Abstract
Background
Candidates with fulminant hepatic failure (Status-1A) receive the highest priority for liver transplantation (LT) in the United States. However, no studies have compared wait-list mortality risk among end-stage liver disease (ESLD) candidates with high Model for End-stage Liver Disease (MELD) scores to those listed as Status-1A. We aimed to determine if there are MELD scores for ESLD candidates at which their wait-list mortality risk is higher than that of Status-1A, and to identify the factors predicting wait-list mortality among Status-1A.
Methods
Data were obtained from the Scientific Registry of Transplant Recipients for adult LT candidates (n=52,459) listed between 09/01/2001 and 12/31/2007. Candidates listed for repeat LT as Status-1 A were excluded. Starting from the date of wait listing, candidates were followed for 14 days or until the earliest of death, transplant, or granting of an exception MELD score. ESLD candidates were categorized by MELD score, with a separate category for those with calculated MELD >40. We compared wait-list mortality between each MELD category and Status-1A (reference) using time-dependent Cox regression.
Results
ESLD candidates with MELD >40 had almost twice the wait-list mortality risk of Status-1A candidates, with a covariate-adjusted hazard ratio of HR=1.96 (p=0.004). There was no difference in wait-list mortality risk for candidates with MELD 36–40 and Status-1A, while candidates with MELD <36 had significantly lower mortality risk than Status-1A candidates. MELD score did not significantly predict wait-list mortality among Status-1A candidates (p=0.18). Among Status-1A candidates with acetaminophen toxicity, MELD was a significant predictor of wait-list mortality (p<0.0009). Post-transplant survival was similar for Status-1A and ESLD candidates with MELD >20 (p=0.6).
Conclusions
Candidates with MELD >40 have significantly higher wait-list mortality and similar post-transplant survival as Status-1A, and therefore, should be assigned higher priority than Status-1A for allocation. Since ESLD candidates with MELD 36–40 and Status-1A have similar wait-list mortality risk and post-transplant survival, these candidates should be assigned similar rather than sequential priority for deceased donor LT.
Keywords: decompensated end-stage liver disease, fulminant hepatic failure, model for end-stage liver disease, Status-1A, Status-1B, survival
Introduction
Liver transplantation (LT) is the only therapeutic option for candidates with fulminant hepatic failure (FHF) and those with decompensated end-stage liver disease (ESLD). Candidates with FHF are listed as Status-1A on the transplant waiting list and receive the highest priority for deceased donor LT 1. These candidates are ordered by their waiting time on the wait-list.
The Organ Procurement and Transplantation Network (OPTN) defines FHF as the onset of hepatic encephalopathy within eight weeks of the first symptoms of liver disease in the absence of pre-existing liver disease, in the intensive care unit, meeting at least one of three criteria: a) ventilator dependence, b) requiring renal replacement therapy or c) international normalized ratio (INR) of prothrombin time >2.0 with a life expectancy of seven days or less without LT 1. FHF is associated with high mortality in the absence of transplant, with variation in prognosis based upon patient age, etiology, and grade of hepatic encephalopathy.
ESLD candidates are prioritized after Status-1A based upon their Model for End-stage Liver Disease (MELD) score 1. MELD score is a predictor of wait-list mortality and is calculated using the candidate’s serum bilirubin, creatinine and INR 2 and was adopted as the basis for deceased-donor liver allocation by the OPTN in February 2002 3, 4. The national allocation policy for donor livers is based upon nested rules pertaining to wait-list mortality and geography. The Status-1 category was further subdivided into Status-1A and Status-1B. This policy went into effect on August 24, 2005 5. Donor livers are first offered to Status-1A candidates with FHF or immediate post-LT graft failure, followed by Status-1B (pediatric candidates with chronic liver disease in an intensive care unit). Candidates with ESLD are allocated organs after Status-1A candidates in descending order of MELD or Pediatric End-stage Liver Disease (PELD) score 5. For allocation purposes, these scores are capped by the OPTN at 40. However, data from previous studies showed that candidates with MELD scores >40 have higher wait-list mortality than candidates with a MELD score of 403,6.
ESLD candidates with higher MELD scores have higher wait-list mortality risk compared to those with lower MELD scores 3. However, there are no studies comparing the wait-list mortality risk of FHF candidates listed as Status-1A to the wait-list mortality risk of ESLD candidates with high MELD scores. Moreover, it has been tacitly assumed that wait-list mortality risk among FHF Status-1A candidates is equal, irrespective of MELD score. In fact, one previous study suggested further stratification of Status-1A by diagnosis to address this issue 6.
The primary objective of the current study was to determine if there are MELD scores at which the wait-list mortality risk of ESLD candidates is higher than that of FHF Status-1A candidates and to identify predictors of wait-list mortality risk among Status-1A candidates.
Patients and Methods
Data Source and Study Population
This study used data from the Scientific Registry of Transplant Recipients (SRTR) submitted by the members of the OPTN. Mortality information was supplemented by data from the Social Security Death Master File.
