Abstract
BACKGROUND
Indoleamine 2,3 dioxygenase 1 (IDO1) is a tryptophan catabolizing enzyme with immunotolerance promoting functions. We sought to determine if increased gut expression of IDO1 in Crohn’s disease (CD) would result in detectable changes in serum levels of tryptophan and the initial IDO1 pathway catabolite, kynurenine.
METHODS
Individuals were prospectively enrolled through the Washington University Digestive Diseases Research Center. Montreal classification was used for disease phenotyping. Disease severity was categorized by physician’s global assessment. Serum tryptophan and kynurenine were measured by high pressure liquid chromatography. IDO1 immunohistochemical staining was performed on formalin-fixed tissue blocks.
RESULTS
25 CD patients and 11 controls were enrolled. 8 CD patients had serum collected at two different time points and levels of disease activity. Strong IDO1 expression exists in both the lamina propria and epithelium during active CD compared to controls. Suppressed serum tryptophan levels and an elevated kynurenine/tryptophan (K/T) ratio were found in individuals with active CD as compared to those in remission or the control population. K/T ratios correlated positively with disease activity as well as with C-reactive protein and erythrocyte sedimentation rate. In the subgroup of CD patients with two serum measurements, tryptophan levels elevated while kynurenine levels and the K/T ratio lowered as the disease activity lessened.
CONCLUSIONS
IDO1 expression in Crohn’s disease is associated with lower serum tryptophan and an elevated K/T ratio. These levels may serve a reasonable objective marker of gut mucosal immune activation and surrogate for Crohn’s Disease activity.
Keywords: Indoleamine 2,3 dioxygenase; tryptophan; Crohn; Biomarker
INTRODUCTION
Crohn’s disease is a chronic inflammatory condition predominantly affecting the colon and small intestine. Current data and leading theory suggests Crohn’s disease arises after environmental triggers influence a genetically susceptible host to develop an overly aggressive immune response to a subset of commensal bacteria.(1) The immune response in active Crohn’s disease appears to result from aberrant handling of luminal bacteria by the innate immune system, resulting in an activated inflammatory cascade involving TH1 and TH17 cells of the adaptive immune system and secretion several inflammatory cytokines including TNFα, IFNγ, IL1β, IL12/23 and IL17.(2, 3) The degree of inflammation, its location and eventual sequelae are expressed both in gastrointestinal symptom severity and often systemic toxicity.
Indoleamine 2,3 dioxygenase-1 (IDO1) is an enzyme expressed in cells of the innate immune system and acts as the initial and rate limiting step in catabolism of the essential amino acid tryptophan along the kynurenine pathway. The primary relevance for IDO1 appears to be immunologic, rather than metabolic or nutritional. Accordingly, toll like receptor (TLR) activation, IFNγ, IFNα, and TNFα are all known to induceIDO1 expression.(4, 5) Acting as an interface between innate and adaptive immune responses, IDO1 expression inmacrophages and dendritic cells promotes immune tolerance by suppressing T-cell proliferation and clonal expansion.(4) IDO1 is expressed at baseline in the gastrointestinal tract and is more highly expressed in both human inflammatory bowel diseases (IBD) and animal models of colitis.(6–8) Experimental approaches from our laboratory suggest that IDO1 expression in the gut serves as a natural brake to the inflammatory response. Where pharmacologic inhibition of IDO exacerbates experimental colitis,(8) pharmacologic induction of IDO1 limits inflammation severity.(9)
Several studies have evaluated the possibility that increased expression of IDO1 in a disease state might lead to detectable systemic changes in quantities of the enzyme’s substrate (tryptophan) and initial catabolite (kynurenine).(10–13) Most of these studies showed that the disease state was associated with a modest reduction in serum tryptophan, increase in serum kynurenine, or a combination of the two. While it is known that IDO1 expression is elevated in intestinal tissues during active IBD, the impact of Crohn’s disease activity on serum markers of IDO1 activity has not yet been systematically evaluated. As a proof of principle for relevance of our investigations into IDO1’s role in chronic intestinal inflammation, we sought to examine whether such changes are detectable in human Crohn’s disease. In this study we compare the serum tryptophan and kynurenine levels in individuals with Crohns to a control population. Moreover, we examine how changes in serum levels reflective of the tryptophan catabolism pathway correlate with clinical disease severity, whereby establishing the potential of these measurements for use as a biomarker of Crohn’s disease activity.
