Abstract
Objective
To provide the first empirical investigation of the association between smoking cessation and indices of physiological and subjective sexual health in men.
Subjects and methods
Male smokers, irrespective of erectile dysfunction status, who were motivated to stop smoking (‘quitters’), were enrolled in an 8-week smoking cessation programme involving a nicotine transdermal patch treatment and adjunctive counselling.
Participants were assessed at baseline (while smoking regularly), at mid-treatment (while using a high-dose nicotine transdermal patch), and at a 4-week post-cessation follow-up.
Physiological (circumferential change via penile plethysmography) and subjective sexual arousal indices (continuous self-report), as well as self-reported sexual functioning were assessed at each visit.
Results
Intent-to-treat analyses indicated that, at follow-up, successful quitters (n = 20), compared with those who relapsed (n = 45), showed enhanced erectile tumescence responses, and faster onset to reach maximum subjective sexual arousal.
Although successful quitters displayed across-session enhancements in sexual function, they did not show a differential improvement compared with unsuccessful quitters.
Conclusion
Smoking cessation significantly enhances both physiological and self-reported indices of sexual health in long-term male smokers, irrespective of baseline erectile impairment.
It is hoped that these results may serve as a novel means to motivate men to stop smoking.
Keywords: sexual arousal, erectile dysfunction, smoking, cessation
Introduction
Tobacco use constitutes the single most preventable cause of disease and death in the world today [1], and is responsible for enormous health and economic burdens. In addition to introducing cardiovascular [2] and respiratory diseases [3], as well as many types of cancer [4], smoking has been associated with elevated rates of erectile dysfunction (ED). ED is considered a significant public health problem and is estimated to affect 34 million men in the USA and > 150 million men worldwide [5, 6]. Large cross-sectional [7–11] and longitudinal [12] epidemiological studies indicate that chronic smokers are about 1.5 to 2-times as likely as nonsmokers to report ED, even after controlling for age and confounding cardiovascular risk factors.
Considering the robust evidence indicating the link between cigarette smoking and ED, an intervention with the broadest health impact is smoking cessation. To the author’s knowledge, only two studies have investigated the effects of stopping smoking on sexual responding. Sighinolfi et al. [13] tested 20 heavy smokers with ED and these participants showed a significant improvement in penile blood flow 24 to 36 h after smoking discontinuation. Similarly, Guay et al. [14] measured nocturnal penile tumescence and rigidity in 10 male smokers. Results showed significant improvement 24 h after smoking cessation for both of these indices. Additionally, four men were assessed 1 month later while adhering to a daily21-mg nicotine transdermal patch regimen, and results indicated a trend for continued improvement.
These studies provide an excellent foundation for examining the putative relationship between smoking and sexual health; however, they raise several questions that remain unanswered. First, these studies have only assessed individuals with clinically diagnosed ED, and therefore it remains unclear how smoking cessation affects sexual arousal responses in nonclinical individuals. Second, although improvements in erectile capacity were shown in these studies, it is unclear how these statistically significant improvements translate to clinically significant enhancements. Incorporating a ‘gold-standard’ measure of sexual functioning would help address this issue and would be a valid way of assessing changes in sexual health.
Although stopping smoking substantially enhances many aspects of health, the positive health benefits of smoking cessation are not sufficient enough for many smokers to consider quitting. Therefore, the primary aim of the present study was to examine whether stopping smoking was associated with sexual health improvements, with the hope that the results could serve as a novel means to influence men to stop smoking. Sexual arousal, measured both physiologically and subjectively, as well a sexual functioning, were assessed at three time intervals: (i) at baseline, while participants were regularly smoking; (ii) at mid-treatment, while using a 21-mg nicotine transdermal patch; and (iii) at follow-up, 4 weeks after nicotine patch cessation.
Subjects and methods
Male participants, who were motivated to stop smoking (quitters), were recruited through online and community advertisements between 2008 and 2010. All prospective participants were screened via telephone for inclusion/exclusion criteria. Participants were eligible for inclusion if they were between the ages of 23 and 60 years, smoked at least 15 cigarettes/day for a minimum of 5 consecutive years, had no self-reported sexual dysfunction before smoking onset, and were sexually active. Exclusion criteria were as follows: (i) use of medications known or thought to affect sexual or vascular functioning, or that are contraindicated by the nicotine patch; (ii) use of non-nicotine smoking cessation medications at time of enrollment (bupropion, varenicline); (iii) medical conditions known to affect sexual functioning, or that could make nicotine administration unsafe (e.g., recent myocardial infarction, stroke, heart arrhythmias, angina); (iv) uncontrolled hypotension or hypertension; (v) history of severe drug or alcohol abuse during the past 12 months (≥16 points on the Alcohol Use Disorders Identification Test (AUDIT) [15] and ≥ 6 on the Drug Abuse Screening Test (DAST-10) [16]); and (vi) self-report of a sexually transmitted infection.
Intervention
Participants received an 8-week nicotine transdermal patch treatment (Habitrol®, Novartis Consumer Health Inc., Summit, NJ, USA) administered in a step-down fashion (21 mg, weeks 1–4; 14 mg, weeks 5–6; 7 mg, weeks 7–8). All participants were monitored weekly for patch compliance, as well as for intra- and post-treatment tobacco and nicotine replacement therapy use. Participants also received adjunctive counselling, which was based upon the tobacco use and dependence clinical practice guidelines [17] and the protocols of the National Cancer Institute [18]. Counselling occurred during a participant’s laboratory visit and lasted 45 min. Participants also received a minimum of ten 10-min weekly telephone counselling sessions.