In order to avoid cohort heterogeneity due to subdivision of Status-1 into Status-1A and 1B in August 2005, our study population included all adult candidates (age ≥18 years) listed as Status-1 (before the policy change) and Status-1A (after the policy change) for FHF, with an initial date of registration for deceased donor LT between September 1, 2001 and December 31, 2007 a priori, and refer to all such candidates hereafter as Status-1A in this paper. The start date of the study corresponded to the initial date of mandatory submission of the three components of the MELD score. Since this policy change for Status-1 mainly affected pediatric candidates, our study excluded candidates <18 years of age listed as Status-1or Status-1B as well as candidates listed for repeat LT as Status-1 or Status-1A for primary non-function.
The primary outcome was wait-list mortality within two weeks of listing. Candidates were followed from the time of listing until the earliest of death, receipt of LT, the granting of exception MELD score, or 14 days after listing. The majority of Status-1A candidates with FHF receive a transplant, recover, or die within 14 days of listing. Moreover, a request to continue a Status-1A listing beyond 14 days results in a review of all local Status-1A liver candidate listings 5. For these reasons and in the light of our objectives, an a priori follow-up period of two weeks after listing was considered to be the most relevant.
FHF candidates listed as Status-1A were stratified as related to acetaminophen toxicity and non-acetaminophen etiology, based upon diagnosis codes. To avoid misclassification, the etiology of FHF was ascertained manually. The etiology of FHF Status-1A candidates was coded as acetaminophen if the primary or secondary diagnosis data field indicated any mention of acetaminophen, (Tylenol® [McNEIL-PPC, Inc., Fort Washington, PA]) or drugs containing acetaminophen (such as Vicodin® [Abbott Laboratories, Abbott Park, Il], Darvocet® [AAIPharma, Wilmington, NC], APAP-hydrocodone, Percocet® [Endo Pharmaceuticals, Chadds Ford, PA] etc.). All other FHF candidates listed as Status-1A were coded as non-acetaminophen.
Statistical Analysis
For descriptive purposes, continuous variables were presented as mean ± standard deviation, while levels of categorical variables were presented as percentages.
Kaplan-Meier analysis was used to estimate unadjusted wait-list survival probabilities. For wait-list survival, candidates were followed from listing to death, LT or 14-days after listing. Note that for this part of the analysis, wait-list candidates were classified based on their MELD/Status at listing, due to the technical (as well as conceptual) difficulties associated with estimating survival curves in the presence of time-dependent covariates; e.g., as discussed in Kalbfleisch and Prentice 7, and in Schaubel et al 8.
Cox regression analysis was used to model wait-list mortality. MELD and Status-1A were coded as time-dependent covariates, such that changes in MELD score and Status-1A were factored into the modeling. Status-1A served as the reference category, to which each MELD category was compared. Mortality contrasts between each MELD category and Status-1A were quantified by the hazard ratio (HR), which (for a particular MELD category) can be interpreted as the covariate-adjusted ratio of death rates, with the death rate for Status-1A (HR=1) serving as the denominator. ESLD candidates with a calculated MELD score >40 were assigned to the ‘MELD >40’ category. Cox models were stratified by OPO and adjusted for age, sex, race, diagnosis, dialysis, albumin, and diabetes and hospitalization status at listing.
Using only the Status-1A candidates, we then fitted Cox models to assess the impact of MELD score on wait-list mortality. First, we fitted a model to all Status-1A candidates; second, we fitted separate models to the Status-1A-acetaminophen and Status-1A-non-acetaminophen candidates. The model was adjusted for age, sex, race, diagnosis, dialysis, albumin, and diabetes and hospitalization status at listing. We also evaluated the interaction between MELD and calendar year of listing, for the wait-list mortality model.
Kaplan-Meier analysis was used to estimate unadjusted post-transplant survival probabilities. Candidates were followed from the time of transplant until the earliest of death, three years post-transplant, or the end of the follow-up period.
Lastly, we fitted Cox models to contrast Status-1A (reference) and the MELD categories with respect to post-transplant mortality. Included in this component of the analysis were all patients from the original study cohort who received a first deceased-donor LT during the observation period. We adjusted for all covariates listed previously, as well as time between wait-listing and transplant, and donor risk index 9. Cox regression analysis was used to compare the post-LT survival analysis among all LT recipients. All statistical analyses were conducted using SAS v9.2 (SAS Institute; Cary, NC, USA).
Results
Description of Cohort
A total of n=52,459 candidates (Status-1A candidates: n=2,128 ESLD candidates: n= 50,331) aged ≥18 years were listed for deceased donor LT between September 1, 2001 and December 31, 2007. Of the Status-1A candidates, 485 (23%) were classified as acetaminophen while the remaining 1,643 (77%) were classified as non-acetaminophen. The baseline characteristics of such candidates before and after the policy change were similar (data not shown). Characteristics of the Status-1A and ESLD candidates are listed in Table 1. The acetaminophen Status-1A candidates tended to be younger, more likely to be white and female with lower body mass index than non-acetaminophen Status-1A candidates (Table 1). Approximately 34% of acetaminophen Status-1A candidates were on renal replacement therapy at listing, compared with 60% of non-acetaminophen Status-1A candidates.
