Abstract
Please cite this paper as: Cwach et al. (2011) Contribution of murine innate serum inhibitors toward interference within influenza virus immune assays. Influenza and Other Respiratory Viruses DOI: 10.1111/j.1750‐2659.2011.00283.x.
Background Prior to detection of an antibody response toward influenza viruses using the hemagglutination inhibition assay (HAI), sera are routinely treated to inactivate innate inhibitors using both heat inactivation (56°C) and recombinant neuraminidase [receptor‐destroying enzyme (RDE)].
Objectives We revisited the contributions of innate serum inhibitors toward interference with influenza viruses in immune assays, using murine sera, with emphasis on the interactions with influenza A viruses of the H3N2 subtype.
Methods We used individual serum treatments: 56°C alone, RDE alone, or RDE + 56°C, to treat sera prior to evaluation within HAI, microneutralization, and macrophage uptake assays.
Results Our data demonstrate that inhibitors present within untreated murine sera interfere with the HAI assay in a manner that is different from that seen for the microneutralization assay. Specifically, the γ class inhibitor α2‐Macroglobulin (A2‐M) can inhibit H3N2 viruses within the HAI assay, but not in the microneutralization assay. Based on these findings, we used a macrophage uptake assay to demonstrate that these inhibitors can increase uptake by macrophages when the influenza viruses express an HA from a 1968 H3N2 virus isolate, but not a 1997 H3N2 isolate.
Conclusions The practice of treating sera to inactivate innate inhibitors of influenza viruses prior to evaluation within immune assays has allowed us to effectively detect influenza virus‐specific antibodies for decades. However, this practice has yielded an under‐appreciation for the contribution of innate serum inhibitors toward host immune responses against these viruses, including contributions toward neutralization and macrophage uptake.
Keywords: Assay, influenza, innate immunity, murine, serology, virus
Introduction
The most frequently used correlate of protective immunity against influenza virus is defined using the hemagglutination inhibition (HAI) assay. Using this assay, antibody titers ≥1:32 are generally considered protective. 1 However, evidence of protection without HAI titers achieving these levels has raised interest in the establishment of more adequate correlates of vaccine‐induced protective immunity. 2 In addition to technical issues facing the HAI assay, 3 results from HAI assays are confounded by the fact that sera must be treated to inactivate innate host inhibitors of influenza viruses prior to evaluation within this assay. 4 Specifically, three classes of serum factors that naturally interact with influenza viruses have been identified, and are characterized within either the α‐, β‐, or γ‐class. 5 , 6 Inhibitors of the α and γ classes express sialic acid residues that specifically bind influenza virus hemagglutinin (HA), 6 , 7 and are not inactivated by heating to 56°C. 8 , 9 Inhibitors of the β class include the mannose‐binding lectins, 6 , 10 and inhibitors in this class are heat labile. 5 Neutralization of viral particles is typically associated with β and γ inhibitors, while α inhibitors, historically referred to as ‘Francis inhibitors’, 6 , 11 are unable to neutralize infectivity. 12 To date, α2‐macroglobulin (A2‐M) is the major inhibitor within the γ class that has been identified. 13
Since these innate inhibitors can interfere with the results of HAI assays, sera are treated with Vibrio cholerae neuraminidase [receptor‐destroying enzyme (RDE)] to inactivate sialylated α and γ inhibitors, 7 , 13 followed by heating at 56°C to inactivate the β inhibitors. 6 , 12 , 14 Inhibitors in the β class are known to specifically interact with influenza A viruses of the H1N1 subtype, 12 while γ class inhibitors interact with influenza A viruses of the H2N2 and H3N2 subtypes. 8 , 14 Inhibitors of the α class have not been studied in decades, but were initially identified based on their ability to interact with influenza B viruses. 11 , 12 , 15 To date, an interaction linking influenza virus and mannose‐binding lectin (β inhibitor) to neutrophil binding has been reported, 16 but similar interactions between innate serum inhibitors and macrophages have not been described.
We developed the hypothesis that sialylated serum inhibitors of the α and/or γ class have the ability to interact with both influenza viruses and host cells in a manner that has not been appreciated, because of the frequent inactivation of these inhibitors prior to evaluation of sera within immune assays. Using a standard HAI assay that incorporated either murine sera or purified murine A2‐M, we were able to demonstrate that murine A2‐M specifically inhibits influenza viruses of the H3N2 subtype that circulated within humans from 1968 to 2004, and that this inhibition is eliminated by RDE treatment. Alternatively, when a standard microneutralization assay was used, we observed that innate murine serum inhibitors do not neutralize viruses expressing H3N2 HAs that circulated in 1968, 1973, and 1975, but that otherwise isogenic viruses expressing HA from later isolates within the H3N2 subtype (1977–2004) were inhibited. The fact that this inhibitor is inactivated by RDE, but not heat, places it in either the α or the γ class. Contrary to our findings within the HAI assay, the innate inhibitor in the microneutralization assay was not A2‐M. Finally, using a macrophage uptake assay, we demonstrate that untreated, non‐immune sera moderately increases uptake of viruses expressing an H3N2 HA from 1968, but not 1997, providing evidence that these innate inhibitors can bridge influenza virus interactions with macrophages. Thus, we report that innate murine serum inhibitors from both the α and the γ class need further evaluation, not only with regard to their contributions toward interference with results obtained from immune assays, but also for their undefined contributions toward host immunity to influenza viruses.
