Abstract
Purpose
This cross-sectional study examines the existence and frequency of functional and structural abnormalities in the adolescent type 1 diabetic retina. We also compare the results to those of adolescents with type 2 diabetes.
Methods
Thirty-two adolescents with type 1 diabetes (5.7 ± 3.6 yrs; mean duration ± SD), 15 with type 2 diabetes (2.1 ± 1.3 yrs) and 26 age-matched control subjects were examined. Multifocal electroretinogram (mfERG) responses from 103 retinal regions were recorded. Optical coherence tomography was used to measure retinal thickness. Vascular diameter around the optic nerve was also assessed.
Results
Nine of the 32 (28%) adolescents with type 1 diabetes and 6 of the 15 (40%) with type 2 diabetes had significant mfERG implicit time delays compared to 2 of the 26 controls (8%). Retinal thicknesses in both patient groups were significantly (p ≤ 0.01) thinner than controls. The type 2 group also showed significant (p ≤ 0.03) retinal venular dilation (235.8 ± 5.9μm) compared to controls (219.6 ± 4.0μm).
Conclusions
The present study illustrates that subtle but significant functional and structural changes occur very early in type 1 diabetes. Adolescents with type 2 diabetes appear to be more affected than those with type 1 diabetes. Further longitudinal examination of the etiology and progression of these abnormalities is warranted.
Keywords: Adolescent, Diabetes, mfERG, OCT, Retina, Type 1 Diabetes, Type 2 Diabetes, Vascular
Diabetic eye disease is one of the major complications affecting adults with diabetes in the United States.1 National trends in adult diabetes mellitus indicate that its prevalence has reached epidemic proportions over the last two decades.2, 3 Similar trends have also been observed in the adolescent population.4-8 As the prevalence of adult and adolescent diabetes increases, these patients can expect to bear greater health care and financial burdens due to diabetes. These trends necessitate the investigation of early diabetes related pathology. The early diagnosis of pathology in the eyes of adults and adolescents with diabetes will improve our monitoring of diabetes-related complications and ultimately improve patient care and outcomes.
Current clinical techniques for diagnosing and monitoring complications in the eye focus largely on the presence of visible vascular related lesions or changes in visual acuity. Recent diagnostic advances have shown that diabetic eye disease can be characterized by both morphological and physiological changes in retinal structures prior to visible lesions and loss of visual acuity.9-11 Using noninvasive retinal electrophysiology, there is considerable evidence that the neural function of the retina can be affected early in diabetes.12-16 The multifocal electroretinogram (mfERG) is an objective measure that allows for the assessment of small discrete regions of neural retinal function. Several studies have shown significant mfERG response delays in small patches of the retina even in the absence of co-localized vascular lesions.9, 15, 16 These local delays have been shown to be indicative of future vascular lesions in the corresponding region.9, 16, 17
Optical coherence tomography (OCT) is an objective measure of retinal structure that has been an effective tool for detecting structural changes in the diabetic retina. OCT allows for cross-sectional analysis of retinal thickness. Studies have shown that retinal thickness can be affected in the absence of retinal vascular lesions.14, 18, 19 Digital imaging and analysis of the retinal vasculature around the optic disk have been used to assess vascular changes in the eyes of adults. Abnormalities in the diameter of these blood vessels have been shown to be associated with major diabetes-related complications throughout the body.20-23
The present study is the first to look at the retinal thickness in adolescents with type 1 diabetes as well as retinal function and retinal vascular caliber. While adolescents with type 1 and type 2 diabetes are both challenged with the task of managing their blood sugar levels in order to minimize the risk of future pathology, the pathologies preceding and/or contributing to the onset of hyperglycemia in adolescents with type 1 diabetes are very different from those with type 2 diabetes mellitus. Those with type 2 diabetes tend to present with higher body mass index (BMI), higher blood pressure, hyperlipidemia and a history of hyperinsulinemia. The purpose of the present study is to examine the existence and frequency of functional and structural retinal abnormalities in adolescents with type 1 diabetes. We also present some previously published data on adolescents with type 2 diabetes so that we can compare the frequency of retinal abnormalities between the two diabetic groups.14 We find that functional and structural abnormalities in the retina can be documented very early in both type 1 and type 2 diabetes and that adolescents with type 2 diabetes present with more abnormalities.
Methods
Subjects
This study comprised 3 subject groups:
26 Control subjects without diabetes mellitus (Table 1);
32 adolescents with type 1 diabetes (type 1 group) with a mean diabetes duration of 5.7 ± 3.6 years (Table 1);
15 adolescents diagnosed with type 2 diabetes (type 2 group) with a mean duration of 2.1 ± 1.3 years (Table 1).
All diabetes diagnoses were made by an endocrinologist at Children’s Hospital, Oakland. Adolescents with type 1 diabetes were diagnosed based on random glucose (BG) levels, HbA1c, and the presence of autoantibodies upon presentation. Adolescents with type 2 diabetes were diagnosed based on random BG levels, HbA1c, oral glucose tolerance tests and the absence of autoantibodies upon presentation.
Table 1.
Group Characteristics and Mean Measurements
| Control | Type 1 | Type 2 | |
|---|---|---|---|
| Group Characteristics | |||
| n | 26 | 32 | 15 |
| Gender (F/M) | 16/10 | 14/18 | 9/6 |
| Age (mean ± SD) | 17.6 ± 3.0 | 15.6 ± 2.0 | 16.0 ± 1.9 |
| Duration (yrs) | N/A | 5.7 ± 3.6 | 2.1 ± 1.3† |
| BG (mg/dL) | 97.0 ± 10.9 | 274.6 ± 120.4* | 197.0 ± 133.8* |
| HbA1c (%) | N/A | 9.6 ± 2.2 | 7.6 ± 3.0† |
| BMI (kg/m2) | 22.6 ± 3.6 | 22.4 ± 2.9 | 34.6 ± 6.4*† |
| Systolic BP (mmHg) | 107.0 ± 10.3 | 107.0 ± 12.4 | 115.7 ± 7.4*† |
| Diastolic BP (mmHg) | 68.2 ± 9.2 | 69.4 ± 7.5 | 72.7 ± 5.6 |
| Refractive Error (Diop.) | -0.6 ± 1.3 | -0.0 ± 1.8 | +0.4 ± 1.0* |
| Retinal Data | |||
| Implicit Time (ms) | 27.1 ± 0.1 | 27.5 ± 0.1* | 27.6 ± 0.1* |
| Amplitude (nV) | 241.4 ± 10.3 | 241.8 ± 10.0 | 219.6 ± 13.5 |
| Arteriole (μm) | 169.4 ± 3.4 | 169.9 ± 3.1 | 163.7 ± 3.6 |
| Venule (μm) | 270.9 ± 4.3 | 269.6 ± 4.1 | 283.4 ± 5.6* |
| AV Ratio | 0.63 ± 0.01 | 0.63 ± 0.01 | 0.58 ± 0.02*† |
| Retinal Thickness (μm) | 253 ± 2.5 | 249.9 ± 2.4 | 243.4 ± 3.4* |
P < 0.05, Control vs Diabetic groups;
P <0.05, type 1 vs type 2
BMI, blood pressure and blood glucose level were also measured at the time of recording in all subject groups. Blood glucose was measured with the One Touch Ultra meter (LifeScan, Inc., Milpitas, CA). Hemoglobin A1c (HbA1c) was also measured at the time of recording in the diabetic patients using A1C At-Home testing kits (FlexSite Diagnostics, Inc., Palm City, FL). HbA1c measurements made at Children’s Hospital and Research Center Oakland were measured with the DCA 2000 (Bayer Diagnostic, Milan, Italy). In order to compare HbA1c values across testing locations, 12 subjects with diabetes were tested at both sites within a one-week period. The relationship between the A1C At-Home and DCA 2000 measurements was linear (y = 1.0x − 1.4, (R2 = 0.97, P < 0.001)). Based on this data, the A1C At-Home measurements were adjusted by −1.4% to compare across A1c testing locations.