Table 1.
Characteristics of Status-1A (Acetaminophen and Non-Acetaminophen) and ESLD Candidates at Listing
| Variables | Status-1A (N=2128) |
ESLD (N=50331) |
|
|---|---|---|---|
| Acetaminophen (N=485) |
Non-Acetaminophen (N=1643) |
||
| Age (years) | 33 ± 11.2 | 40.5 ± 14.1 | 52.5±13.5 |
| % Male | 22% | 35% | 65% |
| White | 81% | 58% | 75% |
| Black | 9% | 21% | 7% |
| Hispanic | 7% | 12% | 14% |
| Others | 3% | 9% | 4% |
| MELD Score | 35 ± 6.7 | 33 ± 7.3 | 16±7.6 |
| Dialysis | 13% | 8% | 3.3% |
| BMI | 24.6 ± 5.1 | 29.3 ±5.4 | 32.5 ±5.8 |
MELD Distribution at the Time of Listing
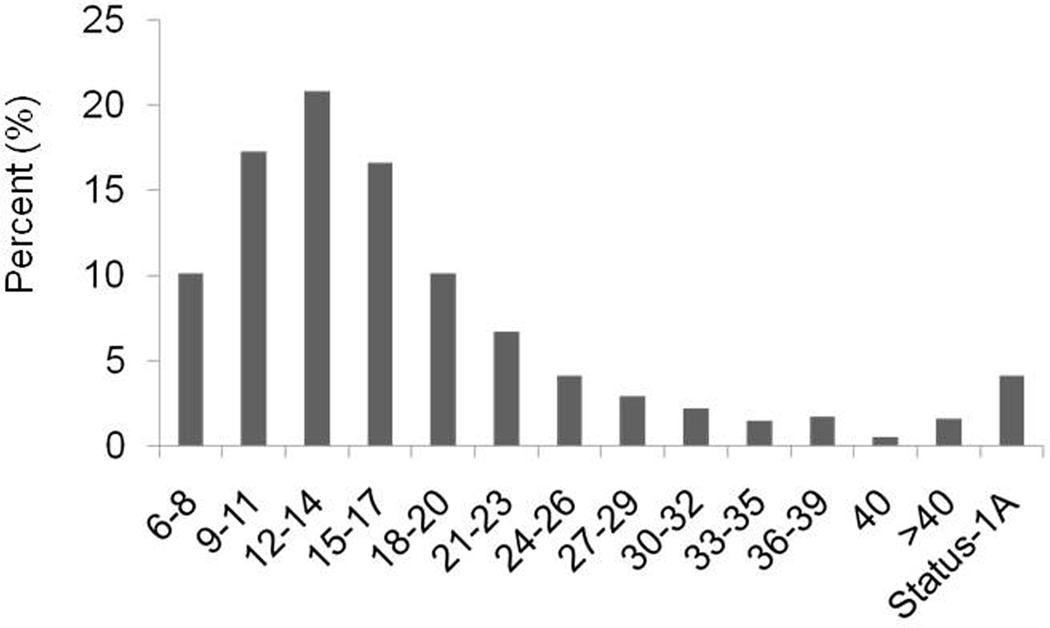
Figure 1 shows the distribution of MELD score at the time of listing among Status-1A and ESLD candidates. A total of 576 (27%) Status-1A candidates had a calculated MELD score >40 at the time of listing whereas 813 (1.6%) ESLD candidates had MELD >40 at listing.
Figure 1.