Methods and materials
Influenza viruses and murine anti‐sera
Influenza viruses used in this study were generated using reverse genetics technology, and have been previously characterized. 17 , 18 The primary viruses used in these studies expressed the HA and NA from either the A/Hong Kong/1/68‐H3N2 isolate or the A/Sydney/5/97‐H3N2 isolate, with the other six influenza genes provided by A/Puerto Rico/8/34 virus (PR8). These viruses will be referred to as ‘HK68’ and ‘SY97’, respectively. Additional viruses were similarly generated by reverse genetics to express the six internal genes from PR8, the NA from the SY97 isolate, and individual HAs from A/Port Chalmers/1/73 (PC73), A/Texas/1/77 (TX77), A/Memphis/6/86 (ME86), A/Memphis/7/90 (ME90), A/Beijing/353/89 (BE89), A/Beijing/32/92 (BE92), A/Wuhan/359/95 (WU95), A/Fujian/411/02 (FU02), and A/California/7/04 (CA04). 17 , 19 Representative isolates of the H1N1 subtype included a virus created with all eight PR8 genes, as well as one expressing seven PR8 genes and the HA from the A/New Caledonia/20/99 virus isolate. 20 Primary influenza B viruses that represent the B/Yamagata/16/88 (B/Yamanashi/166/98) and the B/Victoria/2/87 (B/Memphis/13/03) lineages were used. 20 , 21 All influenza A viruses except FU02 HA‐expressing viruses were propagated in 10‐day‐old embryonated chicken eggs for 3 days at 35°C, while FU02 HA‐expressing viruses and all influenza B virus isolates were propagated in MDCK cells for 3 days at 35°C, as described. 18 , 21
Sera used throughout this study were collected from previous experiments that vaccinated mice with inactivated influenza viruses expressing either HK68 HA or SY97 HA, and were partially characterized as reported previously. 22 Sera from alum‐vaccinated mice collected in the aforementioned study were used as a negative control (unvaccinated). All viruses and sera were kindly provided by Jonathan A. McCullers (St. Jude Children’s Research Hospital, Memphis, TN, USA).
Serum treatment
Untreated murine sera from individual vaccine recipients were pooled and divided into four treatment groups that recapitulate the RDE treatment protocols commonly used. 4 These four groups are untreated, heat inactivation at 56°C (56°C) alone, RDE alone, and RDE + 56°C. For these studies, RDE (Accurate Chemical, Westbury, NY, USA) was resuspended in 0·9% NaCl and used following the manufacturer’s instructions. Specifically, pooled sera (100 μl) were treated with three volumes of RDE (300 μl) by incubation overnight at 37°C. To this solution, three volumes (300 μl) of a 2·5% sodium citrate solution were added, and sera were heated at 56°C. After this treatment, three volumes (300 μl) of PBS were added, to achieve a final dilution of 1:10. Untreated sera were similarly mixed with 0·9% NaCl (overnight at 37°C), 2·5% sodium citrate (30 minutes at 25°C), and PBS. Sera treated by heat‐inactivation alone were incubated with 0·9% NaCl (overnight at 37°C), 2·5% sodium citrate (30 minutes at 56°C), and PBS. Sera treated with RDE alone were incubated with RDE in 0·9% NaCl (overnight at 37°C), 2·5% sodium citrate (30 minutes at 25°C), and PBS.
Hemagglutination inhibition assay
Hemagglutination inhibition assays were performed using standard techniques. 17 Briefly, sera were serially diluted, and four HA units of the virus of interest was added to each well. The virus:sera mixture was incubated for 1 hour at 4°C, and 50 μl of 0·5% chicken red blood cells (RBC) was subsequently added to each well. Assays performed using the FU02 HA‐expressing virus incorporated turkey RBC. 23 Lyophilized, purified murine A2‐M (Lee Biosolutions Inc., St. Louis, MO, USA) was resuspended to 1 mg/ml using buffer supplied by the company.
Microneutralization assay
Microneutralization assays were performed using standard procedures, as described previously. 20 Briefly, either murine sera or purified murine A2‐M were diluted and incubated with 100 TCID50 of each virus prior to adding these mixtures to MDCK cell monolayers (3 × 105 cells per well). Inocula were removed, and MDCK cells were incubated overnight in media supplemented with TPCK‐trypsin (2 μg/ml). Acetone‐fixed cells were stained for detection of influenza virus nucleoprotein (NP) using a murine monoclonal antibody (Fisher Scientific, Pittsburgh, PA, USA), and detected using goat anti‐mouse IgG (Fc‐specific) conjugated with HRP (Sigma, St. Louis, MO, USA), and o‐phenylenediamine dichloride substrate (Sigma). The reaction was stopped using H2SO4, and OD was measured at 490 nm on a BioTek EL808 reader (BioTek, Winooski, VT, USA).