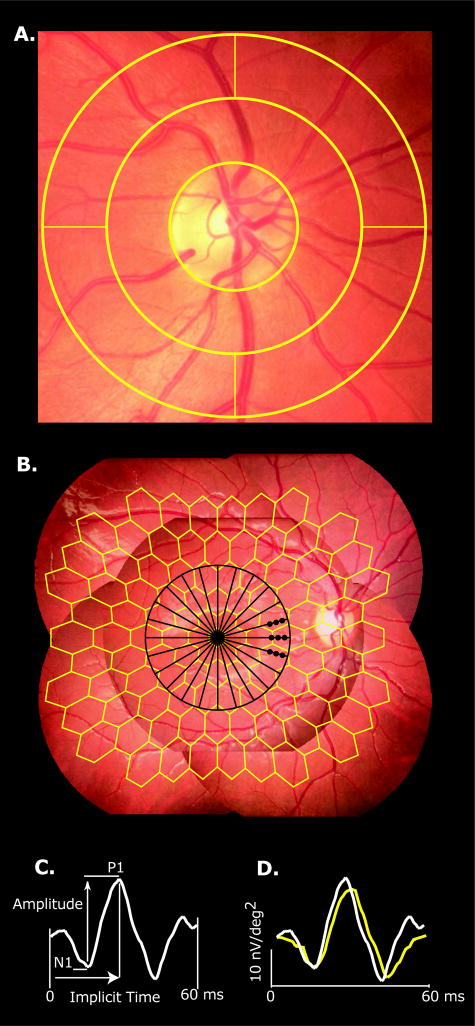
All subjects had best-corrected visual acuity of 20/20 or better, no history of disease unrelated to diabetes, and clear ocular media. The subjects had no refractive errors outside the range of −6.0 to +4.0 D. The mean refractive errors of the three subject groups are listed in table 1. The type 2 group was hyperopic relative to the control group (p = 0.01) but there is no evidence that this is a contributor to the differences seen here. We also found that there were no correlations between refractive error and any other measure presented. Both eyes were tested in the same order for each assessment in this study but only the first eye that was tested (chosen by the subjects) was analyzed in this study. Digital fundus photos (Canon CR-DGi, Canon Inc., U.S.A., Inc, Lake Success, NY), subtending approximately 50 degrees (5 overlapping fields), were taken at the time of testing and were graded for the presence of diabetic retinopathy by a retinal specialist who was masked to the diagnosis and other results. All of the adolescents in the three subject groups were free of retinopathy in both eyes. Retinal blood vessels within ½ to 1 disc diameter around the optic nerve were analyzed with IVAN (University of Wisconsin-Madison, WI), an automated but supervised program that measures and summarizes the diameters of retinal arterioles and venules (Figure 1a). These methods followed a standard protocol described in detail in previous reports.24-26
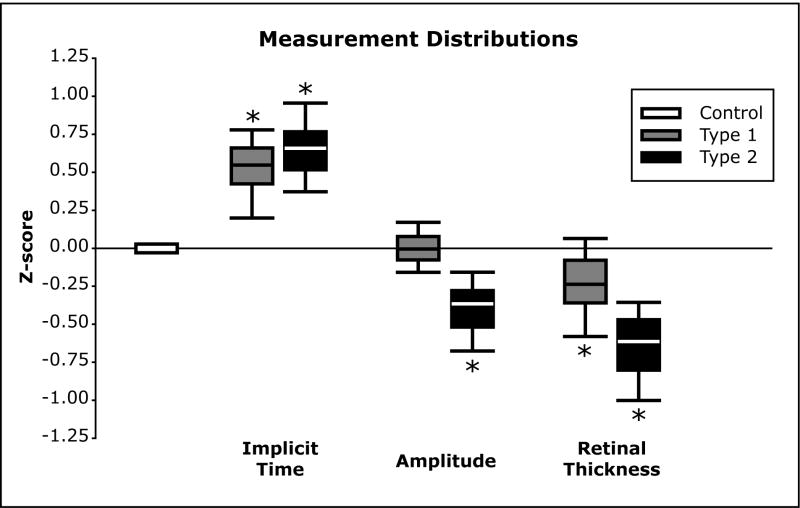
Figure 1.
Retinal Vessel Analysis and Multifocal Electroretinogram (mfERG). (A) Retinal blood vessels within outer annulus (½ to 1 disc diameter around the optic nerve) were analyzed with IVAN (University of Wisconsin-Madison, WI), an automated but supervised program. (B) Five field fundus mosaic depicting the retinal regions measured with the 103 mfERG stimulus pattern (yellow). Black 12 radial scans underlying the retinal thickness map. Black dots indicate the 9 samples used for retinal nerve fiber analysis. (C) Amplitude is the voltage difference between the N1 trough and P1 peak; implicit time is measured from the onset of the local flash to the P1 peak. (D) Example waveforms obtained from a normal control subject (white trace) and a subject with diabetes (yellow trace).
The purposes and potential consequences of the study were explained and informed consent was obtained from all subjects before testing. Procedures adhered to the tenets of the Declaration of Helsinki, and this research was approved by the University of California Committee for the Protection of Human Subjects.