MATERIALS AND METHODS
Subjects and samples
This study was approved by the Washington University Human Research Protection Office. Patients with Crohn’s disease and healthy controls were prospectively recruited into this study and into the Washington University Digestive Diseases Research Center Tissue Procurement Facility and Clinical Database (WU DDRCC TPF) from January 2008–December2010. Patients with Crohn’s disease were recruited in both inpatient and outpatient settings. At enrollment, complete clinical information including Montreal disease location (14), smoking status, gender, race and age of diagnosis, surgical and therapeutic history were acquired and entered into the database. Disease severity was assessed and categorized using the Physicians Global Assessment and confirmed as fitting the American College of Gastroenterology practice guidelines criteria.(15, 16) Patients in symptomatic remission while on corticosteroids were excluded. Blood samples were taken at enrollment, processed for serum isolation and stored at −80°C until analysis. For a subset of patients, clinical data and serum was collected at a second time point. Tissue samples were acquired either at surgery or endoscopy, immediately fixed in formalin and processed for paraffin embedding. C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) were considered when available. The healthy control cohort was composed of individuals voluntarily participating in the TPF who had no existing chronic intestinal or other inflammatory condition. Demographic information and study consent was collected the same as for patients.
Immunohistochemistry
Colon and small intestinal biopsy specimens from healthy controls and patients with active Crohn’s disease and were examined for IDO1 expression by immunohistochemistry. Xylene deparaffinizing and graded alcohol hydration steps were followed by high temperature citrate buffer antigen retrieval. The primary antibody was mouse anti-human IDO-1 (1:50 dilution, Cat# MAB5412, Chemicon International, Temecula, CA) and a biotinylated anti-mouse secondary antibody was used (1:250; Jackson ImmunoResearch, West Grove, PA). Isotype control mouse anti-human IgG was used (CBL600, Chemicon International) to assess staining specificity. Antibody signal was detected by incubation in streptavidin-POD (Roche, Indianapolis, IN), followed by 3,3′-diaminobenzidine tetrahydrochloride.
Measurement of tryptophan and kynurenine
High pressure liquid chromatography (HPLC) (Varian HPLX system) was used to determine serum tryptophan (Trp) and kynurenine (Kyn) levels. The protocol was adopted from published reports and confirmed for suitability in our environment.(17) After trichloroacetic acid deproteination, the filtered (0.20μm) serum sample was spiked with the reference standard 3-nitro-L-tyrosine (3-NT). 3-NT can be detected at both wavelengths of 280nm and 360nm aiding the identification of kynurenine and tryptophan retention time peaks. Standard curves were constructed for L-kynurenine, 3-NT, and L-tryptophan spanning a concentration range of over 2 orders of magnitude (0.5μM to 250μM). Linear regression plots were generated confirming that linearity was maintained over this concentration range. The linear slope analysis was used to determine the concentrations of L-kynurenine and L-tryptophan in biological samples(supplementary figure 1). Injections were separated using a Polaris C-18-A column (150 × 46mm i.d., 3.0μm particle size) with a 40min isocratic gradient from 100% 15mM acetic acid–sodium acetate buffer (pH4.0) to 73% buffer–acetonitrile, and a flow rate of 0.5mL/min. The average retention times were L-kynurenine (~14.3min), 3-NT (~17.0min), and L-tryptophan (~18.5min). A Varian Pro Star UV-V is detector was used to detect L-kynurenine at 360nm, and L-tryptophan at 280nm. These values were within the range of previous reports.(17, 18) Assay reliability and reproducibility was tested prior to and concurrent with patient/control sample analysis by comparing repeated measurements of the same sample. In four unique samples analyzed, no significant variability was identified with an average intra-sample variance of ± 4.1% (p=0.84).