Macrophage uptake assay
Using a technique previously reported by Huber et al., 24 we performed an uptake assay using our murine serum samples and Cy3‐labeled influenza virus. Influenza viruses (either HK68 or SY97) were propagated in embryonated chicken eggs, concentrated, purified over sucrose gradient, and pelleted as described previously. 18 This pellet was resuspended in 0·1 m Carbonate buffer (pH 9.3), and added to Cy3 dye (Fisher). After a 30‐minute incubation, the labeled virus was resuspended in STE buffer, pelleted, and resuspended in PBS prior to storage at −80°C. Labeled viruses were incubated with indicated murine sera for 30 minutes at 37°C with shaking prior to incubation with 106 J774A.1 BALB/c murine macrophages (ATCC, Manassas, VA, USA) for 30 minutes at 37°C with shaking. Trypan blue was added to quench extracellular fluorescence. Cells were collected using an Accuri C6 flow cytometer (Accuri Cytometers Ltd., Ann Arbor, MI, USA), and data were analyzed using CFlow Plus software (Accuri).
Results
Prior to evaluation of immunity toward influenza virus, serum samples are treated to remove innate inhibitors. 4 This treatment typically involves RDE to inactivate α and γ inhibitors and heating at 56°C to inactivate β inhibitors. Evaluating sera from vaccinated mice (either HK68 HA vaccine or SY97 HA vaccine) using an HAI assay (Figure 1), it is clear that these innate inhibitors interfere significantly with the results of this assay. This is evident within both vaccine groups where titers of untreated sera frequently exceed the correlate of protective immunity using this assay (an HAI titer of 1:32). 1 This inhibition is observed for most of the H3N2 viruses tested, representing antigenic clusters that circulated between 1968 and 2004 as defined by Smith et al. 25 , 26 The notable exception is the virus expressing HA from the HK68 isolate, observed in the SY97 HA‐vaccinated group. Treatment with RDE + 56°C inactivated these inhibitors, and only vaccine‐induced HAI titers were detected after treatment. These data demonstrate the importance of serum treatment for accurate evaluation of serum antibodies within the HAI assay. Next, we incorporated pooled sera from unvaccinated mice into our HAI assay (Figure 2). These results demonstrate that for H3N2 viruses in the HAI assay, RDE treatment inactivates the innate inhibition observed, but 56°C does not. Based on the inactivation of these inhibitors by RDE, but not 56°C, we placed these inhibitors in either the α or the γ class. Because α inhibitors interact with influenza B viruses, 15 while γ class inhibitors interact with influenza A viruses of the H2N2 and H3N2 subtypes, 8 we hypothesized that the major inhibitor observed within our HAI assay is from the γ class.
Figure 1.
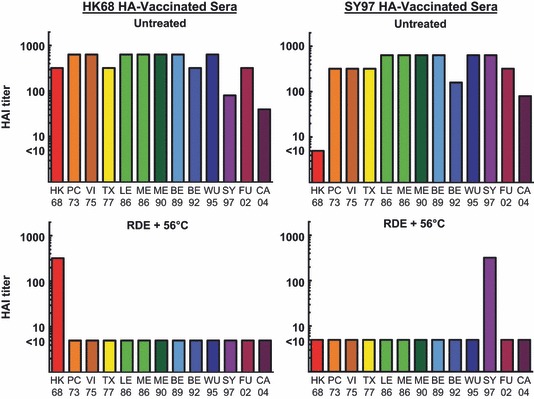
Hemagglutination inhibition (HAI) assay for both untreated and receptor‐destroying enzyme (RDE) + 56°C‐treated sera from vaccinated mice. Pooled sera from 19 individual mice in two vaccine groups (HK68 HA‐vaccinated or SY97 HA‐vaccinated) were used in an HAI assay after either no treatment, or RDE + 56°C treatment. Titers are reported as the reciprocal of the final serum dilution that demonstrates inhibition of hemagglutination, and values lower than the detectable limit of the assay (initial serum dilution of 1:10) were assigned a value of five for graphical representation.
Figure 2.
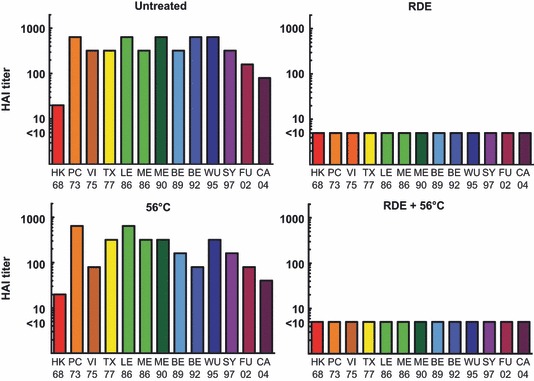
Hemagglutination inhibition (HAI) assay for untreated, receptor‐destroying enzyme (RDE) alone, 56°C alone, and RDE + 56°C‐treated sera from unvaccinated mice. Pooled sera from 20 individual unvaccinated mice were used in an HAI assay with the designated treatment groups. Titers are reported as the reciprocal of the final serum dilution that demonstrates inhibition of hemagglutination, and values lower than the detectable limit of the assay (initial serum dilution of 1:10) were assigned a value of five for graphical representation.
