Abstract
Background
There are multiple benefits to transfusing only ABO identical blood components. Historically our institution routinely transfused ABO non-identical platelets (PLTs) and cryoprecipitate to surgical patients. In April 2005, we implemented a policy of transfusing only ABO identical components whenever feasible, regardless of outdating/logistic considerations.
Methods
Technical staff closely monitored product usage and adjusted blood center orders based on recent utilization and planned transfusions. When unable to provide ABO identical PLTs ABO compatible platelets were washed to remove incompatible plasma. Data on outdating were collected for eighteen months before and after implementation. We compared transfusion reaction and red cell alloimmunization incidence for four years preceding (2001–2004) and subsequent (2006–2009) to implementation.
Results
In the year following implementation, only 11 of 410 surgical patients received ABO non-identical platelets (2.7%). There was a 5.6% increase in outdating of platelets. Transfusing ABO identical components was associated with significant reductions in febrile (−46%; 8.0 to 4.3 per 10,000 components; p<0.0001) and allergic transfusion reactions (−23%; from 7.0 to 5.4 per 10,000 components; p=0.025). A progressive reduction in de novo red cell alloimmunization incidence also occurred (−50% by 2009; p=0.03).
Conclusions
Providing ABO identical platelets to almost all patients was feasible in our setting by changing ordering and inventorying procedures, and making the ABO identical policy a staff priority. Unexpected and striking reductions in febrile and allergic reactions, and red cell alloimmunization were observed, of uncertain causal relationship to this ABO policy change, which will require further study.
Background
There is a growing body of evidence supporting the potential benefits of using ABO identical blood components. Contrary to red cell transfusion where ABO incompatibility can result in fatal outcomes, current transfusion practice often pays little attention to ABO matching in platelet and plasma product transfusions. The sole imperative has been to avoid hemolysis. However, platelets do possess ABO blood group antigens.1 Additionally, plasma components and the plasma contained in platelet products may contain anti-A and anti-B antibodies and soluble ABH antigens. ABO-incompatible platelet transfusions can occasionally result in hemolytic transfusion reactions, occasionally severe or, rarely, fatal. 2 Most transfusion services have a policy concerning the use of platelets containing ABO-incompatible plasma but do not have a proven method to limit the consequences of transfusion of anti-A and anti-B antibodies and, consequently, there is always a small risk of hemolysis when such platelets are transfused. 3
Other consequences of providing ABO minor side incompatible platelets and plasma products are uncertain, but of concern given that soluble antigens and corresponding antibodies could lead to immune complex formation with as unknown, but likely unfavorable consequences. 4 For example, AB plasma, long considered the universal donor plasma, and thought to be compatible with all recipients, contains both A and B soluble antigens. When AB plasma is transfused to an O, A or B type recipient possessing anti-A and/or anti-B antibodies, antibody-antigen interactions occur and may be clinically significant in the case of type O recipients, including an association with a 9% increase in mortality. 5 It seems likely that transfusions of ABO identical components, whenever possible, would carry the lowest risk for the patient.
Older studies have demonstrated a reduction in post transfusion platelet count increments with ABO non-identical platelets when compared to transfusion of ABO identical platelets.6 The transfusion of only ABO identical platelets to patients requiring ongoing platelet support in randomized trials yields better increments, a reduction of overall platelet usage and a decrease in platelet refractoriness. 7,8 Cardiac surgery patients with a ventricular assist device receiving only leukoreduced, ABO identical transfusions have a reduced risk of HLA-A,B allosensitization in one observational study.9 A retrospective, observational cohort study of cardiac surgical patients demonstrated that patients receiving only ABO identical platelets had fewer red cell and platelet transfusions, a shorter length of hospital stay, fewer days of postoperative fever, fewer days on antibiotics, fewer hours in the ICU, one third the in-hospital mortality, and lower hospital costs and charges when compared to patients receiving ABO non-identical platelets. 10 Transfusion of ABO non-identical components, in preliminary reports, has been associated with an increase in red cell transfusions in blunt trauma patients11 and in surgical patients overall. 12 In an in vitro model, ABO mismatched platelets and plasma produced prolonged closure times with the PFA-100 analyzer, decreased platelet aggregation and altered cytokine release. 13 The utilization of red cells in bleeding surgical patients changed dramatically according to recipient ABO blood group after introduction of an ABO identical platelet transfusion policy. 14
Historically our institution transfused ABO identical red cell and FFP or liquid plasma components but less priority was given to transfusion of ABO identical platelets and cryoprecipitate in patients not requiring multiple transfusions. With increasing evidence supporting the potential clinical benefits of providing only ABO identical components, we decided to implement a policy of administering only ABO identical blood components whenever feasible, without regard to inventory management or non-clinical issues. We report here the feasibility of and approach to a policy of transfusing only ABO identical platelets, and data on associated changes in clinical outcomes.