Procedure
During the initial telephone screening, a ‘quit’ date was set, which corresponded to the day after their first experimental session. All men entered the laboratory at their preferred nicotine level, but were not allowed to smoke during any experimental session. Participants were tested individually in a private, internally locked testing room. After providing written informed consent, participants completed a battery of self-report measures assessing demographic variables, mood (via the Positive and Negative Affect Schedule (PANAS) [19]), and several smoking characteristics. All participants provided saliva samples and they were spuriously informed that these samples would be assayed for salivary nicotine content. This was to help ensure valid self-reporting of cigarette consumption. Participants then fit the penile plethysmograph themselves and viewed an erotic film. During film presentation, participants were asked to continuously monitor their level of subjective sexual arousal using a hand-controlled device. Immediately after the film presentation, participants removed the plethysmograph and they were given 28 high-dose patches, and were asked to start nicotine replacement therapy the following morning.
The procedures of visits 2 and 3 were identical to the first session. The second visit occurred during week 4 of patch treatment. At the completion of the session, participants were given the remainder of the patch regimen. At the completion of the third laboratory visit (4 weeks after completing the 8-week patch intervention), participants were provided $30, and were mailed a detailed report of their laboratory assessments. The protocol was approved by the XXX Institutional Review Board.
Primary outcome measures
Genital arousal was assessed via penile circumferential change using a mercury-in-rubber strain gauge (Hokanson, Inc., Bellevue, WA, USA). Penile tumescence is considered the most sensitive index of sexual arousal and the most reliable measure of physiological response [20]. The signal was sampled at a rate of 80 samples/s, bandpass filtered (to 0.5 Hz), and digitized (40 Hz). Indices of physiological sexual arousal included within-session percentage change in penile tumescence, and rate of onset to reach maximum tumescence (slope).
Measures of self-reported sexual arousal were continuously measured using a hand-controlled device [21], which consisted of a mouse mounted on a wooden track divided into seven equally spaced intervals, where zero indicated neutral, and 1–7 reflected increasingly higher levels of feeling sexually aroused. A software program written in MatLab (The MathWorks, Inc, Natick, MA, USA) detected the position of the pointer with respect to the y-axis of the computer’s monitor, and the signal was low-pass filtered (to 0.5 Hz), and digitized (40 Hz). Indices of subjective sexual arousal included within-session percentage change, and rate of onset to reach maximum arousal (slope).
Sexual function was assessed with the International Index of Erectile Function (IIEF) [22], which is the most widely used psychometric index of self-reported erectile function. The IIEF is a 15-item measure assessing five-factor analytically derived areas of male sexual functioning including erectile function, orgasmic function, sexual desire, intercourse satisfaction, and overall satisfaction. Participants reporting an IIEF erectile function score of ≤ 25 were considered to have ED of a clinical nature [23].
Data reduction
Initial physiological and continuous subjective sexual arousal scores for each session were computed by averaging all data collected during the neutral and erotic film segments. The percentage change (from the neutral to erotic film presentations) was calculated for both physiological and continuous subjective sexual arousal. Efficacy of smoking cessation was evaluated with the use of a 1-week point prevalence abstinence rate at week 12 (4 weeks after patch discontinuation). Participants reporting zero cigarettes during the prior 7 days at follow-up were considered successful quitters, whereas individuals reporting ≥1 cigarettes were classified as unsuccessful quitters (relapsers).
The required sample size was calculated to provide at least 80% power with a two-tailed α set at 0.05. Based upon these parameters, a priori power analyses suggested that 18 participants were necessary in each group at each time point to detect across-session differences, and a total sample size of 54 participants was necessary to adequately assess between-group differences. To be conservative, 65 participants were enrolled.
All analyses were conducted on an intent-to-treat basis using full information maximum likelihood estimation [24]. Missing values for each primary outcome variable were successively estimated using several baseline characteristics, discontinuation status, as well as each respective baseline primary outcome value. Additionally, total number of cigarettes smoked throughout the study, total patch use (days), and number of cigarettes smoked during week 12 were estimated in a similar fashion. Group status (successful quitter, relapser) was based on these imputed values. General linear modelling (in the form of 3 × 2 repeated measures analysis of covariance [ANCOVA] models) was used as the primary analytical approach to compare successful and unsuccessful quitters at each time point for all outcome measures. For these analyses, the interaction effect of group × time was of primary interest. In cases where the overall interaction term was statistically significant, planned comparison F-tests for adjusted cell means were used to assess between-group differences at each time point. Pack years, total cigarettes smoked throughout enrolment, baseline erectile functioning, baseline drinking severity, and smoking status at visit 2 (smoke-free, relapsed) were entered as covariates in all analyses. Differences in baseline characteristics between treatment completers and those that discontinued treatment, as well as between successful quitters and relapsers, were compared with t tests or Pearson chi-squared tests, as appropriate. Fisher’s exact tests were used in cases with low cell counts. All analyses were performed using SPSS statistical software version 17.0 (SPSS Inc., Chicago, IL, USA).
