Abstract
The present study compared cortisol and soluble tumor necrosis factor-α receptor II (sTNFαRII) responses provoked by cold pressor, hot water, ischemic, and neutral water (i.e., room temperature) modalities. Oral fluid samples were collected before, immediately after, and during recovery to assess physiological responses. From baseline, the cold pressor, but not hot water or ischemic modalities, produced a significant time-dependent elevation in cortisol, whereas cortisol significantly decreased for the neutral water task. When compared to baseline, the cold pressor, hot water, and ischemic modalities were associated with decreased sTNFαRII responses over time. The sTNFαRII response to neutral water initially decreased but returned to approximate baseline levels. Pain ratings were positively associated with cortisol increase from baseline and the overall cortisol response was negatively associated with the overall sTNFαRII response.
As the neurobiological pathways of pain processing are elucidated, it has become increasingly clear that pain may impact on morbidity and mortality through pain-related changes in neuroendocrine and immune function (Kiecolt-Glaser, Page, Marucha, MacCullum, & Glaser, 1998; Chapman, Tuckett, & Song, 2008; Ren & Torres, 2009). Studies of tissue-damaging painful stimuli, such as surgery, document neuroendocrine and immune changes reflective of hypothalamo-pituitary-adrenal (HPA) axis activation and immune suppression in both animals and humans (Salomaki, Leppaluoto, Laitinen, Vuolteenaho, & Nuutinen, 1993; Page, Ben-Eliyahu, & Liebeskind, 1994; Page, Blakely, Ben-Eliyahu, 2001; Page, 2002). Additionally, animal studies document that non-tissue damaging painful stimuli, such as footshock and tailshock, elicit neuroendocrine (e.g., corticosterone) elevations and suppress immune function, particularly pro-inflammatory cytokine expression (Page & Ben-Eliyahu, 1997; O’Connor et al., 2003). While valuable, there are considerable disadvantages to these models for examining pain-related changes in neuroendocrine and immune function. Clinical models that involve tissue damage, such as surgery, confound changes in neuroendocrine and immune function in response to pain with changes initiated by tissue injury. Further, the focus on painful electrical stimuli in animal studies has serious limitations in its relevance to clinical pain (Rainville, Feine, Bushnell, & Duncan, 1992). Thus, there remains a need for examination of neuroendocrine and immune responses to painful stimulation in humans using standardized, non-tissue damaging models of pain.
Recent experimental studies have examined pain provoked alterations of neuroendocrine and immune responses using complex batteries of various combinations of pain-induction modalities (Roupe van der Voort, Heijnen, Wulffraat, Kuis, & Kavelaars, 2000; Edwards et al., 2008). The inherent trouble with examining physiological responses across a battery of multiple and different experimental pain modalities is that it is not clear whether each modality is similarly or differentially affecting the physiological responses. The cold pressor task (CPT), the hot water task (HWT), and the submaximal effort tourniquet task (i.e., ischemic pain task; IPT) represent viable experimental pain modalities for characterization of neuroendocrine and immune responses to evoked pain. This is because each modality has a substantive affective component, encompasses a sizable anatomic region, and has been shown to produce pain that is tonic and clinically relevant (Edwards, Sarlani, Wesselmann, & Fillingim, 2005; Soyupek, Bozlu, Armagan, Ozorak, & Perk, 2007; Dannecker, Price, O’Connor, & Robinson, 2008). Of these three experimental pain induction modalities, the CPT has been used most consistently to assess neuroendocrine and immune responses to pain. At this time very few studies have specifically examined neuroendocrine and/or immune responses to the IPT or the HWT (Schwabe, Haddad, & Schachinger, 2008). Therefore, whether the CPT, HWT and IPT provoke comparable or differential patterns of neuroendocrine and immune response remains to be adequately addressed. Comparing the ability of these three pain modalities to reliably activate neuroendocrine and immune responses may lend further support (or not) for the use of each as a clinically relevant means of experimental pain induction.
Studies examining immunological changes associated with the HWT and IPT are scant. The CPT is reportedly associated with immunological changes in humans; however, results have been mixed. For instance, exposure to the CPT has been associated with suppression of immune response including decreased sIgA (Ring et al., 2000; Willemsen, Carroll, Ring, & Drayson, 2002; Isowa, Ohira, & Murashima, 2004), decreased lymphocyte proliferation (Delehanty et al., 1996), and decreased natural killer cell cytotoxicity (Delehanty et al., 1996). Conversely, the CPT and other acute physical stressors appear to be associated with enhanced release of pro-inflammatory cytokines. Indeed, pro-inflammatory cytokines such as interleukin 1 beta (IL-1β), interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) have been shown to be enhanced in response to the CPT and other acute stressors (Roupe van der Voort et al., 2000; Johnson et al., 2002; Deinzer et al., 2004; Edwards et al., 2008). In the present study, we chose to focus on one aspect of the immune system, the inflammatory response. TNF-α is a pro-inflammatory cytokine primarily released by activated macrophages that plays an important role in initiating inflammatory processes. The soluble receptor for TNF-α (sTNFαRII) reflects TNF-α activity (Aukrust et al., 1994; Zangerle et al., 1994); we chose to assess sTNFαRII because it is more stable and reliably measured than TNF-α (Diez-Ruiz et al., 1995). We also assessed cortisol response because cytokines have been shown to interact with the HPA axis (Maier & Watkins, 1998), and because the CPT reliably and consistently provokes increases in adrenocortical output (e.g., cortisol) (al’Absi, Petersen, & Wittmers, 2002; Cahill, Gorski, & Le, 2003; al’Absi et al., 2004; Dixon, Thorn, & Ward, 2004). Additionally, cortisol and sTNFαRII were chosen because they can be reliably assessed in oral fluids (Kirschbaum & Hellhammer, 1994; Nishanian, Aziz, Chung, Detels, & Fahey, 1998), which provides a less invasive and reactive means for assessing neuroendocrine and immune activity.
The goal of the present research was to characterize the neuroendocrine and inflammatory responses to multiple experimental pain modalities while remaining tolerable to participants. Specifically, cortisol and sTNFαRII responses provoked by the 3 painful modalities mentioned above, and a non-painful control stimulus (i.e., room temperature water task), were compared. Laboratory-based studies commonly do not provide a non-painful (i.e., control) modality for comparison when evaluating evoked pain sensitivity and resulting physiological responses. The rationale for the use of a non-painful modality in the current study is that the cortisol and sTNFαRII responses produced by the non-painful room temperature water will provide a reference from which the magnitude of cortisol response and sTNFαRII response provoked by the painful modalities can be judged. Several broad aims were addressed by this study. The first aim sought to compare cortisol and sTNFαRII responses among the 3 different pain modalities, and to compare these responses with any response provoked by the non-painful control modality. The second aim was to evaluate whether pain ratings were related to the cortisol and sTNFαRII response, respectively. The third and final aim was to examine the association between the cortisol response and the sTNFαRII response. It was hypothesized that, relative to baseline levels, each of the painful stimuli would be associated with significant provocations of the cortisol and sTNFαRII response, whereas the non-painful stimulus would not provoke any significant change (Aim 1). We further hypothesized that pain ratings would be positively associated with cortisol and sTNFαRII responses (Aim 2). Lastly, it was hypothesized that the cortisol response would be inversely related with the sTNFαRII response (Aim 3).