Materials and Methods
The University of Rochester Medical Center Transfusion Service/Blood Bank, located 5 miles from its primary blood supplier, performs 65,000 transfusions each year in support of a 750 bed hospital. Our blood bank also supports an emergency department that serves more than 96,000 patient visits each year and has the only Level 1 trauma center within a radius of 70 miles. In addition, our institution supports services that perform liver, kidney, stem cell and heart transplants as well as a Cancer Center that currently treats about 10,000 patients each year. Universal leukoreduction of red cell and platelet transfusions was instituted in 2000 and thus did not influence the results reported. Beginning in 1990, all platelet transfusions for patients with hematologic diseases or diseases that might be treated with stem cell transplant were administered as ABO identical. The policy for virtually all other platelet transfusions until spring, 2005 was to give platelet pools that were nearest to outdate, regardless of ABO type.
When ABO identical cellular components are unavailable, it is policy to reduce non-identical plasma by saline washing prior to transfusion if time permits. Washing leads to minimal loss of red cells but about a 20% loss of platelets. A phased in approach was utilized for implementation. Initially in 1990 it became policy to transfuse only ABO identical platelets to patients with hematologic malignancies. However, in April 2005, we established a policy in which ABO identical platelets are routinely given to all patients. Because of inventory capabilities, providing only ABO identical cryoprecipitate was not a difficult change in policy. ABO identical FFP and liquid plasma were always standard practice, although AB plasma was given when emergencies occurred and the patient’s ABO type was not known. ABO identical red cells, platelets, cryoprecipitate and plasma are now routinely utilized for all transfusions except unidentified trauma patients or when ABO identical components are unavailable and the clinical need for transfusion is urgent.
Monitor of Blood Supply
Our staff routinely monitors recent blood utilization and planned transfusions. Based upon the monitor, standing blood orders are adjusted to accommodate anticipated needs. Adjustments are made both with regard to quantity and blood type. In addition, in the time frame data were collected, our institution almost exclusively used whole blood derived pooled platelets. The routine platelet dose consisted of five units of platelets. During times of platelet shortage, however, our institution would compensate by providing a number of ABO identical single donor platelets and reduced doses of whole blood platelets (e.g., pools of three or four platelets) until adequate ABO identical supplies could be attained from blood suppliers.
Blood Suppliers
Our suppliers must have flexibility with standing orders and inventory replenishment to accommodate adjustments of quantity and blood type of components. It is also indispensable to have a blood supplier in close proximity for placing urgent orders. Our primary blood supplier is located just 5 miles from our hospital allowing rapid replenishment of inventory, a major advantage in providing ABO identical components.
Plasma Reduction
Saline washing with one of four 2991 COBE (subsequently Gambro, now Caridian) cell washers, or another method of plasma reduction to remove incompatible plasma and soluble antigen from platelet products, is essential in our setting to avoiding transfusion of incompatible antibody ± soluble antigen. This practice allows the use of plasma depleted ABO compatible platelets when ABO identical products are unavailable.
Physician Communications
We found it essential to have excellent communications with bedside practitioners. In the event of emergent situations, including traumas, when patient survival may be jeopardized by a delay necessitated by external sourcing or washing components, a physician or technologist may approve the use of unwashed O type red cells, unwashed ABO compatible platelets and AB type plasma or cryoprecipitate. When the patient’s blood type is unknown, group O red cells and AB plasma are transfused, and group A platelets are typically administered.
