Abstract
Background
Adipose tissue is an important target for ethanol action. One important effect of ethanol is to reduce the secretion of adiponectin from adipocytes; this decrease is associated with lowered circulating adiponectin in rodent models of chronic ethanol feeding. Adiponectin is an insulin-sensitizing, anti-inflammatory adipokine; decreased adiponectin activity may contribute to tissue injury in response to chronic ethanol. Here we investigated the role of cytochrome P450 2E1 (CYP2E1) and oxidative stress in the mechanism for impaired adiponectin secretion from adipocytes in response to ethanol.
Methods
Male Wistar rats were fed a liquid diet containing ethanol as 36% of calories or pair-fed a control diet for 4 weeks. 3T3-L1 adipocyte cultures, expressing CYP2E1 or not, were exposed to ethanol or 4-hydroxynonenal (4-HNE).
Results
Chronic ethanol feeding to rats suppressed the secretion of adiponectin from isolated epididymal adipocytes. Ethanol feeding induced the expression of CYP2E1 in adipocytes and increased markers of oxidative stress, including 4-HNE and protein carbonyls. Because adiponectin is post-translationally processed in the endoplasmic reticulum and Golgi, we investigated the impact of ethanol on the redox status of high density microsomes. Chronic ethanol decreased the ratio of reduced glutathione to oxidized glutathione (4.6:1, pair-fed; 2.9:1, ethanol-fed) in high density microsomes isolated from rat epididymal adipose tissue. We next utilized the 3T3-L1 adipocyte-like cell model to interrogate the mechanisms for impaired adiponectin secretion. Culture of 3T3-L1 adipocytes overexpressing exogenous CYP2E1, but not those overexpressing anti-sense CYP2E1, with ethanol increased oxidative stress and impaired adiponectin secretion from intracellular pools. Consistent with a role of oxidative stress in impaired adiponectin secretion, challenge of 3T3-L1 adipocytes with 4-HNE also reduced adiponectin mRNA expression and secretion, without affecting intracellular adiponectin concentration.
Conclusions
These data demonstrate that CYP2E1-dependent reactive oxygen species production in response to ethanol disrupts adiponectin secretion from adipocytes.
Keywords: alcohol, adipocytes, adipokines, glutathione, protein trafficking
Introduction
Oxidative stress is characterized by the presence of excess amounts of reactive oxygen species (ROS) and/or reactive nitrogen species (RNS). An imbalance between the production of ROS/RNS and/or the level or activity of antioxidants results in a state of oxidative stress (Schafer and Buettner, 2001). Well-regulated production of ROS/RNS has essential roles in signal transduction pathways that maintain control of cellular and systemic functions, as well as protect against infection (Finkel and Holbrook, 2000). However, excessive ROS/RNS contribute to the development of a number of pathophysiological conditions, including atherosclerosis, diabetes mellitus, obesity, neurodegenerative diseases, cancer (Reuter et al., 2010), as well as tissue injury associated with chronic alcohol exposure (Zima and Kalousová, 2005).
Cellular macromolecules can be attacked by ROS and RNS. For example, increased ROS leads to lipid peroxidation by reacting with double bonds on unsaturated fatty acids, resulting in the formation of multiple electrophilic aldehyde species. These species are capable of forming adducts with proteins, leading to protein dysfunction. Chronic ethanol exposure increases production of ROS, lowers cellular antioxidant levels, and enhances oxidative stress in a number of cells and tissues (Cederbaum, 2001). While many oxidant and anti-oxidant pathways are involved in ethanol-induced oxidative stress, one central pathway for the generation of oxidants is the CYP2E1-dependent microsomal ethanol oxidizing system pathway (Lu and Cederbaum, 2008).
Upregulation of hepatic CYP2E1 occurs in alcoholic liver disease (Tsukamoto and Lu, 2001) and non-alcoholic steatohepatitis (Weltman et al., 1998). Chronic ethanol exposure increases CYP2E1 synthesis and decreases CYP2E1 degradation in the liver (Lu and Cederbaum, 2008). Importantly, CYP2E1 inhibitors prevent ethanol-induced lipid peroxidation in the liver (Morimoto et al., 1995) and cyp2e1-deficient mice exhibit less oxidative stress and lipid peroxidation in liver after chronic ethanol feeding compared to wild-type mice (Lu et al., 2008). Exposure of a human hepatoma HepG2 cell line overexpressing CYP2E1 (E47 cells), but not control HepG2 cells that do not express CYP2E1, to ethanol during culture increases lipid peroxidation (Wu and Cederbaum, 2005). While CYP2E1 is predominantly expressed in the liver, low levels of CYP2E1 are also detected in adipose tissue (Yoshinari et al., 2004).
Adiponectin is a 30-kDa adipokine primarily secreted by adipocytes and is abundant in serum (0.01% of total serum protein) (Kadowaki and Yamauchi, 2005). It is an insulin sensitizer with anti-diabetic, anti-atherogenic, anti-inflammatory and cardioprotective properties (Wang et al., 2008). Adiponectin is present primarily in three species in the circulation: a low molecular weight trimer (~ 67 kDa), a middle molecular weight hexamer (~ 120 kDa), and a high molecular weight multimer (>300 kDa) (Wang et al., 2008). Adiponectin expression is regulated at both the transcriptional and post-transcriptional level (Liu and Liu, 2010). Post-translational modifications, including disulfide bond formation, hydroxylation and subsequent glycosylation, play an important role in adiponectin oligomerization (Wang et al., 2008). Since dysregulation in adiponectin expression and/or function is linked to obesity, insulin resistance, Type 2 diabetes, and other related metabolic diseases (Gil-Campos et al., 2004), understanding the mechanisms regulating transcription and post-translational modifications of adiponectin is critical for the development of new approaches to treat these diseases.
Adiponectin concentrations are decreased in the circulation in mice (Song et al., 2008; Xu et al., 2003; You et al., 2005) and rats (Chen et al., 2007; Chen et al., 2009; Thakur et al., 2006) after chronic ethanol exposure. Reduced plasma adiponectin in rat models of chronic ethanol feeding are associated with suppressed secretion of adiponectin by isolated adipocytes (Chen et al., 2007), as well as an ethanol-induced increase in CYP2E1 expression and the accumulation of markers of oxidative stress in adipose tissue (Chen et al., 2009). Here, making use of pharmacological and genetic manipulations in cultured 3T3-L1 adipocytes, we have tested the hypothesis that CYP2E1-mediated ROS production in adipocytes in response to ethanol exposure impairs adiponectin secretion by adipocytes.
