Abstract
Dynamics are an important and indispensible physical attribute that plays essential roles in RNA function. RNA dynamics are complex, spanning vast timescales and encompassing large number of physical modes. The technique of site-directed spin labeling (SDSL), which derives information on local structural and dynamic features of a macromolecule by monitoring a chemically stable nitroxide radical using electron paramagnetic resonance (EPR) spectroscopy, has been applied to monitor intrinsic dynamics at defined structural states as well as to probe conformational transition dynamics of RNAs. Current state of SDSL studies of RNA dynamics is summarized here. Further SDSL developments promise to open up many more opportunities for probing RNA dynamics and connecting dynamics to structure and function.
Keywords: RNA, Dynamics, EPR, Site-directed spin labeling
Introduction
Our understanding of RNA functions in the cell has been exploding in the past 30 years, as exemplified by discoveries of catalytic RNAs, small interfering RNAs, microRNAs, riboswitches, and many other non-coding RNAs.1 Because RNA participates in all cellular processes associated with the maintenance and expression of genetic information, knowledge of the molecular basis of RNA function is essential for understanding basic biology as well as for identifying new drug targets and devising novel therapeutic strategies. Towards this goal, much has been learned about RNA structure, and many high-resolution structures of functional RNAs are now available.2 In parallel, the importance of RNA dynamics has been increasingly appreciated, and it is now recognized that coordinated and complex conformational transformations of RNA are essential to nearly all RNA mediated processes.3-9
RNA dynamics span vast timescales (i.e., 10-15 − 1 s), encompass different modes of motions (e.g., bond vibrations/rotations, individual nucleotide motions, and helix/domain rearrangements), and are dictated by intimate interplay between intrinsic RNA dynamics and external factors (e.g., metal ions, metabolites, and proteins). To tackle this complex problem requires experimental techniques that provide time-dependent structural information. A number of biophysical methods, including (but not limited to) NMR and fluorescence spectroscopy,3-7, 10 have provided a wealth of information on pathways and kinetics of conformational transformations as well as on intrinsic dynamics at given states in a number of RNAs and RNA/protein complexes. However, current knowledge on RNA dynamics is rather primitive, and much remains to be learned about characteristics of RNA dynamic modes, how they are dictated by structure, and how they influence function.
This article focuses on experimental studies of RNA dynamics using a technique called site-directed spin labeling (SDSL). In SDSL,11, 12 a chemically stable nitroxide radical is covalently attached at specific sites within bio-macromolecules. Electron paramagnetic resonance (EPR) spectroscopy, either in continuous-wave (cw) or pulsed mode, is used to monitor nitroxide behaviors, from which structural (e.g., distance constraints) and dynamic (e.g., motions at the labeling site) information on the parent molecule is derived (Figure 1). SDSL is capable of probing dynamics ranging from nanoseconds to seconds, and can be applied to study high molecular weight systems under physiological conditions using a small amount of samples (∼ 50 μM in 5 μl). It provides more detailed structural and dynamic information as compared to chemical probing, and avoids a number of fundamental issues faced by crystallography (e.g., crystalline sample preparation, interference from lattice packing) and NMR (e.g., limitation on molecule size). The nitroxide is smaller than most fluorophores, and many studies have demonstrated that a covalently attached nitroxide probe minimally perturbs native behaviors of RNA/DNA under investigation. Last but not least, EPR observables can be analyzed based on first principles (e.g., spectral simulations), thus allowing quantitative descriptions of macromolecular behaviors, such as rate and order of RNA motions.
Figure 1.
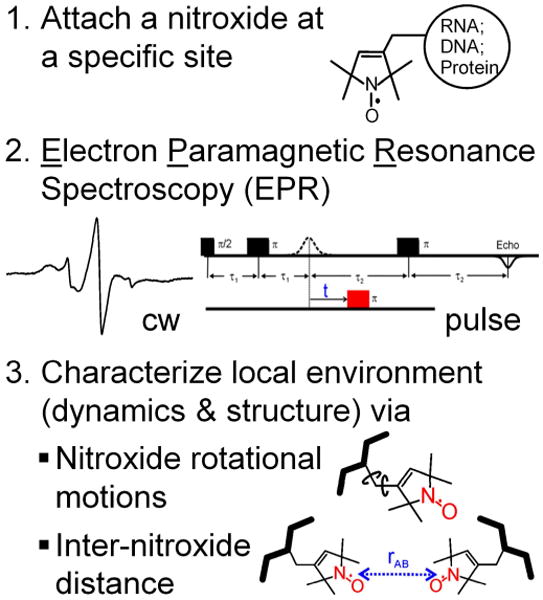
General strategy of site-directed spin labeling.
In the following sections, we will first describe the basic principle of SDSL, and then summarize reported SDSL studies of RNA dynamics. Interested readers may also consult a number of recent reviews12-14 for more in-depth description of SDSL and its application to nucleic acids. While this article focuses exclusively on the use of nitroxide spin labels, we also note that a number of metal ions (e.g., Mn2+) are EPR active and have been used as probes for studying RNA.15
Site-Directed Spin Labeling For Studying Nucleic Acids
The technique of SDSL was pioneered by the Hubble group for studying membrane proteins, and has matured as a powerful tool for studying protein structures and dynamics.16-18 However, nucleic acids differ from proteins in their basic chemical (nucleotides vs. amino acids) and structural (A/B- helices vs. α-helix/β-strand) units. Therefore, SDSL studies of nucleic acids require unique methodologies, particularly for nitroxide labeling and data interpretation.12, 13, 19 In this section, we summarize methods for attaching nitroxides to specific sites within nucleic acids and describe commonly used EPR observables for studying nucleic acids, including RNA dynamics.
Nitroxide attachment to specific sites within nucleic acids
Most nucleic acid SDSL studies employ nitroxides covalently attached to base, 2′-position of sugar, or phosphate of specific nucleotides of a target strand (Figure 2),12, 13 although recently nitroxides inserted non-covalently into specific sites of DNA duplexes have been explored for distance measurements at low temperature.20 Under physiological conditions, the four naturally occurring nucleotides are not reactive enough to allow direct coupling to nitroxides, thus necessitating the use of modified nucleotides containing more reactive functionalities. Solid-phase chemical synthesis is the most commonly used strategy for site-specific incorporation of modified nucleotides, although enzymatic approaches have also been explored.12, 21, 22
Figure 2.
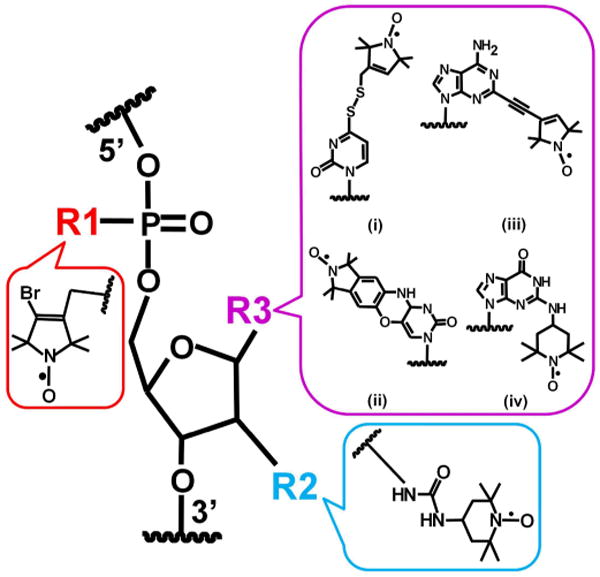
Representative examples of nitroxide attached to backbone (R1), 2′-position of sugar (R2), or various positions of base (R3) of a specific nucleotide within a nucleic acid strand.