MELD Score Distribution at Listing among ESLD and Status-1A Candidates
Abbreviations: ESLD: End-Stage Liver Disease; MELD: Model for End-Stage Liver Disease
Wait-list Mortality Among ESLD and Status-1A Candidates
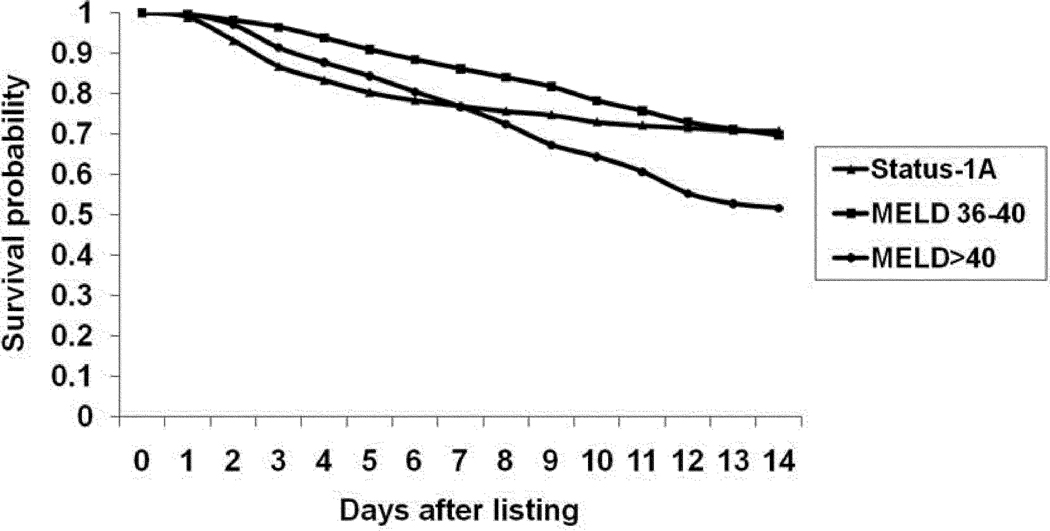
There were a total of 333 and 1,302 wait-list deaths among Status-1A and ESLD candidates, respectively, within two weeks of listing. Figure 2 shows the candidate survival stratified by Status-1A and ESLD candidates with MELD score 36–40 and >40. Fourteen-day wait-list survival probability was estimated at 71% for Status-1A, 70% for MELD score 36–40 and 52% for candidates with MELD >40.
Figure 2.
Kaplan-Meier wait-list survival curves for patients listed at Status-1A, MELD 36–40 and MELD >40.
Abbreviations: MELD: Model for End-Stage Liver Disease
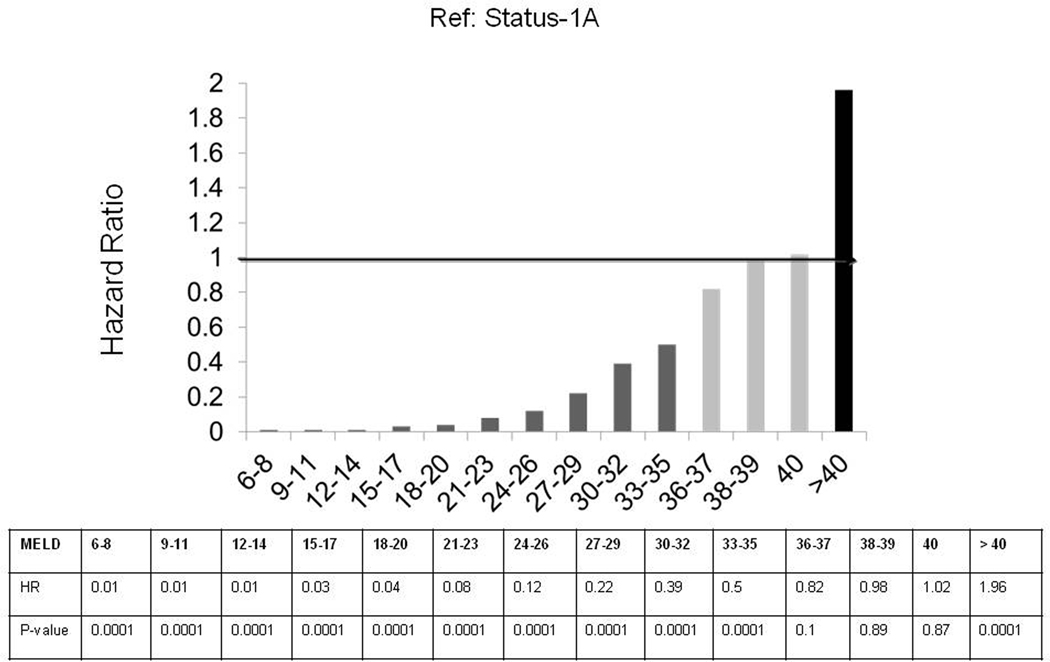
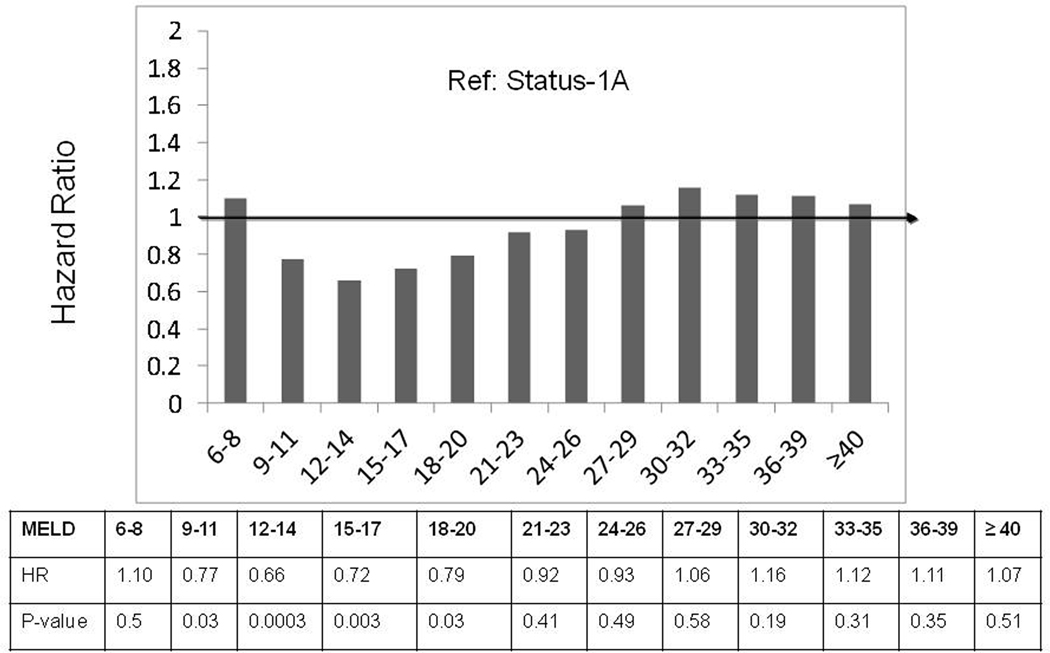
Results from the Cox regression analysis comparing ESLD candidates in each MELD category to Status-1A (reference: HR=1) are shown in Figure 3. Candidates in all MELD score categories less than 36 had significantly lower wait-list mortality risk compared to Status-1A. Candidates with MELD scores 36 to 40 had similar wait-list mortality risk compared to those at Status-1A. Compared to Status-1A candidates, those with MELD score >40 had significantly higher wait-list mortality risk, with a covariate-adjusted hazard ratio [HR] of HR=1.96 (p<0.0001). Hence, candidates with MELD scores >40 (which are capped at 40 by the Organ Procurement and Transplantation Network for allocation purposes) had approximately twice the covariate-adjusted wait-list mortality risk of Status-1A candidates. Wait-list mortality risk among acetaminophen Status-1A candidates was not significantly different than that of non-acetaminophen Status-1A candidates. Similarly, the wait-list mortality risk for Status-1A candidates was not significantly different before versus after the policy change for Status-1 in August 2005 (HR=0.82; p=0.11).
Figure 3.
Comparing Covariate-adjusted Wait-list Mortality Risk between ESLD and Status-1A Candidates