Storage for Increased Quantities of Products
It is crucial to have adequate storage for an increased inventory. We needed to increase our in-house inventory of cryoprecipitate in order to ensure availability of each ABO blood type. With an increase in frozen products, we needed to make arrangements to accommodate these products in monitored freezers that maintain the appropriate temperature. Typically we maintain a two day inventory of group A and O platelets and order B and AB platelets as needed.
Compliance with an ABO Identical Only Policy
Our most challenging cohort to treat was used as an indicator of compliance with our new policy. Transfusion data were collected for one year post implementation on surgical patients requiring at least one platelet transfusion. Patient transfusion records were reviewed from the time the patient had surgery until they were discharged from the hospital. The number of patients receiving at least one transfusion of a non-ABO identical platelet component was tabulated. The proportion of surgical patients receiving any ABO non-identical transfusions was calculated by dividing this population by the total number of surgical patients receiving platelets.
Measure of Wastage
To determine consequences of our policy on blood component outdating, a review of wastage was performed for eighteen months before and after implementation of this policy. This was an arbitrary choice of period. Total numbers of each component ordered from our blood suppliers were tabulated for each eighteen month period. Then, the numbers of components wasted in these time periods were obtained from a blood utilization report that is generated quarterly. Percent wastage per total number of components ordered was calculated for each blood component type for the eighteen months prior to and subsequent to implementation of the ABO identical blood component policy.
Rate of Transfusion Reactions and Red Cell Alloimmunization
We hypothesized that immunologic consequences due to ABO mismatched transfusions might include transfusion reactions and alloimmunization. There is evidence that inflammation, which speculatively might be caused by ABO immune complexes, can facilitate alloimmunization in animal models.15,16 Thus we compared febrile and allergic transfusion reactions for periods of four years preceding and following implementation. The primary rationale for this time period was to have a large enough number of reactions and alloimmunizations to analyze. The number of allergic and febrile transfusion reactions was obtained from the blood utilization quarterly report for the time periods of four years before and after implementation of the ABO identical blood component policy. The total numbers of components transfused were also collected during each of these time periods. The rate of transfusion reactions per total components transfused was calculated.
In 2010, based upon work suggesting that immune complexes and inflammation may alter the likelihood of alloimmunization,15,16 we examined the rate of newly detected red cell alloantibodies in our institution for the four years previous to and after instituting the new policy regarding ABO identical platelets/cryoprecipitate. The primary rationale for choosing these time periods was having a sufficiently large number of events to study. The rate of alloimmunization was calculated per antibody screen (indirect antiglobulin test) performed on patients during a given year. There were no changes in methods of antibody screening or identification during this nine year period, either methodologically or in terms of screening cell number or selection. Upon inspection of the raw data, it appeared that trend analysis was the most appropriate method of displaying the data.
As a surrogate for HLA antibody alloimmunization and platelet transfusion refractoriness, which may be increased by transfusion of ABO non-identical platelets,8 we calculated, as a percentage, the total annual use of HLA matched platelets per total platelet doses transfused during the periods 2001–2004 and 2006–2009. We did not expect to see a substantial difference as our refractoriness rate was already very low prior to the ABO identical policy. This low incidence of refractoriness is likely due to our protocol that from 1990 onward mandated transfusion of only leukoreduced, ABO identical platelets for all patients at high risk of platelet transfusion refractoriness.4,7–9
Statistical Analysis
All data collection was retrospective. Chi-square testing without Yates correction was performed to determine statistical significance of categorical variables, such as the proportion of events per transfused component. For continuous variables, at test was employed for data variables with non-significantly different standard deviations, and the Mann Whitney non-parametric test was employed for analysis of changes in derived numerical data (e.g., percentages of platelets given as HLA matched). Upon inspection of the red cell alloimmunization raw data, it appeared that trend analysis was the most appropriate method of displaying the data. Linear regression was employed to determine trends, either a level trend (slope not significantly different from zero) or increasing or decreasing trend (slope significantly different from zero) A two- sided p value of <0.05 was considered significant for all determinations. Instat 3.1a (GraphPad, La Jolla, CA) was employed for all analyses.
