Abstract
Although schizophrenia and schizoaffective disorders have both similar and differing clinical features, it is not well understood whether similar or differing pathophysiological processes mediate patients’ cognitive functions. Using psychophysical methods, this study compared the performances of schizophrenia (SZ) patients, patients with schizoaffective disorder (SA), and a healthy control group in two face-related cognitive tasks: emotion discrimination, which tested perception of facial affect, and identity discrimination, which tested perception of non-affective facial features. Compared to healthy controls, SZ patients, but not SA patients, exhibited deficient performance in both fear and happiness discrimination, as well as identity discrimination. SZ patients, but not SA patients, also showed impaired performance in a theory-of-mind task for which emotional expressions are identified based upon the eye regions of face images. This pattern of results suggests distinct processing of face information in schizophrenia and schizoaffective disorders.
Introduction
Schizophrenia (SZ) and schizoaffective disorder (SA) are two diagnoses within the psychotic disorder spectrum. It has been long debated whether similar or differing pathophysiological processes underlie schizophrenia and schizoaffective disorder, and more broadly, whether these diagnoses represent a single disorder or two distinct disorders. Evidence supporting the notion of a single disorder emerges from both electrophysiological and cognitive studies, where similar deficits in some aspects of cortical response and cognitive performance were found in both patient groups (Fiszdon et al 2007; Hooper et al 2010; Mathalon et al 2010). On the other hand, these and other studies also found differential deficits in other aspects of electrophysiological and cognitive responses between patients with SZ and with SA, supporting the notion of two distinct disorders (Mathalon et al 2010; Stip et al 1999). It appears that some pathophysiological and cognitive mechanisms overlap in the two disorders while others do not. Thus, identifying the pathophysiological and cognitive processes that distinguish schizophrenia and schizoaffective patients may provide new insights into the relationship between the two psychotic diagnoses.
At the phenomenological level, schizoaffective disorder is associated with prominent mood abnormalities (mania and/or depression) whereas schizophrenia is not. An interesting but poorly explored question is whether the differing clinical abnormalities in these two disorders are related to their cognitive ability to process affective vs. non-affective information. Previous studies have found that the processing of facial emotional stimuli is impaired in schizophrenia (Green et al 2009; Heimberg et al 1992; Norton et al 2009; Walker et al 1984). Because these studies combined patients with SA and SZ into a single group, the findings allow no inference about whether and how clinical abnormalities and impairments in the processing of external emotion information may be related in each of the psychotic disorders.
In this context, comparative studies in the facial processing domain may provide empirical opportunities for distinguishing the cognitive processes involved in schizophrenia and schizoaffective disorder. Using psychophysical methods, this study examined facial emotion discrimination in patients with SZ or SA. This study also included a facial identity discrimination task as a non-emotion comparison and the Eyes Test as a measure of Theory of Mind (Baron-Cohen et al 2001). To establish the relevance to differing clinical variations between the two diagnoses, the perceptual responses to affective and non-affective facial signals were compared with symptom scales (PANSS) (Kay et al 1987).
Methods
Subjects
The sample included schizophrenia (SZ) patients, patients with schizoaffective disorder (SA), and healthy controls (HC). The inclusion criteria for subjects were that they: 1) be between 18 – 65 years old, 2) have no history of drug or alcohol abuse in the six months prior to participation, 3) have no neurological problems such as seizure, stroke or major head injury, and 4) have an IQ > 70.
Patients were recruited from McLean Hospital as well as the Greater Boston area. They were diagnosed using the SCID-IV (First et al 2002b) by independent clinicians who were blind to the purposes of the study, and using all available medical records. Patients with SA had a chronic psychotic illness but were not in an acute mood episode at the time of participating in this study. The strategy of avoiding confounds of acute mood states allowed this study to focus on the trait-related abnormalities which are more closely linked to the underlying pathophysiology of the disorder. The clinical assessment of these patients is summarized in Table 1.
Table 1.
Comparison of Clinical Characteristics between Schizophrenia and Schizoaffective Groups
| SZ (n=19) |
SA (n=15) |
|
|---|---|---|
| Positive Symptom Subscale Scores1 | 15.3 (5.8) |
16.2 (7.1) |
| Negative Symptom Subscale Scores1 * | 19.3 (8.4) |
13.0 (4.9) |
| General Psychopathology Scores1 | 31.8 (7.9) |
29.9 (5.7) |
| Illness duration (year) | 18.1 (10.8) |
15.3 (10.3) |
| Antipsychotic dose (mg)2 | 563.5 (339.6) |
546.3 (372.3) |
| Proportion taking antidepressants (%) | 42.1 | 60.0 |
based upon the PANSS scale (Kay et al 1987)
calculated from chlorpromazine equivalent (Woods 2003)
Two groups differ significantly (p<0.05)
Healthy controls were recruited by posting advertisements in the local community. They were screened for exclusion of psychiatric illness using the SCID (NP version) (First et al 2002a).