Status-1A is the reference group (hazard ratio set to 1). Compared to Status-1A, ESLD candidates with MELD score ≤35 have lower wait-list mortality risk; ESLD candidates with MELD score 36–40 have similar wait-list mortality risk; and ESLD candidates with MELD scores >40 have higher wait-list mortality risk.
Abbreviations: MELD: Model for End Stage Liver Disease
Predictors of Wait-list Mortality Among Status-1A Candidates
In Table 2, we list results from wait-list mortality models fitted for Status-1A candidates only. Separate Cox models were also fitted to acetaminophen and non-acetaminophen Status-1A candidates. MELD score was a significant predictor of wait-list mortality among acetaminophen Status-1A candidates, with a covariate-adjusted hazard ratio of HR=1.071 (p=0.009) indicating that each one point increase in MELD score was associated with a 7.1% increase in wait-list mortality, after adjustment for age, sex, race, diagnosis, dialysis, albumin, and diabetes and hospitalization status. MELD was not found to have a significant association with wait-list mortality among non-acetaminophen Status-1A candidates, with HR=0.998 (p=0.86). Interestingly, the interaction between MELD and listing year on wait-list mortality among non-acetaminophen Status-1A candidates was significant (HR=0.99; p=0.046), suggesting that the effect of MELD on wait-list mortality has been decreasing in more recent years for these candidates.
Table 2.
Effect of MELD on Wait-list Mortality Risk Among Status-1A Candidates
| Model1 | Hazard Ratio* (95% CI) | P-value |
|---|---|---|
| All Status-1A | 1.012 (0.995,1.029) | 0.1837 |
| Acetaminophen Status-1A | 1.070 (1.028, 1.114) | 0.0009 |
| Non-acetaminophen Status-1A | 0.998 (0.980, 1.017) | 0.86 |
Three separate models stratified by OPO.
Adjusted for age, sex, race, diagnosis, dialysis, albumin, diabetes and hospitalization status.
Hospitalization in the intensive care unit (HR=2.06; p=0.001) and receipt of dialysis (HR=1.97, p<0.0001) were the only other significant predictors of wait-list mortality among all Status-1A candidates.