Multifocal Electroretinogram
MfERGs were recorded using VERIS Science 4.3 (EDI, San Mateo, CA). The stimulus consisted of a 103-element scaled hexagonal array (Figure 1b). Each hexagon alternated between white (200 cd/m2) and black (< 2 cd/m2). Pupils were dilated using 2.5% phenylephrine and 1.0% tropicamide, and the cornea was anesthetized with 0.5% proparacaine. Retinal signals were acquired with a standard bipolar contact lens electrode placed on the cornea. Total recording time was approximately 8 minutes per eye. Response amplitude is the voltage difference between the N1 trough and P1 peak (Figure 1c, “Amplitude”) and the implicit time is measured from the onset of the local flash to the P1 peak (Figure 1c, “Implicit Time”). The waveforms in Figure 1d were obtained from a normal control subject and a subject with type 2 diabetes and illustrate both delay and diminished amplitude in the patient’s eye. The mfERG methods have been described previously in detail.9 Supplemental images of the mfERG trace arrays illustrating 6 or more abnormal responses has posted online.
Optical Coherence Tomography
Retinal thickness was measured using OCT (Stratus OCT 3, Carl Zeiss Meditec, INC., Dublin, CA). Subjects fixated on a central target while 12 radial cross-sectional images of the retina were acquired sequentially. Each 6-mm scan was composed of 512 axial samples centered on the fovea. Retinal thickness is defined as the difference between the vitreoretinal interface and the pigment epithelial/photoreceptor outer-segment interface. These measurements formed the basis of a topographic thickness map of the central 20 degrees of the retina.27 This thickness map was then divided into 37 hexagonal regions that corresponded to the central 37 elements of the mfERG stimulus (Figure 1b). An average retinal thickness for each hexagon was then computed from all points falling within the given region, which permitted local analysis of retinal thickness.
Data Analysis
Figure 1b shows the spatial arrangement of the mfERG and OCT regions tested. In order to correct for intrinsic retinotopic and topographic variation in the mfERG and OCT measurements, the mean and standard deviations at each retinal location of the control group were used to generate Z-score values for these measurements. To compare control and diabetic groups, we averaged Z-scores across subjects at each tested retinal location, creating an average eye for each subject group. This allowed for an equal N (mfERG = 103, OCT = 37) for each group. Univariate analysis of variance (ANOVA) was then used to compare the mean differences between the groups’ average eyes. Significant differences using the ANOVA were followed by Tukey WSD post-hoc tests using simultaneous 95% confidence intervals to correct for multiple comparisons (StataCorp. 2007. Stata Statistical Software: Release 10. College Station, TX: StataCorp LP). To examine local abnormalities within subjects, retinal measurements with Z-scores ≥2 were considered abnormal (p < 0.023). We then calculated the number of abnormalities for each subject to determine whether there were more abnormalities than expected by chance alone for each of the retinal measures. Using binomial expansion, the expected frequency of abnormalities within the 103 hexagons (for mfERG) and within the 37 hexagons (for OCT) were calculated using a p-value less than 0.03 as our criterion. An eye with 6 or more abnormal mfERG responses or 3 or more abnormal OCT measurements (both p < 0.03) was deemed abnormal. To compare the number of abnormal eyes between groups, χ2 analyses with Fisher’s Exact (FE) corrections were applied. Univariate ANOVA and multiple linear regression analysis were used to examine relationships between the mfERG, OCT, vascular measurements and clinical covariates (i.e., HbA1c, BG or BMI). Clinical covariates were entered into the regression model and removed systematically until the most statistically significant model remained. Results are presented as means ± SE unless otherwise stated.
Results
Clinical Covariates
Clinical covariates are summarized in Table 1. The duration of diabetes in the type 2 group was significantly less than the type 1 group (P<0.01). The mean BG at the time of testing (without fasting) in the type 1 and type 2 groups were both significantly higher (P < 0.01) than that of the control group. The mean HbA1c of the type 1 group was slightly higher (P < 0.03) than the type 2 group. The HbA1c measurements for both groups were consistent with the yearly average for the type 1 (8.9 ± 1.9%) and type 2 (7.7 ± 2.5%) adolescents at Children’s Hospital. The BMI of the type 2 group was significantly higher (P <0.001) than both the type 1 and control groups. Whereas the BP of the type 2 group was not outside of clinical norms, the systolic pressure was significantly higher (p < 0.001) than both the type 1 and control groups.
Neural Retinal Function
Implicit Time
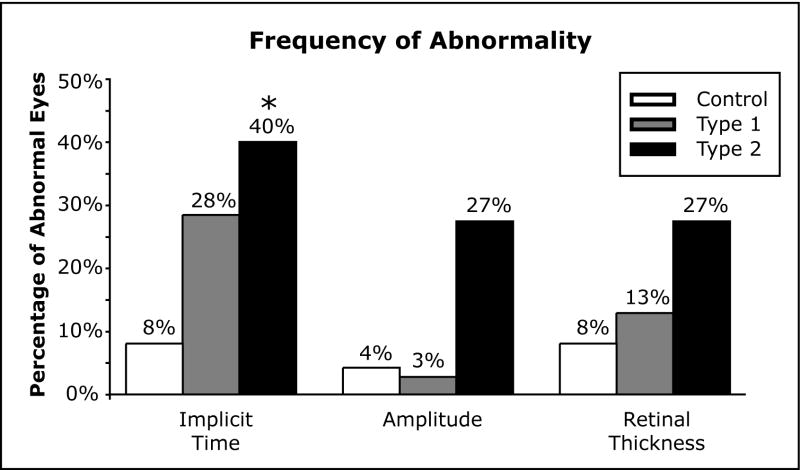
As seen in Table 1, the mean implicit times, averaged across all subject means in the type 1 and type 2 subjects, were subtly but significantly delayed compared to the control group (P < 0.02). Figure 2 (Implicit Time) shows the distributions of implicit time Z-scores of the average eyes. The distribution of responses for the type 1 and type 2 groups were significantly delayed (Tukey WSD) compared to the control group.
Figure 2.
Response Z-score Distributions for Average Eyes. Box plots represent the 5th, 25th, 50th, 75th and 95th percentiles. Control distributions for each measure are centered on a Z-score of zero. (Implicit Time) The implicit time distributions of the type 1 and type 2 groups were all significantly delayed compared to the control distribution (Tukey WSD). (Amplitude) The amplitude distribution of the type 2 group differed significantly from both the type 1 and controls (Tukey WSD). (Retinal Thickness) The retinal thickness distributions of both the type 1 and type 2 groups were significantly thinner than the control group (Tukey WSD). The (*) indicates a significant difference from controls.