Estimated IDO1 activity was determined by calculating the kynurenine/tryptophan (K/T) ratio, a technique shown to correlate with immune activation while averting potential bias generated by differences in dietary intake among individuals. (10, 11, 17, 19) K/T ratios are shown at values ×1000
Statistical Analysis and Data Presentation
All data analysis and graph assembly was completed using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA). Data are presented where dots represent single individuals and horizontal bars represent the group mean. Tabular data is presented as mean ± standard deviation unless otherwise stated. Statistical techniques included Fisher’s Exact test (patient characteristics), Student’s T-Test (paired or unpaired to compare two groups), 1-way ANOVA (difference across ≥3 groups), Wilcoxon Signed Ranks Test (intra-sample)and linear regression analysis(K/T vs. CRP or ESR). For all comparisons, a P-value ≤ 0.05 was considered statistically significant.
RESULTS
Patient Characteristics
11healthy controls and 25 patients with Crohn’s disease (CD) confirmed by endoscopy or histology were enrolled prospectively though the WU DDRCC TPF. Demographics and disease characteristics are presented in Table 1. Differences in age, gender, race or smoking status were not statistically different between groups. Serum was collected at two time points from 8 of the patients with CD to compare examined levels at two different states of disease activity. In patients with active disease medication use included immunomodulators (4), anti-TNFα (7), 5-ASA (2), corticosteroids (3 systemic; 2 budesonide) and antibiotics (3). Therapies among patients in remission included immunomodulators (2), anti-TNFα (2) and post-surgical (2).
Table I.
Patient Demographics
| Controls (n=11) | Patients (n=25) | |
|---|---|---|
| Age at Study Entry (range) | 31.3 (22–53) | 33.4 (19–62) |
| Age at Diagnosis | 28.0 (13–63) | |
| Gender M:F (%) | 4:7 (36:64) | 7:18 (28:72) |
| Race C:Asian:AA (%) | 7:2:2 (64:18:18) | 22:0:3 (82:0:17) |
| Montreal Classification Location N (%) | ||
| L1) Small Bowel Only | 10 (40) | |
| L2) Colon Only | 6 (24) | |
| L3) Colon and Small Bowel | 9 (36) | |
| Current Smoker, N (%) | 1 (9) | 6 (24) |
| History of Surgery, N (%) | 7 (28) | |
Indoleamine 2,3 Dioxygenase-1expressionin Crohns
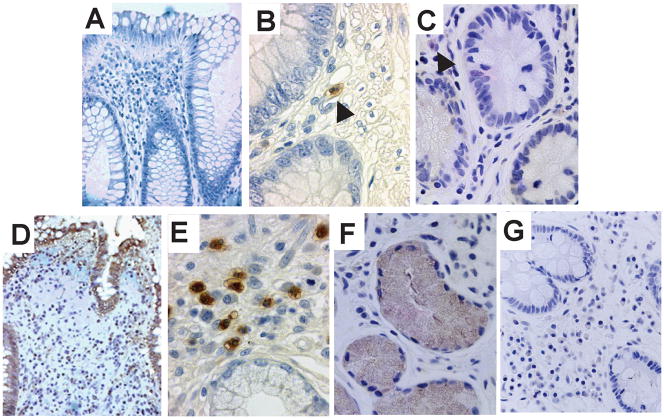
Immunohistochemistry revealed IDO1 staining to be faintly detectable in cells of the intestinal lamina propria in healthy controls (figure 1). In active CD however, elevated IDO1 is expression identified not only in cells of the lamina propria, but also prominently within the colonic and small intestinal epithelium.
Figure 1.
Immunohistochemical staining for IDO1 in human biopsy samples. Selected representative images are shown from healthy controls [A–C] and active Crohn’s disease [D–F]. Staining from control populations showed no significant IDO1 staining in the epithelium (A, 400×) and only faint staining (arrowheads) in lamina propria mononuclear cells of the colon (B, 630×) and small intestine (C, 630×). This was in contrast to the strong staining present in biopsies from Crohn’s disease patients in the colon (D, 400× and E, 630×) and small intestine (F, 630×). Isotype control of inflamed small intestine (G, 400×).