Two general labeling strategies have been employed using solid-phase chemical synthesis.12, 13 In the post-synthesis-modification approach, modified nucleotides containing a reactive functional group (e.g., phosphorothioate,23 2′-amino,24, 25 4-thiouridine,26, 27 and more recently, alkyne28) is incorporated at a specific site, and the oligonucleotide is reacted with an appropriate nitroxide derivative after completion of chemical synthesis. This approach avoids subjecting the nitroxide to potentially damaging conditions during oligonucleotide synthesis and post-synthesis manipulation, and many of the modified oligonucleotides can be obtained commercially. In another approach, nitroxide is attached to the target strand during solid-phase chemical synthesis, either via direct incorporation of a nitroxide containing phosphoroamidite29 or via on-column derivatization.30-32 Using this approach, one may incorporate “designer” nitroxides with sophisticated chemical structures (e.g., “C-spin” (Ç),29 Figure 2, R3(ii)) to tune behaviors of spin labels with respect to the parent macromolecule, which is advantageous for extracting information on the macromolecule. On the other hand, this approach generally requires complex synthetic procedures, and certain analogues (e.g., Ç) have until now been incorporated into DNA strands only.
Monitoring nitroxide behaviors using EPR spectroscopy
A nitroxide contains one unpaired electron (spin quantum number S = ½) that is shared between the nitrogen and the oxygen atoms. The theories describing behaviors of the unpaired electron in the presence of a magnetic field are analogous to that of a proton nuclear spin (quantum number I = ½), although in some instances differences between an electron and a proton (e.g., mass ratio of 1 to 1,836) do result in drastic changes in behaviors. For example, spin relaxation time at room temperature is in the nanosecond range for an unpaired electron vs. that of a millisecond or longer for a proton.33
The energy levels of the unpaired electron depend on interactions between the electron with the external magnetic field (the Zeeman interaction); the nearby nuclear spin(s) (the hyperfine interaction), and if present, other electron spin(s). EPR is used to monitor transitions between these energy levels, from which one can deduce information on behaviors of the nitroxide. Nucleic acid studies have been primarily utilizing two EPR observables (Figure 1): (i) rotational motions of a nitroxide, revealed primarily from measurements of the cw-EPR spectrum;12, 13 and (ii) distance(s) between a pair of nitroxides, obtained by measuring electron spin dipolar coupling using either cw-EPR (for distances between 5 to 20 Å) and more recently pulsed EPR techniques (for distances between 20 to ∼ 80 Å).12, 14, 34 Measurements of distances in nucleic acids in vivo using pulsed EPR have recently been reported.35
A commonly used EPR observable is the rotational re-orientation dynamics of the nitroxide, which can be measured using cw-EPR spectroscopy.12, 13 The observed nitroxide dynamics may be coupled to the overall rotation of the entire molecule (designated as the τR motion), and therefore can be used to monitor size and shape of the parent RNAs as well as to probe interactions with their partners.12, 13 In addition, at the labeling site, the macromolecule dictates the sterically-allowable space available for accommodating the nitroxide ring, and may enable interactions between nitroxide functional groups and the parent molecule. This influences torsional rotations about bonds connecting the nitroxide ring to the macromolecule, which are designated as the internal motion (τi motion). Furthermore, motions of the macromolecule at the labeling site (τb motion) may be transmitted through the connecting bond(s) and influence nitroxide dynamics. The τi and τb motions are potentially very sensitive to structural and dynamic features of the macromolecule at the labeling site, and have been extensively utilized in RNA studies.12, 13 These two motions may also be inter-connected. For example, macro-molecular motions at the labeling site (τb motions) may change the sterically allowed space as well as enable or eliminate interactions between functional groups of the nitroxide and the parent molecule, both of which will alter nitroxide internal motions (τi motions) (Qin et.al, manuscript in preparation).
A number of RNA studies, primarily using model duplexes,25, 31, 32, 36-38 have established methods for correlating measured inter-nitroxide distances to structures of the parent RNAs, thus establishing a basis for using SDSL distance measurements to map RNA global structures and to monitor conformational dynamics. With pulsed EPR, the upper limit of detectable distance is comparable to that measured using FRET, although most EPR measurements are carried out in frozen solution states as constrained by the short electron spin relaxation time.12, 14 It is also notable that instead of a single distance value, EPR measurements yield the entire profile of distance distribution, which reveals the degree of structural ordering (or disordering) at given states of RNAs39 or the presence of multiple RNA conformations32, 40 (see later sections on the hammerhead ribozyme and riboswitches). This provides information on RNA conformational dynamics.
While this article focuses on RNA studies, nitroxide labeling schemes and EPR observables described above are applicable for probing DNAs. SDSL studies of DNAs are closely parallel and mutually beneficial to investigations of RNA. Interested readers may consult a 2008 review article,12 as well as many excellent recent publications.41-48
RNA Dynamic Information Obtained Using SDSL
From monitoring nitroxide rotational motions and measuring inter-nitroxide distances, information has been gained on intrinsic dynamics of individual structural elements within folded RNAs as well as on conformational distributions and transitions, both globally and at the level of individual nucleotides, in RNAs and RNA/protein complexes. Recent studies of RNA dynamics using SDSL and EPR are summarized below, with topics organized according to the systems being investigated.
Intrinsic dynamics of substrate recognition duplex in the Tetrahymena group I ribozyme
The Tetrahymena group I ribozyme is a large RNA (∼ 400-nucleotide, 120 kD) derived from a self-splicing group I intron in the large ribosomal RNA precursor of Tetrahymena thermophila. It catalyzes a site-specific cleavage of an oligonucleotide substrate using a non-covalently bound guanosine, and has been extensively studied for understanding RNA structure, folding, and function.49 Qin and co-workers reported a SDSL study on probing nanosecond intrinsic dynamics of the substrate recognition duplex (the P1 duplex) within the entire Tetrahymena ribozyme.50 Using a phosphorothioate labeling scheme,23, 51 a nitroxide (Figure 2, example shown as R1) was attached to either the Rp- or the Sp-phosphorothioate diastereomer incorporated into the substrate oligonucleotide. The labeled substrates were hybridized with the folded ribozyme to achieve specific labeling within the P1 duplex (Figure 3A). Functional groups within the substrate were modified to direct the ribozyme into two distinct states (Figure 3A): the open complex, in which P1 extends from the ribozyme core through a single-stranded J1/2 linker while makes no tertiary contacts to the ribozyme core; and the closed complex, in which P1 docks into the pre-folded core via multiple tertiary interactions and positions the substrate for subsequent cleavage. The labeling site was chosen to be away from the folded ribozyme core in either states, and the spin label did not perturb ribozyme structure and dynamics.50
Figure 3.
