Acknowledgements
We are grateful to the SEER Program for making its data publicly available.
Funding: This work was supported by grant 1 R03 CA150048 from the National Cancer Institute.
Footnotes
Financial disclosures: None
REFERENCES
- 1.Central Brain Tumor Registry of the United States (CBTRUS) CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2007. CBTRUS; Hinsdale, IL: 2011. [accessed June 16, 2011]. Available from URL: http://www.cbtrus.org/2011-NPCR-SEER/WEB-0407-Report-3-3-2011.pdf. [Google Scholar]
- 2.Barnholtz-Sloan JS, Sloan AE, Schwartz AG. Relative survival rates and patterns of diagnosis analyzed by time period for individuals with primary malignant brain tumor, 1973–1997. J Neurosurg. 2003;99:458–466. doi: 10.3171/jns.2003.99.3.0458. [DOI] [PubMed] [Google Scholar]
- 3.Davis FG, Freels S, Grutsch J, Barlas S, Brem S. Survival rates in patients with primary malignant brain tumors stratified by patient age and tumor histological type: an analysis based on Surveillance, Epidemiology, and End Results (SEER) data, 1973–1991. J Neurosurg. 1998;88:1–10. doi: 10.3171/jns.1998.88.1.0001. [DOI] [PubMed] [Google Scholar]
- 4.Deorah S, Lynch CF, Sibenaller ZA, Ryken TC. Trends in brain cancer incidence and survival in the United States: Surveillance, Epidemiology, and End Results Program, 1973 to 2001. Neurosurg Focus. 2009;20:E1. doi: 10.3171/foc.2006.20.4.E1. [DOI] [PubMed] [Google Scholar]
- 5.Schering-Plough Corporation [accessed March 14, 2011];First new chemotherapy agent for brain tumors in 20 years. Available from URL: http://prnwire.com/cgibin/stories.pl?ACCT=104&STORY=/www/story/08-11-1999/0001001481&EDATE.
- 6.Mason WP, Cairncross JG. Drug insight: temozolomide as a treatment for malignant glioma--impact of a recent trial. Nat Clin Pract Neurolo. 2005;1:88–95. doi: 10.1038/ncpneuro0045. [DOI] [PubMed] [Google Scholar]
- 7.Danson SJ, Middleton MR. Temozolomide: a novel oral alkylating agent. Expert Rev Anticancer Ther. 2001;1:13–19. doi: 10.1586/14737140.1.1.13. [DOI] [PubMed] [Google Scholar]
- 8.Wiemels JL, Wilson D, Patil C, et al. IgE, allergy, and risk of glioma: update from the San Francisco Bay Area Adult Glioma Study in the temozolomide era. Int J Cancer. 2009;125:680–687. doi: 10.1002/ijc.24369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sher DJ, Henson JW, Avutu B, et al. The added value of concurrently administered temozolomide versus adjuvant temozolomide alone in newly diagnosed glioblastoma. J Neurooncol. 2008;88:43–50. doi: 10.1007/s11060-008-9530-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vinjamuri M, Adumala RR, Altaha R, Hobbs GR, Crowell EB., Jr. Comparative analysis of temozolomide (TMZ) versus 1,3-bis (2-chloroethyl)-1 nitrosourea (BCNU) in newly diagnosed glioblastoma multiforme (GBM) patients. J Neurooncol. 2009;91:221–225. doi: 10.1007/s11060-008-9702-6. [DOI] [PubMed] [Google Scholar]
- 11.Mutter N, Stupp R. Temozolomide: a milestone in neuro-oncology and beyond? Expert Rev Anticancer Ther. 2006;6:1187–1204. doi: 10.1586/14737140.6.8.1187. [DOI] [PubMed] [Google Scholar]
- 12.McKoy JM, Samaras A, Kaplan CE, Bennett CL. Temozolomide: applying healthcare fraud and abuse laws to oncology drugs. Community Oncol. 2008;5:553–554. [Google Scholar]
- 13.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 14.Cohen MH, Johnson JR, Pazdur R. Food and Drug Administration Drug approval summary: temozolomide plus radiation therapy for the treatment of newly diagnosed glioblastoma multiforme. Clin Cancer Res. 2005;11:6767–6771. doi: 10.1158/1078-0432.CCR-05-0722. [DOI] [PubMed] [Google Scholar]
- 15.Iwamoto FM, Reiner AS, Panageas KS, Elkin EB, Abrey LE. Patterns of care in elderly glioblastoma patients. Ann Neurol. 2008;64:628–634. doi: 10.1002/ana.21521. [DOI] [PubMed] [Google Scholar]
- 16. [accessed May 7, 2010];Surveillance, Epidemiology and End Results. Available from URL: www.seer.cancer.gov.
- 17.Fritz A, Percy C, Jack A, et al., editors. International Classification of Diseases for Oncology. Third Edition. World Health Organization; Geneva: 2000. U.S. Interim Version 2000. [Google Scholar]
- 18.National Cancer Institute, Surveillance, Epidemiology, and End Results Program. [accessed March 24, 2011];Number of persons by race and Hispanic ethnicity for SEER participants (2000 Census Data) Available from URL: http://seer.cancer.gov/registries/data.html.