Results
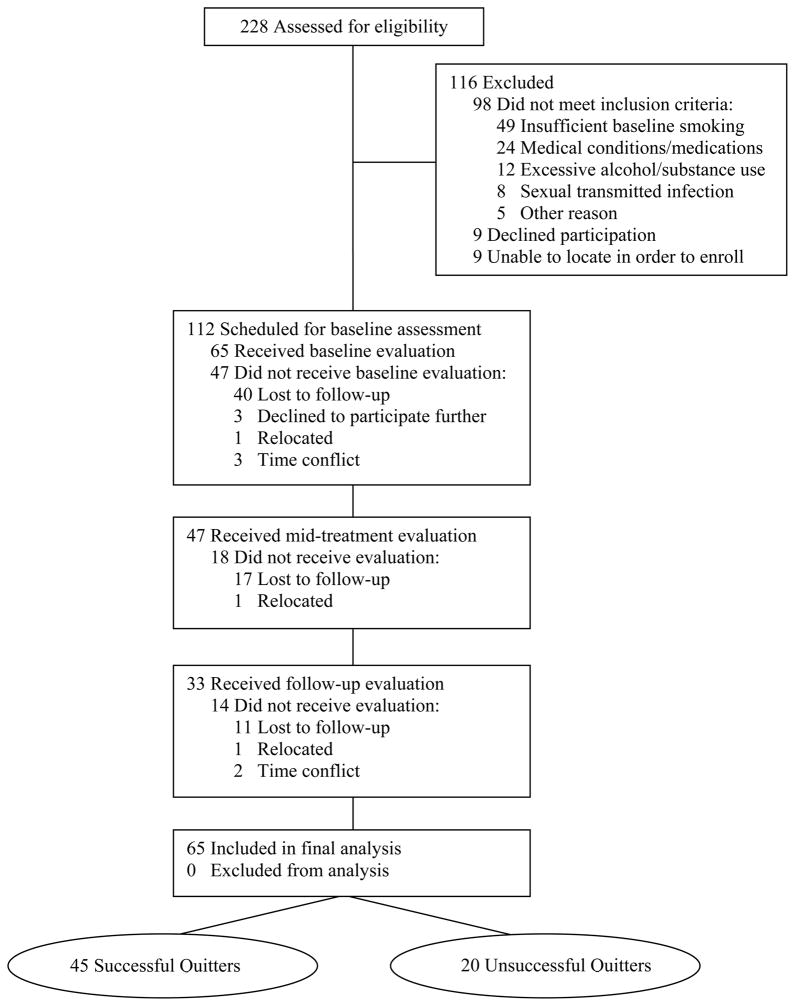
In all, 228 men completed the initial telephone screening. Of these individuals, 116 were ineligible, nine declined to participate, and nine were unable to be contacted further to enrol. Of the 112 men who met inclusion criteria, 47 did not attend their initial evaluation, resulting in a final sample of 65 participants. The discontinuation rate was 28% after the initial visit, and 49% after the second visit, resulting in 51% who completed the study (Figure 1). Study completers vs those that discontinued treatment differed only with respect to education and race. Specifically, those who withdrew reported less years of education (P = 0.02; d = 0.60), and were more likely to be non-White (P = 0.01; ϕ = 0.32) (Table 1). Successful and unsuccessful quitters did not differ significantly on any of the socio-demographic or smoking characteristics. The only variable on which groups differed was baseline drinking severity, with unsuccessful quitters reporting significantly less alcohol use compared with successful quitters (P < 0.01; d = 0.40). For treatment efficacy, 35% (23/65) and 69% (45/65) of men were considered relapsed at mid-treatment and follow-up, respectively. Successful quitters reported smoking less cigarettes (P < 0.001; d = 0.80) and reported a more days using the patch (P = 0.05; d = 0.27) compared with unsuccessful quitters.
Figure 1.
Participant flow.
Table 1.
