Abstract
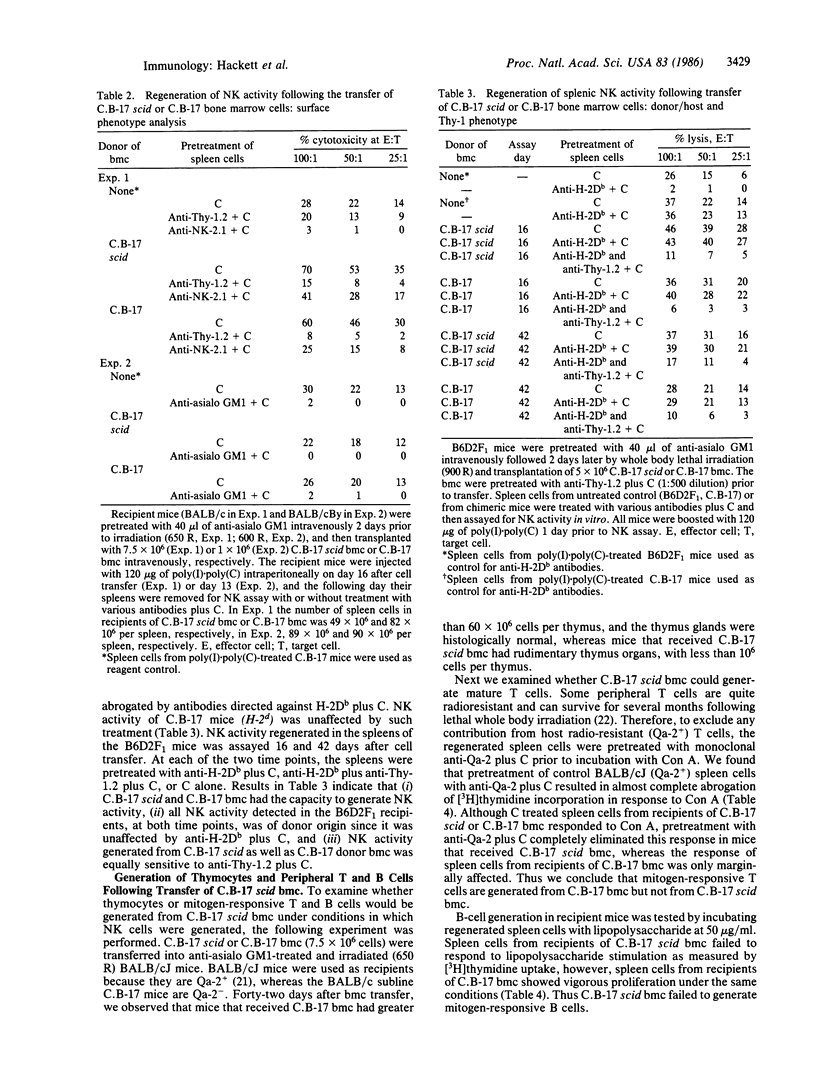
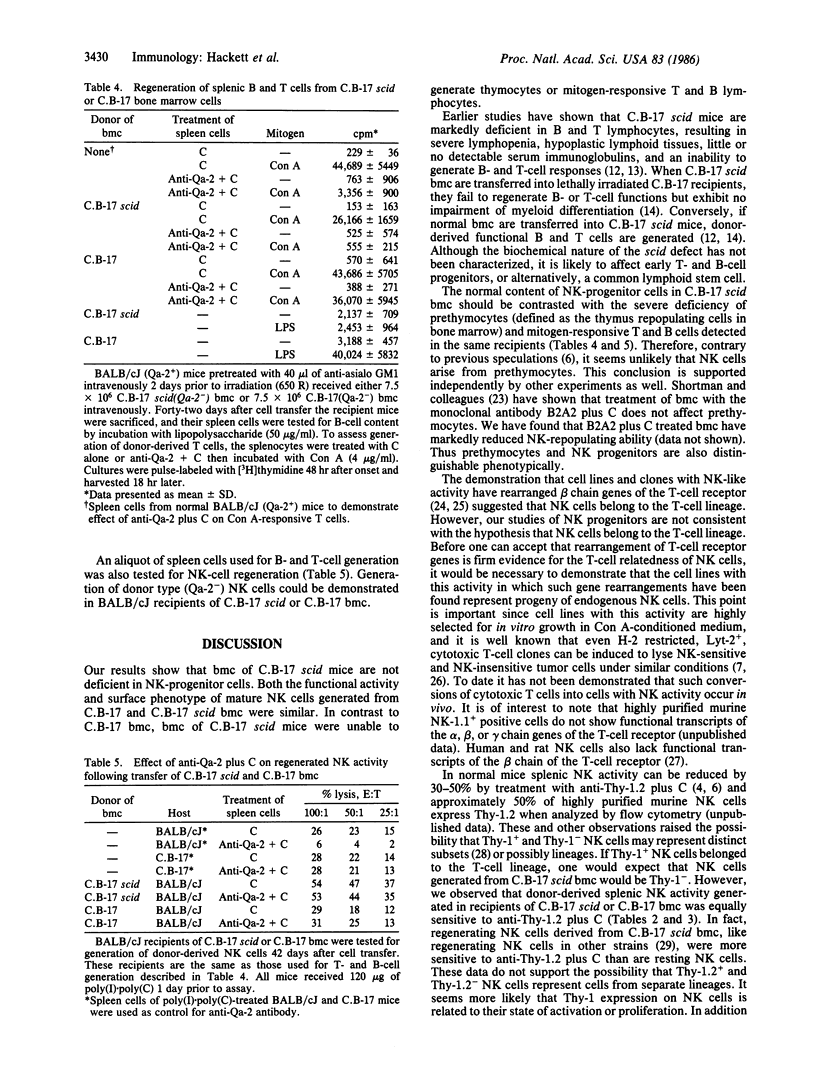
We have utilized a mouse mutant (C.B-17 scid) that lacks functional T and B lymphocytes to examine the relationship among transplantable progenitors of natural killer (NK) cells, T cells, and B cells. The NK-progenitor cells contained in the bone marrow were detected by their ability to generate mature NK cells, following transfer of bone marrow cells into NK cell-depleted and lethally irradiated mice. Regeneration of NK activity in the recipient mice was monitored by two different assays: the ability to rapidly clear infused YAC-1 cells in vivo and the ability of spleen cells to lyse YAC-1 cells in vitro. Recipients were also tested for the presence of mitogen-responsive T and B cells and for prethymocytes (thymus-repopulating cells). We found that the capacity of C.B-17 scid bone marrow cells to generate mature NK cells was equivalent to that of control C.B-17 bone marrow cells. The regenerated NK cells shared similar functional activity and surface phenotype. In contrast, bone marrow cells from C.B-17 scid mice failed to generate thymocytes and peripheral T and B cells. These data indicate that the transplantable NK-progenitor cells are not defective or deficient in C.B-17 scid mice and, therefore, are distinct from the transplantable progenitor(s) of T and B cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett M., Baker E. E., Eastcott J. W., Kumar V., Yonkosky D. Selective elimination of marrow precursors with the bone-seeking isotope 89Sr: implications for hemopoiesis, lymphopoiesis, viral leukemogenesis and infection. J Reticuloendothel Soc. 1976 Jul;20(1):71–87. [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Brooks C. G., Urdal D. L., Henney C. S. Lymphokine-driven "differentiation" of cytotoxic T-cell clones into cells with NK-like specificity: correlations with display of membrane macromolecules. Immunol Rev. 1983;72:43–72. doi: 10.1111/j.1600-065x.1983.tb01072.x. [DOI] [PubMed] [Google Scholar]