HAI assays have been used for decades to evaluate immunity against influenza viruses, 1 but the microneutralization assay is a more recent tool for determining immunity. 27 , 28 To date, a correlate for protective immunity using the microneutralization assay has not been defined. Despite a lack of publications on the role of innate inhibitor activity within the microneutralization assay, the WHO Manual on Animal Influenza Diagnosis and Surveillance 4 recommends using RDE‐treated sera for this assay. Using similar serum treatments, we evaluated the contributions of innate inhibitors within this assay using sera from vaccinated mice (either HK68 HA‐ or SY97 HA‐expressing viruses) (Figure 3).
Figure 3.
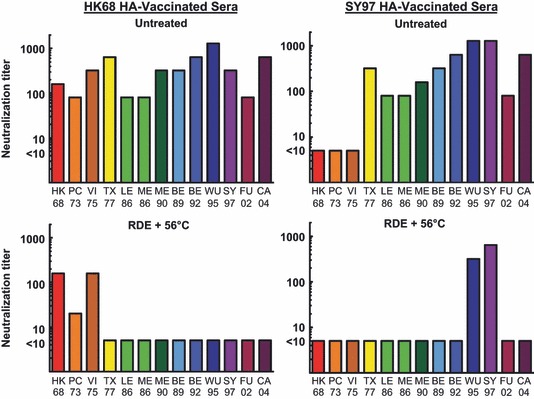
Microneutralization assay for both untreated and receptor‐destroying enzyme (RDE) + 56°C‐treated sera from vaccinated mice. Pooled sera from 19 individual mice in two vaccine groups (HK68 HA‐vaccinated or SY97 HA‐vaccinated) were used in a microneutralization assay after either no treatment, or RDE + 56°C treatment. Endpoint titers are reported as the final serum dilution with an OD490 value of less than half the OD detected in control infected cells. Serum samples that did not inhibit virus infection of MDCK cells (titer <10) were assigned a value of 5.
These results demonstrate that innate inhibitors behave differently within a microneutralization assay than they do in the HAI assay. Specifically, untreated murine sera were only able to inhibit viruses expressing HA from HK68, PC73, and VI75 isolates when HK68 HA vaccine‐induced antibodies were present. Untreated sera from SY97 HA‐vaccinated mice did not inhibit viruses expressing HK68, PC73, or VI75 HAs in the microneutralization assay, while the RDE + 56°C‐treated sera from SY97 HA‐vaccinated mice inhibited viruses expressing WU95 and SY97. These data demonstrate an increased breadth of reactivity within the microneutralization assay, as sera were able to neutralize viruses from a distance of 1 or 2 antigenic clusters, 25 which was not seen within the HAI assay (Figure 1). Evaluation of sera from unvaccinated mice using all four treatment conditions (Figure 4) demonstrated that the innate inhibitors that interfere with the microneutralization assay are inactivated by RDE, but not 56°C. Interestingly, viruses expressing HAs from the HK68, PC73, and VI75 isolates were not neutralized by inhibitors present in sera from unvaccinated mice when the microneutralization assay was used. Thus, similar to the HAI assay, our data from the microneutralization assay demonstrate that a γ class inhibitor is likely interfering with these viruses. This conclusion is based on the viral HA expressed (H3), but it should be noted that there were differences in reactivity depending on the H3N2 isolate that was used. It is important to note that these viruses were specifically generated to differ only in the HA expressed, so other viral genes are less likely to be contributing to this phenomenon.
Figure 4.
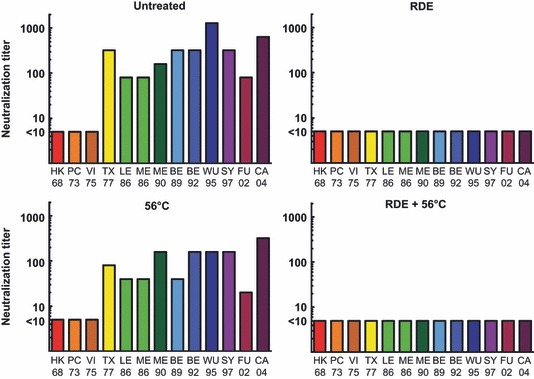
Microneutralization assay for untreated, receptor‐destroying enzyme (RDE) alone, 56°C alone, and RDE + 56°C‐treated sera from unvaccinated mice. Pooled sera from 20 individual unvaccinated mice were used in microneutralization assay with the designated treatment groups. Endpoint titers are reported as the final serum dilution with an OD490 value of less than half the OD detected in control infected cells. Serum samples that did not inhibit virus infection of MDCK cells (titer <10) were assigned a value of 5.
Based on the evidence that the dominant serum inhibitors for both assays were likely from the γ class, we identified A2‐M as a specific serum γ class inhibitor that interferes with H3N2 viruses. 7 Thus, we performed similar HAI and microneutralization assays where we incorporated purified murine A2‐M instead of murine sera (Table 1). Using the HAI assay, we demonstrate that a concentration of A2‐M as low as 31 μg/ml has the ability to inhibit representative isolates of H3N2 antigenic clusters from 1968 through 2004, with the exception of the BE92 and FU02 isolates. Confirming the γ class inhibitor phenotype of A2‐M, neither H1N1 nor influenza B viruses were inhibited within the HAI assay. Alternatively, using the microneutralization assay, we demonstrate that A2‐M only modestly inhibits two of the H3N2 HA‐expressing viruses (SY97 and FU02).