Kynurenine, Tryptophan and the K/T ratio as markers of Crohn’s Disease activity
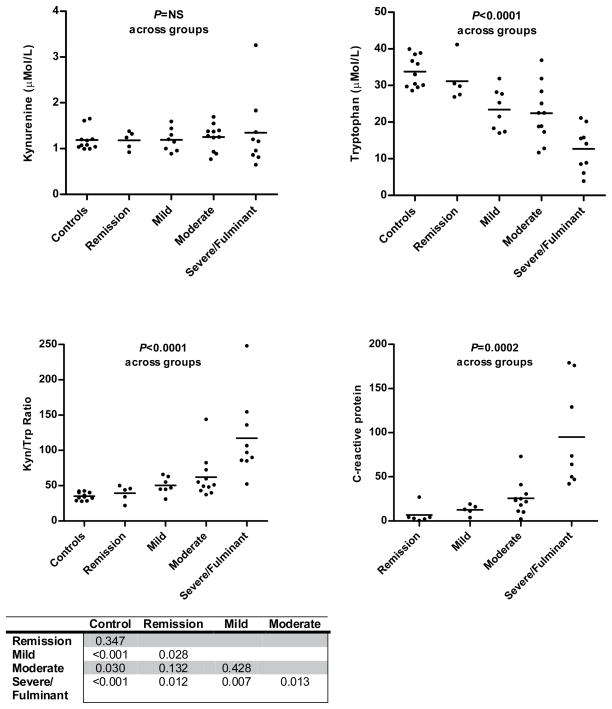
To examine the possibility that the elevatedIDO1 expression in active CD could be detected in serum we measured serum levels of the enzyme’s substrate, tryptophan, and its first metabolic product, kynurenine. These levels were then used to calculate the serum kynurenine/tryptophan (K/T) ratio, a demonstrated functional estimate of IDO1 activity. These levels as well as C-reactive protein (CRP) were measured for CD patients and plotted based on physician global assessment of disease activity along with levels for the 11 healthy controls (Figure 2). While kynurenine levels did not significantly differ between controls and across groups, tryptophan levels were significantly lower in patients with active CD compared to controls. Furthermore, tryptophan levels were more depressed as disease activity increased. In severe/fulminant CD mean tryptophan levels were 12.7 μM compared to 31.2μM CD in remission or 33.8 μM for healthy controls. The changes in tryptophan levels largely accounted for the significant rise in K/T ratio along with disease severity assessment. The mean K/T ratio was highest in CD patients with severe or fulminant disease activity, 117.4; a ratio three times that of CD patients in remission (39.2) or healthy controls (35.3). Individuals with moderate or severe CD involving the colon had higher K/T ratio than for those with only small bowel involvement (Montreal L2 or L3 vs. L1; Avg K/T = 100.4 vs.50.6, P=0.03).
Figure 2.
Serum IDO substrate/metabolite stratified by Global Physician Assessment of Disease Activity. P-values across patient groups were calculated by 1-way ANOVA. Table shows P-value for K/T ratio difference between groups calculated by unpaired Student’s T-test.
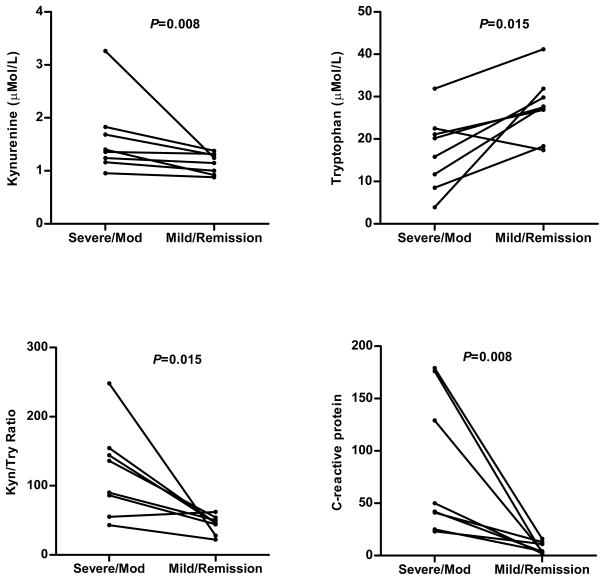
To further examine whether the K/T ratio or its component amino acids track with CD activity we measured levels at two different time points and stages of disease activity in the same patient. One assessment was made when the patient had at least moderately active disease and the other was made when they were in steroid free remission or with only mild disease activity and off steroids. The range of time between assessments was 3–12 months. When an individual served as his/her own internal control, the differences in kynurenine levels became significant as well as the tryptophan levels and K/T ratio (Figure 3). Overall the changes followed with the predicted decrease in CRP. One of the patient’s K/T ratio went up when going from moderate CD activity to remission (55 to 62); however despite the remission of symptoms, her CRP was elevated (10 mg/L) and she presented with a flare one month later. Moreover, when considering all patients with CD, linear regression analysis showed the K/T ratio to positively and significantly correlate with acute phase proteins considered relevant to Crohn’s disease activity, CRP and ESR (Figure 4).