The verbal component of the WAIS-R (Wechsler 1981) was administered to all subjects as a measure of general intelligence. This sample represents a group of subjects who participated in two previous studies with this research group (Chen et al 2009; Norton et al 2009). Demographic information of the sample is presented in Table 2.
Table 2.
Summary of Demographic Information of the Sample
| Group | Sex (M-male, F-female) |
Age (year) |
Verbal IQ* | Education (year) |
|---|---|---|---|---|
| Schizophrenia (n = 19) |
M = 11 F =8 |
42.6 (9.4) |
93.9 (10.9) |
12.6 (2.3) |
| Schizoaffective (n=15) |
M =7 F =8 |
38.9 (10.9) |
103.4 (12.9) |
15.3 (2.5) |
| Healthy Control (n = 30) |
M =13 F = 17 |
41.0 (14.4) |
110.4 (9.0) |
14.1 (3.0) |
Groups differ significantly (p<0.001).
Procedure
The procedure included the administration of two psychophysical tasks – emotion discrimination (Norton et al 2009) and identity discrimination (Chen et al 2009) – and of one standardized cognitive task known as the Eyes Test (Baron-Cohen et al 2001).
Emotion Discrimination
The targets were face images generated from the NimStim Face Stimulus Set (Tottenham et al 2009). The images contained various intensities of happy or fearful expressions, ranging from a neutral expression to a highly emotive one. The variation of emotion intensity was achieved by morphing between a facial image with a highly emotive expression (extreme happiness or extreme fearfulness) and one with a neutral expression. Morphing was done using FantaMorphPro 1.0 (2007) (Abrosoft Co.). Images were cropped to minimize the presence of hair and other non-face information. The extent to which an image contained information from the emotionally salient face (i.e. 100% emotional) was considered the emotional signal strength.
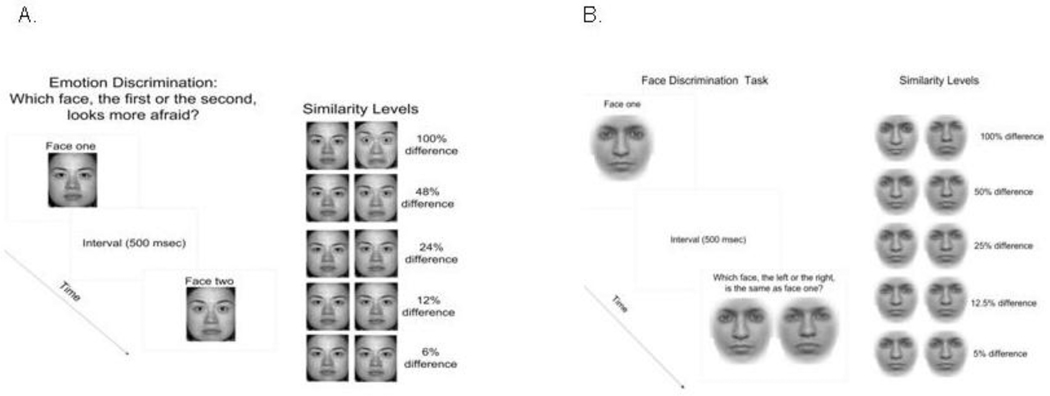
The task was to indicate which of two sequentially presented face images, one with a specific emotional intensity and the other with a neutral expression, was either happier or more fearful (Figure 1a). The time interval between two presentations was 500 msec. The critical measure was the just noticeable difference (JND) between the emotional and neutral faces at which subjects performed at the criterion of 80% correct. This JND can be extracted from a psychometric function of the percent correct scores, and is defined as the perceptual discrimination threshold (Chen et al 2005). The order in which the neutral and emotive images were presented was randomized. A two-alternative-forced-choice procedure was utilized to eliminate subjective bias in participants’ responses. The presentation of the face images was blocked such that a given session contained only one type of emotion (e.g. happiness). In each testing session, eight trials were repeated for each of the six emotion intensity levels (0, 6, 12, 24, 48, or 100%). There were four testing sessions in total: two identities (male and female)×two emotions (fear and happiness), which were presented in quasi-random order among subjects. The data derived from all emotional intensity conditions including 0% were used for threshold calculations. The 0% emotion intensity data were not used for accuracy analyses, since this condition compares two identical images, and is invalid as an accuracy measure. Accuracy was analyzed using mixed-model 3 (group: SA, SZ and HC)×5 (emotion intensity level: 6, 12, 24, 48 and 100 %) ANOVA. Separate ANOVAs were used for the two emotion conditions – one for fear discrimination and one for happiness discrimination. Thresholds were analyzed first using ANOVAs and further using Student’s t-tests.