Materials and Methods
Materials
Adult male Wistar rats weighing 140 to 150 g were purchased from Harlan Laboratories, Inc. (Indianapolis, IN). Female C57BL/6 mice were from the Jackson Laboratory (Bar Harbor, ME). Lieber-DeCarli ethanol diet (regular, no.710260) was purchased from Dyets Inc. (Bethlehem, PA). The murine 3T3-L1 fibroblast cell line was purchased from the American Type Culture Collection (ATCC, Rockville, MD). Cell culture reagents were obtained from GIBCO (Grand Island, NY). Insulin, dexamethasone, 3-isobutyl-1-methylxanthine solutions were purchased from Cayman Chemicals (Ann Arbor, MI). Antibodies were purchased from the following sources: anti-adiponectin (AbCam, Cambridge, MA), anti-CYP2E1 (AbCam), anti-HSC70 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-4-HNE (Cayman Chemicals), anti-syntaxin 6 (BD Bioscience, San Jose, CA), anti-ERK1/2 (Upstate, Charlorttesville, VA), anti-phospho-eIF2α (Cell Signaling Technology Inc., Danvers, MA), and anti-eIF2α (Cell Signaling Technology Inc.). Alexa fluor-488 conjugated secondary antibody was purchased from Invitrogen (Carlsbad, CA). EZ-link biotin hydrazide and poly-HRP streptavidin were purchased from Pierce (Rockford, IL). Protease inhibitor cocktail (Complete EDTA free™) was purchased from Roche (Indianapolis, IN). Paraformaldehyde (4%) was purchased from Bioscience (San Diego, CA). VECTASHIELD was purchased from Vector Laboratories, Inc. (Burlingame, CA). General research chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Chronic ethanol feeding
All procedures involving animals were approved by the Institutional Animal Care and Use Committee at the Cleveland Clinic. Chronic ethanol feeding to rats was performed as previously described (Rachdaoui et al., 2003). Rats were randomly assigned to pair-fed or ethanol-fed groups. Ethanol-fed group were given free access to Lieber-DeCarli complete liquid diets containing ethanol as 36% of total caloric value for 4 weeks. Control rats were pair fed a liquid diet with maltose dextrin isocalorically substituted for ethanol. Final body weights for the animals used in this study were 300 ± 9 g for pair-fed and 288 ± 6 g for ethanol-fed. Epididymal adipose depots were 4.4 ± 0.3 g in pair-fed and 3.4 ± 0.2 g in ethanol-fed rats (n=16, p<0.05).
Cell culture and treatments
3T3-L1 fibroblasts were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (high glucose) with 10% fetal bovine serum (FBS) at 37 °C in a 10% CO2 atmosphere for 4 days. 3T3-L1 cells were then cultured in DMEM-FBS containing 10 μg/ml insulin, 0.25 μM dexamethasone, and 0.5 mM 3-isobutyl-1-methylxanthine for 3 days to initiate differentiation. Medium was then changed every 2 days to DMEM-FBS with 10 μg/ml insulin alone for 7 days. Cells were then cultured in DMEM medium containing 10% bovine serum for 2–3 days before treatment. Appropriate differentiation was confirmed by accumulation of lipid droplets. Cells were incubated in the culturing medium containing 4-HNE for 4 hours. In some experiments, cells were exposed to acetaldehyde vapor generated from a 10 mM acetaldehyde solution placed in neighboring wells on the same plate at 37°C for 18 hours, as described previously (Rao, 1998).. Cells were washed with sterile phosphate buffered saline (PBS) buffer, and medium was then changed to DMEM. Media samples were collected over time for measurements of adiponectin secretion. Cells were lysed after 2 hours for determination of intracellular adiponectin concentration.
Rat adipocyte isolation, incubation and lysis
Adipocytes were isolated as previously described (Sebastian et al., 2008). After isolation, adipocytes were diluted to 0.25~0.5 × 106 cells/ml in wash buffer. Cells were incubated for 1h, extracellular media collected over time, as indicated in the figure legends and then lysed, as previously described (Sebastian et al., 2008).
Adiponectin enzyme-linked immunosorbent assay (ELISA)
Adiponectin concentrations were measured by ELISA (B-Bridge, San Diego CA) according to the manufacturer’s protocol. Each set of experiments were carried out in a single lot of ELISA reagents.
Western blotting, protein concentration assay and slot blot analysis
Western blot was performed using enhanced chemiluminescence. Images were collected and signal intensities were quantified using Kodak Image Station 4000R (New Haven, CT). Protein concentrations were measured using a DC kit (Bio-Rad, Hercules, CA). Immunoreactivity of 4-HNE was measured using slot blot analysis. Adipocytes were isolated and lysed as described above, extracted 1 hour on ice and centrifuged 3 min at 8,000 × g. Protein was loaded onto a Bio-Dot SF apparatus (BioRad) and transferred to nitrocellulose membrane. Membranes were blocked with 3% bovine serum albumin and probed with anti-4-HNE antibody (1:1000). Detection of 4-HNE immunoreactivity was performed using enhanced chemiluminescence and visualized using film. Densitometric analysis was performed using Image J software (NIH).
Real-time polymerase chain reaction (RT-PCR)
Total RNA was isolated from 3T3-L1 adipocytes using the RNeasy mini kit (Qiagen, Valencia, CA), following the manufacturer’s instructions. Total RNA was reverse transcribed using a RETROscript kit (Ambion, Austin, TX). RT-PCR amplification was performed using the Brilliant SYBR green QPCR Master Mix (Applied Biosystems, Warrington, UK ) in an Mx3000p PCR machine (Stratagene, La Jolla, CA) as previously described (McMullen et al., 2005). The primer sequences used were: adiponectin (forward: 5′–GAGACGCAGGTGTTCTTG-3′, reverse: 5′-CCTACGCTGAATGCTGAG-3′),18s (forward: 5′-ACGGAAGGGCACCACCAGGA-3′, reverse: 5′-CACCACCACCCACGGAATCG-3′), CHOP (forward: 5′-TCCCTGCCTTTCACCTTG-3′, reverse: 5′-GCCCTGGCTCCTCTGTCA-3′), and Grp78 (forward: 5′-AGACTGCTGAGGCGTATTTG-3′, reverse: 5′-TGGTGAGAAGAGACACATCG-3′).
Biotin hydrazide modification and detection of protein carbonylation
Adipose proteins were extracted and modified with biotin hydrazide in order to assess protein carbonylation, as previously described (Grimsrud et al., 2007). Samples were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and visualized with HRP-conjugated streptavidin (1:10,000 dilution). The biotin-avidin interaction was detected using enhanced chemiluminescence.