Table 1.
HAI and microneutralization assays using purified murine α2‐macroglobulin (1000 μg/ml starting concentration)
| H3N2 Subtype | |||
|---|---|---|---|
| Hemagglutinin | Cluster | α2‐macroglobulin inhibitory concentration (μg/ml) | |
| HAI | Microneutralization | ||
| A/Hong Kong/1/68 | HK68 | 500 | ≥1000 |
| A/Port Chalmers/1/73 | EN72 | 31 | ≥1000 |
| A/Victoria/3/75 | VI75 | 125 | ≥1000 |
| A/Texas/1/77 | TX77 | 31 | ≥1000 |
| A/Leningrad/360/86 | LE86 | 31 | ≥1000 |
| A/Memphis/6/86 | ME86 | 31 | ≥1000 |
| A/Memphis/7/90 | ME90 | 31 | ≥1000 |
| A/Beijing/353/89 | BE89 | 63 | ≥1000 |
| A/Beijing/32/92 | BE92 | ≥1000 | ≥1000 |
| A/Wuhan/359/95 | WU95 | 250 | ≥1000 |
| A/Sydney/5/97 | SY97 | 250 | 500 |
| A/Fujian/411/02 | FU02 | ≥1000 | 1000 |
| A/California/7/04 | CA04 | 63 | ≥1000 |
| H1N1 Subtype | |||
| Hemagglutinin | α2‐macroglobulin inhibitory concentration (μg/ml) | ||
| HAI | Microneutralization | ||
| A/Puerto Rico/8/34 | ≥1000 | ND | |
| A/New Caledonia/20/99 | ≥1000 | ≥1000 | |
| Influenza B Virus | |||
| Hemagglutinin | Lineage | α2‐macroglobulin inhibitory concentration (μg/ml) | |
| HAI | Microneutralization | ||
| B/Yamanashi/166/98 | B/Yamagata/16/88 | ≥1000 | ND |
| B/Memphis/13/03 | B/Victoria/2/87 | ≥1000 | ND |
ND, Not done; HAI, hemagglutination inhibition.
Since A2‐M has the ability to interact with the low‐density lipoprotein‐related receptor protein/A2‐M receptor (LRP/A2‐MR), which is expressed by the murine J774A.1 monocyte/macrophage cell line, 29 we determined whether uptake of fluorescently labeled influenza virus is increased in the presence of innate inhibitors (Table 2). Specifically, we incorporated untreated and RDE + 56°C‐treated serum samples from mice that were either unvaccinated or vaccinated (HK68 or SY97 HA‐expressing viruses). These data demonstrate that vaccine‐induced immunity increases uptake of labeled virus, regardless of the treatment used, corroborating earlier data from Huber et al. 24 Of note, viruses expressing HK68 HA demonstrated an increase in uptake by macrophages when incubated with untreated sera, compared with RDE + 56°C‐treated sera. A similar difference was not seen with SY97 HA‐expressing viruses. Samples analyzed using singly treated serum samples (either 56°C alone or RDE alone) did not provide conclusive evidence for which class of inhibitors are associated with this uptake (data not shown), likely due to the fact that the differences between untreated and RDE + 56°C‐treated samples themselves were not statistically significant. These data imply that innate serum inhibitors contribute to macrophage‐mediated uptake of influenza viruses, but further experimentation is needed to define this interaction and its role in host immunity.
Table 2.
Macrophage uptake assay using either untreated or RDE + HI‐treated sera from unvaccinated and vaccinated mice
| HK68 HA‐expressing virus | SY97 HA‐expressing virus | ||||||
|---|---|---|---|---|---|---|---|
| No vaccine | HK68 HA vaccine | No vaccine | SY97 HA vaccine | ||||
| Untreated | RDE + 56°C | Untreated | RDE + 56°C | Untreated | RDE + 56°C | Untreated | RDE + 56°C |
| 1·01 ± 0·09 (n = 3) | 0·77 ± 0·12 (n = 4) | 1·17 ± 0·12 (n = 3) | 1·25 ± 0·12 (n = 4) | 0·79 ± 0·13 (n = 3) | 0·66 ± 0·05 (n = 6) | 1·14 ± 0·12 (n = 3) | 0·99 ± 0·11 (n = 6) |
RDE, receptor‐destroying enzyme.
Mean fluorescence intensity (MFI) values for samples from individual experiments were standardized using the fluorescence of control cells incubated with virus alone, and data are reported as the mean ± SEM for these relative fluorescent units.
Discussion
We designed experiments to evaluate the contributions of innate murine serum inhibitors toward results obtained in HAI and microneutralization assays. Our data demonstrate that H3N2 viruses are inhibited by γ class serum inhibitors within the HAI assay, and that murine A2‐M specifically contributes to this inhibition. Alternatively, when a microneutralization assay was used, H3N2 viruses expressing HA from HK68, PC73, and VI75 were not inhibited, while viruses expressing HAs starting with the TX77 cluster 25 were inhibited by untreated sera. The inhibitors of these viruses, like in the HAI assay, were inactivated by RDE treatment, but not 56°C. Using purified A2‐M, we were able to demonstrate that this γ class inhibitor does not directly contribute to the neutralization observed. Finally, a macrophage uptake assay demonstrated a trend toward increased uptake of HK68 viruses by macrophages using untreated sera, but a similar trend was not observed with SY97 HA‐expressing viruses. These findings are discussed in the context of assays for detection of influenza virus immunity, and the potential for these inhibitors to mediate interactions between influenza viruses and host macrophages.