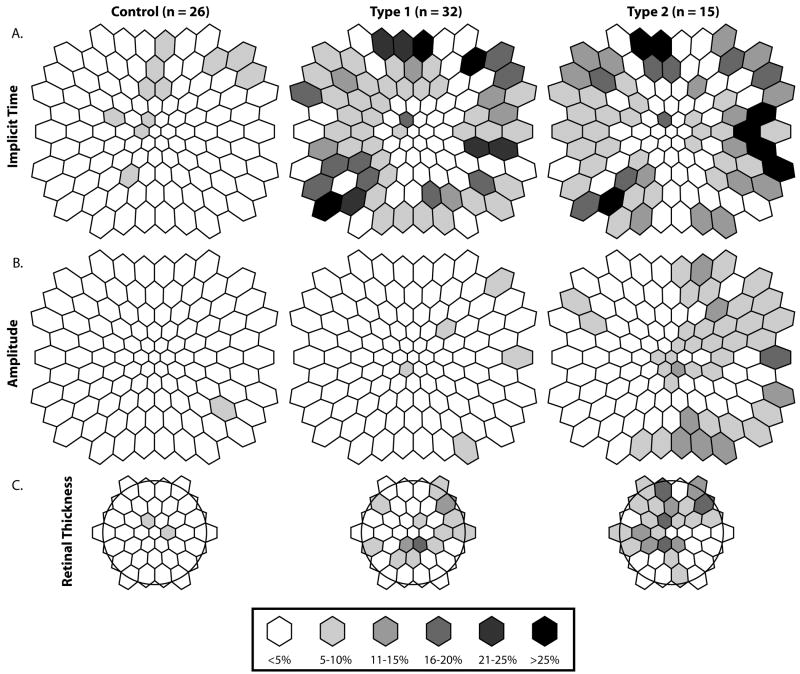
As shown in Figure 3 (Implicit time), 9 out of 32 eyes (28%) of the type 1 group and 6/15 (40%) eyes in the type 2 group had 6 or more abnormally delayed IT responses (abnormal eye) compared to 2/26 (8%) in the control group. However, the difference in the percentage of abnormal eyes between the type 1 and control groups failed to reach statistical significance based on the χ2 analysis (FE: χ2 = 3.90, p = 0.09). After correcting for multiple comparisons, only the type 2 (FE: χ2 = 6.32, p = 0.035) group approached a significant number of abnormal eyes when compared to the control group but did not differ from the type 1 group (FE: χ2 = 0.66, p = 0.51). Figure 4 (Implicit time) shows the percentage of Implicit time abnormalities at each retinal location in all 3 groups.
Figure 3.
Retinal distributions of abnormalities. Percentages of subjects with a response or measurement equal to or beyond ±2.0 Z-scores at each retinal location are coded gray to black from <5% to >25%. A. Frequency of implicit time abnormalities at each retinal location. B. Frequency of amplitude abnormalities at each retinal location. C. Frequency of abnormal retinal thickness at each retinal location.
Figure 4.
Frequency of Abnormality. (Implicit Time) Percentage of subjects with an eye with 6 or more abnormally delayed Implicit Time response Z-scores (p < 0.03). (Amplitude) Percentage of subjects with an eye with 6 or more abnormal smaller Amplitude response Z-scores. (Retinal Thickness) Percentage of subjects with an eye with 3 or more abnormal thin Retinal Thickness Z-scores. The (*) indicates a significant difference from controls.
Amplitude
As seen in Table 1, the mean amplitude averaged across all subjects in the type 1 and type 2 groups did not differ significantly when compared to the control group (P > 0.2). However, when looking at the distribution of amplitude Z-scores for each group’s average eye, the type 2 group was significantly smaller (N = 103, p < 0.0001) than both the control and type 1 groups (Figure 2, Amplitude). The type 1 group amplitude distributions did not differ from the controls. As shown in Figure 3 (Amplitude), 1/32 eyes (3%) of the type 1 group and 4/15 eyes (27%) in the type 2 group had 6 or more abnormally small amplitude responses (abnormal eye) compared to 1/26 (4%) in the control group. However, the difference in the percentage of abnormal eyes between the type 1 and control groups was not significant based on the χ2 analysis (FE: χ2 = 0.02, p = 0.88). The type 2 group approached a significant number of abnormal eyes compared to the control group (FE: χ2 = 4.63, p = 0.05) and type 1 group (FE: χ2 = 5.95, p = 0.03). Figure 4 (Amplitude) shows the percentage of amplitude abnormalities at each retinal location in all 3 groups.
Retinal Structure
Vessel Diameter
As seen in Table 1, the mean arteriolar diameter did not differ (P>0.05) significantly between the diabetic groups and controls, although the type 2 group had smaller diameters. However, the mean venular diameter of the type 2 group was significantly larger (p < 0.03) than controls. The type 1 group did not differ from controls in venular diameter. The arteriolar/venular ratio (AVR) of the type 2 group differed (P < 0.03) from both the control and type 1 groups.
Retinal Thickness
As seen in Table 1 the mean retinal thickness across all subjects in the type 1 group did not differ when compared to the control group (P > 0.05). In contrast, the type 2 group had significantly thinner retinas than the control group (P < 0.03). As seen in Figure 2 (Retinal Thickness), the distribution of retinal thickness Z-scores showed that both the type 1 and type 2 group retinal thicknesses were significantly smaller (N = 37, P < 0.001) than measures obtained from the control group. As shown in Figure 3 (Retinal Thickness), 4/32 (13%) eyes of the type 1 group and 4/15 (27%) of the type 2 group had 3 or more abnormally thin retinal locations compared to 2/26 (8%) in the control group. Neither the type 1 (FE: χ2 = 0.36, P = 0.68) nor the type 2 (FE: χ2 = 2.74, P = 0.16) groups differed from the control group in frequency of abnormality, nor from each other (FE: χ2 = 1.45, P = 0.25). Figure 4 (Retinal Thickness) shows the percentage of Retinal Thickness abnormalities at each retinal location in all 3 groups. Supplemental illustrative images of the mean retinal thickness have been posted online.
In order to examine the retinal nerve fiber layer contribution to the overall retinal thickness, the nerve fiber and retinal thicknesses were sampled and examined in 9 discrete locations proximal to the optic disk (Figure 1b, Black Dots). As seen in Table 2, only the mean retinal nerve fiber (RNFL) thickness of the type 2 group was significantly (P = 0.01) thinner than that of the control group. Thus, consistent with the total retinal thickness differences mentioned earlier, the type 2 group RNFL was also thinner than that of the controls in the same locations.
Table 2.