Preparation of high density microsomes
Epididymal adipose fat pads from pair- or ethanol-fed rats were weighed and cut evenly into two parts. Fat pad was washed with PBS buffer with/without 70 mM iodoacetamide (pH 8.5) and minced. Two grams of minced fat from pair- or ethanol-fed rats were homogenized using a 40 mL glass dounce in 10 mL of TSE/PI buffer (20 mM Tris, 1 mM ethylenediaminetetraacetic acid (EDTA), 225 mM sucrose, and protease inhibitor cocktail at 1 tablet/2.6 mL, pH 8.5) with/without 70 mM iodoacetamide. The homogenate was centrifuged at 500 × g for 5 min at 4°C. The infranatant was filtered through a 250 μm nylon mesh, and centrifuged at 16,000 × g for 30 min at 4°C. The fat layer was removed, and the supernatant was then centrifuged at 48,000 × g for 30 min at 4°C. The pellet was washed with 5 mL of TSE/PI buffer and centrifuged at 48,000 × g for 30 min at 4°C. The final pellet was resuspended in 300 μL TSE/PI buffer for the measurement of reduced glutathione/oxidized glutathione (GSH/GSSG) ratio.
GSH/GSSG measurements
A modified version of high-performance liquid chromatography (HPLC) method as previously described (Jacobsen et al., 1989; Jacobsen et al., 1994.) was used to measure GSH/GSSG ratio. In brief, samples (100 μL each) were mixed with 2.5 μL of amyl alcohol. The mixtures were treated with 20 μL of 1.43 M sodium borohydride in 0.1 M sodium hydroxide, and mixed and treated with 20 μL of 1.0 M hydrogen chloride. Derivatization of GSH was performed by adding 25 μL of 7.0 mM mono-bromobimane in 5.0 mM disodium EDTA, pH 8.0. The mixtures were incubated at 42°C for 15 minutes. Ice-cold 1.5 M perchloric acid (25 μL) was added. Samples were centrifuged at 12,500 × g for 10 min at 4°C. Ice-cold 2 M Trizma base (12.5 μL) was added. Samples were centrifuged at 12,500 × g for 10 min at 4°C. Separation of 70 μL of the final supernatant was performed on a LUNA 5μ C18 reverse-phase column (Phenomenex) using gradient separation as described previously (Jacobsen et al., 1994.). Fluorescence was measured at 390 nm excitation and 475 nm emission.
Release of lactate dehydrogenase
Measurement of lactate dehydrogenase in culturing medium and lysed cells was carried out using a CytoTox 96 Non-Radioactive Cytotoxicity Assay kit (Promega, Madison, WI), according to the manufacturer’s instructions.
Transfection of differentiated 3T3-L1 adipocytes
Plasmids pCI-2E1 (sense-cyp2e1) and pCI-as-2E1 (antisense-cyp2e1) are gift of Dr. A. I. Cederbaum (Chen and Cederbaum, 1998). Differentiated 3T3-L1 adipocytes were transfected using the Amaxa 3T3-L1 adipocytes Nucleofector kit (Lonza, Cologne, Germany) according to the manufacturer’s instructions using the A-033 program, except for the following modifications. The entire transfection procedure for each sample was completed in less than 5 min. One hundred microliters of mixture containing 2 × 106 cells and 4 μg DNA was transferred into the electroporation cuvette, and placed in the Nucleofector device (Lonza). Cells were immediately removed from the cuvette after transfection and plated in a 24-well plate containing pre-warmed DMEM-FBS medium (37 °C). Each well contains 0.5 × 106 cells. After 24 hours, the culturing medium was replaced with fresh medium with or without 100 mM ethanol for an additional 24 hours at 37 °C. The culturing medium was collected and cells were lysed for the measurement of adiponectin concentration.
Immunohistochemistry
3T3-L1 adipocytes were plated on a chamber slide (Nagle Nunc International, Rochester, NY) with cell density of 0.1 × 106 cells/mL, 0.5 mL/well. After 4-HNE or ethanol treatment, cells were washed with ice-cold PBS buffer and fixed with 4% paraformaldehyde for 20 min at room temperature (0.5 mL/well). Cells were washed with 25 mM glycine (two times, 1 min each, 0.5 mL/well), and followed by two washes in PBS buffer (1 min each, 0.5 mL/well). Cells were blocked with 1% fish gelatin containing 2% BSA and 0.1% Triton-X-100 for 1 h and incubated overnight with polyclonal rabbit anti 4-HNE (diluted 1:500 in blocking buffer) antibody at 4 °C in a humidified chamber. After three washes in PBS buffer (3 times 5 min each), cells were incubated with the fluorochrome-conjugated secondary antibody (Alexa fluor 488 labeled goat-anti-rabbit IgG, 1:600 diluted in blocking buffer) for 1 h at room temperature. Cells were then washed three times in PBS buffer and mounted with VECTASHIELD. A slide without primary antibody was used as negative control. Fluorescence images were acquired using a LEICA confocal microscope.
Statistical analysis
Values reported are mean ± standard error of mean. Data were analyzed by Student’s t-test or general linear models procedure (SAS Institute, Cary, IN). Data were log transformed, if needed, to obtain a normal distribution.
Results
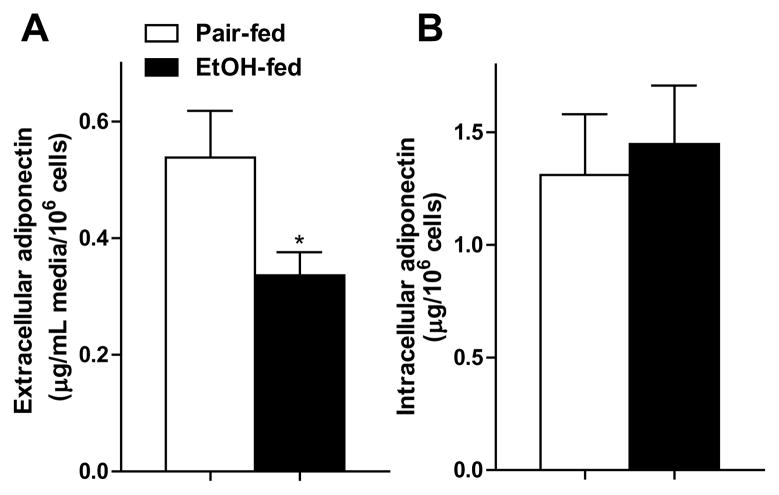
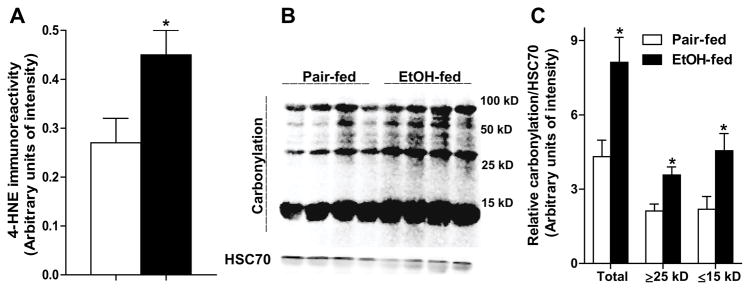
Chronic ethanol feeding lowers adiponectin secretion from isolated subcutaneous adipocytes in rats (Chen et al., 2007). Here, we found a similar suppression in secretion of adiponectin (Fig. 1A) from rat epididymal adipocytes after chronic ethanol feeding. Ethanol feeding did not decrease intracellular adiponectin (Fig. 1B), suggesting an impaired secretion of adiponectin by adipocytes after ethanol feeding. Chronic ethanol feeding also induced oxidative stress in epididymal adipose tissue in rats, evidenced by the accumulation of 4-HNE protein adducts in adipocytes (Fig. 2A) and increased carbonylation in adipose proteins (Fig 2B and C).