Figure 1.
Illustrations of the face perception paradigms used in this study. Panel A is for emotion discrimination and Panel B is for identity discrimination.
Identity Discrimination
The targets were face images with neutral expressions, either the original images of two individuals or images morphed between them (Figure 1b). Morphing for identity discrimination was done using the software program Morph (Version 1.0) (1992) San Diego, CA: Gryphon Software.
The task was to determine which of two simultaneously presented face images was identical to a face image presented immediately prior. Each trial contained two presentations. The first presentation contained a single face image, while the second contained two face images side by side: one of which was identical to that of the first presentation, the other differing from it by varying degrees. The time interval between two presentations was 500 msec. During the testing session, sixteen trials were used for each of the five facial identity difference levels (5, 10, 20, 40, or 100%) and were distributed in a random order. The critical measure for identity discrimination was the just noticeable difference (JND) between the two comparison face images at which performance reached the criterion of 80% correct, which was extracted from the same psychometric function used in emotion discrimination. Accuracy was analyzed using mixed-model 3 (group: SA, SZ and HC)×5 (facial difference level: 5, 10, 20, 40 and 100 %) ANOVA. Perceptual thresholds of the groups were analyzed using Student’s t-tests.
Eyes Test
This is a widely used cognitive task which measures a person’s capacity to discriminate the mental states of others, or Theory of Mind, based on expressions in the eye region of the face (Baron-Cohen et al 2001). The targets were 36 images of actors’ and actress’s eyes depicting different types of emotions. The task was to view each of the serially presented images and select for each image which of four accompanying words best described the emotion that was being conveyed. Performance was measured by the proportion of the correct responses produced by each subject. Group differences in these percent-correct scores were analyzed using Student’s ttests.
Results
Fear Discrimination
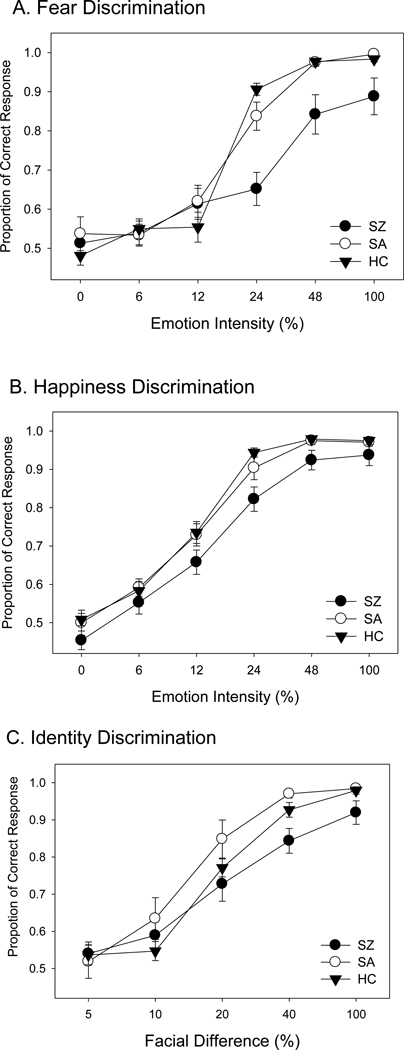
A two-way ANOVA of accuracy (group by emotion intensity) showed a significant group difference (F=12.8, p<0.001). The main effect for emotion intensity was also significant (F=109.8, p<0.001). The group-stimulus saliency interaction was significant as well (F=4.6, p<0.001). Post hoc tests showed significant effects between the SZ and SA groups (F=12.8, p<0.001) and between the SZ and HC groups (F=19.8, p<0.001) (Figure 2 A). However, the SA and HC groups were not significantly different (F=0.02, p=0.88).
Figure 2.