Post-LT Survival Rates Among Status-1A and ESLD Recipients
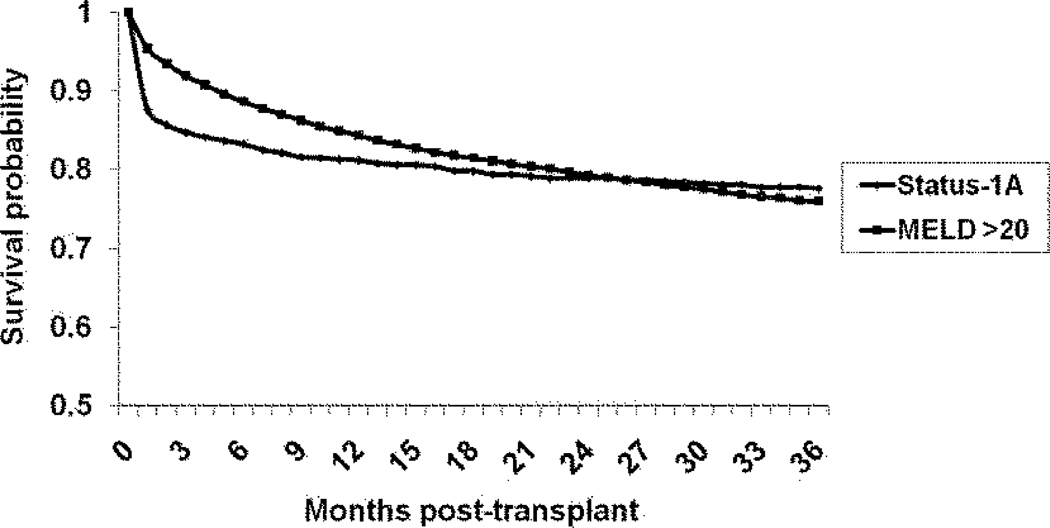
Figure 4 shows the unadjusted post-transplant survival for Status-1A and MELD >20 recipients. The 1-, 2- and 3-year post-transplant survival was 81%, 79% and 78% for Status-1A and 84%, 79% and 76% for recipients with MELD score >20. In the Cox regression analysis, there were no significant differences in post-LT mortality risk between Status-1A and ESLD recipients in any subgroup of MELD >20 (Figure 5).
Figure 4.
Kaplan-Meier post-transplant survival curves for recipients transplanted at Status-1A, and MELD >20.
Abbreviations: MELD: Model for End Stage Liver Disease
Figure 5.
Comparing Covariate-adjusted Post-Transplant Mortality Risk between ESLD and Status-1A Candidates.
Status-1A is the reference group (hazard ratio set to 1). Compared to Status-1A, ESLD candidates with MELD score ≤ 20 have lower overall post-transplant mortality risk; ESLD candidates with MELD scores 21–40 and >40 have similar post-transplant mortality risk.
Abbreviations: ESLD: End Stage Liver Disease; MELD: Model for End Stage Liver Disease
Discussion
This is the first study to compare wait-list mortality risk among Status-1A and ESLD candidates with high MELD scores. Our analysis showed that ESLD candidates with MELD scores 36 to 40 had similar wait-list mortality risk as Status-1A candidates, and those with MELD scores >40 had significantly higher wait-list mortality risk than Status-1A candidates. Importantly, post-LT survival was similar among Status-1A and all groups of ESLD candidates with MELD scores >20, suggesting that futility concerns for those with the highest MELD scores are largely unfounded, given current practices. Our results also showed that wait-list mortality risk is not homogeneous among Status-1A candidates; MELD was a significant independent predictor of wait-list mortality in the acetaminophen Status-1A subgroup.
Although the MELD score was adopted in February 2002 as the measure of wait-list urgency utilized to allocate donor liver allografts to ESLD candidates, the allocation process for Status-1A candidates has remained unchanged and still uses waiting time as the primary allocation criterion 4, 5. Previous studies evaluating the wait-list mortality risk of ESLD candidates excluded Status-1A candidates 2, 3, 10. For ESLD candidates, Merion et al. showed that wait-list mortality risk increased exponentially with increasing MELD score; the unadjusted wait-list mortality rates were 4,364 per 1000 patient years at MELD 30–39 and 13,153 per 1,000 patient years at MELD 40 10. In addition, Wiesner et al. and Kamath et al., in their MELD validation studies, estimated the three-month death probability to be 60%–83% and 79%–100% for ESLD candidates with MELD scores 30–39 and 40, respectively 2, 3.
Given the sequential nature of the current allocation system, our study results, showing similar wait-list mortality risk and similar early, as well as overall post-LT mortality risk among Status-1A and high MELD candidates, have important implications for ESLD candidates with high MELD scores. For instance, in 2007, the median time to LT among Status-1A candidates was five days, compared to 15 days for candidates with MELD ≥30. Candidates with high MELD scores (36–40) wait longer than candidates at Status-1A to receive an allograft 11, 12, prolonging their exposure to the risk of wait-list death. Thus, despite similar risk of wait-list death per unit time, the longer exposure for the ESLD candidates of necessity results in a lower proportion transplanted and a higher proportion who die on the wait-list. Since the intent of current liver allocation policy is to reduce wait-list mortality, ESLD candidates with MELD scores 36–40 should receive similar priority to Status-1A for deceased donor LT.