Fig. 1.
Chronic ethanol reduced adiponectin secretion by isolated adipocytes without affecting intracellular adiponectin content. Rats were allowed free access to an ethanol-containing diet or pair-fed a control diet for 4 weeks. Adipocytes were isolated from epididymal fat pads and incubated at 37°C for 1 hour. (A) Extracellular and (B) intracellular adiponectin concentrations were measured by ELISA. Values represent means ± SEM. n=7. Values with an asterisk are significantly different from each other, p< 0.05.
Fig. 2.
Chronic ethanol feeding increased oxidative stress in adipocytes and adipose tissue. (A) 4-HNE protein adducts were quantified by slot blot analysis in isolated epididymal adipocytes. n=7. (B) Proteins were extracted from rat epididymal adipose tissue, coupled with biotin hydrazide, resolved by SDS-PAGE, transferred to PVDF membrane, and blotted with HRP-streptavidin. HSC70 was used as loading control. (C) Results of densitometry after normalization to HSC70. Values represent means ± SEM. n=4. Values with an asterisk are significantly different from each other, p< 0.05.
Adiponectin is extensively modified at the post-translational level in the endoplasmic reticulum (ER) before it is secreted into the extracellular medium (Wang et al., 2008). Maintenance of appropriate redox balance in the ER and Golgi is critical for the appropriate post-translational modifications of proteins (Schröder and Kaufman, 2005). Determination of GSH/GSSG ratio is a very useful approach to evaluate ER function. However, only a few studies have attempted to directly quantify GSH/GSSG redox state in the ER of tissues and cells (Bass et al., 2004; Dixon et al., 2008; Hwang et al., 1992). To date, no study has ever been conducted determining adipose microsomal redox state. Hwang et al. initially measured the general ER redox environment in a hybridoma cell line and reported a ratio of GSH to GSSG between 1.5:1 to 3.3:1 (Hwang et al., 1992). In isolated rat liver microsomes, the GSH/GSSG ratio was estimated to be 3:1 (Bass et al., 2004). Recently, Dixon et al reported an artifactual oxidation occurring during microsome isolation with the methods used by previous researchers. When this oxidation was controlled by the alkylation of the thiols using iodoacetic acid, isolated rat liver microsomes yielded a higher GSH/GSSG ratio between 4.7:1 to 5.5:1 compared to a ratio of 0.7:1 to 1.2:1 microsomes in which artifactual oxidation was not controlled (Dixon et al., 2008).
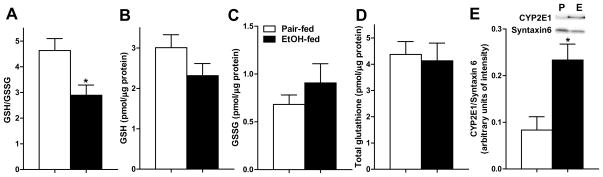
In order to determine GSH/GSSG ratios specifically in high density microsomes of adipose tissue, which are enriched in the ER and Golgi, we modified the protocol of Dixon et al to control the artifactual oxidation during high density microsomes isolation. Alkylation of thiols was carried out at a more physiological pH by using iodoacetamide (pH 8.5). In order to obtain a single form of thiol derivant, mono-bromobimane was used as the thiol derivatizing agent. Using this method, adipose high density microsomes from pair-fed rats yielded a GSH/GSSG ratio of 4.6:1(Fig. 3A), comparable to that of Dixon et al in liver. High density microsomes isolated from ethanol-fed rats exhibited a lower ratio of 2.9:1 (Fig. 3A), indicating increased oxidative stress after ethanol feeding. This ethanol-induced shift in GSH/GSSG ratio occured independently of a depletion of total glutathione (Fig. 3D). Reduced GSH/GSSG ratio was associated with increased CYP2E1 expression in high density microsomes isolated from epididymal adipose tissue after ethanol feeding (Fig. 3E).
Fig. 3.
Chronic ethanol feeding decreased GSH/GSSG ratio in high density microsomes isolated from adipose tissue, associated with an increase in CYP2E1 expression. Rats were allowed free access to an ethanol-containing diet or pair-fed a control diet for 4 weeks. Epididymal adipose tissue was homogenized, high density microsomes were isolated and lysed. (A) GSH/GSSG, (B) GSH, (C) GSSG, and (D) total glutathione were determined by a HPLC-based method as described in methods. n=6. (E) Immunoreactive CYP2E1 was measured by western blot in high density microsome lysates. n=4–5. Images are representative of 4–5 rats per treatment group. Values represent means ± SEM. Values with an asterisk are significantly different from each other, p< 0.05.
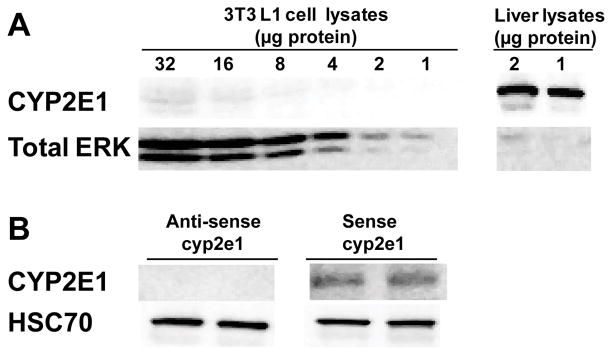
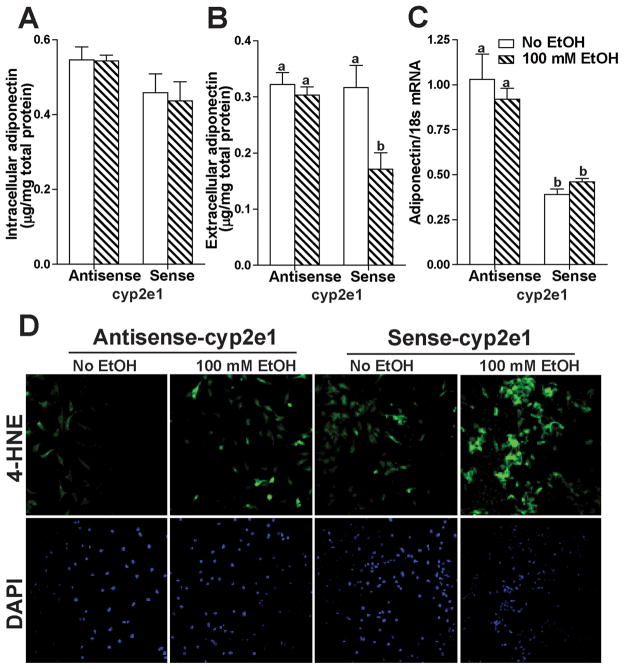
In order to determine the causal relationship between CYP2E1, oxidative stress and adiponectin secretion, mouse 3T3-L1 adipocytes, an adipocyte-like cell line, were utilized as an in vitro model of adipocytes. Challenge of wild-type 3T3-L1 adipocytes with ethanol did not impair adiponectin secretion (data not shown); CYP2E1 was not detectable by western blot in wild-type 3T3-L1 adipocytes (Fig. 4A). Because of the association between ethanol, CYP2E1 expression in adipocytes and impaired adiponectin secretion in rat models of chronic ethanol exposure, we hypothesized that expression of CYP2E1 would be required for ethanol to suppress adiponectin secretion from 3T3-L1 adipocytes. To test this hypothesis, 3T3-L1 adipocytes were transfected with a CYP2E1 overexpression plasmid (Chen and Cederbaum, 1998). CYP2E1 was overexpressed in 3T3-L1 adipocytes transfected with sense-cyp2e1 plasmid, not in the ones transfected with antisense-cyp2e1 plasmid (Fig 4B).