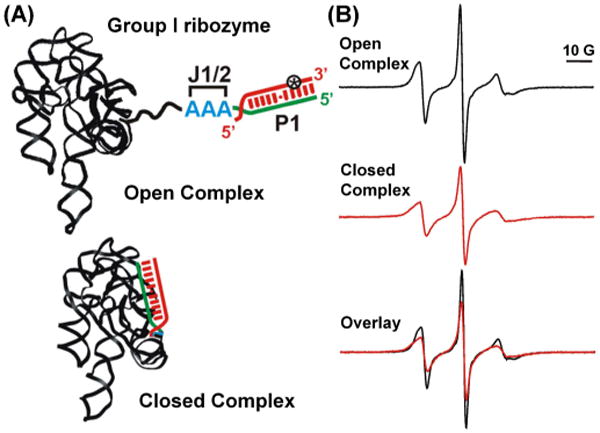
SDSL studies of nanosecond motions of the P1 duplex in the Tetrahymena group I ribozyme. (A) Schematic representation of two ribozyme states: the open and closed complexes. Star indicates the nitroxide label shown in the R1 panel of Figure 2. (B) X-band cw-EPR spectra of open and closed complexes acquired in aqueous buffer at 25 °C. The broader spectrum of the closed complex indicates slower motions, which arises from reduced P1 mobility in the closed complex. Figure adopted from Ref. 50 with permission.
Nitroxide dynamics were monitored using X-band cw-EPR. At X-band (∼ 9.4 GHz), cw-EPR spectral lineshape changes drastically as the nitroxide motions vary between 0.5 ns – 50 ns.12, 13 Information about nitroxide dynamics can be either assessed semi-quantitatively using simple lineshape parameters such as the linewidths (Figure 3B), or can be quantitatively described by simulations based on the first principle using defined models of nitroxide motions.12, 13 In the group I ribozyme study, the closed complex gave clearly broader cw-EPR spectra than the open complex (Figure 3B), indicating lower nitroxide mobility in the closed complex. With a number of control experiments,50 it was established that the observed lower nitroxide mobility in the closed complex reports reduced P1 motions upon docking into the ribozyme core. Therefore, although the nitroxide was not rigidly fused to the P1 duplex, it remains sufficiently coupled to the RNA to report on P1 dynamics in the nanosecond regime.
Ribozymes with a mutated J1/2 element (Figure 3A) were then studied to understand how RNA structure modulates intrinsic dynamics.50 In the open complex, observed spectra were found to vary upon altering J1/2 length (Figure 4). From lineshape comparisons and spectral simulations, it was concluded that lengthening J1/2 reduces P1 motional ordering and gives rise to higher intrinsic dynamics (or flexibility), while deleting J1/2 results in more ordered P1 motion and thus lower flexibility. Such changes in RNA intrinsic dynamics were correlated to observed phenotypes of these mutants, namely weaker substrate binding and cryptic cleavage. Specifically, an extended J1/2 gives higher P1 mobility and increases flexibility, thus raising the entropic cost of docking, while in the J1/2 deletion mutant, P1 mobility is more restricted, and docking occurs at the energetic expense of distorting the ribozyme. While the two mutations affect P1 conformational sampling in opposite manners, thermodynamically both tilt the equilibrium towards the open complex,50 thus affecting tertiary interactions between substrate and the ribozyme core and overall weakening substrate binding. Alterations in P1 flexibility may also differentially affect the registers in which the P1 duplex docks, giving rise to cryptic cleavage with mutation-specific patterns.
Figure 4.
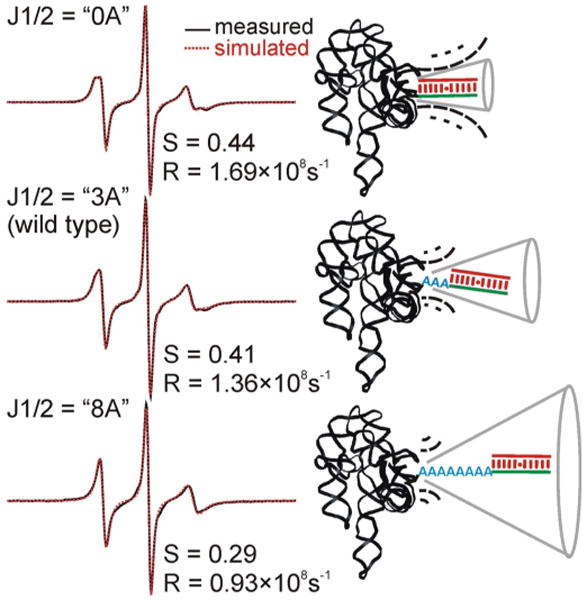
The J1/2 element modulates nanosecond dynamics of the P1 duplex in the open complex of the Tetrahymena group I ribozyme. Simulations of the measured spectra indicate that lengthening J1/2 (from 0, 3, to 8 adenosines) results in decreases in motional ordering (lower order parameter S) and slower rotational diffusion rate (smaller R). This reveals changes in intrinsic dynamics of the P1 duplex upon alteration of J1/2. Figure adopted from Ref. 50 with permission.
We note that P1 docking (i.e. transition between open and closed complexes) serves as a model for studying RNA folding.49 Noticeably, the rate of docking has been measured to be ∼ 1 s-1,52 much slower than that expected of a simple conformational search to position a duplex into a pre-folded core. It has been proposed that intrinsic dynamics of P1 in the open complex, which control the ability of P1 to sample different conformations (e.g., open & closed complex), may be one of the factors responsible for the observed slow docking rate.50, 52, 53 However, virtually nothing is known about intrinsic dynamics of any individual states in large folded RNAs such as the Tetrahymena ribozyme. The SDSL studies described above was the first example in which intrinsic dynamics of P1 in the nanosecond regime were probed experimentally in the context of the entire ribozyme, and the data revealed that intrinsic dynamics are modulated by structure (i.e., J1/2) and may influence function (i.e., substrate binding affinity and cleavage fidelity). We expect that expanded SDSL investigations of the Tetrahymena ribozyme will further advance our understanding of the relationship between RNA dynamics, structure, and function.
Conformational dynamics in the hammerhead ribozyme
The hammerhead ribozyme (HHRz) is another class of catalytic RNA that catalyzes a specific phosphodiester bond isomerization reaction.54, 55 They are found in a wide range of organisms,56 and a variant of HHRz has recently been found embedded in mammalian mRNAs.57 HHRz self-cleavage motifs consist of three A-form helices (stems) flanking a junction comprised of invariant or mostly conserved nucleotides.54, 55 SDSL studies on HHRz have been focused on probing RNA conformational dynamics that are associated with Mg2+ ion binding.