Demographic characteristics of the participant sample
| Characteristic | Successful quitters (n = 20) | Unsuccessful quitters (n = 45) | P | ||
|---|---|---|---|---|---|
| Mean (SD) | n (%) | Mean (SD) | n (%) | ||
| Demographics | |||||
| Age, yearsa | 34.9 (9.65) | 40.4 (10.9) | 0.06 | ||
| Education, yearsb | 15.5 (2.04) | 14.4 (2.25) | 0.07 | ||
| Ethnicity: | |||||
| White | 19 (95.0) | 37 (82.2) | |||
| African-American | 0 | 3 (6.7) | |||
| Latino/a | 0 | 3 (6.7) | 0.19 | ||
| Asian | 0 | 2 (4.4) | |||
| Other | 1 (5.0) | 0 | |||
| Income, USA$: | |||||
| < 25 000 | 6 (30.0) | 7 (15.6) | |||
| 25 000–49 999 | 6 (30.0) | 18 (40.0) | |||
| 50 000–74 999 | 6 (30.0) | 9 (20.0) | 0.45 | ||
| 75 000–99 000 | 2 (10.0) | 6 (13.3) | |||
| ≥100 000 | 0 (0.0) | 5 (11.1) | |||
| Marital status: | |||||
| Single | 15 (75.0) | 24 (53.3) | |||
| Married/cohabiting | 5 (25.0) | 21 (46.7) | 0.53 | ||
| Substance use: | 0.80 | ||||
| Alcohol usec | 6.9 (3.13) | 4.1 (3.18) | <0.01 | ||
| Drug used | 0.7 (0.98) | 0.6 (0.78) | 0.66 | ||
| Smoking characteristics: | |||||
| Age started smoking, yearsa | 16.1 (2.37) | 16.8 (5.3) | 0.57 | ||
| Smoking duration, yearsa | 17.9 (10.03) | 22.6 (11.4) | 0.12 | ||
| Pack years | 18.2 (14.02) | 23.1 (16.9) | 0.26 | ||
| Smoking frequency, cigerettes/daya | 19.0 (5.09) | 22.8 (8.66) | 0.07 | ||
| Nicotine dependencea,e | 5.4 (1.84) | 5.5 (2.04) | 0.78 | ||
| Quit attempts, na | 3.4 (2.78) | 3.5 (2.66) | 0.81 | ||
| Sexual functioningf: | |||||
| Erectile function | 27.0 (4.75) | 26.5 (5.19) | 0.72 | ||
| Orgasmic function | 8.7 (1.87) | 9.0 (1.42) | 0.45 | ||
| Sexual desire | 6.8 (1.52) | 7.4 (1.74) | 0.13 | ||
| Intercourse satisfaction | 11.9 (2.13) | 11.6 (3.08) | 0.67 | ||
| Overall satisfaction | 7.9 (1.76) | 7.6 (1.95) | 0.63 | ||
| EDg | 4 (20.0) | 15 (33.3) | 0.28 | ||
Data were missing for one participant;
Data were missing for three participants;
Assessed with the Alcohol Use Disorders Identification Test. Possible score range from 0 to 40, with higher scores reflecting increasing levels of problematic drinking;
Assessed with the Drug Abuse Screening Test, 10-item. Possible score range from 0 to 10, with higher scores indicating greater substance abuse severity;
As per the Fagerström Test of Nicotine Dependence [30]. Possible score range from 0 to 10, with higher scores indicating greater dependency to nicotine;
Assessed with the IIEF;
According to the IIEF erectile functioning threshold score of 25.
Analyses of physiological sexual arousal
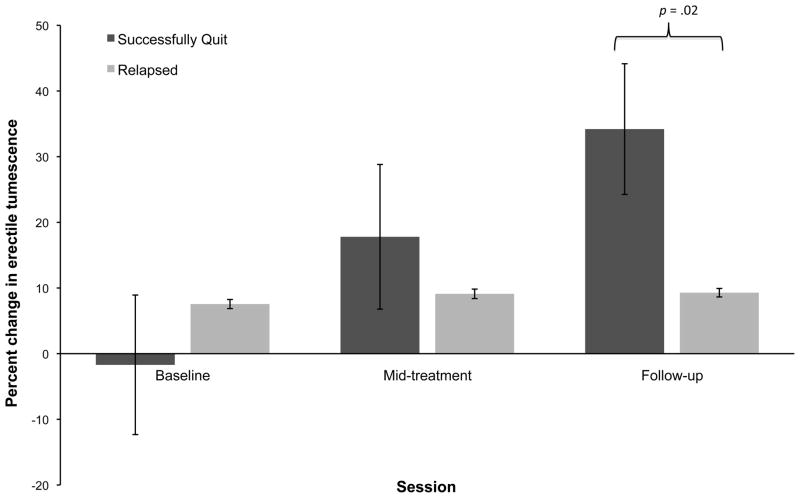
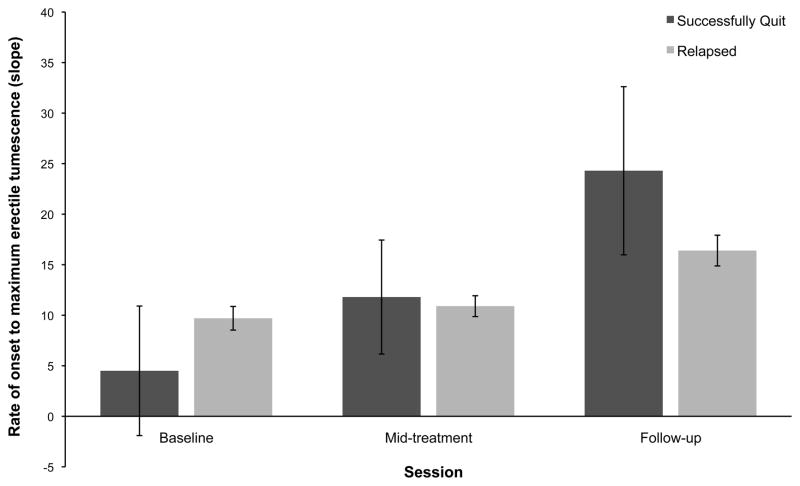
Results of the 2 (group: successful vs unsuccessful quitter) × 3 (time: baseline, mid-treatment, follow-up) repeated measures ANCOVA showed a significant group × time interaction effect for both percentage change in penile tumescence [F(4124) = 8.93, P < 0.001, η2 = 0.22] and rate of onset to reach maximum erection [F(4124) = 4.74, P = 0.001, η2 = 0.13]. Post hoc tests of between-subjects contrasts showed significantly greater within-session percentage increases in erectile tumescence among successful quitters compared with unsuccessful quitters at follow-up (P = 0.02, d = 0.31). There were no between-group differences at mid-treatment (Figure 2). For erectile onset, both groups had significant within-group improvements across time, with both groups showing increased erectile onset at follow-up compared with baseline (successful quitters: P = 0.04; d = 0.50; unsuccessful quitters: P < 0.001, d = 0.54). There were no differential enhancements across time as a function of group status, and therefore groups did not differ from one another at mid-treatment or follow-up (Figure 3).
