Abstract
Background
Plasma is vital for the resuscitation of injured patients and to restore necessary pro-coagulants, especially factors II, V, VII, X and XIII; however, female plasma has been implicated in the majority of transfusion-related acute lung injury (TRALI) cases and male-only plasma transfusion regimens have significantly decreased the incidence of TRALI. Little is known about the Human plasma proteome, and no comparisons have been made between male and female plasma; therefore, we hypothesize that there are significant differences between plasma from male and female donors.
Methods
5 units of fresh frozen plasma (FFP) each were collected from nulliparous female donors and male donors and the proteome was analyzed by depleting the 14 most common proteins by immunoaffinity columns followed by protein separation by one dimension gel electrophoresis, tryptic digestion of the proteins, analysis of the peptides by liquid chromatography tandem mass spectrometry and identification employing human protein sequence databases.
Results
female plasma, vs. males contained pregnancy zone protein (419-580-fold), factor V (2-fold), α1-antitrypsin (2-fold), β2-microglobulin (2-fold), and complement factors H and C4B (1.5-2-fold) at significantly higher concentrations than males and males contained significant increases in Fc binding protein (2-fold), protein Z-dependent protease inhibitor (2-fold), phosphatidylinositol-glycan specific phospholipase (4-fold), protein S-100 (3-fold) and transgelin-2 (14-fold) vs. females (p<.005). The increases in factor V, α1-antitrypsin, and β2-microglobulin were confirmed by an activity assay or immunoblots. We conclude that there are proteomic differences between male and female plasma which could be exploited to improve clinical outcomes in transfused patients.
Introduction
Plasma is used for the resuscitation of patients with inherent, factor XI deficiency, or acquired coagulopathies, and is vital for resuscitation of injured patients especially those requiring massive transfusions.1-6 For resuscitation of the injured, the administration of plasma is especially important to restore coagulation factors, especially factors II, V, VII, and XIII, and in which levels of 20% of normal are required to provide appropriate hemostasis for surgical bleeding.2,4,7 Although vital for resuscitation of trauma patients, plasma has been considered to be the “most dangerous” blood product due to untoward effects and its relationship with poor outcomes with liberal use. 8
Plasma and plasma-containing blood products are inordinately implicated in transfusion-related acute lung injury (TRALI) the leading cause of transfusion mortality world-wide.9,10 Female plasma has been linked to the majority of TRALI reactions due to fetal:maternal alloimmunization resulting in the production of antibodies that recognize the Human Lymphocyte Antigens (HLA), both class I and class II, which have been implicated in TRALI.9,11,12 Recently, male-only transfusion practices have resulted in a significant decrease in both the total number of, and fatalities from, transfusion-related acute lung injury (TRALI) in both the United States and the United Kingdom.13-15 We hypothesize that there are differences in coagulation factors and other proteins between plasma from female and male donors which may affect the transfused host.
Materials and Methods
Reagents
Bovine serum albumin (BSA), ammonium bicarbonate, dithiothreitol (DTT), and iodoacetamide were all purchased from Sigma-Aldrich. Formic acid (FA) was obtained from Fluka (Buchs, Switzerland), and acetonitrile was from Burdick and Jackson (Morristown, NJ). Trypsin (sequencing grade, l-1-tosylamido-2-phenylethyl chloromethyl ketone-treated) was from Promega (Madison, WI). Antibodies for immunoblotting were purchased from Santa Cruz (Santa Cruz, CA).
Human Blood Plasma Samples
Units of FDA-licensed plasma (FP24) were collected from 5 healthy male donors (all A+, age 59.8 years, range 45-73) and 5 healthy antibody-negative female, nulliparous, donors (3 O+ and 2 A+, age 41 years, range 27-52) per industry standards via the Standard Operating Procedures of Bonfils Blood Center. Aliquots of plasma were drawn through sterile couplers from the original plasma unit prior to freezing, and freezing was completed 10 hours of collection with all samples remaining at −80°C until use. All proteomic analyses were complete within 2 months of storage.
Immunoaffinity Depletion of High-Abundance Proteins
The 14 most abundant proteins (albumin, IgG, α1-anti-trypsin, IgA, transferrin, haptoglobin, fibrinogen, α2-macroglobulin, α1-acid glycoprotein, IgM, apolipoprotein AI, apolipoprotein AII, complement C3, and transthyretin) were depleted from plasma using the antibody-based multiple affinity removal spin cartridge (Agilent Technologies, Santa Clara, CA, USA). Plasma (10 μl) was diluted with 190 μl of buffer A and centrifuged through a 0.22 μm filter at 5,000 × g for 5 minutes to remove particulates. The filtered sample was loaded onto the Multiple Affinity Removal Spin Cartridge. The sample was incubated at room temperature for 5 min and passed into the column at slow speed (100 × g for 1.5 minutes). The column was washed twice with 400 μL of equilibration buffer A, and centrifuged (2.5 min, 100 × g) to collect the total unbound and wash fractions. The bound fraction was eluted with 2.5 mL of manufacturer's elution buffer B. The cartridge was recycled for further use by washing with 5 mL of buffer A. Both the flow-through fraction containing unbound proteins and wash fractions were pooled and concentrated with a 5 kDa MWCO spin concentrator at 3000 rpm for 30 min at 4°C to a volume of 200 μL. Total protein content was determined by the BCA protein assay at 595 nm.