Summary of performance data (accuracy) from three groups of subjects: SZ (schizophrenia patients), SA (patients with schizoaffective disorder), and HC (healthy controls). The x-axis indicates the emotional intensity or facial difference level used for discrimination, and the y-axis indicates the performance accuracy. The error bars represent ±1 standard error. Panel A is for fear discrimination, Panel B is for happiness discrimination, and Panel C is for identity discrimination.
A one-way ANOVA of thresholds showed a significant group difference (F=20.49, p<0.001). The significant group difference remained (F=12.25, p<0.001) after the IQ scores were included as a covariate. Post hoc t-tests showed that patients with schizoaffective disorder (SA) had significantly lower thresholds (better performance) in fear discrimination than patients with schizophrenia (SZ) (t=3.74, p=0.002). HC also had significantly lower thresholds than SZ (t=5.91, p<0.001). Between SA and HC, however, there was no significant group difference (t=1.25, p=0.175). To evaluate the influence of the negative symptoms (the only PANNS subscale in which the patient groups differed) within the patient groups, a separate ANOVA of threshold including only the SZ and SA groups was performed with negative symptom scores in the model. The significant group difference remained (F=7.34, p=0.01) after the negative PANNS scores were included as a covariate.
Happiness Discrimination
A two-way ANOVA of accuracy (group by emotion intensity) showed a significant group difference (F=11.2, p<0.001). The stimulus saliency effect was also significant (F=150.4, p<0.001), but the group-stimulus saliency interaction was not (F=0.7, p=0.69). Post hoc tests showed significant effects between the SZ and SA groups (F=10.2, p=0.002) and between the SZ and HC groups (F=19.2, p<0.001) (Figure 2 B). However, the SA and HC groups were not significantly different (F=0.45, p=0.50).
A one-way ANOVA of threshold showed a significant group difference (F=6.02, p=0.004). The significant group difference remained (F=3.75, p=0.03) after the IQ scores were included as a covariate. A post hoc t-test showed that patients with SA had significantly lower thresholds (better performance) in happiness discrimination than patients with SZ (t=2.34, p=0.035). HC also had significantly lower thresholds than SZ (t=3.25, p=0.002). Between the SA and HC groups, however, there was no significant group difference (t=0.06, p=0.989). A separate ANOVA of thresholds in the SZ and SA groups including negative PANNS scores as a covariate yielded an insignificant effect for group (F=2.13, p=0.16).
Identity Discrimination
A two-way ANOVA (group by stimulus saliency) of accuracy showed a significant group difference (F=3.99, p=0.02). The main effect for stimulus saliency was also significant (F=95.0, p<0.001). The group-stimulus saliency interaction was not significant (F=1.3, p=0.27). Post hoc tests showed significant effects between the SZ and SA groups (F=6.5, p<0.012) (Figure 2 C). However, no significant effect was found either between the SA and HC groups (F=3.9, p=0.05) or between the SZ and HC groups (F=2.34, p=0.13). Note that as seen in Figure 2, SA performed slightly better than HC, and SZ performed worse.
A one-way ANOVA of threshold showed a significant group difference (F=6.23, p=0.004). The group difference became insignificant (F=2.77, p=0.07) after the IQ scores were included as a covariate. A post hoc t-test showed that patients in the SA group had marginally lower thresholds in identity discrimination than patients in the SZ group (t=2.77, p=0.009). HC also had significantly lower thresholds than SZ (t=1.96, p=0.04). Between the SA and HC groups, however, there was no significant group difference (t=0.04, p=0.971). The group difference became insignificant (F=1.84, p=0.19) after the negative PANNS scores were included as a covariate.
Eyes Test
Patients in the SA group had significantly higher scores (better performance) in the Eyes Test than patients in the SZ group (t=2.73, p=0.006). HC also had significantly higher scores than SZ (t=3.12, p=0.007). Between SA and HC, however, there was no significant group difference (t=1.21, p=0.25).
Correlations between Affective and Non-affective Facial Discrimination and Other Variables
In patients (SZ and SA), the thresholds for fear discrimination were moderately correlated with the PANSS scores for negative symptoms (r30=0.40, p=0.02) and for general symptoms (r30=0.38, p=0.03), but not with the scores for positive symptoms (r30=0.08, p>0.05). The thresholds for happiness discrimination were moderately correlated with the PANSS scores for positive symptoms (r30=0.40, p=0.02), but not with the scores for negative symptoms (r30=−0.16, p>0.05) or general symptoms (r30=0.14, p>0.05). The thresholds for identity discrimination were significantly correlated with negative symptoms (r30=0.55, p=0.002), but not with the scores for positive symptoms (r30=0.04, p>0.05) or for general symptoms (r30=0.04, p>0.05).