Method
Participants
Healthy, pain-free college students of both sexes were eligible for study enrollment. Relative to a clinical sample, inclusion of healthy, pain-free participants removes potential confounding variables such as prescription medication use and pre-existing alterations in pain sensitivity that may affect neuroendocrine and immunologic function. Individuals were unable to participate if they met any of the following criteria: (a) age less than 18 or over 45 years; (b) ongoing chronic pain problems; (c) diagnosed with hypertension or taking medication for blood pressure; (d) circulatory disorders; (e) history of cardiac events; (f) history of metabolic disease or neuropathy; (g) pregnant; (h) currently using prescription analgesics, tranquilizers, antidepressants, or other centrally acting agents; (i) use of nicotine, (j) use of prescription medication (e.g., corticosteriods, oral contraceptives), (k) psychiatric disorders (e.g., depression), or (l) chronic or acute health problems that affect the neuroendocrine or immune system. Individuals were successively assigned to experimental stimulus modalities in the following manner: IPT (N = 16), CPT (N = 10), HWT (N = 10), and NWT (N = 10). All participants were previously exposed to at least one type of experimental pain modality prior to participation in the current study. Individuals generally familiar with laboratory pain induction procedures were included to minimize the potential for anxiety and distress, which often accompanies the novelty of initial exposure to experimental pain, and that, could plausibly confound cortisol and sTNFαRII responses. This study was carried out in accordance with the 1975 Declaration of Helsinki (and its subsequent revisions) guidelines for ethical conduct of research. Informed consent was obtained in accordance with approved protocol guidelines of an Institutional Review Board. As part of the informed consent, all participants were told of which experimental stimulus modality (CPT, HWT, IPT, or NWT) they would be exposed. All participants were compensated for their participation.
Painful and non-painful stimulus modalities
Cold pressor task
The CPT procedure involved a NESLAB RTE-10 liter water bath (Thermo Electron Corporation, Portsmouth, New Hampshire) filled with circulating cold water maintained at approximately 4°C (± 0.2°C). Participants were instructed to place their dominant hand into the cold water up to their wrist. Standardized instructions indicated that participants should keep their hands immersed “for the entire duration of the procedure or until the pain becomes intolerable”, which is a typical measure of cold pain tolerance. Unbeknownst to participants, the maximum allowable duration of the CPT was 300 seconds (though participants were permitted to terminate the task at any time if the sensations become intolerable). While prior research has used different cutoff times, our 300 second cutoff is consistent with many previous studies (Walsh, Schoenfeld, Ramamurth, & Hoffman, 1989).
Hot water task
The hot water task is a tonic pain model similar in procedure to the CPT, but the temperature of the water is increased to 47°C (Rainville et al., 1999; Greenspan, Roy, Caldwell, & Farooq, 2003). A NESLAB RTE-10 liter water bath (Thermo Electron Corporation, Portsmouth, New Hampshire) was filled with circulating hot water maintained at approximately 47°C (± 0.2°C). Just as with the CPT, participants were instructed to place their dominant hand into the hot water up to their wrist and to keep their hands immersed “for the entire duration of the procedure or until the pain becomes intolerable”. The maximum allowable duration of the HWT was 300 seconds (though participants were permitted to terminate the task at any time if the sensations become intolerable).
Ischemic pain task
The IPT consisted of a modified submaximal effort tourniquet procedure that involved exercising the hand as blood flow to the arm was occluded, evoking ischemic pain. As is the standard IPT procedure, maximum grip strength of the dominant hand was determined using a Lafayette hand-held dynamometer (Lafayette Instrument Co., Lafayette, IN). Participants then elevated the dominant arm above heart level for 30 seconds to drain blood from the arm. The arm was then occluded with a standard blood pressure cuff positioned proximal to the elbow and inflated to 240 mm Hg using a Hokanson E20 rapid cuff inflator (D.E. Hokanson, Inc., Bellevue, WA). Participants then performed 20 handgrip exercises of 2 seconds duration with 4 second intervals at 50% of their maximum grip strength. Participants were instructed to say “stop” if they felt that the pain had become intolerable; otherwise, the task was carried out to 900 seconds. The 900 second duration for IPT is common and consistent with previous research (Pertovaara, Nurmikko, & Pontinen, 1984; Fillingim et al., 2005).
Neutral water task
The NWT is a non-painful stimulus modality similar in procedure to the CPT and HWT, but the temperature of the water is maintained at room temperature. The NESLAB RTE-10 liter water bath (Thermo Electron Corporation, Portsmouth, New Hampshire) was filled with circulating room temperature water maintained at approximately 25°C (± 2°C). Just as with the CPT and HWT, participants were instructed to place their dominant hand into the room temperature water up to their wrist, and to keep their hands immersed “for the entire duration of the procedure or until any pain they might experience becomes intolerable”. The maximum allowable duration of the NWT was 300 seconds.
Measures
Pain reports
Throughout each stimulus modality (CPT, HWT, IPT, NWT), separate ratings of pain intensity and pain unpleasantness were collected at 30 second intervals following task initiation, or until the task was discontinued due to pain intolerance. Ratings were also obtained at the exact time the task was terminated. A description of the difference between pain intensity (“How strong the pain feels”) and pain unpleasantness (“How unpleasant or disturbing the pain is for you”) was read to all subjects. Following this, pain intensity and pain unpleasantness were assessed by asking subjects for verbal self-reports on 0–100 scales, with 0 = “No pain” (or “Not at all unpleasant”) and 100 = “Pain as intense as I can imagine” (or “Pain as unpleasant as I can imagine). Numeric rating scales of pain intensity and pain unpleasantness have demonstrated validity through their ability to detect treatment effects, as well as their strong association to other measures of pain intensity and unpleasantness (Jensen & Karoly, 1992). Pain intensity and unpleasantness ratings were averaged across the pain task and mean values were included in statistical analyses, respectively. If participants were subjected to the entire task, or terminated their respective task prior to the allotted maximum time of exposure, the duration of exposure was recorded and classified as pain tolerance.
Neuroendocrine and immune response
Consistent with the procedures incorporated by Dickerson and colleagues (2004), the biological parameters in this study were obtained from oral fluids, which provide an established method for assessing cortisol (Kirschbaum & Hellhammer, 1994) and has been validated for assessing certain inflammatory products (Nishanian et al., 1998). Particularly, it has been demonstrated that levels of sTNFαRII in oral fluids are significantly and highly correlated with those obtained from plasma (Nishanian et al., 1998). Therefore, oral fluid collection seems to be a less reactive and invasive means for reliably measuring neuroendocrine and immune activity relative to a needle stick with blood draws.
An OraSure collection device (Epitope, Beaverton, OR) was placed into the mouth, between the lower cheek and gum, for 2.5 minutes per sampling time-point; this placement collects samples mainly containing oral mucosal transudate (OMT). Along with the OraSure device, salivettes (Sarsted, Leicester, UK) were concurrently placed into the mouth, on top of the tongue, for saliva collection. OMT and saliva are both oral fluids; OMT is a filtrate of blood plasma while saliva contains enzymes and other particles from the parotid and salivary glands. Cortisol in saliva is in its unbound, biologically active form, and its concentration is independent of saliva flow rate. OMT was collected for sTNFαRII rather than saliva because sTNFαRII is more readily available in OMT.