Figure 3.
Individuals’ serum levels of IDO1 substrate/metabolite change with Crohn’s disease severity. P-value calculated by paired Student’s T-test.
Figure 4.
Kynurenine/Tryptophan ratio correlates with inflammation biomarkers in Crohn’s disease. Statistical comparison performed by linear regression analysis.
DISCUSSION
Indoleamine 2,3 dioxygenase-1 is a metabolically active enzyme with immunomodulatory properties positioned at the interface between innate and adaptive immunity. Expression of IDO1 is induced by cytokines overexpressed in Crohn’s Disease. In this study we demonstrate that in active Crohns, elevated gut IDO1 expression is associated with significant depression in serum levels of its substrate, tryptophan. The serum Kynurenine/Tryptophan (K/T) ratio, a marker of IDO1 activity which minimizes the influence of dietary variation, increased with disease severity assessment and positively correlated with both CRP and ESR. Measurement of serum K/T ratios may serve a reasonable objective marker of gut mucosal immune activation and surrogate biomarker for Crohn’s Disease activity. Furthermore, the recognition that active Crohns can alter serum levels of tryptophan and kynurenine within individuals may have implications for disease associated extraintestinal morbidities.
Study into the importance of IDO1 enzyme activity rapidly expanded after the seminal observation of its critical role in mediating immune tolerance at the maternal-fetal interface.(20) The expression of IDO1 by professional antigen presenting cells(APC), macrophages and plasmacytoid dendritic cells exerts potent suppressive effects on T-cell proliferation.(4, 21, 22) Tolerance at the maternal-fetal interface parallels that of the gastrointestinal tract, as the context in which foreign antigens are presented dictates the tolerogenic response. In active CD we found increased expression of IDO1 not only in cells of the lamina propria, but also prominently in epithelium. IDO1 has known antimicrobial properties which may be particularly important in light of the epithelial barrier dysfunction associated with IBD.(23, 24) While it has not been specifically evaluated as such, it is possible that IDO1 expression by LP APCs would promote immune tolerance by suppressing T-cell responses while IDO1 activity in epithelial cells serves to limit microbial invasion; together these IDO1 expressing cell types would function to limit ongoing inflammation. Though epithelial IDO1 expression has not been uniformly described,(7)our findings in CD support those which identified high expression of IDO1 in epithelial cells flanking intestinal ulceration and in active disease.(25, 26)Differences in antibody selection or antigen retrieval methods may account for such differences.
The finding of altered serum levels of tryptophan and kynurenine attributable to IDO1 activity has been described in other inflammation associated disease states.(10–13, 18, 27) Table 2 summarizes these reports and shows the relative fold difference in these levels compared to the control population reported in the same study. Though variance exists in the reported absolute values for control subjects among studies, most disorders are associated with approximately a 25% decrease in serum tryptophan and 50% increase in the K/T ratio. In the current study, compared to our own controls, patients with severely active Crohn’s disease had a remarkable 73% decrease in tryptophan and >3.3 fold increase in K/T ratio(Table 2 bottom row). The serum K/T ratio is recognized as the best marker of IDO1 activity by immune activation limiting the variability seen when tryptophan is used alone (10); thus these profound changes in serum K/T ratio may be attributable to the gut’s large surface are a and the presence of multiple IDO1 expressing cell types during active CD. However, reduced protein intake or absorption in patients with high disease CD activity may also contribute.(28) Only individuals who died of septic shock have been found to have higher K/T ratios than severely active CD patients. While the study current study was underpowered to assess, it is also possible that medication-effects may impact K/T ratios independent of inflammation severity.
Table II.