For decades, interference by innate serum inhibitors in immune assays has been observed. 8 , 30 In fact, during each pandemic since the isolation of influenza virus in 1933, a re‐visiting of serum treatment protocols has been necessary because of changes in viral interactions with these serum inhibitors. Specifically, treatment at 56°C was the first modification tested, and this worked to eliminate the β inhibitors that interfere with H1N1 viruses. 12 When the H2N2 viruses, and subsequently H3N2 viruses, emerged, the dominant serum inhibitors were resistant to 56°C, but sensitive to neuraminidase (RDE). 31 It is worth noting that influenza viruses isolated during the H1N1 pandemic of 2009–2010 do not interact with the influenza virus β class inhibitor, mannose‐binding lectin, 32 and that human A2‐M inhibits hemagglutination by these H1N1 viruses. 33 These historical and recent findings support a re‐evaluation of the contribution of innate serum inhibitors from all three classes toward influenza virus immunity in humans.
The dominant γ class inhibitor identified to date, A2‐M, naturally functions as a protease inhibitor that can be produced by monocyte/macrophages, 34 including alveolar macrophages. 35 Within serum, A2‐M is present at 2–4 mg/ml, 36 and its expression is modulated by pro‐inflammatory cytokines, including IL‐6 and TNF‐α. 37 , 38 The internalizing receptor for A2‐M, identified as the LRP/A2‐MR, is expressed on fibroblasts, hepatocytes, and monocyte/macrophages, and uptake via this receptor leads to degradation of A2‐M and the associated protease. 39 A second receptor for A2‐M has been identified on macrophages, but the contribution of this A2‐M signaling receptor (A2‐MSR), 40 has not been extensively characterized. A2‐M is of particular interest to us because of its unique ability to interact with both influenza viruses and host macrophages, and we propose an evaluation of A2‐M and other γ class inhibitors as potential mediators of host:virus interactions, including uptake of viruses 41 and immunity.
Innate serum inhibitors mediate species‐specific interactions with influenza viruses. In fact, the sialic acid residues expressed by A2‐M may even limit the cross‐species transmission of H3 HA‐expressing influenza viruses, 5 , 7 , 13 , 42 and specific amino acid substitutions have been associated with sensitivity toward these inhibitors. 43 Interestingly, the topic of innate human serum inhibitors against influenza A viruses of the H3N2 subtype was revisited when an H3N2 isolate that circulated in humans (A/Los Angeles/2/87) demonstrated particular sensitivity to inhibitors in human sera. 9 Interactions between this virus isolate and these inhibitors interfered with detection of antibody using the HAI assay, and recommendations made at the time were to standardize serum treatment protocols and viruses used in the HAI assay. 9 To date, binding of influenza viruses to neutrophils, mediated by the β inhibitor mannose‐binding lectin, has been demonstrated, 16 but the consequences of similar interactions between macrophages and the three classes of innate inhibitors are largely undefined. Based on our data with murine sera, we are planning similar experiments to define the interactions between human A2‐M, influenza viruses, and the LRP/A2‐MR expressed on human macrophages. 44
We evaluated the contributions of innate murine serum inhibitors against influenza viruses toward inhibition of HAI and microneutralization assays, with emphasis on their role in host immunity toward these viruses. Our data demonstrate that murine serum γ class inhibitors, including A2‐M, interfere with the results of HAI assays. Alternatively, when a microneutralization assay is employed, RDE‐sensitive inhibitors (characteristic of either the α or the γ class) interfere with H3N2 HAs that circulated both during and after 1977, and A2‐M alone does not play a major role in this inhibition. Finally, using a macrophage uptake assay, we demonstrate that untreated sera from unvaccinated mice can enhance uptake of an HK68 HA‐expressing virus by murine macrophages, and this uptake is reduced by treatment with RDE + 56°C. These findings represent the first evidence to our knowledge that different RDE‐sensitive murine serum inhibitors interfere with HAI and microneutralization assays, and that these inhibitors may mediate host immune cell functions, like uptake by macrophages. We conclude that our current practice of treating sera prior to analysis within immune assays, which is critical for detecting influenza virus‐specific antibodies using the HAI assay, has left us uncertain of the direct contributions of these three classes of innate inhibitors toward host immunity. Future evaluation of these innate serum inhibitors will consider drift variants as the H3 HA continues to evolve, as well as the role of these inhibitors within human sera.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Acknowledgements
The authors acknowledge technical assistance from Joseph Carrington as well as the contributions of the Flow Cytometry Core Facility at Sanford Research, in particular Satoshi Nagata and John Lee. We also thank Jonathan A. McCullers for providing influenza viruses and murine sera for these studies. Portions of this manuscript were presented at the Immune Correlates of Protection Against Influenza: Challenges for Licensure of Seasonal and Pandemic Influenza Vaccines Conference (March, 2010) in Miami, FL (Abstract Number P‐012) and the Options for the Control of Influenza VII (September, 2010) in Hong Kong SAR (Abstract Number P‐415). Financial support: SD‐BRIN Undergraduate Fellows Program: NIH Grant Number 2 P20 RR016479 from the INBRE Program of the National Center for Research Resources; Sanford School of Medicine Medical Summer Research Program (JMK); Division of Basic Biomedical Sciences, USD.