Figure 2.
The mean within-session percentage changes in erectile tumescence across time for successful and unsuccessful quitters. Error bars represent standard errors of the means. The means have been adjusted for pack years, total cigarette consumption throughout enrolment, baseline erectile functioning, and smoking status at mid-treatment.
Figure 3.
Across-session changes in rate of onset to reach maximum erectile tumescence for successful and unsuccessful quitters. Error bars represent standard errors of the means. Means have been adjusted for pack years, total cigarette consumption throughout enrolment, baseline erectile functioning, and smoking status at mid-treatment. Means were not significantly different from one another (All P > 0.05).
Analyses of continuous subjective sexual arousal
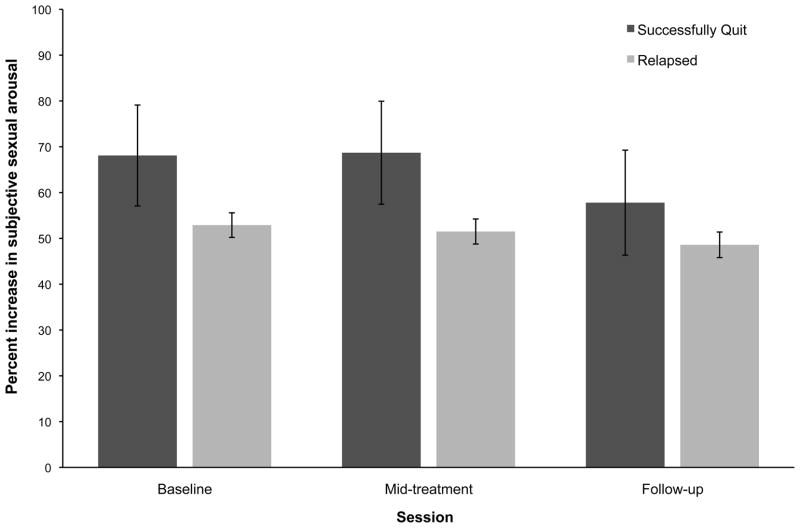
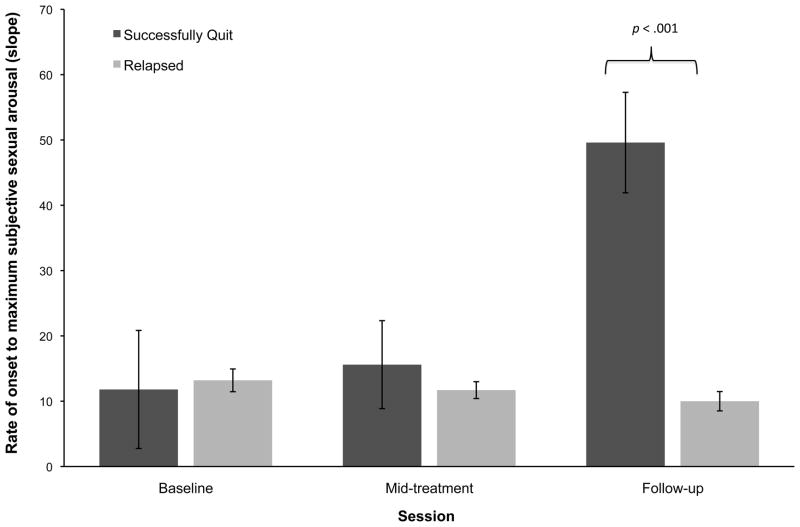
Results of the repeated measures ANCOVAs did not show any significant group × time interaction effects for within-session percentage change in subjective sexual arousal [F(4124) = 1.47, P = 0.22, η2 = 0.05]. As can be seen in Figure 4, neither group displayed any significant across-session changes. However, there was a statistically significant group × time interaction for rate of onset to reach maximum subjective sexual arousal [F(4124) = 4.64, P < 0.01, η2 = 0.13). Post hoc tests of between-subjects contrasts showed significantly greater rate of onset at follow-up among successful quitters compared with unsuccessful quitters (P < 0.001, d = 0.50). Similar to analyses of physiological sexual arousal, there were no between-group differences at mid-treatment (Figure 5).
Figure 4.
The mean within-session percentage changes in continuous subjective sexual arousal across time for successful and unsuccessful quitters. Error bars represent standard errors of the means. Means have been adjusted for pack years, total cigarette consumption throughout enrolment, baseline erectile functioning, and smoking status at mid-treatment. Means were not significantly different from one another (All P > 0.05).
Figure 5.