Comparison of serum tryptophan and kynurenine levels in normal and disease states*
| Patient Population | Tryptophan [μmol/L] (fold difference from healthy controls) | Kynurenine [μmol/L] (fold difference from healthy controls) | K/T ratio [×1000] (fold difference from healthy controls) |
|---|---|---|---|
| Healthy Controls17 | 73 ± 14.9 | 1.92 ± 0.58 | 26.9 ± 8.1 |
| Systemic Lupus Erythematosus13 | 53.9 ± 8.2 (0.77X) | 2.45 ± 0.7 (1.36X) | 43 ± 15 (1.59X) |
| Rheumatoid Arthritis11 | 58 ± 19.3 (0.79X) | 2.2 ±.82 (1.14X) | 37.9 (1.41X) |
| Primary Sjogren’s Syndrome33 | 75 ± 8 (0.94X) | 2.41 ± 0.7 (1.29X) | 34 ± 9 (1.41X) |
| Celiac Disease18 | 35.8 ± 1.3 (0.73X) | 4.2 ± 0.27 (1.62X) | 115 ± 10.1 (1.77X) |
| Septic Shock26 | |||
| Deceased | 46.5 (38–59) | 8.76 (5.1–12.26) | 193.7 (124–253) |
| Survivors | 51.1 (38–64) | 4.27 (2.9–6.7) | 82.4 (51–138) |
| Severe/Fulminant Crohn’s | 12.7 ± 2.0 (0.37X) | 1.34 ± 0.26 (1.13X) | 117.4 ± 19.1 (3.33X) |
The comparison was generated from published literature as cited. The fold difference from healthy controls (bold) was relative to levels to the control populations from the same report. No control population was reported for the study on septic shock so a fold difference is not listed. Values are shown as mean ± standard deviation for all studies except references 18 and 26 (SEM and median quartiles, respectively).
Several studies evaluating IDO1 activation found patient populations with inflammatory diseases to have significant elevations in serum kynurenine compared to control population values. Within individuals, we found serum kynurenine levels to be elevated during times of greater disease severity (figure 3); however, as a group, levels in Crohn’s patients were not significantly higher than the control population (figure 2). In active Crohn’s disease significant reduction in substrate (tryptophan) availability due to IDO1 catabolism and nutritional depletion during states of chronic intestinal inflammation, combined with ongoing kynurenine metabolism, may represent a possible explanation for this finding.
The correlation between gut inflammation and activation of IDO1 mediated tryptophan catabolism may also have implication to the mood disorders associated with active IBD. The neurotransmitter serotonin is derived from tryptophan via a short biochemical pathway. Within the central nervous system serotonin must be synthesized from tryptophan or the intermediary 5-hydroxy-tryptophan since the blood brain barrier excludes serotonin transport. Some investigations have found tryptophan levels to be low in acutely depressed patients and acute tryptophan depletion can lead to depressive relapse.(29, 30) New onset or worsening anxiety and depression, have been temporally linked to IBD diagnosis and disease flares.(31) Mood disorders are a well recognized side effect of hepatitis C directed immunotherapy with recombinant cytokines known to stimulate IDO1 activity (IFNα).(32) In IFNα treated patients it is suggested that elevation in CSF tryptophan metabolites have a greater impact on depressive symptoms than relative lack of tryptophan itself, though the depression in serum tryptophan in these patients was modest compared to that found in active CD.(33) Whether the more robust decrease of serum tryptophan levels observed in active CD may contribute to mood disorders remains to be causally demonstrated.
Herein we have shown that active Crohn’s disease is associated with expression of intestinal IDO1 and depression of serum tryptophan. Further investigation into IDO1 tryptophan catabolism pathway and its impact on both intestinal inflammation and extraintestinal morbidities of Crohn’s disease activity is warranted. The surrogate marker of IDO1 activity, serum K/T ratio, correlates positively with level of disease severity and recognized inflammatory markers. With confirmative study, measurement of the serum K/T ratio has the potential to be useful in both therapeutic trials and clinical practice trials as a novel biomarker of Crohn’s disease activity.