After obtaining the OMT and saliva samples, the samples were immediately refrigerated before being transferred and stored at −80°C until batch assayed. Cortisol was measured using high sensitivity salivary cortisol immunoassay kits (Salimetrics, State College, PA). Intra- and interassay variability was less than 8%. sTNFαRII was measured using Quantikine Human sTNFαRII enzyme immunoassay kits (R&D Systems, Minneapolis, MN). The Bradford method (Bradford, 1976) was used to quantify protein in the oral fluids, using the Bio-Rad protein assay kit with bovine plasma albumin as the standard. The sTNFαRII results are reported using the ratio of the experimental value for the analyte to the protein concentration in the test sample. This ratio controls for changes in salivary flow rate, which can be altered by experimental procedures or vary between individuals. The ratio values are more reliable than the analyte values alone (Nishanian et al., 1998). The intra- and interassay variability was less than 5%.
Procedures
Prior to the laboratory session, participants were asked to not use nonprescription medications or alcohol within 24 hours of their appointment. Participants were asked to refrain from exercise and consumption of caffeine for at least 2 hours prior to the testing session. To minimize potential error associated with the collection of oral fluid samples, participants were asked to not eat foods that may cause bleeding of the gums (e.g, potato chips) or brush their teeth for at least 2 hours prior to the testing session. This is because blood leakage from microinjuries of the oral mucosa may confound the measurement of salivary cortisol and OMT sTNFαRII (Kivlighan et al., 2004). All study procedures were carried out between the hours of 4 P.M. and 7 P.M. to control for diurnal variations in neuroendocrine parameters and because afternoon sessions have been associated with greater cortisol responses (Dickerson & Kemeny, 2004). Upon arrival to the laboratory, participants were escorted to a small room where all procedures were facilitated by 2 research assistants extensively trained according to study guidelines and protocols. After resting comfortably in a chair for 15 minutes to adapt to the experimental setting, participants provided saliva and OMT samples for cortsiol and sTNFαRII assessment (initial sample). The initial sample was intended to familiarize participants with the saliva collection procedures and is not included in data analysis. Participants then rested an additional 15 minutes at which time a second saliva and OMT sample (baseline sample) was collected prior to the initiation of the experimental (non-) painful task. Additional cortisol and sTNFαRII samples were collected immediately following termination of the (non-) painful modality and at various intervals during recovery from these modalities (10, 15, 20, 25, and 35 minutes after termination). These sampling time-points were chosen based on a meta-analysis of prior research showing that peak changes in cortisol occur at approximately 30 minutes following stressor onset (Dickerson & Kemeny, 2004). Additionally, a recent study has reported that sTNFαRII responses to acute stress have been found to occur at that same time-point (Dickerson, Kemeny, Aziz, Kim, & Fahey, 2004).
Statistical analyses
A significant amount of skew in the distributions of cortisol and sTNFαRII data was discovered. Accordingly, these data were subjected to logarithmic transformation using a log10 function, which was effective for reducing skew according to Shapiro-Wilk’s tests (p’s > 0.05). Subsequent statistical analysis of cortisol and sTNFαRII responses was completed using transformed values; however, study figures show anti-log mean values for easier interpretation of results. Inter-relationships among continuous study variables were evaluated using Pearson-product moment correlations. Chi-square was used to examine relationships between categorical variables. Reports of pain intensity and pain unpleasantness from the painful and non-painful stimuli were compared using analysis of variance (ANOVA). Changes in levels of salivary cortisol and OMT sTNFαRII were evaluated using mixed-model repeated measures analysis of variance (RM-ANOVA) with Greenhouse-Geisser corrections for violations of spherecity. Significant interaction and main effects were further analyzed by Bonferroni adjusted post-hoc tests. Analyses include the partial η2 as a measure of effect size where appropriate. Following the conventions of Cohen (1988), partial η2 = 0.01 is considered a small effect, partial η2 = 0.06 a medium-sized effect and partial η2 = 0.14 a large effect. Per the specifications of Pruessner and colleagues (2003), measures of area under the curve with respect to ground (AUCG) and area under the curve with respect to increase (AUCI) were calculated to summarize overall levels and time-dependent change of cortisol and sTNFαRII, respectively. With endocrine and immune data, AUCG is assumed to be a measure more related to total cortisol/sTNFαRII output, whereas AUCI is a parameter that emphasizes the changes over time and is more related to sensitivity of the system. Finally, the relationships between pain reports and these summary indicators of physiological reactivity to pain were assessed using zero-order correlations and partial correlations when indicated. SPSS for windows (version 19.0) was used for all analyses.
Results
Participant characteristics
The final sample for data analysis consisted of 46 individuals that included 52% female and 48% male participants (mean age = 20.2 years, SD = 2.7); 44% self-identified as non-Hispanic white, 17% were African American, and 39% were Asian American. Results of chi-square analyses revealed that the painful and non-painful experimental tasks did not significantly differ as a function of participants’ sex (χ2(3) = 3.52, p = 0.32) or ethnicity (χ2(6) = 8.29, p = 0.22). Further, neither participants’ sex or ethnicity (dummy-coded with non-Hispanic white as the referent) was significantly correlated with pain intensity and unpleasantness reports or any of the AUC summary scores for cortisol and sTNFαRII (all p’s > 0.05). Accordingly, participants’ sex and ethnicity were not included in any additional analyses.
Pain reports
Differences in pain reports among the different stimuls modalities are shown in Table 1. Results demonstrated sigificant differences in duration of exposure (i.e., pain tolerance) to the painful tasks (F(3,42) = 24.8, p < 0.001). Tukey’s post-hoc analyses revealed that the duration of exposure to the IPT was much longer than that of the CPT, HWT, or NWT (all p’s < 0.001). This difference in pain tolerance was expected given that the maximum allowed duration of exposure to the CPT, HWT, NWT, and IPT was 300 sec, 300 sec, 300 sec, and 900 sec, respectively. None of the individuals exposed to the non-painful NWT task reported any onset of pain, and each individual was exposed for the full 300 sec allowed. Accordingly, the pain tolerance variable was included as a covariate in analyses examining pain ratings. There were significant differences by stimulus modality for ratings of pain intensity (F(3,42) = 22.1, p < 0.001) and pain unpleasantness (F(3,42) = 26.7, p < 0.001). Tukey’s post-hoc analyses revealed that intensity and unpleasantness ratings were greater for each of the painful tasks compared to the non-painful task (p’s < 0.001); however, ratings of intensity and unpleasantness did not significantly differ among the CPT, HWT, and IPT (p’s > 0.15).
Table 1.
Mean differences in pain tolerance, intensity, and unpleasantness across CPT, HWT, IPT, and NWT.
| Cold Pressor Task N = 10 |
How Water Task N = 10 |
Ischemic Pain Task N = 16 |
Neutral Water Task N = 10 |
|
|---|---|---|---|---|
| Duration of Exposure (Pain Tolerance)* | 248.7 sec, SD = 108.7 | 246.7 sec, SD = 78.2 | 675.0 sec, 236.3 | 300.0 sec, SD = 0.0 |
| Pain Intensity** | 62.9, SD = 24.4 | 67.2, SD = 22.4 | 48.4, SD = 23.5 | 0.0, SD = 0.0 |
| Pain Unpleasantness*** | 63.2, SD = 24.6 | 67.8, SD = 22.1 | 50.6, SD = 18.5 | 0.7, SD = 1.7 |
IPT > CPT, HWT, NWT; p’s < 0.001
NWT < CPT, HWT, IPT; p’s < 0.001
NWT < CPT, HWT, IPT; p’s < 0.001
Biological outcomes
First, the baseline measures for cortisol and sTNFαRII were compared between each of the experimental pain tasks. Mean baseline values for cortisol were not found to be statistically different across the four experimental modalities (F(3,42) = 1.64, p = 0.19). The absence of statistical differences among baseline cortisol across the four modalities suggests that participants had equivalent cortisol levels prior to initiation of each stimulus modality. Conversely, mean baseline values for sTNFαRII were statisically different (F(3,42) = 3.15, p = 0.03), such that the mean baseline of sTNFαRII for those participants subjected to the HWT was significantly less than the baseline of those subjected to the IPT (p = 0.04). Accordingly, baseline sTNFαRII values were included in subsquent analyses as a statistical control when appropriate.