In our study, ESLD candidates with actual MELD scores >40 had the highest risk of dying on the wait-list and had similar post-LT survival as Status-1A, suggesting that liver transplant survival benefit among MELD >40 candidates was greater than that of Status-1A patients. However, these candidates are grouped together with those ESLD candidates whose MELD scores are exactly 40. Given the higher wait-list mortality risk among those with MELD scores >40, the arbitrary capping of MELD score at 40 appears to have harmed these candidates and further increased the overall mortality among this sickest group of candidates. Rank ordering by calculated MELD score beyond 40 may improve the chances of getting an offer in a timely manner for MELD>40 candidates and may decrease their wait-list mortality.
Status-1A candidates currently benefit from broader sharing of organs. This mechanism is not available to high MELD patients. Moreover, the impact of broader sharing upon wait-list mortality among high MELD patients is not known. Given the comparable mortality risk of Status-1A and MELD>40 candidates, admixture of these candidate pools into the current regional sharing tier of organ distribution would also be reasonably expected to have a salutary effect on mortality. This change in allocation policy could be studied by simulation modeling or by a regionally based pilot study as was conducted for MELD ≥29 patients in Region 813.
Although the MELD score is not currently considered in the allocation of liver allografts to Status-1A candidates, several studies have suggested prognostic value of MELD score in certain subsets of candidates with acute liver failure 6, 14–17. In one study, a higher MELD score was predictive of the development of FHF, but once FHF developed, the MELD score was not any more accurate at predicting survival than either the King's College criteria or INR alone 17. In another study from the pre-MELD era of 720 adult Status-1A candidates, non-acetaminophen, FHF candidates had the poorest overall survival on the liver transplant wait-list, and higher MELD score was highly correlated with lower survival in this group 6. Our study from the MELD era, found that every unit increase in MELD score was associated with 7% increase in wait-list mortality risk among acetaminophen FHF Status-1A candidates. This is a novel finding.
The effect of MELD score on wait-list mortality risk was not seen in non-acetaminophen FHF Status-1A candidates, in distinction to the Kremers et al. study 6. The significant interaction between MELD and listing year on wait-list mortality among non-acetaminophen FHF Status-1A candidates in our study suggests that the impact of MELD in this subgroup significantly diminished as calendar time progressed. Our study and that by Kremers et al. 6 are markedly different. The present study utilized a cohort five times larger than Kremers et al. (N=1643 vs. N=312), analyzed outcomes since the collection of MELD components was initiated, and included patients with Wilson’s disease. Changes in practice patterns, including earlier referral of fulminant liver failure patients to liver transplant centers and improved ICU care may account for the lack of association between MELD and wait-list mortality outcome for non-acetaminophen FHF candidates.
The main limitation of our study relates to its retrospective observational design, which results in the potential for bias due to unmeasured patient characteristics. Although we ascertained the acetaminophen cases manually in order to minimize the misclassification as described in the Methods section, misclassification of acetaminophen and non-acetaminophen Status-1A cases is still plausible due to the incomplete information, missing data, and the inability to confirm the cause of FHF with the submitting centers. The results of our study cannot necessarily be applied to candidates awaiting second liver transplants after primary graft non-function or to children, as these groups were excluded from our study cohort. Despite these limitations, our study is the first to demonstrate the comparability of wait-list mortality risk of Status-1A and ESLD candidates with high MELD scores.
MELD score may serve as a reasonable tool in ranking acetaminophen FHF Status-1A candidates on the transplant waiting list. However, further studies using the cross-classifications of acetaminophen FHF Status-1A and ESLD candidates with MELD score ≥36 for wait-list mortality risk stratification must be done to validate MELD score as an evidence-based allocation tool among these candidates. The MELD score was not a significant predictor of wait-list mortality among non-acetaminophen FHF Status-1A candidates. Another approach would be allocation of deceased donor livers by survival benefit 8, however, this may require additional investigation and further validation.
In conclusion, our study has shown that ESLD candidates with MELD >40 have higher wait-list mortality rates than Status-1A candidates. In addition, ESLD candidates with MELD scores 36 to 40 have wait-list mortality similar to that of Status-1A. Regardless of their wait-list mortality, the LT recipients with MELD scores >20, including those with MELD >40, have similar post-transplant survival as Status-1A recipients. These results imply that an evidence-based modification in the current allocation scheme may further reduce overall wait-list mortality without compromising post-transplant survival.