Retinal Thickness and Retinal Nerve Fiber Layer Thickness
| Retinal Thickness (μm) | Retinal Nerve Fiber Layer (μm) | |
|---|---|---|
| Group | ||
| Control | 277.2 ± 3.3 | 35.1 ± 0.8 |
| Type 1 | 270.7 ± 3.2 | 33.5 ± 0.8 |
| Type 2 | 267.2 ± 3.9* | 31.8 ± 0.8* |
P < 0.05, Control vs Diabetic groups
Associations between measures
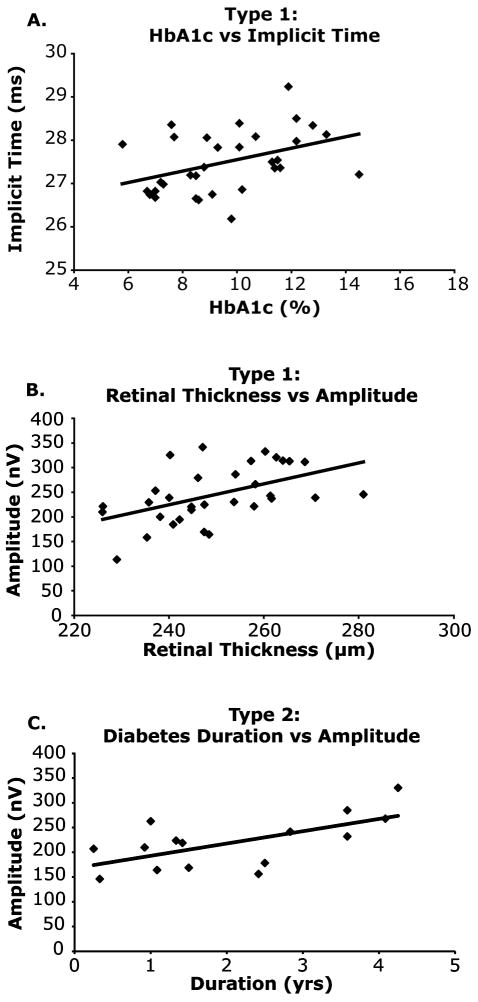
In the control group, there were no significant relationships between the clinical covariates (Age, BG, BMI and BP) and either mean implicit time, amplitude or vascular diameter. After systematically removing clinical covariates from the multiple linear regression models, univariate regression was the most statistically significant model for describing the relationships between HbA1c and mean implicit time in the type 1 group (Figure 5A), indicating that as HbA1c increased there was a significant increase in mfERG implicit time (r2 = 0.17, P < 0.03). In order to look at the relationship between a subjects’ mean functional measure and their mean retinal thickness, we averaged across the central 37 elements of the mfERG that corresponded to the retinal thickness map. We then compared the subjects’ mean retinal thickness to both their mean implicit time and mean amplitude. There was a significant (r2 = 0.25, p < 0.01) association between retinal thickness and amplitude in the type 1 group, showing a 21nV amplitude increase per 10micrometer increase in retinal thickness (Figure 5B). In adolescents with type 2 diabetes there was a significant relationship between mean amplitude and diabetes duration in the type 2 group (Figure 5C), showing that as diabetes duration lengthened there was a trend towards larger amplitudes (r2 = 0.41, P < 0.03). There were no other significant relationships between the remaining clinical covariates and retinal measures for the diabetes groups.
Figure 5.
Significant Associations in Adolescents with Diabetes. (A) An association between HbA1c and mean implicit time in the type 1 group suggesting that as HbA1c increased there was a significant increase in implicit time (y = 0.13x + 26.21, R2 = 0.17, P < 0.01). To make the relationship between HbA1c and implicit time visible, the range of the Y-axis is limited to 25-30 ms. (B) Retinal Thickness and Amplitude (C) An association between type 2 diabetes duration and mfERG amplitude suggesting that as duration increases mfERG increases (y = 24.85x + 168.05, R2 = 0.41, P = 0.01).
Discussion
While adolescents with type 1 and type 2 diabetes are challenged to manage blood sugar levels in order to minimize the risk of future pathology, the pathologies preceding and/or contributing to the onset of hyperglycemia can be very different. The data presented here show that their retinas are affected very early in the disease. Additionally, the results illustrate that the presentation of retinal abnormalities can also be very different between adolescent diabetes types.
28% of the subjects in the type 1 group presented with 6 or more abnormal implicit time responses compared to 8% of the control subjects and were thus considered to have functionally abnormal retinas. The percentage of abnormal eyes increased to 40% in the type 2 group. This was also evident when looking at the mean distribution differences between the model eyes for each group. In the adult diabetic population, delayed implicit times have been associated with increased risk of developing retinopathy and have been predictive of future development of retinopathy in given retinal locations.9, 17, 28
Consistent with the results from the adult diabetic population, in the present study, mfERG implicit time abnormalities are more common than the amplitude abnormalities.15, 29 The adolescent type 1 mfERG amplitude responses reported here are consistent with adult diabetic data presented elsewhere.9 However, the amplitude abnormalities in the type 2 group were unexpected because, in the adult population, it takes significant visible vascular pathology such as edema to present with amplitude abnormalities.30 The number of subjects presenting with an abnormal eye based on mfERG amplitude was not large. However, the distribution of amplitude responses in the type 2 group were significantly smaller in amplitude than that of the controls as well as the type 1 group (Figure 2, Amplitude). Overall, the amplitude responses in the present study were not affected to the same degree as implicit time responses.
On average, the overall retinal thickness of the type 2 group was 10.3 ± 0.4μm (−4%) thinner than the Control group compared to the 3.0 ± 0.5μm (−1%) difference in the type 1 group. These differences are small but statistically significant and similar to results seen in the adult diabetic population.18, 19 Retinal thinning has been found in both type 1 and type 2 diabetes in adults with little or no retinopathy.18, 19 However, there are also reports of retinal thickening in patients without retinopathy.10, 11 It has been suggested that retinal thinning in the adult diabetic population is due to neural tissue loss, but this is an unlikely explanation for the present results, given the short duration of diabetes in our adolescent population.10, 31-33 The retinal thickness differences we found were unexpected and concerning but their basis is currently unknown. Further investigation with a larger sample is warranted.
In order to examine the RNFL thickness contribution the retinal thickness differences seen in the adolescents with diabetes, we measured the RNFL and total retinal thicknesses in 9 discrete points proximal to the disc. The RNFL thickness in this region is normally the greatest and the most reliably delineated within in our scanned area. The RNFL layer of the type 2 group was 9.4% thinner than that of the control group compared to 4.6% thinner in the type 1 group. These differences were larger than the percentage difference in retinal thickness (minus the RNFL) in these locations, where the type 2 retina was 2.8% thinner than controls and the type 1 group was 2.0% thinner. While this doesn’t account for the total retinal thinning seen in the subjects with diabetes, it suggests that the RNFL is affected proportionally to a greater degree than total retinal thickness.
Future studies using the spectral domain OCT and advances in image analysis will allow for better identification and segmentation of the individual retinal layers. This will hopefully contribute to our understanding of the relationship between retinal thickness and the mfERG abnormalities presented here.