Across-session changes in rate of onset to reach maximum subjective sexual arousal for successful and unsuccessful quitters. Error bars represent standard errors of the means. Means have been adjusted for pack years, total cigarette consumption throughout enrolment, baseline erectile functioning, and smoking status at mid-treatment.
Analyses of sexual function
Although successful quitters displayed across-session enhancements in sexual function, there were no statistically significant group × time interaction effects for any of the sexual function indices: erectile function [F(4124) = 0.51, P = 0.66, η2 = 0.02]; orgasmic function [F(4124) = 1.28, P = 0.29, η2 = 0.04], sexual desire [F(4124) = 1.01, P = 0.36, η2 = 0.03], intercourse satisfaction [F(4124) = 0.55, P = 0.65, η2 = 0.02], and overall satisfaction [F(4124) = 0.51, P = 0.73, η2 = 0.02]. This indicated that quitting smoking had no discernable effects on self-reported sexual functioning. For changes in ED status as a function of smoking cessation, 20% (four of 20) of successful quitters met criteria for ED at baseline, and 20% (four of 20) and 5% (one of 19) met criteria for ED at mid-treatment and post-cessation follow-up, respectively. Among, unsuccessful quitters, 33% (15 of 45) met criteria for ED at baseline, and 22% (10 of 45) and 13% (six of 45) met criteria for ED at mid-treatment and follow-up, respectively. This corresponded to an ED remission rate of 75% for successful quitters and 61% for unsuccessful quitters. Although successful quitters had a larger rate of ED remission, there were no statistically significant between-group differences at any time point.
Analyses of effect moderators
Whether subjective ratings of mood covaried with the three outcome measures were also explored. Difference scores between the first and last session were separately derived within participants for each outcome measure, as well as for positive affect (PA) and negative affect (NA) scores of the PANAS. These sets of corresponding difference scores were then entered into separate regression models. There was no association between PA and NA change scores and erectile tumescence change scores for either successful or unsuccessful quitters. Similarly, there was no association between changes in PA or NA for self-reported sexual arousal and sexual function for either of the two groups of quitters. Taken together, these results indicate that changes in sexual arousal were not an epiphenomenon of affect change as a result of smoking status.
Discussion
The present study examined the association between quitting smoking and indices of physiological and subjective sexual health in long-term male smokers, irrespective of baseline erectile functioning. Results indicated that successful quitters, compared with unsuccessful quitters, exhibited significantly greater penile tumescence at follow-up (4 weeks after nicotine patch discontinuation), but there were no differences at mid-treatment (while using a high-dose nicotine patch). Although rate of onset to reach maximum erectile capacity showed the same pattern of improvement, there were no differential enhancements across time as a function of group status. The overall pattern of results was similar to previous studies showing significant improvements in penile blood flow [13], as well as rigidity and tumescence [14], as a result of smoking cessation. Furthermore, results suggested that cessation-induced improvements in genital responses were attributable primarily to nicotine elimination (as evidenced by between-group differences in physiological outcome measures at follow-up when successful quitters were nicotine and smoke free), rather than tobacco smoke discontinuation alone (i.e., lack of statistical between-group differences at mid-treatment). In fact, effect sizes for between-group comparisons at follow-up (range 0.12 to 0.33) were about three-times larger than the effect sizes for mid-treatment comparisons (range 0.01 to 0.09). These results are similar with previous studies showing that isolated nicotine hinders male genital responses [25].
Although all participants had large and reliable increases in subjective sexual arousal during each experimental session, stopping smoking had no differential group effect on the percentage increase in self-reported sexual arousal. However, there was an association between stopping smoking and rate of onset to reach maximum subjective sexual arousal. Specifically, successful quitters vs unsuccessful quitters had significant across-session improvements, which resulted in significantly faster rates of onset at follow-up. In fact, 4 weeks after discontinuing the nicotine patch, successful quitters had a five-fold enhancement in rate of onset to reach maximum subjective sexual arousal compared with participants who relapsed (effect size 0.50 vs 0.09).
Unlike results of physiological and subjective sexual arousal, men did not show statistically significant improvements in self-reported sexual function as a result of smoking cessation. However, it deserves mention that successful quitters had a 75% remission rate of ED, and at follow-up only 5% met criteria for ED, which is below age-associated norms [26, 27]. In fact, relapsed participants were nearly three-times as likely to report ED at follow-up compared with successful quitters.
It is unclear why statistical changes in laboratory measures of erectile responding did not correspond with clinically significant enhancements among successful quitters. One possibility is that participants may have displayed subtle, albeit statistically significant, changes in erectile capacities that were not of sufficient magnitude to noticeably affect their sexual performances in real-life sexual settings (e.g., vaginal penetration, erectile maintenance). It is also possible that a 4-week follow-up period is not of sufficient duration to fully capture improvements in real-life sexual performance and function. It is feasible that stopping smoking enhances genital responses relatively quickly and in an automatic fashion, whereas men’s perceptions of their sexual function (which is, in part, a result of partner feedback) may take longer to be reflected as significant augmentations via a self-report measure.