The IQ scores of patients were also moderately correlated with the thresholds for fear discrimination (r31=−0.49, p=0.01) and for happiness discrimination (r31=−0.36, p=0.04).
Discussion
This study found distinct facial emotion discrimination between schizophrenia patients and patients with schizoaffective disorder, with an impairment occurring in the former but not in the latter group. The SA patients performed similarly to healthy controls on both fear and happiness discrimination tasks. The impaired performance in SZ patients also occurs in the identity discrimination task, a non-affective face perception task, and the Eyes Test, an emotional perception task based upon the eye regions of face images.
Affective and Non-affective Face Perception and Schizophrenia
Impaired affective and non-affective face perception in schizophrenia has been widely reported (Butler et al 2008; Chen 2011; Chen et al 2008; Marwick & Hall 2008; Phillips & David 1995; Silverstein et al 2010; Zivotofsky et al 2008). Yet it is not completely understood how the processing of facial information is related to clinical dimensions in this mental disorder. Some studies have linked deficient performance in perceiving non-affective face images to positive psychotic symptoms such as delusions (Phillips & David 1995; Walther et al 2009). Others, meanwhile, have found that deficient perception of facial emotions is related to negative symptoms (Kohler et al 2003). After patients in this study were divided into SZ and SA groups, the SZ group showed significantly worse scores than the SA group in negative symptoms but not in positive symptoms (Table 1). The SZ group also showed deficient performance on the facial emotion discrimination and facial identity discrimination tasks and in the Eyes Test, whereas the SA group did not. The comparisons between groups and across perceptual abilities and clinical manifestations are consistent with the notion that poor processing of facial emotion information is associated with the negative symptoms of schizophrenia.
On the other hand, the correlations between emotion discrimination and PANSS scores only provide partial support for the negative symptom interpretation, since patients’ negative symptom scores were moderately correlated with fear discrimination (r=0.40), but not with happiness discrimination (r=−0.16). These correlational results suggest that other factors may also be involved in the different face perception performances of the two groups of patients.
Affective and Non-affective Face Perception in Schizoaffective Disorder
It was unexpected that patients with schizoaffective disorder would show similar performance in affective and non-affective face perception to healthy controls. In addition to their positive psychotic symptoms, this group of patients also experienced various mood problems. Patients with mood disturbances tend to be impaired in their social interactions (Schoeyen et al 2011; Wingo et al 2010). Since affective and non- affective face information plays a major role in social interactions (Haxby et al 2002), it would be expected that face perception and mood disturbances would be associated with each other as well, and thus that patients with schizoaffective disorder would be more deficient in face perception than schizophrenia patients. Yet, SA patients exhibited significantly better performance than SZ patients on almost all face perception tasks.
One may thus raise a paradoxical question as to whether mood problems have any beneficial effect on the processing of facial information. In fact, community outcomes do appear to be better in schizoaffective disorder than in schizophrenia (First et al 1994). One study found that the presence of depressive episodes is associated with favorable functional outcomes in patients with schizoaffective disorder, while a diagnosis of schizophrenia is associated with unfavorable functional outcomes (Steinmeyer et al 1989). Another study reported that elderly patients with bipolar disorder who had mood but not psychotic symptoms had better functional outcomes than elderly patients with schizophrenia or schizoaffective disorder (Masand & Gupta 2003). When viewing face images, patients with affective disorders showed a similar visual scanning pattern to healthy controls whereas schizophrenia patients showed a restricted scanpath style (Loughland et al 2002), suggesting that mood problems were at least not a disturbing factor in the processing of face information. Future study should directly assess the relationship between the perceptual ability to process facial information and the mood statuses in patients.
Other Cognitive and Clinical Variables
In this sample, SA patients performed similarly to healthy controls in identity discrimination as well as in the Eye Test, whereas SZ patients showed impaired performances. The Eyes Test results from the two patient groups are consistent with a previous study, which also showed a better performance on a different theory-of-mind task in SA than in SZ patients (Fiszdon et al 2007). These results, together with the emotion discrimination performances, suggest distinct processing of affective and non-affective facial information in schizophrenia and schizoaffective disorder. Given that both affective and non-affective face perception involve a specialized brain network (Adolphs et al 1996; Kanwisher et al 1997), this study highlights the notion that the brain mechanisms for processing socially important face information are implicated primarily in schizophrenia. However, it is also possible that impairment of a broad mechanism for general cognitive processing, commonly referenced as the ‘generalized deficit’ factor (Chapman & Chapman 1973), is responsible for the deficient perceptual performance in schizophrenia. In schizoaffective disorder, these brain mechanisms, either specific to the social domain or general for cognitive processing, are likely preserved or compensated for by other concurrent pathophysiological processes such as those involved in mood disturbance.