Acknowledgments
Funding Sources: This research was presented, in part, as a free communication at the American Transplant Congress, 2009, held in Boston, Massachusetts. Dr. Sharma was the recipient of an American Society of Transplantation/Roche Clinical Science Faculty Development grant for 2008–10. Dr. Sharma is also supported by National Institutes of Health (NIH) grant KO8 DK-088946. The statistical methodology development and analysis for this investigation was supported in part by National Institutes of Health (NIH) grant 2 R01 DK070869 to Dr. Schaubel. Drs. Sharma, Schaubel, and Gong are also supported by Michigan Institute for Health and Clinical Research NIH-Clinical and Translational Sciences Award UL1RR024986. The Scientific Registry of Transplant Recipients is funded by contract number 231-00-0116 from the Health Resources and Services Administration (HRSA), US Department of Health and Human Services. The views expressed herein are those of the authors and not necessarily those of the US Government. This study was approved by HRSA's SRTR project officer. HRSA has determined that this study satisfies the criteria for the IRB exemption described in the “Public Benefit and Service Program” provisions of 45 CFR 46.101(b)(5) and HRSA Circular 03. The authors would like to thank Ms. Shauna Leighton, Medical Editor, Arbor Research Collaborative for Health, Ann Arbor, Michigan, funded by the Scientific Registry of Transplant Recipients for providing editorial assistance.
Abbreviations
- FHF
Fulminant Hepatic Failure
- HR
Hazard Ratio
- INR
International Normalized Ratio
- LT
Liver Transplantation
- MELD
Model for End-Stage Liver Disease
- SRTR
Scientific Registry of Transplant Recipients
References
- 1.UNOS/OPTN. 3.6 Organ Distribution: Allocation of Livers. Policies. 2002;Volume 2011 [Google Scholar]
- 2.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 3.Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, Kremers W, Lake J, Howard T, Merion RM, Wolfe RA, Krom R. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91–96. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 4.Wiesner RH, McDiarmid SV, Kamath PS, Edwards EB, Malinchoc M, Kremers WK, Krom RA, Kim WR. MELD and PELD: application of survival models to liver allocation. Liver Transpl. 2001;7:567–580. doi: 10.1053/jlts.2001.25879. [DOI] [PubMed] [Google Scholar]
- 5.UNOS/OPTN. 3.6 Allocation of Livers. Volume 2005 [Google Scholar]
- 6.Kremers WK, van IM, Kim WR, Freeman RB, Harper AM, Kamath PS, Wiesner RH. MELD score as a predictor of pretransplant and posttransplant survival in OPTN/UNOS status 1 patients. Hepatology. 2004;39:764–769. doi: 10.1002/hep.20083. [DOI] [PubMed] [Google Scholar]
- 7.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. 2nd ed. New York: Wiley; 2002. [Google Scholar]
- 8.Schaubel DE, Guidinger MK, Biggins SW, Kalbfleisch JD, Pomfret EA, Sharma P, Merion RM. Survival benefit-based deceased-donor liver allocation. Am J Transplant. 2009;9:970–981. doi: 10.1111/j.1600-6143.2009.02571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, Greenstein SM, Merion RM. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–790. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 10.Merion RM, Schaubel DE, Dykstra DM, Freeman RB, Port FK, Wolfe RA. The survival benefit of liver transplantation. Am J Transplant. 2005;5:307–313. doi: 10.1111/j.1600-6143.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- 11.Berg CL, Steffick DE, Edwards EB, Heimbach JK, Magee JC, Washburn WK, Mazariegos GV. Liver and intestine transplantation in the United States 1998–2007. Am J Transplant. 2009;9:907–931. doi: 10.1111/j.1600-6143.2009.02567.x. [DOI] [PubMed] [Google Scholar]
- 12.SRTR. SRTR/OPTN Annual Report. 2009;Volume 2010
- 13.Hunsicker LGCTW, Voigt MD. Outcomes of Region-Wide Sharing to Candidates with MELD >29 in Region 8, Abstract #63. Am J Transplant. 2010;10:58. [Google Scholar]
- 14.Yantorno SE, Kremers WK, Ruf AE, Trentadue JJ, Podesta LG, Villamil FG. MELD is superior to King's college and Clichy's criteria to assess prognosis in fulminant hepatic failure. Liver Transpl. 2007;13:822–828. doi: 10.1002/lt.21104. [DOI] [PubMed] [Google Scholar]
- 15.Dhiman RK, Jain S, Maheshwari U, Bhalla A, Sharma N, Ahluwalia J, Duseja A, Chawla Y. Early indicators of prognosis in fulminant hepatic failure: an assessment of the Model for End-Stage Liver Disease (MELD) and King's College Hospital criteria. Liver Transpl. 2007;13:814–821. doi: 10.1002/lt.21050. [DOI] [PubMed] [Google Scholar]
- 16.Taylor RM, Davern T, Munoz S, Han SH, McGuire B, Larson AM, Hynan L, Lee WM, Fontana RJ. Fulminant hepatitis A virus infection in the United States: Incidence, prognosis, and outcomes. Hepatology. 2006;44:1589–1597. doi: 10.1002/hep.21439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt LE, Larsen FS. MELD score as a predictor of liver failure and death in patients with acetaminophen-induced liver injury. Hepatology. 2007;45:789–796. doi: 10.1002/hep.21503. [DOI] [PubMed] [Google Scholar]