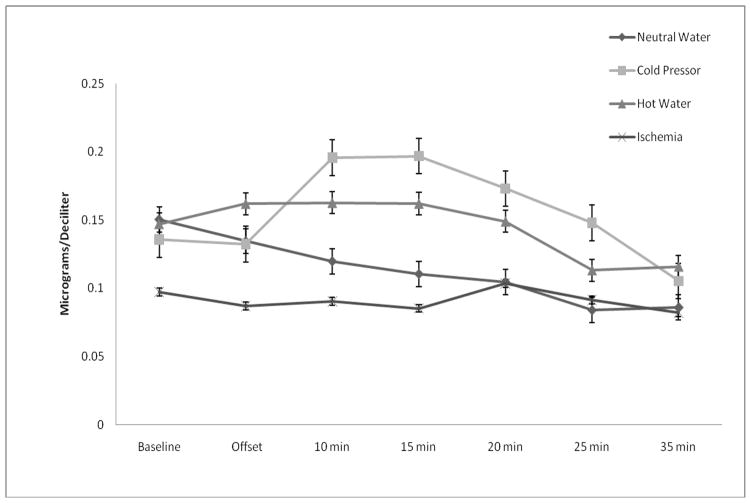
Cortisol responses are shown in Figure 1. Results of RM-ANOVA revealed a significant main effect of time (F(6,252) = 6.19, p < 0.01, η2 = 0.128) and a significant interaction of stimulus modality X time for cortisol (F(18,252) = 2.31, p = 0.03, η2 = 0.142). This interaction effect remained significant after adjustment for differences in pain tolerance across the four experimental modalities (F(18,246) = 2.13, p = 0.04, η2 =0.135). The main effect of stimulus modality was non-significant (p > 0.10, η2 = 0.124). When compared to baseline, post-hoc tests showed that the CPT induced significant increases in cortisol at 10 min and 15 min following completion (Bonferroni adjusted post-hoc tests: p’s < 0.025). The CPT-induced elevation at 15 min was significantly greater than that of the IPT and NWT (Bonferroni adjusted post-hoc tests: p’s < 0.017), but not the HWT. Neither the HWT nor IPT produced significant increases in cortisol relative to their respective baselines. The cortisol response for the NWT was found to be marginally decreased from baseline at termination (p = 0.03), but then became significantly diminished at each successive sampling point (Bonferroni adjusted post-hoc tests: p’s < 0.001). Results for salivary cortisol response do not appear to have been meaningfully influenced by heterogeneity of covariance or variance according to Box’s test and Levene’s tests, respectively (p’s > 0.05).
Figure 1.
Cortisol responses to painful and non-painful modalities.
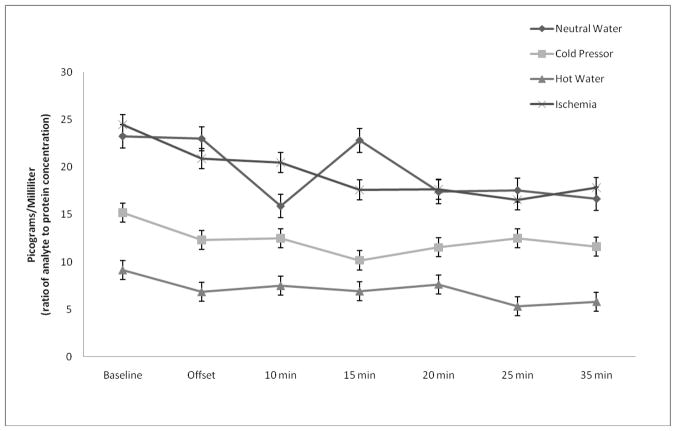
sTNFαRII responses are shown in Figure 2. An additional RM-ANOVA demonstrated significant main effects of time (F(6,252) = 4.80, p < 0.01, η2 =0.102) and stimulus modality (F(3,42) = 3.69, p = 0.02, η2 = 0.209); however, the stimulus modality X time interaction was non-significant (F(18,252) = 1.15, p = 0.32, η2 = 0.076). When compared to baseline, the three painful tasks were associated with decreased sTNFαRII responses that were most robust, on average, at 25 min and 35 min following task initiation (Bonferroni adjusted post-hoc tests: p’s < 0.017). Although there was a significant decrease in sTNFαRII at 10 min following termination of the non-painful task (p < 0.01); thereafter, sTNFαRII returned to levels that were non-significantly different from baseline. After adjusting for differences in pain tolerance, the main effect of time was no longer significant (F(6,246) = 1.27, p = 0.28, η2 = 0.030); however, the main effect of stimulus modality remained significant (F(3,41) = 3.28, p = 0.03, η2 =0.193). Post-hoc analysis of the significant main effect for stimulus modality showed that overall sTNFαRII production for the HWT was significantly less than the IPT (Bonferroni adjusted post-hoc tests: p < 0.01). The main effect of stimulus modality for overall sTNFαRII production was no longer significant when baseline sTNFαRII levels were controlled (p > 0.05). Box’s test and Levene’s tests were non-significant (p’s > 0.05).
Figure 2.
sTNFαRII responses to painful and non-painful modalities.