DeRose and co-workers used SDSL to study Mg2+ induced conformational transitions in an HHRz derived from Schistosoma mansoni (Figure 5).39, 58 These studies employed an extended HHRz construct that includes the catalytic core and the extended loop-loop interactions between stems I and II. Using singly-labeled nitroxides (Figure 2, R2) attached to stem I, it was observed that nitroxide dynamics, as reported by the cw-EPR spectrum, change upon additions of Mg2+.58 Such changes were interpreted as reporting docking between stems I and II resulted from interactions between the extended loops. Furthermore, inter-spin distances between a pair of nitroxides attached to stems I and II were measured using double electron-electron resonance (DEER) spectroscopy (Figure 5).39 In the absence of Mg2+, the measured inter-spin distance distribution is broad and uniform between 2 – 5 nm, with fluctuations comparable to those of the single-labeled control (Figure 5). This indicates a lack of defined inter-spin distance, and was interpreted as reporting a conformational ensemble in which the two stems are randomly oriented. As Mg2+ concentrations increases, a defined population with an inter-nitroxide distance around 2.5 nm was observed (Figure 5). The measured distance is consistent with what is expected from a crystal structure in which stem I docks with stem II. The studies provide an example in which Mg2+ induces formation of a specific RNA conformation, thus reducing RNA conformational dynamics during folding. Interestingly, in both studies, the [Mg2+] ½ of RNA conformational transition was reported to be lower than that estimated from cleavage rate measurements, indicating Mg2+ requirements are different for HHRz structure, conformational dynamics, and catalysis.
Figure 5.
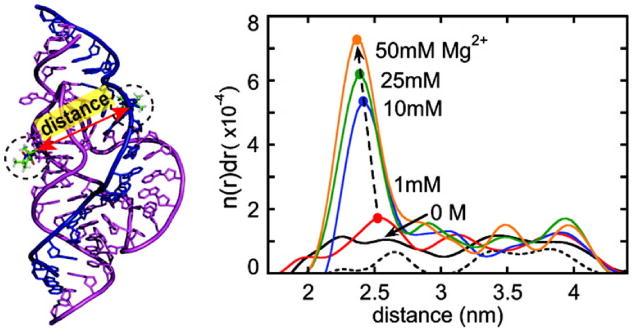
SDSL distance measurements reveal changes of conformational disordering in the hammerhead ribozyme upon addition of Mg2+. A schematic of the hammerhead ribozyme being studied is shown on the left. Measured distance distribution profiles at various Mg2+ concentrations are shown on the right, with the x-axis being the inter-spin distance and the y-axis representing the probability of finding a given distance. Figure adopted from Ref. 39 with permission.
In addition to the extended HHRz, Sigurdsson and co-workers used SDSL to investigate folding dynamics in a truncated hammerhead.59 A nitroxide was site-specifically incorporated at the 2′-position of the sugar of a uridine near the catalytic core, and cw-EPR spectra were used to monitor RNA conformational changes in response to variations in metal ions, pH, and ribozyme inhibitors. The EPR data revealed a two-step folding pathway with one folding event occurred at low Mg2+ concentration (0.25 mM) and another one at 10 mM Mg2+. This is consistent with other studies.
RNA conformational transitions in the GAAA tetraloop-receptor interaction
Qin and co-workers used SDSL to probe conformational changes upon formation of an RNA tertiary interaction motif – a GAAA tetraloop and its RNA receptor in solution.27, 51, 60 The GAAA tetraloop/receptor interaction is the most studied member of the GNRA (N: any nucleotide; R: purine) tetraloop/receptor family, which is one of the most frequently occurring tertiary interaction motifs in RNA.61 To characterize the GAAA tetraloop/receptor interaction without interference of other RNA elements, solution SDSL studies were carried out using two molecules: a hairpin with the GAAA tetraloop (TL) and an RNA containing the 11-nucleotide tetraloop receptor (TLR). By measuring changes in τR motions using an nitroxide attached to TL, the dissociation constant between the tetraloop and receptor was first determined to reveal the intrinsic interaction strength of this important motif.51 Furthermore, nitroxides were attached, one at a time, to specific uridine bases within TLR (Figure 2, example shown as R3(i)). Nitroxide dynamics, deduced from lineshape parameters of the measured cw-EPR spectra, were found to report on dynamics and structure state of the attached bases.27 In the unbound TLR with the presence of Mg2+, dynamics and structure state of each of the nitroxide-labeled uridine correlate well with the NMR structure of the free TLR obtained in the absence of Mg2+,27 indicating that Mg2+ alone does not alter the receptor structure. In the presence of Mg2+ and the tetraloop, a large increase of nitroxide mobility was observed at one of the labeled bases, which was interpreted as reporting unstacking of the base upon tetraloop docking.60 This stacked to unstacked transition results in a solution bound receptor conformation that is similar to that observed in the crystal structure of a group I ribozyme domain. The SDSL data indicate that in solution both the tetraloop and Mg2+ are required to stabilize the unstacked conformation of the bound receptor.60 This suggests energetic coupling between receptor conformational change, coordination of specific Mg2+ ions, and tetraloop binding, which may provide a means to achieve specificity in RNA tertiary interaction.
HIV trans-activation response element: RNA dynamics and structure at the level of individual nucleotides
The trans-activation response (TAR) element is a structural motif located at the 5′-end of the HIV RNA. The interaction between TAR RNA and the Tat protein during viral transcription is important for an efficient replication of the HIV virus.62. Therefore, the TAR-Tat complex has been extensively studied as a target site for HIV therapy.62 Sigurdsson and co-workers applied SDSL to investigate structure and dynamics of the TAR RNA as it interacts with a host of ligands (Figure 6).63-67 These studies employed nitroxides attached to the 2′-position of the sugar (Figure 6B) at four specific uridine sites (Figure 6A), and lineshape variations in the observed cw-EPR spectra were used to characterize nitroxide dynamics and to deduce information on TAR. In the absence of ligands,63 the flexible bulge region of the TAR (U23 and U25, Figure 6A) shows narrow spectra, indicating high mobility similar to that of a single strand; while the base-paired sites (U38 and U40, Figure 6A) display more complex spectra with broad central lines and clear hyperfine splitting, indicating reduced nitroxide motions. The observed spectral variations were attributed to the difference in the structural states and intrinsic dynamics of the labeled nucleotide, demonstrating the ability of nitroxides to monitor dynamic features at specific sites of TAR.63
Figure 6.