Fig. 4.
CYP2E1 expression in 3T3-L1 adipocytes transfected with a cyp2e1 expression plasmid. Mature 3T3-L1 adipocytes were transfected using Amaxa Nucleofection system. Immunoreactive CYP2E1 was measured by western blot in (A) Non-transfected 3T3-L1 adipocytes (2, 4, 8, 16, and 32 μg total proteins) and (B) 3T3-L1 adipocytes transfected with anti-sense cyp2e1 or sense-cyp2e1 plasmid. Mouse liver homogenates (1 and 2 μg of protein) were used as a positive control (A), total ERK1/2 or HSC70 was used as a loading control.
When CYP2E1 was overexpressed in 3T3-L1 adipocytes, culture with ethanol had no effect on intracellular adiponectin concentration (Fig. 5A), but suppressed the release of adiponectin into the extracellular medium (Fig. 5B). In 3T3-L1 adipocytes transfected with anti-sense cyp2e1, ethanol had no effect on either intracellular or extracellular adiponectin concentration (Fig. 5A and B). Interestingly, overexpression of CYP2E1 decreased adiponectin mRNA in both the absence or presence of ethanol (Fig. 5C). Because ROS is generated during oxidation of ethanol via the CYP2E1 pathway, ethanol would be expected to increase oxidative stress in 3T3-L1 adipocytes overexpressing CYP2E1. Accumulation of 4-HNE protein adducts was assessed by immunofluorescence. In 3T3-L1 adipocytes overexpressing CYP2E1, 4-HNE adducts were accumulated in both the presence and absence of ethanol, with a more robust signal in the presence of ethanol (Fig. 5D).
Fig. 5.
Ethanol decreased adiponectin secretion from 3T3-L1 adipocytes overexpressing CYP2E1. 3T3-L1 adipocytes were transiently transfected with sense-or anti-sense cyp2e1. After 24 h, cells were then cultured with or without 100 mM ethanol for a further 24 h. (A) Intracellular and (B) extracellular adiponectin in 3T3-L1 cells were measured by ELISA. (C) mRNA expression level of adiponectin was evaluated by RT-PCR. Values represent means ± SEM. n=4–6. Values with different superscripts are significantly different from each other, p< 0.05. (D) 4-HNE adducts were detected in 3T3-L1 adipocytes by immunofluoresence using an antibody against 4-HNE protein adducts. Nuclei were stained with DAPI. All images were acquired using a 10X objective. Figures are representative of 4–6 images per experimental condition.
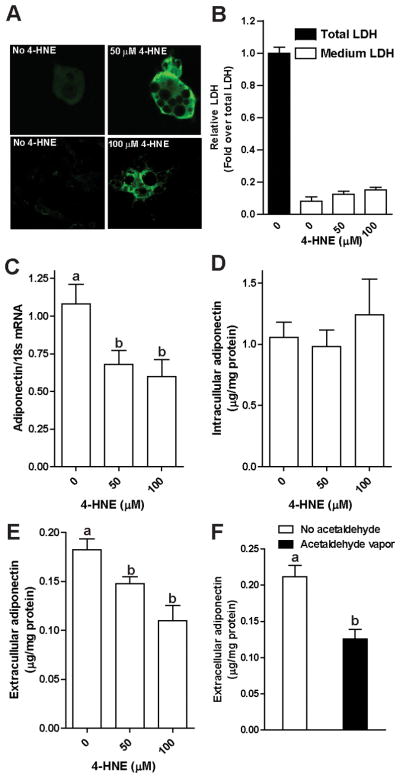
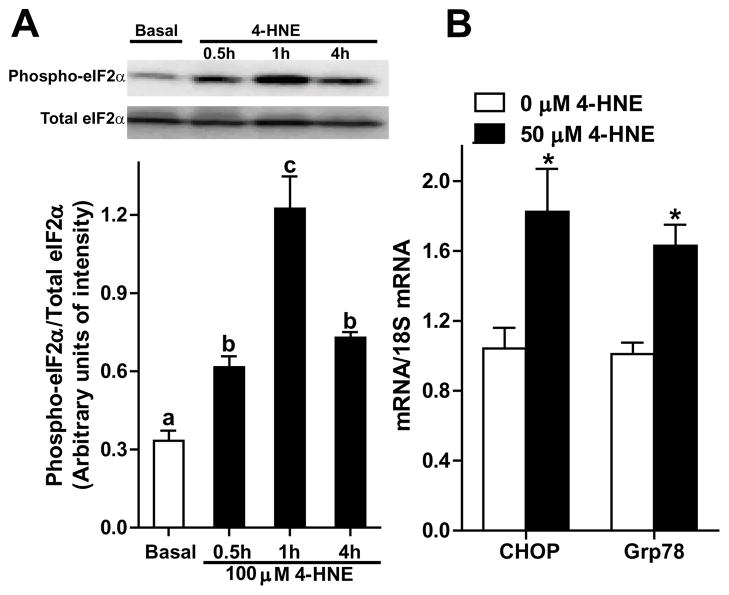
Adiponectin secretion by adipocytes decreased in parallel to ethanol-induced oxidative stress in adipocytes. Therefore, we hypothesized that oxidative stress per se would impair adiponectin secretion by adipocytes. Direct addition of oxidants, such as 4-HNE, to 3T3-L1 adipocytes in culture allowed us to investigate the impact of oxidative stress on adipocytes without the interference from other cell types present in adipose tissue. 3T3-L1 adipocytes were treated with increasing concentrations of 4-HNE. 4-HNE adducts were detected in a punctate distribution throughout cells, apparently concentrated in multiple vesicular compartments in the cells (Fig. 6A). Exposure to 4-HNE (up to 100 μM) had no detectable cytotoxicity in 3T3-L1 adipocytes, assessed by lactate dehydrogenase in the extracellular medium (Fig. 6B). Adiponectin mRNA expression was reduced after 4-HNE treatment (Fig. 6C); however, the intracellular concentration of adiponectin was not affected (Fig. 6D). Challenge with 50 and 100 μM 4-HNE decreased the accumulation of adiponectin in the extracellular medium (Fig. 6E). Culture of 3T3-L1 adipocytes in the presence of vaporized acetaldhehyde also decreased adiponectin secretion. Challenge of 3T3-L1 adipocytes with 4-HNE also induced the expression of typical markers of ER stress, including an increase in the phosphorylation of eukaryotic initiating factor-2α (eIF2α) at 0.5, 1, and 4 hours (Fig. 7A) and an increase in the mRNA levels of CHOP and Grp78 after 4 hours (Fig. 7B).