One-dimensional gel electrophoresis
A portion of the sample (30 μg) was diluted into NuPAGE® LDS Sample Buffer (4X) (Invitrogen, Paisley, Renfrewshire, UK) and heated for 10 minutes at 70°C. The samples were loaded onto a 1 mm thick NuPage 4-12% Bis-Tris gel (Invitrogen). The BenchMark™ Protein Ladder (Invitrogen) was used as a protein molecular mass marker. The gel was electrophoresed, using MES SDS running buffer, in an X-Cell II mini gel system (Invitrogen) at 200 V, 120 mA, 25 W per gel for 30 minutes. Proteins were visualized using Coomassie Blue (Invitrogen) followed by destaining in 10% ethanol and 7.5% acetic acid. Each lane of the gel was divided into 10 equal-sized pieces and proteins in the gel were digested as follows.
In-gel tryptic digestion
Excised gel pieces were destained in 200 μl of 25 mM ammonium bicarbonate in 50% v/v ACN for 15 min, and then 200 μl of 100% ACN was applied for 15 min at room temperature. Reduction of disulfide bonds was achieved by addition of 10 mm DTT for 30 min at 65 °C. After cooling to room temperature, the free cysteine residues were alkylated with 20 mM iodoacetamide in the dark at room temperature for 45 min. The iodoacetamide was then removed, and washes were performed with 200 μl of distilled water followed by addition of 100 μl of ACN. Then ACN was removed, and 50 μl of the 0.01 μg/μl trypsin solution was added to each plug and allowed to rehydrate the gel plugs at 4°C for 30 min and then incubated at 37°C overnight. The tryptic mixtures were acidified with formic acid up to a final concentration of 1%. Peptides were extracted three times from the gel plugs using 50% ACN, 1% FA, concentrated by SpeedVac™ to a desired volume (∼18 μl), and subjected to LC-MS/MS analysis. If necessary, they were stored at −20 °C
Liquid Chromatography–Tandem Mass Spectrometry
Digested samples were analyzed with a capillary HPLC system (Agilent 1200, Palo Alto, CA) coupled with a linear ion trap mass spectrometer LTQ-XL Linear Ion Trap Mass Spectrometer (Thermo Fisher; San Jose, CA) through an in-house built a nanoelectrospray ionization source. Two microliters of tryptic digest sample was injected onto a reverse phase C18 column (75 μm ID × 360 μm OD × 100 mm length) packed in-house with 4 μm, 100 Å pore (Synergy, Phenomenex, Torrance, CA) kept at a constant 40°C using an in-house built column heater with a flow rate of 120 μL/min before the T-split and 380 nL/min postsplit (Agilent, Santa Clara, CA). Mobile-phase A was 5% acetonitrile with 0.1% formic, and mobile-phase B was 95% acetonitrile with 0.1% formic acid. A 90-min linear gradient from 5 to 50% B was employed for peptide separation. Data acquisition was performed using the instrument supplied Xcalibur (version 2.0.6) software. The LC runs were monitored in positive ion mode by sequentially recording survey MS scans (m/z 400-2000), MS2 were obtained in the ICR cell. The spray voltage was set at 2 kV; the ion transfer capillary temperature was set at 200 °C; and the normalized collision energy for MS/MS decomposition of peptides was set at 35%. With regard to sensitivity, it is typical for this analytical platform to yield 150-400 protein identifications from 30 μg of total protein from complex samples such as plasma.
Database searching, protein identification
MS/MS spectra were extracted from raw data files and converted into .mgf files using PAVA (UCSF, MSF, San Francisco, CA). Mascot (version 2.2; Matrix Science Inc., London, UK) was used to perform database searches against the human subset SwissProt database of the extracted MS/MS data. Peptide tolerance was set at ± 10 ppm with MS/MS tolerance set at ± 0.6 Da. Trypsin specificity was used allowing for 1 missed cleavage. The modifications of Met oxidation, protein N-terminal acetylation, and peptide N-terminal pyroglutamic acid formation were allowed for, and Cys-carbamidomethylation was set as a fixed modification.
Scaffold (version 2, Proteome Software, Portland, OR, USA) was used to validate MS/MS based peptide and protein identifications. Peptide identifications were accepted if they could be established at greater than 95.0% probability as specified by the Peptide Prophet algorithm. Protein identifications were accepted if they could be established at greater than 99.0% probability and contained at least two identified unique peptides.
Both unique peptides and total peptides per protein were calculated for each patient and normalized to total unique and total identified peptides per sample. These values were used to perform spectral counting for a semi-quantitative comparison between groups. A Student's t-test (2-tailed) was used to determine statistical significance (p<.005) between the two groups for each protein.
Factor V activity assays
Factor V activity assays were performed In the Hematology Laboratory at The Children's Hospital Aurora, CO. Factor V activity was measured on a STA-R Evolution coagulation analyzer (Diagnostica Stago, Asnieres sur Seine, France), which employs Factor V deficient plasma (Precision BioLogic, Nova Scotia, Canada) and lyophilized thromboplastin (Diagnostica Stago, Asnieres sur Seine, France). Briefly, Factor V deficient plasma is mixed with the patient's plasma and calcium thromboplastin to measure the clotting time, the degree of correction of the PT is proportional to the level of Factor V in the patient plasma and is compared with that of a normal standard curve (the clotting times are on Y axis and the percentages of Factor V activity are on x-axis) to obtain a quantitative activity of Factor V of the patient. These data were completed in a blinded fashion so that the Medical Technologist did not know the donor gender of the plasma sample.