The relationship between performances in the affective and non-affective face perception tasks and the neurocognitive (IQ) or clinical symptom levels lends some support to the notion that socially specific mechanisms play a primary role here. In this sample, SA patients had higher IQ scores than SZ patients had. However, only moderate correlations were found between affective face perception performance and general intelligence (with IQ accounting for approximately 13–24% of variances in the perceptual performance). In addition, the performance difference between SZ and SA groups in emotion discrimination remained significant after the IQ scores were included as covariate. This analysis suggests that different neurocognitive levels in the two patient groups may be a non-negligible, but not a sole, factor for the difference in the processing of affective face information between these two groups. Vice versa, it may discount the generalized deficit as a primary factor here. If verified in a larger sample of patients, this relationship would implicate the socially important brain mechanisms in schizophrenia but not in schizoaffective disorder.
The face perception performance difference in the two groups of patients is also hardly attributable solely to the clinical factors, as they had similar levels of positive and general symptoms. However, the negative symptoms appear to have a significant influence on both affective and non-affective face perception, as the inclusion of this factor reduced group difference levels between the SA and SZ patients,
Lastly, the two patient groups received similar antipsychotic treatments and thus the medications do not appear to play a role in their different perceptual performances.
Concluding Remarks
In summary, this study found distinct processing of facial information between schizophrenia and schizoaffective disorders. This finding provides empirical support for the notion that differential pathophysiological processes underlie social cognition in the two psychotic disorders. Further study is needed to more completely understand how the relatively better face perception ability of schizoaffective patients might assist them in achieving better clinical and functional outcomes than schizophrenia patients. Nonetheless, the development of therapeutic interventions should consider this distinction in perceptual and cognitive processing while tackling different behavioral impairments.
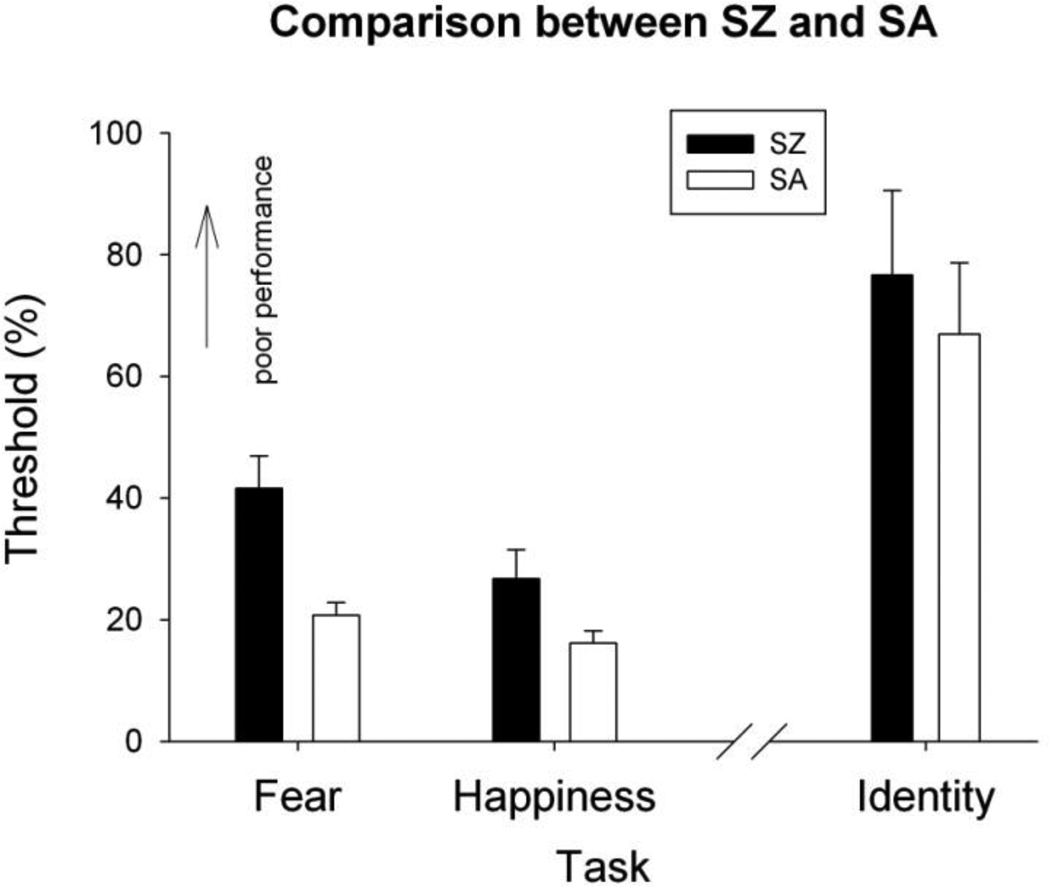
Figure 3.