Results
Compliance
Surgical patients had a very low incidence of protocol violations with the ABO identical component protocol. In the year following implementation, only 11 of 410 surgical patients received ABO non-identical platelets (2.7%), compared with 135 of 335 (40.3%) from the previous one year time frame. All of these protocol violations post spring 2005 were for group AB and B recipients.
Wastage
Table 1 indicates modest or no changes in percent wastage between the pre and post implementation time frames for red cells, plasma and apheresis platelet products. Whole blood PLT wastage increased by 5.6%. With further staff experience monitoring of platelet transfusion needs by ABO type, and use of prestorage leukoreduced, pooled whole blood platelets, our current wastage rate has been below 5% for both apheresis and whole blood platelet pools for 2010.
Table 1.
Product Wastage, Percent of Total Received from Suppliers
| 18 Months Prior Oct. 2003--March 2005 |
18 Months After June 2005–Oct. 2006 |
p value | |
|---|---|---|---|
| Whole Blood PLTs | 10.8 | 16.4 | <0.0001 |
| Apheresis PLTs | 5.4 | 5.6 | 0.7898 |
| RBC | 1.5 | 1.7 | NS |
| plasma | 11.2 | 11.0 | NS |
Transfusion Reaction
In the four year period before implementing the ABO identical policy, there were a total of 370 febrile and allergic transfusion reactions reported. In this time frame, our institution transfused 247,778 blood components. In the comparable time period following introduction of the ABO identical policy, 280 reactions were reported following transfusion of 289,399 blood components. Transfusing only ABO identical components, as shown in Table 2, coincided with an overall decrease in these minor transfusion reactions by 35% (p<0.0001 by Chi square). Regression analysis demonstrated that the changes in trends of transfusion reactions prior to the change in ABO policy and afterward were each not significantly different from zero. This suggests that the observed alterations in transfusion reaction incidence associated with the change in ABO policy were essentially immediate, in contrast to the changes seen in red cell alloimmunization incidence (as detailed in the next section).
Table 2.
Febrile and Allergic Transfusion Reactions Per Components Transfused
| 2001–2004 | 2006–2009 | p value | |
|---|---|---|---|
| Components Transfused | 247,778 | 289,399 | --- |
| Platelet Transfusions | 17,481 | 23,298 | --- |
| Febrile Reactions | 197 (0.080%) | 123 (0.043%) | <0.0001 |
| Allergic Reactions | 173 (0.070%) | 157 (0.054%) | 0.025 |
Red Cell Alloimmunization Incidence
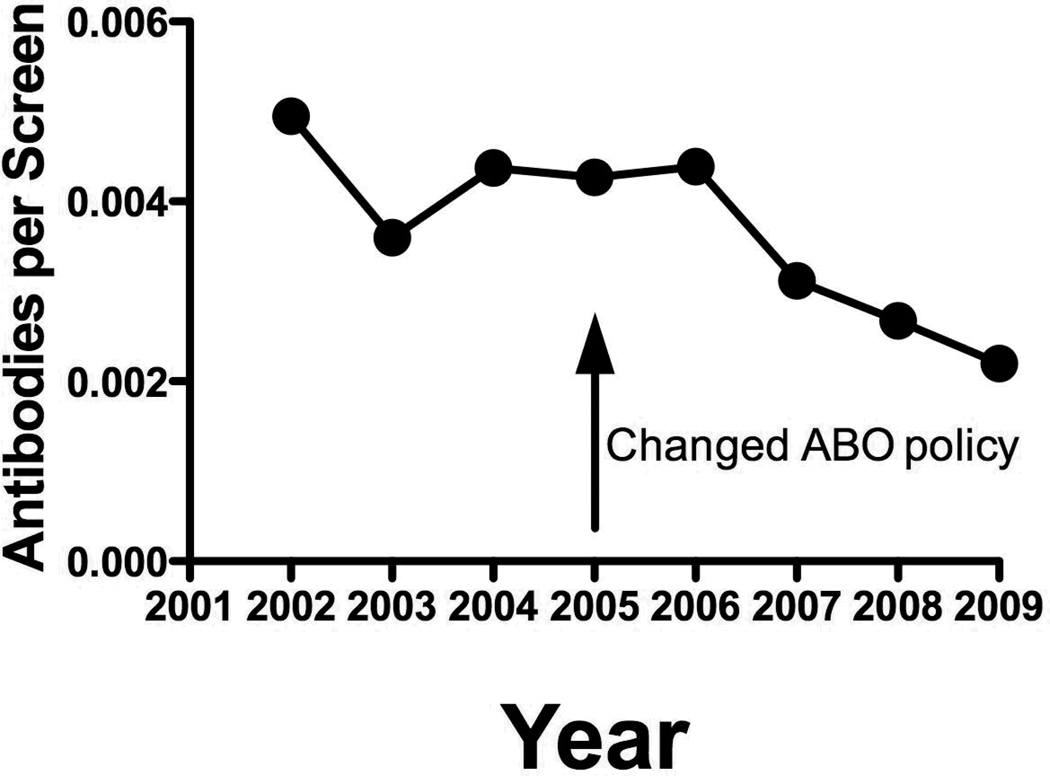