Western Blotting of plasma proteins
Plasma samples (25 μl) were digested in sodium dodecyl sulfate (SDS)-digestion buffer with protease inhibitors, loaded onto 10% or 15% SDS-polyacrylamide gels, and the proteins separated.16 The separated proteins were transferred to nitrocellulose and immunoblotted with antibodies specific for α1-antitrypsin and β2-microglobulin, as previously described.16 Densitometry was completed using Image J software (National Institutes of Health) to determine the average amounts of the protein in plasma units from male vs. female donors.
Results
Proteomic Analysis
Plasma from 5 males and 5 nulliparous (never been pregnant) females has been analyzed using a label-free differential proteomics approach. The 14 most abundant proteins in human plasma, albumin & globulins, were removed via affinity chromatography prior to analysis to maximize the number of identifications. Mass spectrometry analysis resulted in the identification of 231 total proteins (please see Supplementary Table 1), and proteins that were differentially present in female vs. male plasma appear in Table 1. These proteins include significant increases in females (vs. males, p<.005) of pregnancy zone protein (419- to 580-fold), coagulation factor V (2-fold), α1-antitrypsin (2-fold), β2-microglobulin (2-fold), and complement factors H and C4B (1.5-fold). In males (vs. females, p<.005) there were increases in the Fc-binding protein (2-fold), protein Z-dependent protease inhibitor (2-fold), phosphatidylinositol-glycan specific phospholipase (4-fold), protein S-100 (3-fold) and transgelin-2 (14-fold). Therefore, transfusion of male plasma would appear to result in decreased levels of factor V and decreased anti-protease activity when compared to female plasma. Importantly, all the male donors were A+, and the female donors consisted of 3 O+ and 2 A+ individuals. No differences were observed when A+ and O+ females were compared (data not shown).
Table 1. The Plasma Proteome: Female vs. Male.
| Female (fold increase over Male) |
Male (fold increase over Female) |
|
|---|---|---|
| Pregnancy Zone Protein | 419-580 | |
| Coagulation Factor V | 2.0 | |
| α1-antitrypsin | 2.0 | |
| β2-microglobulin | 2.0 | |
| Complement Factors H and C4B | 1.5-2 | |
| Fc Binding Protein | 2.0 | |
| Protein Z-dependent Protease Inhibitor | 2.0 | |
| Phosphatidylinositol-glycan specific phospholipase | 4.0 | |
| Protein S-100 | 3.0 | |
| Transgelin-2 | 14.0 | |
| p<0.005 for all | ||
Factor V activity assays
To validate our findings for Factor V, an activity assay was used that is based on the use of Factor V deficient plasma, activation with thromboplastin, and the use of a standard curve of known Factor V activity. The results confirmed significantly higher Factor V activity in the female samples 101±5 vs. males 77±3% activity. The lowest concentration of factor V in female plasma (88%) was greater than the highest concentration in male plasma (86%).
Western blotting of α1-antitrypsin and β2-microglobulin
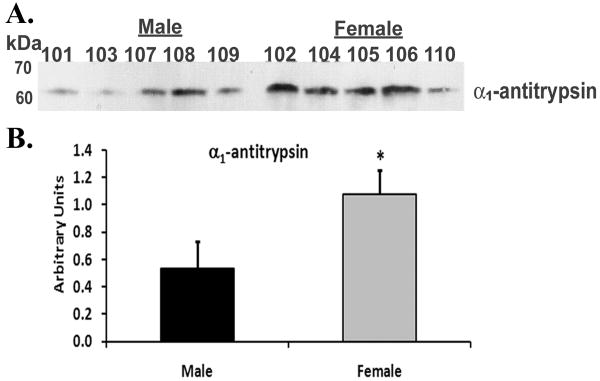
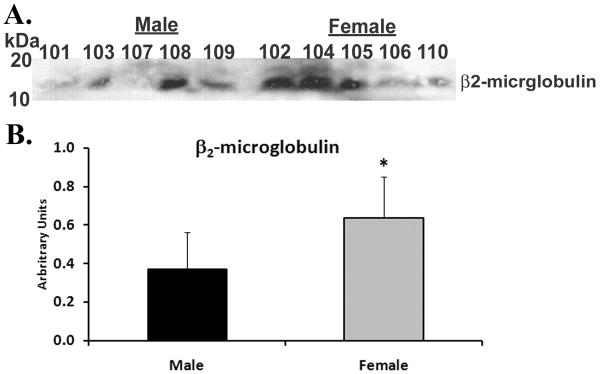
Immunoblotting of unmodified plasma demonstrated that there was more α1-antitrypsin immunoreactivity in the plasma from female donors than in male donors (female 1.1±0.2* vs. male 0.54±0.2, *=p<.05, Fig.1). Similarly, the Western blot signal for β2-microglobulin was also elevated in the plasma from female vs. male donors (female: 0.64±0.2* vs. male: 0.37±0.2, *=p<.05, Figs.2). These data provide confirmation of the differences seen via our label-free proteomic approach using standard biochemical techniques.