Comparison of the thresholds in fear, happiness and identity discrimination. The x-axis indicates the type of face perception tasks and the y-axis indicates the threshold values. The higher value a threshold has, the worse performance it represents. The error bars represent ±1 standard error.
Acknowledgment
This study was supported in part by grants from NIH and Harvard University. We thank Ryan McBain and Jejoong Kim for their help during various phases of this study. We also thank two anonymous reviewers for the helpful comments on an early version of the paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R, Damasio H, Tranel D, Damasio AR. Cortical systems for the recognition of emotion in facial expressions. J. Neurosci. 1996;16:7678–7687. doi: 10.1523/JNEUROSCI.16-23-07678.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The "Reading the Mind in the Eyes" Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J. Child Psychol. Psychiatry. 2001;42:241–251. [PubMed] [Google Scholar]
- Butler PD, Tambini A, Yovel G, Jalbrzikowski M, Ziwich R, et al. What's in a face? Effects of stimulus duration and inversion on face processing in schizophrenia. Schizophr. Res. 2008;103:283–292. doi: 10.1016/j.schres.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman L, Chapman J. Disordered Thought in Schizophrenia. New York: Apple Century Crofts; 1973. [Google Scholar]
- Chen Y. Face Perception in Schizophrenia Spectrum Disorders: Interface Between Cognitive and Social Cognitive Functioning. In: Ritsner M, editor. Handbook of Schizophrenia Spectrum Disorders. Dordrecht Heidelberg London New York: Springer; 2011. pp. 111–120. [Google Scholar]
- Chen Y, Bidwell LC, Holzman PS. Visual motion integration in schizophrenia patients, their first-degree relatives, and patients with bipolar disorder. Schizophr. Res. 2005;74:271–281. doi: 10.1016/j.schres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Chen Y, Norton D, McBain R, Ongur D, Heckers S. Visual and cognitive processing of face information in schizophrenia: detection, discrimination and working memory. Schizophr. Res. 2009;107:92–98. doi: 10.1016/j.schres.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Norton D, Ongur D, Heckers S. Inefficient face detection in schizophrenia. Schizophr. Bull. 2008;34:367–374. doi: 10.1093/schbul/sbm071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, William JB. Structure Clinical Interview for DSM -IV-TR Axis I Disorders - Non-patient Edition (SCID-I/NP, 11/2002 revision) New York, NY: Biometric Research Department, New York State Psychiatric Institute; 2002a. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Disorders (SCID) Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV-TR Axis I Disorders -Patient Edition (SCID - I/P, 11/2002 revision) New York, NY: Biometrics Research Department; 2002b. [Google Scholar]
- Fiszdon JM, Richardson R, Greig T, Bell MD. A comparison of basic and social cognition between schizophrenia and schizoaffective disorder. Schizophrenia Research. 2007;91:117–121. doi: 10.1016/j.schres.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Green MF, Butler PD, Chen Y, Geyer MA, Silverstein S, et al. Perception Measurement in Clinical Trials of Schizophrenia: Promising Paradigms From CNTRICS. Schizophr. Bull. 2009;35:163–181. doi: 10.1093/schbul/sbn156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. Human neural systems for face recognition and social communication. Biol. Psychiatry. 2002;51:59–67. doi: 10.1016/s0006-3223(01)01330-0. [DOI] [PubMed] [Google Scholar]
- Heimberg C, Gur RE, Erwin RJ, Shtasel DL, Gur RC. Facial emotion discrimination: III. Behavioral findings in schizophrenia. Psychiatry Res. 1992;42:253–265. doi: 10.1016/0165-1781(92)90117-l. [DOI] [PubMed] [Google Scholar]