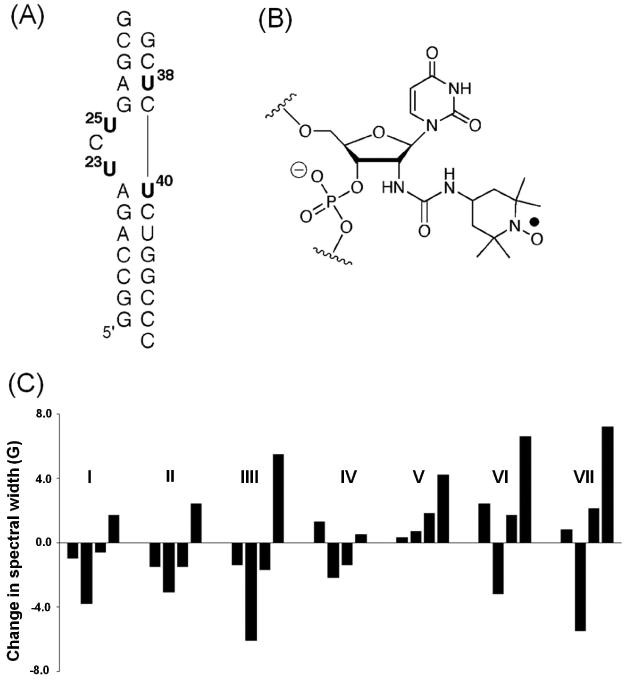
SDSL studies of the TAR RNA. (A) The secondary structure of the TAR RNA construct used in the study, with the four labeled uridines shown in bold. (B) Chemical structure of the nitroxide-labeled uridine. (C) An example of variations of dynamic signatures, which are represented by differences in the effective hyperfine splitting obtained from measured X-band cw-EPR spectra, upon TAR binding to various small molecule ligands (I: Hoechst; II: DAPI; III: berenil; IV: CGP40336A; V: neomycin; VI: guanidine neomycin; VII: arginiamide). Figure adopted from Ref. 19 with permission.
Further studies were carried out to investigate how the TAR RNA dynamic features vary as it binds to various ligands, including small molecules65, peptides64, 67, and metal ions64, 66. These studies used lineshape parameters (central-line width or effective hyperfine splitting) obtained from the four labeled sites to construct RNA “dynamic signatures”, which were used to assess intrinsic dynamics of the TAR RNA and their responses to various ligands. For example, investigations of small molecule inhibitors65 (Figure 6C) revealed different patterns of dynamic signature for multicyclic dyes (Hoechst, DAPI and berenil) that binds in the major groove pocket created by the trinucleotide bulge; the antiviral CGP 40336A compound that binds to the G26•C39 base pair without distorting the RNA; neomycin that binds to the minor groove of the lower stem; and argininamide that mimics the Tat protein and induces a conformational change at the trinucleotide bulge. The data suggest that each particular class of RNA-ligand complexes may have a specific pattern of intrinsic dynamics. As summarized in a recent review,68 SDSL/EPR studies on TAR not only yielded results that are consistent or later confirmed by other studies (e.g., the neomycin binding site also revealed by solution NMR69), but also provided unique insights on ligand interactions (e.g., identifying binding sites for a new ligand, guanidino neomycin65).
SDSL studies of riboswitches
Riboswitches are cis-acting RNA regulatory elements most commonly found in the 5′-untranslated region of bacterial mRNAs. They consist of two domains: a highly structured aptamer domain that binds to the cognate ligand with high affinity and specificity; and an expression platform that transduces the binding event into a change in the structure of downstream sequence and affects gene expression.70 Towards understanding ligand-dependent RNA conformational dynamics that serves as the linchpin of riboswitch function, a variety of biophysical techniques, including EPR and spin labeling, have been applied to study conformational switches in riboswitch aptamer domain in response to ligand binding.
Prisner and co-workers applied EPR techniques to investigate inter-conversions between conformational states of a 27-nucleotide neoymycin-responsive riboswitch that is generated by a combination of in vitro selection and in vivo screening.71 The nitroxide derivative 2,2,5,5-tetramethyl-pyrrolin-1-oxyl-3-acetylene was labeled at specific uridines. The global conformation of the riboswitch, assessed by pulsed EPR measurement of inter-nitroxide distances ranging from 20 – 40 Å, was found to be unchanged upon ligand binding. However, dynamics of nucleotides within the binding pocket, as assessed by dynamics of singly-labeled nitroxides, were observed to vary in response to ligand binding. Taken together, the EPR data indicate that neoymycin may bind to a pre-organized RNA binding pocket via a conformational selection mechanism. Similar conclusions were drawn from NMR studies.72
Steinhoff and co-workers recently reported SDSL studies of a synthetic tetracycline aptamer, which has been used as an RNA switch for conditional gene expression.40 Nitroxides were attached via post-synthetic modification schemes to selective 2′-positions of the sugar (Figure 2, R2) and C4-postions of uridines (Figure 2, R3(i)), and RNA global conformations were monitored via inter-spin distance measurements. The data suggest that the free aptamer populates at least two conformations. One of them is trapped in the presence of tetracycline, and the resulting ligand-bound apatmer conformation resembles the one observed in the X-ray structure. The study thus provides another example in which the conformational capture mechanism is used in RNA/ligand recognition.
SDSL studies of RNA/protein complexes
In addition to the Tat-TAR system, SDSL has been applied to study a number of RNA/peptide and RNA/protein complexes. The Scholes group performed a series of studies to understand interactions between the 20-mer HIV-I RNA stem loop 3 and the HIV-1 nucleocapsid Zn-finger protein (NCp7) (Figure 7).73, 74 A nitroxide attached to the 5′ terminus of stem loop 3 was used to report the overall tumbling dynamics of the RNA as it interacts with NCp7. The data reveal the NCp7 stoichiometry within the protein/RNA complex, which varies depending on NCp7 concentrations, ionic conditions, and the presence of the Zn2+ ion. Importantly, NCp7/RNA association kinetics were studied from milli-seconds to seconds using a specialized micro-mixer stopped-flow EPR system, and the results uncovered multiple kinetic events consistent with an initial rapid NCp7/RNA binding followed by a slower complex forming process (Figure 7C).73
Figure 7.

SDSL studies of NCp7 protein binding to HIV-I RNA stem loop 3. (A) Schematics of NCp7/RNA complex formation. (B) Detection of NCp7/RNA complex formation by NCp7 induced X-band EPR spectral broadening, which reports reduction of the overall tumbling of the nitroxide labeled RNA stem loop. (C) Stopped-flow measurements of EPR spectral amplitude reveal a bi-phasic behavior during formation of the NCp7/RNA complex. Figure adopted from Ref. 73 with permission.
Qin and co-workers recently reported studies of conformational distributions at the interface between a 22-amino acid peptide (called the N-peptide) and a stem-loop RNA element (called boxB) in bacteriophage λ.75 The N-peptide/boxB complex plays a critical role in transcriptional anti-termination, and has been used as a paradigm for understanding mechanisms of protein/RNA recognition. By monitoring X-band cw-EPR spectra of nitroxides attached at different locations of the N-peptide, it was concluded that the C-terminal fragment of bound N-peptide adopts multiple discrete conformations that are in slow exchange at the nanosecond timescale. The results support a dynamic two-state model of N-peptide/boxB recognition that was previously proposed based on studies monitoring picosecond fluorescence decay of labels attached to the boxB RNA, and demonstrate a connection between picosecond and nanosecond dynamics in a biological complex.
Conclusion
Available reports in the literature have demonstrated the ability of SDSL for providing information on various aspects of RNA dynamics that is complementary and, in some cases inaccessible, to other methods. Further developments, such as paramagnetic relaxation enhancement76 and orientation dependent measurement,77 promise to open up many more opportunities for probing RNA dynamics and connecting dynamics to structure and function.