Figure 1. Immunoblot of α1-antitrypsin from plasma of females vs. males.
Plasma proteins were separated by 10% SDS polyacrylamide electrophoresis, transferred to nitrocellulose and α1-antitrypsin immunoreactivity (panel A) was visualized by western blotting. In the aggregate there is more total immunoreactivity for α1-antitrypsin in female plasma, vs. male plasma, as documented by the densitometry of the resulting bands of immunoreactivity depicted in the bar graph (panel B). This figure is representative of two separate experiments.
Figure 2. Immunoblot of β2-microglobulin from plasma of females vs. males.
Plasma proteins were separated by 10% SDS polyacrylamide electrophoresis, transferred to nitrocellulose β2-microglobulin immunoreactivity (panel A) was visualized by western blotting. In the aggregate there is more total immunoreactivity for β2-microglobulin in female plasma vs. male plasma as documented by the densitometry of the bands of immunoreactivity presented in the bar graph (panel B). This figure is representative of two separate experiments.
Discussion
The presented data identified approximately 231 distinct proteins after the 14 most abundant proteins, albumin, etc., were removed by affinity chromatography. Of no surprise, female plasma had higher levels of pregnancy zone protein, which is directly related to the sex hormones responsible for gender differences. Surprisingly, females also had significantly more factor V and increased amounts of α1-antitrypsin and β2-microglobulin. These differences were confirmed by standard biochemical assays, including: factor V activity in a CAP, CLIA and FDA-certified clinical coagulation laboratory and western blotting, respectively. Males plasma showed an increase in Fc-binding protein, protein Z-dependent protease inhibitor, phosphatidylinositol-glycan specific phospholipase, protein S-100, and transgelin-2. One limitation, other than small sample size, is that this study includes data from only 5 A+ males and 3 A+ and 2 O+ females. However, none of the proteins that demonstrated numerical distinctions between male and female donors have been shown to differ among ABO or Rh blood groups, similar to factor VIII or von Willebrand factor, and there were no significant differences noted between the plasma from A+ or O+ females.4 In addition, the complement factors H and C4B, demonstrated a 1.5- to 2-fold difference in our analysis, the smallest fold difference of the proteins analyzed with a statistical difference between the spectral counts of p<.005.
Factor V is a vital coagulation co-factor that requires Ca2+ and a platelet phospholipid surface to accelerate the formation of factor Xa from hours to seconds, and allows for the productive recruitment of pro-thrombin to the platelet membrane for efficient thrombin generation. 4 In addition, free Factor Va markedly facilitates clearance of the Va:Xa complex to decrease widespread thrombin generation.4,17 Factor V levels increase with age 6-7.6% per decade of life.18,19 Deficiencies in Factor V results in minor to major bleeding and the treatment involves the infusion of frozen plasma; however, factor V is the most labile of the coagulation factors in frozen plasma such that one should transfuse plasma of 1-2 months storage age to treat deficiency.4,17,20 The presented proteomic data demonstrates that factor V procoagulant levels are higher in the donated plasma units from 5 females than 5 males despite the males being significantly older (Males: 59.8 years vs. females: 41 years, p=0.02). This difference was confirmed by a factor V activity assay employing factor V-deficient plasma. However, one would expect that factor V levels in male plasma, especially from significantly older males, would be greater or equal to factor V levels in the plasma from younger female donors, which was not the case. Furthermore, the factor V levels are from plasma units that were stored for identical times and not from freshly isolated plasma samples as was the case for previous studies that concluded that there is not a difference in factor V from males and females.18,19 Further studies with a large population of donors is required to corroborate these findings.
α-1-antitrypsin is the best known serine protease inhibitor of the serpin family which has a structural conformation that allows it to bind tightly and inhibit serine proteases, including: neutrophil elastase, cathepsin G, and proteinase 3.21-23 α-1-antitrypsin deficiency is manifested by protease-induced bronchiectasis, lung injury, and liver damage including: cholestasis, cirrhosis, hepatocellular carcinoma, and neutrophil cutaneous panniculitis. 21-23 α-1-antitrypsin inhibits neutrophil elastase both in vitro and in vivo and has been successful in treating the pulmonary but not the liver manifestations of α-1-antirypsin deficiency, and adults with cystic fibrosis in a small clinical study.21-23 From our previous experience with immunoaffinity depletion (ID) of plasma for MS analysis it was not surprising to observe peptides arising from proteins that the ID column was directed against. In general, we observe 85 to 98% depletion efficiency of the intact protein as long as the binding capacity of the column is not exceeded. While the antibodies used are usually polyclonal there is the possibility that cleavage or modifications could prevent epitope recognition. To validate the difference observed in α1-antitrypsin levels we performed Immunoblotting of unmodified (undepleted) plasma and found the aforementioned differences in female vs. male plasma.