The present study has several strengths. First, to the author’s knowledge, this is the first study of the association between smoking cessation and sexual health that recruited men irrespective of ED status. This is especially important, considering that most smokers (aged < 60 years) would not be expected to have erectile difficulties, whereas after 60 years of age, the natural age-associated rate of ED increases substantially, with most individuals reporting clinically significant erectile difficulties [27]. Second, the present study is the first to examine cessation-induced changes in subjective indices of sexual health. Complementing physiological laboratory assessments with a well-validated measure of sexual functioning provided a means of assessing clinically significant changes. Moreover, including measures of continuous subjective sexual arousal responses provided a means to assess the interplay between cognitive aspects of sexual arousal and physiological responses. Third, this was the first study that has incorporated a comparison control group (unsuccessful quitters), thereby enhancing internal validity.
A number of limitations warrant mention. First, this was not a clinical trial with randomization, and therefore results should be interpreted cautiously. However, the study did use a between-group design, comparing relapsed participants (which served as a quasi-control group) to successful quitters. A second limitation is that smoking abstinence was determined by self-report rather than by objective biochemical verification. It should be noted that all participants provided saliva samples at each visit and they were spuriously informed that these samples would be assayed for cotinine content (a by-product of nicotine that has a relatively long half-life), and verified with their self-report. This technique has been shown to produce reliable and accurate estimates of smoking [28]. A third limitation is that ED was assessed via a self-report measure, and therefore participants did not undergo a medical evaluation. Considering that the present study was concerned with the classification of ED as a primary endpoint, rather than understanding the aetiology and type of erectile difficulty, using the IIEF in this regard was appropriate.
Results of the present study have important clinical and public health implications. In addition to discussing traditional acute (e.g., enhanced cardiovascular and pulmonary functioning) and long-term (e.g., reduced morbidity and mortality from cancer, lung disease, and heart disease) benefits of stopping smoking with patients, healthcare providers may find it useful to discuss the benefits of quitting smoking in terms of enhanced erectile performance. This may serve as a more motivating and relevant means for men to consider the cessation process. An important point to note is that these results suggest that men who do not complain of erectile problems may still benefit sexually from quitting smoking. In fact, previous studies suggest that smoking may have a stronger deleterious effect on sexual functioning in younger compared with older men [29]. Therefore, these results may serve as an important motivator, particularly for young men who may put considerable importance on their erectile performance, to consider quitting smoking. Enhancing successful smoking cessation in men would significantly enhance quality of life, substantially reduce premature death, and alleviate enormous economic burdens caused by smoking-related diseases such as cardiovascular disease, respiratory disease, and cancer.
In conclusion, this is the first study that shows that smoking cessation significantly enhances both physiological and self-reported indices of sexual health in long-term male smokers, irrespective of baseline erectile impairment. It is hoped that these results may serve as a novel means to influence men to quit smoking.
Acknowledgments
This project was supported by Award Number F31DA026276 from the National Institute on Drug Abuse (NIDA) to Christopher Harte. The contents of this work are solely the responsibility of the author and do not necessarily represent the official views of the NIDA or the National Institutes of Health. Portions of this work were also made possible by Grant Number 1 RO1 HD051676-01 A1 to Cindy M. Meston from the National Institute of Child Health and Human Development (NICHD). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NICHD. This project was also supported by the following fellowships/awards to the first author: The Sexual Medicine Society of North America Student Research Grant; two Graduate Dean’s Prestigious Fellowship Supplements, and a College of Liberal Arts Graduate Research Fellowship, all from the University of Texas at Austin; the American Psychological Association Dissertation Research Award; and The Kinsey Institute Student Research Grant. Portions of this study have been presented at the 2010 annual meeting of the Society for Behavioral Medicine, Seattle, WA; the 2010 annual meeting of the Association for Psychological Science, Boston, MA; and the 2011 annual meeting of the Society for Research on Nicotine and Tobacco, Toronto, Canada. The authors thank Tyler Watts, Hillary Perlman, Olivia Bentkowski, Alicia Whitaker, Gail Dalton, and Katy Siciliano for help with participant recruitment, screening, and data management.