- 19.Davis FG, Malmer BS, Aldape K, et al. Issues of diagnostic review in brain tumor studies: from the Brain Tumor Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev. 2008;17:484–489. doi: 10.1158/1055-9965.EPI-07-0725. [DOI] [PubMed] [Google Scholar]
- 20.Chang SM, Barker FG., 2nd. Marital status, treatment, and survival in patients with glioblastoma multiforme: a population based study. Cancer. 2005;104:1975–1984. doi: 10.1002/cncr.21399. [DOI] [PubMed] [Google Scholar]
- 21.Wrensch M, Rice T, Miike R, et al. Diagnostic, treatment, and demographic factors influencing survival in a population-based study of adult glioma patients in the San Francisco Bay Area. Neuro-oncol. 2006;8:12–26. doi: 10.1215/S1522851705000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mrugala MM, Chamberlain MC. Mechanisms of disease: temozolomide and glioblastoma--look to the future. Nat Clin Pract Oncol. 2008;5:476–486. doi: 10.1038/ncponc1155. [DOI] [PubMed] [Google Scholar]
- 23.Friedman HS. State-of-the-art therapy for glioblastoma multiforme. US Oncological Dis. 2007:16–17. [Google Scholar]
- 24.Weller M, Felsberg J, Hartmann C, et al. Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: a prospective translational study of the German Glioma Network. J Clin Oncol. 2009;27:5743–5750. doi: 10.1200/JCO.2009.23.0805. [DOI] [PubMed] [Google Scholar]
- 25.van Genugten JA, Leffers P, Baumert BG, Tjon-A-Fat H, Twijnstra A. Effectiveness of temozolomide for primary glioblastoma multiforme in routine clinical practice. J Neurooncol. 2010;96:249–257. doi: 10.1007/s11060-009-9956-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bauchet L, Mathieu-Daudé H, Fabbro-Peray P, et al. Oncological patterns of care and outcome for 952 patients with newly diagnosed glioblastoma in 2004. Neuro-oncol. 2010;12:725–735. doi: 10.1093/neuonc/noq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scoccianti S, Magrini SM, Ricardi U, et al. Patterns of care and survival in a retrospective analysis of 1059 patients with glioblastoma multiforme treated between 2002 and 2007: a multicenter study by the Central Nervous System Study Group of AIRO (Italian Association of Radiation Oncology) Neurosurg. 2010;67:446–458. doi: 10.1227/01.NEU.0000371990.86656.E8. [DOI] [PubMed] [Google Scholar]
- 28.Stummer W, van den Bent MJ, Westphal M. Cytoreductive surgery of glioblastoma as the key to successful adjuvant therapies: new arguments in an old discussion. Acta Neurochir. 2011;153:1211–1218. doi: 10.1007/s00701-011-1001-x. [DOI] [PubMed] [Google Scholar]
- 29.Piepmeier JM. The future of neuro-oncology. Acta Neurochir. 2009;151:1343–1348. doi: 10.1007/s00701-009-0471-6. [DOI] [PubMed] [Google Scholar]
- 30.Asthagiri AR, Pouratian N, Sherman J, Ahmed G, Shaffrey ME. Advances in brain tumor surgery. Neurol Clin. 2007;25:975–1003. doi: 10.1016/j.ncl.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Sanai N, Berger MS. Operative techniques for gliomas and the value of extent of resection. Neurother. 2009;6:478–486. doi: 10.1016/j.nurt.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ, The ALA-Glioma Study Group Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 33.Grossman SA, Ye X, Piantadosi S, et al. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res. 2010;16:2443–2449. doi: 10.1158/1078-0432.CCR-09-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chamberlain MC. What role should cilengitide have in the treatment of glioblastoma? J Clin Oncol. 2010;28:e695. doi: 10.1200/JCO.2010.31.2371. [DOI] [PubMed] [Google Scholar]
- 35.Narayana A, Yamada J, Berry S, et al. Intensity-modulated radiotherapy in high-grade gliomas: clinical and dosimetric results. Int J Radiat Oncol Biol Phys. 2006;64:892–897. doi: 10.1016/j.ijrobp.2005.05.067. [DOI] [PubMed] [Google Scholar]
- 36.Scheithauer BW. Development of the WHO classification of tumors of the central nervous system: a historical perspective. Brain Pathol. 2009;19:551–564. doi: 10.1111/j.1750-3639.2008.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siker ML, Wang M, Porter K, et al. Age as an independent prognostic factor in patients with glioblastoma: a radiation therapy oncology group and American College of Surgeons National Cancer Data Base comparison. J Neurooncol. doi: 10.1007/s11060-010-0500-6. published online January 9, 2011, DOI 10.1007/s11060-010-0500-6. [DOI] [PubMed] [Google Scholar]
- 38.Barnholtz-Sloan JS, Williams VL, Maldonado JL, et al. Patterns of care and outcomes among elderly individuals with primary malignant astrocytoma. J Neurosurg. 2008;108:642–648. doi: 10.3171/JNS/2008/108/4/0642. [DOI] [PubMed] [Google Scholar]