β2-microglobulin, which is the light or β-chain of the HLA class I molecule, is present in serum, urine, and synovial fluid, and because of its purely renal excretion has been used as a marker of renal function.24,25 β2-microglobulin is increased in the serum or plasma of patients with chronic kidney disease, hematological malignancies, auto-immune disease, especially systemic lupus erythematosis, infections and in lymphoproliferative disorders, e.g. myeloma; however, little data exists about plasma levels in healthy adults.24-30 In the sera, adolescent males are reported to have higher levels of β2-microglobulin than females; however, β2-microglobulin levels in the sera from adults demonstrate no increase in males and a mild increase with age.29,31 To the best of our knowledge there are no reports of relative β2-microglobulin plasma levels in males versus females.
Increases in the complement proteins C4 and factor H have not been linked to clinical disease; however deficiencies of C4 result in immune complex disease and deficiencies of factor H have been linked to recurrent pyogenic infections.32 C4 is the substrate for C1 to activate the classical pathway of complement activation and Factor H, along with Factor I, controls activation of the alternative pathway.32 There are no data with regard to gender differences in the complement proteins.
In FDA-licensed plasma from male donors a number of proteins were increased vs. the proteins from female plasma. Protein S-100 is a protein expressed by neural glia in the central nervous system and is used as a marker for brain tumors and other malignancies and lymphoproliferative diseases.33,34 The function of S-100 proteins are multifaceted and have not been clearly defined.33,34
Protein Z-dependent protease inhibitor (ZPI) is a member of the serpin superfamily of protease inhibitors which inhibits both Factor Xa and Factor Xia activity with the former requiring protein Z binding factor Xa in a Ca2+-dependent fashion.35,36 ZPI is consumed during coagulation; however, it forms weak complexes with factor Xa and Xia and serves as a co-factor with protein Z to dampen coagulation.35,36
Fc-binding protein is widely expressed in the tissues of human and on the epithelial surface, especially the mucosal epithelium, and it is functionally intact in mucus, saliva, and other fluids and appears to be an important part of mucosal membrane defenses.37-39 In addition, Fc-binding proteins can inhibit complement mediated interactions and help protect the host from foreign immunoglobulins.37-39
Transgelin-2 is a structural protein from smooth muscle that has been reported to be a marker of lung adenocarcinoma.40,41 Furthermore, transgelin-2 appears to be synthesized by B-1 and not B-2 lymphocytes from the spleen.40. The function of transgelin-2 remains unknown, and although it is reported to be a structural protein of smooth muscle, its presence in epithelial stroma, B-1 lymphocytes, and pulmonary adenocarcinoma remain undefined.40,41
Lastly, phosphatidylinositol-glycan specific phospholipase is an intracellular phospholipase responsible for the generation of second messengers from phosphatidylinositol 4,5-bisphosphate (PIP2) most notably inositol 1,4,5-trisphosphate (IP3) and inositol 1,3,4,5-tetrakisphosphate (IP4).42-44 Its significance in male plasma is entirely unknown but it appeared in all 5 plasma samples and was significantly increased in the plasma units from male versus female donors.
Recent data has demonstrated differences between patients with solid tumors and healthy controls to some of the more abundant proteins removed by affinity depletion in these studies, namely α-1-antitrypsin, transthyretin, and apolipoprotein AI.45 Follow-up studies demonstrated that both transthyretin and apolipoprotein AI (both decreased) used in combination with the tumor marker CA125 were reliable predictors of early-stage ovarian cancer; however none of these proteins alone were sensitive or specific enough to predict ovarian cancer. Follow-up data has not demonstrated the specificity or the sensitivity of the earlier studies.46-48 In addition, both transthyretin and α-1-antitrypsin have been implicated as sensitive and specific tumor markers of pancreatic cancer; conversely, newer data has demonstrated that when the studies accounted for obstructive jaundice, only transthyretin remained as a sensitive and specific tumor marker.45,49,50 Further work is needed to ascertain the best tumor markers for specific age groups, it is notable that differences in α-1-antitrypsin via gender in healthy adult blood donors were found in the presented data set.
Our data indicate that despite the small sample size used for discovery there are protein differences in FDA-licensed plasma that reflect donor gender. Several differences were corroborated by standard biochemical quantification: factor V, β2-microglobulin, and α-1-antitrypsin. It is possible that our observed differences are artifacts due to the small sample size, and studies with larger populations need to be completed to validate these data. The roles of proteins found at higher abundance in either male or female plasma remain undefined; nevertheless, these results were not foreseen. Plasma infusions are important to replace coagulation factors, especially factors II, V, and VII and a case may be made for factor X in injured patients. In addition, the role of proteases in acute organ injury and multiple organ failure have been suggested, and if correct, female plasma could more efficiently be used as part of resuscitation to augment factor V levels, to increase circulating anti-protease concentrations, and to potentially decrease indiscriminant alternative pathway activation of complement. More boldly stated, this study suggests that there are “trade-offs” with every policy including the transfusion of male-only plasma such that while a specific policy may help increase blood safety with some patients, e.g. TRALI, it may reduce the optimal transfusion benefits for other patient types. Clearly, though, these advantages are theoretical and would require careful outcome analyses in well-matched patient cohorts before any definitive statement could be made.