- Hooper SR, Giuliano AJ, Youngstrom EA, Breiger D, Sikich L, et al. Neurocognition in early-onset schizophrenia and schizoaffective disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49:52–60. doi: 10.1097/00004583-201001000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J. Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kohler CG, Turner TH, Bilker WB, Brensinger CM, Siegel SJ, et al. Facial emotion recognition in schizophrenia: intensity effects and error pattern. Am. J. Psychiatry. 2003;160:1768–1774. doi: 10.1176/appi.ajp.160.10.1768. [DOI] [PubMed] [Google Scholar]
- Loughland CM, Williams LM, Gordon E. Schizophrenia and affective disorder show different visual scanning behavior for faces: a trait versus state-based distinction? Biol. Psychiatry. 2002;52:338–348. doi: 10.1016/s0006-3223(02)01356-2. [DOI] [PubMed] [Google Scholar]
- Marwick K, Hall J. Social cognition in schizophrenia: a review of face processing. British Medical Bulletin. 2008;88:43–58. doi: 10.1093/bmb/ldn035. [DOI] [PubMed] [Google Scholar]
- Masand PS, Gupta S. Long-acting injectable antipsychotics in the elderly: guidelines for effective use. Drugs Aging. 2003;20:1099–1110. doi: 10.2165/00002512-200320150-00003. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Hoffman RE, Watson TD, Miller RM, Roach BJ, Ford JM. Neurophysiological Distinction between Schizophrenia and Schizoaffective Disorder. Front Hum Neurosci. 2010;3:70. doi: 10.3389/neuro.09.070.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton D, McBain R, Holt DJ, Ongur D, Chen Y. Association of impaired facial affect recognition with basic facial and visual processing deficits in schizophrenia. Biol. Psychiatry. 2009;65:1094–1098. doi: 10.1016/j.biopsych.2009.01.026. [DOI] [PubMed] [Google Scholar]
- Phillips M, David A. Facial processing in schizophrenia and delusional misidentification: cognitive neuropsychiatric approaches. Schizophr. Res. 1995;17:109–114. doi: 10.1016/0920-9964(95)00035-k. [DOI] [PubMed] [Google Scholar]
- Schoeyen HK, Birkenaes AB, Vaaler AE, Auestad BH, Malt UF, et al. Bipolar disorder patients have similar levels of education but lower socio-economic status than the general population. J. Affect. Disord. 2011;129:68–74. doi: 10.1016/j.jad.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Silverstein SM, All SD, Kasi R, Berten S, Essex B, et al. Increased fusiform area activation in schizophrenia during processing of spatial frequency-degraded faces, as revealed by fMRI. Psychol. Med. 2010;40:1159–1169. doi: 10.1017/S0033291709991735. [DOI] [PubMed] [Google Scholar]
- Steinmeyer EM, Marneros A, Deister A, Rohde A, Junemann H. Long-term outcome of schizoaffective and schizophrenic disorders: a comparative study. II. Causal-analytical investigations. Eur. Arch. Psychiatry Neurol. Sci. 1989;238:126–134. doi: 10.1007/BF00450999. [DOI] [PubMed] [Google Scholar]
- Stip E, Lussier I, Lalonde P, Luyet A, Fabian J. Atypical neuroleptics and selective attention. Encephale. 1999;25:260–264. [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E, McGuire M, Bettes B. Recognition and identification of facial stimuli by schizophrenics and patients with affective disorders. Br. J. Clin. Psychol. 1984;23:37–44. doi: 10.1111/j.2044-8260.1984.tb00624.x. [DOI] [PubMed] [Google Scholar]
- Walther S, Federspiel A, Horn H, Bianchi P, Wiest R, et al. Encoding deficit during face processing within the right fusiform face area in schizophrenia. Psychiatry Res. 2009;172:184–191. doi: 10.1016/j.pscychresns.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Adult Intelligence Scale-Revised. New York: Psychological Corporation; 1981. [Google Scholar]
- Wingo AP, Baldessarini RJ, Compton MT, Harvey PD. Correlates of recovery of social functioning in types I and II bipolar disorder patients. Psychiatry Res. 2010;177:131–134. doi: 10.1016/j.psychres.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J. Clin. Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Zivotofsky AZ, Oron L, Hibsher-Jacobson L, Weintraub Y, Strous RD. Finding the hidden faces: schizophrenic patients fare worse than healthy subjects. Neuropsychologia. 2008;46:2140–2144. doi: 10.1016/j.neuropsychologia.2008.02.024. [DOI] [PubMed] [Google Scholar]