Abbreviations
- ED
erectile dysfunction
- IIEF
International Index of Erectile Function
- (P)(N)A
(positive) (negative) affect
- ANCOVA
analysis of covariance
References
- 1.Centers for Disease Control and Prevention. Annual smoking-attributable mortality, years of potential life lost, and economic costs – United States, 1995–1999. MMWR Morb Mort Wkly Rep. 2002;51:300–3. [PubMed] [Google Scholar]
- 2.Fielding JE, Husten CG, Eriksen MP. Tobacco: Health effects and control. In: Maxcy KF, Rosenau MJ, Last JM, Wallace RB, Doebbling BN, editors. Public Health and Preventative Medicine. New York: McGraw-Hill; 1998. pp. 817–45. [Google Scholar]
- 3.Fagerström K. The epidemiology of smoking: Health consequences and benefits of cessation. Drugs. 2002;62:1–9. doi: 10.2165/00003495-200262002-00001. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Department of Health and Human Services. The health consequences of smoking: A report of the surgeon general. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004. [Google Scholar]
- 5.Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible key consequences. BJU Int. 1999;84:50–6. doi: 10.1046/j.1464-410x.1999.00142.x. [DOI] [PubMed] [Google Scholar]
- 6.Young JM, Bennett C, Gilhooly P, Wessells H, Ramos DE. Efficacy and safety of sildenafil citrate (Viagra) in Black and Hispanic American men. Urology. 2002;60:39–48. doi: 10.1016/s0090-4295(02)01689-8. [DOI] [PubMed] [Google Scholar]
- 7.Tengs TO, Osgood ND. The link between smoking and impotence: Two decades of evidence. Prev Med. 2001;32:447–52. doi: 10.1006/pmed.2001.0830. [DOI] [PubMed] [Google Scholar]
- 8.Dorey G. Is smoking a cause of erectile dysfunction? A literature review. Br J Nurs. 2001;10:455–65. doi: 10.12968/bjon.2001.10.7.5331. [DOI] [PubMed] [Google Scholar]
- 9.Lam TH, Abdullah AS, Ho LM, Yip AW, Fan S. Smoking and sexual dysfunction in Chinese males: Findings from men’s health survey. Int J Impot Res. 2006;18:364–9. doi: 10.1038/sj.ijir.3901436. [DOI] [PubMed] [Google Scholar]
- 10.Mannino DM, Klevens RM, Flanders WD. Cigarette smoking: An independent risk factor for impotence? Am J Epidemiol. 1994;140:1003–8. doi: 10.1093/oxfordjournals.aje.a117189. [DOI] [PubMed] [Google Scholar]
- 11.He J, Reynolds K, Chen J, et al. Cigarette smoking and erectile dysfunction among Chinese men without clinical vascular disease. Am J Epidemiol. 2007;166:803–9. doi: 10.1093/aje/kwm154. [DOI] [PubMed] [Google Scholar]
- 12.Feldman HA, Johannes CB, Derby CA, et al. Erectile dysfunction and coronary risk factors: Prospective results from the Massachusetts male aging study. Prev Med. 2000;30:328–38. doi: 10.1006/pmed.2000.0643. [DOI] [PubMed] [Google Scholar]
- 13.Sighinolfi MC, Mofferdin A, De Stefani S, Micali S, Cicero AF, Bianchi G. Immediate improvement in penile hemodynamics after cessation of smoking: previous results. Urology. 2007;69:163–5. doi: 10.1016/j.urology.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 14.Guay AT, Perez JB, Heatley GJ. Cessation of smoking rapidly decreases erectile dysfunction. Endocr Pract. 1998;4:23–6. doi: 10.4158/EP.4.1.23. [DOI] [PubMed] [Google Scholar]
- 15.Saunders J, Aasland O, Babor T, de la Fuente J, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 16.Skinner H. The drug abuse screening test. Addict Behav. 1982;7:363–71. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- 17.Fiore M, Bailey W, Cohen S, et al. Clinical practice guideline: Treating tobacco use and dependence. Rockville, MD: US Department of Health and Human Services. Public Health Service; 2000. [Google Scholar]
- 18.Glynn TJ, Manley M. NIH publication 90–3064. Washington, DC: Smoking and Tobacco Control Program, Division of Cancer Prevention, National Cancer Institute, US Dept of Health and Human Services; Nov, 1990. How to help your patients stop smoking: a National Cancer Institute manual for physicians; p. 1990. [Google Scholar]
- 19.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 20.Rosen RC, Keefe FJ. The measurement of human penile tumescence. Psychophysiology. 1978;15:366–76. doi: 10.1111/j.1469-8986.1978.tb01393.x. [DOI] [PubMed] [Google Scholar]
- 21.Rellini AH, McCall KM, Randall PK, Meston CM. The relationship between women’s subjective and physiological sexual arousal. Psychophysiology. 2005;42:116–24. doi: 10.1111/j.1469-8986.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 22.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): A multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–30. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 23.Cappelleri JC, Rosen RC, Smith MD, Mishra A, Osterloh IH. Diagnostic evaluation of the erectile function domain of the International Index of Erectile Function. Urology. 1999;54:346–51. doi: 10.1016/s0090-4295(99)00099-0. [DOI] [PubMed] [Google Scholar]
- 24.Little RJ, Rubin DB. Statistical analysis with missing data. 2. Hoboken, NJ: Wiley–Interscience; 2002. [Google Scholar]
- 25.Harte CB, Meston CM. Are the inhibitory effects of nicotine on erectile response in nonsmokers generalizable to long-term smokers? A reply J Sex Med. 2008;5:2003–4. doi: 10.1111/j.1743-6109.2008.00891.x. [DOI] [PubMed] [Google Scholar]
- 26.Selvin E, Burnett E, Platz E. Prevalence and risk factors for erectile dysfunction in the US. Am J Med. 2007;120:151–7. doi: 10.1016/j.amjmed.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: Prevalence and predictors. JAMA. 1999;281:537–44. doi: 10.1001/jama.281.6.537. [DOI] [PubMed] [Google Scholar]
- 28.Murray D, O’Connell C, Schmid L, Perry C. The validity of smoking self-reports by adolescents: A reexamination of the bogus pipeline procedure. Addict Behav. 1987;12:7–15. doi: 10.1016/0306-4603(87)90003-7. [DOI] [PubMed] [Google Scholar]
- 29.Gades NM, Nehra A, Jacobson DJ, et al. Association between smoking and erectile dysfunction: A population-based study. Am J Epidemiol. 2005;161:346–51. doi: 10.1093/aje/kwi052. [DOI] [PubMed] [Google Scholar]
- 30.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence:A revision of the Fagerstrom tolerance questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]