Acknowledgments
The authors would like to acknowledge support from the National Institute of Health (GM069557) and the National Science Foundation (MCB 054652).
Contributor Information
Phuong Nguyen, Department of Chemistry, University of Southern California, Los Angeles, California 90089-0744, USA.
Peter Z. Qin, Email: pzq@usc.edu, Department of Chemistry, University of Southern California, Los Angeles, California 90089-0744, USA.
References
- 1.Gesteland RF, Atkins JF, Cech TR, editors. RNA world. 3rd. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2006. [Google Scholar]
- 2.Holbrook SR. Structural Principles From Large RNAs. Annual Review of Biophysics. 2008;37:445–464. doi: 10.1146/annurev.biophys.36.040306.132755. [DOI] [PubMed] [Google Scholar]
- 3.Al-Hashimi HM. Dynamics-Based Amplification of RNA Function and Its Characterization by Using NMR Spectroscopy. ChemBioChem. 2005;6:1506–1519. doi: 10.1002/cbic.200500002. [DOI] [PubMed] [Google Scholar]
- 4.Shajani Z, Deka P, Varani G. Decoding RNA motional codes. Trends Biochem Sci. 2006;31:421–424. doi: 10.1016/j.tibs.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Hall KB. RNA in motion. Current Opinion in Chemical Biology. 2008;12:612–618. doi: 10.1016/j.cbpa.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Hashimi HM, Walter NG. RNA dynamics: it is about time. Curr Opin Struct Biol. 2008;18:321–329. doi: 10.1016/j.sbi.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia T. Taking femtosecond snapshots of RNA conformational dynamics and complexity. Current Opinion in Chemical Biology. 2008;12:604–611. doi: 10.1016/j.cbpa.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 8.Bevilacqua PC, Russell R. Editorial overview: exploring the vast dynamic range of RNA dynamics. Current Opinion in Chemical Biology. 2008;12:601–603. doi: 10.1016/j.cbpa.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walter NG. The blessing and curse of RNA dynamics: past, present, and future. Methods. 2009;49:85–86. doi: 10.1016/j.ymeth.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Alemán EA, Lamichhane R, Rueda D. Exploring RNA folding one molecule at a time. Current Opinion in Chemical Biology. 2008;12:647–654. doi: 10.1016/j.cbpa.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Altenbach C, Flitsch SL, Khorana HG, Hubbell WL. Structural studies on transmembrane proteins. 2.Spin labeling of bacteriorhodopsin mutants at unique cysteines. Biochemistry. 1989;28:7806–7812. doi: 10.1021/bi00445a042. [DOI] [PubMed] [Google Scholar]
- 12.Sowa GZ, Qin PZ. Site-directed spin labeling studies on nucleic acid structure and dynamics. Prog Nucleic Acids Res Mol Biol. 2008;82:147–197. doi: 10.1016/S0079-6603(08)00005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Cekan P, Sigurdsson ST, Qin PZ. Studying RNA using site-directed spin-labeling and continuous-wave electron paramagnetic resonance spectroscopy. Method Enzymol. 2009;469:303–328. doi: 10.1016/S0076-6879(09)69015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiemann O. Methods in Enzymology. Vol. 469. Academic Press; 2009. Mapping Global Folds of Oligonucleotides by Pulsed Electron-Electron Double Resonance; pp. 329–351. [DOI] [PubMed] [Google Scholar]
- 15.Hunsicker-Wang L, Vogt M, Derose VJ. EPR methods to study specific metal-ion binding sites in RNA. Methods Enzymol. 2009;468:335–367. doi: 10.1016/S0076-6879(09)68016-2. [DOI] [PubMed] [Google Scholar]
- 16.Hubbell WL, Cafiso DS, Altenbach C. Identifying conformational changes with site-directed spin labeling. Nat Struct Biol. 2000;7:735–739. doi: 10.1038/78956. [DOI] [PubMed] [Google Scholar]
- 17.Fanucci GE, Cafiso DS. Recent Advances and applications of site-directed spin labeling. Curr Opin Struct Biol. 2006;16:644–653. doi: 10.1016/j.sbi.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Klug CS, Feix JB. Methods and applicants of site-directed spin labeling EPR spectroscopy. Methods in Cell Biology. 2008;84:617–658. doi: 10.1016/S0091-679X(07)84020-9. [DOI] [PubMed] [Google Scholar]
- 19.Qin PZ, Dieckmann T. Application of NMR and EPR methods to the study of RNA. Curr Opin Struct Biol. 2004;14:350–359. doi: 10.1016/j.sbi.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Shelke SA, Sigurdsson ST. Noncovalent and site-directed spin labeling of nucleic acids. Angew Chem Int Ed Engl. 2010;49:7984–7986. doi: 10.1002/anie.201002637. [DOI] [PubMed] [Google Scholar]
- 21.Grant GPG, Qin PZ. A facile method for attaching nitroxide spin labels at the 5′ terminus of nucleic acids. Nucl Acids Res. 2007;35:e77. doi: 10.1093/nar/gkm240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Obeid S, Yulikov M, Jeschke G, Marx A. Enzymatic Synthesis of Multiple Spin-Labeled DNA. Angewandte Chemie International Edition. 2008;47:6782–6785. doi: 10.1002/anie.200802314. [DOI] [PubMed] [Google Scholar]
- 23.Qin PZ, Haworth IS, Cai Q, Kusnetzow AK, Grant GPG, Price EA, Sowa GZ, Popova A, Herreros B, He H. Measuring nanometer distances in nucleic acids using a sequence-independent nitroxide probe. Nat Protocols. 2007;2:2354–2365. doi: 10.1038/nprot.2007.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edwards TE, Sigurdsson ST. Site-specific incorporation of nitroxide spin-labels into 2′-positions of nucleic acids. Nature Protocols. 2007;2:1954–1962. doi: 10.1038/nprot.2007.273. [DOI] [PubMed] [Google Scholar]
- 25.Kim N, Murali A, DeRose VJ. A distance ruler for RNA using EPR and site-directed spin labeling. Chem Biol. 2004;11:939–948. doi: 10.1016/j.chembiol.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Ramos A, Varani G. A new method to detect long-range protein-RNA contacts: NMR detection of electron-proton relaxation induced by nitroxide spin-labeled RNA. J Am Chem Soc. 1998;120:10992–10993. [Google Scholar]
- 27.Qin PZ, Hideg K, Feigon J, Hubbell WL. Monitoring RNA base structure and dynamics using site-directed spin labeling. Biochemistry. 2003;42:6772–6783. doi: 10.1021/bi027222p. [DOI] [PubMed] [Google Scholar]
- 28.Jakobsen U, Shelke SA, Vogel S, Sigurdsson ST. Site-directed spin-labeling of nucleic acids by click chemistry: detection of abasic sites in duplex DNA by EPR spectroscopy. J Am Chem Soc. 2010;132:10424–10428. doi: 10.1021/ja102797k. [DOI] [PubMed] [Google Scholar]