- Burton R. C., Winn H. J. Studies on natural killer (NK) cells. I. NK cell specific antibodies in CE anti-CBA serum. J Immunol. 1981 May;126(5):1985–1989. [PubMed] [Google Scholar]
- Chervenick P. A., Boggs D. R. Decreased neutrophils and megakaryocytes in anemic mice of genotype W/W. J Cell Physiol. 1969 Feb;73(1):25–30. doi: 10.1002/jcp.1040730104. [DOI] [PubMed] [Google Scholar]
- Claësson M. H., Olsson L., Martinsen L., Brix-Poulsen P. Bone marrow derived diffuse colonies: their cytotoxic potential, morphology, and antigenic phenotype. Exp Hematol. 1982 Sep;10(8):708–721. [PubMed] [Google Scholar]
- Custer R. P., Bosma G. C., Bosma M. J. Severe combined immunodeficiency (SCID) in the mouse. Pathology, reconstitution, neoplasms. Am J Pathol. 1985 Sep;120(3):464–477. [PMC free article] [PubMed] [Google Scholar]
- Dorshkind K., Keller G. M., Phillips R. A., Miller R. G., Bosma G. C., O'Toole M., Bosma M. J. Functional status of cells from lymphoid and myeloid tissues in mice with severe combined immunodeficiency disease. J Immunol. 1984 Apr;132(4):1804–1808. [PubMed] [Google Scholar]
- Dorshkind K., Pollack S. B., Bosma M. J., Phillips R. A. Natural killer (NK) cells are present in mice with severe combined immunodeficiency (scid). J Immunol. 1985 Jun;134(6):3798–3801. [PubMed] [Google Scholar]
- Galli S. J., Dvorak A. M., Ishizaka T., Nabel G., Der Simonian H., Cantor H., Dvorak H. F. A cloned cell with NK function resembles basophils by ultrastructure and expresses IgE receptors. Nature. 1982 Jul 15;298(5871):288–290. doi: 10.1038/298288a0. [DOI] [PubMed] [Google Scholar]
- Hackett J., Jr, Bennett M., Kumar V. Origin and differentiation of natural killer cells. I. Characteristics of a transplantable NK cell precursor. J Immunol. 1985 Jun;134(6):3731–3738. [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Holden H. T. Low density of Thy 1 antigen on mouse effector cells mediating natural cytotoxicity against tumor cells. J Immunol. 1978 Jul;121(1):304–309. [PubMed] [Google Scholar]
- Hirokawa K., Sado T., Kubo S., Kamisaku H., Hitomi K., Utsuyama M. Intrathymic T cell differentiation in radiation bone marrow chimeras and its role in T cell emigration to the spleen. An immunohistochemical study. J Immunol. 1985 Jun;134(6):3615–3624. [PubMed] [Google Scholar]