Along with functional abnormalities and retinal thinning, adolescents with type 2 diabetes presented with significant venular dilation compared to controls. Several studies have shown that changes in vascular diameter can precede diabetes onset in adults.34 In children aged 6-8 yrs old, Cheung et al (2007) also found that elevated BMI was significantly associated with retinal venular dilation.35 This suggests that the venular dilation, in adolescents with type 2 diabetes, could have preceded the presentation of both hyperglycemia and the functional delays seen our sample. Longitudinal analysis of the causal relationships is needed. While there was no correlation found between the retinal vasculature and mfERG responses in the present study, there is evidence in the adult diabetic population that such a relationship exists. Luu et al. (2010) found that as retinal arteriolar caliber decreased, there was a decrease in the full-field ERG oscillatory potential 4 amplitude in adults with type 2 diabetes.36
Several studies have shown that changes in vascular caliber can present in type 1 diabetes of short duration and can be linked to future pathology.37-39 Although the adolescents with type 1 diabetes in the present study did not show signs of arteriolar dilation relative to the control group, Cheung et al. (2008) found that adolescents with type 1 diabetes and larger retinal arterioles had a three fold higher risk of developing vascular lesions compared to those diabetic patients with smaller arterioles within a median time span of 2.5 years from baseline.39 In adults with type 1 diabetes, Grauslund et al. (2009) found that arteriolar narrowing was associated with nephropathy as well as systemic macrovascular complications.38
The fact that implicit time delays were associated with elevated HbA1c in adolescents with type 1 diabetes suggests that aggressive diabetes management early in the pathogenesis of this disease has the potential to play a significant role in preventing neural retinal dysfunction. While there is a significant relationship between HbA1c and implicit time, the correlation only describes 17% (r2 = 0.17) of the variation in implicit time. Further analysis with a larger sample might clarify the additional effects of other underlying factors such as duration of the disease on implicit time. This also raises questions of whether these retinal functional differences are permanent or whether function can be improved with better diabetes management, behavioral modification or other prophylactic measures.
The relationship between retinal thickness and electroretinography has been examined most often in the case of sight threatening diabetic macular edema. These studies consistently reveal that decreased amplitude and delayed implicit times are associated with areas of significant retinal thickening.30, 40-42 In contrast, the present study, where no edema is present, shows a different relationship. As the mean retinal thickness of the type 1 group increased there was an associated increase in the subject’s mean mfERG amplitude. The reason for the difference from previous literature could be due to the fact that our subjects were at a different pathological stage of eye disease. This relationship between mean retinal thickness and mfERG amplitude in adolescents with type 1 diabetes suggests that as the retina thins, there is a reduction of neural generator activity that produces a loss of mfERG amplitude. While we did not find this relationship in the adolescents with type 2 diabetes, they did show both significant retinal thinning and significantly lower mfERG amplitude. These results merit further longitudinal investigation.
The lack of an association between diabetes duration and implicit time measures was somewhat unexpected but might be due to the relatively small range of durations. The mean duration of diabetes in those subjects who had abnormal eyes based on implicit time was 5.0 ± 3.3 years for the type 1 group and 2.0 ± 1.4 years for the type 2 group. There were significant numbers of implicit time abnormalities found in eyes with as little as 1-year diabetes duration in both groups. This clearly illustrates that neural retinal function can be affected very early in the pathogenesis of diabetes mellitus. The relationship between type 2 diabetes duration and amplitude seen in figure 5C also illustrates that function might be affected very early and prior to diagnosis in diabetes. We have previously speculated that the mechanisms underlying the mfERG amplitude could be affected prior to the onset of the hyperglycemic state in type 2 diabetes.14 It could be that the pre-diabetic hyperinsulinemia and presence of inflammatory factors are toxic to the neural retina and thus affect the amplitudes early in the disease.43, 44 The slow recovery of the amplitude response towards a “normal state” as duration lengthens could be the result of diabetes treatment and decreased insulin production due to either decreased demand or beta cell dysfunction.
The amplitudes and implicit times measured here are largely determined by responses from bipolar cells within the middle retinal layers. These cells are non-myelinated and respond to stimulation with a graded potential. The Muller cell is a major glial support mechanism within the retina and has been shown to play a critical role in the development and progression of diabetic eye disease.45, 46 Damage to or altered function of these cells would likely contribute to both abnormal neural as well as vascular function because Muller cells act as a bridge between the vascular and neural tissue. This suggests that the abnormal neural function and vascular changes presented here could be the result of a dysregulation secondary to the effects of diabetes on regulatory mechanism such as Muller cells. Through improved diabetes management along with early intervention normal function might be recovered. This possibility should be investigated in a longitudinal study.
While adolescents with type 1 diabetes are faced with the significant challenge of regulating their blood sugar levels, adolescents with type 2 diabetes often present with other comorbidities that compound the challenges they face. In adults, dilated retinal venules have been shown to be associated with nephropathy, cardiovascular disease and increased risk of stroke.47-51 There has been suggestion that retinal venular dilation in type 2 diabetes could be due to systemic inflammation associated with elevated BMI.35, 52-54 This inflammatory vascular response in conjunction with the hyperinsulinemia and hyperglycemia that are inherent to type 2 diabetes might also contribute to the functional differences in the retina between type 1 and type 2 diabetes that we have described here.
One limitation of this study is that a relatively small number of subjects were examined. This might have limited the number of statistically significant findings in our study. This might also limit the generalizability of the study to the population of adolescent patients as a whole.
As a result of recent advances in the assessment of both neural retinal functioning and vascular health, we have been able to detect subtle but significant differences early in the pathogenesis of diabetes duration in adolescents. The present study is the first look at retinal thickness in adolescents with type 1 diabetes. The present study shows early indications of focal retinal neuropathy in adolescents with type 1 diabetes mellitus. In fact, there were significant numbers of implicit time abnormalities found in eyes with as little as 1-year diabetes duration in both type 1 and type 2 groups. This clearly illustrates that the neural retinal function can be affected very early in the pathogenesis of diabetes and prior to presentation of clinical vasculopathy. The established relationship between delayed implicit time and future vascular damage in the adult diabetic population raises an alarm for the young retinopathy-free subjects examined here. The association between implicit time and HbA1c in the type 1 group also raises the hope of preventing or minimizing retinopathy development. Perhaps of greatest concern is our finding that adolescents with type 2 diabetes not only have focal retinal neuropathy, but also significant retinal thinning, venular enlargement and elevated BMI. Further longitudinal examination of these two populations is needed in order to fully understand the etiology of these functional and structural abnormalities.
Supplementary Material
Acknowledgments
Financial Support: National Eye Institute Grants EY02271 (AJA) and T32 TY07043 (KBC), Prevent Blindness America (MES) and Juvenile Diabetes Research Foundation International (MAB)
Footnotes
None of the authors have any proprietary interest.