Supplementary Material
Acknowledgments
This grant was supported in part by Bonfils Blood Center, grant GM-49222 from NIGMS, P30 CA046934-17 through the University of Colorado Comprehensive Cancer Center Core Support and S10RR023015 from the NCRR, NIH, and the Departments of Pediatrics and Surgery, School of Medicine University of Colorado Denver
References
- 1.Gonzalez EA, Moore FA, Holcomb JB, Miller CC, Kozar RA, Todd SR, Cocanour CS, Balldin BC, McKinley BA. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007 Jan;62(1):112–9. doi: 10.1097/01.ta.0000250497.08101.8b. [DOI] [PubMed] [Google Scholar]
- 2.Hardy JF, de M P, Samama M. Massive transfusion and coagulopathy: pathophysiology and implications for clinical management. Can J Anaesth. 2004 Apr;51(4):293–310. doi: 10.1007/BF03018233. [DOI] [PubMed] [Google Scholar]
- 3.Hardy JF, de M P, Samama CM. The coagulopathy of massive transfusion. Vox Sang. 2005 Oct;89(3):123–7. doi: 10.1111/j.1423-0410.2005.00678.x. [DOI] [PubMed] [Google Scholar]
- 4.Hathaway WE, Goodnight SH. Disorders of Hemostasis and Thrombosis: A Clinical Guide. First. New York: McGraw-Hill Inc.; 1993. pp. 3–20. [Google Scholar]
- 5.Hess JR. Blood and coagulation support in trauma care. Hematology Am Soc Hematol Educ Program. 2007;2007:187–91. doi: 10.1182/asheducation-2007.1.187. [DOI] [PubMed] [Google Scholar]
- 6.Kashuk JL, Moore EE, Johnson JL, Haenel J, Wilson M, Moore JB, Cothren CC, Biffl WL, Banerjee A, Sauaia A. Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma. 2008 Aug;65(2):261–70. doi: 10.1097/TA.0b013e31817de3e1. [DOI] [PubMed] [Google Scholar]
- 7.Deitcher SR. Interpretation of the international normalised ratio in patients with liver disease. Lancet. 2002 Jan 5;359(9300):47–8. doi: 10.1016/S0140-6736(02)07282-3. [DOI] [PubMed] [Google Scholar]
- 8.MacLennan S, Williamson LM. Risks of fresh frozen plasma and platelets. J Trauma. 2006 Jun;60(6 Suppl):S46–S50. doi: 10.1097/01.ta.0000199546.22925.31. [DOI] [PubMed] [Google Scholar]
- 9.Bux J. Antibody-mediated (immune) transfusion-related acute lung injury. Vox Sang. 2011 Jan;100(1):122–8. doi: 10.1111/j.1423-0410.2010.01392.x. [DOI] [PubMed] [Google Scholar]
- 10.Holness L, Knippen MA, Simmons L, Lachenbruch PA. Fatalities caused by TRALI. Transfus Med Rev. 2004 Jul;18(3):184–8. doi: 10.1016/j.tmrv.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Looney MR, Gropper MA, Matthay MA. Transfusion-related acute lung injury: a review. Chest. 2004 Jul;126(1):249–58. doi: 10.1378/chest.126.1.249. [DOI] [PubMed] [Google Scholar]
- 12.Shaz BH, Stowell SR, Hillyer CD. Transfusion-related acute lung injury: from bedside to bench and back. Blood. 2011 Feb 3;117(5):1463–71. doi: 10.1182/blood-2010-04-278135. [DOI] [PubMed] [Google Scholar]
- 13.Chapman CE, Stainsby D, Jones H, Love E, Massey E, Win N, Navarrete C, Lucas G, Soni N, Morgan C, et al. Ten years of hemovigilance reports of transfusion-related acute lung injury in the United Kingdom and the impact of preferential use of male donor plasma. Transfusion. 2009 Mar;49(3):440–52. doi: 10.1111/j.1537-2995.2008.01948.x. [DOI] [PubMed] [Google Scholar]
- 14.Eder AF, Herron RM, Jr, Strupp A, Dy B, White J, Notari EP, Dodd RY, Benjamin RJ. Effective reduction of transfusion-related acute lung injury risk with male-predominant plasma strategy in the American Red Cross (2006-2008) Transfusion. 2010 Aug;50(8):1732–42. doi: 10.1111/j.1537-2995.2010.02652.x. [DOI] [PubMed] [Google Scholar]
- 15.Williamson LM, Stainsby D, Jones H, Love E, Chapman CE, Navarrete C, Lucas G, Beatty C, Casbard A, Cohen H. The impact of universal leukodepletion of the blood supply on hemovigilance reports of posttransfusion purpura and transfusion-associated graft-versus-host disease. Transfusion. 2007 Aug;47(8):1455–67. doi: 10.1111/j.1537-2995.2007.01281.x. [DOI] [PubMed] [Google Scholar]
- 16.McLaughlin NJ, Banerjee A, Kelher MR, Gamboni-Robertson F, Hamiel C, Sheppard FR, Moore EE, Silliman CC. Platelet-activating factor-induced clathrin-mediated endocytosis requires beta-arrestin-1 recruitment and activation of the p38 MAPK signalosome at the plasma membrane for actin bundle formation. J Immunol. 2006 Jun 1;176(11):7039–50. doi: 10.4049/jimmunol.176.11.7039. [DOI] [PubMed] [Google Scholar]