Fig. 6.
4-HNE decreased adiponectin mRNA expression and adiponectin secretion, but not intracellular adiponectin content in 3T3-L1 adipocytes. Mature 3T3-L1 cells were cultured in media with 0, 50, and 100 μM 4-HNE at 37°C for 4 hours (Panels A–E) or cultured in acetaldehyde vapor generated from 10 mM acetaldehyde solution in neighboring wells at 37°C for 18 hours (Panel F). Cells were then incubated in fresh medium. After 2 hours, media was removed and cells were lysed. (A) 4-HNE adducts were detected in 3T3-L1 adipocytes by immunofluoresence using an antibody against 4-HNE protein adducts (green flourescence). Nuclei were stained with DAPI. All images were acquired using a 40X objective. (B) Released LDH in media and cell lysates were measured with a coupled enzymatic assay, the absorbance of the final products was read at 490 nm. n=4; (C) adiponectin mRNA expression was measured by RT-PCR and then normalized to 18S; (D) intracellular and (E/F) extracellular adiponectin concentration in 3T3-L1 cells were measured by ELISA. n=4–12. Values represent means ± SEM. Values with different superscripts are significantly different from each other, p< 0.05.
Fig. 7.
4-HNE induced ER stress in 3T3-L1 adipocytes. Mature 3T3-L1 cells were cultured in media with or without 50 or 100 μM 4-HNE at 37°C for up to 4h. (A) Immunoreactive phospho-eIF2α and total eIF2α were detected by western blot. A representative image from 3 independent experiments is shown. (B) CHOP and Grp78 mRNA level in 3T3-L1 adipocytes were measured by RT-PCR and then normalized to 18S mRNA level. n=6. Values represent means ± SEM. Values with different superscripts (A) or asterisks (B) are significantly different from each other, p< 0.05.
Discussion
In this work we showed that induction of CYP2E1 expression in rat epididymal adipose tissue after chronic ethanol feeding was associated with increased oxidative stress, as well as decreased adiponectin secretion. Importantly, we reported for the first time that chronic ethanol feeding decreased the ratio of GSH to GSSG in high density microsomes of adipose tissue, resulting in a disruption in the thiol balance in ER and Golgi. Making use of 3T3-L1 adipocytes, which do not express endogenous CYP2E1, we found that overexpression of CYP2E1 was sufficient for ethanol-induced suppression of adiponectin secretion, associated with increased oxidative stress. The impact of CYP2E1 expression/ethanol exposure was modeled by challenge of 3T3-L1 adipocytes with 4-HNE, an end product of lipid peroxidation that is detectable in adipose tissue of ethanol-fed rats. Taken together, these data demonstrate that CYP2E1 plays a key role in ethanol-induced oxidative stress and disruption of adiponectin secretion by adipocytes after ethanol exposure.
Chronic ethanol feeding to rats decreased adiponectin secretion from adipocytes (Chen et al., 2007) (Figure 1A). Because adiponectin plays a critical role in the regulation of metabolism and immunity, it is important to understand the mechanisms by which ethanol impairs adiponectin secretion. While the complex molecular mechanisms regulating the maturation and oligomerization of adiponectin within adipocyte ER and Golgi are not completely understood, the formation of inter- and intra molecular disulfide bonds, catalyzed by oxidized protein disulfide isomerase, is essential for the oligomerization of adiponectin in the ER. Therefore, we hypothesized that disruption of the thiol redox balance in the ER during ethanol exposure could contribute to impaired adiponectin secretion.
Chronic ethanol exposure induces CYP2E1 and increases oxidative stress in liver (Lu and Cederbaum, 2008). CYP2E1 is localized to high density microsomes (Lieber, 1999). Here we found that chronic ethanol exposure increased expression of CYP2E1 in high density microsomes isolated from rat epididymal adipose. This increase was associated with a decrease in GSH/GSSG ratio, suggesting that CYP2E1 is involved in the maintenance of redox balance in ER/Golgi. Perturbations in the ER thiol redox balance have been also observed in diabetic rats (Nardai et al., 2005), as well as protein aggregation diseases (Schröder and Kaufman, 2005). Chronic ethanol feeding decreased the ratio of GSH to GSSG in adipose high density microsomes to 62% of pair-fed animals. Thus, ethanol feeding is associated with a more oxidizing environment in the ER/Golgi of adipose tissue. Reduced GSH/GSSG ratio stabilizes reactive free radicals, facilitating the oxidative modification of proteins. Perturbations in redox status greatly influence activities of resident ER enzymes that catalyze protein folding, leading to protein misfolding (Hwang et al., 1992). Thus, reduced GSH/GSSG ratio after chronic ethanol feeding may contribute to the disruption of adiponectin secretion by impairing post-translational maturation of adiponectin. Identification of the thiol redox balance in ER and Golgi as a target of ethanol exposure offers a new understanding of the complex mechanisms by which ethanol can disrupt protein trafficking in cells.
4-HNE is thought to be a mediator of cellular damage in response to oxidative stress. 4-HNE is detectable in 3T3-L1 adipocytes under basal conditions and its concentration is increased by oxidative stress generated by inclusion of glucose oxidase in the cell culture medium (Zarrouki et al., 2007). When 3T3-L1 adipocytes were treated with glucose oxidase to generate hydrogen peroxide and oxidative stress, there is an inverse correlation between 4-HNE and adiponectin production by 3T3-L1 adipocytes (Soares et al., 2005). Here we reported that 4-HNE decreased adiponectin mRNA expression, as well as adiponectin secretion by 3T3-L1 adipocytes, without affecting intracellular adiponectin protein content. These effects of 4-HNE are similar to the effect of ethanol feeding on decreasing adiponectin mRNA, as well as adiponectin secretion, without affecting the intracellular concentration of adiponectin in adipocytes from subcutaneous adipose tissue (Chen et al., 2007). Both the rate of synthesis of adiponectin protein and its rate of secretion by adipocytes determine intracellular adiponectin concentration. Thus, the maintenance of a normal intracellular pools of adiponectin protein content after challenge of 3T3-L1 adipocytes with 4-HNE is likely due to the combined impact of reduced adiponectin protein synthesis, due to the 4-HNE-mediated decrease in adiponectin mRNA, and reduced adiponectin secretion.