There was a progressive 50% decrease in the incidence of new red cell alloantibodies noted over the four years after instituting an ABO identical platelet transfusion policy in 2005 (Figure 1). There was no change in the incidence of alloantibodies in 2006, but over the next three years, a continued and gradual decrease in red cell alloimmunization rate was observed.
Figure 1.
Incidence of newly detected red cell alloantibodies per antibody screen annually. The slope of the linear regression line for years 2001–2005 is not significantly different from zero (p=0.70). The slope of the linear regression line for years 2006–2009 is significantly different from zero (p=0.037).
Use of HLA Matched Platelets
There was a non-significant decrease in the number of HLA matched platelet doses required annually, and a nearly significant decrease in the proportion of total annual platelet doses (varying from approximately 4,000 to 6,000 total platelet transfusions annually) given as HLA matched. The mean number of HLA matched platelets required annually during 2001–2004 was 63 ± 16. During 2006–2009 the mean was 44 ± 16 (p=0.15 by t test). As a proportion of total platelet doses given each year, HLA matched platelets constituted a mean of 1.43 % ± 0.37 in 2001–2004 as compared with 0.79 % ± 0.30 in 2006–2009 (p = 0.06 by Mann-Whitney test). Regression analysis demonstrated that the changes in trends of utilization of HLA matched platelets prior to the change in ABO policy and afterward were not significantly different from zero. This suggests that the modest observed differences in HLA matched platelet use occurred immediately after the institution of the ABO identical policy.
Discussion
The major concern of implementing an ABO identical policy is blood component wastage creating shortages, and related expenses when inventory must be increased to supply patients with blood products ABO identical to their own type. Whereas plasma components and cryoprecipitate have an extended shelf life based on frozen storage,platelet components have a maximum of 5 days of shelf life and pose the greatest risk for wastage. We observed a modest but statistically significant increase in whole blood platelet wastage. However, we view the increase in wastage and related expenses as minimal when weighed against the potential detriment of transfusing ABO non-identical products. Furthermore, our current outdating rate is below 5% for both apheresis and whole blood platelets pools during the year 2010. Given our data that use of ABO identical platelets (and FFP and cryoprecipitate) is associated with decreased red cell transfusion needs in bleeding, predominately massively transfused surgical patients,14 any increase in platelet costs due to outdating has the potential to be counterbalanced by a reduction in the need for red cell transfusion and its costs. This speculation will require additional investigation.