Associations between pain reports and biological outcomes
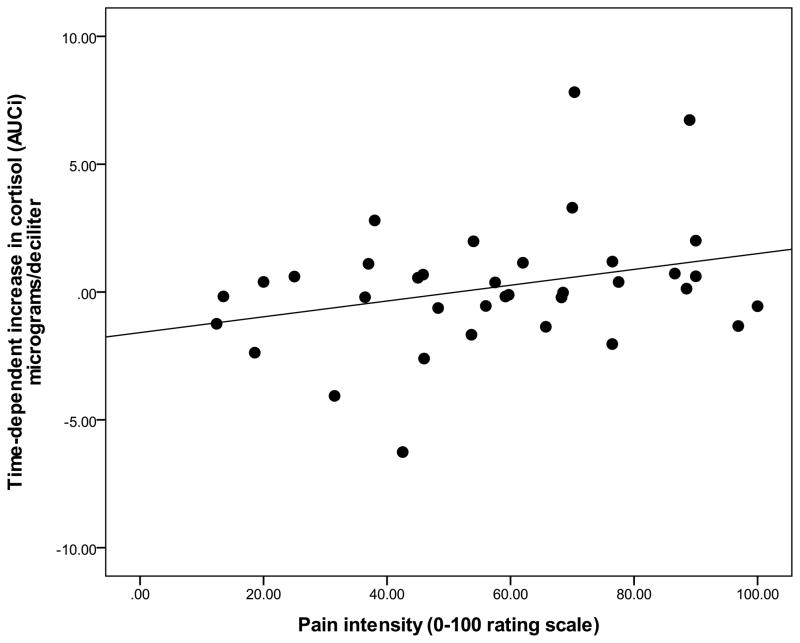
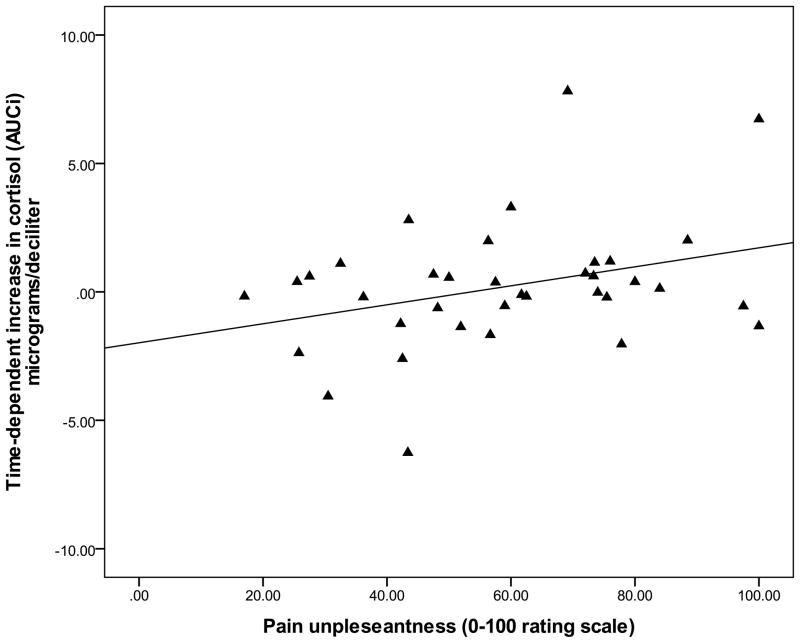
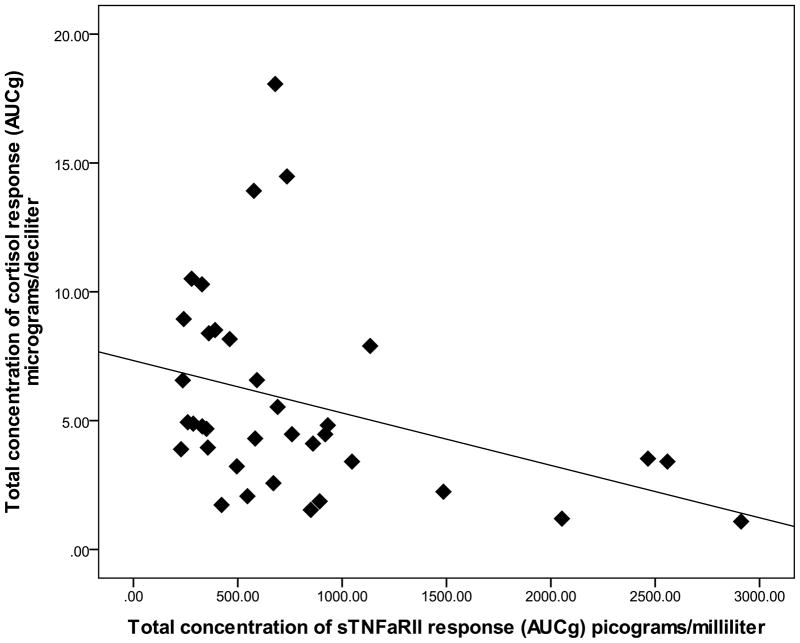
Since pain was not reported for the NWT modality, examination of relations among pain reports and biological outcomes was completed using data that was collapsed over the CPT, HWT, and IPT cohorts. Zero-order correlations (Table 2) demonstrated that the summary measure for time-dependent change in cortisol (AUCI) was significantly and positively associated with average pain ratings of intensity (r = .33, p = 0.04) and unpleansantness (r = .37, p = 0.02). Specifially, greater intensity and unpleasantness pain ratings were associated with greater cortisol increase from baseline (see Figure 3 and Figure 4). The summary measure for total cortisol concentration (AUCG) was not significantly associated with any pain ratings (p’s > 0.05). Controlling for baseline differences in sTNFαRII across pain modalities, partial correlations revealed that neither time-dependent change in sTNFαRII (AUCI) or total sTNFαRII concentration (AUCG) was significantly related to pain ratings of intensity or unpleasantness (all p’s > 0.05). However, the summary measures (AUCG) for total concentration of the cortisol response and total concentration of the sTNFαRII response were significantly and negatively correlated (r = −.31, p = 0.04), which suggests that greater overall cortisol production is potentially related to diminished overall sTNFαRII production in association with the painful modalities (Figure 5).
Table 2.
Zero-order correlations among pain sensitivity indicators and pain-related physiological responses.
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| 1) Duration of Exposure (Pain Tolerance) | — | |||||
| 2) Pain Intensity | −.51** | — | ||||
| 3) Pain Unpleasantness | −.44** | .95*** | — | |||
| 4) Cortisol AUCG | −.22 | .01 | .01 | — | ||
| 5) Cortisol AUCI | −.21 | .33* | .37* | .41* | — | |
| 6) sTNFαRII AUCG | .26 | −.11 | −.13 | −.36* | −.10 | — |
| 7) sTNFαRII AUCI | −.32 | −.04 | −.04 | −.02 | .02 | −.40* |
Note: Pain and physiological data was collapsed across CPT, HWT, and IPT only
p < 0.05,
p < 0.01,
p < 0.001
Figure 3.
Relationship between time-dependent increase in salivary cortisol and ratings of pain intensity (data collapsed across CPT, HWT, and IPT).
Figure 4.
Relationship between time-dependent increase in salivary cortisol and ratings of pain unpleasantness (data collapsed across CPT, HWT, and IPT).
Figure 5.
Relationship between overall salivary cortisol response and overall sTNFαRII response (data collapsed across CPT, HWT, and IPT).
Discussion
Pain and cortisol differences across pain modalties
Results showed that the NWT did not produce pain; however, the adminstration of the CPT, HWT, and IPT successfully produced painful experiences that were comparable in terms of ratings of intensity and unpleasantness. Additionally, our data suggest that the CPT was the modality most capable of eliciting significant elevations in salivary cortisol that were greater than the responses to the IPT and NWT. Interestingly, compared with their basal levels, neither the HWT nor IPT provoked significant increases in cortisol, and cortisol significantly decreased over time in relation to the NWT. As expected, ratings of pain intensity and pain unpleasantess were signficantly correlated with the summary measure of time-dependent cortisol increase (AUCI) in response to the painful tasks. These correlations suggest that ratings of greater pain intensity and greater pain unpleasnantness were both associated with greater increases of cortisol in response to the painful modalities.