This study showed that 4-HNE decreased adiponectin secretion by adipocytes. However, the molecular mechanism by which oxidative stress disrupts adiponectin secretion by adipocytes is not clear. Ethanol caused accumulation of 4-HNE protein adducts in adipocytes (Fig. 2A) and adipose tissue (Chen et al., 2009). Ethanol feeding to mice (Song et al., 2008), as well as challenge of 3T3-L1 adipocytes with 4-HNE induced ER stress in adipocytes(Fig. 7), suggesting that ethanol-induced oxidative stress may decrease adiponectin secretion via the disruption in protein folding capacity in the ER. Further, adipose proteins were oxidatively modified after chronic ethanol exposure (Fig. 2B). It has been suggested that dysregulation in the post-translational modification process and intracellular trafficking disrupts adiponectin secretion (Wang et al., 2008). 4-HNE alters a number of proteins in making covalent adducts, leading to protein dysfunction (Bennaars-Eiden et al., 2002; Carbone et al., 2005a; Carbone et al., 2005b; Grimsrud et al., 2007; Grimsrud et al., 2008; Mark et al., 1997; Yang et al., 2004). Therefore, 4-HNE likely disrupts adiponectin secretion by forming adducts with proteins that are involved in post-translational modification process and intracellular trafficking of adiponectin. While we have not yet identified the 4-HNE-modified targets in adipocytes, there are several likely candidates, including protein disulfide isomerase and other redox-sensitive proteins in the ER and Golgi. Identification of these protein adducts in adipose tissue will be an important future area of investigation.
In other pathophysiological conditions, such as obesity, adiponectin expression is sensitive to oxidative stress (Furukawa et al., 2004). Similarly, compounds with anti-oxidant activity, such as taurine or betaine, protect rats and mice from ethanol-induced oxidative stress in adipose tissue and restore circulating concentrations of adiponectin (Chen et al., 2009; Song et al., 2008). The current study demonstrates, for the first time, an important role for CYP2E1 in reduced adiponectin secretion by adipocytes. CYP2E1 plays important roles in a variety of diseases with its ability to stimulate lipid peroxidation (Leclercq et al., 2000). As adipose tissue is the major source of adiponectin, and adiponectin has many beneficial biological functions, inhibition of CYP2E1 in adipose tissue may be a useful strategy to prevent ethanol-induced reduction of adiponectin secretion and subsequent injury.
Acknowledgments
We thank Dr. Arthur. I. Cederbaum for his generous gift of pCI-2E1 and pCI-as-2E1 plasmids, Dr. Sanjoy Roychowdhury for help with confocal microscopy, and Dr. Palash Mandal for help with transfection. We are also grateful to Brian T. Pratt for technical support. This work was supported by National Institutes of Health R37AA011876 and P20AA17069 (to L.E.N) and RO1AA17671 (to D.W.J).
References
- Bass R, Ruddock LW, Klappa P, Freedman RB. A major fraction of endoplasmic reticulum-located glutathione is present as mixed disulfides with protein. J Biol Chem. 2004;279(7):5257–5262. doi: 10.1074/jbc.M304951200. [DOI] [PubMed] [Google Scholar]
- Bennaars-Eiden A, Higgins L, Hertzel AV, Kapphahn RJ, Ferrington DA, Bernlohr DA. Covalent modification of epithelial fatty acid-binding protein by 4-hydroxynonenal in vitro and in vivo. Evidence for a role in antioxidant biology. J Biol Chem. 2002;277(52):50693–50702. doi: 10.1074/jbc.M209493200. [DOI] [PubMed] [Google Scholar]
- Carbone DL, Doorn JA, Kiebler Z, Ickes BR, Petersen DR. Modification of heat shock protein 90 by 4-hydroxynonenal in a rat model of chronic alcoholic liver disease. J Pharmacol Exp Ther. 2005a;315(1):8–15. doi: 10.1124/jpet.105.088088. [DOI] [PubMed] [Google Scholar]
- Carbone DL, Doorn JA, Kiebler Z, Petersen DR. Cysteine modification by lipid peroxidation products inhibits protein disulfide isomerase. Chem Res Toxicol. 2005b;18(8):1324–1331. doi: 10.1021/tx050078z. [DOI] [PubMed] [Google Scholar]
- Cederbaum AI. Introduction-serial review: alcohol, oxidative stress and cell injury. 2001;31(12):1524–1526. doi: 10.1016/s0891-5849(01)00741-9. [DOI] [PubMed] [Google Scholar]
- Chen Q, Cederbaum AI. Cytotoxicity and apoptosis produced by cytochrome P450 2E1 in Hep G2 cells. Mol Pharmacol. 1998;53(4):638–648. doi: 10.1124/mol.53.4.638. [DOI] [PubMed] [Google Scholar]
- Chen X, Sebastian BM, Nagy LE. Chronic ethanol feeding to rats decreases adiponectin secretion by subcutaneous adipocytes. Am J Physiol Endocrinol Metab. 2007;292(2):E621–628. doi: 10.1152/ajpendo.00387.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Sebastian BM, Tang H, McMullen MM, Axhemi A, Jacobsen DW, Nagy LE. Taurine supplementation prevents ethanol-induced decrease in serum adiponectin and reduces hepatic steatosis in rats. Hepatology. 2009;49(5):1554–1562. doi: 10.1002/hep.22811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon BM, Heath SH, Kim R, Suh JH, Hagen TM. Assessment of endoplasmic reticulum glutathione redox status is confounded by extensive ex vivo oxidation. Antioxid Redox Signal. 2008;10(5):963–972. doi: 10.1089/ars.2007.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Campos M, Canete RR, Gil A. Adiponectin, the missing link in insulin resistance and obesity. Clin Nutr. 2004;23:963–974. doi: 10.1016/j.clnu.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Grimsrud PA, Picklo MJS, Griffin TJ, Bernlohr DA. Carbonylation of adipose proteins in obesity and insulin resistance: identification of adipocyte fatty acid-binding protein as a cellular target of 4-hydroxynonenal. Mol Cell Proteomics. 2007;6(4):624–637. doi: 10.1074/mcp.M600120-MCP200. [DOI] [PubMed] [Google Scholar]
- Grimsrud PA, Xie H, Griffin TJ, Bernlohr DA. Oxidative stress and covalent modification of protein with bioactive aldehydes. J Biol Chem. 2008;283(32):21837–21841. doi: 10.1074/jbc.R700019200. 18445586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang C, Sinskey AJ, Lodish HF. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992;257(5076):1496–1502. doi: 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- Jacobsen DW, Gatautis VJ, Green R. Determination of plasma homocysteine by high-performance liquid chromatography with fluorescence detection. Analytical Biochemistry. 1989;178:208–214. doi: 10.1016/0003-2697(89)90381-3. [DOI] [PubMed] [Google Scholar]