References
- 1. Kilbourne ED, Butler WT, Rossen RD. From the National Institutes of Health. J Infect Dis 1973; 127:220–236. [Google Scholar]
- 2. Eichelberger M, Golding H, Hess M et al. FDA/NIH/WHO public workshop on immune correlates of protection against influenza A viruses in support of pandemic vaccine development, Bethesda, Maryland, US, December 10–11, 2007. Vaccine 2008; 26:4299–4303. [DOI] [PubMed] [Google Scholar]
- 3. Stephenson I, Das RG, Wood JM, Katz JM. Comparison of neutralising antibody assays for detection of antibody to influenza A/H3N2 viruses: an international collaborative study. Vaccine 2007; 25:4056–4063. [DOI] [PubMed] [Google Scholar]
- 4. WHO . WHO Manual on Animal Influenza Diagnosis and Surveillancein Webster RG, Cox N, Stohr K. (eds) Geneva, Switzerland: World Health Organization, 2002; 1–135. [Google Scholar]
- 5. Matrosovich M, Gao P, Kawaoka Y. Molecular mechanisms of serum resistance of human influenza H3N2 virus and their involvement in virus adaptation in a new host. J Virol 1998; 72:6373–6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anders EM, Hartley CA, Jackson DC. Bovine and mouse serum beta inhibitors of influenza A viruses are mannose‐binding lectins. Proc Natl Acad Sci USA 1990; 87:4485–4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pritchett TJ, Paulson JC. Basis for the potent inhibition of influenza virus infection by equine and guinea pig alpha 2‐macroglobulin. J Biol Chem 1989; 264:9850–9858. [PubMed] [Google Scholar]
- 8. Krizanova O, Rathova V. Serum inhibitors of myxoviruses. Curr Top Microbiol Immunol 1969; 47:125–151. [DOI] [PubMed] [Google Scholar]
- 9. Subbarao EK, Kawaoka Y, Ryan‐Poirier K, Clements ML, Murphy BR. Comparison of different approaches to measuring influenza A virus‐specific hemagglutination inhibition antibodies in the presence of serum inhibitors. J Clin Microbiol 1992; 30:996–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hartley CA, Jackson DC, Anders EM. Two distinct serum mannose‐binding lectins function as beta inhibitors of influenza virus: identification of bovine serum beta inhibitor as conglutinin. J Virol 1992; 66:4358–4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smith W, Westwood JC. Influenza virus haemagglutination; the mechanism of the Francis phenomenon. Br J Exp Pathol 1950; 31:725–738. [PMC free article] [PubMed] [Google Scholar]
- 12. Konno J. Studies on several inhibitors against influenza viruses. 2. beta‐Inhibitor, its biological and physicochemical properties with particular emphasis on the differences from alpha‐inhibitor, immune serum and properdin. Tohoku J Exp Med 1958; 67:391–405. [DOI] [PubMed] [Google Scholar]
- 13. Ryan‐Poirier KA, Kawaoka Y. Alpha 2‐macroglobulin is the major neutralizing inhibitor of influenza A virus in pig serum. Virology 1993; 193:974–976. [DOI] [PubMed] [Google Scholar]
- 14. Cohen A, Belyavin G. Hemagglutination inhibition of Asian influenza viruses: a new pattern of response. Virology 1959; 7:59–74. [DOI] [PubMed] [Google Scholar]
- 15. Francis T. Dissociation of hemagglutinating and antibody‐measuring capacities of influenza virus. J Exp Med 1947; 85:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hartshorn KL, White MR, Shepherd V, Reid K et al. Mechanisms of anti‐influenza activity of surfactant proteins A and D: comparison with serum collectins. Am J Physiol 1997; 273:L1156–L1166. [DOI] [PubMed] [Google Scholar]
- 17. Huber VC, Thomas PG, McCullers JA. A multi‐valent vaccine approach that elicits broad immunity within an influenza subtype. Vaccine 2009; 27:1192–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huber VC, McKeon RM, Brackin MN, Miller LA et al. Distinct contributions of vaccine‐induced immunoglobulin G1 (IgG1) and IgG2a antibodies to protective immunity against influenza. Clin Vaccine Immunol 2006; 13:981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Halasa N, Englund JA, Nachman S, Weinberg GA et al. Safety of live attenuated influenza vaccine in mild to moderately immunocompromised children with cancer. Vaccine 2011; 29:4110–4115. [DOI] [PubMed] [Google Scholar]
- 20. Huber VC, McCullers JA. Live attenuated influenza vaccine is safe and immunogenic in immunocompromised ferrets. J Infect Dis 2006; 193:677–684. [DOI] [PubMed] [Google Scholar]
- 21. Huber VC, Kleimeyer LH, McCullers JA. Live, attenuated influenza virus (LAIV) vehicles are strong inducers of immunity toward influenza B virus. Vaccine 2008; 26:5381–5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huber VC, Peltola V, Iverson AR, McCullers JA. Contribution of vaccine‐induced immunity toward either the HA or the NA component of influenza viruses limits secondary bacterial complications. J Virol 2010; 84:4105–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Widjaja L, Ilyushina N, Webster RG, Webby RJ. Molecular changes associated with adaptation of human influenza A virus in embryonated chicken eggs. Virology 2006; 350:137–145. [DOI] [PubMed] [Google Scholar]