The finding that salivary cortisol was moderately, yet significantly, elevated from baseline following exposure to the CPT is consisent with previous reports (al’Absi et al., 2002; Cahill et al., 2003; Gluck, Geliebter, Hung, & Yahav, 2004; Andreano & Cahill, 2006). The lack of cortisol response to the HWT in the present study is also consistent with that of a previous study by Schwabe and colleagues (2008). It does not appear that cortisol responses were related to the duration of exposure (i.e., pain tolerance) to the specific modalities. This is because differences in cortisol response remained significant after controlling for pain tolerance. Although reports of pain intensity and unpleasantness were significantly and positively correlated with elevations in cortisol, pain ratings did not significantly different across the painful modalities. Therefore, differences in cortisol response among the CPT, HWT, and IPT do not appear to be purely a function of the pain experience. Compared to the HWT and IPT, it may be that the pain quality and other stress-related features (e.g., sympathetic activation) characteristic of the CPT made it the most effective at eliciting elevations in cortisol. The elevated cortisol response to the CPT in the current study’s non-clinical sample needs to be reconciled with previous clinical studies demonstrating that hypoactivity of the HPA axis (e.g., blunted cortisol responses) can also be associated with enhanced pain sensitivity and more severe pain ratings (McBeth et al., 2005). One potential factor affecting the pattern of cortisol responses to pain might be whether the pain experienced is experimental or the result of a clinical condition. For instance, in our sample of healthy, pain-free individuals, the CPT may be more likely to produce elevations in cortisol (i.e., a normal stress response) that were subsequently related to greater ratings of pain as a consequence of this acute pain modality. Alternatively, in clinical samples of individuals afflicted with painful conditions, chronic pain that threatens physical well-being has been shown to be related with flat diurnal profiles of cortisol release and diminished cortisol responses to painful stressors (Chrousos, 2000). Despite these differences, excessive activation of the HPA axis associated with chronic stress has been implicated in the development of chronic pain conditions (Blackburn-Munro & Blackburn-Munro, 2003) and pain-related comorbidities such as depression (Blackburn-Munro, 2004). Therefore, the CPT may be the most clinically relevant experimental pain modality for future studies seeking to examine how excessive HPA axis activations contribute to chronic pain development.
That the cortisol response was significantly decreased from baseline in association with the NWT may be explained by participants’ anticipatory appraisals and distress related to completion of the non-painful modality (Gaab, Rohleder, Nater, & Ehlert, 2005). For instance, all participants may have experienced some level of distress in anticipation of their respective experimental modality, but compared to the CPT, HWT, and IPT, those participants assigned to the NWT were more able to relax upon being told that they would be subjected to a non-painful modality. Therefore, without the provocation of pain, cortisol response diminished over time for the NWT (Rohrmann, Henning, & Netter, 1999; Smyth, Litcher, Hurewitz, & Stone, 2001). It also cannot be ruled out that the gradual decrease in cortisol associated with the NWT was related to the circadian rhythm of cortisol, which tends to decline even in the late afternoon and evening (Straub & Cutolo, 2007).
sTNFαRII differences across pain modalities
Contrary to previous reports of enhanced pro-inflammatory cytokine responses following administration of acute painful/stressful stimuli (Edwards et al., 2008; Roupe van der Voort et al., 2000; Deinzer et al., 2004), our results showed that sTNFαRII in oral fluid significantly decreased from baseline in relation to all three experimental pain modalities. At this time it cannot be said with complete certainty that diminished sTNFαRII following the CPT, HWT, and IPT was a specific response to our study’s painful modalities. This is because sTNFαRII was also found to be significantly decreased following completion of the non-painful NWT; however, sTNFαRII did eventually return to a level indistinguishable from baseline for the NWT. Methodological differences may explain the discrepancy between this study and prior studies that found enhanced pro-inflammatory cytokine responses to acute pain. For example, in the studies conducted by Edwards et al., 2008 and Deinzer et al., 2004, the most significant enhancements in inflammatory cytokines following experimental pain induction were demonstrated 60 min post-pain and beyond. Unfortunately, nothing can be discerned about sTNFαRII responses beyond the 35 min mark in the present study. Our study may have benefited from a time-course devised to examine patterns of change in sTNFαRII response that occurred beyond the 35 min mark following acute painful stimulation. Another possible explanation for the discrepancy is that, in the present study, the summary indicator (AUCG) for the overall concentration of the cortisol response was significantly and negatively associated with the overall concentration of the sTNFαRII response. This finding is similar to that of a previous study (Kunz-Ebrecht, Mohamed-Ali, Feldman, Kirschbaum, & Steptoe, 2003) and consistent with the evidence that glucocorticoids such as cortisol down-regulate cytokine levels and receptor expression (Franchimont, Kino, Galon, Meduri, & Chrousos, 2002; Franchimont, 2004). Since cortisol can also act on cytokine recptors, the negative assocation between the cortisol and sTNFαRII responses could also be explained by the interference of cortisol with the activation of the soluble TNF-α receptor (Sorrells & Sapolsky, 2007). On balance, the sTNFαRII responses in the current study make it difficult to determine which experimental pain modality might be most clinically useful for demonstrating pain-related changes in pro-inflammatory cytokines.
Limitations of present study
The present study possesses several limitations and qualifications that merit caution when interpreting the findings. First, individuals were not randomly assigned to experimental modality and the sample size within each modality was relatively small. It may be that randomization of a greater number of people to the painful and non-painful modalities would have helped to minimize baseline differences in sTNFαRII across modalities. Another limitation is that all study participants were college students. Although great efforts were made to ensure that student participants did not matriculate through the study during times of academic examination or other acute psychosocial stressors that could influence HPA axis and immune response, this cannot be guaranteed. As a consequence, the generalizability of these findings is not perfectly clear and replication of these effects in other samples will be quite pertinent. Lastly, the current study was only able to sample the soluble receptor of a single inflammatory cytokine (sTNFαRII). Simultaneous assessment of many cytokines in a biological sample provides more comprehensive information rather than assessing a single cytokine (Prabhakar et al., 2004). Hence, systemic measurement of multiple cytokines in oral fluids, plasma, and/or serum may provide a more detailed indication of any underlying pro-inflammatory responses and also yield information on the skewness of the T-helper responses (Th1 or Th2) in response to acute painful stimulation (Sachdeva et al., 2007).
Conclusions and future prospects
In conclusion, the current study extends prior research suggesting that the CPT might be the most effective experimental acute pain modality for reliably inducing elevated cortisol responses. A particular strength of the current study was the inclusion of the non-painful NWT. The majority of studies that have investigated neuroendocrine and immune responses to acute experimentally-induced pain have not included a non-painful modality for comparison. The finding that cortisol was significantly diminished from baseline following completion of the NWT helps to more comprehensively characterize patterns of HPA axis activity in response to multiple modalities of experimental stimulation. For reasons mentioned above, the inflammatory cytokine response (sTNFαRII) to multiple modalities of acute experimental pain remains less clear, and it appears that additional research into this matter is warranted. Further characterization of inflammatory cytokine responses to experimental pain modalities may help refine and validate the clinical usefulness of these modalities. Technically speaking, it is also proposed that future studies more extensively examine the link between experimental pain and the induction of HPA axis and inflammatory cytokine activity up to 60 min or more following exposure to the experimental pain modality. This proposed time-line is based upon the current study’s results and a growing literature indicating that the cortisol response, the inflammatory response, and their interactions, can be detected in the periphery up to 60 min and longer following an acute painful stressor (Sorrells & Sapolsky, 2007; Steptoe, Willemsen, Owen, Flower, & Mohamed-Ali, 2001).
Acknowledgments
Funding and support for this study and manuscript preparation was provided by NIH/NCCAM Grant R21AT003250-01A1 (L.McGuire) and by an NIH Training Grant T32NS045551-06 (B. Goodin). Neither the NIH nor the University of Florida had any further role in study design; the collection, analysis and interpretation of data; in the writing of this report; nor in the decision to submit the paper for publication.