Shortly after these data were collected, our institution began providing pre-storage pooled whole blood platelets as the primary platelet components for transfusion. Apheresis platelets were used as a supplementary component. The use of this approach has had minimal impact on our processes. Our institution has implemented procedures to allow “splitting” of such products when necessary to provide adequate doses of ABO identical platelets. Based on the multiple strategies we have employed, our institution has infrequently experienced platelet shortages. Hence, the use of split platelet products has been a rare occurrence.
Initially there was a concern that obtaining blood group AB cryoprecipitate would prove challenging. It took some time for our primary blood supplier to attain the required number of components to allow us to reach maximum inventory levels. In addition, at times the supplier requires additional time to obtain replenishment. However, we believe that our maximum number reflects an adequate inventory level that allows our blood suppliers appropriate time for re-stocking.
Another concern our institution expected to face was one of compliance, especially during trauma or massive surgical bleeds. Only a small proportion (2.7%) of our surgical patients received ABO non-identical platelets in the year after our policy change, exclusively patients of blood groups AB and B.14 These results suggest that dedicated implementation of an ABO identical transfusion policy can be achieved via multiple strategies including close monitoring of platelet usage, plasma depletion of platelet products by washing, and flexibility of blood suppliers to adjust orders for ABO identical platelets. We acknowledge that having our regional blood center only 10 minutes distant greatly increases the feasibility of obtaining ABO identical platelets (or other components) on short notice.
There are a number of limitations to the interpretation of our data. The policy for ABO identical transfusions did not change for patients with hematologic malignancies, but it was not possible to exclude these patients from analysis. Thus the true magnitude of the possible association of the new ABO matching policy with transfusion reactions and alloimmunization may be smaller or larger than the one reported. We did not have patient level data for the number of unique patients who were transfused, number of unique patients with antibody screens, etc. Thus our use of event level data (transfusion reactions, HLA matched platelet use, new red cell alloimmunizations) is compared with a denominator of total antibody screens or transfusions, not number of patients having antibody screens or transfusions. This may alter the quantitative nature of the association we found, but we think highly unlikely to change the interpretation of the data. Almost all (>90%) the transfusion reactions, use of HLA matched platelets and new red cell alloimmunizations in our hospital occur de novo in unique patients, with a very small number of patients having more than one reaction or more than one new red cell alloantibody. Thus use of patient level data on transfusions and antibody screens would likely alter only the absolute ratios, not the relative ratios observed.
These data also raise the novel hypotheses that ABO non-identical platelet transfusions may contribute to allergic and febrile reactions, and increase red cell alloimmunization rates. Our results also are compatible with previous work8 that transfusing ABO non-identical platelets increases HLA alloimmunization rates. Further study in larger populations, animal models and mechanistic studies will be needed to assess these hypotheses.
Acknowledgments
Grant Support
Supported in part by NIH grants HL078603, HL095467, HL100051.
Footnotes
Disclosure of Conflicts of Interest
NB has received lecture honoraria, consulting fees and research support from Pall Biomedical and Fenwal, manufacturers of leukoreduction filters, as well as from Caridian, manufacturers of cell washing equipment and supplies. The other authors declare they have no potential conflicts of interest.