- 17.Soff GA, Rosenberg RD. Physiology of Hemostasis: the Fluid Phase. In: Nathan DG, Oski FA, editors. Hematology of Infancy and Childhood. 4th. Philadelphia: W. B. Saunders; 1993. pp. 1534–60. [Google Scholar]
- 18.Brozovic M, Chakrabarti R, Stirling Y, Fenton S, North WR, Meade TW. Factor V in an industrial population. Br J Haematol. 1976 Aug;33(4):543–50. doi: 10.1111/j.1365-2141.1976.tb03573.x. [DOI] [PubMed] [Google Scholar]
- 19.Kamphuisen PW, Rosendaal FR, Eikenboom JC, Bos R, Bertina RM. Factor V antigen levels and venous thrombosis: risk profile, interaction with factor V leiden, and relation with factor VIII antigen levels. Arterioscler Thromb Vasc Biol. 2000 May;20(5):1382–6. doi: 10.1161/01.atv.20.5.1382. [DOI] [PubMed] [Google Scholar]
- 20.Webster WP, Roberts HR, Penick GD. Hemostasis in Factor V Deficiency. Am J Med Sci. 1964 Aug;248:194–202. doi: 10.1097/00000441-196408000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Bals R. Alpha-1-antitrypsin deficiency. Best Pract Res Clin Gastroenterol. 2010 Oct;24(5):629–33. doi: 10.1016/j.bpg.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Griese M, Kappler M, Gaggar A, Hartl D. Inhibition of airway proteases in cystic fibrosis lung disease. Eur Respir J. 2008 Sep;32(3):783–95. doi: 10.1183/09031936.00146807. [DOI] [PubMed] [Google Scholar]
- 23.Mulgrew AT, Taggart CC, McElvaney NG. Alpha-1-antitrypsin deficiency: current concepts. Lung. 2007 Jul;185(4):191–201. doi: 10.1007/s00408-007-9009-y. [DOI] [PubMed] [Google Scholar]
- 24.Cunningham BA, Berggard I. Structure, evolution and significance of beta2-microglobulin. Transplant Rev. 1974;21(0):3–14. doi: 10.1111/j.1600-065x.1974.tb01543.x. [DOI] [PubMed] [Google Scholar]
- 25.Drueke TB, Massy ZA. Beta2-microglobulin. Semin Dial. 2009 Jul;22(4):378–80. doi: 10.1111/j.1525-139X.2009.00584.x. [DOI] [PubMed] [Google Scholar]
- 26.Drueke TB. Beta2-microglobulin and amyloidosis. Nephrol Dial Transplant. 2000;15 1:17–24. doi: 10.1093/oxfordjournals.ndt.a027958. [DOI] [PubMed] [Google Scholar]
- 27.Kim HA, Jeon JY, Yoon JM, Suh CH. Beta 2-microglobulin can be a disease activity marker in systemic lupus erythematosus. Am J Med Sci. 2010 Apr;339(4):337–40. doi: 10.1097/MAJ.0b013e3181d26dfb. [DOI] [PubMed] [Google Scholar]
- 28.Kristensen K, Lindstrom V, Schmidt C, Blirup-Jensen S, Grubb A, Wide-Swensson D, Strevens H. Temporal changes of the plasma levels of cystatin C, beta-trace protein, beta2-microglobulin, urate and creatinine during pregnancy indicate continuous alterations in the renal filtration process. Scand J Clin Lab Invest. 2007;67(6):612–8. doi: 10.1080/00365510701203488. [DOI] [PubMed] [Google Scholar]
- 29.Matrai Z, Nemeth J, Miklos K, Szabo Z, Masszi T. Serum beta2-microglobulin measured by immunonephelometry: expression patterns and reference intervals in healthy adults. Clin Chem Lab Med. 2009;47(5):585–9. doi: 10.1515/CCLM.2009.137. [DOI] [PubMed] [Google Scholar]
- 30.Ohashi K. Pathogenesis of beta2-microglobulin amyloidosis. Pathol Int. 2001 Jan;51(1):1–10. doi: 10.1046/j.1440-1827.2001.01156.x. [DOI] [PubMed] [Google Scholar]
- 31.Satoh T, Brown LM, Blattner WA, Maloney EM, Kurman CC, Nelson DL, Fuchs D, Wachter H, Tollerud DJ. Serum neopterin, beta2-microglobulin, soluble interleukin-2 receptors, and immunoglobulin levels in healthy adolescents. Clin Immunol Immunopathol. 1998 Aug;88(2):176–82. doi: 10.1006/clin.1998.4568. [DOI] [PubMed] [Google Scholar]
- 32.Geha RS, Rosen FS, Chatila T. Primary Immunodeficiency Diseases. In: Nathan DG, Oski FA, editors. Hematology of Infancy and Childhood. 4th. Philadlephia: WB Saunders Company; 1993. pp. 1033–57. [Google Scholar]
- 33.Isobe T, Nakajima T, Okuyama T. Reinvestigation of extremely acidic proteins in bovine brain. Biochim Biophys Acta. 1977 Sep 27;494(1):222–32. doi: 10.1016/0005-2795(77)90150-7. [DOI] [PubMed] [Google Scholar]
- 34.Tiulenev VI, Kapralov AA, Belik I. Role of S-100 protein in the function of brain cell nuclei. Ukr Biokhim Zh. 1996 May;68(3):3–13. [PubMed] [Google Scholar]
- 35.Han X, Fiehler R, Broze GJ., Jr Characterization of the protein Z-dependent protease inhibitor. Blood. 2000 Nov 1;96(9):3049–55. [PubMed] [Google Scholar]