- 29.Barhate N, Cekan P, Massey Archna P, Sigurdsson Snorri T. A Nucleoside That Contains a Rigid Nitroxide Spin Label: A Fluorophore in Disguise. Angew Chem Int Ed. 2007;46:2655–2658. doi: 10.1002/anie.200603993. [DOI] [PubMed] [Google Scholar]
- 30.Schiemann O, Piton N, Plackmeyer J, Bode BE, Prisner TF, Engels JW. Spin labeling of oligonucleotides with the nitroxide TPA and use of PELDOR, a pulse EPR method, to measure intramolecular distances. Nat Protoc. 2007;2:904–923. doi: 10.1038/nprot.2007.97. [DOI] [PubMed] [Google Scholar]
- 31.Piton N, Mu Y, Stock G, Prisner TF, Schiemann O, Engels JW. Base-specific spin-labeling of RNA for structure determination. Nucl Acids Res. 2007;35:3128–3143. doi: 10.1093/nar/gkm169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sicoli G, Wachowius F, Bennati M, Hobartner C. Probing secondary structures of spin-labeled RNA by pulsed EPR spectroscopy. Angew Chem Int Ed Engl. 2010;49:6443–6447. doi: 10.1002/anie.201000713. [DOI] [PubMed] [Google Scholar]
- 33.Schweiger A, Jeschke G. Principles of pulse electron paramagnetic resonance. Oxford: Oxford University Press; 2001. [Google Scholar]
- 34.Schiemann O, Prisner TF. Long-range distance determinations in biomacromolecules by EPR spectroscopy. Q Rev Biophys. 2007;40:1–53. doi: 10.1017/S003358350700460X. [DOI] [PubMed] [Google Scholar]
- 35.Krstic I, Hansel R, Romainczyk O, Engels JW, Dotsch V, Prisner TF. Long-Range Distance Measurements on Nucleic Acids in Cells by Pulsed EPR Spectroscopy. Angew Chem Int Ed Engl. 2011;50:5070–5074. doi: 10.1002/anie.201100886. [DOI] [PubMed] [Google Scholar]
- 36.Schiemann O, Weber A, Edwards TE, Prisner TF, Sigurdsson ST. Nanometer distance measurements on RNA using PELDOR. J Am Chem Soc. 2003;125:3334–3335. doi: 10.1021/ja0274610. [DOI] [PubMed] [Google Scholar]
- 37.Cai Q, Kusnetzow AK, Hideg K, Price EA, Haworth IS, Qin PZ. Nanometer Distance Measurements in RNA Using Site-Directed Spin Labeling. Biophys J. 2007;93:2110–2117. doi: 10.1529/biophysj.107.109439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romainczyk O, Endeward B, Prisner TF, Engels JW. The RNA-DNA hybrid structure determined by EPR, CD and RNase H1. Mol Biosyst. 2011 doi: 10.1039/c0mb00258e. [DOI] [PubMed] [Google Scholar]
- 39.Kim NK, Bowman MK, DeRose VJ. Precise mapping of RNA tertiary structure via nanometer distance measurements with double electron-electron resonance spectroscopy. J Am Chem Soc. 2010;132:8882–8884. doi: 10.1021/ja101317g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wunnicke D, Strohbach D, Weigand JE, Appel B, Feresin E, Suess B, Muller S, Steinhoff HJ. Ligand-induced conformational capture of a synthetic tetracycline riboswitch revealed by pulse EPR. Rna. 2011;17:182–188. doi: 10.1261/rna.2222811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakano S, Wu L, Oka H, Karimata HT, Kirihata T, Sato Y, Fujii S, Sakai H, Kuwahara M, Sawai H, et al. Conformation and the sodium ion condensation on DNA and RNA structures in the presence of a neutral cosolute as a mimic of the intracellular media. Mol Biosyst. 2008;4:579–588. doi: 10.1039/b718806d. [DOI] [PubMed] [Google Scholar]
- 42.Popova AM, Kálai T, Hideg K, Qin PZ. Site-Specific DNA Structural and Dynamic Features Revealed by Nucleotide-Independent Nitroxide Probes. Biochemistry. 2009;48:8540–8550. doi: 10.1021/bi900860w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Popova AM, Qin PZ. A nucleotide-independent nitroxide probe reports on site-specific stereomeric environment in DNA. Biophysical Journal. 2010;99:2180–2189. doi: 10.1016/j.bpj.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cekan P, Jonsson EO, Sigurdsson ST. Folding of the cocaine aptamer studied by EPR and fluorescence spectroscopies using the bifunctional spectroscopic probe C. Nucleic Acids Res. 2009;37:3990–3995. doi: 10.1093/nar/gkp277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cekan P, Sigurdsson ST. Identification of single-base mismatches in duplex DNA by EPR spectroscopy. J Am Chem Soc. 2009;131:18054–18056. doi: 10.1021/ja905623k. [DOI] [PubMed] [Google Scholar]
- 46.Sicoli G, Mathis G, Aci-Seche S, Saint-Pierre C, Boulard Y, Gasparutto D, Gambarelli S. Lesion-induced DNA weak structural changes detected by pulsed EPR spectroscopy combined with site-directed spin labelling. Nucleic Acids Res. 2009;37:3165–3176. doi: 10.1093/nar/gkp165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith AL, Cekan P, Brewood GP, Okonogi TM, Alemayehu S, Hustedt EJ, Benight AS, Sigurdsson ST, Robinson BH. Conformational Equilibria of Bulged Sites in Duplex DNA Studied by EPR Spectroscopy. J Phys Chem B. 2009;113:2664–2675. doi: 10.1021/jp808260b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh V, Azarkh M, Exner TE, Hartig JS, Drescher M. Human telomeric quadruplex conformations studied by pulsed EPR. Angew Chem Int Ed Engl. 2009;48:9728–9730. doi: 10.1002/anie.200902146. [DOI] [PubMed] [Google Scholar]
- 49.Hougland JL, Piccirilli JA, Forconi M, Lee J, Herschlag D. How the Group I Intron Works: A Case Study of RNA Structure and Function. In: Gesteland RF, Cech TR, Atkins JF, editors. RNA World. 3rd. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2006. pp. 133–205. [Google Scholar]
- 50.Grant GPG, Boyd N, Herschlag D, Qin PZ. Motions of the Substrate Recognition Duplex in a Group I Intron Assessed by Site-Directed Spin Labeling. J Am Chem Soc. 2009;131:3136–3137. doi: 10.1021/ja808217s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qin PZ, Butcher SE, Feigon J, Hubbell WL. Quantitative analysis of the GAAA tetraloop/receptor interaction in solution: A site-directed spin labeling study. Biochemistry. 2001;40:6929–6936. doi: 10.1021/bi010294g. [DOI] [PubMed] [Google Scholar]
- 52.Bartley LE, Zhuang X, Das R, Chu S, Herschlag D. Exploration of the Transition State for Tertiary Structure Formation between an RNA Helix and a Large Structured RNA. J Mol Biol. 2003;328:1011–1026. doi: 10.1016/s0022-2836(03)00272-9. [DOI] [PubMed] [Google Scholar]