- Hurme M., Sihvola M. High expression of the Thy-1 antigen on natural killer cells recently derived from bone marrow. Cell Immunol. 1984 Apr 1;84(2):276–284. doi: 10.1016/0008-8749(84)90099-6. [DOI] [PubMed] [Google Scholar]
- Kasai M., Iwamori M., Nagai Y., Okumura K., Tada T. A glycolipid on the surface of mouse natural killer cells. Eur J Immunol. 1980 Mar;10(3):175–180. doi: 10.1002/eji.1830100304. [DOI] [PubMed] [Google Scholar]
- Koo G. C., Jacobson J. B., Hammerling G. J., Hammerling U. Antigenic profile of murine natural killer cells. J Immunol. 1980 Sep;125(3):1003–1006. [PubMed] [Google Scholar]
- Kumar V., Ben-Ezra J., Bennett M., Sonnenfeld G. Natural killer cells in mice treated with 89strontium: normal target-binding cell numbers but inability to kill even after interferon administration. J Immunol. 1979 Oct;123(4):1832–1838. [PubMed] [Google Scholar]
- Lohmann-Matthes M. L., Domzig W., Roder J. Promonocytes have the functional characteristics of natural killer cells. J Immunol. 1979 Oct;123(4):1883–1886. [PubMed] [Google Scholar]
- Lust J. A., Bennett M., Kumar V. Lysis of FLD-3 Friend erythroleukemia cells in vitro and in vivo: effect of 89Sr treatment and Friend virus infection. Int J Cancer. 1984 Jan 15;33(1):107–113. doi: 10.1002/ijc.2910330117. [DOI] [PubMed] [Google Scholar]
- MCCULLOCH E. A., SIMINOVITCH L., TILL J. E. SPLEEN-COLONY FORMATION IN ANEMIC MICE OF GENOTYPE WW. Science. 1964 May 15;144(3620):844–846. doi: 10.1126/science.144.3620.844. [DOI] [PubMed] [Google Scholar]
- Meyer D., McKee P. A., Hoyer L. W., Zimmerman T. S., Gralnick H. R. Molecular biology of factor VIII/von Willebrand Factor. Thromb Haemost. 1978 Oct 31;40(2):245–251. [PubMed] [Google Scholar]
- Minato N., Reid L., Bloom B. R. On the heterogeneity of murine natural killer cells. J Exp Med. 1981 Sep 1;154(3):750–762. doi: 10.1084/jem.154.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccardi C., Barlozzari T., Santoni A., Herberman R. B., Cesarini C. Transfer to cyclophosphamide-treated mice of natural killer (NK) cells and in vivo natural reactivity against tumors. J Immunol. 1981 Apr;126(4):1284–1289. [PubMed] [Google Scholar]