References
- 1.Cooling LL, Kelly K, Barton J, Hwang D, Koerner TA, Olson JD. Determinants of ABH expression on human blood platelets. Blood. 2005;96:3356–3364. doi: 10.1182/blood-2004-08-3080. [DOI] [PubMed] [Google Scholar]
- 2.Sadani DT, Urbaniak SJ, Bruce M, Tighe JE. Repeat ABO-incompatible platelet transfusions leading to haemolytic transfusion reaction. Transfus Med. 2006 Oct;16(5):375–379. doi: 10.1111/j.1365-3148.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- 3.Fung MK, Downes KA, Shulman IA. Transfusion of platelets containing ABO-incompatible plasma: A survey of 3156 north american laboratories. Arch Pathol Lab Med. 2007 Jun;131(6):909–916. doi: 10.5858/2007-131-909-TOPCAP. [DOI] [PubMed] [Google Scholar]
- 4.Heal JM, Liesveld JL, Phillips GL, Blumberg N. What would karl landsteiner do? the ABO blood group and stem cell transplantation. Bone Marrow Transplant. 2005 Nov;36(9):747–755. doi: 10.1038/sj.bmt.1705101. [DOI] [PubMed] [Google Scholar]
- 5.Shanwell A, Andersson TM, Rostgaard K, Edgren G, Hjalgrim H, Norda R, et al. Post-transfusion mortality among recipients of ABO-compatible but non-identical plasma. Vox Sang. 2009 May;96(4):316–323. doi: 10.1111/j.1423-0410.2009.01167.x. [DOI] [PubMed] [Google Scholar]
- 6.Jimenez TM, Patel SB, Pineda AA, Tefferi A, Owen WG. Factors that influence platelet recovery after transfusion: Resolving donor quality from ABO compatibility. Transfusion. 2003 Mar;43(3):328–334. doi: 10.1046/j.1537-2995.2003.00326.x. [DOI] [PubMed] [Google Scholar]
- 7.Heal JM, Rowe JM, McMican A, Masel D, Finke C, Blumberg N. The role of ABO matching in platelet transfusion. Eur J Haematol. 1993 Feb;50(2):110–117. doi: 10.1111/j.1600-0609.1993.tb00150.x. [DOI] [PubMed] [Google Scholar]
- 8.Carr R, Hutton JL, Jenkins JA, Lucas GF, Amphlett NW. Transfusion of ABO-mismatched platelets leads to early platelet refractoriness. Br J Haematol. 1990 Jul;75(3):408–413. doi: 10.1111/j.1365-2141.1990.tb04356.x. [DOI] [PubMed] [Google Scholar]
- 9.Coppage M, Baker M, Fialkow L, Meehan D, Gettings K, Chen L, et al. Lack of significant de novo HLA allosensitization in ventricular assist device recipients transfused with leukoreduced, ABO identical blood products. Hum Immunol. 2009 Jun;70(6):413–416. doi: 10.1016/j.humimm.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blumberg N, Heal JM, Hicks GL, Jr, Risher WH. Association of ABO-mismatched platelet transfusions with morbidity and mortality in cardiac surgery. Transfusion. 2001 Jun;41(6):790–793. doi: 10.1046/j.1537-2995.2001.41060790.x. [DOI] [PubMed] [Google Scholar]
- 11.Fialkow LB, Zucchiatti A, Cheng J, Piotrowski ES, Lentz C, King R, et al. ABO non-identical transfusions and red blood cell usage in blunt trauma patients. 2007;47(S3):195. (abstract) [Google Scholar]
- 12.Blumberg N, Fialkow LB, Gettings K, Zucchiatti A, Heal JM, Bankey PE. Blood group O and ABO non-identical platelet transfusions are associated with increased red cell utilization in non-trauma surgical patients. Blood. 2007;110:4012. (abstract) [Google Scholar]
- 13.Refaai M, Masel E, Gettings K, Phipps R, Spinelli S, Corsetti J, et al. The effects of anti-A and anti-B on platelet function: An in vitro model of ABO non-identical transfusion. Transfusion. 2008;48(s2):218. (abstract) [Google Scholar]
- 14.Refaai M, Fialkow LB, Heal JM, Henrichs KF, Spinelli SL, Phipps RP, et al. An association of ABO non-identical platelet and cryoprecipitate transfusions with altered red cell transfusion needs in surgical patients. Vox Sanguinis. doi: 10.1111/j.1423-0410.2010.01464.x. (published on-line March 18, 2011 doi: 10.1111/j.1423-0410.2010.01464.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimring JC, Hendrikson JE. The role of inflammation in alloimmunization to antigens on transfused red blood cells. Curr Opin Hematol. 2008:631–635. doi: 10.1097/MOH.0b013e328313695e. [DOI] [PubMed] [Google Scholar]
- 16.Heal JM, Masel D, Rowe JM, Blumberg N. Circulating immune complexes involving the ABO system after platelet transfusion. Br J Haematol. 1993:566–572. doi: 10.1111/j.1365-2141.1993.tb03349.x. [DOI] [PubMed] [Google Scholar]