- 53.Shi X, Mollova ET, Pljevaljčić G, Millar DP, Herschlag D. Probing the Dynamics of the P1 Helix within the Tetrahymena Group I Intron. Journal of the American Chemical Society. 2009;131:9571–9578. doi: 10.1021/ja902797j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu Q, Huang L, Zhang Y. The structure and function of catalytic RNAs. Science in China Series C: Life Sciences. 2009;52:232–244. doi: 10.1007/s11427-009-0038-z. [DOI] [PubMed] [Google Scholar]
- 55.Scott WG, Martick M, Chi YI. Structure and function of regulatory RNA elements: Ribozymes that regulate gene expression. Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms. 2009;1789:634–641. doi: 10.1016/j.bbagrm.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 56.de la Pena M, Garcia-Robles I. Ubiquitous presence of the hammerhead ribozyme motif along the tree of life. RNA. 2010;16:1943–1950. doi: 10.1261/rna.2130310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martick M, Horan LH, Noller HF, Scott WG. A discontinuous hammerhead ribozyme embedded in a mammalian messenger RNA. Nature. 2008;454:899–902. doi: 10.1038/nature07117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim NK, Murali A, DeRose VJ. Separate metal requirements for loop interactions and catalysis in the extended hammerhead ribozyme. J Am Chem Soc. 2005;127:14134–14135. doi: 10.1021/ja0541027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Edwards TE, Sigurdsson ST. EPR spectroscopic analysis of U7 hammerhead ribozyme dynamics during metal ion induced folding. Biochemistry. 2005;44:12870–12878. doi: 10.1021/bi050549g. [DOI] [PubMed] [Google Scholar]
- 60.Qin PZ, Feigon J, Hubbell WL. Site-directed spin labeling studies reveal solution conformational changes in a GAAA tetraloop receptor upon Mg2+-dependent docking of a GAAA tetraloop. J Mol Biol. 2005;351:1–8. doi: 10.1016/j.jmb.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 61.Jaeger L, Michel F, Westhof E. Involvement of a GNRA tetraloop in long-range RNA tertiary interactions. J Mol Biol. 1994;236:1271–1276. doi: 10.1016/0022-2836(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 62.Frankel AD, Young JAT. HIV-1: fifteen proteins and an RNA. Annu Rev Biochem. 1998;67:1–25. doi: 10.1146/annurev.biochem.67.1.1. [DOI] [PubMed] [Google Scholar]
- 63.Edwards TE, Okonogi TM, Robinson BH, Sigurdsson ST. Site-specific incorporation of nitroxide spin-labels into internal sites of the TAR RNA. Structure-dependent dynamics of RNA by EPR spectroscopy. J Am Chem Soc. 2001;123:1527–1528. doi: 10.1021/ja005649i. [DOI] [PubMed] [Google Scholar]
- 64.Edwards TE, Okonogi TM, Sigurdsson ST. Investigation of RNA-protein and RNA-metal ion interactions by electron paramagnetic resonance spectroscopy: The HIV TAR-Tat motif. Chem Biol. 2002;9:699–706. doi: 10.1016/s1074-5521(02)00150-3. [DOI] [PubMed] [Google Scholar]
- 65.Edwards TE, Sigurdsson ST. Electron paramagnetic resonance dynamic signatures of TAR RNA-small molecule complexes provide insight into RNA structure and recognition. Biochemistry. 2002;41:14843–14847. doi: 10.1021/bi026299a. [DOI] [PubMed] [Google Scholar]
- 66.Edwards TE, Sigurdsson ST. EPR spectroscopic analysis of TAR RNA-metal ion interactions. Biochem Biophys Res Commun. 2003;303:721–725. doi: 10.1016/s0006-291x(03)00411-x. [DOI] [PubMed] [Google Scholar]
- 67.Edwards TE, Robinson BH, Sigurdsson ST. Identification of amino acids that promote specific and rigid TAR RNA-Tat protein complex formation. Chem Biol. 2005;12:329–337. doi: 10.1016/j.chembiol.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 68.Sigurdsson ST. EPR spectroscopy for the study of RNA-ligand interactions. In: Wanunu M, Tor Y, editors. Methods for Studying Nucleic Acid/Drug Interactions. Francis and Taylor; 2011. in press. [Google Scholar]
- 69.Faber C, Sticht H, Schweimer K, Rosch P. Structural rearrangements of HIV-1 Tat-responsive RNA upon binding of neomycin B. J Biol Chem. 2000;275:20660–20666. doi: 10.1074/jbc.M000920200. [DOI] [PubMed] [Google Scholar]
- 70.Serganov A. The long and the short of riboswitches. Current Opinion in Structural Biology. 2009;19:251–259. doi: 10.1016/j.sbi.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krstic I, Frolow O, Sezer D, Endeward B, Weigand JE, Suess B, Engels JW, Prisner TF. PELDOR spectroscopy reveals preorganization of the neomycin-responsive riboswitch tertiary structure. J Am Chem Soc. 2010;132:1454–1455. doi: 10.1021/ja9077914. [DOI] [PubMed] [Google Scholar]
- 72.Duchardt-Ferner E, Weigand JE, Ohlenschläger O, Schmidtke SR, Suess B, Wöhnert J. Highly Modular Structure and Ligand Binding by Conformational Capture in a Minimalistic Riboswitch. Angewandte Chemie International Edition. 2010;49:6216–6219. doi: 10.1002/anie.201001339. [DOI] [PubMed] [Google Scholar]
- 73.Xi X, Sun Y, Karim CB, Grigoryants VM, Scholes CP. HIV-1 Nucleocapsid Protein NCp7 and Its RNA Stem Loop 3 Partner: Rotational Dynamics of Spin-Labeled RNA Stem Loop 3. Biochemistry. 2008;47:10099–10110. doi: 10.1021/bi800602e. [DOI] [PubMed] [Google Scholar]
- 74.Zhiwen Z, Xiangmei X, Charles PS, Christine BK. Rotational dynamics of HIV-1 nucleocapsid protein NCp7 as probed by a spin label attached by peptide synthesis. Biopolymers. 2008;89:1125–1135. doi: 10.1002/bip.21064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang X, Lee SW, Zhao L, Xia T, Qin PZ. Conformational distributions at the N-peptide/boxB RNA interface studied using site-directed spin labeling. RNA. 2010;16:2474–2483. doi: 10.1261/rna.2360610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leulliot v, Quevillon-Cheruel S, Graille M, van Tilbeurgh H, Leeper TC, Godin KS, Edwards TE, Sigurdsson ST, Rozenkrants N, Nagel RJ, et al. A new alpha-helical extension promotes RNA binding by the dsRBD of Rnt1p RNAse III. EMBO J. 2004;23:2468–2477. doi: 10.1038/sj.emboj.7600260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schiemann O, Cekan P, Margraf D, Prisner TF, Sigurdsson ST. Relative orientation of rigid nitroxides by PELDOR: beyond distance measurements in nucleic acids. Angew Chem Int Ed Engl. 2009;48:3292–3295. doi: 10.1002/anie.200805152. [DOI] [PubMed] [Google Scholar]