- 24. Huber VC, Lynch JM, Bucher DJ, Le J, Metzger DW. Fc receptor‐mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J Immunol 2001; 166:7381–7388. [DOI] [PubMed] [Google Scholar]
- 25. Smith DJ, Lapedes AS, De Jong JC, Bestebroer TM et al. Mapping the antigenic and genetic evolution of influenza virus. Science 2004; 305:371–376. [DOI] [PubMed] [Google Scholar]
- 26. Kang S, Yang IS, Lee JY, Park Y et al. Epidemiologic study of human influenza virus infection in South Korea from 1999 to 2007: origin and evolution of A/Fujian/411/2002‐like strains. J Clin Microbiol 2010; 48:2177–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Frank AL, Puck J, Hughes BJ, Cate TR. Microneutralization test for influenza A and B and parainfluenza 1 and 2 viruses that uses continuous cell lines and fresh serum enhancement. J Clin Microbiol 1980; 12:426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rowe T, Abernathy RA, Hu‐Primmer J, Thompson WW et al. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol 1999; 37:937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Caceres LC, Bonacci GR, Sanchez MC, Chiabrando GA. Activated alpha(2) macroglobulin induces matrix metalloproteinase 9 expression by low‐density lipoprotein receptor‐related protein 1 through MAPK‐ERK1/2 and NF‐kappaB activation in macrophage‐derived cell lines. J Cell Biochem 2010; 111:607–617. [DOI] [PubMed] [Google Scholar]
- 30. Brans LM, Hertzberger E, Binkhorst JL. Studies on the elimination of non‐specific inhibitors in sera against influenza viruses with the aid of filtrates of Vibrio cholerae . Antonie Van Leeuwenhoek 1953; 19:309–323. [DOI] [PubMed] [Google Scholar]
- 31. Shortridge KF, Lansdell A. Serum inhibitors of A 2 ‐Hong Kong influenza virus haemagglutination. Microbios 1972; 6:213–219. [PubMed] [Google Scholar]
- 32. Tokunaga H, Ushirogawa H, Ohuchi M. The pandemic (H1N1) 2009 influenza virus is resistant to mannose‐binding lectin. Virol J 2011; 8:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen CH, Zhang XQ, Lo CW, Liu PF et al. The essentiality of alpha‐2‐macroglobulin in human salivary innate immunity against new H1N1 swine origin influenza A virus. Proteomics 2010; 10:2396–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hovi T, Mosher D, Vaheri A. Cultured human monocytes synthesize and secrete alpha2‐macroglobulin. J Exp Med 1977; 145:1580–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. White R, Janoff A, Godfrey HP. Secretion of Alpha‐2‐macroglobulin by human alveolar macrophages. Lung 1980; 158:9–14. [DOI] [PubMed] [Google Scholar]
- 36. Armstrong PB. Proteases and protease inhibitors: a balance of activities in host‐pathogen interaction. Immunobiology 2006; 211:263–281. [DOI] [PubMed] [Google Scholar]
- 37. Geiger T, Andus T, Klapproth J, Hirano T et al. Induction of rat acute‐phase proteins by interleukin 6 in vivo . Eur J Immunol 1988; 18:717–721. [DOI] [PubMed] [Google Scholar]
- 38. Gresser I, Delers F, Tran QN, Marion S et al. Tumor necrosis factor induces acute phase proteins in rats. J Biol Regul Homeost Agents 1987; 1:173–176. [PubMed] [Google Scholar]
- 39. Chu CT, Howard GC, Misra UK, Pizzo SV. Alpha 2‐macroglobulin: a sensor for proteolysis. Ann NY Acad Sci 1994; 737:291–307. [DOI] [PubMed] [Google Scholar]
- 40. Misra UK, Chu CT, Gawdi G, Pizzo SV. Evidence for a second alpha 2‐macroglobulin receptor. J Biol Chem 1994; 269:12541–12547. [PubMed] [Google Scholar]
- 41. Hofer F, Gruenberger M, Kowalski H, Machat H et al. Members of the low density lipoprotein receptor family mediate cell entry of a minor‐group common cold virus. Proc Natl Acad Sci USA 1994; 91:1839–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Suzuki Y, Ito T, Suzuki T, Holland RE Jr et al. Sialic acid species as a determinant of the host range of influenza A viruses. J Virol 2000; 74:11825–11831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ryan‐Poirier KA, Kawaoka Y. Distinct glycoprotein inhibitors of influenza A virus in different animal sera. J Virol 1991; 65:389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kurdowska A, Alden SM, Noble JM, Stevens MD, Carr FK. Involvement of alpha‐2‐macroglobulin receptor in clearance of interleukin 8‐alpha‐2‐macroglobulin complexes by human alveolar macrophages. Cytokine 2000; 12:1046–1053. [DOI] [PubMed] [Google Scholar]