References
- 1.NIDDK. National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK); Diabetes Fact Sheet. http://diabetes.niddk.nih.gov/dm/pubs/statistics/ [DOI] [PubMed] [Google Scholar]
- 2.Mokdad AH, Bowman BA, Ford ES, et al. The continuing epidemics of obesity and diabetes in the United States. Jama. 2001;286(10):1195–1200. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
- 3.Mokdad AH, Ford ES, Bowman BA, et al. The continuing increase of diabetes in the US. Diabetes Care. 2001;24(2):412. doi: 10.2337/diacare.24.2.412. [DOI] [PubMed] [Google Scholar]
- 4.Pinhas-Hamiel O, Dolan LM, Daniels SR, et al. Increased incidence of non-insulin-dependent diabetes mellitus among adolescents. J Pediatr. 1996;128(5 Pt 1):608–615. doi: 10.1016/s0022-3476(96)80124-7. [DOI] [PubMed] [Google Scholar]
- 5.Fagot-Campagna A, Pettitt DJ, Engelgau MM, et al. Type 2 diabetes among North American children and adolescents: an epidemiologic review and a public health perspective. J Pediatr. 2000;136(5):664–672. doi: 10.1067/mpd.2000.105141. [DOI] [PubMed] [Google Scholar]
- 6.Pinhas-Hamiel O, Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr. 2005;146(5):693–700. doi: 10.1016/j.jpeds.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 7.Diabetes Care. 3. Vol. 23. American Diabetes Association; 2000. Type 2 diabetes in children and adolescents; pp. 381–389. [DOI] [PubMed] [Google Scholar]
- 8.Liese AD, D’Agostino RB, Jr, Hamman RF, et al. The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics. 2006;118(4):1510–1518. doi: 10.1542/peds.2006-0690. [DOI] [PubMed] [Google Scholar]
- 9.Bearse MA, Jr, Adams AJ, Han Y, et al. A multifocal electroretinogram model predicting the development of diabetic retinopathy. Prog Retin Eye Res. 2006;25(5):425–448. doi: 10.1016/j.preteyeres.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugimoto M, Sasoh M, Ido M, et al. Detection of early diabetic change with optical coherence tomography in type 2 diabetes mellitus patients without retinopathy. Ophthalmologica. 2005;219(6):379–385. doi: 10.1159/000088382. [DOI] [PubMed] [Google Scholar]
- 11.Cunha-Vaz J, Bernardes R. Nonproliferative retinopathy in diabetes type 2. Initial stages and characterization of phenotypes. Prog Retin Eye Res. 2005;24(3):355–377. doi: 10.1016/j.preteyeres.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Juen S, Kieselbach GF. Electrophysiological changes in juvenile diabetics without retinopathy. Arch Ophthalmol. 1990;108(3):372–375. doi: 10.1001/archopht.1990.01070050070033. [DOI] [PubMed] [Google Scholar]
- 13.Lovasik JV, Spafford MM. An electrophysiological investigation of visual function in juvenile insulin-dependent diabetes mellitus. Am J Optom Physiol Opt. 1988;65(4):236–253. doi: 10.1097/00006324-198804000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Bronson-Castain KW, Bearse MA, Jr, Neuville J, et al. Adolescents with type 2 diabetes: early indications of focal retinal neuropathy, retinal thinning, and venular dilation. Retina. 2009;29(5):618–626. doi: 10.1097/IAE.0b013e31819a988b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bearse MA, Jr, Han Y, Schneck ME, Adams AJ. Retinal function in normal and diabetic eyes mapped with the slow flash multifocal electroretinogram. Invest Ophthalmol Vis Sci. 2004;45(1):296–304. doi: 10.1167/iovs.03-0424. [DOI] [PubMed] [Google Scholar]
- 16.Han Y, Schneck ME, Bearse MA, Jr, et al. Formulation and evaluation of a predictive model to identify the sites of future diabetic retinopathy. Invest Ophthalmol Vis Sci. 2004;45(11):4106–4112. doi: 10.1167/iovs.04-0405. [DOI] [PubMed] [Google Scholar]
- 17.Ng JS, Bearse MA, Jr, Schneck ME, et al. Local diabetic retinopathy prediction by multifocal ERG delays over 3 years. Invest Ophthalmol Vis Sci. 2008;49(4):1622–1628. doi: 10.1167/iovs.07-1157. [DOI] [PubMed] [Google Scholar]
- 18.Biallosterski C, van Velthoven ME, Michels RP, et al. Decreased optical coherence tomography-measured pericentral retinal thickness in patients with diabetes mellitus type 1 with minimal diabetic retinopathy. Br J Ophthalmol. 2007;91(9):1135–1138. doi: 10.1136/bjo.2006.111534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Dijk HW, Kok PHB, van Velthoven MEJ, Baillosterski C, Schlingemann RO, Michels BPJ, de Vries HH, Verbraak FD. Preliminary OCT Findings: Macular thicknes in patients with diabetes mellitus type 1, with or without minimal diabetic retinopathy. Invest Ophthalmol Vis Sci. 2007;48:E-Abstract 1404. [Google Scholar]
- 20.Wong TY, Klein R, Sharrett AR, et al. Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The Atherosclerosis Risk in Communities Study. Jama. 2002;287(9):1153–1159. doi: 10.1001/jama.287.9.1153. [DOI] [PubMed] [Google Scholar]
- 21.Wong TY, Klein R, Couper DJ, et al. Retinal microvascular abnormalities and incident stroke: the Atherosclerosis Risk in Communities Study. Lancet. 2001;358(9288):1134–1140. doi: 10.1016/S0140-6736(01)06253-5. [DOI] [PubMed] [Google Scholar]
- 22.Sharrett AR, Hubbard LD, Cooper LS, et al. Retinal arteriolar diameters and elevated blood pressure: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 1999;150(3):263–270. doi: 10.1093/oxfordjournals.aje.a009997. [DOI] [PubMed] [Google Scholar]
- 23.Klein R, Klein BE, Moss SE, Wong TY. Retinal vessel caliber and microvascular and macrovascular disease in type 2 diabetes: XXI: the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ophthalmology. 2007;114(10):1884–1892. doi: 10.1016/j.ophtha.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 24.Hubbard LD, Brothers RJ, King WN, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106(12):2269–2280. doi: 10.1016/s0161-6420(99)90525-0. [DOI] [PubMed] [Google Scholar]
- 25.Wong TY, Klein R, Klein BE, et al. Retinal vessel diameters and their associations with age and blood pressure. Invest Ophthalmol Vis Sci. 2003;44(11):4644–4650. doi: 10.1167/iovs.03-0079. [DOI] [PubMed] [Google Scholar]
- 26.Knudtson MD, Lee KE, Hubbard LD, et al. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003;27(3):143–149. doi: 10.1076/ceyr.27.3.143.16049. [DOI] [PubMed] [Google Scholar]