- Riccardi C., Puccetti P., Santoni A., Herberman R. B. Rapid in vivo assay of mouse natural killer cell activity. J Natl Cancer Inst. 1979 Oct;63(4):1041–1045. [PubMed] [Google Scholar]
- Ritz J., Campen T. J., Schmidt R. E., Royer H. D., Hercend T., Hussey R. E., Reinherz E. L. Analysis of T-cell receptor gene rearrangement and expression in human natural killer clones. Science. 1985 Jun 28;228(4707):1540–1543. doi: 10.1126/science.2409597. [DOI] [PubMed] [Google Scholar]
- Scollay R., Bartlett P., Shortman K. T cell development in the adult murine thymus: changes in the expression of the surface antigens Ly2, L3T4 and B2A2 during development from early precursor cells to emigrants. Immunol Rev. 1984 Dec;82:79–103. doi: 10.1111/j.1600-065x.1984.tb01118.x. [DOI] [PubMed] [Google Scholar]
- Shah P. D., Gilbertson S. M., Rowley D. A. Dendritic cells that have interacted with antigen are targets for natural killer cells. J Exp Med. 1985 Aug 1;162(2):625–636. doi: 10.1084/jem.162.2.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A., Shortman K. Degradation of specificity in cytolytic T lymphocyte clones: two broad specificity, H-2-independent recognition systems, one natural killer-like, develop during culture, in addition to the clonally distributed antigen-specific receptor. Eur J Immunol. 1985 Sep;15(9):899–905. doi: 10.1002/eji.1830150907. [DOI] [PubMed] [Google Scholar]
- Yanagi Y., Caccia N., Kronenberg M., Chin B., Roder J., Rohel D., Kiyohara T., Lauzon R., Toyonaga B., Rosenthal K. Gene rearrangement in cells with natural killer activity and expression of the beta-chain of the T-cell antigen receptor. Nature. 1985 Apr 18;314(6012):631–633. doi: 10.1038/314631a0. [DOI] [PubMed] [Google Scholar]
- Young H. A., Ortaldo J. R., Herberman R. B., Reynolds C. W. Analysis of T cell receptors in highly purified rat and human large granular lymphocytes (LGL): lack of functional 1.3 kb beta-chain mRNA. J Immunol. 1986 Apr 1;136(7):2701–2704. [PubMed] [Google Scholar]