References
- al’Absi M, Petersen KL, Wittmers LE. Adrenocortical and hemodynamic predictors of pain perception in men and women. Pain. 2002;96:197–204. doi: 10.1016/S0304-3959(01)00447-X. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Wittmers LE, Ellestad D, Nordehn G, Kim SW, Kirschbaum, et al. Sex differences in pain and hypothalamic-pituitary-adrenocortical responses to opioid blockade. Psychosomatic Medicine. 2004;66:198–206. doi: 10.1097/01.psy.0000116250.81254.5d. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Glucocorticoid release and memory consolidation in men and women. Psychological Science. 2006;17:466–470. doi: 10.1111/j.1467-9280.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- Aukrust P, Liabakk N, Muller F, Lien E, Espevik T, Froland SS. Serum levels of tumor necrosis factor-alpha (TNF-α) and soluble TNF receptors in human immunodeficiency virus type 1 infection—correlations to clinical, immunologic, and virologic parameters. Journal of Infectious Diseases. 1994;169:420–424. doi: 10.1093/infdis/169.2.420. [DOI] [PubMed] [Google Scholar]
- Blackburn-Munro G. Hypothalamic-pituitary-adrenal axis dysfunction as a contributory factor to chronic pain and depression. Current Pain and Headache Reports. 2004;8:116–124. doi: 10.1007/s11916-004-0025-9. [DOI] [PubMed] [Google Scholar]
- Blackburn-Munro G, Blackburn-Munro R. Pain in the brain: Are hormones to blame? TRENDS in Endocrinology and Metabolism. 2003;14:20–27. doi: 10.1016/S1043-2760(02)00004-8. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cahill L, Gorski L, Le K. Enhanced human memory consolidation with post-learning stress: interaction with the degree of arousal at encoding. Learning and Memory. 2003;10:270–274. doi: 10.1101/lm.62403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman CR, Tuckett RP, Song CW. Pain and stress in a systems perspective. Reciprocal neural, endocrine, and immune interactions. Journal of Pain. 2008;9:122–145. doi: 10.1016/j.jpain.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos G. Stress, chronic inflammation, and emotional and physical well-being: Concurrent effects and chronic sequelae. Journal of Allergy and Clinical Immunology. 2000;106:S275–S291. doi: 10.1067/mai.2000.110163. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Lawrence Erlbaum; Hillsdale: 1988. [Google Scholar]
- Dannecker EA, Price DD, O’Connor PD, Robinson ME. Appraisals of pain from controlled stimuli: Relevance to quantitative sensory testing. British Journal of Health Psychology. 2008;13:537–550. doi: 10.1348/135910707X230985. [DOI] [PubMed] [Google Scholar]
- Deinzer R, Granrath N, Stuhl H, Twork L, Idel H, Waschul B, et al. Acute stress effects on local IL-1beta responses to pathogens in a human in vivo model. Brain, Behavior, and Immunity. 2004;18:458–467. doi: 10.1016/j.bbi.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Delahanty DL, Dougall AL, Schmitz JB, Hawken L, Trakowski JH, Jenkins FJ, et al. Time course of natural killer cell activity and lymphocyte proliferation in response to two acute stressors in healthy men. Health Psychology. 1996;15:48–55. doi: 10.1037/0278-6133.15.1.48. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME, Aziz N, Kim KH, Fahey JL. Immunological effects of induced shame and guilt. Psychosomatic Medicine. 2004;66:124–131. doi: 10.1097/01.PSY.0000097338.75454.29. [DOI] [PubMed] [Google Scholar]
- Diez-Ruiz A, Tilz GP, Zangerle R, Baier-Bitterlich G, Wachter H, Fuchs D. Soluble receptors for tumor necrosis factor in clinical laboratory diagnosis. European Journal of Haematology. 1995;54:1–8. doi: 10.1111/j.1600-0609.1995.tb01618.x. [DOI] [PubMed] [Google Scholar]
- Dixon KE, Thorn BE, Ward LC. An evaluation of sex differences in psychological and physiological responses to experimentally-induced pain: a path analytic description. Pain. 2004;112:188–196. doi: 10.1016/j.pain.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Kronfli T, Haythornthwaite JA, Smith MT, McGuire L, Page GG. Association of catastrophizing with interleukin-6 responses to acute pain. Pain. 2008;140:135–144. doi: 10.1016/j.pain.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RR, Sarlani E, Wesselmann U, Fillingim RB. Quantitative assessment of experimental pain perception: multiple domains of clinical relevance. Pain. 2005;114:315–319. doi: 10.1016/j.pain.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Ness TJ, Glover TL, Campbell CM, Hastie BA, Price DD, et al. Morphine responses and experimental pain: Sex differences in side effects and cardiovascular responses but not analgesia. Journal of Pain. 2005;6:116–124. doi: 10.1016/j.jpain.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Franchimont D. Overview of the actions of glucocorticoids on the immune response: A good model to characterize new pathways of immunosuppression for new treatment strategies. Annals of the New York Academy of Science. 2004;1024:124–137. doi: 10.1196/annals.1321.009. [DOI] [PubMed] [Google Scholar]
- Franchimont D, Kino T, Galon J, Meduri GU, Chrousos GP. Glucocorticoids and inflammation revisited: The state of the art. NeuroImmunoModulation. 2002;10:247–260. doi: 10.1159/000069969. [DOI] [PubMed] [Google Scholar]
- Gaab J, Rohleder N, Nater UM, Ehlert U. Psychological determinants of the cortisol stress response: The role of anticipatory cognitive appraisal. Psychoneuroendocrinology. 2005;30:599–610. doi: 10.1016/j.psyneuen.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Gluck ME, Geliebter A, Hung J, Yahav E. Cortisol, hunger, and desire to binge eat following a cold stress in obese woman with binge eating disorder. Psychosomatic Medicine. 2004;66:876–881. doi: 10.1097/01.psy.0000143637.63508.47. [DOI] [PubMed] [Google Scholar]
- Greenspan JD, Roy EA, Caldwell PA, Farooq NS. Thermosensory intensity and affect throughout the perceptible range. Somatosensory and Motor Research. 2003;20:19–26. doi: 10.1080/0899022031000083807. [DOI] [PubMed] [Google Scholar]
- Isowa T, Ohira H, Murashima S. Reactivity of immune, endocrine and cardiovascular parameters to active and passive acute stress. Biological Psychology. 2004;65:101–120. doi: 10.1016/S0301-0511(03)00115-7. [DOI] [PubMed] [Google Scholar]
- Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R, editors. Handbook of Pain Assessment. New York, NY: Guilford Press; 1992. pp. 135–151. [Google Scholar]
- Johnson JD, O’Connor KA, Deak T, Spencer RL, Watkins LR, Maier SF. Prior stressor exposure primes the HPA axis. Psychoneuroendocrinology. 2002;27:353–365. doi: 10.1016/S0306-4530(01)00057-9. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Page GG, Marucha PT, MacCallum RC, Glaser R. Psychological influences on surgical recovery. Perspectives from psychoneuroimmunology. American Psychologist. 1998;53:1209–1218. doi: 10.1037/0003-066X.53.11.1209. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DC. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology. 1994;19:313–333. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Kivlighan KT, Granger DA, Schwartz EB, Nelson V, Curran M, Shirtcliff EA. Quantifying blood leakage into the oral mucosa and its effects on the measurement of cortisol, dehydroepiandrosterone, and testosterone in saliva. Hormones and Behavior. 2004;46:39–46. doi: 10.1016/j.yhbeh.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Kunz-Ebrecht SR, Mohamed-Ali V, Feldman PJ, Kirschbaum C, Steptoe A. Cortisol responses to mild psychological stress are inversely associated with proinflammatory cytokines. Brain, Behavior, and Immunity. 2003;17:373–383. doi: 10.1016/S0889-1591(03)00029-1. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Cytokines for psychologists: Implications of bidirectional immune-to-brain communication for understanding behavior, mood and cognition. Psychological Review. 1998;105:83–107. doi: 10.1037/0033-295X.105.1.83. [DOI] [PubMed] [Google Scholar]