- 27.Neuville JM, Bronson-Castain K, Bearse MA, Jr, et al. OCT reveals regional differences in macular thickness with age. Optom Vis Sci. 2009;86(7):E810–816. doi: 10.1097/OPX.0b013e3181adff59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han Y, Bearse MA, Jr, Schneck ME, et al. Multifocal electroretinogram delays predict sites of subsequent diabetic retinopathy. Invest Ophthalmol Vis Sci. 2004;45(3):948–954. doi: 10.1167/iovs.03-1101. [DOI] [PubMed] [Google Scholar]
- 29.Fortune B, Schneck ME, Adams AJ. Multifocal electroretinogram delays reveal local retinal dysfunction in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 1999;40(11):2638–2651. [PubMed] [Google Scholar]
- 30.Greenstein VC, Holopigian K, Hood DC, et al. The nature and extent of retinal dysfunction associated with diabetic macular edema. Invest Ophthalmol Vis Sci. 2000;41(11):3643–3654. [PubMed] [Google Scholar]
- 31.Ozdek S, Lonneville YH, Onol M, et al. Assessment of nerve fiber layer in diabetic patients with scanning laser polarimetry. Eye. 2002;16(6):761–765. doi: 10.1038/sj.eye.6700207. [DOI] [PubMed] [Google Scholar]
- 32.Lonneville YH, Ozdek SC, Onol M, et al. The effect of blood glucose regulation on retinal nerve fiber layer thickness in diabetic patients. Ophthalmologica. 2003;217(5):347–350. doi: 10.1159/000071350. [DOI] [PubMed] [Google Scholar]
- 33.Lopes de Faria J, HR VPC. Retinal nerve fibre layer loss in patients with type 1 diabetes mellitus without retinopathy. Br J Opthalmol. 2002;86:725–728. doi: 10.1136/bjo.86.7.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsueh WA, Law RE. Diabetes is a vascular disease. J Investig Med. 1998;46(8):387–390. [PubMed] [Google Scholar]
- 35.Cheung N, Saw SM, Islam FM, et al. BMI and retinal vascular caliber in children. Obesity (Silver Spring) 2007;15(1):209–215. doi: 10.1038/oby.2007.576. [DOI] [PubMed] [Google Scholar]
- 36.Luu CD, Szental JA, Lee SY, et al. Correlation between retinal oscillatory potentials and retinal vascular caliber in type 2 diabetes. Invest Ophthalmol Vis Sci. 2010;51(1):482–486. doi: 10.1167/iovs.09-4069. [DOI] [PubMed] [Google Scholar]
- 37.Sasongko MB, Wang JJ, Donaghue KC, et al. Alterations in retinal microvascular geometry in young type 1 diabetes. Diabetes Care. 2010;33(6):1331–1336. doi: 10.2337/dc10-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grauslund J, Hodgson L, Kawasaki R, et al. Retinal vessel calibre and micro- and macrovascular complications in type 1 diabetes. Diabetologia. 2009;52(10):2213–2217. doi: 10.1007/s00125-009-1459-8. [DOI] [PubMed] [Google Scholar]
- 39.Cheung N, Rogers SL, Donaghue KC, et al. Retinal arteriolar dilation predicts retinopathy in adolescents with type 1 diabetes. Diabetes Care. 2008;31(9):1842–1846. doi: 10.2337/dc08-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma J, Yao K, Jiang J, et al. Assessment of macular function by multifocal electroretinogram in diabetic macular edema before and after vitrectomy. Doc Ophthalmol. 2004;109(2):131–137. doi: 10.1007/s10633-004-2890-2. [DOI] [PubMed] [Google Scholar]
- 41.Moschos MM, Moschos M. Intraocular bevacizumab for macular edema due to CRVO. A multifocal-ERG and OCT study. Doc Ophthalmol. 2008;116(2):147–152. doi: 10.1007/s10633-007-9110-9. [DOI] [PubMed] [Google Scholar]
- 42.Shetty R, Pai SA, Vincent A, et al. Electrophysiological and structural assessment of the central retina following intravitreal injection of bevacizumab for treatment of macular edema. Doc Ophthalmol. 2008;116(2):129–135. doi: 10.1007/s10633-007-9090-9. [DOI] [PubMed] [Google Scholar]
- 43.Caballero AE, Bousquet-Santos K, Robles-Osorio L, et al. Overweight Latino children and adolescents have marked endothelial dysfunction and subclinical vascular inflammation in association with excess body fat and insulin resistance. Diabetes Care. 2008;31(3):576–582. doi: 10.2337/dc07-1540. [DOI] [PubMed] [Google Scholar]
- 44.Festa A, D’Agostino R, Jr, Howard G, et al. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102(1):42–47. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 45.Gardner TW, Antonetti DA, Barber AJ, et al. Diabetic retinopathy: more than meets the eye. Surv Ophthalmol. 2002;47(Suppl 2):S253–262. doi: 10.1016/s0039-6257(02)00387-9. [DOI] [PubMed] [Google Scholar]
- 46.Barber AJ, Antonetti DA, Gardner TW. Altered expression of retinal occludin and glial fibrillary acidic protein in experimental diabetes. The Penn State Retina Research Group. Invest Ophthalmol Vis Sci. 2000;41(11):3561–3568. [PubMed] [Google Scholar]
- 47.Wong TY, Shankar A, Klein R, Klein BE. Retinal vessel diameters and the incidence of gross proteinuria and renal insufficiency in people with type 1 diabetes. Diabetes. 2004;53(1):179–184. doi: 10.2337/diabetes.53.1.179. [DOI] [PubMed] [Google Scholar]
- 48.Ikram MK, de Jong FJ, Vingerling JR, et al. Are retinal arteriolar or venular diameters associated with markers for cardiovascular disorders? The Rotterdam Study. Invest Ophthalmol Vis Sci. 2004;45(7):2129–2134. doi: 10.1167/iovs.03-1390. [DOI] [PubMed] [Google Scholar]
- 49.Ikram MK, De Jong FJ, Van Dijk EJ, et al. Retinal vessel diameters and cerebral small vessel disease: the Rotterdam Scan Study. Brain. 2006;129(Pt 1):182–188. doi: 10.1093/brain/awh688. [DOI] [PubMed] [Google Scholar]
- 50.Ikram MK, de Jong FJ, Bos MJ, et al. Retinal vessel diameters and risk of stroke: the Rotterdam Study. Neurology. 2006;66(9):1339–1343. doi: 10.1212/01.wnl.0000210533.24338.ea. [DOI] [PubMed] [Google Scholar]
- 51.Wong TY, Kamineni A, Klein R, et al. Quantitative retinal venular caliber and risk of cardiovascular disease in older persons: the cardiovascular health study. Arch Intern Med. 2006;166(21):2388–2394. doi: 10.1001/archinte.166.21.2388. [DOI] [PubMed] [Google Scholar]
- 52.Zaldivar F, McMurray RG, Nemet D, et al. Body fat and circulating leukocytes in children. Int J Obes (Lond) 2006;30(6):906–911. doi: 10.1038/sj.ijo.0803227. [DOI] [PubMed] [Google Scholar]
- 53.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96(9):939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 54.Lyon CJ, Law RE, Hsueh WA. Minireview: adiposity, inflammation, and atherogenesis. Endocrinology. 2003;144(6):2195–2200. doi: 10.1210/en.2003-0285. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.