- Jacobsen DW, Gatautis VJ, Green R, Robinson K, Savon SR, Secic M, Ji J, Otto JM, Taylor LM. Rapid HPLC determination of total homocysteine and other thiols in serum and plasma: sex differences and correlation with cobalamin and folate concentrations in healthy subjects. Clinical Chemistry. 1994;40:873–881. [PubMed] [Google Scholar]
- Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26(3):439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest. 2000;105(8):1067–1075. doi: 10.1172/JCI8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Liu F. Transcriptional and post-translational regulation of adiponectin. Biochem J. 2010;425:41–52. doi: 10.1042/BJ20091045. [DOI] [PubMed] [Google Scholar]
- Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med. 2008;44(5):723–738. doi: 10.1016/j.freeradbiomed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Zhuge J, Wang X, Bai J, Cederbaum AI. Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology. 2008;47(5):1483–1494. doi: 10.1002/hep.22222. [DOI] [PubMed] [Google Scholar]
- Mark RJ, Pang Z, Geddes JW, Uchida K, Mattson MP. Amyloid beta-peptide impairs glucose transport in hippocampal and cortical neurons: involvement of membrane lipid peroxidation. J Neurosci. 1997;17(3):1046–1054. doi: 10.1523/JNEUROSCI.17-03-01046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullen MR, Pritchard MT, Wang Q, Millward CA, Croniger CM, Nagy LE. Early growth response-1 transcription factor is essential for ethanol-induced fatty liver injury. Gastroenterology. 2005;128(7):2066–2076. doi: 10.1053/j.gastro.2005.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto M, Hagbjörk AL, Wan YJ, Fu PC, Clot P, Albano E, Ingelman-Sundberg M, French SW. Modulation of experimental alcohol-induced liver disease by cytochrome P450 2E1 inhibitors. Hepatology. 1995;21(6):1610–1617. [PubMed] [Google Scholar]
- Nardai G, Stadler K, Papp E, Korcsmáros T, Jakus J, Csermely P. Diabetic changes in the redox status of the microsomal protein folding machinery. Biochem Biophys Res Commun. 2005;334(3):787–795. doi: 10.1016/j.bbrc.2005.06.172. [DOI] [PubMed] [Google Scholar]
- Rachdaoui N, Sebastian BM, Nagy LE. Chronic ethanol feeding impairs endothelin-1-stimulated glucose uptake via decreased G alpha 11 expression in rat adipocytes. Am J Physiol Endocrinol Metab. 2003;285(2):E303–E310. doi: 10.1152/ajpendo.00547.2002. [DOI] [PubMed] [Google Scholar]
- Rao RK. Acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Alcohol Clin Exp Res. 1998;22(8):1724–1730. [PubMed] [Google Scholar]
- Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic Biol Med. 2010 doi: 10.1016/j.freeradbiomed.2010.09.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30(11):1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- Schröder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569(1–2):29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- Sebastian BM, Kang L, Chen X, Nagy LE. Methods to investigate the effects of chronic ethanol on adipocytes. Methods Mol Biol. 2008;447:357–366. doi: 10.1007/978-1-59745-242-7_23. [DOI] [PubMed] [Google Scholar]
- Soares AFMG, Cozzone D, Bernoud-Hubac N, Bouzaïdi-Tiali N, Lagarde M, Géloën A. Effects of oxidative stress on adiponectin secretion and lactate production in 3T3-L1 adipocytes. Free Radic Biol Med. 2005;38(7):882–889. doi: 10.1016/j.freeradbiomed.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Song Z, Zhou Z, Deaciuc I, Chen T, McClain CJ. Inhibition of adiponectin production by homocysteine: a potential mechanism for alcoholic liver disease. Hepatology. 2008;47(3):867–879. doi: 10.1002/hep.22074. [DOI] [PubMed] [Google Scholar]
- Thakur V, Pritchard MT, McMullen MR, Nagy LE. Adiponectin normalizes LPS-stimulated TNF-alpha production by rat Kupffer cells after chronic ethanol feeding. Am J Physiol Gastrointest Liver Physiol. 2006;290(5):G998–1007. doi: 10.1152/ajpgi.00553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto H, Lu SC. Current concepts in the pathogenesis of alcoholic liver injury. FASEB J. 2001;15(8):1335–1349. doi: 10.1096/fj.00-0650rev. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lam KS, Yau MH, Xu A. Post-translational modifications of adiponectin: mechanisms and functional implications. Biochem J. 2008;409(3):623–633. doi: 10.1042/BJ20071492. [DOI] [PubMed] [Google Scholar]
- Weltman MD, Farrell GC, Hall P, Ingelman-Sundberg M, Liddle C. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology. 1998;27(1):128–133. doi: 10.1002/hep.510270121. [DOI] [PubMed] [Google Scholar]
- Wu D, Cederbaum AI. Oxidative stress mediated toxicity exerted by ethanol-inducible CYP2E1. Toxicol Appl Pharmacol. 2005;207(2 Suppl):70–76. doi: 10.1016/j.taap.2005.01.057. [DOI] [PubMed] [Google Scholar]
- Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112(1):91–100. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JH, Yang ES, Park JW. Inactivation of NADP+-dependent isocitrate dehydrogenase by lipid peroxidation products. Free Radic Res. 2004;38(3):241–249. doi: 10.1080/10715760310001657712. [DOI] [PubMed] [Google Scholar]
- Yoshinari K, Sato T, Okino N, Sugatani J, Miwa M. Expression and induction of cytochromes p450 in rat white adipose tissue. J Pharmacol Exp Ther. 2004;311(1):147–154. doi: 10.1124/jpet.104.067066. [DOI] [PubMed] [Google Scholar]
- You M, Considine RV, Leone TC, Kelly DP, Crabb DW. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology. 2005;42(3):568–577. doi: 10.1002/hep.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrouki B, Soares AF, Guichardant M, Lagarde M, Géloën A. The lipid peroxidation end-product 4-HNE induces COX-2 expression through p38MAPK activation in 3T3-L1 adipose cell. FEBS Lett. 2007;581(13):2394–2400. doi: 10.1016/j.febslet.2007.04.048. [DOI] [PubMed] [Google Scholar]
- Zima T, Kalousová M. Oxidative stress and signal transduction pathways in alcoholic liver disease. Alcohol Clin Exp Res. 2005;33 (11):110S–115S. doi: 10.1097/01.alc.0000189288.30358.4b. [DOI] [PubMed] [Google Scholar]