Supplementary Material
Linear regression plots for Tryptophan and Kynurenine assay by HPLC. Graphs show standards based plots for L-kynurenine and L-tryptophan spanning a concentration range of over 2 orders of magnitude shown in complete (left) and with an expanded lower dose range (right). Best fit slope: Tryptophan (43,774); Kynurenine (36,123).
Acknowledgments
Grant Funding: This work was supported in part by a Crohn’s and Colitis Foundation of America Career Development Award (MAC), National Institutes of Health Grants DK075713(WFS), DK089016 and L30-RR030244 (MAC), and P30-DK52574 (Washington University Digestive Diseases Research Core). NKG was the recipient of a WUSM Dept of Internal Medicine Mentors in Medicine grant. AIT was a Howard Hughes Medical Institute Medical Research Training Fellow.
References
- 1.Sartor RB. Genetics and environmental interactions shape the intestinal microbiome to promote inflammatory bowel disease versus mucosal homeostasis. Gastroenterology. 2010;139:1816–1819. doi: 10.1053/j.gastro.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 2.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 3.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 5.Penberthy WT. Pharmacological targeting of IDO-mediated tolerance for treating autoimmune disease. Curr Drug Metab. 2007;8:245–266. doi: 10.2174/138920007780362545. [DOI] [PubMed] [Google Scholar]
- 6.Dieckgraefe BK, Stenson WF, Korzenik JR, et al. Analysis of mucosal gene expression in inflammatory bowel disease by parallel oligonucleotide arrays. Physiol Genomics. 2000;4:1–11. doi: 10.1152/physiolgenomics.2000.4.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Wolf AM, Wolf D, Rumpold H, et al. Overexpression of indoleamine 2,3-dioxygenase in human inflammatory bowel disease. Clin Immunol. 2004;113:47–55. doi: 10.1016/j.clim.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Gurtner GJ, Newberry RD, Schloemann SR, et al. Inhibition of indoleamine 2,3-dioxygenase augments trinitrobenzene sulfonic acid colitis in mice. Gastroenterology. 2003;125:1762–1773. doi: 10.1053/j.gastro.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 9.Ciorba MA, Bettonville EE, McDonald KG, et al. Induction of IDO-1 by immunostimulatory DNA limits severity of experimental colitis. J Immunol. 2010;184:3907–3916. doi: 10.4049/jimmunol.0900291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schrocksnadel K, Wirleitner B, Winkler C, et al. Monitoring tryptophan metabolism in chronic immune activation. Clin Chim Acta. 2006;364:82–90. doi: 10.1016/j.cca.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Schroecksnadel K, Winkler C, Duftner C, et al. Tryptophan degradation increases with stage in patients with rheumatoid arthritis. Clin Rheumatol. 2006;25:334–337. doi: 10.1007/s10067-005-0056-6. [DOI] [PubMed] [Google Scholar]
- 12.Widner B, Leblhuber F, Walli J, et al. Tryptophan degradation and immune activation in Alzheimer’s disease. J Neural Transm. 2000;107:343–353. doi: 10.1007/s007020050029. [DOI] [PubMed] [Google Scholar]
- 13.Widner B, Sepp N, Kowald E, et al. Enhanced tryptophan degradation in systemic lupus erythematosus. Immunobiology. 2000;201:621–630. doi: 10.1016/S0171-2985(00)80079-0. [DOI] [PubMed] [Google Scholar]
- 14.Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19:9A–13A. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 15.Hanauer SB, Sandborn W. Management of Crohn’s disease in adults. Am J Gastroenterol. 2001;96:635–643. doi: 10.1111/j.1572-0241.2001.3671_c.x. [DOI] [PubMed] [Google Scholar]
- 16.Lichtenstein GR, Hanauer SB, Sandborn WJ. Management of Crohn’s disease in adults. Am J Gastroenterol. 2009;104:465–483. doi: 10.1038/ajg.2008.168. quiz 464, 484. [DOI] [PubMed] [Google Scholar]
- 17.Widner B, Werner ER, Schennach H, et al. Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clin Chem. 1997;43:2424–2426. [PubMed] [Google Scholar]