- McBeth J, Chiu YH, Silman AJ, Ray D, Morriss R, Dickens C, et al. Hypothalamic-pituitary-adrenal stress axis function and the relationship with chronic widespread pain and its antecedents. Arthritis Research & Therapy. 2005;7:R992–R1000. doi: 10.1186/ar1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishanian P, Aziz N, Chung J, Detels R, Fahey JL. Oral fluids as an alternative to serum for measurement of markers of immune activation. Clinical and Diagnostic Laboratory Immunology. 1998;5:507–512. doi: 10.1128/cdli.5.4.507-512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor KA, Johnson JD, Hansen MK, Wieseler Frank JL, Maksimova E, Watkins LR, et al. Peripheral and central proinflammatory cytokine response to a severe acute stressor. Brain Research. 2003;991:123–132. doi: 10.1016/j.brainres.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Page GG. Analgesia administration attenuates surgery-induced tumor promotion. Regional Anesthesia and Pain Medicine. 2002;27:197–199. doi: 10.1097/00115550-200203000-00014. [DOI] [PubMed] [Google Scholar]
- Page GG, Ben-Eliyahu S. Increased surgery-induced metastasis and suppressed natural killer cell activity during proestrus/estrus in rats. Breast Cancer Research and Treatment. 1997;45:159–167. doi: 10.1023/A:1005826403235. [DOI] [PubMed] [Google Scholar]
- Page GG, Ben-Eliyahu S, Liebeskind JC. The role of LGL/NK cells in surgery-induced promotion of metastasis and its attenuation by morphine. Brain Behavior and Immunity. 1994;8:241–250. doi: 10.1006/brbi.1994.1022. [DOI] [PubMed] [Google Scholar]
- Pertovaara A, Nurmikko T, Pontinen PJ. Two separate components of pain produced by the submaximal effort tourniquet test. Pain. 1984;20:53–58. doi: 10.1016/0304-3959(84)90810-8. [DOI] [PubMed] [Google Scholar]
- Prabhakar U, Eirikis E, Reddy M, Silvestro E, Spitz S, Pendley C, et al. Validation and comparative analysis of a multiplexed assay for the simultaneous quantitative measurement of Th1/Th2 cytokines in human serum and human peripheral blood mononuclear cell culture supernatants. Journal of Immunological Methods. 2004;291:27–38. doi: 10.1016/j.jim.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/S0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Rainville P, Carrier B, Hofbauer RK, Bushnell MC, Duncan GH. Dissociation of sensory and affective dimensions of pain using hypnotic modulation. Pain. 1999;82:159–171. doi: 10.1016/S0304-3959(99)00048-2. [DOI] [PubMed] [Google Scholar]
- Rainville P, Feine JS, Bushnell MC, Duncan GH. A psychophysical comparison of sensory and affective responses to four modalities of experimental pain. Somatosensory and Motor Research. 1992;9:265–277. doi: 10.3109/08990229209144776. [DOI] [PubMed] [Google Scholar]
- Ren K, Torrres R. Role of interleukin-1β during pain and inflammation. Brain Research Reviews. 2009;60:57–64. doi: 10.1016/j.brainresrev.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring C, Harrison LK, Winzer A, Carroll D, Drayson M, Kendall M. Secretory immunoglobulin A and cardiovascular reactions to mental arithmetic, cold pressor, and exercise: Effects of alpha-adrenergic blockade. Psychophysiology. 2000;37:634–643. doi: 10.1111/1469-8986.3750634. [DOI] [PubMed] [Google Scholar]
- Rohrmann S, Hennig J, Netter P. Changing psychobiological stress reactions by manipulating cognitive processes. International Journal of Psychophysiology. 1999;33:149–161. doi: 10.1016/S0167-8760(99)00036-7. [DOI] [PubMed] [Google Scholar]
- Roupe van der Voort C, Heijnen CJ, Wulffraat N, Kuis W, Kavelaars A. Stress induces increases in IL-6 production by leucocytes of patients with the chronic inflammatory disease juvenile rheumatoid arthritis: a putative role for alpha(1)-adrenergic receptors. Journal of Neuroimmunology. 2000;110:223–229. doi: 10.1016/S0165-5728(00)00328-3. [DOI] [PubMed] [Google Scholar]
- Sachdeva N, Yoon HS, Oshima K, Garcia D, Gookdin K, Asthana D. Biochip array-based analysis of plasma cytokines in HIV patients with immunological and virological discordance. Scandinavian Journal of Immunology. 2007;65:549–554. doi: 10.1111/j.1365-3083.2007.01906.x. [DOI] [PubMed] [Google Scholar]
- Salomaki TE, Leppaluoto J, Laitinen JO, Vuolteenaho O, Nuutinen LS. Epidural versus intravenous fentanyl for reducing hormonal, metabolic, and physiologic responses after thoracotomy. Anesthesiology. 1993;79:672–679. doi: 10.1097/00000542-199310000-00007. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Haddad L, Schachinger H. HPA axis activation by a socially evaluated cold-pressor test. Psychoneuroendocrinology. 2008;33:890–895. doi: 10.1016/j.psyneuen.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Smyth J, Litcher L, Hurewitz A, Stone A. Relaxation training and cortisol secretion in adult asthmatics. Journal of Health Psychology. 2001;6:217–227. doi: 10.1177/135910530100600202. [DOI] [PubMed] [Google Scholar]
- Sorrells SF, Sapolsky RM. An inflammatory review of glucocorticoid actions in the CNS. Brain, Behavior, and Immunity. 2007;21:259–272. doi: 10.1016/j.bbi.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyupek S, Bozlu M, Armagan A, Ozorak A, Perk H. Does experimental pain assessment before biopsy predict for pain during transrectal ultrasound-guided prostate biopsy? Urology. 2007;70:681–684. doi: 10.1016/j.urology.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Willemsen G, Owen N, Flower L, Mohamed-Ali V. Acute mental stress elicits delayed increases in circulating inflammatory cytokine levels. Clinical Science. 2001;101:185–192. doi: 10.1042/CS20010038. [DOI] [PubMed] [Google Scholar]
- Straub RH, Cutolo M. Circadian rhythms in rheumatoid arthritis: Implications for pathophysiology and therapeutic management. Arthritis & Rheumatism. 2007;56:399–408. doi: 10.1002/art.22368. [DOI] [PubMed] [Google Scholar]
- Walsh N, Schoenfeld L, Ramamurth S, Hoffman J. Normative model for cold pressor test. American Journal of Physical Medicine and Rehabilitation. 1989;68:6–11. doi: 10.1097/00002060-198902000-00003. [DOI] [PubMed] [Google Scholar]
- Willemsen G, Carroll D, Ring C, Drayson M. Cellular and mucosal immune reactions to mental and cold stress: Associations with gender and cardiovascular reactivity. Psychophysiology. 2002;39:222–228. doi: 10.1111/1469-8986.3920222. [DOI] [PubMed] [Google Scholar]
- Zangerle R, Gallati H, Sarcletti M, Weiss G, Denz H, Wachter H, et al. Increased serum concentrations of soluble tumor necrosis factor receptors in HIV-infected individuals are associated with immune activation. Journal of Acquired Immunodeficiency Syndrome. 1994;7:79–85. [PubMed] [Google Scholar]