- 36.Rau JC, Beaulieu LM, Huntington JA, Church FC. Serpins in thrombosis, hemostasis and fibrinolysis. J Thromb Haemost. 2007 Jul;5 1:102–15. doi: 10.1111/j.1538-7836.2007.02516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi K, Ogata H, Morikawa M, Iijima S, Harada N, Yoshida T, Brown WR, Inoue N, Hamada Y, Ishii H, et al. Distribution and partial characterisation of IgG Fc binding protein in various mucin producing cells and body fluids. Gut. 2002 Aug;51(2):169–76. doi: 10.1136/gut.51.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raeder R, Faulmann EL, Boyle MD. Evidence for functional heterogeneity in IgG Fc-binding proteins associated with group A streptococci. J Immunol. 1991 Feb 15;146(4):1247–53. [PubMed] [Google Scholar]
- 39.Tashiro M, Montelione GT. Structures of bacterial immunoglobulin-binding domains and their complexes with immunoglobulins. Curr Opin Struct Biol. 1995 Aug;5(4):471–81. doi: 10.1016/0959-440x(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 40.Frances R, Tumang JR, Kaku H, Gurdak SM, Rothstein TL. B-1 cells express transgelin 2: unexpected lymphocyte expression of a smooth muscle protein identified by proteomic analysis of peritoneal B-1 cells. Mol Immunol. 2006 May;43(13):2124–9. doi: 10.1016/j.molimm.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 41.Rho JH, Roehrl MH, Wang JY. Tissue proteomics reveals differential and compartment-specific expression of the homologs transgelin and transgelin-2 in lung adenocarcinoma and its stroma. J Proteome Res. 2009 Dec;8(12):5610–8. doi: 10.1021/pr900705r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamisaka Y, Toyoshima S, Osawa T. Phosphatidylinositol-specific phospholipase C of murine lymphocytes. Arch Biochem Biophys. 1986 Sep;249(2):569–78. doi: 10.1016/0003-9861(86)90035-4. [DOI] [PubMed] [Google Scholar]
- 43.Saleh SN, Albert AP, Large WA. Activation of native TRPC1/C5/C6 channels by endothelin-1 is mediated by both PIP3 and PIP2 in rabbit coronary artery myocytes. J Physiol. 2009 Nov 15;587(Pt 22):5361–75. doi: 10.1113/jphysiol.2009.180331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang P, Toyoshima S, Osawa T. Physical and functional association of cytosolic inositolphospholipid-specific phospholipase C of calf thymocytes with a GTP-binding protein. J Biochem. 1987 Nov;102(5):1275–87. doi: 10.1093/oxfordjournals.jbchem.a122166. [DOI] [PubMed] [Google Scholar]
- 45.Diamandis EP, van der Merwe DE. Plasma protein profiling by mass spectrometry for cancer diagnosis: opportunities and limitations. Clin Cancer Res. 2005 Feb 1;11(3):963–5. [PubMed] [Google Scholar]
- 46.Cramer DW, Bast RC, Jr, Berg CD, Diamandis EP, Godwin AK, Hartge P, Lokshin AE, Lu KH, McIntosh MW, Mor G, et al. Ovarian cancer biomarker performance in prostate, lung, colorectal, and ovarian cancer screening trial specimens. Cancer Prev Res (Phila) 2011 Mar;4(3):365–74. doi: 10.1158/1940-6207.CAPR-10-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore LE, Fung ET, McGuire M, Rabkin CC, Molinaro A, Wang Z, Zhang F, Wang J, Yip C, Meng XY, et al. Evaluation of apolipoprotein A1 and posttranslationally modified forms of transthyretin as biomarkers for ovarian cancer detection in an independent study population. Cancer Epidemiol Biomarkers Prev. 2006 Sep;15(9):1641–6. doi: 10.1158/1055-9965.EPI-05-0980. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Z, Bast RC, Jr, Yu Y, Li J, Sokoll LJ, Rai AJ, Rosenzweig JM, Cameron B, Wang YY, Meng XY, et al. Three biomarkers identified from serum proteomic analysis for the detection of early stage ovarian cancer. Cancer Res. 2004 Aug 15;64(16):5882–90. doi: 10.1158/0008-5472.CAN-04-0746. [DOI] [PubMed] [Google Scholar]
- 49.Matsubara J, Ono M, Honda K, Negishi A, Ueno H, Okusaka T, Furuse J, Furuta K, Sugiyama E, Saito Y, et al. Survival prediction for pancreatic cancer patients receiving gemcitabine treatment. Mol Cell Proteomics. 2010 Apr;9(4):695–704. doi: 10.1074/mcp.M900234-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yan L, Tonack S, Smith R, Dodd S, Jenkins RE, Kitteringham N, Greenhalf W, Ghaneh P, Neoptolemos JP, Costello E. Confounding effect of obstructive jaundice in the interpretation of proteomic plasma profiling data for pancreatic cancer. J Proteome Res. 2009 Jan;8(1):142–8. doi: 10.1021/pr800451h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.