- 18.Torres MI, Lopez-Casado MA, Lorite P, et al. Tryptophan metabolism and indoleamine 2,3-dioxygenase expression in coeliac disease. Clin Exp Immunol. 2007;148:419–424. doi: 10.1111/j.1365-2249.2007.03365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brandacher G, Winkler C, Aigner F, et al. Bariatric surgery cannot prevent tryptophan depletion due to chronic immune activation in morbidly obese patients. Obes Surg. 2006;16:541–548. doi: 10.1381/096089206776945066. [DOI] [PubMed] [Google Scholar]
- 20.Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 21.Mellor AL, Keskin DB, Johnson T, et al. Cells expressing indoleamine 2,3-dioxygenase inhibit T cell responses. J Immunol. 2002;168:3771–3776. doi: 10.4049/jimmunol.168.8.3771. [DOI] [PubMed] [Google Scholar]
- 22.Mellor AL, Baban B, Chandler PR, et al. Cutting edge: CpG oligonucleotides induce splenic CD19+ dendritic cells to acquire potent indoleamine 2,3-dioxygenase-dependent T cell regulatory functions via IFN Type 1 signaling. J Immunol. 2005;175:5601–5605. doi: 10.4049/jimmunol.175.9.5601. [DOI] [PubMed] [Google Scholar]
- 23.MacKenzie CR, Heseler K, Muller A, et al. Role of indoleamine 2,3-dioxygenase in antimicrobial defence and immuno-regulation: tryptophan depletion versus production of toxic kynurenines. Curr Drug Metab. 2007;8:237–244. doi: 10.2174/138920007780362518. [DOI] [PubMed] [Google Scholar]
- 24.Zeissig S, Burgel N, Gunzel D, et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barcelo-Batllori S, Andre M, Servis C, et al. Proteomic analysis of cytokine induced proteins in human intestinal epithelial cells: implications for inflammatory bowel diseases. Proteomics. 2002;2:551–560. doi: 10.1002/1615-9861(200205)2:5<551::AID-PROT551>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 26.Ferdinande L, Demetter P, Perez-Novo C, et al. Inflamed intestinal mucosa features a specific epithelial expression pattern of indoleamine 2,3-dioxygenase. Int J Immunopathol Pharmacol. 2008;21:289–295. doi: 10.1177/039463200802100205. [DOI] [PubMed] [Google Scholar]
- 27.Huttunen R, Syrjanen J, Aittoniemi J, et al. High activity of indoleamine 2,3 dioxygenase enzyme predicts disease severity and case fatality in bacteremic patients. Shock. 2010;33:149–154. doi: 10.1097/SHK.0b013e3181ad3195. [DOI] [PubMed] [Google Scholar]
- 28.Beeken WL. Serum tryptophan in Crohn’s disease. Scand J Gastroenterol. 1976;11:735–740. [PubMed] [Google Scholar]
- 29.DeMyer MK, Shea PA, Hendrie HC, et al. Plasma tryptophan and five other amino acids in depressed and normal subjects. Arch Gen Psychiatry. 1981;38:642–646. doi: 10.1001/archpsyc.1981.01780310042003. [DOI] [PubMed] [Google Scholar]
- 30.Booij L, van der Does AJ, Haffmans PM, et al. Acute tryptophan depletion as a model of depressive relapse: behavioural specificity and ethical considerations. Br J Psychiatry. 2005;187:148–154. doi: 10.1192/bjp.187.2.148. [DOI] [PubMed] [Google Scholar]
- 31.Kurina LM, Goldacre MJ, Yeates D, et al. Depression and anxiety in people with inflammatory bowel disease. J Epidemiol Community Health. 2001;55:716–720. doi: 10.1136/jech.55.10.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asnis GM, De La Garza R., 2nd Interferon-induced depression in chronic hepatitis C: a review of its prevalence, risk factors, biology, and treatment approaches. J Clin Gastroenterol. 2006;40:322–335. doi: 10.1097/01.mcg.0000210099.36500.fe. [DOI] [PubMed] [Google Scholar]
- 33.Raison CL, Dantzer R, Kelley KW, et al. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry. 2010;15:393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Linear regression plots for Tryptophan and Kynurenine assay by HPLC. Graphs show standards based plots for L-kynurenine and L-tryptophan spanning a concentration range of over 2 orders of magnitude shown in complete (left) and with an expanded lower dose range (right). Best fit slope: Tryptophan (43,774); Kynurenine (36,123).